Celiac disease (CD) is a complex intestinal disorder with autoimmune features that develops in genetically susceptible individuals expressing HLA-DQ8 or HLA-DQ2 molecules.1 The presence of anti–tissue transglutaminase 2 (TG2) and anti–deamidated gluten peptide antibodies represent strong disease markers.2 Inflammation and villous blunting in the small intestine result from an abnormal immune response to gluten proteins. CD is characterized by the loss of oral tolerance to gluten manifested by HLA-DQ2– or HLA-DQ8–restricted antigluten inflammatory CD4 T cells and a massive expansion of cytotoxic CD8+ intraepithelial lymphocytes (IELs) that mediate the killing of intestinal epithelial cells (IECs).3 These observations have led to the general idea that CD is primarily a T-cell–mediated immune disorder. However, studies in human suggest that B cells could play a central role in CD pathogenesis.4 We have recently engineered a mouse model of CD that develops villous atrophy (VA) in a gluten, HLA-DQ8, and TG2-dependent manner. Intestinal tissue destruction in this model is also dependent on CD4+ and CD8+ T cells and requires interferon-γ. We now demonstrate that B cells are required for the development of VA and the associated full licensing of cytotoxic IELs in this pathophysiologically relevant mouse model of CD, providing support for the exploration of B-cell–directed therapies for the treatment of CD.
Methods
DQ8-Dd-villin-IL-15tg mice developing the main features of CD upon ingestion of gluten3 and DQ8-Dd-villin-IL-15tg crossed with muMT mice lacking mature B cells were used. B-Cell depletion was achieved by treating DQ8-Dd-villin-IL-15tg mice with 250 μg anti–mouse CD20 (clone 5D2, isotype IgG2a; Genentech). Villous height-to-crypt depth ratio was quantitatively determined on cross-sections of the terminal ileum, which is the primary site where VA develops in our mouse model.3 The acquisition of cytotoxic properties by IELs was assessed by measuring the expression of the activating natural killer cell (NK) receptor NKG2D, and cytolytic granules (granzyme B, perforin) in IELs with the use of flow cytometry and quantitative polymerase chain reaction.3 Detailed methods are described in the Supplementary Methods.
Results
To determine whether B cells were required for the development of tissue damage, we analyzed the development of VA in our DQ8-Dd-villin-IL-15tg mouse model maintained on a gluten-free diet (sham), fed with gluten, or fed with gluten and treated with an anti-CD20 depleting antibody (Supplementary Figure 1 and Figure 1A and B). Despite B-cell depletion (Supplementary Figure 1B–D) preventing the development of antigluten IgA and IgG antibodies upon introduction of gluten (Figure 1E and F), intestinal IgA-producing plasma cells (Supplementary Figure 1G) were still present after anti-CD20 treatment. Strikingly, the development of VA was impaired when B cells were depleted in gluten-fed mice (Figure 1A and B) demonstrating the requirement of gluten-specific humoral responses to promote a pathogenic response. Likewise, the development of VA was abrogated in DQ8-Dd-villin-IL-15tg-muMT mice lacking mature B cells (Supplementary Figure 2A and B).
Figure 1.
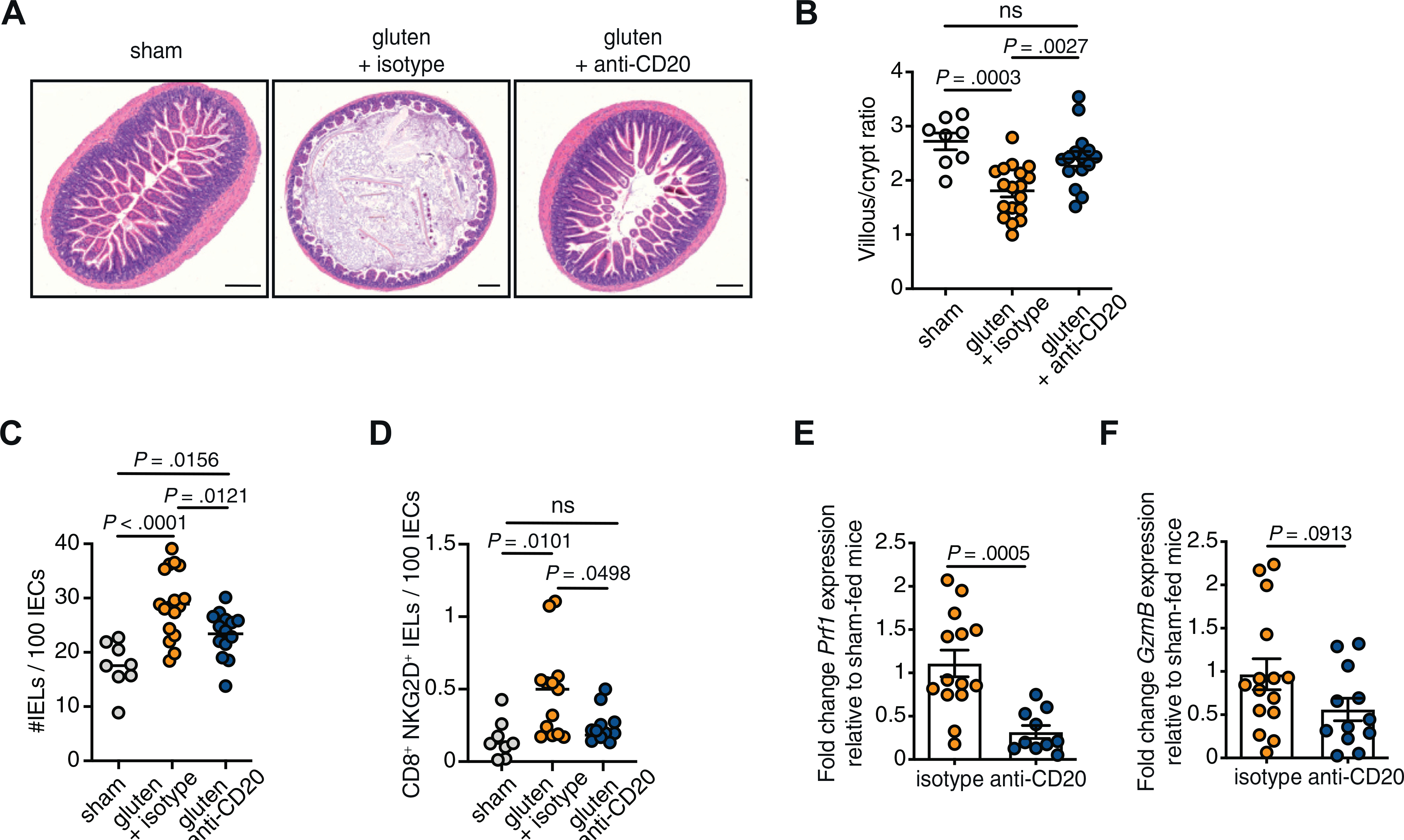
The development of villous atrophy and the acquisition of cytotoxic properties by intraepithelial CD8+ T cells in DQ8-Dd-villin-IL-15tg mice is impaired in the absence of B cells. DQ8-Dd-villin-IL-15tg mice were maintained on a gluten free diet (sham), or fed with gluten every other day for 30 days and treated with 250 μg anti-CD20 antibody (gluten + anti-CD20) or its isotype control (gluten + isotype) every 2 weeks. (A) Hematoxylin-stained ileum sections showing villous atrophy—as evidenced by villous height-to-crypt depth ratio ≤2—in the gluten-fed DQ8-Dd-villin-IL-15tg mice. Scale bar = 250 μm. (B) Morphometric assessment of the villous height-to-crypt depth ratio demonstrating villous atrophy in gluten-fed mice (ratio ≤2) compared with sham-fed mice and gluten-fed mice treated with the anti-CD20 antibody. (C) Quantification of intraepithelial lymphocytes (IELs) among intestinal epithelial cells (IECs); mean. (D) The intestinal epithelium was isolated and analyzed by flow cytometry. IELs were identified as TCRβ+ CD4− CD8+ cells, and the frequency of CD8+ NKG2D+ TCRαβ+ cells were obtained. The total amount of IELs per 100 IECs was determined on hematoxylin and eosin–stained slides. NKG2D+ NKG2− IELs are indicated by absolute number per 100 IECs. (E) The expression of perforin (prf1) in the epithelial compartment was measured by quantitative polymerase chain reaction. Relative expression levels in gluten-fed mice were normalized to the expression levels observed in sham-fed mice. (F) The expression of granzyme B (GzmB) was measured as in E. Data are representative of 5 (B, C) or 4 (D–F) independent experiments, shown as mean ± SEM; ANOVA/Tukey multiple comparison was performed for B, C, and D. Unpaired Student t test was used for E and F.
Given that ablation of B cells significantly reduced the development of villous atrophy and that studies in humans and in DQ8-Dd-villin-IL-15tg mice3 have demonstrated that IELs infiltrating the celiac lesion are critically involved in tissue damage, we next assessed the impact of B-cell depletion on IELs. As shown in Figure 1C, the reduction of intestinal tissue damage in the absence of B lymphocytes was associated with a decrease in the number of IELs. Intestinal tissue destruction arises from the killing of IECs by IELs that have acquired a cytolytic phenotype. We found that the amount of cytotoxic CD8+ IELs expressing the activating NK receptor NKG2D in the absence of inhibitory CD94/NKG2A receptors (Figure 1D) as well as the levels of the cytotoxic molecules granzyme B and perforin (Figure 1E and F and Supplementary Figure 2C and D) were decreased in mice lacking B cells, while the expression of the mouse NKG2D ligand, rae1, and the nonclassical major histocompatibility complex (MHC) class I molecule qa1 remained unchanged (Supplementary Figure 2E and 2F). Altogether, these results indicate that B cells play a role in CD pathogenesis by promoting the cytotoxic potential of IELs and the development of VA.
Discussion
B Cells as antigen-presenting cells have been involved in the development of organ-specific disorders.5 In the context of CD, in vitro assays have shown that such cooperation between B cells and gluten-specific CD4 T cells can take place.4 The strict dependence on gluten exposure for the production of anti-TG2 antibodies suggests that an interaction between gluten-specific CD4+ T cells and TG2-specific B cells having internalized TG2-gluten complexes is required for their generation (as reviewed by Iversen and Sollid4). In addition, a transcriptional B-cell signature was associated with the extent of tissue damage in CD.6 Finally, plasma cells were found to be the most abundant gluten peptide MHC-expressing cells in the lamina propria of CD patients.7
The present study demonstrates that B cells play a role in the activation of IELs and tissue damage, both of which are key features of active CD.1,2 Although B-cell depletion did not restore the villous height-to-crypt depth ratio and the levels of cytotoxic IELs to steady-state level, our results demonstrate that B cells significantly contribute to both the development of VA and the acquisition of cytotoxic properties by CD8+ IELs, which are known to be responsible for enterocytes cytolysis. These results in combination with our previous work highlighting a role for TG2 activation and CD4+ T cells in promoting tissue damage and IEL activation,3 suggest that B cells may be involved in the amplification of the CD4+ T-cell response to a magnitude sufficient to affect the activation of cytotoxic IELs and the ensuing tissue destruction. While providing evidence for a role for B cells in the activation of IELs and tissue damage, this study does not rule out that antibodies also could play a role in CD pathogenesis.8 Interestingly, the critical role of antigen presentation by B cells to CD4+ T cells for enhancing destructive immune reactions was also suggested in the context of experimental autoimmune encephalomyelitis and lupus.5 Future studies will determine the role of HLA-DQ2/DQ8 expression on B cells and assess their role as antigen-presenting cells in CD pathogenesis.
Supplementary Material
Acknowledgments
Funding
This work was supported by a grant from the SickKids Foundation (NI15–040), a Canadian Institute of Health Research operating grant (376783), a Pilot and Feasibility award from the Digestive Diseases Research Core Center (P30 DK42086), and a Young Investigator Award from the Celiac Disease Foundation to Valérie Abadie, and awards from the Wallonie-Bruxelles International-World Excellence and the Fonds de Recherche du Québec—Nature et Technologies to Thomas Lejeune.
Abbreviations used in this paper:
- CD
celiac disease
- IELs
intraepithelial lymphocytes
- IECs
intestinal epithelial cells
- TG2
tissue transglutaminase 2
- VA
villous atrophy
Footnotes
Conflicts of Interest
The authors declare no conflicts.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2021.02.063.
References
- 1.Abadie V, Sollid LM, Barreiro LB, et al. Integration of genetic and immunological insights into a model of celiac disease pathogenesis. Annu Rev Immunol 2011;29:493–525. [DOI] [PubMed] [Google Scholar]
- 2.Husby S, Koletzko S, Korponay-Szabo I, et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J Pediatr Gastroenterol Nutr 2020;70:141–156. [DOI] [PubMed] [Google Scholar]
- 3.Abadie V, Kim SM, Lejeune T, et al. IL-15, gluten and HLA-DQ8 drive tissue destruction in coeliac disease. Nature 2020;578:600–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iversen R, Sollid LM. Autoimmunity provoked by foreign antigens. Science 2020;368:132–133. [DOI] [PubMed] [Google Scholar]
- 5.Getahun A, Cambier JC. Non–antibody-secreting functions of b cells and their contribution to autoimmune disease. Annu Rev Cell Dev Biol 2019;35:337–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garber ME, Saldanha A, Parker JS, et al. A B-cell gene signature correlates with the extent of gluten-induced intestinal injury in celiac disease. Cell Mol Gastroenterol Hepatol 2017;4:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoydahl LS, Richter L, Frick R, et al. Plasma cells are the most abundant gluten peptide MHC-expressing cells in inflamed intestinal tissues from patients with celiac disease. Gastroenterology 2019;156:1428–1439.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maglio M, Troncone R. Intestinal anti-tissue transglutaminase2 autoantibodies: pathogenic and clinical implications for celiac disease. Front Nutr 2020;7:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


