Abstract
Purpose:
Several definitive treatment options are available for prostate cancer, but geographic access to those options is not uniform. We created maps illustrating provider practice patterns relation to patients and assessed the influence of distance to treatment receipt.
Patients and Methods:
The patient cohort was created by searching the National Medicare Database for patients diagnosed and treated for prostate cancer in 2011–2014. The provider cohort was created by querying the AMA Physician Masterfile to identify physicians who had treated with prostatectomy, intensity-modulated radiation therapy (IMRT), brachytherapy, stereotactic body radiation therapy (SBRT), or proton therapy. Maps detailing the location of providers were created for each modality. Multivariate multinomial logistic regressions were used to assess the association between patient-provider distance and probability of treatment.
Results:
Cohorts consisted of 89,902 patients treated by 5,518 physicians. Substantial numbers of providers practicing established modalities (IMRT, prostatectomy, and brachytherapy) was noted in major urban centers, whereas provider numbers were reduced in rural areas, most notably for brachytherapy. Ninety percent of prostate cancer patients lived within 35.1, 28.9, and 55.6 miles of a practitioner of prostatectomy, IMRT, and brachytherapy, respectively. Practitioners of emerging modalities (SBRT and proton therapy) were predominantly concentrated in urban locations, with 90% of patients living within 128 miles (SBRT) and 374.5 miles (proton). Greater distance was associated with decreased probability of treatment (IMRT −3.8% per 10 miles; prostatectomy −2.1%; brachytherapy −2%; proton therapy −1.6%; and SBRT −1.1%).
Conclusions:
Geographic disparities were noted for analyzed treatment modalities, and these disparities influenced delivery.
INTRODUCTION
There exists multiple options for the definitive treatment of prostate cancer.(1) Although efficacy has been shown to be equivalent among modalities,(2) evidence supports differences in resultant quality of life.(3–5) A multidisciplinary discussion is generally agreed to be in the patient’s best interests and reduces provider bias in decision-making.(6–9) However, substantial regional differences in treatment utilization have been identified.(10,11) We hypothesized that one of the strongest influences on the choice of prostate cancer treatment is geographic proximity to physicians who practice specific techniques. We analyzed the National Medicare Database coupled with the AMA Physician Masterfile to create a comprehensive, and to the best of our knowledge, the first map visualizing individual physician practice patterns across the United States referenced to the number of prostate cancer patients and then assessed the influence of provider distance on treatment receipt.
PATIENTS AND METHODS
Study Datasets
Study cohorts were derived from national fee-for-service Medicare claims data inclusive of patients with an ICD-9 diagnosis code of prostate cancer (185) from 2011 through 2014. Two cohorts were created, a patient and a provider cohort. For the patient cohort, we identified all patients with prostate cancer diagnosed and treated from 2011 through 2014 with one of five treatment modalities. We then used the patient cohort to create a provider cohort that identified all radiation oncologists and urologists who treated members of the patient cohort. The data were derived from the Chronic Conditions Warehouse, and the study was approved by the Centers for Medicare and Medicaid Privacy Board and our institutional review board.
Patient Cohort Definition
Cohorts of treated prostate cancer patients were defined based on receipt of definitive treatment: (1) prostatectomy, (2) intensity-modulated radiation therapy (IMRT), (3) brachytherapy, (4) stereotactic body radiation therapy (SBRT), and (5) proton therapy. Patients were included in the prostatectomy group if they received definitive prostatectomy regardless of technique, in the IMRT or proton therapy cohort if they received ≥20 fractions within 90 days of starting radiation, in the SBRT cohort if they received ≥3 fractions of SBRT within 30 days of starting radiation, and in the brachytherapy cohort if they received either low- or high-dose-rate brachytherapy. If a patient was coded as receiving prostatectomy and radiation therapy, that patient was coded as receiving prostatectomy. If a patient received IMRT and another radiation therapy modality, then that patient was coded as receiving the other radiation therapy modality. Additional inclusion criteria were receipt of a prostate biopsy from 2011 through 2014 and survival >1 year after biopsy (Supplementary Table S1).
Claims data from the year before diagnosis were used to calculate Charlson comorbidity score, and claims data from the year after diagnosis were used to identify androgen deprivation therapy receipt (Supplementary Table S2).(12)
Provider Cohort Definition
Physician information from the AMA Physician Master File was associated with claims data from the National Medicare Database utilizing National Provider Identification (NPI) numbers. Physician provider cohorts were created to identify individual physicians who treated Medicare patients with one of the five modalities studied (prostatectomy, IMRT, SBRT, brachytherapy, and proton therapy). Physicians who used radiation for IMRT, SBRT, brachytherapy, or proton therapy were identified based on association with RT (radiation therapy) planning claims (Supplementary Table S2) filed ±1 month from radiation treatment start. Physicians performing prostatectomy were identified via association with prostatectomy procedure codes (Supplementary Table S2). All codes were required to have prostate cancer within the first two diagnosis fields and all identified providers must have been listed by the National Medicare Database or AMA master files as specializing in urology or radiation oncology. We considered the treatment of 4 patients with a single modality as the threshold number to identify a physician who practiced that modality. Physician location data was available at the zip code level and plotted on a county-level map of the United States, where a dot was placed in each zip code if at least one physician was identified to practice the treatment of interest. The density of treated prostate cancer patients overall and those treated with individual modalities was assessed at the county level and displayed on maps that concurrently plotted physician location.
Statistical Analysis
Minimum patient-modality distance was defined as the minimum geodetic distance between a treated prostate cancer patient (as defined in the patient cohort section) and the nearest provider practicing a specific modality (as defined in the physician cohort section), using the centroid of the patient and provider zip code(s). When constructing physician provider maps, we included all physicians practicing within the 50 states, but when calculating minimum patient-modality distances, we excluded patients and providers in Alaska and Hawaii. We utilized a multinomial logistic regression to assess the association between minimum patient-modality distance and probability of treatment with that modality adjusted for age at biopsy, year of biopsy, race, Charlson comorbidity score, minimum patient-modality distance for other modalities, and state buy-in.
For visualization purposes minimum patient-modality distance was grouped into intervals, with cutpoints selected based on inspection of the distribution. For each interval, a predicted probability to receive a given treatment was calculated with a 95% confidence interval. To estimate the magnitude of association between distance and the probability of treatment, we created a second multinomial regression model in which distance was treated as an ordinal variable with 5-mile increments. A linear regression was fit to data points from this second model and the slope of the fitted line was calculated to assess the magnitude of association. This analysis was limited to patients living within 60 miles of a practitioner, as 90% of patients treated with the 3 most common modalities (prostatectomy, IMRT, and brachytherapy) lived within this range. Statistical analyses were done with SAS version 9.4 (SAS Institute, Cary, NC), and R version 3.6.1.
RESULTS
The final cohorts included 89,902 patients treated by 5,518 physicians who practiced one (n=4,664) or more (n=854) of the 5 treatment modalities. Patients were predominantly white (85%), and the median age was 71 years (Table 1). The most common treatment modality was IMRT (n=49,788) followed by prostatectomy (n=27,776), brachytherapy (n=8,121), SBRT (n=2,368), and proton therapy (n=1,849). In terms of practitioners, most practiced IMRT (n=2,761) followed by prostatectomy (n=2,674), brachytherapy (n=769), SBRT (n=171), and proton therapy (n=61; Table 2). Practitioners were predominantly male (89%) and trained in the United States (89%).
Table 1.
Baseline Patient Characteristics by Treatment Groups
| Characteristics | Prostatectomy N = 27,776 |
IMRT N = 49,788 |
Brachytherapy N = 8,121 |
Proton N=1,849 |
SBRT N = 2,368 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | |||||
| Age, years | ||||||||||||||
| 66–69 | 14,503 | 52 | 11,594 | 23 | 2,726 | 34 | 640 | 35 | 671 | 28 | ||||
| 70–74 | 10,913 | 39 | 18,727 | 38 | 3,332 | 41 | 760 | 41 | 933 | 39 | ||||
| 75–79 | 2,176 | 8 | 13,494 | 27 | 1,664 | 18 | 337 | 18 | 556 | 23 | ||||
| ≥80 | 184 | 1 | 5,973 | 12 | 399 | 5 | 112 | 6 | 208 | 9 | ||||
| Year of first biopsy | ||||||||||||||
| 2011 | 12,191 | 44 | 20,041 | 40 | 3,827 | 47 | 788 | 43 | 834 | 35 | ||||
| 2012 | 7,160 | 26 | 13,165 | 26 | 2,139 | 26 | 509 | 28 | 620 | 26 | ||||
| 2013 | 5,368 | 19 | 10,640 | 21 | 1,465 | 18 | 358 | 19 | 585 | 25 | ||||
| 2014 | 3,057 | 11 | 5,942 | 12 | 690 | 8 | 194 | 10 | 329 | 14 | ||||
| Race | ||||||||||||||
| White | 24,356 | 88 | 41,216 | 83 | 6,867 | 85 | 1,661 | 90 | 2,049 | 87 | ||||
| Black | 2,215 | 8 | 6,147 | 12 | 931 | 11 | 120 | 6 | 222 | 9 | ||||
| Hispanic | 248 | 1 | 702 | 1 | 43 | 1 | <11 | NAa | 15 | 1 | ||||
| Other | 957 | 3 | 1,723 | 3 | 280 | 3 | >20 | NAa | 82 | 3 | ||||
| Charlson Index | ||||||||||||||
| 0 | 18,634 | 67 | 28,059 | 56 | 4,930 | 61 | 1,245 | 67 | 1,449 | 61 | ||||
| 1 | 4,480 | 16 | 10,409 | 21 | 1,550 | 19 | 278 | 15 | 418 | 18 | ||||
| ≥2 | 1,917 | 7 | 7,340 | 15 | 831 | 10 | 160 | 9 | 316 | 13 | ||||
| Unknown | 2745 | 10 | 3,980 | 8 | 810 | 10 | 166 | 9 | 185 | 8 | ||||
| Region | ||||||||||||||
| New England | 1,288 | 5 | 2,507 | 5 | 337 | 4 | 27 | 1 | 102 | 4 | ||||
| Mid Atlantic | 2,313 | 8 | 7,794 | 16 | 724 | 9 | 141 | 8 | 528 | 22 | ||||
| East North Central | 4,361 | 16 | 7,275 | 15 | 1363 | 17 | 178 | 10 | 251 | 11 | ||||
| West North Central | 2,435 | 9 | 2,879 | 6 | 619 | 8 | 46 | 2 | 120 | 5 | ||||
| South Atlantic | 4,930 | 18 | 11,252 | 23 | 2,263 | 28 | 498 | 27 | 496 | 21 | ||||
| East South Central | 2,385 | 9 | 2,816 | 6 | 696 | 9 | 102 | 6 | 161 | 7 | ||||
| West South Central | 2,906 | 10 | 5,879 | 12 | 469 | 6 | 271 | 15 | 96 | 4 | ||||
| Mountain | 1,821 | 7 | 2,666 | 5 | 341 | 4 | 1115 | 6 | 244 | 10 | ||||
| Pacific | 3,834 | 14 | 4,355 | 9 | 821 | 10 | 362 | 20 | 275 | 12 | ||||
| Unknown | 1,503 | 5 | 2,365 | 5 | 489 | 6 | 109 | 6 | 95 | 4 | ||||
| ADT use | ||||||||||||||
| No | 25,128 | 90 | 25,154 | 51 | 5,439 | 67 | 1,299 | 70 | 1955 | 83 | ||||
| Yes | 2,648 | 10 | 24634 | 49 | 2,682 | 33 | 550 | 30 | 413 | 17 | ||||
| State buy-in | ||||||||||||||
| Partial / no | 26,531 | 96 | 45,846 | 92 | 7,711 | 95 | 1,818 | 98 | 2,255 | 95 | ||||
| Full | 1,245 | 4 | 3,942 | 8 | 410 | 5 | 31 | 2 | 113 | 5 | ||||
Cell sizes <11 have been suppressed in accordance with Centers for Medicare and Medicaid Services privacy policies
Abbreviations: IMRT, intensity-modulated radiation therapy; SBRT, stereotactic body radiation therapy; ADT, androgen deprivation therapy.
Table 2.
Characteristics of Physicians Treating at least 4 Patients with the Indicated Modality from 2011 through 2014
| Characteristics | Prostatectomy N = 2,674 |
IMRT N = 2,761 |
Brachytherapy N = 769 |
Proton N=61 |
SBRT N = 171 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | |||||
| US-trained | ||||||||||||||
| Yes | 2,444 | 91 | 2,375 | 86 | 672 | 87 | 56 | 92 | 151 | 88 | ||||
| No | 230 | 9 | 386 | 14 | 97 | 13 | 5 | 8 | 20 | 12 | ||||
| Board Certified | ||||||||||||||
| Yes | 2,577 | 96 | 2,668 | 97 | 755 | 98 | 60 | 98 | 159 | 99 | ||||
| No | 97 | 4 | 93 | 3 | 14 | 2 | 1 | 2 | 2 | 1 | ||||
| MD or DO | ||||||||||||||
| MD | 2,610 | 98 | 2,708 | 98 | 757 | 98 | 60 | 98 | 169 | 99 | ||||
| DO | 64 | 2 | 53 | 2 | 12 | 2 | 1 | 2 | 2 | 1 | ||||
| Year of Medical School Graduation | ||||||||||||||
| Before 1980 | 393 | 15 | 503 | 18 | 142 | 18 | 5 | 8 | 19 | 11 | ||||
| 1980 – 1989 | 710 | 27 | 908 | 33 | 290 | 38 | 17 | 28 | 56 | 33 | ||||
| After 1990 | 1,571 | 59 | 1,350 | 49 | 337 | 44 | 39 | 64 | 96 | 56 | ||||
| Metro | ||||||||||||||
| Large metro (>1 mill) | 1,474 | 55 | 1,583 | 57 | 420 | 55 | 54 | 89 | 120 | 70 | ||||
| Metro (250K – 1 mill) | 547 | 20 | 540 | 20 | 159 | 21 | 2 | 3 | 24 | 14 | ||||
| Urban-Rural (<250K) | 347 | 13 | 315 | 11 | 108 | 14 | 5 | 8 | 16 | 9 | ||||
| Unknown | 306 | 11 | 323 | 12 | 82 | 11 | 11 | 6 | ||||||
| Region | ||||||||||||||
| Northeast | 360 | 13 | 501 | 18 | 125 | 16 | 11 | 18 | 36 | 21 | ||||
| Midwest | 667 | 25 | 640 | 23 | 176 | 23 | 8 | 13 | 29 | 17 | ||||
| South | 1,005 | 38 | 1,047 | 38 | 333 | 43 | 26 | 43 | 67 | 39 | ||||
| West | 638 | 24 | 564 | 20 | 132 | 17 | 16 | 26 | 39 | 23 | ||||
| Sex | ||||||||||||||
| Male | 2,597 | 97 | 2,253 | 82 | 714 | 93 | 52 | 85 | 148 | 87 | ||||
| Female | 77 | 3 | 508 | 18 | 55 | 7 | 9 | 15 | 23 | 33 | ||||
Abbreviations: IMRT, intensity-modulated radiation therapy; SBRT, stereotactic body radiation therapy.
Physician Practice Map
Geographic distribution of providers was plotted on a map of the United States based on physician zip code in relation to location of patients from the patient cohort (Fig. 1). Multiple providers of IMRT (n=2,761) and prostatectomy (n=2,674) were identified in every state (red dots on Fig. 1). Brachytherapy practitioners were substantially more limited (n=769), with no or limited providers (limited to one metro area) in New Mexico, Alaska, Nevada, and Hawaii (Fig. 1).
Fig. 1. Patient and provider maps showing prostate cancer treatment providers in relationship to all treated prostate cancer patients.
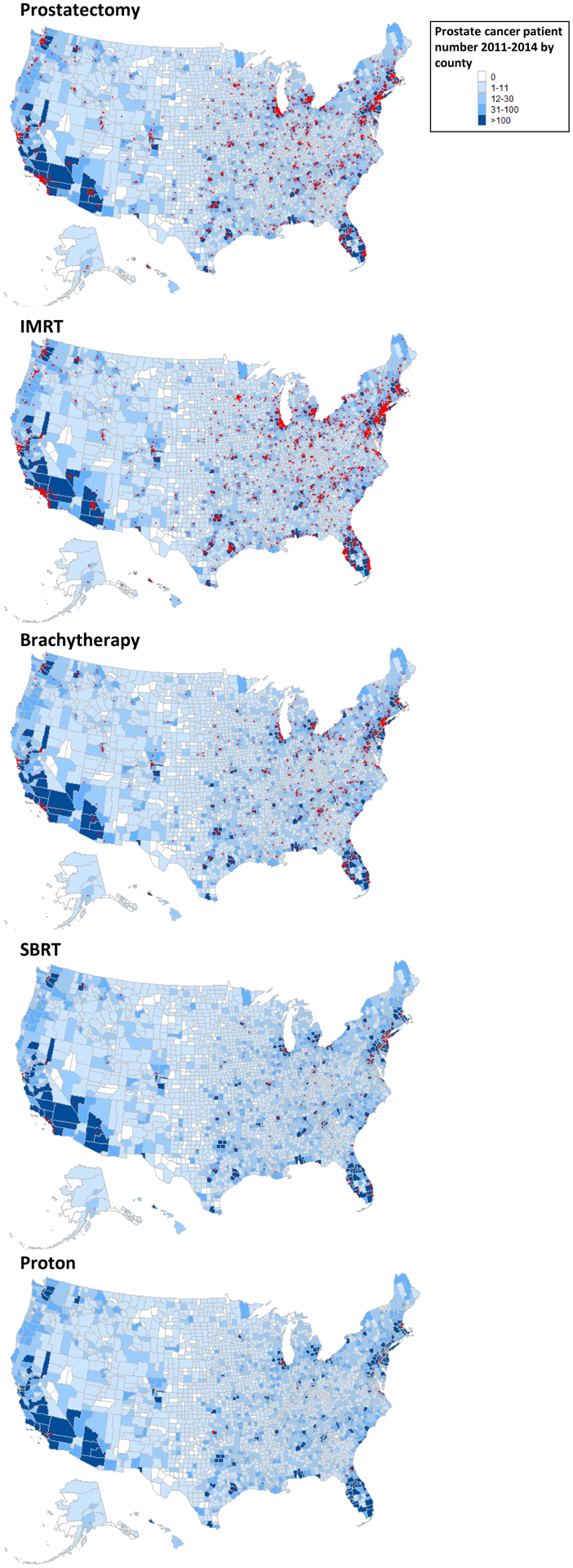
Maps of the United States showing distribution of providers of specific prostate cancer treatment modalities relative to the location of all definitive treated prostate cancer patients at the county level. Counties with a provider utilizing a specific modality are indicated by red dots.
On the county level, 421 counties had 31–100 treated prostate cancer patients, of which 159 counties (37.8%) had no provider of prostatectomy and 108 counties (25.7%) had no provider of IMRT, in comparison with 256 counties (60.8%) that had no brachytherapy providers. When assessing counties with >100 treated prostate cancer patients (n=188), 17 (9%) had no providers of prostatectomy and 4 (2.1%) had no providers of IMRT, in comparison with 47 counties (25%) without a brachytherapy provider.
SBRT practitioners were predominantly clustered in southern California, southern Florida, and the northeast corridor. (Fig. 1). The location of proton therapy providers was the most limited, with locations corresponding to 10 known proton therapy centers (Fig. 1). During the study period (2011–2014), 11 proton therapy centers were operational before 2013, and 4 more became operational in 2013–2014.(13) Of counties with >100 prostate cancer patients, we identified 116 (61.7%) with no provider of SBRT and 171 (91%) with no provider of proton therapy.
Assessing the number of active physician, defined as treat at least 1 case within a specific year with a particular modality identified equal numbers of IMRT (2011: 2,471 vs 2014: 2,319), proton therapy (2011: 43 vs 2014: 46), and SBRT (2011: 121 vs 214: 120) providers throughout the study period. However declines were noted in the number of prostatectomy (2011: 2,456 vs 2014: 1,970) and brachytherapy providers (2011: 703 vs. 2014: 542).
Minimum Patient-Modality Distance Analyses
Patient rank (indicated by the right Y-axis) and their minimum patient-modality distances from shortest to longest are shown by blue curves in Figure 2a. All prostate cancer patients were located relatively close to a provider practicing prostatectomy and IMRT, with 90% of patients living within 35.1 miles (prostatectomy) and 28.9 miles (IMRT) from a practitioner and 99% living within 82.4 miles (prostatectomy) and 70.9 miles (IMRT) from a practitioner (Fig. 2a). Minimum distances to practitioners of brachytherapy and SBRT were considerably higher, with 90% of patients living within 55.6 miles (brachytherapy) and 128 miles (SBRT) of a practitioner, whereas 99% lived within 156.5 miles (brachytherapy) and 275.1 miles (SBRT) (Fig. 2a). Minimum patient-modality distance was longest for proton therapy, with 90% of patients living within 374.5 miles and 99% living within 952.7 miles of a proton therapy practitioner (Fig. 2a).
Fig. 2. Relationship between distance and patient choice and patient/provider maps showing prostate cancer treatment providers in relationships to prostate cancer patients treated with specific modalities.
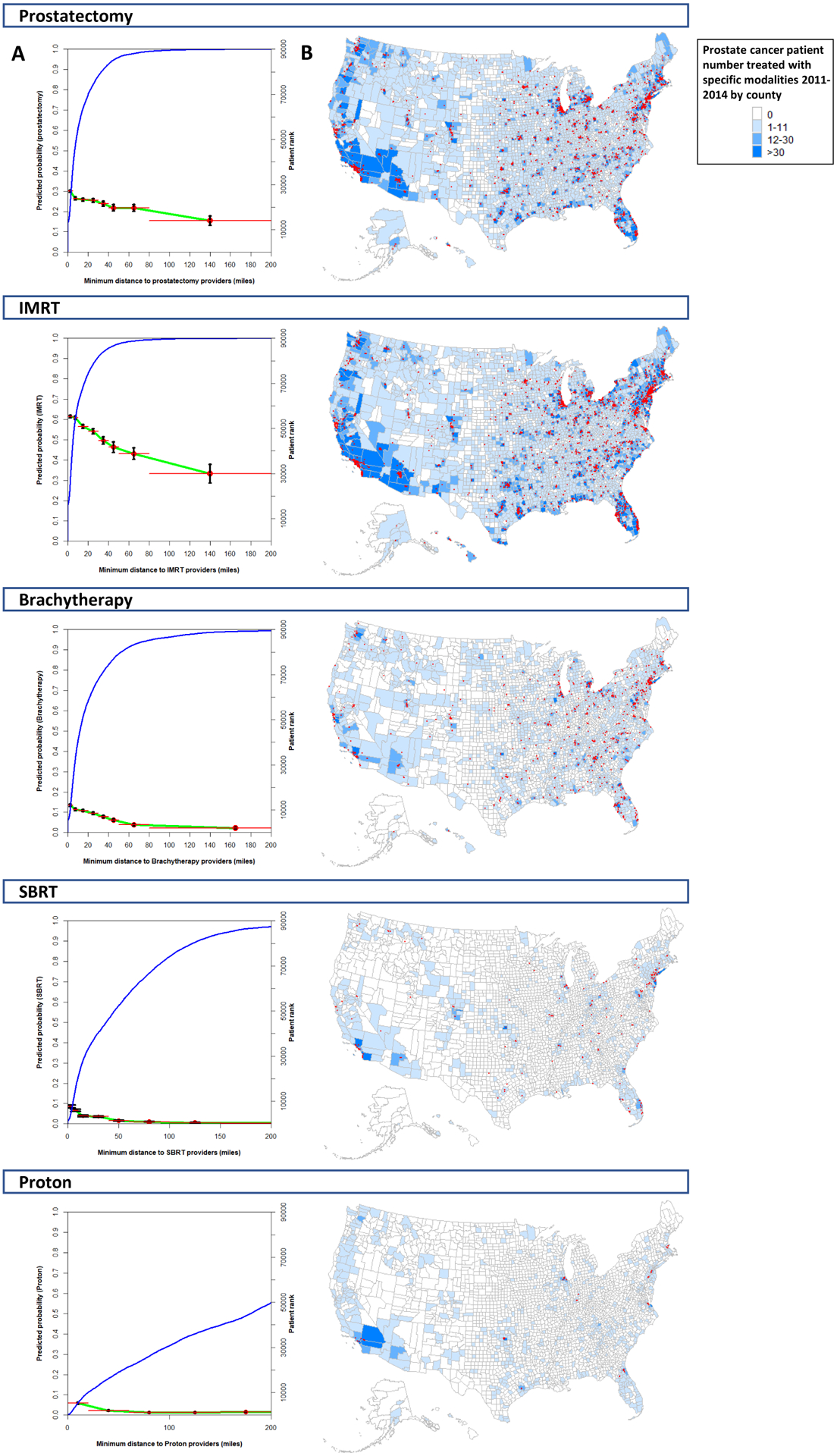
Ranked minimum patient-modality distances for all patients treated with a specific modality (blue line; right y-axis; A). Association between minimum patient-modality distance and predicted probability of receiving that modality (green line with associated 95% confidence intervals; left y-axis; A). Distances are shown up to 200 miles. Maps of the United States showing distribution of providers of specific definitive prostate cancer treatment modalities relative to the location of prostate cancer patients treated by that modality at the county level (B). Counties with a provider utilizing a specific modality are indicated by red dots.
The predicted probability of receiving each treatment modality as a function of minimum patient-modality distance is also presented in Figure 2a. A linear relationship was observed between the probability of receiving a modality and the minimum patient-modality distance of 0–60 miles. The magnitude of association between minimum patient-modality distance and probability of receiving that modality was highest for IMRT, with every 10-mile increase in the minimum patient-modality distance resulting in a 3.8% decrease in the chance of receiving IMRT (95% confidence interval [CI] 3.7–3.8%; Table 3). The next highest magnitude was for prostatectomy (2.1% decrease per 10 miles, 95% CI 2.1–2.2%) and brachytherapy (2% decrease per 10 miles, 95% CI 1.7–2.4%; Table 3). The lowest magnitude of association was observed for proton therapy (1.6% decrease per 10 miles, 95% CI 1.2–2%) and SBRT (1.1% decrease per 10 miles, 95% CI 0.9–1.4%; Table 3).
Table 3:
Linear regression estimates based on the predicted probabilities of receiving a modality in relation to minimum patient-modality distance
| Treatment | Slope Estimate | 95% Lower CL | 95% Upper CL |
|---|---|---|---|
| Prostatectomy | −2.1% | −2.2% | −2.1% |
| IMRT | −3.8% | −3.8% | −3.7% |
| Hypofractionated IMRT | −0.6% | −0.5% | −0.7% |
| Brachytherapy | −2.0% | −2.4% | −1.7% |
| Proton Therapy | −1.6% | −2.0% | −1.2% |
| SBRT | −1.1% | −1.4% | −0.9% |
Abbreviations: CL, confidence limit; IMRT, intensity-modulated radiation therapy; SBRT, stereotactic radiation therapy.
As Figure 1b plotted the location of providers in relationship to all treated prostate patients, to better visualize the influence of provider-patient distance on treatment choice we constructed a separate set of maps displaying provider locations in relationship to prostate cancer patients treated with a specific modality (Fig. 2b).
Impact of Hypofractionation on Probability of IMRT Treatment
We investigated the impact of hypofractionation (defined as 20–28 fractions) on the predicted probability of receiving IMRT. Of the 2,761 IMRT physicians, 1,759 treated at least one patient with hypofractionated IMRT for a total of 3,020 patients (6% of IMRT patients) treated with hypofractionation. In contrast 2,701 physicians utilized conventional fractionation IMRT (defined as 39+ fractions) with 42,972 receiving conventional IMRT (86%). For conventional fractionated IMRT, every 10-mile increase in distance from a provider resulted in a 3.8% (95% CI 3.8–3.9%) decrease in the probability of receiving this modality. In contrast, for hypofrationated IMRT every 10 mile increase in distance resulted in a decrease in the predicted probability by 0.6% (95% CI 0.5–0.7%) (Table 3). To investigate whether “end of the year” effect led to a spurious increase in the number of hypofractionated radiation patients, we identified the median number of patients that started radiation in any given month to be 169. In Jan 2011 this number was 248 and in November and December 2014, this number was 404 and 269, respectively, thus suggesting a potential modest over-estimation.
DISCUSSION
Understanding the geographic proximity of physicians to patients with prostate cancer allows an assessment of the influence of location on patient choice, a mechanism to assess the underlying drivers of provider distribution, and an opportunity to evaluate areas of need. The modalities considered here include both established modalities (IMRT, prostatectomy, and brachytherapy), which have been in widespread use for at least 20 years,(14,15) and emerging modalities (SBRT and proton therapy), which have had limited but increasing use over the past 10 years.(16,17) We present here the first set of provider maps detailing the location of practitioners of specific definitive prostate cancer treatment modalities. This map illustrates significant numbers of prostatectomy, IMRT, and brachytherapy providers (the three established modalities) in urban areas (Fig. 1). Of these three modalities, patient access was most limited for brachytherapy. On the other hand, SBRT and proton therapy (the 2 emerging modalities) were practiced in a limited number of urban areas with limited rural penetration.
Literature evaluating the geographic location of prostate cancer patients has demonstrated that rural status is associated with restricted treatment choices and higher likelihood of deviations from national guidelines.(11,18,19) The practitioner maps created here (Fig. 1) provide context for these analyses, demonstrating lower provider availability in rural areas, especially in western states. Among the three established treatment modalities (prostatectomy, EBRT, and brachytherapy), lack of patient access outside of central urban areas was most prominent for brachytherapy (Fig. 1). Furthermore, analyses of the National Cancer Data Base (NCDB) have found that increased provider-patient distance was associated with decreased IMRT use when compared with prostatectomy or SBRT.(18,20) Greater patient-practitioner distance has also been associated with decreased use of time-intensive treatment options, in part because of financial strain.(21) Examples include reduced adjuvant radiation for high-risk prostate cancers and decreased use of breast-conservation therapy versus mastectomy for early-stage breast cancer.(22)
The two prostate cancer treatment modalities practiced most frequently by practitioners were prostatectomy and IMRT (Table 2). Practitioner maps demonstrated significant numbers of providers in urban areas for these two modalities, with substantially lower provider availability in rural areas (Fig. 1). In every state providers of both modalities were represented in at least two geographic areas (Fig. 1), and 99% of patients lived within 70–85 miles of a provider of either modality (Figs. 2a). Analysis of minimum patient-provider distance found increased distance to an IMRT provider to have the strongest association with decreased probability of IMRT treatment (3.8% reduction per 10 miles). This magnitude was almost double than that for prostatectomy (2.1% reduction per 10 miles; Table 3). Because IMRT was delivered predominantly through conventional fractionation (86%), which requires a commitment of ≥8 weeks, these results are consistent with prior analyses suggesting that treatments requiring longer time commitments may be especially problematic for patients traveling longer distances.(18,20) The strength of the association between probability of treatment and distance was mitigated through the use of hypofractionated IMRT (Table 3).(23,24) In addition, the identified association between patient-provider distance and probability of treatment receipt suggests that distance variables could be further explored as instrumental variables for comparative effectiveness analyses that assess efficacy and toxicity, as has been done in other diseases.(25)
Brachytherapy us has been in decline because of decreased relative reimbursement and reduced emphasis during residency training.(26–28) Brachytherapy represents a high-quality alternative to prostatectomy and IMRT, allowing 1-day outpatient treatment for men with intermediate- or low-risk prostate cancer.(5,29) Although all radiation oncologists are required to demonstrate competency in prostate brachytherapy during residency,(27) the number of prostate brachytherapy practitioners identified was approximately a quarter that of IMRT practitioners (769 vs 2,761). Reduced numbers of brachytherapy practitioners was also noted in rural areas, with 99% of patients living within 156.5 miles of a brachytherapy provider (Fig. 2a). Moreover, among the 188 counties with >100 treated prostate cancer patients, 47 (25%) did not have a brachytherapy provider compared with 17 (9%) with a prostatectomy provider and 4 (2.1%) with an IMRT provider. These data are concordant with past NCDB analyses that identified brachytherapy use to have reached a peak of 17% of all prostate cancer cases in 2002 and a decline to a low of 8% in 2010.(27,28) The current analysis confirms this trends with the number of active brachytherapy providers decreasing from 703 in 2011 to 542 in 2014. Although it’s possible that this trend may also be related to other nation-wide trends during this time period including increased emphasis on active surveillance especially for older men.(6) Upcoming policies may alter this trend, with reimbursement equalization via the radiation oncology alternative payment model (RO-APM) and the upcoming “300 in 10” brachytherapy training initiative from the American Brachytherapy Society.(30)
No randomized comparisons exist comparing proton therapy versus conventional techniques, and randomized comparisons between SBRT and IMRT are limited by short follow-up.(31,32) Given the expense of proton therapy facilities, proton therapy providers were found to cluster around a limited number of predominately academic centers, with 99% of patients living within 952.7 miles of a proton therapy provider (Fig. 2a). SBRT, on the other hand, does not require the same startup and therefore exhibited more diffuse availability. SBRT providers were found to be clustered in urban centers, with a significant amount of SBRT practitioners in more rural areas (99% of patients living within 275.1 miles of a SBRT practitioner; Fig. 2a). Among counties with >100 treated prostate cancer patients, 116 (61.7%) did not have an SBRT provider and 171 (91%) did not have a proton therapy provider within that county. For both modalities, it is likely that a significant number of patients were referred from a larger catchment area into specialty centers (Fig. 2b). In support of this hypothesis, we observed the influence of minimum provider-patient distance on treatment receipt to be less than that observed for established modalities (proton 1.6% and SBRT 1.1% reduction per 10 miles; Table 3).
Various limitations deserve mention. First, all patients must have been at least 65 years old in 2011 to be included in this dataset and must have been enrolled in fee-for-Service Medicare throughout the entirety of the study period, thus excluding patients using Medicare Advantage plans and patients traveling for care from foreign countries. Because older patients are more likely to receive noninvasive treatments such as IMRT,(6) the potential exists to underrepresent the practice of more invasive procedures such as prostatectomy or brachytherapy. Second, the analysis dataset spanned 2011–2014 and thus does not reflect the most current geographic locations of the prostate cancer providers. Third, the analyzed dataset does not include information on disease characteristics such as stage. Fourth, the AMA Physician Masterfile does not capture multiple practice locations if a provider has multiple offices and thus may overestimate minimum provider-patient distances. Fifth, data on changes in treatment utilization over time should be interpreted with caution. To be included in this dataset patients must have had uninterrupted Medicare coverage throughout the entire study period, thus potentially reducing the number of treated patients during later years of the study period. Finally, the created provider access maps display provider location at the end of the study period and doesn’t reflect the interim location of providers if they changed locations during the study period.
Overall market and treatment practice forces have resulted in a prostate cancer treatment landscape that facilitates relative ease in geographic access of patients to IMRT and prostatectomy practitioners within the United States. However, these same forces have resulted in considerably less brachytherapy providers, especially in rural areas. Regarding emerging modalities, demand for treatments that may be less toxic (proton therapy) or be more convenient for patients (SBRT) has driven the rise of both emerging techniques in limited urban centers. In the second part of this analysis, minimum patient-provider distance was found to be inversely proportional to the probability of treatment receipt between 0–60 miles. The association between minimum patient-modality distance and treatment receipt was strongest for IMRT, highlighting one of the key limitations of IMRT, extensive patient time commitment. This association between distance was attenuated for radiation modalities that require shorter treatment duration, such as SBRT, brachytherapy and hypofractionated IMRT. In conclusion these data demonstrate significant heterogeneity in the access of definitive prostate cancer treatment modalities across the United States, decreased active brachytherapy and prostatectomy providers over time, and quantification of the inverse association between provider-patient distance and utilization of specific modalities.
Supplementary Material
Acknowledgements
We would like to acknowledge Christine F. Wogan for her help in editing the manuscript.
Funding: Dr. C Tang is supported by a Radiation Oncology Institute (ROI) grant and the Cancer Prevention & Research Institute of Texas (RP180140) and is an Andrew Sabin Family Fellow. Dr. B Smith is supported by NIH R01 CA207216, the Cancer Prevention & Research Institute of Texas (RP160674) and is an Andrew Sabin Family Fellow. Dr. YT Shih is supported by NIH R01 CA207216. This work was supported by The University of Texas MD Anderson Cancer Center under the Cancer Center Support Core Grant (NCI P30 CA016672).
Research data are available at: https://data.medicare.gov/ and https://www.ama-assn.org/practice-management/masterfile/ama-physician-masterfile
Conflict of Interest: None
REFERENCES
- 1.Prostate cancer (ver 1.2020). In: Editor, editorêditors. Book Prostate cancer (ver 1.2020): National Comprehensive Cancer Network; 2020. [Google Scholar]
- 2.Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016;375:1415–1424. [DOI] [PubMed] [Google Scholar]
- 3.Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 2016;375:1425–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffman KE, Penson DF, Zhao Z, et al. Patient-reported outcomes through 5 years for active surveillance, surgery, brachytherapy, or external beam radiation with or without androgen deprivation therapy for localized prostate cancer. JAMA 2020;323:149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen RC, Basak R, Meyer AM, et al. Association between choice of radical prostatectomy, external beam radiotherapy, brachytherapy, or active surveillance and patient-reported quality of life among men with localized prostate cancer. JAMA 2017;317:1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang C, Hoffman KE, Allen PK, et al. Contemporary prostate cancer treatment choices in multidisciplinary clinics referenced to national trends. Cancer 2020;126:506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patrikidou A, Maroun P, Patard JJ, et al. Helping patients make informed decisions. Two-year evaluation of the gustave roussy prostate cancer multidisciplinary clinic. Clin Transl Radiat Oncol 2018;12:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aizer AA, Paly JJ, Zietman AL, et al. Multidisciplinary care and pursuit of active surveillance in low-risk prostate cancer. J Clin Oncol 2012;30:3071–6. [DOI] [PubMed] [Google Scholar]
- 9.Cushman TR, Mashni J, Roberts T, et al. The impact of a 1-day multidisciplinary prostate cancer clinic on patients’ choice of treatment. J Am Coll Radiol 2018;15:1745–1748. [DOI] [PubMed] [Google Scholar]
- 10.Scherzer ND, DiBiase ZS, Srivastav SK, et al. Regional differences in the treatment of localized prostate cancer: An analysis of surgery and radiation utilization in the united states. Adv Radiat Oncol 2019;4:331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagley AF, Anscher MS, Choi S, et al. Association of sociodemographic and health-related factors with receipt of nondefinitive therapy among younger men with high-risk prostate cancer. JAMA Netw Open 2020;3:e201255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000;53:1258–67. [DOI] [PubMed] [Google Scholar]
- 13.Mohan R, Grosshans D. Proton therapy - present and future. Adv Drug Deliv Rev 2017;109:26–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purdy JA. Intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys 1996;35:845–6. [DOI] [PubMed] [Google Scholar]
- 15.Zaorsky NG, Davis BJ, Nguyen PL, et al. The evolution of brachytherapy for prostate cancer. Nat Rev Urol 2017;14:415–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahal BA, Chen YW, Efstathiou JA, et al. National trends and determinants of proton therapy use for prostate cancer: A national cancer data base study. Cancer 2016;122:1505–12. [DOI] [PubMed] [Google Scholar]
- 17.Kapoor DA, Zimberg SH, Ohrin LM, et al. Utilization trends in prostate cancer therapy. J Urol 2011;186:860–4. [DOI] [PubMed] [Google Scholar]
- 18.Muralidhar V, Rose BS, Chen YW, et al. Association between travel distance and choice of treatment for prostate cancer: Does geography reduce patient choice? Int J Radiat Oncol Biol Phys 2016;96:313–317. [DOI] [PubMed] [Google Scholar]
- 19.Cetnar JP, Hampton JM, Williamson AA, et al. Place of residence and primary treatment of prostate cancer: Examining trends in rural and nonrural areas in wisconsin. Urology 2013;81:540–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahal BA, Chen YW, Sethi RV, et al. Travel distance and stereotactic body radiotherapy for localized prostate cancer. Cancer 2018;124:1141–1149. [DOI] [PubMed] [Google Scholar]
- 21.Vetterlein MW, Loppenberg B, Karabon P, et al. Impact of travel distance to the treatment facility on overall mortality in us patients with prostate cancer. Cancer 2017;123:3241–3252. [DOI] [PubMed] [Google Scholar]
- 22.Acharya S, Hsieh S, Michalski JM, et al. Distance to radiation facility and treatment choice in early-stage breast cancer. Int J Radiat Oncol Biol Phys 2016;94:691–9. [DOI] [PubMed] [Google Scholar]
- 23.Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 chhip trial. Lancet Oncol 2016;17:1047–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan SC, Hoffman K, Loblaw DA, et al. Hypofractionated radiation therapy for localized prostate cancer: An astro, asco, and aua evidence-based guideline. J Clin Oncol 2018:JCO1801097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Presley CJ, Soulos PR, Herrin J, et al. Patterns of use and short-term complications of breast brachytherapy in the national medicare population from 2008–2009. J Clin Oncol 2012;30:4302–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang C, Lei X, Smith GL, et al. Costs and complications after a diagnosis of prostate cancer treated with time-efficient modalities: An analysis of national medicare data. Pract Radiat Oncol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcrom SR, Kahn JM, Colbert LE, et al. Brachytherapy training survey of radiation oncology residents. Int J Radiat Oncol Biol Phys 2019;103:557–560. [DOI] [PubMed] [Google Scholar]
- 28.Martin JM, Handorf EA, Kutikov A, et al. The rise and fall of prostate brachytherapy: Use of brachytherapy for the treatment of localized prostate cancer in the national cancer data base. Cancer 2014;120:2114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris WJ, Tyldesley S, Rodda S, et al. Androgen suppression combined with elective nodal and dose escalated radiation therapy (the ascende-rt trial): An analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys 2017;98:275–285. [DOI] [PubMed] [Google Scholar]
- 30.Petereit DG Vision 2020. In: Editor, editorêditors. Book Vision 2020; 2019. pp. https://www.americanbrachytherapy.org/ABS/document-server/?cfp=ABS/assets/file/public/brachynews/ABS_BrachyNews_Summer_2019.pdf. [Google Scholar]
- 31.Brand DH, Tree AC, Ostler P, et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (pace-b): Acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol 2019;20:1531–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Widmark A, Gunnlaugsson A, Beckman L, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the hypo-rt-pc randomised, non-inferiority, phase 3 trial. Lancet 2019;394:385–395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


