Abstract
The contents of working memory must be maintained in the face of distraction, but updated when appropriate. To manage these competing demands of stability and flexibility, maintained representations in working memory are complemented by distinct gating mechanisms that selectively transmit information into and out of memory stores. The operations of such dopamine-dependent gating systems in the midbrain and striatum, and their complementary dopamine-dependent memory maintenance operations in cortex, may therefore be dissociable. If true, selective increases in cortical dopamine tone should preferentially enhance maintenance over gating mechanisms. To test this hypothesis, tolcapone, a catechol-O-methyltransferase inhibitor that preferentially increases cortical dopamine tone, was administered in randomized, double-blind, placebo-controlled, within-subject fashion to 49 subjects who completed a hierarchical working memory task that varied maintenance and gating demands. Tolcapone improved performance in a condition with higher maintenance requirements and reduced gating demands, reflected in a reduction in the slope of response times across the distribution. Resting state fMRI data demonstrated that the degree to which tolcapone improved performance in individual subjects correlated with increased connectivity between a region important for first-order stimulus-response mappings (left dorsal premotor cortex) and cortical areas implicated in visual working memory, including the intraparietal sulcus and fusiform gyrus. Together these results provide evidence that augmenting cortical dopamine tone preferentially improves working memory maintenance.
Keywords: working memory, dopamine, maintenance, gating, hierarchy
Introduction:
The ability to selectively update the maintained contents of working memory is critical to working memory function (D’Esposito & Postle, 2015). Memoranda must be amenable to change as sensory inputs and goals evolve, but they must also be resistant to distraction; thus, deciding when to update those memoranda, and when to simply maintain them, is essential. To render maintenance more responsive to such inputs and goals, past computational modeling has argued for the presence of input and output gating mechanisms (Frank, Loughry, & O’Reilly, 2001; Frank & O’Reilly, 2006). When an input gate is open, the contents of working memory can be updated; when an input gate is closed, those contents are maintained and updates are suppressed. Similarly, the opening of an output gate selects an item (or items) maintained in working memory to be emitted to influence behavior. The maintenance process is itself an active one, and this process will complement the gating of memoranda in and out.
Over the past decade neural evidence for the existence of input and output gates has accumulated (Badre & Frank, 2012; Chatham, Frank, & Badre, 2014; D’Ardenne et al., 2012; Frank & Badre, 2012). Current findings suggest that gating is controlled by the striatum through its connections with the frontal cortex. In particular, activity in the striatum increases when information is gated into working memory areas within the dorsolateral prefrontal cortex (PFC), and transcranial magnetic stimulation of the PFC disrupts this gating of new items into working memory (D’Ardenne et al., 2012). Similarly, increases in selection demands from within working memory, as instantiated by output gating, correlate with increases in activity within the caudate, as well as an increase in caudate connectivity with the prefrontal cortex (Chatham et al., 2014). These findings complement results indicating that maintenance is primarily a cortical process (D’Esposito & Postle, 2015). Work in both macaques (M. Wang, Vijayraghavan, & Goldman-Rakic, 2004) and humans (Lorenc, Lee, Chen, & D’Esposito, 2015), for example, has demonstrated that causal interventions in specific lateral PFC regions can degrade the performance of working memory maintenance, and more recent work has demonstrated the role of lateral PFC in maintaining representations in posterior cortical regions that encode relevant stimuli (Rose et al., 2016).
These different neural substrates share a link to the neuromodulator dopamine. In computational models that include gating mechanisms, a signal representing the actions of dopamine is responsible for opening and closing the gates (Frank et al., 2001). Moreover, in humans, phasic activity within the dopaminergic midbrain, where the striatal dopaminergic signal presumably originates, correlates with input gating (D’Ardenne et al., 2012). With respect to working memory maintenance, neural evidence for the role of cortical dopamine signaling has come from experiments in nonhuman primates in which dopamine agonists and antagonists were infused directly into lateral PFC (Cai & Arnsten, 1997; Vijayraghavan, Wang, Birnbaum, Williams, & Arnsten, 2007; M. Wang et al., 2004; Y. Wang & Goldman-Rakic, 2004). Depending on the dose of such infusions, working memory performance could either improve or decline, supporting the now-classic inverted U-shaped influence of dopamine on behavior, such that behavior is optimized for intermediate dopamine tone (Cools & D’Esposito, 2011).
Based on the above findings, the specific locus of dopaminergic effects should determine the nature of their influence on working memory function. In particular, changes in cortical dopamine tone should influence maintenance, but should not differentially impact input and output gating. To our knowledge, this hypothesis has not been tested. To address this idea directly, here we take advantage of the unique neuroanatomy and pharmacology of the catechol-O-methyltransferase (COMT) enzyme. Dopamine metabolism is regulated differentially in the frontal cortex and striatum: while termination of dopamine’s effect in the striatal synapse is predominantly mediated by reuptake via the dopamine transporter, the action of synaptic dopamine in the frontal cortex is terminated primarily via degradation by the COMT enzyme (Chen et al., 2004; Gogos et al., 1998). The brain-penetrant COMT inhibitor tolcapone might therefore preferentially augment cortical dopamine tone (Tunbridge, Bannerman, Sharp, & Harrison, 2004) and thereby enhance working memory maintenance, potentially by increasing connectivity of frontal regions with the posterior cortical regions important for representing maintained stimuli (Mueller, Krock, Shepard, & Moore, 2020; Noudoost & Moore, 2011). A previous study of tolcapone in humans has shown modest enhancements of working memory (Apud et al., 2007); however, the working memory task employed in that study, the N-back, confounds encoding, maintenance, and retrieval processes on single trials, and therefore cannot easily differentiate input gating, output gating, and maintenance demands. Here we propose that tolcapone’s effects should be expressed primarily in maintenance, not gating.
To test our hypothesis, we take advantage of a paradigm that has previously been used to assess hierarchical working memory maintenance and gating (Chatham et al., 2014) via independent manipulations of working memory load (primarily placing demands on maintenance processes) and task context (primarily impacting gating). In the task, subjects are required to maintain one or two stimuli – a letter, a symbol, or both – across a trial, based on a context cue (a number) that can be provided either before or after the other items. We hypothesize that tolcapone should lead to the greatest behavioral improvements when the demand on memory maintenance is greater. Moreover, we argue that this effect should be most prominent when output gating demands are low, thereby reducing response time variability induced by context-contingent selection from working memory. Thus, we specifically predict that we will find behavioral improvement when maintenance demands are high but gating demands are low. Similarly, administration of tolcapone should have limited effects on performance as a function of gating demands when maintenance demands are held constant.
Methods:
60 healthy subjects with no history of medical, psychiatric, or neurological contraindications were recruited and ultimately eligible to participate in the study. All subjects gave written informed consent in accordance with the Declaration of Helsinki and the Committee for the Protection of Human Subjects at the University of California, San Francisco and University of California, Berkeley; they were compensated for their participation. Subjects first underwent a history and physical exam, as well as blood testing for liver function and urine screening for drugs of abuse, to ensure there were no medical contraindications to tolcapone use or magnetic resonance imaging (MRI) scanning. All subjects were right-handed and had normal or corrected-to-normal vision. Before testing sessions, subjects were trained on the task in order to familiarize them with task procedures. Subjects then underwent two separate behavioral sessions, each consisting of 180 task trials, as well as resting state functional MRI (fMRI) that was part of a larger study. For those sessions, subjects were randomized in double-blind, counterbalanced, placebo-controlled fashion to receive either a single 200mg dose of tolcapone or matched placebo on their first visit, and the alternative treatment on their second visit. The tolcapone dose was based upon our previously published findings that a single 200mg dose has measurable behavioral effects (Kayser, Allen, Navarro-Cebrian, Mitchell, & Fields, 2012; Kayser, Mitchell, Weinstein, & Frank, 2015; Saez, Zhu, Set, Kayser, & Hsu, 2015).
Overall, 11 subjects were excluded prior to final behavioral data analysis: four because they only participated in one day of behavioral testing, four because they did not complete all study procedures within each testing day, and three because their task accuracy did not exceed chance. The remaining 49 subjects contributed to all behavioral data. Ages ranged from 18-33 years old (mean 21.6 ± 3.1 (sd)); 26 of 49 were women. An additional four subjects were removed from the resting state data set because of excessive motion (translation greater than 3 mm), leaving 45 subjects for imaging analyses.
Task.
Details of the task have been published elsewhere (Chatham & Badre, 2013; Chatham et al., 2014). Briefly, each trial of the task consisted of three separate visual stimuli – a number (1, 2, or 3), a letter (A or B), and a symbol (a snowflake or a sun) – that could be presented sequentially in any order (Figure 1). Each of the first two stimuli for that trial were presented for 0.5 seconds, separated by an inter-stimulus interval (ITI) of 1.5 – 5.0 seconds (drawn from a uniform distribution). Following a second ITI, the final stimulus remained on the screen until the subject had chosen one of the two accompanying response options (see below). Subjects were required to maintain both the context, as cued by the number, and at least one of the letter and symbol stimuli across the trial. Specifically, numbers served as a “context” that conveyed information about which of the two other stimuli were relevant for a given trial: for the number 1, subjects were required to selectively remember the symbol (“selective” context); for the number 2, subjects were required to selectively remember the letter (“selective” context); and for the number 3, subjects were required to remember both the symbol and the letter (“global” context). Trials in which both the letter and the symbol were admitted into working memory were considered to be high load trials, while those trials in which only one of the two was maintained were considered to be low load trials (Figure 1). Accompanying the third visual stimulus (whether number, letter, or symbol) were two choices consisting of both a letter and a symbol; subjects were required to make a left or right button press to identify the choice with the appropriate memorandum/a. For global trials, subjects were informed that the two choice options could share one of the memoranda, requiring subjects to remember both items to make the correct decision.
Figure 1.
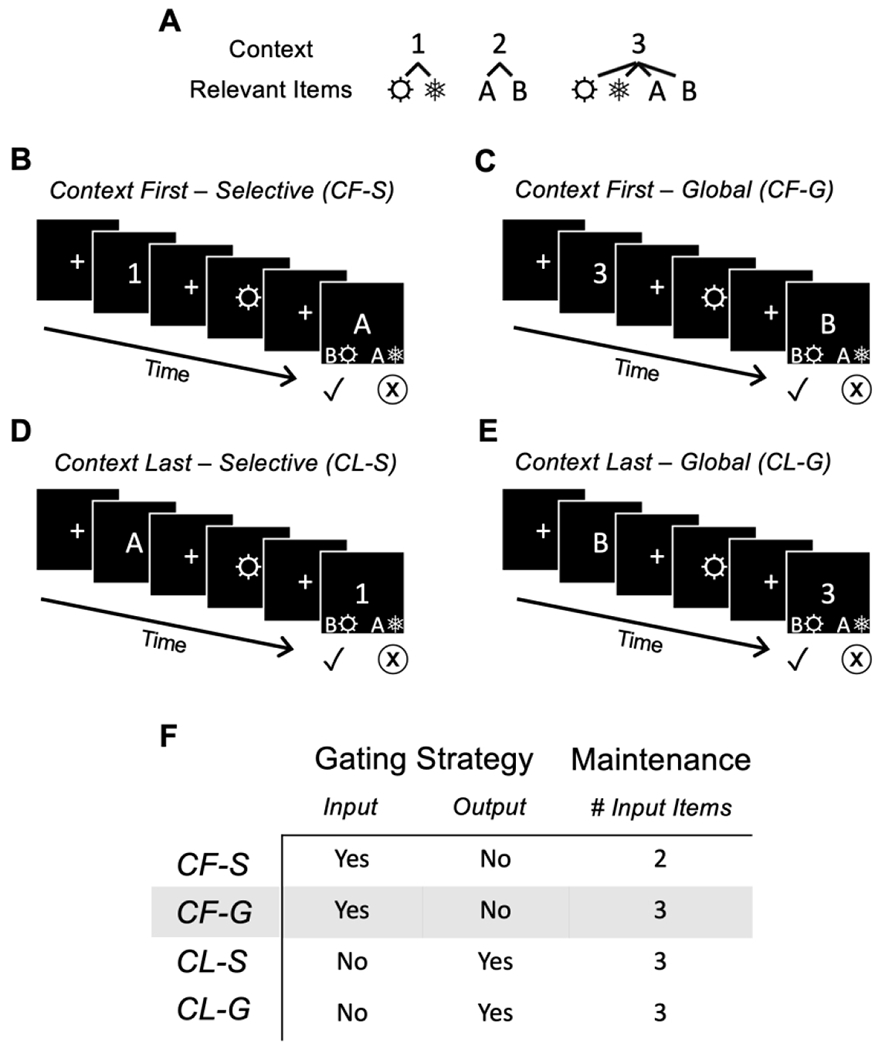
Task
A. In this task, numbers define the context of each trial. The numbers 1 and 2 indicate that only the symbols or the letters, respectively, are relevant to the response. These “selective” contexts are differentiated from the “global” context defined by the number 3, which indicates that both symbols and letters are relevant to the response. B-E. All trials conclude with a screen containing two response options, one of which includes the correct item (for the selective contexts) or the correct items (for the global context). In all cases, only one of the two responses is correct, here indicated by the check mark. Importantly, the order of presentation of the three stimuli in each trial can vary. When the number representing the context is presented first (panels B and C), subjects can update working memory with only the relevant item(s), thereby taxing only input gating. In contrast, when the context is presented last, subjects must have already gated both memoranda into working memory, placing greater demands on selection of the relevant output from memory and more strongly taxing output gating. F. The four trial types differ in both the strategy required and the number of encoded stimuli. Our prediction that tolcapone’s effect should be most visible in conditions with increased maintenance requirements and decreased gating demands suggests that behavioral effects should be seen most clearly in the CF-G condition (highlighted).
Gating demands were manipulated by varying the order in which the three stimuli were presented. Trials in which the number was presented first placed primary demands on input gating: subjects needed to select the appropriate visual stimulus/stimuli to input and maintain across the delay, but output gating demands were reduced, as all maintained memoranda were behaviorally relevant. In contrast, trials in which the number was presented last not only placed demands on input gating, but also placed significantly greater demands on output gating: subjects updated and maintained all visually presented stimuli in working memory, because the identity of the behaviorally relevant stimuli was not yet specified, but they then needed to select for output only the appropriate choice from the contents of working memory. For these trials in which the number was presented last, note that output gating demands were higher for the “selective” contexts, compared with the “global” context, because working memory contained items that were not behaviorally relevant. Lastly, trials in which the context (i.e. the number) was presented as the second of the three visual stimuli were included in the behavioral task for completeness, to ensure that subjects needed to attend to all stimulus positions equally. However, because these trials more strongly confound input gating, output gating, and maintenance demands, they were not analyzed further. In sum, four task conditions were analyzed: context first, selective (CF-S); context first, global (CF-G); context last, selective (CL-S); and context last, global (CL-G).
Importantly, context-first (CF) and context-last (CL) trials, irrespective of whether they are selective or global, are not distinguished by other factors, such as conflict during response selection. For example, the CF-G and CL-G conditions both include a correct response that contains the symbol and the letter presented during the trial. Additionally, as noted previously some global trials contain the same item in both the target and foil responses to ensure that subjects cannot simply focus on one, rather than both, items.
Behavioral Analysis.
In keeping with previous studies (Chatham & Badre, 2013; Chatham et al., 2014), we focused primarily on response time (RT), rather than accuracy. Accuracy, as a binary (right/wrong) outcome measure, is relatively insensitive to changes in task efficiency. While true maintenance and gating failures could be reflected in changes in accuracy, inefficiencies would not; instead, responses would simply be slowed. To address the hypothesis that tolcapone should preferentially reduce the number of inefficient trials, even if the proportion of ultimately-correct trials remains unchanged, we used a measure sensitive to the distribution of responses across trials, and in particular to the number of inefficient (long RT) responses. Of note, while RT reflects a combination of factors, including early visual processing and motor preparation, early visual processing demands are matched across the task, and our previous work has confirmed that tolcapone does not significantly speed motor responses (Furman et al., 2020; Kayser et al., 2012; Kayser et al., 2015). Thus, early visual processing and motor preparation demands should not distinguish task conditions based on RT-related measures.
All behavioral data were preprocessed prior to analysis. Because the primary focus was on reaction times, data that impacted stable RT measurements were removed. As noted previously, 3 of the 11 excluded subjects were eliminated for failing to respond with greater than chance accuracy across all trials. For each of the 49 retained subjects, the first 10 trials of each session were removed from all analyses; in addition, all incorrect trials and any trials with RTs greater than 5 standard deviations outside of the mean RT for that subject were excluded from analysis of RT (Chatham & Badre, 2013; Chatham et al., 2014). This outlier threshold was chosen to balance two concerns: the desire to avoid censoring inefficient RTs, but also the goal of avoiding very long RTs confounded by factors unrelated to the task (e.g. due to failure to attend to the computer screen). Across all subjects, only 1 trial was removed for falling outside the desired RT range.
A linear mixed effects model was used to address tolcapone-related changes in mean RT. The model was additionally constructed to test for tolcapone-related effects on the RT distribution (see below) for each task condition (Chatham et al., 2014), as measuring the mean RT does not address potentially more subtle changes in the distribution of RTs across experimental manipulations. Conceptually, changes in the efficiency of maintenance or gating may not be reflected in trials for which these processes are already optimized. Trials with very fast RTs, for example, may reflect strong maintenance and gating processes for which any manipulation may have little observable beneficial effect. In contrast, trials with very slow RTs may reflect inefficient maintenance and gating processes that might improve with drug. Similarly, if tolcapone worsened the efficiency of gating or maintenance, these effects might be most visible at the fast end of the RT distribution. To measure any such effects, we took an approach utilized previously with this task (Chatham et al., 2014) to divide the RT data for each participant and condition into 10 deciles, sorted by RT from fastest to slowest, and to use the mean RT values per decile as the dependent variable in our analysis. This approach permitted us to evaluate drug-related changes in slope across the deciles (“RT slope”), as well as the mean change in RT. In the model, factors included drug (tolcapone or placebo; treatment coded), task condition (CF-S, CF-G, CL-S, or CL-G; sum coded), and decile (1-10; ordinal), as well as all interactions. To account for potential nonlinear effect of tolcapone on RT distribution, a comparable set of interaction terms was included for decile2 (“decile squared”). Finally, interactions with drug session order (drug first or drug last; sum coded) were included as a control measure. Initially, a maximal random effects structure was constructed to minimize Type I error (Barr, Levy, Scheepers, & Tily, 2013). Random effects included the intercept of subject, as well as the slopes of drug, task condition, and decile/decile2 and their interactions, and the correlation between random slopes and subject intercept. This model failed to converge; thus, following the protocol outlined in (Bates, Kliegl, Vasishth, & Baayen, 2015), we removed the correlation between random slopes and intercept. F-tests were computed for fixed effects using the Satterthwaite method for approximating degrees of freedom. Analyses were carried out using the “lme4” (Bates et al., 2015) and “afex” (Singmann et al., 2018) libraries in R (R Core Team, 2017). Estimation of marginal means and trends, as well as follow-up z-tests, were conducted using the “emmeans” package (Lenth, 2018).
For completeness, trial-wise accuracy was also analyzed. A binomial generalized mixed effects model included the fixed factors drug, task condition, and their interaction. After dropping terms to enable convergence and avoid singular fit, the final random effects structure included random intercepts for subject and random slopes of drug within subject. Likelihood-ratio tests were used to determine the significance of fixed effects terms.
MRI Parameters.
MRI scanning was conducted on a Siemens MAGNETOM Trio 3T MR Scanner at the Henry H. Wheeler, Jr. Brain Imaging Center at the University of California, Berkeley. Anatomical images consisted of 160 slices acquired using a T1-weighted MP-RAGE protocol (TR = 2300 ms, TE = 2.98 ms, FOV = 256 mm, matrix size = 256 x 256, voxel size = 1 mm3). Resting state functional images were obtained while subjects were lying quietly with eyes open, and consisted of 35 slices acquired with a gradient echoplanar imaging protocol (TR = 1900 ms, TE = 24 ms, FOV = 225 mm, matrix size = 96 x 96, voxel size = 3.0 mm x 3.0 mm x 3.5 mm).
fMRI preprocessing.
fMRI preprocessing was performed using both the AFNI (http://afni.nimh.nih.gov) and FSL (http://www.fmrib.ox.ac.uk/fsl/) software packages. Resting state functional images were converted to 4D NIfTI format and corrected for slice-timing offsets. Motion correction was carried out using the AFNI program 3dvolreg, with the reference volume set to the mean image. Co-registration with the anatomical scan was performed using the AFNI program 3dAllineate, and anatomical images were normalized to a standard volume (MNI_N27) using the FSL program fnirt. The same normalization parameters were later applied to native-space statistical maps to generate group statistical maps.
Resting state connectivity analysis.
Resting state data were smoothed by a 5mm FWHM Gaussian kernel prior to temporal bandpass filtering between 0.009 Hz and 0.08 Hz to reduce the influence of cardiac and respiratory artifact (Fox et al., 2005). Movement parameters and the white matter and ventricular time series, but not the global mean signal, were included as regressors of no interest during preprocessing, independently of the subsequent connectivity analyses. Regions of interest (ROIs) within the lateral prefrontal cortex were then selected, based on (a) their increased activity and central role in this and related tasks (Badre, Kayser, & D’Esposito, 2010; Chatham et al., 2014), and (b) the hypothesis that on tolcapone these regions, particularly those more proximate to the motor response, would demonstrate increased connectivity with visual areas in posterior cortex. Specifically, these regions were located in the left and right dorsal premotor cortex (PMd, with MNI coordinates ±30, −12, 66) and left and right pre-premotor cortex (pPMd, with MNI coordinates ±36, 8, 34) (Badre et al., 2010; Chatham et al., 2014).
Each ROI was defined by a set of MNI coordinates that formed the center for a sphere with 8mm radius. Time courses defined by averaging across voxels in each of these regions were then correlated separately with all other voxels in the brain, and correlation coefficients were Fisher-transformed to allow for the application of parametric statistical tests. The resulting individual brain maps were normalized to the MNI template prior to the application of group-level statistics. To examine the relationship between drug effects on behavioral performance and drug-related changes in functional connectivity, we first calculated the difference between placebo and tolcapone connectivity maps for each participant and seed region, and then computed the correlation between these differences maps and the random effect variables corresponding to subject-wise drug × decile effect (“overall RT slope”) and drug x decile x CF-G effect (computed as the additive effect of “drug × decile” and “drug × decile x CF-G”; hereafter referred to as “RT slope for the CF-G condition”) estimated in our behavioral model (see Behavioral Analysis). Map-wise significance (p < 0.001, corrected for multiple comparisons) was determined by applying a cluster-size correction (20 voxels) derived from the AFNI programs 3dFWHMx and 3dClustSim to data initially thresholded at a value of p < 0.0001, uncorrected.
Results:
49 subjects completed a hierarchical working memory task in which they were required to use context cues, indicated by numbers, to recall symbols and/or letters across the duration of a trial (Figure 1A–E). Consistent with prior work (Chatham et al., 2014), four task conditions were evaluated: context first, selective (CF-S); context first, global (CF-G); context last, selective (CL-S); and context last, global (CL-G). Notably, each of these conditions places differential strategic demands on input gating, output gating, and maintenance (Methods, and Figure 1F). For these conditions we evaluated both the mean RTs and the change in the distribution of RTs across ten ordered deciles for each task condition (Chatham et al., 2014). This “RT slope” value better reflects the distribution of reaction times for each condition; specifically, in distinction from mean RT or accuracy, it addresses the possibility that enhancing cortical dopamine tone may not improve maintenance across all trials, but instead may preferentially improve inefficient maintenance, or disrupt efficient maintenance, across trial subtypes (see Methods).
Though accuracy varied by task condition (X2(3)=174.23, p<0.0001), there was no significant effect of drug (X2(1)=0.03, p=0.87), nor interaction of drug and condition (X2(3)=1.83, p=0.61), on task accuracy (see Table 1). Our analysis of RT revealed a significant main effect of task condition on RT (F[3,114.79]=420.87, p < 0.0001), consistent with previous work using this paradigm (Chatham et al., 2014). Interactions of condition x decile (F[3,80.1]=26.19, p < 0.0001) and of condition x decile2 (F[3,57.87]=17.07, p < 0.0001), and the hypothesized 3-way interactions of condition x decile x drug (F[3,59.65]=3.50, p = 0.02), and of condition x decile2 x drug (F[3,83.22]=3.05, p = 0.03) were also identified (see Table 1). Of note, these 3-way interactions persisted despite a 4-way interaction of condition x decile x drug x session order (F[3,59.65]=2.96, p=0.04; the comparable term “condition x decile2 x drug x session order” was not significant, F[3,83.22]=1.59, p=0.2). There was no simple effect of drug on RT (F[1,49.68]=0.03, p=0.86), and the interactions of drug x decile (F[1,47.36]=0.34, p = 0.56), drug x decile2 (F[1,63.41]=1.36, p = 0.25), and drug x condition (F[3,76.84] = 0.76, p = 0.52) were all insignificant. As expected, the simple effects of decile (F[l,58.43]=1078.76, p < 0.0001) and decile2 (F[1,44.22]=485.78, p < 0.0001) were significant, but these effects are a direct consequence of the analysis design and were not explored further.
Table 1:
Estimated marginal means and trends, by task condition and drug
| PLACEBO | TOLCAPONE | ||||
|---|---|---|---|---|---|
| Task | Estimate | 95% CI | Estimate | 95% CI | |
| Accuracy (proportion) | CF-G | 0.94 | (0.93, 0.96) | 0.95 | (0.93, 0.96) |
| CF-S | 0.93 | (0.91, 0.94) | 0.92 | (0.91, 0.94) | |
| CL-G | 0.93 | (0.91, 0.94) | 0.93 | (0.91, 0.95) | |
| CL-S | 0.86 | (0.84, 0.88) | 0.85 | (0.83, 0.87) | |
| RT (ms) | CF-G | 808.95 | (774.19, 843.7) | 789 | (746.54, 831.46) |
| CF-S | 645.66 | (609.46, 681.85) | 643.98 | (601.2, 686.76) | |
| CL-G | 897.38 | (855.5, 939.25) | 898.68 | (846.3, 951.07) | |
| CL-S | 1029.89 | (992.58, 1067.2) | 1044.22 | (999.08, 1089.36) | |
| RT slope (ms/decile) | CF-G | 65.31 | (60.31, 70.31) | 59.07 | (52.33, 65.82) |
| CF-S | 64.78 | (59.75, 69.82) | 64.86 | (57.68, 72.04) | |
| CL-G | 59.73 | (53.3, 66.17) | 59.4 | (50.32, 68.47) | |
| CL-S | 79.18 | (74.28, 84.07) | 81.67 | (75.53, 87.81) | |
| RT quadratic term (ms/decile2) | CF-G | 7.31 | (6.23, 8.38) | 6.05 | (4.72, 7.37) |
| CF-S | 9.3 | (8.16, 10.44) | 8.23 | (6.74, 9.72) | |
| CL-G | 4.77 | (3.35, 6.19) | 4.52 | (2.66, 6.38) | |
| CL-S | 3.74 | (2.74, 4.74) | 4.73 | (3.59, 5.86) | |
Estimated marginal means for condition, and condition-specific trends across decile and decile2, for both placebo and tolcapone sessions are provided in Table 1. Follow-up z-tests determined that the 3-way interaction of interest (drug x condition x decile) was driven, at least in part, by a significant effect of tolcapone (vs. placebo) on RT slope for CF-G trials (trend estimate = −6.2, SE = 2.7, z = −2.3, p = 0.02). This effect on RT slope was also evident in the CF-G condition in the raw data (Figure 2B) and consistent with our hypothesis that the effect of tolcapone should be most evident when maintenance demands are high and (output) gating demands are low (Figure 1F). In addition, because optimized behavioral responses should have shorter RTs, this reduction in RT slope is consistent with the hypothesis that tolcapone should improve the efficiency of maintenance processes such that the proportion of trials with longer RTs should decrease.
Figure 2.
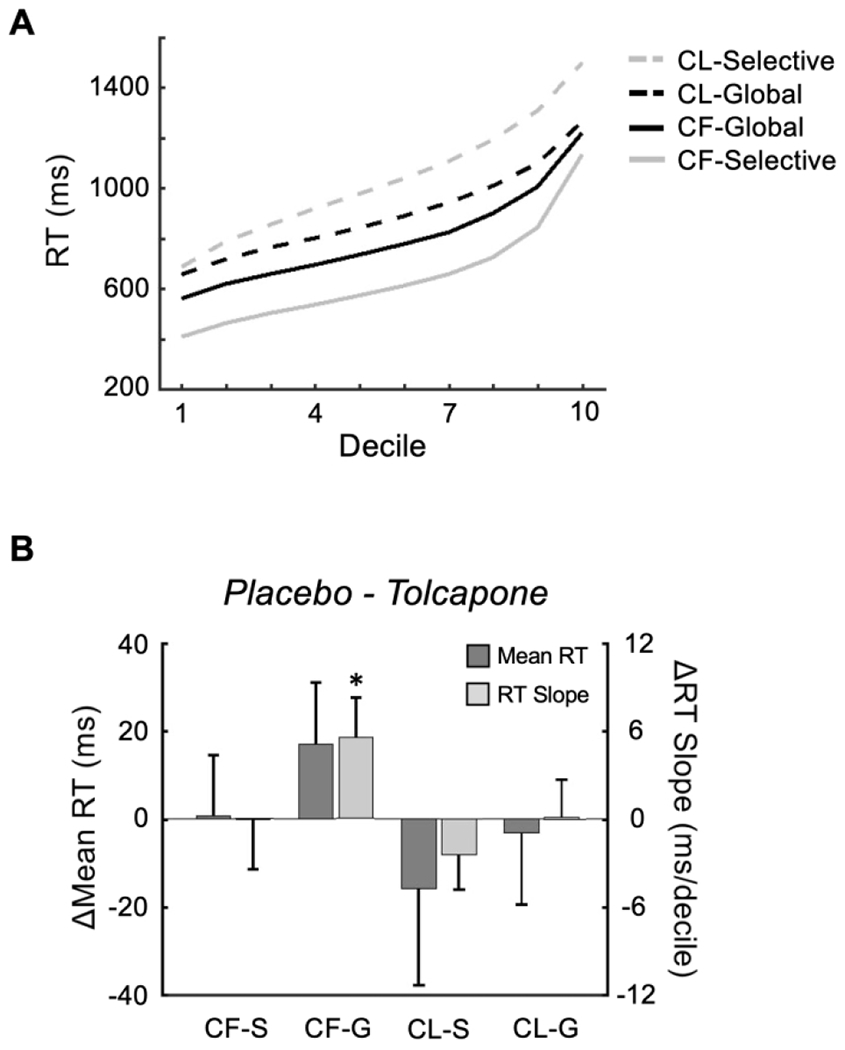
Behavior
A. Collapsed across drug condition, the raw RTs divided by decile demonstrate differences in both offset and slope for the four task conditions. B. The decline in RT slope on tolcapone versus placebo is evident in the model-free data for CF-G (* p < 0.05).
Drug x decile effects did not reach statistical significance for any of the other task conditions (CF-S: 0.08±2.9, z = 0.03; CL-S: 2.49±2.3, z=1.08; CL-G: −.33±3.5, z=−0.09), though notably, the trend for CL-S was numerically opposed to that observed for CF-G (i.e., greater slope on tolcapone). Indeed, upon directly comparing drug effects (drug x decile) between task conditions, we found a difference between CL-S and CF-G (8.7±3.05, z=2.73, p = 0.008, Bonferroni adjusted for 6 tests) but no significant difference between any other two conditions. Importantly, these two trial types are matched on working memory load and differ only in selective gating demands (Chatham et al., 2014). Thus, this comparison suggests that tolcapone may have opposing effects on the maintenance of information in WM and the ability to selectively gate information out of WM. Further, the specificity of this finding for the CF-G condition argues against a broader effect of tolcapone on some other, more general factor, such as the speed of motor responding.
Post-hoc examination of the decile2 x drug effect by condition revealed a pattern consistent with that described above: tolcapone decreased the magnitude of the quadratic trend in the CF-G condition but increased it in the CL-S condition. Though drug did not significantly change the quadratic trend within any condition (CF-S: −1.07±0.72, z=−1.48, p = 0.14; CF-G: −1.26± 0.66, z=−1.91, p=0.06; CL-S: 0.98±0.60, z=1.64, p=0.10; CL-G: −0.25±0.81, z=−0.31, p=0.75), direct comparison between task conditions again demonstrated a significant difference between CL-S and CF-G (2.24±0.84, z=2.69, p=0.04, Bonferroni adjusted for 6 tests).
To determine whether the significant drug x decile effect on WM maintenance reflected the function of a more stable underlying neural process (i.e. one on the order of minutes or hours rather than seconds), we took advantage of resting state data obtained from the same participants on tolcapone and placebo. Because resting state data are more likely to reflect an underlying state than a task-specific response, we focused on overall RT slope (i.e., drug x decile parameter from our model), though we also evaluated the additive, more condition-specific effects of RT slope for the CF-G condition (see Methods). Brain areas in the lateral frontal cortex that are sensitive to level of task abstraction and strongly linked to performance on this task, including the dorsal premotor cortex (PMd) and pre-premotor cortex (pPMd) (Badre & D’Esposito, 2009; Badre et al., 2010; Chatham et al., 2014), were used as seed regions for an individual differences analysis of resting state connectivity.
Notably, when evaluating connectivity between left PMd and the rest of the brain, we found changes in connection strength that correlated with the strength of the effect of tolcapone on overall RT slope within brain areas including the left fusiform cortex, right intraparietal sulcus, and the right lateral prefrontal cortex (Figure 3 and Table 2). We also found changes in left PMd <-> right fusiform cortex connectivity that were more specifically correlated with the drug-related change in CF-G behavior (Figure 3, right panel, and Table 2). No significant changes in connectivity between our PFC ROIs and the striatum were found for either analysis, nor for the comparable analyses with decile2 parameters. These results were not driven by outliers; tolcapone-induced increases in connectivity values, as shown for right middle intraparietal sulcus (mIPS; overall RT slope) and right fusiform gyrus (Figure 3, bottom; RT slope for the CF-G condition), correlated with tolcapone-induced flattening of RT slope across a broad range of connectivity values. (Data were very similar for the other significant regions listed in Table 2). No significant relationships emerged for the right PMd ROI.
Figure 3.
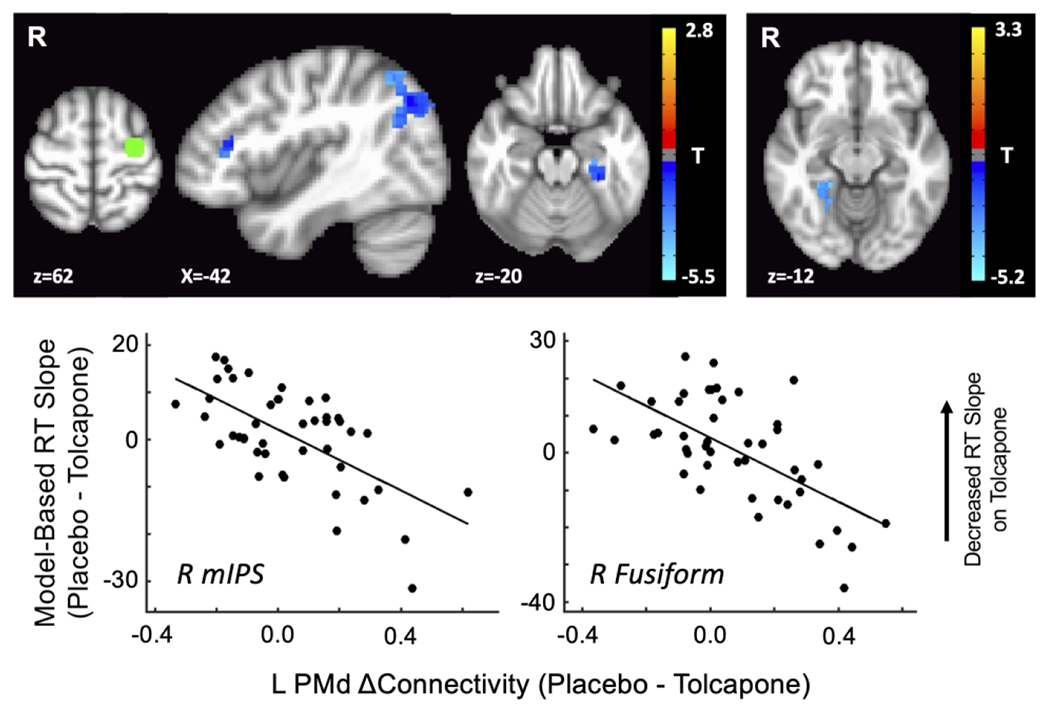
Resting state fMRI results: left dorsal premotor cortex
The strength of connectivity between a seed in left dorsal premotor cortex (L PMd; green region, upper left image) and every voxel in the brain was correlated with the subject-wise estimate of tolcapone’s effect on overall RT slope (left panel) or on RT slope for the CF-G condition (right panel). Significant regions (p < 0.001, corrected) for the former analysis include the right inferior frontal gyrus (IFG), right middle intraparietal sulcus (mIPS), and the left fusiform cortex; for the latter analysis, the right fusiform cortex was found. Representative plots of the datapoints for two regions, right mIPS and right fusiform cortex, are shown to demonstrate that outliers do not drive these effects.
Table 2:
Left Dorsal Premotor (PMd) Connectivity (p < 0.001, corrected)
| Region | Hemisphere | X | Y | Z | Peak T | Num Voxels |
|---|---|---|---|---|---|---|
| mIPS | R | −41 | 66 | 41 | −4.94 | 121 |
| Fusiform | L | 29 | 30 | −19 | −4.97 | 55 |
| IFG | R | −41 | −33 | 14 | −4.45 | 35 |
| CF-G Specific | ||||||
| Fusiform | R | −25 | 48 | −11 | −4.39 | 67 |
In a secondary analysis, we also evaluated changes in connectivity between a more anterior prefrontal region linked to performance on this task, the pre-PMd (Chatham et al., 2014), and the rest of the brain. We observed a significant change in connectivity between the left pre-PMd and bilateral primary somatomotor cortex that tracked the behavioral effect of tolcapone on overall RT slope; connectivity with a subset of left PSMC voxels was also sensitive to the drug-related change in RT slope for the CF-G condition (Figure 4 and Table 3). These changes were also not driven by outliers; tolcapone-induced increases in connectivity values between left pre-PMd and left precentral gyrus, as well as the left supplementary motor area (SMA), correlated with tolcapone-induced flattening of overall RT slope across a broad range of connectivity values. (Data were very similar for the other regions in Table 3). In contrast, suprathreshold regions in a connectivity analysis of right pre-PMd were driven by outlier subjects (data not shown), and thus were unrevealing. Lastly, no significant findings were seen for the decile2 parameters.
Figure 4.
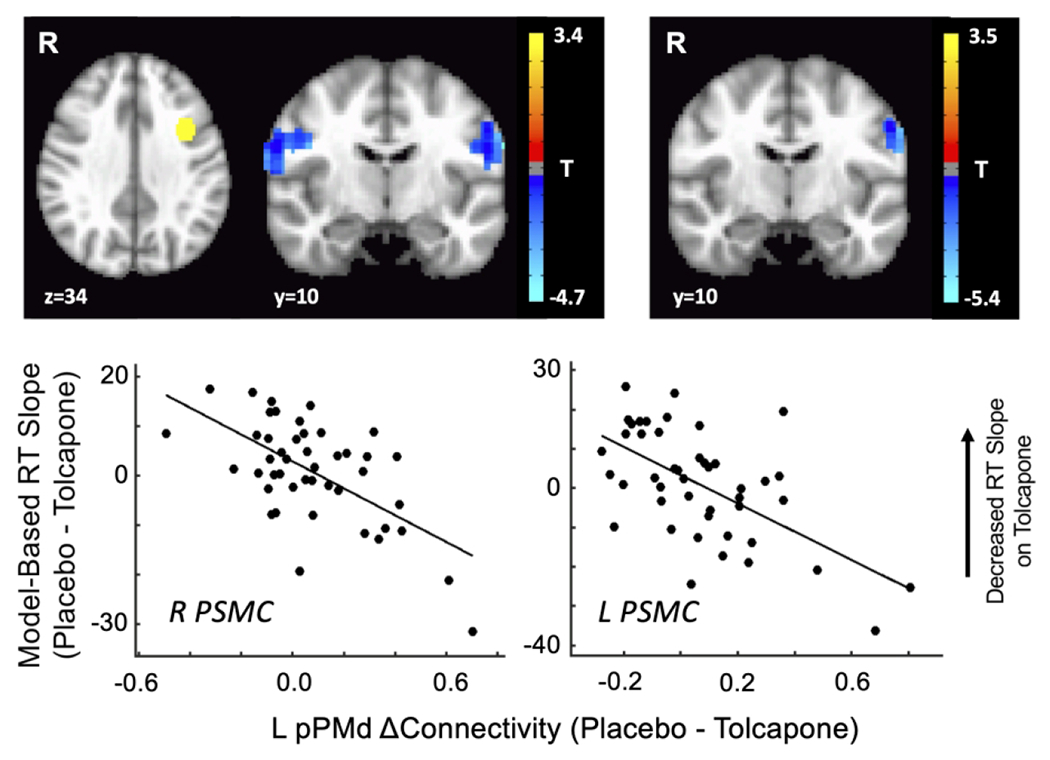
Resting state fMRI results: left pre-premotor cortex
The strength of connectivity between a seed in left pre-premotor cortex (L pPMd; yellow region, upper left image) and every voxel in the brain was correlated with the subject-wise estimate of tolcapone’s effect on overall RT slope. Significant regions (p < 0.001, corrected) for the overall effect of RT slope (left panel) include areas extending over the precentral and postcentral gyri bilaterally (primary somatomotor cortex, or PSMC). A subset of the L PSMC voxels was correlated with RT slope for the CF-G condition. Representative plots of the datapoints for two regions, right PSMC and left PSMC, are shown to demonstrate that outliers do not drive these effects.
Table 3:
Left Dorsal Pre-Premotor (pPMd) Connectivity (p < 0.001, corrected)
| Region | Hemisphere | X | Y | Z | Peak T | Num Voxels |
|---|---|---|---|---|---|---|
| Primary SMC | R | −60 | 8 | 25 | −4.63 | 145 |
| Primary SMC | L | 58 | 9 | 28 | −5.11 | 94 |
| CF-G Specific | ||||||
| Primary SMC | L | 59 | 11 | 33 | −4.82 | 55 |
Discussion:
Here we present convergent evidence that tolcapone significantly improves working memory maintenance without demonstrable effects on gating. Specifically, tolcapone reduces RT slope in a task condition that maximizes maintenance requirements and minimizes selective input and output gating demands (CF-G), but has no statistically significant effect on other task conditions. Moreover, this effect in CF-G is significantly different from the condition that most heavily taxes output gating (CL-S). Across subjects, the degree to which tolcapone reduces overall RT slope (i.e., collapsed across conditions) correlates directly with increases in connectivity between left PMd, a prefrontal region important for linking stimulus with response (Badre & D’Esposito, 2009), and posterior cortical areas previously implicated in visual working memory function, including the intraparietal sulcus and fusiform cortex. In complementary fashion, the degree to which tolcapone reduces RT slope across conditions also correlates with increases in connectivity between a prefrontal region important for more abstract task representations, left pre-PMd, and motor areas including the bilateral primary somatomotor cortex. No individual differences in the functional correlations between these cortical regions and the striatum were found to significantly track drug effects on behavior, as might be expected if gating function were affected. Together these results substantiate the hypothesis that cortical dopamine preferentially supports working memory maintenance rather than gating processes, consistent with theoretical and empirical accounts of working memory function (Cools & D’Esposito, 2011; D’Esposito & Postle, 2015; Frank & Badre, 2012; Frank & O’Reilly, 2006; M. Wang et al., 2004).
As noted above, tolcapone appears to primarily improve the efficiency of maintenance rather than gating. However, the context last (CL) conditions, which preferentially increase demands on output gating, also include a maintenance component, and yet did not show any effect of drug. The most likely explanation has to do with the relative influence of maintenance and gating on overall reaction time. On placebo, increased maintenance demands alone, when gating demands are minimal and constant, increase reaction time (as seen in the RT difference between the “context first” conditions, CF-S and CF-G; Table 1). However, stronger gating demands, and specifically output gating demands, drive a significantly larger increase in reaction time (Chatham et al., 2014): both conditions in which the context is presented last (CL-S, CL-G) have significantly longer reaction times than either of the context first conditions. In addition, the efficiency of output gating directly impacts the motor report used to infer the success of maintenance. Thus, while CL-S and CL-G also have relatively high maintenance requirements, the greater demands on output gating, especially in the selective (CL-S) condition, likely obscure any effects that tolcapone might have on maintenance. As a result, the effect of tolcapone is only significant in the CF-G condition. Alternatively, the tolcapone-induced increase in cortical dopamine tone might actively interfere with the function of the striatally-mediated output gate. In this case, the gate would function more inefficiently, and the effects of tolcapone on maintenance may be indistinguishable in these conditions, regardless of other task manipulations. Consistent with this possibility, we show a significant difference between the effects of tolcapone on the CF-G and CL-S conditions, reducing RT slope in the former but relatively increasing it in the latter (Figure 2B and Table 1).
Notably, in this task we do not strongly distinguish between maintenance of context and maintenance of content. Previous work has demonstrated that subjects can access the contents of working memory via distinct mechanisms, supporting the differentiation of context from content (Gehring, Bryck, Jonides, Albin, & Badre, 2003). Additional experiments have shown that context and content can be accessed relatively independently (Linares & Pelegrina, 2018), or that they may be retrieved together, as composites (Bialkova & Oberauer, 2010). Here, context (the number) is presented explicitly in each trial along with the target / non-target (letter and/or symbol). Our neural hypothesis – that maintenance operations are based in the cortex – does not directly speak to the context / content distinction. Similarly, our work does not speak to whether tolcapone influences a particular subprocess instantiated during maintenance, or the overall maintenance state per se. Future work (e.g. to determine the cortical locus for each of these context and content representations, or to place differential demands on hypothesized maintenance subprocesses) might address to what extent these factors are linked neurally. Additionally, complementing differences in the type of maintained information with parametric gating demands – e.g. by increasing variability in the number of items to be selected from working memory – would further clarify how different corticostriatal circuits support working memory function.
A second particularity of our results concerns the influence of increased frontal dopamine tone on the RT distribution (slope across deciles), but not the mean RT. Given that the lateral frontal cortex is thought to exert top-down control to maintain stimulus representations within posterior structures (D’Esposito & Postle, 2015; Rose et al., 2016), one potential explanation concerns the efficiency of this control. Because task demands are identical for all CF-G trials, but RTs in the last decile are more than 1.5 times the RTs in the first decile (Figure 2A), something other than external task demands must explain the discrepancy. Increased frontal dopamine tone may increase the efficiency of this top-down communication, stabilizing trial-wise top-down control and thereby increasing the proportion of trials for which control is optimized. Such a mechanism would reduce the frequency of trials in which top-down communication is inefficient, decreasing the number of RTs at the slower end of the distribution and leading to a decline in RT slope.
More generally, previous work suggests that a reduction in intra-individual variability can be linked to the optimization of both frontal and dopaminergic function (MacDonald, Li, & Backman, 2009). In a seminal study of patients with brain lesions of various etiologies, Stuss and colleagues demonstrated that lateral frontal lesions increase intra-individual RT variability in a visual shape selection task (Stuss, Murphy, Binns, & Alexander, 2003). Macdonald and colleagues subsequently showed that, in a task pitting number identity against number position, diminished D1 receptor binding in the dorsolateral prefrontal cortex, parietal cortex, and anterior cingulate cortex is likewise associated with increasing intra-individual RT variability for incongruent trials (MacDonald, Karlsson, Rieckmann, Nyberg, & Backman, 2012). Perhaps most directly, in a study linking behavior with the function of the COMT gene, Stefanis and colleagues (Stefanis et al., 2005) found that subjects with greater Met loading at the COMT Val158Met polymorphism demonstrated reduced intra-individual RT variability in the identical pairs version of the continuous performance task (CPT). Because the Met allele for this polymorphism reduces the dopamine metabolizing activity of the enzyme, it is thought to increase dopamine tone; thus, COMT inhibition by tolcapone would also be predicted to reduce intraindividual RT variability, as was seen here.
As demonstrated by the resting state functional MRI data, the behavioral effect of tolcapone, indexed by the model’s overall RT slope parameter for each subject, is reflected in connectivity changes within networks that differ across the lateral frontal cortex. Specifically, drug-related changes in functional connectivity between the pMD, implicated in linking stimulus with response, and left fusiform cortex, right IPS, and right inferior frontal gyrus were correlated with changes in overall RT slope, such that greater enhancement of connectivity tracked greater reduction of RT slope by tolcapone. The combination of fusiform cortex and IPS is frequently seen in the context of visual working memory tasks, in which visual association regions (such as the fusiform gyrus) and frontoparietal control regions (including the IPS) are co-active (D’Esposito & Postle, 2015; Xu, 2017). Although consistent, these findings are only suggestive given that a direct link to visual working memory activity is not possible with resting state data (as it would be with task-active fMRI of a working memory task). Caution should thus be used in extrapolating from brain region to cognitive process (Poldrack, 2011). Nonetheless, changes induced by dopamine in frontal networks have been well-established in previous resting state data (Dang, O’Neil, & Jagust, 2012; Kahnt & Tobler, 2017; Kelly et al., 2009), and we add to the functional relevance of such changes here.
Irrespective of their specific function, however, it is curious that dopaminergic changes in the functional connectivity of a more anterior prefrontal region, pre-PMd, involved brain areas typically associated with motor function – i.e. bilateral primary somatomotor cortex. One might instead expect, given the nature of our task and the observed effect of drug, that tolcapone would alter the more anterior lateral frontal region’s association with those supporting working memory maintenance, and the more posterior lateral frontal region’s association with those subserving its motor implementation. A potential explanation is based on the nature of the task itself. Task performance across the conditions is not distinguished by more abstract control requirements, but rather by load and gating demands. As a result, working memory demands are instead placed on the particular stimulus (e.g. the letter or the symbol) necessary for the response; the demands placed on more abstract task representations (e.g. of the context, as represented by the number) are consistent across tasks and are necessary only to the extent that they lead to the appropriate motor response.
As a caveat, while our primary behavioral result concerns an interaction between drug, decile, and condition, behavioral correlations with resting state fMRI data were primarily driven by the drug x decile parameter, collapsed across conditions. Because resting state functional connectivity is more likely to reflect an underlying state or process than a task-specific response, the overall RT slope parameter may better capture changes in this process (e.g. working memory maintenance) because it includes this change across all task conditions, despite the fact that the behavioral change only reaches significance for CF-G. That said, we did identify more focused areas of resting state connectivity that significantly correlated with the RT slope effect specific to the CF-G condition, suggesting that condition-specific effects may be present, though perhaps with less power. Future fMRI data obtained during task performance both on and off tolcapone would be better able to address the condition-specific nature of connectivity changes.
Given that these results demonstrate an effect of tolcapone on working memory maintenance, future work might also focus on complementary drug manipulations that more strongly impact input and output gating. While many mechanisms have been proposed for global gates that can update all items (or no items) to working memory, selective gating, whether at input or output, is thought to benefit most from striatal mechanisms (Chatham & Badre, 2015). As a result, striatally-acting D2 receptor agonists such as bromocriptine or cabergoline, in contrast to tolcapone, would be expected to impact selective input and output gating. More speculatively, the different posterior areas demonstrating tolcapone-induced changes in functional connectivity with left PMd and left pre-PMd suggest that disruption of activity in either of these two lateral frontal regions – e.g. by transcranial magnetic stimulation – might differentially diminish cognitive control, and thus task performance. If TMS of left PMd disrupts working memory maintenance, for example, accuracy should decrease in CF-G. On the other hand, if TMS of left pre-PMd disrupts motor activity, accuracy should remain unchanged, while RT should increase across all conditions. Together, an improved understanding of the brain networks responsible for optimizing working memory maintenance and gating may provide a better foundation for understanding their intermittent impairments in both control and patient populations.
Acknowledgments:
This work was supported by funding from the National Institute of Mental Health (R01 112775 to MH & AK) and the Office of Naval Research (MURI N00014-16-1-2832 to DB). The authors thank the research subjects whose generous participation allowed this study to be completed.
References
- Apud JA, Mattay V, Chen J, Kolachana BS, Callicott JH, Rasetti R, et al. (2007). Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology, 32(5), 1011–1020. [DOI] [PubMed] [Google Scholar]
- Badre D, & D’Esposito M (2009). Is the rostro-caudal axis of the frontal lobe hierarchical? Nat Rev Neurosci, 10(9), 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, & Frank MJ (2012). Mechanisms of hierarchical reinforcement learning in corticostriatal circuits 2: evidence from fMRI. Cereb Cortex, 22(3), 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Kayser AS, & D’Esposito M (2010). Frontal cortex and the discovery of abstract action rules. Neuron, 66(2), 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DJ, Levy R, Scheepers C, & Tily HJ (2013). Random effects structure for confirmatory hypothesis testing: Keep it maximal. J Mem Lang, 68(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Kliegl R, Vasishth S, & Baayen H (2015). Parsimonious mixed models. arXiv, 1506.04967. [Google Scholar]
- Bialkova S, & Oberauer K (2010). Direct access to working memory contents. Exp Psychol, 57(5), 383–389. [DOI] [PubMed] [Google Scholar]
- Cai JX, & Arnsten AF (1997). Dose-dependent effects of the dopamine D1 receptor agonists A77636 or SKF81297 on spatial working memory in aged monkeys. J Pharmacol Exp Ther, 283(1), 183–189. [PubMed] [Google Scholar]
- Chatham CH, & Badre D (2013). Working memory management and predicted utility. Front Behav Neurosci, 7, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatham CH, & Badre D (2015). Multiple gates on working memory. Curr Opin Behav Sci, 1, 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatham CH, Frank MJ, & Badre D (2014). Corticostriatal output gating during selection from working memory. Neuron, 81(4), 930–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, et al. (2004). Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet, 75(5), 807–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, & D’Esposito M (2011). Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry, 69(12), e113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ardenne K, Eshel N, Luka J, Lenartowicz A, Nystrom LE, & Cohen JD (2012). Role of prefrontal cortex and the midbrain dopamine system in working memory updating. Proc Natl Acad Sci U S A, 109(49), 19900–19909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, & Postle BR (2015). The cognitive neuroscience of working memory. Annu Rev Psychol, 66, 115–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang LC, O’Neil JP, & Jagust WJ (2012). Dopamine supports coupling of attention-related networks. J Neurosci, 32(28), 9582–9587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, & Raichle ME (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A, 102(27), 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, & Badre D (2012). Mechanisms of hierarchical reinforcement learning in corticostriatal circuits 1: computational analysis. Cereb Cortex, 22(3), 509–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Loughry B, & O’Reilly RC (2001). Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn Affect Behav Neurosci, 1(2), 137–160. [DOI] [PubMed] [Google Scholar]
- Frank MJ, & O’Reilly RC (2006). A mechanistic account of striatal dopamine function in human cognition: psychopharmacological studies with cabergoline and haloperidol. Behav Neurosci, 120(3), 497–517. [DOI] [PubMed] [Google Scholar]
- Furman DJ, White RL 3rd, Naskolnakorn J, Ye J, Kayser A, & D’Esposito M (2020). Effects of Dopaminergic Drugs on Cognitive Control Processes Vary by Genotype. J Cogn Neurosci, 32(5), 804–821. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Bryck RL, Jonides J, Albin RL, & Badre D (2003). The mind’s eye, looking inward? In search of executive control in internal attention shifting. Psychophysiology, 40(4), 572–585. [DOI] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, et al. (1998). Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A, 95(17), 9991–9996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahnt T, & Tobler PN (2017). Dopamine Modulates the Functional Organization of the Orbitofrontal Cortex. J Neurosci, 37(6), 1493–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser AS, Allen DC, Navarro-Cebrian A, Mitchell JM, & Fields HL (2012). Dopamine, corticostriatal connectivity, and intertemporal choice. J Neurosci, 32(27), 9402–9409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser AS, Mitchell JM, Weinstein D, & Frank MJ (2015). Dopamine, locus of control, and the exploration-exploitation tradeoff. Neuropsychopharmacology, 40(2), 454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, de Zubicaray G, Di Martino A, Copland DA, Reiss PT, Klein DF, et al. (2009). L-dopa modulates functional connectivity in striatal cognitive and motor networks: a double-blind placebo-controlled study. J Neurosci, 29(22), 7364–7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares R, & Pelegrina S (2018). Focus Switching in Working Memory. Exp Psychol, 65(3), 115–127. [DOI] [PubMed] [Google Scholar]
- Lorenc ES, Lee TG, Chen AJ, & D’Esposito M (2015). The Effect of Disruption of Prefrontal Cortical Function with Transcranial Magnetic Stimulation on Visual Working Memory. Front Syst Neurosci, 9, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald SW, Karlsson S, Rieckmann A, Nyberg L, & Backman L (2012). Aging-related increases in behavioral variability: relations to losses of dopamine D1 receptors. J Neurosci, 32(24), 8186–8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald SW, Li SC, & Backman L (2009). Neural underpinnings of within-person variability in cognitive functioning. Psychol Aging, 24(4), 792–808. [DOI] [PubMed] [Google Scholar]
- Mueller A, Krock RM, Shepard S, & Moore T (2020). Dopamine Receptor Expression Among Local and Visual Cortex-Projecting Frontal Eye Field Neurons. Cereb Cortex, 30(1), 148–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noudoost B, & Moore T (2011). Control of visual cortical signals by prefrontal dopamine. Nature, 474(7351), 372–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA (2011). Inferring mental states from neuroimaging data: from reverse inference to large-scale decoding. Neuron, 72(5), 692–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose NS, LaRocque JJ, Riggall AC, Gosseries O, Starrett MJ, Meyering EE, et al. (2016). Reactivation of latent working memories with transcranial magnetic stimulation. Science, 354(6316), 1136–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez I, Zhu L, Set E, Kayser A, & Hsu M (2015). Dopamine modulates egalitarian behavior in humans. Curr Biol, 25(7), 912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanis NC, van Os J, Avramopoulos D, Smyrnis N, Evdokimidis I, & Stefanis CN (2005). Effect of COMT Val158Met polymorphism on the Continuous Performance Test, Identical Pairs Version: tuning rather than improving performance. Am J Psychiatry, 162(9), 1752–1754. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Murphy KJ, Binns MA, & Alexander MP (2003). Staying on the job: the frontal lobes control individual performance variability. Brain, 126(Pt 11), 2363–2380. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Bannerman DM, Sharp T, & Harrison PJ (2004). Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci, 24(23), 5331–5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, & Arnsten AF (2007). Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci, 10(3), 376–384. [DOI] [PubMed] [Google Scholar]
- Wang M, Vijayraghavan S, & Goldman-Rakic PS (2004). Selective D2 receptor actions on the functional circuitry of working memory. Science, 303(5659), 853–856. [DOI] [PubMed] [Google Scholar]
- Wang Y, & Goldman-Rakic PS (2004). D2 receptor regulation of synaptic burst firing in prefrontal cortical pyramidal neurons. Proc Natl Acad Sci U S A, 101(14), 5093–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y (2017). Reevaluating the Sensory Account of Visual Working Memory Storage. Trends Cogn Sci, 21(10), 794–815. [DOI] [PubMed] [Google Scholar]


