Abstract
Roseburia intestinalis is an anaerobic, Gram-positive, slightly curved rod-shaped flagellated bacterium that produces butyrate in the colon. R. intestinalis has been shown to prevent intestinal inflammation and maintain energy homeostasis by producing metabolites. Evidence shows that this bacterium contributes to various diseases, such as inflammatory bowel disease, type 2 diabetes mellitus, antiphospholipid syndrome, and atherosclerosis. This review reveals the potential therapeutic role of R. intestinalis in human diseases. Patients with inflammatory bowel disease exhibit significant changes in R. intestinalis abundance, and they may benefit a lot from modulations targeting R. intestinalis. The data reviewed here demonstrate that R. intestinalis plays its role in regulating barrier homeostasis, immune cells, and cytokine release through its metabolite butyrate, flagellin and other. Recent advancements in the application of primary culture technology, culture omics, single-cell sequencing, and metabonomics technology have improved research on Roseburia and revealed the benefits of this bacterium in human health and disease treatment.
Keywords: Roseburia, Roseburia intestinalis, probiotic, inflammatory bowel disease (IBD), microbiome
Introduction
Roseburia spp. have been brought to the fore because of their novel role in modulating the gut microbial ecology, immune responses, and the development of human disorders (Tamanai-Shacoori et al., 2017). Roseburia was named in honor of Theodor Rosebury, an American microbiologist, for his groundbreaking contributions to the field of oral microbiome. Roseburia sp. belongs to the phylum Firmicutes, class Clostridia, order Clostridiales, and family Lachnospiraceae (Stackebrandt, 2014). The Roseburia genus has five well-characterized species (Roseburia intestinalis, Roseburia hominis, Roseburia inulinivorans, Roseburia faecis, and Roseburia cecicola), all of which produce short-chain fatty acids (SCFAs), such as acetate, propionate, and butyrate (Tamanai-Shacoori et al., 2017). In 2002, Duncan et al. isolated a strain of anaerobic, Gram-positive, slightly curved rod-shaped, and flagellated bacteria from human fecal samples. Subsequent phylogenetic analysis revealed its intimate similarity with R. cecicola and the members of cluster XIVa of Clostridium subphylum; thus, it was named Roseburia intestinalis (Duncan et al., 2002). Among the top 20 most abundant bacteria in the gut microbiome, the R. intestinalis cluster usually accounts for 0.9%–5.0% (mean = 2.3%) of the total microbiota (Hold et al., 2003; Hiippala et al., 2018).
R. intestinalis are obligate anaerobes that are difficult to culture. They were firstly isolated from human feces in an M2 medium-based culture system (Louis et al., 2004). It was reported that R. intestinalis XB6B4 can be cultivated under anaerobic conditions at 37°C in a complex medium containing clarified rumen fluid (described in Chassard et al., 2007) and 0%–5% complex substrates (oat spelt xylan, wheat or corn bran, pea fiber, cabbage, and leek) or 0%–3% sugar (xylose or glucose) (Chassard et al., 2007; Mirande et al., 2010). R. intestinalis DSM 14610 was reported to degrade and utilize oligofructose as the sole energy source, but only when acetate was added to a special medium for colon bacteria (Falony et al., 2006). This bacterium can also grow anaerobically at 37°C in lytic/10 anaerobic/F medium or LYBHI medium supplemented with yeast extract, cellobiose, maltose, and cysteine (Zhu, C. et al., 2018; Cornuault et al., 2020). Butyryl-CoA:acetate CoA transferase is the key enzyme that produces butyrate in butyrate-producing bacteria. This enzyme has been detected in R. intestinalis, which endows it with the ability to utilize acetate in order to produce butyrate (Pryde et al., 2002). Previous studies have found that R. intestinalis and Faecalibacterium prausnitzii are the most abundant butyrate-producing bacteria in human feces (Hold et al., 2003; Duncan et al., 2004). R. intestinalis can ferment fibers to produce butyrate in an M2 medium supplemented with wheat starch (Louis et al., 2004). Of note is that butyrate has been reported to exert pervasive anti-inflammatory and metabolic modulation effects in different disease models (Canfora et al., 2015; McNabney and Henagan, 2017). Therefore, R. intestinalis can be applied as a potential probiotic given its ability to produce butyrate. Enteric bacteria use fibers as a source of carbon to produce butyrate. Martinez et al. reported that a whole-grain diet increases the intestinal abundance of R. intestinalis and improves the concentration of interleukin-6 (IL-6, which is linked to metabolic dysfunctions) (Martinez et al., 2013). R. intestinalis also has the ability to ferment xylan and β-mannan, which are common fiber ingredients in the diet (Mirande et al., 2010; Leth et al., 2018; La Rosa et al., 2019). β-mannans and xylans are important components of the plant cell wall, and they are acetylated to protect them from degradation by glycoside hydrolases. β-mannans are widely present in human and animal diets. RiCE2 and RiCE17, two carbohydrate esterases from R. intestinalis, remove acetylations from all positions in complex β-mannans in a complementary manner (Michalak et al., 2020). The growth of R. intestinalis is influenced by the carbon source, symbiont, and anoxia, and also by the pH. It has been discovered that iron can modulate the ability of R. intestinalis to produce butyrate through the pyruvate mechanism: ferredoxin oxidoreductase (PFO) can convert pyruvate to acetyl-CoA (Dostal et al., 2015). In addition, PFO is sensitive to reducing ferredoxin (Dostal et al., 2015). These data show that R. intestinalis uses different carbohydrates as the source of energy and that its growth is susceptible to iron. The human regenerating family member 3 alpha (hREG3α) promotes the growth of R. intestinalis by inhibiting reactive oxygen species (ROS) signaling and induces resistance in mice with dextran sulfate sodium (DSS)-induced colitis (Darnaud et al., 2018). Colonic bacteria compete for the colonic ecological niche through nutrition plunder and preempt niche advantage (Leth et al., 2020). Several external factors, such as diet or antibiotic use, and endogenous factors may affect the ecological niche of R. intestinalis. It has been reported that ultravirulent phage mutants can drive the disruption of R. intestinalis and the ecological niche occupied by other resistant bacteria (Cornuault et al., 2020). This phenomenon reveals that the interactions among bacteria in the gut environment are complex. The structural characteristic of the flagella in R. intestinalis regulates its motion. The flagella help R. intestinalis to penetrate the colonic mucus layer in order to interact with the epithelium. Therefore, R. intestinalis is one of the most abundant butyrate-producing bacteria that adhere to intestinal mucin, facilitating its significant advantages for the probiotic role (Van den Abbeele et al., 2013).
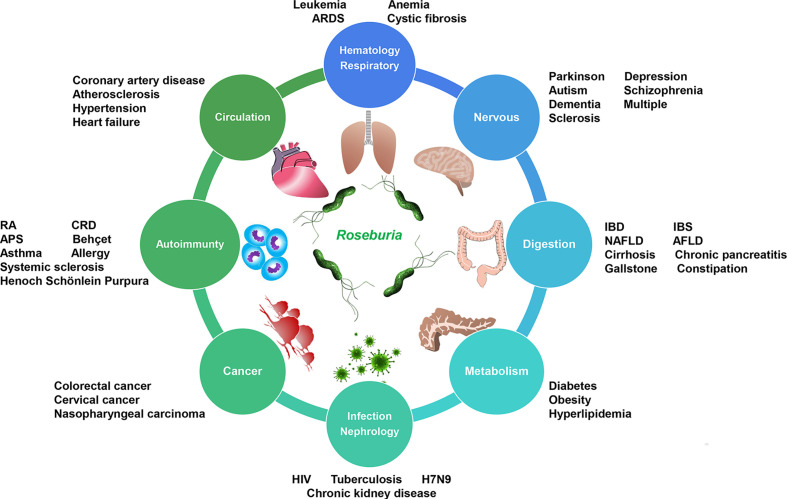
According to the guidelines from the World Health Organization (WHO) on the Evaluation of Probiotics in Food issued in 2002, probiotics are viable microorganisms in dietary supplements that, when consumed at certain levels, stabilize the gastrointestinal tract microflora and confer health benefits to the consumer. In addition to our attempts to expand knowledge about R. intestinalis ( Table 1 and Figure 1 ), multiple trials and experiments based on animal models have elaborated the complicated and tight relationship between R. intestinalis and the occurrence of human diseases ( Figure 2 ). Accumulating evidence also supports the probiotic effect of R. intestinalis in the human body, which demonstrates its fundamental benefits in maintaining a healthy homeostasis. However, the vast majority of the studies described the role of R. intestinalis through microbiome and metabolomics analyses ( Table 2 ). R. intestinalis frequently appears in the list of significantly variable bacteria of different diseases and has been shown to have a beneficial role. Numerous studies have also elucidated the effect of R. intestinalis on immunity and pathophysiology. As such, there is a need to clarify the role of R. intestinalis within separate systems.
Table 1.
Prior bacterial discoveries associated with Roseburia intestinalis.
| Study | Nationality | Year | Journal | Findings |
|---|---|---|---|---|
| (Duncan et al., 2002) | UK | 2002 | IJSEM | Roseburia intestinalis sp. nov., a novel saccharolytic, butyrate-producing bacterium from human feces. |
| (Hold et al., 2003) | UK | 2003 | A.E.M | Roseburia intestinalis: 2.3% of fecal microbiota populations at mean. The genus Roseburia is among the most abundant known butyrate-producing bacteria in human feces. |
| (Duncan et al., 2004) | UK | 2004 | A.E.M | Roseburia spp. seem to have a consistent contribution for acetate to butyrate formation. |
| (Mirande et al., 2010) | France | 2009 | J Appl Microbiol | Roseburia intestinalis is a dominant xylanolytic bacterium in dietary fiber degradation and fermentation. |
| (Van den Abbeele et al., 2013) | Belgium | 2012 | ISME J | Roseburia intestinalis colonizes mucins most specifically with flagella and may allow for penetration into the mucus layer. |
| (Martinez et al., 2013) | USA | 2012 | ISME J | Whole-grain barley enriches Roseburia intestinalis and improves metabolic dysfunctions. |
| (Qin et al., 2012) | China | 2012 | Nature | Roseburia intestinalis is one of the markers that might be useful for classifying type 2 diabetes. |
| (Russell et al., 2013) | UK | 2013 | MNFR | Roseburia intestinalis can ferment tryptophan indole-3-carboxylic acid. |
| (Arpaia et al., 2013) | USA | 2013 | Nature | Butyrate from colonic microorganisms promotes the differentiation of Tregs and influences the pro- and anti-inflammatory balance. |
| (Dostal et al., 2015) | Switzerland | 2015 | J Nutr | Iron modulates butyrate production in Roseburia intestinalis. |
| (Hoffmann et al., 2016) | France | 2016 | ISME J | Roseburia intestinalis induced a decreased IFNγ and IL-17 production with increased IL-22 production. |
| (Hegazy et al., 2017) | UK | 2017 | Gastroenterology | Roseburia intestinalis reactive CD4+ T cells support intestinal homeostasis, and their function is altered during inflammation. |
| (Kasahara et al., 2018) | USA | 2018 | Nat Microbial | Roseburia intestinalis interacts with dietary plant polysaccharides to provide protection against atherosclerosis. |
| (Shen et al., 2018) | China | 2018 | JGH | Roseburia intestinalis alleviates experimental colitis pathology by inducing anti-inflammatory responses. |
| (Quan et al., 2018) | China | 2018 | BBRC | Roseburia intestinalis-derived flagellin is a negative regulator of intestinal inflammation. |
| (Zhu C. et al., 2018) | China | 2018 | Mol Med Rep | Roseburia intestinalis inhibits interleukin-17 excretion and promotes regulatory T-cell differentiation. |
| (Seo et al., 2020) | Korea | 2019 | Cell Host Microbe | Roseburia intestinalis improves the gut ecosystem, leading to ameliorated alcoholic fatty livers. |
| (Tan et al., 2019) | China | 2019 | Scandinavian J.G | Roseburia intestinalis inhibits oncostatin M and maintains tight junction integrity in a murine model. |
| (Luo et al., 2019) | China | 2019 | Mol Med Rep | Roseburia intestinalis supernatant ameliorates colitis induced in mice by regulating the immune response. |
| (La Rosa et al., 2019) | Norway | 2019 | Nat Com | Roseburia intestinalis is a primary degrader of dietary β-mannans. |
| (Ruff et al., 2019) | USA | 2019 | Cell Host Microbe | Roseburia intestinalis cross-reacts with T and B cells to induce auto-APS antibody. |
| (Kellermayer, 2019) | USA | 2019 | Gastroenterology | Roseburia species: prime candidates for microbial therapeutics in inflammatory bowel disease. |
| (Xu et al., 2021) | China | 2021 | Therap Adv Gastroenterol | Roseburia intestinalis modulates the gut homeostasis by the gut–brain axis. |
| (Montalban-Arques et al., 2021) | Switzerland | 2021 | Cell Host Microbe | Roseburia intestinalis exerts synergic therapeutical effects in anti-PD-1 therapy of colorectal cancer. |
Figure 1.
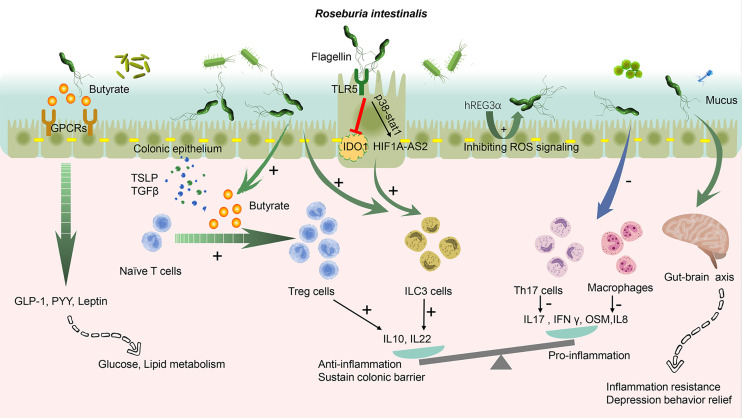
Roseburia intestinalis modulation in the colonic tract. The butyrate produced by R. intestinalis exerts an extensive effect on energy metabolism, gut barrier, and anti-inflammation. R. intestinalis stimulates enteric cells, thereby excreting cytokines, promoting the differentiation of regulatory T cells (Tregs), and activating type 3 innate lymphoid cells (ILC3). It also suppresses Th17 and macrophages. Its flagellin displays an anti-inflammation effect through TLR5. The biological effect induced by R. intestinalis exhibits a significant probiotic-like role. R. intestinalis also influences the energy metabolism and the gut–brain axis. A plus sign indicates promote and a minus sign indicates inhibit. GPCRs, G-protein coupled receptors; TSLP, thymic stromal lymphopoietin; GLP-1, glucagon-like peptide-1; PYY, peptide YY; OSM, oncostatin M; hREG3α, human regenerating family member 3 alpha; TLR5, Toll-like receptor 5; IDO1, indoleamine 2,3-dioxygenase-1; HIF1A-AS2, lncRNA (HIF1A-AS2).
Figure 2.
Roseburia dysbiosis-associated diseases exist in different systems. IBD, inflammatory bowel diseases; IBS, irritable bowel syndrome; NAFLD, non-alcoholic fatty liver disease; AFLD, alcoholic fatty liver disease; CRD, chronic rheumatoid disease; APS, antiphospholipid syndrome; HIV, human immunodeficiency virus; H7N9, avian influenza A (H7N9); ARDS, acute respiratory distress syndrome.
Table 2.
Studies associated with Roseburia spp. in different diseases of the different systems.
| Study | Country | System | Disease | Source | Size | Sample | Method | Findings |
|---|---|---|---|---|---|---|---|---|
| (Gevers et al., 2014) | USA. | Digestion | Crohn’s | Human | 668 | Feces | 16S rRNA | Roseburia intestinalis ↓ |
| (Morgan et al., 2012) | USA. | Digestion | Crohn’s | Human | 223 | Feces | 16S rDNA V3–V5 | Roseburia ↓ |
| (Kumari et al., 2013) | India | Digestion | UC | Human | 40 | Feces | FISH–flow cytometry | Roseburia ↓ |
| (Rajilic-Stojanovic et al., 2013) | Serbia | Digestion | UC | Human | 45 | Feces | 16S rRNA | Roseburia spp. ↓ |
| (Chassard et al., 2012) | France | Digestion | IBS | Human | 26 | Feces | FISH | Roseburia ↓ |
| (Gobert et al., 2016) | France | Digestion | IBS | Human | 91 | Feces | 16S rRNA V5–V6 | Roseburia ↓ |
| (Parthasarathy et al., 2016) | USA. | Digestion | Constipation | Female | 50 | Mucosa feces | 16S rRNA V3–V5 | Roseburia correlated with faster colonic transit |
| (Zhu et al., 2013) | USA. | Digestion | NAFLD | Human | 53 | Feces | 16S rRNA | Roseburia ↓ |
| (Saltzman et al., 2018) | Australia | Digestion | NAFLD | Human | Review | Roseburia ↓ | ||
| (Raman et al., 2013) | UK | Digestion | NAFLD | Human | 60 | Feces | 16S rRNA | Roseburia ↑ |
| (Seo et al., 2020) | Korea | Digestion | AFLD | Human | 212 | Feces | 16S rRNA | Roseburia spp. ↓ |
| (Bajaj et al., 2018) | USA. | Digestion | Cirrhosis | Human | 180 | Feces | 16S rRNA, 16S rDNA | Roseburia was protective against hospitalizations in the DNA model |
| (Bajaj et al., 2012) | USA. | Digestion | Cirrhosis | Human | 53 | Mucosa | 16S rRNA | Roseburia ↓ in hepatic encephalopathy mucosal microbiome |
| (Forbes et al., 2018) | Canada | Autoimmunity | Rheumatoid arthritis | Human | 44 | Feces | 16S rRNA V4 | Roseburia ↓ |
| (Consolandi et al., 2015) | Italy | Autoimmunity | Behçet syndrome | Human | 38 | Feces | 16S rRNA | Roseburia ↓ |
| (Patrone et al., 2017) | Italy | Autoimmunity | Systemic sclerosis | Human | 18 | Feces | 16S rRNA V3–V4 | Roseburia ↓ in patient with gastrointestinal involvement |
| (Salem et al., 2019) | France | Autoimmunity | CRD | Human | 1084 | Feces | Review | Roseburia ↓ |
| (Ruff et al., 2019) | USA. | Autoimmunity | APS | Human | 35 | Feces | 16S rRNA V4 | Roseburia intestinalis involved APS occuring |
| (Qin et al., 2012) | China | Metabolism | Diabetes | Human | 345 | Feces | MGWAS | Roseburia intestinalis ↓ |
| (Tilg and Moschen, 2014) | Australia | Metabolism | Diabetes | Human | Review | Roseburia intestinalis ↓ | ||
| (Leiva-Gea et al., 2018) | Spain | Metabolism | Diabetes | Human | 43 | Feces | 16S rRNA V2–V3 | Roseburia ↓ |
| (Jamshidi et al., 2019) | Iran | Metabolism | Diabetes | Review | The most common bacterial alterations included Roseburia spp. | |||
| (Tims et al., 2013) | Netherlands | Metabolism | Obesity | Human | 80 | Feces | 16S rRNA | Roseburia intestinalis ↑ |
| (Gargari et al., 2018) | Italy | Metabolism | Hyperlipidemia | Human | 30 | Feces | 16S rRNA | Roseburia ↓ |
| (Keshavarzian et al., 2015) | USA. | Nervous | Parkinson’s | Human | 72 | Feces | 16S rRNA V4 | Roseburia ↓ |
| (Sampson et al., 2016) | USA. | Nervous | Parkinson’s | Human | 12 | Feces | 16S rRNA V4 | Roseburia spp. ↑ |
| (Zheng et al., 2016) | China | Nervous | Depression | Human | 121 | Feces | 16S rRNA V4–V5 | Roseburia ↓ |
| (Jiang et al., 2015) | China | Nervous | Depression | Human | 76 | Feces | 16S rRNA V3–V4 | Roseburia ↑ |
| (Liu et al., 2016) | China | Nervous | Depression | Human | 100 | Feces | 16S rRNA | Roseburia as a feature of depression compared with D-IBS |
| (Karlsson et al., 2012) | Sweden | Circulation | Atherosclerosis | Human | 25 | Feces | Sequencing | Roseburia ↓ |
| (Kasahara et al., 2018) | USA. | Circulation | Atherogenesis | Mice | 342 | Ceca | 16S rRNA | Roseburia intestinalis ↓ |
| (Liu et al., 2019) | China | Circulation | CAD | Human | 201 | Feces | 16S rRNA V3–V4 | Bacterial co-abundance group at different stages of CAD was represented by Roseburia. |
| (Zhu Q. et al., 2018) | China | Circulation | CAD | Human | 168 | Feces | 16S rRNA V4 | Roseburia ↓ |
| (Yan et al., 2017) | China | Circulation | Hypertension | Human | 120 | Feces | MGWAS | Roseburia spp. ↓ |
| (Qin et al., 2018) | China | Circulation | Hypertension | Rat | 16 | Feces | 16S rRNA | Roseburia ↓ |
| (Zheng et al., 2019) | China | Circulation | Heart failure | Rat | 30 | Feces | Sequencing | Roseburia ↓ in the 4w-HF group |
| (Rajagopala et al., 2016) | USA. | Hematology | Leukemia | Human | 51 | Feces | 16S rRNA | Roseburia ↓ |
| (Bindels et al., 2015) | Belgium | Hematology | Leukemia | Mice | 33 | Feces | 16S rRNA | Increased Roseburia spp. ameliorated leukemia progression. |
| (McClorry et al., 2018) | Peru | Hematology | Anemia | Infant | 95 | Feces | 16S rRNA V4 | Roseburia ↓ |
| (Dostal et al., 2012) | Switzerland | Hematology | Anemia | Rat | 40 | Feces | 16S rRNA V2–V3 | Roseburia spp. ↓ |
| (Dillon et al., 2017) | USA. | Infection | HIV | Human | 32 | Mucosa | 16S rRNA | Roseburia intestinalis ↓ |
| (McHardy et al., 2013) | USA. | Infection | HIV | Human | 60 | Mucosa | 16S rRNA V4 | Roseburia ↓ in those not receiving anti-retroviral therapy. |
| (Guillen et al., 2019) | Spain | Infection | HIV | Human | 156 | Feces | 16S rRNA | Roseburia intestinalis predicts the presence of gut dysbiosis. |
| (Maji et al., 2018) | India | Infection | Tuberculosis | Human | 12 | Feces | 16S rRNA V3 | Roseburia ↑ |
| (Hu et al., 2019) | China | Infection | Tuberculosis | Human | 61 | Feces | Sequencing | Roseburia ↓ |
| (Liang et al., 2017) | China | Cancer | CRC | Human | 439 | Feces | 16S rRNA | Roseburia intestinalis ↓ |
| (Yu et al., 2017) | China | Cancer | CRC | Human | 168 | Feces | MGWAS | Roseburia intestinalis ↓ |
| (Chen et al., 2013) | China | Cancer | CRC | Human | 100 | Feces | 16S rRNA V1–V3 | Roseburia ↓ |
| (Wang et al., 2012) | China | Cancer | CRC | Human | 94 | Feces | 16S rRNA V3 | Roseburia ↓ |
| (Jiang et al., 2019) | China | Cancer | Nasopharyngeal carcinoma | Human | 59 | Feces | 16S rRNA V3–V4 | Roseburia spp. ↓ |
| (Wang et al., 2019) | China | Cancer | Cervical cancer | Human | 13 | Feces | 16S rRNA V4 | Roseburia spp. were more abundant in the CCa group. |
| (Li et al., 2014) | China | Respiration | ARDS | Rat | 16 | Feces | 16S rRNA V4 | Roseburia ↓ |
| (Xu et al., 2017) | China | Urinary | CKD | Human | 64 | Feces | 16S rRNA V4 | Roseburia ↓ |
| (Jiang et al., 2017) | China | Urinary | CKD | Human | 112 | Feces | qPCR, 16S rRNA | Roseburia ↓ |
| (Wu, I. W. et al., 2020) | China | Urinary | CKD | Human | 130 | Feces | 16S rRNA V4 | Roseburia was negatively correlated with CKD severity. |
Arrows ↑ ↓ : the Roseburia changes in specific disease patients compared with controls.
UC, ulcerative colitis; IBS, irritable bowel syndrome; FISH, fluorescence in situ hybridization; MGWAS, metagenome-wide association study; NAFLD, non-alcoholic fatty liver disease; AFLD, alcoholic fatty liver disease; CRD, chronic rheumatoid disease; APS, antiphospholipid syndrome; CAD, coronary artery disease; HIV, human immunodeficiency virus; H7N9, avian influenza A (H7N9); CRC, colorectal cancer; ARDS, acute respiratory distress syndrome; NMOSD, neuromyelitis optical spectrum disorders.
Digestive System
The Role of R. intestinalis in Digestive Diseases
R. intestinalis, as a digestive tract resident, plays a role in metabolic reprogramming, immune activation, and in sustaining the gut barrier. Notable prominent features of inflammatory bowel disease (IBD) [encompassing Crohn’s disease (CD) and ulcerative colitis (UC)] include microbiota dysbiosis, impaired innate immunity, and extensive tissue damage (mainly in the colon). Global cohorts ( Table 2 ) have uncovered the dysbiosis microbiome profile of IBD with significantly low numbers of Roseburia. A large sample size-based cohort study of the microbiome research project involving 668 CD patients and healthy subjects revealed a low number of R. intestinalis in CD patients (Gevers et al., 2014). Functional enrichment analysis further demonstrated more oxidative stress-activated signaling pathways and fewer carbohydrate and amino acid metabolism pathways associated with nutrition transportation and absorption (Gevers et al., 2014). Another pediatric CD study reported a low abundance of core bacteria, including Faecalibacterium and Roseburia, in patients with ileal CD (Morgan et al., 2012). Antibiotic exposure was demonstrated to magnify the above-mentioned intestinal dysbiosis in CD patients. Previous investigations by our research group revealed similar results of reduced Roseburia in CD patients (Shen et al., 2018). UC and CD have similar changes in Roseburia, although their signatures in genetics and pathophysiology are widely different. For instance, Kumari revealed lower abundance of R. intestinalis and decreased levels of fecal SCFAs in UC patients compared to the healthy group, particularly for butyrate (Kumari et al., 2013). Elsewhere, a study conducted in Serbia involving 15 UC patients and 15 healthy controls reported decreased levels of Roseburia sp. in the microbiota of UC patients, and the dysbiosis in UC was persistent in time and segment dimensions (Rajilic-Stojanovic et al., 2013). Therefore, it is increasingly becoming apparent that Roseburia is depleted in IBD via a mechanism associated with the anti-inflammatory effect, barrier protective effect, and the close connection of R. intestinalis with innate immunity. Irritable bowel syndrome (IBS) is a colonic transit and sensory disorder characterized by constipation, diarrhea, or mixed subgroups. Results from 16S ribosomal RNA (rRNA) sequencing and fluorescence in situ hybridization (FISH) analysis in a French cohort showed that constipated-IBS (C-IBS) patients had lesser Roseburia abundance than did the controls (Chassard et al., 2012; Gobert et al., 2016). These findings demonstrate that both IBS and constipation are likely associated with low Roseburia abundance and impaired colonic transit. For instance, a previous investigation of the colonic mucosal microbiota, fecal microbiota, and the transit signature between constipated patients and healthy controls revealed a correlation of the Roseburia genus with faster colonic transit (Parthasarathy et al., 2016). Butyrate, mainly metabolized in the liver, exerts a local inhibitory effect against liver adipogenesis and adipose accumulation (Canfora et al., 2015). A high-fat supplement elevates the triglyceride content and decreases β-oxidation within the liver. Studies have demonstrated that butyrate or butyrate-producing bacterial strains are related with reduced ectopic lipids in a non-alcoholic fatty liver disease (NAFLD) animal model (Mattace Raso et al., 2013; Jin et al., 2015). Based on existing factors, there is strong evidence that Roseburia is more underrepresented in NAFLD patients than in the control group (Zhu et al., 2013; Saltzman et al., 2018). However, some researchers reported divergent results and proposed that the altered diet and habitat effects may explain the discrepancy in the results (Raman et al., 2013). Seo et al. (2020) reported that alcoholic fatty liver disease (AFLD) displayed a significant depletion of R. intestinalis, and such dysbiosis correlates with a medical history of alcohol intake in a cohort of Korean twins (Seo et al., 2020). R. intestinalis potentially ameliorated the experimental AFLD model by restoring the gut barrier integrity. The flagellin of R. intestinalis is an active microbial ingredient that elevates tight junction protein and modulates immune response via the TLR5 receptor (Seo et al., 2020). In other digestive disorders such as cirrhosis, the fecal microbial sequencing results of cirrhosis patients showed that Roseburia protected against hospitalization. This study used fecal RNA instead of DNA for microbial 16S rRNA sequencing in order to better understand the bacterial metabolic activity (Bajaj et al., 2018). Hepatic encephalopathy is a severe complication of hepatic cirrhosis, commonly accompanied by impaired gut permeability. In a past study, lower Roseburia abundance was reported in sigmoid mucosal microbiota (Bajaj et al., 2012). Of note is that the majority of studies support the view that Roseburia exerts a protective role in most digestive diseases. R. intestinalis, together with the metagenome, plays beneficial functions in maintaining health and preventing diseases in animal and cell models. However, the primary culture of R. intestinalis strains remains a major hindrance, and there is a lack of well-designed clinical trials to confirm the protective role of R. intestinalis.
Mechanisms of R. intestinalis in Digestive Diseases
Extensive effects of R. intestinalis and its derivatives have been implicated in immune modulation and inflammatory regulation. R. intestinalis primarily functions as a primary butyrate-producing bacterium. Butyrate produced by commensal microbes was revealed to promote the proliferation of extrathymic regulatory T cells (Tregs) through intrinsic enhancer conserved non-coding sequence 1 (CNS1) (Arpaia et al., 2013). The mechanism was essential for extrathymic Treg differentiation, but dispensable for thymic Tregs. Tregs, as vital anti-inflammatory lymphocytes, produce interleukin-10 (IL-10), transforming growth factor beta (TGF-β), and interferon gamma (IFNγ). Microbial butyrate has been demonstrated to contribute to inner pro- and anti-inflammatory balance by influencing the differentiation of Tregs (Hegazy et al., 2017). In view of these findings, our research group investigated the role of Tregs in mouse models induced by bacterial suspension gavage and cell line co-culture. The results demonstrated that R. intestinalis inhibited the secretion of interleukin-17 (IL-17) and promoted the differentiation of Tregs in 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis (Zhu C. et al., 2018). Recent evidence showed that 5-hydroxyindoleacetic acid (5-HIAA) produced via butyrate stimulation can bind the aryl hydrocarbon receptor (AhR) in regulatory B cells (Bregs), inducing suppressive effects (Michaudel and Sokol, 2020). In memory T cells, butyrate activates β-oxidation and significantly influences the state and function of cells (Bachem et al., 2019). It is possible that an impaired production of butyrate may contribute to the pro-inflammatory polarization of intestinal macrophages. In addition, butyrate potentially regulates the intestinal macrophage function via histone deacetylase inhibition, and this causes a dysfunction of the immune response (Michaudel and Sokol, 2020). Evidence shows that gut microbiota-derived butyrate modulates the functions of type 2 innate lymphoid cells (ILC2), which block their uncontrolled activation; as such, they consequently exert a negative role in lung inflammation and asthma (Lewis et al., 2019). Notably, the involvement of intracellular metabolism is supported by the butyrate-mediated induction of changes in mitochondrial ROS (mROS) production and glycolysis (Lewis et al., 2019). Additionally, the preferential use of Fas over glucose by ILC2 to maintain their function in infection or nutritional stress suggests a direct role of butyrate in fueling the tricarboxylic acid cycle (Michaudel and Sokol, 2020). Consequently, butyrate derived from R. intestinalis may exert a profound immune effect in the gut and establish the unique status of R. intestinalis.
Together with similar models, our study demonstrated another mechanism of R. intestinalis in decreasing the secretion of the macrophage-derived oncostatin M (OSM) and maintaining intestinal mucosal permeability to alleviate DSS-induced colitis (Tan et al., 2019). We elucidated Crohn’s bacterial composition signature with reduced R. intestinalis in CD through 16S rRNA sequencing of fecal samples obtained from CD patients and healthy controls (Yang et al., 2020). In a subsequent experiment, we discovered that R. intestinalis alleviates intestinal inflammation by enhancing the proliferation of Tregs and promoting the secretion of anti-inflammatory cytokines [IL-10, TGF-β, and thymic stromal lymphopoietin (TSLP)] (Shen et al., 2018). Through gas chromatography–mass spectrometry (GC-MC) analysis, we affirmed that SCFAs were present in the R. intestinalis non-protein supernatant. Elsewhere, the R. intestinalis non-protein supernatant was found to alleviate TNBS- and DSS-induced colitis by reducing the percentages of colonic inflammatory macrophages and Th17 cells. Such shifts downregulated the IL-6 and STAT3 in both mouse models and cell lines (Luo et al., 2019). The above-described findings suggest a significant anti-inflammatory and immune-modulating effect of the butyrate produced by R. intestinalis exhibited in the gut. Our novel findings are consistent with the results reported by (Arpaia et al., 2013 and Hegazy et al., 2017).
A previous investigation by (Patterson et al., 2017) demonstrated that R. hominis potentially strengthens the function of the gut barrier and enhances the expansion of the population of Tregs, possibly via flagellin/Toll-like receptor 5 (TLR5) signaling. Also, (Vijayan et al., 2018) found that activated TLR5 could stimulate type 3 innate lymphoid cells (ILC3) and promote the production of interleukin-22 (IL-22), which sustains the gut barrier function. In our research, for a clear definition of the physiological role of flagellin, we used the recombinant flagellin of R. intestinalis. The analysis revealed that flagellin played an inflammatory inhibitory role by upregulating lncRNA (HIF1A-AS2) via p38/STAT1 signaling, exhibiting an anti-inflammatory role (Quan et al., 2018). At the same time, R. intestinalis-derived flagellin ameliorated colitis by targeting the miR-223-3p-mediated activation of NLRP3 inflammasome and pyroptosis. The method combined miRDB and TargetScan database prediction and experiment validation in order to ascertain the beneficial role of R. intestinalis in colitis (Wu, I. W. et al., 2020). ILC3s is a member of innate lymphatic cells, and its widespread effects in pathogen resistance, regulation of autoimmune inflammation, tissue remodeling, cancer, and metabolic homeostasis have been reported (Klose and Artis, 2016). R. intestinalis can ferment tryptophan into indole-3-carboxylic acid, a member of the indoles (Russell et al., 2013). In addition, some tryptophan metabolites (e.g., indoles including indole-3-carboxylic acid) may activate the AhR of ILC3. For instance, Hoffmann et al. (2016), using gnotobiotic mice transplanted with R. intestinalis, investigated the physiological impact on the host from the aspects of colonic histology, SCFAs, immune signature, transcriptome, and bile acid metabolism. They revealed that R. intestinalis could induce the upregulation of IL-22 and the downregulation of IL-17 and IFNγ in bacteria-transplanted gnotobiotic mice (Hoffmann et al., 2016). Corresponding findings also support an essential role of ILC3 in the immune modulation of R. intestinalis. In our recent study, we have evaluated whether R. intestinalis could significantly influence the activity of indoleamine 2,3-dioxygenase-1 (IDO1, an enzyme dominating tryptophan metabolism) and compared the results to other glycolysis lipolytic and amino acid metabolic enzymes (unpublished). In another recent project in 2019, we aimed to investigate the balancing mechanism between intracellular and extracellular tryptophan metabolism. The results of the study demonstrated that intracellular IDO1-mediated tryptophan metabolism potentially altered R. intestinalis flagellin–TLR5 signaling. Also, extracellular tryptophan metabolic indoles were suggested to activate ILC3 and IL-22 production via the AhR. Notably, the combined effect may induce colonic and systemic anti-inflammatory effects by immunometabolism. More and more evidence had accumulated to support the potential role the gut–brain axis plays in digestive disorders. For example, R. intestinalis may function in the modulation of the gut–brain axis by reducing the level of colonic 5-hydroxytryptamine (5-HT), inhibiting the expression of glial fibrillary acidic protein (GFAP), and alleviating the depression-like behaviors in colitis model mice (Xu et al., 2021). These pieces of evidence provide a new perspective on the therapeutic role of R. intestinalis in inflammatory bowel diseases.
Autoimmune Disorders
Autoimmune diseases usually involve multiple systems, including the intestine. Bacteria interact with enteric mucosal immune cells by presenting antigen and chemical signals to organs, such as the thymus, brain, liver, and the pancreas. It is universally accepted that the immune system mounts a response to invaders. Usually, autoimmune diseases have complicated pathogenesis associated with gut microbes. According to the sequencing results of the fecal samples obtained from 44 Canadian rheumatic arthritis (RA) patients, Forbes et al. found a decreased Roseburia abundance, which was used to distinguish RA patients from the healthy group in a random forest classification model (Forbes et al., 2018). In addition, the Roseburia genus was a potential marker of health given its butyrate-producing and anti-inflammatory properties (Forbes et al., 2018). Notably, identical lower gut Roseburia abundance phenomena were found in adults with Behçet syndrome and systemic sclerosis, particularly in systemic sclerosis patients with gastrointestinal involvement (Consolandi et al., 2015; Patrone et al., 2017). However, these studies were limited by the small sample size of the cohorts. Elsewhere, a systematic literature review of studies involving the collection of fecal samples from 1,084 patients with chronic rheumatic diseases and healthy controls revealed a decreased number of Roseburia species in enrolled patients (Salem et al., 2019).
Bacteria sometimes imitate autologous antigens to induce autoimmune antibodies. In antiphospholipid syndrome (APS), researchers have discovered cross-reactivity between non-orthologous mimotopes expressed by R. intestinalis and the autoantigen β2-glycoprotein (β2GPI) (Ruff et al., 2019). Besides, the anti-R. intestinalis mimotope immunoglobulin G (IgG) was significantly elevated in APS patients and was correlated with anti-β2GPI IgG autoantibodies. Meanwhile, R. intestinalis gavage could trigger an autoimmune pathology in susceptible mice (Ruff et al., 2019). However, due to R. intestinalis appearing as a type of common gut-friendly bacteria, the specific autoimmune phenotype transformation needs stricter consideration in such a mouse model. Otherwise, the on–off switch may hide in a particular microenvironment. These findings provide strong evidence that R. intestinalis displays beneficial effects in most patients with autoimmune diseases despite the occasionally complicated effects. As such, further exploration is warranted to elucidate the mechanism of the interaction of R. intestinalis with the immune system, an adaptation of immune cells, perception, and evolution with intestinal microbes.
Metabolic Diseases
Through dysbiosis-associated Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis, researchers have demonstrated that intestinal microbiome dysbiosis alters the commensal remolding and shifts the nutritional metabolism. Accumulating evidence reveals the tight association of butyrate with glucose homeostasis, insulin resistance, and appetite (Canfora et al., 2015). Metabolic diseases are typically multi-nutrition-utilizing disorders and abnormalities of immune-induced hormones. As mentioned above, Roseburia spp. are a critical butyrate-producing bacteria cluster. Several researchers have reported that the potential role of butyrate is by inhibiting histone deacetylase (HDAC) or interacting with G protein-coupled receptors (GPCRs) such as free fatty acid receptors 2 (FFAR2) and 3 (FFAR3) in the control of body weight and insulin sensitivity (Canfora et al., 2015; Morrison and Preston, 2016; McNabney and Henagan, 2017). Butyrate can increase energy expenditure and fat oxidation, thereby exerting a further influence on energy homeostasis and lipid metabolism. Studies have also reported that FFAR 2/3-associated signaling exerts a potential regulatory role for butyrate in glucose homeostasis (Canfora et al., 2015). To date, there is strong evidence on the relationship between R. intestinalis and type 2 diabetes mellitus (T2DM) ( Table 2 ). A metagenome-wide association study (MGWAS) in a large-scale population-based cohort study in China based on deep shotgun sequencing of the gut microbial DNA from 345 Chinese individuals. discovered a decreased trend in the abundance of R. intestinalis in T2DM patients (Qin et al., 2012). These results were confirmed in subsequent studies (Tilg and Moschen, 2014). However, the big question is whether different types of diabetes determine the diversity of the gut Roseburia population. Another investigation that evaluated the gut microbiota and metabolism in patients with type 1 diabetes mellitus (T1DM) reported lower R. intestinalis abundance (Leiva-Gea et al., 2018). Furthermore, a systemic review involving 26 studies, including 2,600 children and 189 adults, revealed that the most significant bacterial alterations in T1DM patients involved Roseburia spp. (Jamshidi et al., 2019). Taken together, these findings coincide with the essential role of butyrate in glucose homeostasis. Meanwhile, obesity shares the same difference in gut commensal compositions. A study by (Tims et al., 2013) recruited 40 monozygotic twin pairs and explored the association of microbial signatures with body mass index (BMI). The results revealed the positive correlation between R. intestinalis and BMI; that is, individuals with higher BMI carried more abundant butyrate producers. Another study reported that children and adolescents with primary hyperlipidemia had a lower abundance of Roseburia, suggesting the correlation of the Roseburia genus with the changes in lipidemic parameters (Gargari et al., 2018). These pieces of research demonstrate that R. intestinalis plays an extensively instrumental role in metabolic disorders. However, further studies are warranted to elucidate whether R. intestinalis plays a more pivotal role in metabolic diseases than do widely studied microorganisms, such as Akkermansia muciniphila and Christensenellaceae. Some of the significant shortcomings of metabolism research include the lack of an effective design for primary bacterial culture, identification of animal and cell experimental function, and necessary clinical trials. Perhaps, R. intestinalis will be included in mixed probiotic prescriptions for metabolic disorders in the future.
Nervous System
The microbiota–gut–brain axis modulates the interaction between the nervous system and the intestinal environment. Bacterial composition and bacterial metabolites influence the function of both the enterocytes and exocrine glands. The effects of exocrine molecules on the enteric nervous system are either direct or indirect. Compelling evidence shows that immune cells such as ILC3 can interact with the colonic glia to form a glia–ILC3 axis, whereas microbes, including Roseburia, transmit signals to the colonic glia to stimulate the IL-22 production of ILC3 mediated by glial neurotrophic factors (Bernink et al., 2016; Ibiza et al., 2016). These complicated interaction axes involve microbes, immunity, and nerve. Therefore, gut bacteria such as Roseburia exert a potential impact on the pathogenetic process of nervous system diseases ( Table 2 ). An investigation by Prof. Wei demonstrated that R. intestinalis influenced the 5-HT level and GFAP expression in colonic tissue, alleviating the depression-like behavior in mice (Xu et al., 2021). This piece of evidence supports the role of Roseburia in neural disorders. In another study, the mucosal and fecal 16S rRNA-based assay of patients with Parkinson’s disease (PD) and controls showed that Roseburia was significantly more abundant in controls than in PD patients (Keshavarzian et al., 2015). Additionally, when the fecal microbiota obtained from PD patients or healthy controls were transplanted into germ-free mice via oral gavage, the results indicated that the abundance of Roseburia spp. increased in animals colonized with microbiota from PD patients (Keshavarzian et al., 2015). It was speculated that Roseburia spp.-associated gut dysbiosis promoted the aggregation of α-synuclein via colonic motor defect, and the subsequent specific changes enhanced neuroinflammation and the progression of PD (Sampson et al., 2016). Considering the impact of the transplant on the actual dysbiosis of PD patients, the divergent results should be analyzed carefully and cautiously. Aside from PD, depression is another global neurological concern. Previous evidence indicated a lower abundance of Roseburia in major depressive disorder (MDD) patients than in the matched healthy group (Zheng et al., 2016). In the same study, fecal microbiota transplantation (FMT) from MDD patients to germ-free recipient mice could induce depression-like behaviors. Moreover, the transplanted depression mice were characterized by an enriched carbohydrate and amino acid metabolism (Zheng et al., 2016). Nevertheless, cohort data from a study by Jiang et al. revealed a relatively more abundant Roseburia in active MDD patients than in the healthy group (Jiang et al., 2015). Liu et al. confirmed that the reduced Roseburia abundance could be an independent label for MDD compared to diarrhea-predominant IBS (D-IBS) (Liu et al., 2016). Therefore, the specific microbial profile of nervous disorders should be defined and clarified cautiously in consideration of the differences in the nationalities of the subjects, control baselines, fecal storage, digestive tract problems, and the detection methods used. With FMT, one can partly prove the primary function of gut Roseburia. However, interactions between bacteria and neurons are more complex than are other interactions such as bacteria–epithelium or bacteria–immunity; mechanism studies are lacking. The advancement in the culture techniques of the human microbiota and culturomics has provided a platform to elucidate and uncover new details on the explicit role of Roseburia in neural disorders.
Circulation and Hematology
Metabolites derived from R. intestinalis can infiltrate the gut barrier and circulate in the artery and the vein. As such, it is imperative to investigate the effects of R. intestinalis in circulatory diseases ( Table 2 ). A classic role of Roseburia has been proven strongly in the modulation of atherogenesis. In a Swedish study, patients with symptomatic atherosclerosis had less abundant Roseburia than did healthy subjects (Karlsson et al., 2012). Moreover, gut metagenomes are enriched in genes encoding metabolic pathways forming atherosclerotic plaques. Kasahara et al. revealed a negative correlation of Roseburia spp. with the development of atherosclerotic plaques in a mouse model. The study established a murine model colonized with R. intestinalis and fed the transplanted gnotobiotic mice with a high concentration of plant polysaccharides (Kasahara et al., 2018). The results showed that R. intestinalis contributed to the reprogramming of metabolism from glycolysis to fatty acid utilization and the reduction of systemic inflammation. In addition, R. intestinalis inhibited atherosclerotic plaque formation and ameliorated atherosclerosis (Kasahara et al., 2018). A similar phenomenon had been observed in coronary artery disease (CAD) reported by two published large-scale population-based studies by Liu et al., who recruited 161 CAD patients and 40 healthy controls, and Zhu et al., who enrolled 70 CAD patients and 98 healthy controls. These two studies characterized bacterial co-abundance group changes at different disease stages and demonstrated a significant depletion of Roseburia in the CAD group (Liu et al., 2019; Zhu, Q. et al., 2018). In another research group, MGWAS analysis showed a lower abundance of Roseburia spp. in hypertension patients than in healthy controls (Yan et al., 2017). A related animal study also reported reduced Roseburia abundance in a two-kidney-one-clip (2K1C) hypertensive rat model (Qin et al., 2018). Furthermore, Zheng et al. demonstrated a disrupted abundance of bacteria, including Roseburia, in experimental rats from a 4-week heart failure rat model (Zheng et al., 2019). Collectively, these findings suggest a close correlation of the diseases of the circulatory system with Roseburia.
Regarding hematology, studies on disease microbiome are scanty. In one of the investigations, decreased Roseburia abundance was detected in the fecal samples of lymphoblastic leukemia patients (Rajagopala et al., 2016). Another study revealed that mice fed with pectic oligosaccharides had increased intestinal Roseburia spp., and the diet ameliorated leukemia progression in a leukemia mouse model (Bindels et al., 2015). These findings may unravel the role of Roseburia in leukemia. Moreover, studies conducted in Peru and Switzerland reported that iron-deficient anemia patients had a lower fecal Roseburia abundance than did the controls (Dostal et al., 2012; McClorry et al., 2018). These findings provide an insight into the interaction between hematology and microbiology. In this view, we believe that R. intestinalis sustains the stability of the gut ecology and provides persistent indirect benefits in circulation and hematology; the effect is slow, but persistent. It is undeniable that multiomics and single-cell sequencing technologies have greatly improved the breadth and depth of bacteria–circulation research. These new technologies will guide the examination of circulation and the blood effects of germ-free animals after R. intestinalis intervention.
Infection and Cancer
Microbiota-reactive CD4+ T cells are essential in intestinal homeostasis because they produce permeability-sustaining cytokines and provide a large pool of T-cell alternatives that repel pathogens (Hegazy et al., 2017). Human immunodeficiency virus (HIV) impacts vast global populations, posing heavy public burdens and life-threatening risks. Evidence shows that HIV attacks the immune system (including the enteric defending barrier) whereby it infects and depletes CD4+ T cells. The consequently low CD4+ T cells induce gut dysbiosis, systemic inflammation, and clinical complications in chronic HIV patients. Researchers have detected low R. intestinalis abundance in the colonic mucosa of HIV patients compared to that in uninfected patients. In a recent investigation, the abundance of R. intestinalis was found to be inversely correlated with bacterial translocation, immune activation, and vascular inflammation (Dillon et al., 2017). Of note is that data from rectal mucosal microbiota rather than feces indicated depleted Roseburia in HIV patients who did not receive antiretroviral therapy (McHardy et al., 2013). However, the mechanism of dysbiosis induced by low CD4+ T cells is complex. In feces of HIV patients, low microbial gene counts (LGCs) but enrichment in R. intestinalis correlated with low nadir CD4+ T-cell counts, which could predict gut dysbiosis in HIV (Guillen et al., 2019). In addition, a significantly enriched Roseburia abundance was reported in active tuberculosis (TB) patients, and Roseburia promoted TB pathophysiology by enhancing the anti-inflammatory milieu in the host (Maji et al., 2018). However, a contrary report of a Chinese trial confirmed a decrease in Roseburia abundance in TB patients (Hu et al., 2019). Therefore, further investigation is warranted to address these divergent results.
The cancer-associated microbiome has always been a preference in cancer research. It is worth noting that the microbiota contributes to carcinogenesis and tumor progression in colorectal cancer (CRC), hepatocellular carcinoma, pancreatic tumor, and cervical cancer. Therefore, the microbiota can serve as early diagnostic biomarkers to guide precise cancer treatment. A previous investigation by Liang et al. (2017) reported that R. intestinalis abundance was decreased in CRC patients compared to healthy controls. Notably, the change in R. intestinalis abundance is consistently significant among cancer-associated microbiome studies. In a study by Yu et al. using MGWAS, the abundances of some marked microbes, including R. intestinalis, were decreased in 74 Chinese patients with CRC compared to the controls. These results were consistent with validated data from cohorts in Denmark, France, and Austria (Yu et al., 2017). Similar findings were also documented in independent studies (Wang et al., 2012; Chen et al., 2013). After a careful evaluation of the literature reports on CRC microbiota based on their large size and consistent results, we provide evidence that R. intestinalis may be a definite protective factor and a reliable biomarker in CRC. However, for other cancers (nasopharyngeal carcinoma and cervical cancer), divergent results were found (Jiang et al., 2019; Wang et al., 2019). It is notable that both studies displayed connections between Roseburia abundance and cancer risk, although the results were not related to the beneficial effect of Roseburia. Generally, cancer-associated microbiota is relevant to the cancer microenvironment. R. intestinalis may contribute to the dysfunction of the immune microenvironment and metabolic reprogramming of cancer cells. Recent evidence demonstrated an association of the Clostridiales members of the gut microbiota with a lower tumor burden in mouse models of CRC. Notably, these commensal species, including R. intestinalis, were also significantly reduced in CRC patients compared to healthy controls (Montalban-Arques et al., 2021). A mix of four Clostridiales strains (CC4) in mice prevented and successfully cured CRC as a stand-alone treatment in anti-PD-1 therapy. Also, the bacterial treatment induced a high infiltration of CD8+ T cells within the tumor, making them more immunogenic (Montalban-Arques et al., 2021). A single application of R. intestinalis showed higher efficacy than that of CC4 (Montalban-Arques et al., 2021). This section has shown a potential synergy between tumor immunotherapy and microbes. Thus, further cancer-associated microbial studies are warranted to elucidate the role of R. intestinalis in tumor immunotherapy. Although metagenome results could not distinguish the above role, germ-free animals and primary bacterial culture of tumors have great application prospects.
Respiratory, Nephrology, and Others
Acute respiratory distress syndrome (ARDS) is an acute lung injury that occurs mostly following an infection, chemical airway burn, or other factors. ARDS is characterized by a disruption of the alveolar epithelial and endothelial barrier, inflammatory effusion, ventilation defect, and severe respiratory failure. Intestinal microbial dysbiosis may aggravate ARDS. A previous study conducted using an acute lung injury rat model (rats were intratracheally instilled with lipopolysaccharides) demonstrated a higher diversity index and a lower number of Roseburia spp. in the gut flora of model rats compared to that of the control group (Li et al., 2014).
In most cases, chronic kidney disease (CKD) is comorbid with metabolic and cardiovascular complications, and most patients can progress into end-stage renal disease. In addition, a deteriorated internal environment frequently accompanies an impaired gut barrier and low-grade inflammation. CKD patients have been shown to harbor shrunken percentages of beneficial microbes, including Roseburia (Xu et al., 2017). In another study, a reduced abundance of Roseburia was reported in the end-stage renal disease group and was negatively correlated with C-reactive protein and cystatin C (Jiang et al., 2017). Some studies have shown a negative correlation of Roseburia with CKD severity at different stages of the disease (Wu, I. W. et al., 2020), and related studies continue to emerge. This review has repeatedly confirmed the beneficial role of Roseburia in different diseases. However, the precise role of Roseburia spp. could hardly be defined because dysbiosis is expected in such diseases. Nevertheless, we are more convinced that Roseburia is a potential probiotic.
R. intestinalis as a Probiotic Candidate: The Roads to Explore
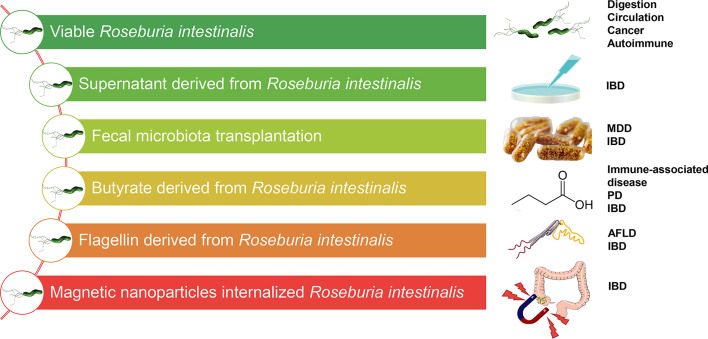
Many clinical studies of human cohorts using established disease models have documented the protective role of Roseburia spp., especially R. intestinalis. Nearly all the data demonstrate a probiotic role of R. intestinalis in preventing infection and in relieving and reversing the pathological process. Notably, R. intestinalis has displayed a beneficial role in different modes, such as butyrate, supernatant, flagellin, and organism effect. In combination with other emerging evidence, findings from our research group have revealed the effect of R. intestinalis on Tregs, ILC3, Th17, and macrophages and the stimulation of anti-inflammatory cytokines (IL-22, IL-17, IFNγ, TSLP, and OSM). There is evidence that different technologies have been applied to modulate R. intestinalis-associated disease treatment ( Figure 3 ), and the significance of FMT as a microbial treatment choice in diseases is well highlighted. For example, Clostridium difficile colitis is associated with microbiota dysbiosis caused by the overuse of antibiotics. FMT, as standard therapy, reverses microbiota dysbiosis and maintains the natural microbiota. FMT also demonstrates favorable potential in managing chronic inflammatory disorders, including UC. A randomized, double-blinded controlled trial, for instance, demonstrated that the application of FMT to manage active UC patients improved the microbial diversity and altered the bacterial taxa. Furthermore, patients in the remission group displayed more abundant Roseburia spp. and higher SCFA levels than those who experienced no remission after FMT (Paramsothy et al., 2019). Another investigation proposed that Roseburia species could be the prominent candidates for the probiotic treatment of patients with IBD based on the consistent results in the experimental center (Kellermayer, 2019). In our previous study (Yang et al., 2020), CD patients who received FMT showed significant improvement in the number of operational taxonomic units (OTUs) and Shannon diversity index than did donors, 2 weeks after FMT, which included R. intestinalis. Collectively, R. intestinalis might play a beneficial role in the recovery of the gut microenvironment and the improvement of disease pathophysiology through FMT.
Figure 3.
Different therapeutic methods targeting Roseburia intestinalis in potential diseases. IBD, inflammatory bowel disease; MDD, major depressive disorder; PD, Parkinson’s disease; AFLD, alcoholic fatty liver disease.
It is well known that the butyrate produced by R. intestinalis is a prebiotic. Researchers have attempted to directly or indirectly administer butyrate or butyrate “carriers” such as tributyrin (Wächtershäuser and Stein, 2000; Tuleu et al., 2001; McNabney and Henagan, 2017). For effective absorption, rectal butyrate enema had been previously introduced in UC treatment (Hove et al., 1995). Similarly, our research group revealed the therapeutic effect of R. intestinalis by suspension gavage in mice. In this view, we strongly suggest a need to develop viable probiotics based on R. intestinalis in medicine. Whole-grain diets with abundant fiber can also be fermented using R. intestinalis. Of note is that other special prebiotic supplements, such as acetate, xylan, β-mannans, and protein hREG3α, alter the abundance of R. intestinalis and butyrate production and promote niche superiority. As such, the application of R. intestinalis can benefit from advancement in the development of materials. Recently, in our laboratory, we developed a magnetic iron oxide nanoparticle (MION) internalized R. intestinalis, and the magnetic field can be directed both in vitro and in vivo (Xiao et al., 2019). Additionally, a careful investigation of the viability and cytotoxicity of MION internalized R. intestinalis and a custom-made magnetic guide facility were conducted in vitro. The results demonstrated good orienteering and the anti-inflammatory effect of MIONs in the TNBS-induced colitis mouse model (Xiao et al., 2019).
More findings from our laboratory indicated that the flagellin from R. intestinalis displayed a favorable anti-inflammatory effect via intraperitoneal injection (Quan et al., 2018; Wu, X. et al., 2020). These results strongly suggest that purified flagellin may be a safe and effective therapy for intestinal immune modulation. Furthermore, some tryptophan metabolites, including indoles, may be a new choice targeting R. intestinalis-associated mechanisms, for example, IL-22 production and ILC3 activation by the AhR. Therefore, further well-designed studies and credible clinical trials should be conducted to clarify the beneficial effects of R. intestinalis.
Regarding the safety of R. intestinalis, our laboratory is evaluating its risk in mouse symptoms, circulating system, and organs systemically. Further data will estimate its safety. Evidence should be accumulated in its safety and proper uptake dose. As a probiotic candidate, preclinical evaluation and clinical trials are urgently needed to prove its therapeutic potential in diseases.
Discussion
In 2014, the International Scientific Association for Probiotics and Prebiotics consensus statement updated the scope and appropriate use of the term probiotic. The reinforced concept defines probiotics as live microorganisms that, when administered in adequate amounts, confer a health benefit to the host. The expert consensus listed several probiotic candidates, including A. muciniphila, F. prausnitzii, Roseburia spp., and Eubacterium hallii (Hill et al., 2014). It is expected that therapeutic approaches targeting R. intestinalis will significantly improve specific human pathogenic status. Notably, inflammatory bowel disease exhibits significant changes in the abundance of R. intestinalis and benefits the most from its modulations. Recent breakthroughs also suggest the probiotic role of R. intestinalis, which has a widespread effect in preventing intestinal inflammation and maintaining energy homeostasis based on its metabolites, and noumenon. R. intestinalis could impact the functions of immune cells and the release of cytokines through its metabolites, butyrate and flagellin, and other unknown supernatant components. Advancements in primary culture technology, culture omics, single-cell sequencing, and metabonomics technology are expected to help researchers in elucidating the role of Roseburia spp. in order to lay a solid foundation in the maintenance of human health and treatment strategies for diseases in the future. Based on the available evidence, focused, well-designed randomized controlled trials, and future high-quality systematic reviews and meta-analyses should be urgently conducted to uncover the probiotic role of R. intestinalis. In conclusion, as a significant butyrate producer, R. intestinalis, which belongs to the Roseburia species, has great application prospects in the development of novel probiotics. It is possible that R. intestinalis can exert a specific and a significant therapeutic influence in different diseases associated with microbial dysbiosis.
Author Contributions
KN reviewed all the literature and wrote the manuscript. KM, WL, ZS, ZY, TT, and XM undertook most of the group’s research work and published their findings. They also discussed each part of the manuscript and helped form our viewpoints. YY funded us and edit the manuscript. XW conducted the research group including above authors and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This project was supported by the National Natural Science Foundation of China (NSFC nos. 81970494 and 81800500) 81970494 was grant to XW and 81800500 to YY.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
IBD, inflammatory bowel diseases; UC, ulcerative colitis; IBS, irritable bowel syndrome; GPCRs, G-protein coupled receptors; TSLP, thymic stromal lymphopoietin; GLP-1, glucagon-like peptide-1; PYY, peptide YY; OSM, oncostatin M; hREG3α, human regenerating family member 3 alpha; TLR5, Toll-like receptor 5; IDO1, indoleamine2,3-dioxygenase-1; HIF1A-AS2, lncRNA (HIF1A-AS2); FISH, fluorescence in situ hybridization; MGWAS, metagenome-wide association study; NAFLD, non-alcoholic fatty liver disease; AFLD, alcoholic fatty liver disease; CRD, chronic rheumatoid disease; APS, antiphospholipid syndrome; CAD, coronary artery disease; HIV, human immunodeficiency virus; CRC, colorectal cancer; ARDS, acute respiratory distress syndrome.
References
- Arpaia N., Campbell C., Fan X., Dikiy S., van der Veeken J., deRoos P., et al. (2013). Metabolites Produced by Commensal Bacteria Promote Peripheral Regulatory T-Cell Generation. Nature 504 (7480), 451–455. doi: 10.1038/nature12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachem A., Makhlouf C., Binger K. J., de Souza D. P., Tull D., Hochheiser K., et al. (2019). Microbiota-Derived Short-Chain Fatty Acids Promote the Memory Potential of Antigen-Activated CD8(+) T Cells. Immunity 51 (2), 285–297 e285. doi: 10.1016/j.immuni.2019.06.002 [DOI] [PubMed] [Google Scholar]
- Bajaj J. S., Hylemon P. B., Ridlon J. M., Heuman D. M., Daita K., White M. B., et al. (2012). Colonic Mucosal Microbiome Differs From Stool Microbiome in Cirrhosis and Hepatic Encephalopathy and is Linked to Cognition and Inflammation. Am. J. Physiol. Gastrointest Liver Physiol. 303 (6), G675–G685. doi: 10.1152/ajpgi.00152.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj J. S., Thacker L. R., Fagan A., White M. B., Gavis E. A., Hylemon P. B., et al. (2018). Gut Microbial RNA and DNA Analysis Predicts Hospitalizations in Cirrhosis. JCI Insight 3 (5). doi: 10.1172/jci.insight.98019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernink J. H., Spits H., de Jonge W. J. (2016). A New Edge to Immune Surveillance by the Neural System. Cell Res. 26 (11), 1178–1179. doi: 10.1038/cr.2016.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindels L. B., Neyrinck A. M., Salazar N., Taminiau B., Druart C., Muccioli G. G., et al. (2015). Non Digestible Oligosaccharides Modulate the Gut Microbiota to Control the Development of Leukemia and Associated Cachexia in Mice. PloS One 10 (6), e0131009. doi: 10.1371/journal.pone.0131009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfora E. E., Jocken J. W., Blaak E. E. (2015). Short-Chain Fatty Acids in Control of Body Weight and Insulin Sensitivity. Nat. Rev. Endocrinol. 11 (10), 577–591. doi: 10.1038/nrendo.2015.128 [DOI] [PubMed] [Google Scholar]
- Chassard C., Dapoigny M., Scott K. P., Crouzet L., Del'homme C., Marquet P., et al. (2012). Functional Dysbiosis Within the Gut Microbiota of Patients With Constipated-Irritable Bowel Syndrome. Aliment Pharmacol. Ther. 35 (7), 828–838. doi: 10.1111/j.1365-2036.2012.05007.x [DOI] [PubMed] [Google Scholar]
- Chassard C., Goumy V., Leclerc M., Del'homme C., Bernalier-Donadille A. (2007). Characterization of the Xylan-Degrading Microbial Community From Human Faeces. FEMS Microbiol. Ecol. 61 (1), 121–131. doi: 10.1111/j.1574-6941.2007.00314.x [DOI] [PubMed] [Google Scholar]
- Chen H. M., Yu Y. N., Wang J. L., Lin Y. W., Kong X., Yang C. Q., et al. (2013). Decreased Dietary Fiber Intake and Structural Alteration of Gut Microbiota in Patients With Advanced Colorectal Adenoma. Am. J. Clin. Nutr. 97 (5), 1044–1052. doi: 10.3945/ajcn.112.046607 [DOI] [PubMed] [Google Scholar]
- Consolandi C., Turroni S., Emmi G., Severgnini M., Fiori J., Peano C., et al. (2015). Behçet's Syndrome Patients Exhibit Specific Microbiome Signature. Autoimmun. Rev. 14 (4), 269–276. doi: 10.1016/j.autrev.2014.11.009 [DOI] [PubMed] [Google Scholar]
- Cornuault J. K., Moncaut E., Loux V., Mathieu A., Sokol H., Petit M. A., et al. (2020). The Enemy From Within: A Prophage of Roseburia Intestinalis Systematically Turns Lytic in the Mouse Gut, Driving Bacterial Adaptation by CRISPR Spacer Acquisition. ISME J. 14 (3), 771–787. doi: 10.1038/s41396-019-0566-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnaud M., Dos Santos A., Gonzalez P., Augui S., Lacoste C., Desterke C., et al. (2018). Enteric Delivery of Regenerating Family Member 3 Alpha Alters the Intestinal Microbiota and Controls Inflammation in Mice With Colitis. Gastroenterology 154 (4), 1009–1023 e1014. doi: 10.1053/j.gastro.2017.11.003 [DOI] [PubMed] [Google Scholar]
- Dillon S. M., Kibbie J., Lee E. J., Guo K., Santiago M. L., Austin G. L., et al. (2017). Low Abundance of Colonic Butyrate-Producing Bacteria in HIV Infection is Associated With Microbial Translocation and Immune Activation. AIDS 31 (4), 511–521. doi: 10.1097/QAD.0000000000001366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostal A., Chassard C., Hilty F. M., Zimmermann M. B., Jaeggi T., Rossi S., et al. (2012). Iron Depletion and Repletion With Ferrous Sulfate or Electrolytic Iron Modifies the Composition and Metabolic Activity of the Gut Microbiota in Rats. J. Nutr. 142 (2), 271–277. doi: 10.3945/jn.111.148643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostal A., Lacroix C., Bircher L., Pham V. T., Follador R., Zimmermann M. B., et al. (2015). Iron Modulates Butyrate Production by a Child Gut Microbiota in Vitro . mBio 6 (6), e01453–e01415. doi: 10.1128/mBio.01453-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S. H., Hold G. L., Barcenilla A., Stewart C. S., Flint H. J. (2002). Roseburia Intestinalis Sp. Nov., a Novel Saccharolytic, Butyrate-Producing Bacterium From Human Faeces. Int. J. Systematic Evolutionary Microbiol. 52 (5), 1615–1620. doi: 10.1099/ijs.0.02143-0 [DOI] [PubMed] [Google Scholar]
- Duncan S. H., Holtrop G., Lobley G. E., Calder A. G., Stewart C. S., Flint H. J. (2004). Contribution of Acetate to Butyrate Formation by Human Faecal Bacteria. Br. J. Nutr. 91 (6), 915–923. doi: 10.1079/BJN20041150 [DOI] [PubMed] [Google Scholar]
- Falony G., Vlachou A., Verbrugghe K., De Vuyst L. (2006). Cross-Feeding Between Bifidobacterium Longum BB536 and Acetate-Converting, Butyrate-Producing Colon Bacteria During Growth on Oligofructose. Appl. Environ. Microbiol. 72 (12), 7835–7841. doi: 10.1128/AEM.01296-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes J. D., Chen C. Y., Knox N. C., Marrie R. A., El-Gabalawy H., de Kievit T., et al. (2018). A Comparative Study of the Gut Microbiota in Immune-Mediated Inflammatory Diseases-Does a Common Dysbiosis Exist? Microbiome 6 (1), 221. doi: 10.1186/s40168-018-0603-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargari G., Deon V., Taverniti V., Gardana C., Denina M., Riso P., et al. (2018). Evidence of Dysbiosis in the Intestinal Microbial Ecosystem of Children and Adolescents With Primary Hyperlipidemia and the Potential Role of Regular Hazelnut Intake. FEMS Microbiol. Ecol. 94 (5). doi: 10.1093/femsec/fiy045 [DOI] [PubMed] [Google Scholar]
- Gevers D., Kugathasan S., Denson L. A., Vazquez-Baeza Y., Van Treuren W., Ren B., et al. (2014). The Treatment-Naive Microbiome in New-Onset Crohn's Disease. Cell Host Microbe 15 (3), 382–392. doi: 10.1016/j.chom.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobert A. P., Sagrestani G., Delmas E., Wilson K. T., Verriere T. G., Dapoigny M., et al. (2016). The Human Intestinal Microbiota of Constipated-Predominant Irritable Bowel Syndrome Patients Exhibits Anti-Inflammatory Properties. Sci. Rep. 6:39399. doi: 10.1038/srep39399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillen Y., Noguera-Julian M., Rivera J., Casadella M., Zevin A. S., Rocafort M., et al. (2019). Low Nadir CD4+ T-Cell Counts Predict Gut Dysbiosis in HIV-1 Infection. Mucosal Immunol. 12 (1), 232–246. doi: 10.1038/s41385-018-0083-7 [DOI] [PubMed] [Google Scholar]
- Hegazy A. N., West N. R., Stubbington M. J. T., Wendt E., Suijker K. I. M., Datsi A., et al. (2017). Circulating and Tissue-Resident CD4(+) T Cells With Reactivity to Intestinal Microbiota are Abundant in Healthy Individuals and Function is Altered During Inflammation. Gastroenterology 153 (5), 1320–1337 e1316. doi: 10.1053/j.gastro.2017.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiippala K., Jouhten H., Ronkainen A., Hartikainen A., Kainulainen V., Jalanka J., et al. (2018). The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation. Nutrients 10 (8). doi: 10.3390/nu10080988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C., Guarner F., Reid G., Gibson G. R., Merenstein D. J., Pot B., et al. (2014). Expert Consensus Document. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 11 (8), 506–514. doi: 10.1038/nrgastro.2014.66 [DOI] [PubMed] [Google Scholar]
- Hoffmann T. W., Pham H. P., Bridonneau C., Aubry C., Lamas B., Martin-Gallausiaux C., et al. (2016). Microorganisms Linked to Inflammatory Bowel Disease-Associated Dysbiosis Differentially Impact Host Physiology in Gnotobiotic Mice. ISME J. 10 (2), 460–477. doi: 10.1038/ismej.2015.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hold G. L., Schwiertz A., Aminov R. I., Blaut M., Flint H. J. (2003). Oligonucleotide Probes That Detect Quantitatively Significant Groups of Butyrate-Producing Bacteria in Human Feces. Appl. Environ. Microbiol. 69 (7), 4320–4324. doi: 10.1128/aem.69.7.4320-4324.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hove H., Holtug K., Jeppesen P. B., Mortensen P. B. (1995). Butyrate Absorption and Lactate Secretion in Ulcerative Colitis. Dis. colon rectum 38 (5), 519–525. doi: 10.1007/BF02148853 [DOI] [PubMed] [Google Scholar]
- Hu Y., Feng Y., Wu J., Liu F., Zhang Z., Hao Y., et al. (2019). The Gut Microbiome Signatures Discriminate Healthy From Pulmonary Tuberculosis Patients. Front. Cell Infect. Microbiol. 9:90. doi: 10.3389/fcimb.2019.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibiza S., García-Cassani B., Ribeiro H., Carvalho T., Almeida L., Marques R., et al. (2016). Glial-Cell-Derived Neuroregulators Control Type 3 Innate Lymphoid Cells and Gut Defence. Nature 535 (7612), 440–443. doi: 10.1038/nature18644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamshidi P., Hasanzadeh S., Tahvildari A., Farsi Y., Arbabi M., Mota J. F., et al. (2019). Is There Any Association Between Gut Microbiota and Type 1 Diabetes? A Systematic Review. Gut Pathog. 11, 49. doi: 10.1186/s13099-019-0332-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Ling Z., Zhang Y., Mao H., Ma Z., Yin Y., et al. (2015). Altered Fecal Microbiota Composition in Patients With Major Depressive Disorder. Brain Behav. Immun. 48, 186–194. doi: 10.1016/j.bbi.2015.03.016 [DOI] [PubMed] [Google Scholar]
- Jiang H., Li J., Zhang B., Huang R., Zhang J., Chen Z., et al. (2019). Intestinal Flora Disruption and Novel Biomarkers Associated With Nasopharyngeal Carcinoma. Front. Oncol. 9:1346. doi: 10.3389/fonc.2019.01346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S., Xie S., Lv D., Wang P., He H., Zhang T., et al. (2017). Alteration of the Gut Microbiota in Chinese Population With Chronic Kidney Disease. Sci. Rep. 7 (1), 2870. doi: 10.1038/s41598-017-02989-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C. J., Sellmann C., Engstler A. J., Ziegenhardt D., Bergheim I. (2015). Supplementation of Sodium Butyrate Protects Mice From the Development of non-Alcoholic Steatohepatitis (NASH). Br. J. Nutr. 114 (11), 1745–1755. doi: 10.1017/S0007114515003621 [DOI] [PubMed] [Google Scholar]
- Karlsson F. H., Fak F., Nookaew I., Tremaroli V., Fagerberg B., Petranovic D., et al. (2012). Symptomatic Atherosclerosis is Associated With an Altered Gut Metagenome. Nat. Commun. 3, 1245. doi: 10.1038/ncomms2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara K., Krautkramer K. A., Org E., Romano K. A., Kerby R. L., Vivas E. I., et al. (2018). Interactions Between Roseburia Intestinalis and Diet Modulate Atherogenesis in a Murine Model. Nat. Microbiol. 3 (12), 1461–1471. doi: 10.1038/s41564-018-0272-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermayer R. (2019). Roseburia Species: Prime Candidates for Microbial Therapeutics in Inflammatory Bowel Disease. Gastroenterology 157 (4), 1164–1165. doi: 10.1053/j.gastro.2019.05.073 [DOI] [PubMed] [Google Scholar]
- Keshavarzian A., Green S. J., Engen P. A., Voigt R. M., Naqib A., Forsyth C. B., et al. (2015). Colonic Bacterial Composition in Parkinson's Disease. Mov Disord. 30 (10), 1351–1360. doi: 10.1002/mds.26307 [DOI] [PubMed] [Google Scholar]
- Klose C. S., Artis D. (2016). Innate Lymphoid Cells as Regulators of Immunity, Inflammation and Tissue Homeostasis. Nat. Immunol. 17 (7), 765–774. doi: 10.1038/ni.3489 [DOI] [PubMed] [Google Scholar]
- Kumari R., Ahuja V., Paul J. (2013). Fluctuations in Butyrate-Producing Bacteria in Ulcerative Colitis Patients of North India. World J. Gastroenterol. 19 (22), 3404–3414. doi: 10.3748/wjg.v19.i22.3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa S. L., Leth M. L., Michalak L., Hansen M. E., Pudlo N. A., Glowacki R., et al. (2019). The Human Gut Firmicute Roseburia Intestinalis is a Primary Degrader of Dietary Beta-Mannans. Nat. Commun. 10 (1), 905. doi: 10.1038/s41467-019-08812-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiva-Gea I., Sanchez-Alcoholado L., Martin-Tejedor B., Castellano-Castillo D., Moreno-Indias I., Urda-Cardona A., et al. (2018). Gut Microbiota Differs in Composition and Functionality Between Children With Type 1 Diabetes and MODY2 and Healthy Control Subjects: A Case-Control Study. Diabetes Care 41 (11), 2385–2395. doi: 10.2337/dc18-0253 [DOI] [PubMed] [Google Scholar]
- Leth M. L., Ejby M., Madland E., Kitaoku Y., Slotboom D. J., Guskov A., et al. (2020). Molecular Insight Into a New Low-Affinity Xylan Binding Module From the Xylanolytic Gut Symbiont Roseburia Intestinalis. FEBS J. 287 (10), 2105–2117. doi: 10.1111/febs.15117 [DOI] [PubMed] [Google Scholar]
- Leth M. L., Ejby M., Workman C., Ewald D. A., Pedersen S. S., Sternberg C., et al. (2018). Differential Bacterial Capture and Transport Preferences Facilitate Co-Growth on Dietary Xylan in the Human Gut. Nat. Microbiol. 3 (5), 570–580. doi: 10.1038/s41564-018-0132-8 [DOI] [PubMed] [Google Scholar]
- Lewis G., Wang B., Shafiei Jahani P., Hurrell B. P., Banie H., Aleman Muench G. R., et al. (2019). Dietary Fiber-Induced Microbial Short Chain Fatty Acids Suppress Ilc2-Dependent Airway Inflammation. Front. Immunol. 10:2051. doi: 10.3389/fimmu.2019.02051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Q., Chiu J., Chen Y., Huang Y., Higashimori A., Fang J., et al. (2017). Fecal Bacteria Act as Novel Biomarkers for Noninvasive Diagnosis of Colorectal Cancer. Clin. Cancer Res. 23 (8), 2061–2070. doi: 10.1158/1078-0432.CCR-16-1599 [DOI] [PubMed] [Google Scholar]
- Li Y., Liu X. Y., Ma M. M., Qi Z. J., Zhang X. Q., Li Z., et al. (2014). Changes in Intestinal Microflora in Rats With Acute Respiratory Distress Syndrome. World J. Gastroenterol. 20 (19), 5849–5858. doi: 10.3748/wjg.v20.i19.5849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Chen X., Hu X., Niu H., Tian R., Wang H., et al. (2019). Alterations in the Gut Microbiome and Metabolism With Coronary Artery Disease Severity. Microbiome 7 (1):68. doi: 10.1186/s40168-019-0683-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhang L., Wang X., Wang Z., Zhang J., Jiang R., et al. (2016). Similar Fecal Microbiota Signatures in Patients With Diarrhea-Predominant Irritable Bowel Syndrome and Patients With Depression. Clin. Gastroenterol. Hepatol. 14 (11), 1602–1611 e1605. doi: 10.1016/j.cgh.2016.05.033 [DOI] [PubMed] [Google Scholar]
- Louis P., Duncan S. H., McCrae S. I., Millar J., Jackson M. S., Flint H. J. (2004). Restricted Distribution of the Butyrate Kinase Pathway Among Butyrate-Producing Bacteria From the Human Colon. J. Bacteriol 186 (7), 2099–2106. doi: 10.1128/JB.186.7.2099-2106.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W., Shen Z., Deng M., Li X., Tan B., Xiao M., et al. (2019). Roseburia Intestinalis Supernatant Ameliorates Colitis Induced in Mice by Regulating the Immune Response. Mol. Med. Rep. 20 (2), 1007–1016. doi: 10.3892/mmr.2019.10327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maji A., Misra R., Dhakan D. B., Gupta V., Mahato N. K., Saxena R., et al. (2018). Gut Microbiome Contributes to Impairment of Immunity in Pulmonary Tuberculosis Patients by Alteration of Butyrate and Propionate Producers. Environ. Microbiol. 20 (1), 402–419. doi: 10.1111/1462-2920.14015 [DOI] [PubMed] [Google Scholar]
- Martinez I., Lattimer J. M., Hubach K. L., Case J. A., Yang J., Weber C. G., et al. (2013). Gut Microbiome Composition is Linked to Whole Grain-Induced Immunological Improvements. ISME J. 7 (2), 269–280. doi: 10.1038/ismej.2012.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattace Raso G., Simeoli R., Russo R., Iacono A., Santoro A., Paciello O., et al. (2013). Effects of Sodium Butyrate and its Synthetic Amide Derivative on Liver Inflammation and Glucose Tolerance in an Animal Model of Steatosis Induced by High Fat Diet. PloS One 8 (7), e68626. doi: 10.1371/journal.pone.0068626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClorry S., Zavaleta N., Llanos A., Casapia M., Lonnerdal B., Slupsky C. M. (2018). Anemia in Infancy is Associated With Alterations in Systemic Metabolism and Microbial Structure and Function in a Sex-Specific Manner: An Observational Study. Am. J. Clin. Nutr. 108 (6), 1238–1248. doi: 10.1093/ajcn/nqy249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHardy I. H., Li X., Tong M., Ruegger P., Jacobs J., Borneman J., et al. (2013). HIV Infection is Associated With Compositional and Functional Shifts in the Rectal Mucosal Microbiota. Microbiome 1 (1):26. doi: 10.1186/2049-2618-1-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNabney S. M., Henagan T. M. (2017). Short Chain Fatty Acids in the Colon and Peripheral Tissues: A Focus on Butyrate, Colon Cancer, Obesity and Insulin Resistance. Nutrients 9 (12). doi: 10.3390/nu9121348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak L., La Rosa S. L., Leivers S., Lindstad L. J., Rohr A. K., Lillelund Aachmann F., et al. (2020). A Pair of Esterases From a Commensal Gut Bacterium Remove Acetylations From All Positions on Complex Beta-Mannans. Proc. Natl. Acad. Sci. U.S.A. 117 (13), 7122–7130. doi: 10.1073/pnas.1915376117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaudel C., Sokol H. (2020). The Gut Microbiota at the Service of Immunometabolism. Cell Metab. 32 (4), 514–523. doi: 10.1016/j.cmet.2020.09.004 [DOI] [PubMed] [Google Scholar]
- Mirande C., Kadlecikova E., Matulova M., Capek P., Bernalier-Donadille A., Forano E., et al. (2010). Dietary Fibre Degradation and Fermentation by Two Xylanolytic Bacteria Bacteroides Xylanisolvens XB1A and Roseburia Intestinalis XB6B4 From the Human Intestine. J. Appl. Microbiol. 109 (2), 451–460. doi: 10.1111/j.1365-2672.2010.04671.x [DOI] [PubMed] [Google Scholar]
- Montalban-Arques A., Katkeviciute E., Busenhart P., Bircher A., Wirbel J., Zeller G., et al. (2021). Commensal Clostridiales Strains Mediate Effective Anti-Cancer Immune Response Against Solid Tumors. Cell Host Microbe 29 (10), 1573–1588 e1577. doi: 10.1016/j.chom.2021.08.001 [DOI] [PubMed] [Google Scholar]
- Morgan X. C., Tickle T. L., Sokol H., Gevers D., Devaney K. L., Ward D. V., et al. (2012). Dysfunction of the Intestinal Microbiome in Inflammatory Bowel Disease and Treatment. Genome Biol. 13 (9), R79. doi: 10.1186/gb-2012-13-9-r79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. J., Preston T. (2016). Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 7 (3), 189–200. doi: 10.1080/19490976.2015.1134082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paramsothy S., Nielsen S., Kamm M. A., Deshpande N. P., Faith J. J., Clemente J. C., et al. (2019). Specific Bacteria and Metabolites Associated With Response to Fecal Microbiota Transplantation in Patients With Ulcerative Colitis. Gastroenterology 156 (5), 1440–1454 e1442. doi: 10.1053/j.gastro.2018.12.001 [DOI] [PubMed] [Google Scholar]
- Parthasarathy G., Chen J., Chen X., Chia N., O'Connor H. M., Wolf P. G., et al. (2016). Relationship Between Microbiota of the Colonic Mucosa vs Feces and Symptoms, Colonic Transit, and Methane Production in Female Patients With Chronic Constipation. Gastroenterology 150 (2), 367–379 e361. doi: 10.1053/j.gastro.2015.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrone V., Puglisi E., Cardinali M., Schnitzler T. S., Svegliati S., Festa A., et al. (2017). Gut Microbiota Profile in Systemic Sclerosis Patients With and Without Clinical Evidence of Gastrointestinal Involvement. Sci. Rep. 7 (1), 14874. doi: 10.1038/s41598-017-14889-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson A. M., Mulder I. E., Travis A. J., Lan A., Cerf-Bensussan N., Gaboriau-Routhiau V., et al. (2017). Human Gut Symbiont Roseburia Hominis Promotes and Regulates Innate Immunity. Front. Immunol. 8:1166. doi: 10.3389/fimmu.2017.01166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryde S. E., Duncan S. H., Hold G. L., Stewart C. S., Flint H. J. (2002). The Microbiology of Butyrate Formation in the Human Colon. FEMS Microbiol. Lett. 217 (2), 133–139. doi: 10.1111/j.1574-6968.2002.tb11467.x [DOI] [PubMed] [Google Scholar]
- Qin J., Li Y., Cai Z., Li S., Zhu J., Zhang F., et al. (2012). A Metagenome-Wide Association Study of Gut Microbiota in Type 2 Diabetes. Nature 490 (7418), 55–60. doi: 10.1038/nature11450 [DOI] [PubMed] [Google Scholar]
- Qin L., Wang Z. L., Feng Y., Chen Z. Z., Yu H. (2018). Analysis of Structural Features of Gut Microbiota in Two-Kidney-One-Clip Hypertensive Rats Based on High-Throughput Sequencing Technology. Zhonghua xin xue guan bing za zhi 46 (9), 706–712. doi: 10.3760/cma.j.issn.0253-3758.2018.09.007 [DOI] [PubMed] [Google Scholar]
- Quan Y., Song K., Zhang Y., Zhu C., Shen Z., Wu S., et al. (2018). Roseburia Intestinalis-Derived Flagellin is a Negative Regulator of Intestinal Inflammation. Biochem. Biophys. Res. Commun. 501 (3), 791–799. doi: 10.1016/j.bbrc.2018.05.075 [DOI] [PubMed] [Google Scholar]
- Rajagopala S. V., Yooseph S., Harkins D. M., Moncera K. J., Zabokrtsky K. B., Torralba M. G., et al. (2016). Gastrointestinal Microbial Populations can Distinguish Pediatric and Adolescent Acute Lymphoblastic Leukemia (ALL) at the Time of Disease Diagnosis. BMC Genomics 17 (1), 635. doi: 10.1186/s12864-016-2965-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajilic-Stojanovic M., Shanahan F., Guarner F., de Vos W. M. (2013). Phylogenetic Analysis of Dysbiosis in Ulcerative Colitis During Remission. Inflammation Bowel Dis. 19 (3), 481–488. doi: 10.1097/MIB.0b013e31827fec6d [DOI] [PubMed] [Google Scholar]
- Raman M., Ahmed I., Gillevet P. M., Probert C. S., Ratcliffe N. M., Smith S., et al. (2013). Fecal Microbiome and Volatile Organic Compound Metabolome in Obese Humans With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 11 (7), 868–875 e861-863. doi: 10.1016/j.cgh.2013.02.015 [DOI] [PubMed] [Google Scholar]
- Ruff W. E., Dehner C., Kim W. J., Pagovich O., Aguiar C. L., Yu A. T., et al. (2019). Pathogenic Autoreactive T and B Cells Cross-React With Mimotopes Expressed by a Common Human Gut Commensal to Trigger Autoimmunity. Cell Host Microbe 26 (1), 100–113 e108. doi: 10.1016/j.chom.2019.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W. R., Duncan S. H., Scobbie L., Duncan G., Cantlay L., Calder A. G., et al. (2013). Major Phenylpropanoid-Derived Metabolites in the Human Gut can Arise From Microbial Fermentation of Protein. Mol. Nutr. Food Res. 57 (3), 523–535. doi: 10.1002/mnfr.201200594 [DOI] [PubMed] [Google Scholar]
- Salem F., Kindt N., Marchesi J. R., Netter P., Lopez A., Kokten T., et al. (2019). Gut Microbiome in Chronic Rheumatic and Inflammatory Bowel Diseases: Similarities and Differences. United Eur. Gastroenterol. J. 7 (8), 1008–1032. doi: 10.1177/2050640619867555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman E. T., Palacios T., Thomsen M., Vitetta L. (2018). Intestinal Microbiome Shifts, Dysbiosis, Inflammation, and non-Alcoholic Fatty Liver Disease. Front. Microbiol. 9:61. doi: 10.3389/fmicb.2018.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson T. R., Debelius J. W., Thron T., Janssen S., Shastri G. G., Ilhan Z. E., et al. (2016). Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson's Disease. Cell 167 (6), 1469–1480 e1412. doi: 10.1016/j.cell.2016.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo B., Jeon K., Moon S., Lee K., Kim W. K., Jeong H., et al. (2020). Roseburia Spp. Abundance Associates With Alcohol Consumption in Humans and its Administration Ameliorates Alcoholic Fatty Liver in Mice. Cell Host Microbe 27 (1), 25–40 e26. doi: 10.1016/j.chom.2019.11.001 [DOI] [PubMed] [Google Scholar]
- Shen Z., Zhu C., Quan Y., Yang J., Yuan W., Yang Z., et al. (2018). Insights Into Roseburia Intestinalis Which Alleviates Experimental Colitis Pathology by Inducing Anti-Inflammatory Responses. J. Gastroenterol. Hepatol. 33 (10), 1751–1760. doi: 10.1111/jgh.14144 [DOI] [PubMed] [Google Scholar]
- Stackebrandt E. (2014). “The Family Lachnospiraceae,” in The Prokaryotes. Berlin, Heidelberg: Springer. pp. 197–201. [Google Scholar]
- Tamanai-Shacoori Z., Smida I., Bousarghin L., Meuric V., Jolivet-Gougeon A. (2017). Roseburia Spp.: A Marker of Health? Future Microbiol. 12 (2), 157–170. doi: 10.2217/fmb-2016-0130 [DOI] [PubMed] [Google Scholar]
- Tan B., Luo W., Shen Z., Xiao M., Wu S., Meng X., et al. (2019). Roseburia Intestinalis Inhibits Oncostatin M and Maintains Tight Junction Integrity in a Murine Model of Acute Experimental Colitis. Scand. J. Gastroenterol. 54 (4), 432–440. doi: 10.1080/00365521.2019.1595708 [DOI] [PubMed] [Google Scholar]
- Tilg H., Moschen A. R. (2014). Microbiota and Diabetes: An Evolving Relationship. Gut 63 (9), 1513–1521. doi: 10.1136/gutjnl-2014-306928 [DOI] [PubMed] [Google Scholar]
- Tims S., Derom C., Jonkers D. M., Vlietinck R., Saris W. H., Kleerebezem M., et al. (2013). Microbiota Conservation and BMI Signatures in Adult Monozygotic Twins. ISME J. 7 (4), 707–717. doi: 10.1038/ismej.2012.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuleu C., Andrieux C., Cherbuy C., Darcy-Vrillon B., Duée P. H. (2001). And Chaumeil, J.C Colonic Delivery of Sodium Butyrate via Oral Route: Acrylic Coating Design of Pellets and in Vivo Evaluation in Rats. Methods findings Exp. Clin. Pharmacol. 23 (5), 245–253. doi: 10.1358/mf.2001.23.5.662119 [DOI] [PubMed] [Google Scholar]
- Van den Abbeele P., Belzer C., Goossens M., Kleerebezem M., De Vos W. M., Thas O., et al. (2013). Butyrate-Producing Clostridium Cluster Xiva Species Specifically Colonize Mucins in an in Vitro Gut Model. ISME J. 7 (5), 949–961. doi: 10.1038/ismej.2012.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan A., Rumbo M., Carnoy C., Sirard J. C. (2018). Compartmentalized Antimicrobial Defenses in Response to Flagellin. Trends Microbiol. 26 (5), 423–435. doi: 10.1016/j.tim.2017.10.008 [DOI] [PubMed] [Google Scholar]
- Wächtershäuser A., Stein J. (2000). Rationale for the Luminal Provision of Butyrate in Intestinal Diseases. Eur. J. Nutr. 39 (4), 164–171. doi: 10.1007/s003940070020 [DOI] [PubMed] [Google Scholar]
- Wang T., Cai G., Qiu Y., Fei N., Zhang M., Pang X., et al. (2012). Structural Segregation of Gut Microbiota Between Colorectal Cancer Patients and Healthy Volunteers. ISME J. 6 (2), 320–329. doi: 10.1038/ismej.2011.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Wang Q., Zhao J., Gong L., Zhang Y., Wang X., et al. (2019). Altered Diversity and Composition of the Gut Microbiome in Patients With Cervical Cancer. AMB Express 9 (1), 40. doi: 10.1186/s13568-019-0763-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu I. W., Lin C. Y., Chang L. C., Lee C. C., Chiu C. Y., Hsu H. J., et al. (2020). Gut Microbiota as Diagnostic Tools for Mirroring Disease Progression and Circulating Nephrotoxin Levels in Chronic Kidney Disease: Discovery and Validation Study. Int. J. Biol. Sci. 16 (3), 420–434. doi: 10.7150/ijbs.37421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Pan S., Luo W., Shen Z., Meng X., Xiao M., et al. (2020). Roseburia Intestinalisderived Flagellin Ameliorates Colitis by Targeting Mir2233pmediated Activation of NLRP3 Inflammasome and Pyroptosis. Mol. Med. Rep. 22 (4), 2695–2704. doi: 10.3892/mmr.2020.11351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao M., Shen Z., Luo W., Tan B., Meng X., Wu X., et al. (2019). A New Colitis Therapy Strategy via the Target Colonization of Magnetic Nanoparticle-Internalized Roseburia Intestinalis. Biomater Sci. 7 (10), 4174–4185. doi: 10.1039/c9bm00980a [DOI] [PubMed] [Google Scholar]
- Xu F., Cheng Y., Ruan G., Fan L., Tian Y., Xiao Z., et al. (2021). New Pathway Ameliorating Ulcerative Colitis: Focus on Roseburia Intestinalis and the Gut-Brain Axis. Therap Adv. Gastroenterol. 14:17562848211004469. doi: 10.1177/17562848211004469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K. Y., Xia G. H., Lu J. Q., Chen M. X., Zhen X., Wang S., et al. (2017). Impaired Renal Function and Dysbiosis of Gut Microbiota Contribute to Increased Trimethylamine-N-Oxide in Chronic Kidney Disease Patients. Sci. Rep. 7 (1), 1445. doi: 10.1038/s41598-017-01387-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Bu C., Yuan W., Shen Z., Quan Y., Wu S., et al. (2020). Fecal Microbiota Transplant via Endoscopic Delivering Through Small Intestine and Colon: No Difference for Crohn's Disease. Dig Dis. Sci. 65 (1), 150–157. doi: 10.1007/s10620-019-05751-y [DOI] [PubMed] [Google Scholar]
- Yan Q., Gu Y., Li X., Yang W., Jia L., Chen C., et al. (2017). Alterations of the Gut Microbiome in Hypertension. Front. Cell Infect. Microbiol. 7:381. doi: 10.3389/fcimb.2017.00381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Feng Q., Wong S. H., Zhang D., Liang Q. Y., Qin Y., et al. (2017). Metagenomic Analysis of Faecal Microbiome as a Tool Towards Targeted non-Invasive Biomarkers for Colorectal Cancer. Gut 66 (1), 70–78. doi: 10.1136/gutjnl-2015-309800 [DOI] [PubMed] [Google Scholar]
- Zheng A., Yi H., Li F., Han L., Yu J., Cheng X., et al. (2019). Changes in Gut Microbiome Structure and Function of Rats With Isoproterenol-Induced Heart Failure. Int. Heart J. 60 (5), 1176–1183. doi: 10.1536/ihj.18-194 [DOI] [PubMed] [Google Scholar]
- Zheng P., Zeng B., Zhou C., Liu M., Fang Z., Xu X., et al. (2016). Gut Microbiome Remodeling Induces Depressive-Like Behaviors Through a Pathway Mediated by the Host's Metabolism. Mol. Psychiatry 21 (6), 786–796. doi: 10.1038/mp.2016.44 [DOI] [PubMed] [Google Scholar]
- Zhu L., Baker S. S., Gill C., Liu W., Alkhouri R., Baker R. D., et al. (2013). Characterization of Gut Microbiomes in Nonalcoholic Steatohepatitis (NASH) Patients: A Connection Between Endogenous Alcohol and NASH. Hepatology 57 (2), 601–609. doi: 10.1002/hep.26093 [DOI] [PubMed] [Google Scholar]
- Zhu Q., Gao R., Zhang Y., Pan D., Zhu Y., Zhang X., et al. (2018). Dysbiosis Signatures of Gut Microbiota in Coronary Artery Disease. Physiol. Genomics 50 (10), 893–903. doi: 10.1152/physiolgenomics.00070.2018 [DOI] [PubMed] [Google Scholar]
- Zhu C., Song K., Shen Z., Quan Y., Tan B., Luo W., et al. (2018). Roseburia Intestinalis Inhibits Interleukin17 Excretion and Promotes Regulatory T Cells Differentiation in Colitis. Mol. Med. Rep. 17 (6), 7567–7574. doi: 10.3892/mmr.2018.8833 [DOI] [PMC free article] [PubMed] [Google Scholar]