Abstract
Objectives:
To examine whether asymptomatic ultrasonographic abnormalities in the Achilles and patellar tendons in runners are associated with an increased risk of pain development.
Methods:
This is a longitudinal, prospective cohort study with 139 runners recruited at a half and full marathon race. Ultrasound examination of the Achilles and patellar tendons was performed bilaterally the day prior to the race. Self-reported injury data were collected at 1, 3, 6 and 12 months. 104 (74.8%) runners were included in the data analysis.
Results:
Ultrasonographic tendon abnormalities were found in 24.1% of the Achilles and in 23.1% of the patellar tendons prior to the race. Runners with tendon abnormality were 2–3 times more likely to develop pain within 12 months than those without (relative risk = 3.14, p = 0.010 for Achilles; relative risk = 2.52, p = 0.008 for patellar tendon). After adjusting for gender, age, years of running, average miles per week of running over a year, and pre-race pain, runners with ultrasound abnormality were about 3 times (hazard ratio = 2.89, p = 0.039 for Achilles; hazard ratio = 2.73, p = 0.030 for patellar tendon) more likely to develop pain after the race. Tendon delamination was most strongly associated with pain in both the Achilles (relative risk = 6.00; p = 0.001) and patellar tendons (relative risk = 3.81; p = 0.001).
Conclusions:
Structural changes in asymptomatic tendons were found in almost 25% of runners. Presence of structural changes was associated with increased development of Achilles and patellar tendon pain within one year.
Keywords: sonography, overuse, primary prevention, tendinopathy, ultrasonography, runners
INTRODUCTION
Tendon injuries are one of the most common overuse injuries in sports, with two tendons, the patellar and Achilles tendons, comprising a significant proportion of these injuries.[1–7] These tendon injuries are prevalent in the general population as well as in athletes,[1,8,9] and are particularly common in sports involving more ballistic running and jumping, such as basketball and football (soccer).[2,9–11] Treatment for these injuries is prolonged,[12,13] and many individuals will have persistent or recurrent symptoms[14–16]. It is generally believed that these tendon injuries are related to abnormal loading due to fatigue and abnormal biomechanics.[17,18] Tendon loading can result in structural tendon changes which typically progress with continued loading along a continuum,[19,20] though the critical quantity of loading for structural alteration is unclear.[21–23] As structural changes indicative of degeneration have been seen in areas of injured tendons,[24–26] such structural changes could conceivably be identified prior to pain development. Ultrasound is an ideal choice to potentially identify these structural precursors, as it is portable, rapid, and radiation-free, which places athletes at no physical risk, and is low in cost. Ultrasound has repeatedly been demonstrated to identify degenerative and/or structural changes within a tendon.[22,23,27,28] These structural changes include increased tendon thickness and cross-sectional area, calcifications, areas of hypoechogenicity, presence of delamination, paratenon blurring, neovascularization and intratendinous softening on sonoelastography.[8,29–36]
The clinical significance of the presence of similar structural changes in asymptomatic athletes remains unclear.[37] Studies on athletes in various sports, including diverse sports such as football (soccer) [30,31,38], Australian rules football[39,40], ballet[29], fencing[32], badminton[41], volleyball[42–44] and basketball[19,45] have identified structural tendon abnormalities in asymptomatic athletes, occurring in up to 11–52% of asymptomatic athletes,[30,31,39] depending on diagnostic criteria. Within this cohort of non-running athletes, asymptomatic athletes who had ultrasound abnormalities had an increased risk of becoming symptomatic throughout the season.[30,31,38,39,46,47]
Similar findings have been reported in the running population. Asymptomatic competitive distance runners have been shown to have structural changes in the Achilles tendon on ultrasound evaluation including increased tendon thickness, changes in echogenicity and collagen alignment.[48–50] Prospective studies assessing the ultrasonographic structure of the Achilles[51,52] and patellar tendons in collegiate distance runners have shown that the tendons undergo structural changes during the season.[53,54] Interestingly, despite demonstrable changes in tendon structure during the season, few athletes developed symptomatic tendinopathy.[54] Prospective evaluation of previously asymptomatic adolescent runners with normal tendons has shown that development of ultrasonographic tendon changes is associated with clinical tendinopathy in the patellar tendon.[55] Thus it is suggested that the structural changes present a spectrum of adaptation to load, which may ultimately progress to clinical pathology in the form of symptomatic tendinopathy if the ability to adapt is insufficient. Although runners have high rates of clinically-symptomatic Achilles and patellar tendinopathy,[4,6,56,57] prior studies have shown that cross-sectional ultrasonographic examination of tendons without knowledge of clinical symptoms could not identify distinctions between asymptomatic abnormalities and symptomatic abnormalities.[4,40,49,55,58] The predictive ability of ultrasound in identifying tendon abnormalities leading to risk of injury has been studied in runners looking at the Achilles tendon only[59] and found a 6.9 fold increased risk of developing symptoms in participants with presence of neovascularization in the tendon at time of ultrasound evaluation.
The purpose of this study was to prospectively assess whether ultrasonographic abnormalities in the patellar and Achilles tendons in a cohort of recreational runners increase the risk of development of tendon-related pain at 1, 3, 6, and 12 months post-race.
MATERIALS AND METHODS
Study Design:
Asymptomatic runners 18 years of age or over participating in the Salt Lake City marathon were recruited at the pre-race exposition. Subjects with prior rupture of, or surgery to, the patellar or Achilles tendons were excluded. All subjects provided informed written consent. Written consent for the study was obtained from the race director. Institutional review board approval was obtained.
Age, sex, height (m), mass (kg), smoking history, and running history (years running, average miles run per week in the past year) were obtained for each participant. As marathons appear to acutely induce ultrasonographic change[60,61], all ultrasound examinations were performed prior to running the race. Each patient had four separate areas scanned: patellar tendon (left and right) and Achilles tendon (left and right) as described below. Participants were contacted at 1, 3, 6, and 12 months after the running event via email, and completed a secure online self-reported injury survey. Injury was defined as subject-reported pain in the patellar or Achilles tendon (including their insertion points) with activity, yes or no. Each survey described the locations and definitions of injury verbally and pictorially for the subjects. VISA-A[62] and VISA-P[63] values were obtained if the subject developed pain in the respective tendon, as well as a numeric pain score at rest.
Image acquisition and analysis:
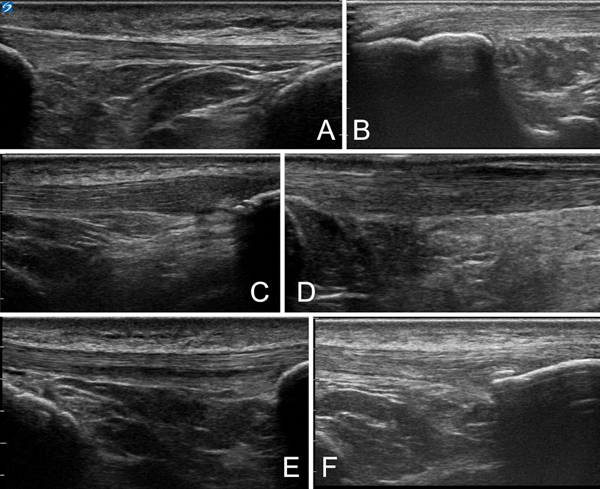
For evaluation of the patellar tendon and Achilles tendon, ultrasonography was performed with a 12-MHz linear transducer, 3cm length, with a Sonosite X-porte ultrasound machine. To limit inter-rater variability, this was performed by only one of the study sports medicine physicians with several years of experience with musculoskeletal ultrasound, who was blinded to the subject’s clinical symptoms, training history, and social history. Images for every subject were saved without identifiable information. Runners were placed prone with the feet hanging over the edge of the table with the ankles flexed to 90° passively for evaluation of the Achilles tendons. The patellar tendons were then examined with the runner in a supine position, with the quadriceps muscle activated. The sonographer did not readily identify areas of abnormalities at the time of scanning, and the subjects were not informed of potential abnormalities. Short-axis images were saved at the tendon location at its greatest width while longitudinal assessment was made in the midline tendon, centered over the area of maximum thickness. To standardize all measurements, the following measurement techniques and definitions were used, as previously described.[31,39,64] At a later time-point, all saved static tendon images were reviewed in a blinded manner by a physician with several years’ experience in musculoskeletal ultrasound. Pilot data revealed an intra-rater reliability kappa value of 0.66 (95% CI 0.47–0.85). Each imaged tendon was classified as ultrasonographically normal or abnormal. Abnormal findings were further categorized for presence of 1) hypoechogenicity, 2) intratendinous delamination (consisting of intratendinous defects with discrete separation of tendon fibers)[39], 3) paratenon blurring[38], 4) calcification, and 5) tendon thickening (defined as barrel-shaped thickening greater than 0.5mm in the Achilles or greater than 1.0mm in the patellar tendon as compared to the normal distal portion)[31]. See figure 1.
Figure 1 –
Demonstration of tendon findings. A) Normal patellar tendon. B) Normal Achilles tendon at insertion. C) Hypoechogenicity and thickening on right side of image. D) Paratenon blurring seen at superficial portion of tendon. E) Delamination seen within middle of tendon. F) Intratendinous calcification.
Data Analysis:
Descriptive statistics were calculated for the demographics, pre-race ultrasound findings, and pain after the race in the Achilles and patellar tendons of the runners. A two-by-two contingency table analysis was performed to examine the association between ultrasound abnormality and development of pain in the Achilles and patellar tendons at 1, 3, 6, and 12 months post-race. In addition, the association of a specific type of ultrasound abnormality to post-race pain at any time point was examined using a two-by-two contingency table analysis. Fisher’s exact test was used for significance tests, and a relative risk (RR) and its 95% confidence interval (CI) were calculated to aid in interpretations. Further, as multivariate analysis, binary logistic regression analysis was conducted to examine the relationships of demographic variables, training, and pre-race pain to ultrasound abnormality in the Achilles and patellar tendons. Lastly, the Cox proportional hazards model was used to examine the risk of developing pain in the Achilles and patellar tendon after the race by ultrasound abnormality, while using gender, age, years of running, average miles per week of training over a year, and pre-race pain as covariates. The assumption of proportional hazards was tested and ensured based on the Schoenfeld residuals[65]. Adjustments for missing data points during statistical analysis were treated as follows: No survey responses on post-race pain at each time point were treated as missing data; whereas, for the variable on pain at any time point, no survey responses were treated as absence of pain.
RESULTS
Of the 139 runners who were examined with ultrasound before the race, 104 responded to the post-race survey for at least one time-point (74.8%), and were included in the analysis for this study. Survey response rate at each time was: 79.8% (n = 83) at 1 month post-race, 59.6% (n = 62) at 3 months post-race, 53.8% (n = 56) at 6 months post-race, and 60.6% (n = 63) at 12 months post-race. Demographics, pre-race ultrasound findings, and post-race pain at any time point are summarized in Table 1. To evaluate for loss of follow-up bias, the populations were compared at each time point, and there was no significant difference in any demographic variable between pre-race and any time point at post-race (p > 0.05). The proportion of runners found to have an ultrasound tendon abnormality prior to the race were 24.0% (n = 25) in the Achilles tendon and 23.1% (n = 24) in the patellar tendon. Overall, 16.4% (n = 17) and 25.0% (n = 26) of runners had pain in the Achilles and patellar tendons, respectively, at some time after the race. Severity of pain in those reporting symptoms for the Achilles were 3.3 ± 1.0, 2.9 ± 1.0, 1.5 ± 0.7, and 1.0 ± 0.5 at 1, 3, 6, and 12 months respectively. Values for the patellar tendon were 3.2 ± 1.2, 2.5 ± 1.1, 1.9 ± 1.0, and 2.7 ± 1.4. VISA-A scores for the same time points were 46.2 ± 10.8, 46.4 ± 10.0, 50.0 ± 9.2, and 53.5 ± 5.7. Finally, VISA-P scores were 59.4 ± 5.0, 54.9 ± 10.2, 61.0 ± 5.1, 61.6 ± 5.1. Of the runners reporting pain, 35% of those with Achilles pain and 38% of those with patellar pain reported pain at multiple time points in the study.
Table 1.
Demographic characteristics of study participants included in data analysis (N = 104).
| Variable | f (%) | ||
|---|---|---|---|
|
| |||
| Gender | Male | 59 (56.7) | |
| Female | 45 (43.3) | ||
| Median age category | 31–40 years | ||
| Height (m) [mean (SD)] | 1.72 (0.1) | ||
| Mass (kg) [mean (SD)] | 71.4 (13.1) | ||
| Body mass index (kg/m2) [mean (SD)] | 24.0 (3.5) | ||
| Smoking status | Never smoked | 91 (87.5) | |
| Former Smoker | 12 (11.5) | ||
| Current Smoker | 1 (1.0) | ||
| Race | Half-marathon | 68 (65.4) | |
| Marathon | 25 (24.0) | ||
| Other | 11 (10.6) | ||
| Years of running | Started this year | 9 (8.6) | |
| 1–5 years | 37 (35.6) | ||
| 6–10 years | 27 (26.0) | ||
| 11+ years | 31 (29.8) | ||
| Average miles per week of running over a year | 0–10 miles | 26 (25.0) | |
| 11–20 miles | 41 (39.4) | ||
| 21–30 miles | 23 (22.1) | ||
| > 30 miles | 14 (13.5) | ||
| Ultrasound abnormality (left or right) | Achilles tendon | Yes | 25 (24.0) |
| No | 79 (76.0) | ||
| Patellar tendon | Yes | 24 (23.1) | |
| No | 80 (76.9) | ||
| Post-race pain at any time point | Achilles tendon | Yes | 17 (16.4) |
| No | 87 (83.6) | ||
| Patellar tendon | Yes | 26 (25.0) | |
| No | 78 (75.0) | ||
Types and frequencies of ultrasound abnormality in the Achilles and patellar tendons are described in Table 2. The association of type of ultrasound abnormality to pain in the Achilles and patellar tendons at any time point are summarized in Tables 3a and 3b. Delamination was significantly associated with development of pain in both Achilles and patellar tendons (p = 0.001 for both tendons). Further, there was a significant association between paratenon blurring or calcification and pain in the patellar tendon (p = 0.035 and 0.008), however this association was not statistically significant for the Achilles tendon (p = 0.081 and 0.067 for paratenon blurring and calcification, respectively). Presence of tendon thickening and areas of hypoechogenicity were not significantly associated with the development of pain within the 12 months after the race.
Table 2.
Ultrasonographic findings in Achilles and patellar tendons pre-race (N = 104, 208 tendons total).
| Type of ultrasound abnormality [f (%) out of 208] | |||||
|---|---|---|---|---|---|
|
|
|||||
| Tendon | Hypoechogenicity | Delamination | Paratenon blur | Thickening | Calcification |
| Achilles tendon (left and right) | 18 (8.7) | 13 (6.3) | 5 (2.4) | 8 (3.8) | 10 (4.8) |
| Patellar tendon (left and right) | 15 (7.2) | 15 (7.2) | 5 (2.4) | 7 (3.4) | 20 (9.6) |
Table 3a.
Achilles tendon: Analysis of type of ultrasonographic abnormality in the Achilles tendon and development of pain at any time point (N = 104 runners, 208 tendons total).
| Pain, f (%) | |||||
|---|---|---|---|---|---|
|
|
|||||
| Type of ultrasound abnormality | Yes | No | Fisher’s exact p | Relative risk (95% CI) | |
| Hypoechogenicity | Abnormal | 2 (11.1) | 16 (88.9) | 0.700 | 1.11 (0.28, 4.39) |
| Normal | 19 (10.0) | 171 (90.0) | |||
| Delamination | Abnormal | 6 (46.2) | 7 (53.8) | 0.001* | 6.00 (2.80, 12.86) |
| Normal | 15 (7.7) | 180 (92.3) | |||
| Paratenon Blurring | Abnormal | 2 (40.0) | 3 (60.0) | 0.081 | 4.27 (1.35, 13.57) |
| Normal | 19 (9.4) | 184 (90.6) | |||
| Thickening | Abnormal | 1 (12.5) | 7 (87.5) | 0.580 | 1.25 (0.19, 8.19) |
| Normal | 20 (10.0) | 180 (90.0) | |||
| Calcification | Abnormal | 3 (30.0) | 7 (70.0) | 0.067 | 3.30 (1.16, 9.38) |
| Normal | 18 (9.1) | 180 (90.9) | |||
CI = confidence interval.
Significant association.
Table 3b.
Patellar tendon: Analysis of type of ultrasonographic abnormality in the patellar tendon and development of pain at any time point (N = 104 runners, 208 tendons total).
| Pain, f (%) | |||||
|---|---|---|---|---|---|
|
|
|||||
| Type of ultrasound abnormality | Yes | No | Fisher’s exact p | Relative risk (95% CI) | |
| Hypoechogenicity | Abnormal | 5 (33.3) | 10 (66.7) | 0.142 | 2.14 (0.98, 4.71) |
| Normal | 30 (15.5) | 163 (84.5) | |||
| Delamination | Abnormal | 8 (53.3) | 7 (46.7) | 0.001* | 3.81 (2.12, 6.87) |
| Normal | 27 (14.0) | 166 (86.0) | |||
| Paratenon Blurring | Abnormal | 3 (60.0) | 2 (40.0) | 0.035* | 3.81 (1.74, 8.33) |
| Normal | 32 (15.8) | 171 (84.2) | |||
| Thickening | Abnormal | 1 (14.3) | 6 (85.7) | 0.999 | 0.84 (0.13, 5.32) |
| Normal | 34 (16.9) | 167 (83.1) | |||
| Calcification | Abnormal | 8 (40.0) | 12 (60.0) | 0.008* | 2.79 (1.47, 5.28) |
| Normal | 27 (14.4) | 161 (85.6) | |||
CI = confidence interval.
Significant association.
Tables 4a and 4b show the associations of ultrasound findings to pain in the Achilles and patellar tendons after the race. At 3 months post-race, runners with any ultrasound abnormality were about 5.5 times (RR = 5.49; 95% CI = 1.60, 18.86; p = 0.011) and 4.7 times (RR = 4.72; 95% CI = 1.87, 11.92; p = 0.004) more likely to develop pain in the Achilles and patellar tendons, respectively, compared with runners without ultrasound abnormality. Further, the risk of having pain in the Achilles tendon 6 months after the race was 8.7 times (RR = 8.70; 95% CI = 2.30, 33.23; p = 0.003) higher for runners with ultrasound abnormality than those without ultrasound abnormality. At 12 months post-race, runners with an ultrasound abnormality in the patellar tendon were about 4.0 times (RR = 3.98; 95% CI = 1.55, 10.22; p = 0.010) more likely to have pain in the patellar tendon than those without ultrasound abnormality. When all time points were combined, runners with ultrasound abnormality in the Achilles and patellar tendons were 2–3 times more likely to develop pain in the respective tendons than those without ultrasound abnormality (RR = 3.14; 95% CI = 1.41, 7.01; p = 0.010 for Achilles tendon; RR = 2.52; 95% CI = 1.37, 4.62; p = 0.008 for patellar tendon).
Table 4a.
Association between pre-race ultrasonographic findings in the Achilles tendons and pain at 1, 3, 6, and 12 months post-race (N = 104 runners, 208 tendons total).
| Pain, f (%) | |||||
|---|---|---|---|---|---|
|
|
|||||
| Post-race time point | Pre-race Ultrasound | Yes | No | Fisher’s exact p | Relative risk (95% CI) |
| 1 month post-race | Abnormal | 3 (10.0) | 27 (90.0) | 0.112 | 3.40 (0.80, 14.40) |
| Normal | 4 (2.9) | 132 (97.1) | |||
|
| |||||
| 3 months post-race | Abnormal | 5 (21.7) | 18 (78.3) | 0.011* | 5.49 (1.60, 18.86) |
| Normal | 4 (4.0) | 97 (96.0) | |||
|
| |||||
| 6 months post-race | Abnormal | 5 (27.8) | 13 (72.2) | 0.003* | 8.70 (2.30, 33.23) |
| Normal | 3 (3.2) | 91 (96.8) | |||
|
| |||||
| 12 months post-race | Abnormal | 3 (15.0) | 17 (85.0) | 0.153 | 2.65 (0.72, 9.73) |
| Normal | 6 (5.7) | 100 (94.3) | |||
CI = confidence interval.
Significant association.
Table 4b.
Association between pre-race ultrasonographic findings in the patellar tendons and pain at 1, 3, 6, and 12 months post-race (N = 104 runners, 208 tendons total).
| Pain, f (%) | |||||
|---|---|---|---|---|---|
|
|
|||||
| Post-race time point | Pre-race Ultrasound | Yes | No | Fisher’s exact p | Relative risk (95% CI) |
| 1 month post-race | Abnormal | 2 (8.0) | 23 (92.0) | 0.999 | 1.13 (0.26, 4.84) |
| Normal | 10 (7.1) | 131 (92.9) | |||
|
| |||||
| 3 months post-race | Abnormal | 6 (35.3) | 11 (64.7) | 0.004* | 4.72 (1.87, 11.92) |
| Normal | 8 (7.5) | 99 (92.5) | |||
|
| |||||
| 6 months post-race | Abnormal | 2 (11.1) | 16 (88.9) | 0.999 | 1.16 (0.27, 4.93) |
| Normal | 9 (9.6) | 85 (90.4) | |||
|
| |||||
| 12 months post-race | Abnormal | 6 (30.0) | 14 (70.0) | 0.010* | 3.98 (1.55, 10.22) |
| Normal | 8 (7.5) | 98 (92.5) | |||
CI = confidence interval.
Significant association.
The results of the binary logistic regression analysis analysis are summarized in Table 5 showing no statistically significant association between any collected demographic and training characteristics, except male sex with the development of patellar tendon pain, with an odds ratio of 3.70 (95% CI = 1.17, 11.74).
Table 5.
Binary logistic regression models on ultrasound abnormality by demographic characteristics.
| Outcome | Predictor | Odds ratio (95% CI) | p | |
|---|---|---|---|---|
| Ultrasound abnormality at Achilles tendon (left or right)* | Gender (reference: female) | Male | 2.61 (0.85, 8.01) | 0.094 |
| Age (reference: 18–30 years old) | 31–40 years | 0.80 (0.17, 3.77) | 0.774 | |
| 41–50 years | 3.30 (0.69, 15.87) | 0.136 | ||
| 51+ years | 3.72 (0.89, 15.64) | 0.073 | ||
| Years of running (reference: started this year) | 1–5 years | 1.18 (0.19, 7.46) | 0.861 | |
| 6–10 years | 0.49 (0.06, 3.66) | 0.483 | ||
| 11+ years | 0.60 (0.08, 4.54) | 0.621 | ||
| Average miles per week of running over a year (reference: > 30 miles) | 0–10 miles | 2.21 (0.32, 15.20) | 0.421 | |
| 11–20 miles | 1.08 (0.16, 7.25) | 0.937 | ||
| 21–30 miles | 5.66 (0.81, 39.45) | 0.080 | ||
| Pre-race pain (reference: no) | Yes | 1.69 (0.42, 6.82) | 0.462 | |
|
| ||||
| Ultrasound abnormality at patellar tendon (left or right)** | Gender (reference: female) | Male | 3.70 (1.17, 11.74) | 0.026 |
| Age (reference: 18–30 years old) | 31–40 years | 0.73 (0.18, 2.90) | 0.655 | |
| 41–50 years | 0.65 (0.14, 3.05) | 0.583 | ||
| 51+ years | 0.59 (0.12, 2.88) | 0.510 | ||
| Years of running (reference: started this year) | 1–5 years | 4.75 (0.41, 54.97) | 0.212 | |
| 6–10 years | 1.69 (0.13, 21.19) | 0.688 | ||
| 11+ years | 2.21 (0.18, 27.53) | 0.537 | ||
| Average miles per week of running over a year (reference: > 30 miles) | 0–10 miles | 0.36 (0.04, 3.38) | 0.372 | |
| 11–20 miles | 2.00 (0.35, 11.30) | 0.434 | ||
| 21–30 miles | 3.05 (0.49, 19.01) | 0.232 | ||
| Pre-race pain (reference: no) | Yes | 1.03 (0.29, 3.65) | 0.966 | |
CI = confidence interval.
Model χ2 = 18.06, p = 0.080
Model χ2 = 15.95, p = 0.143.
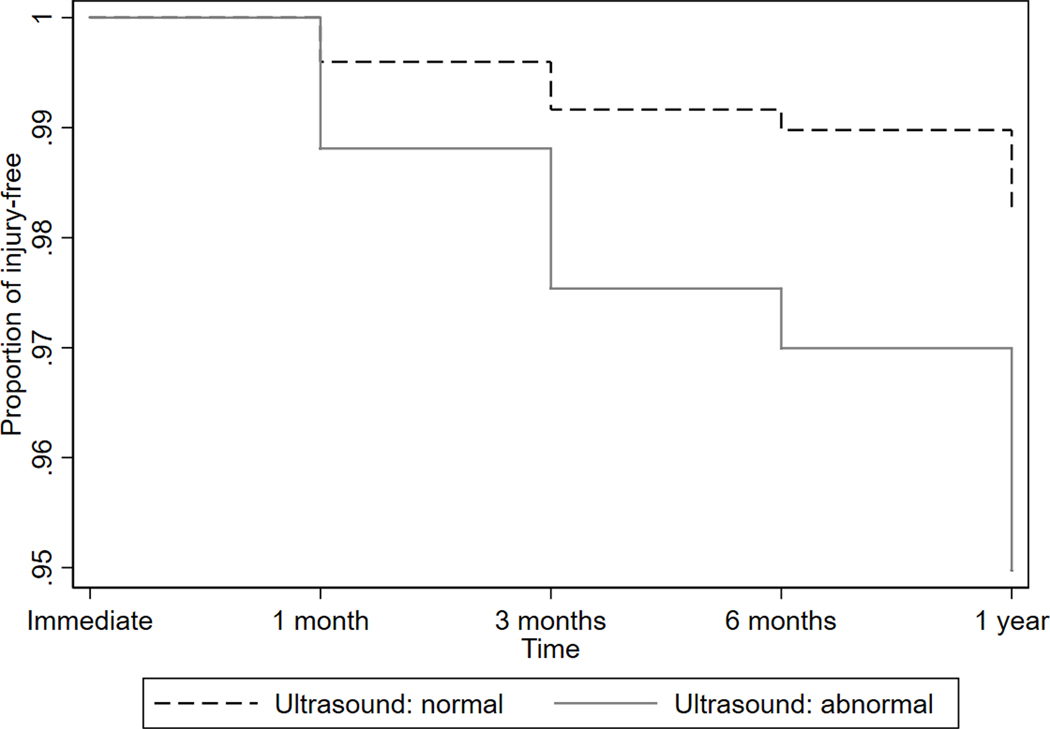
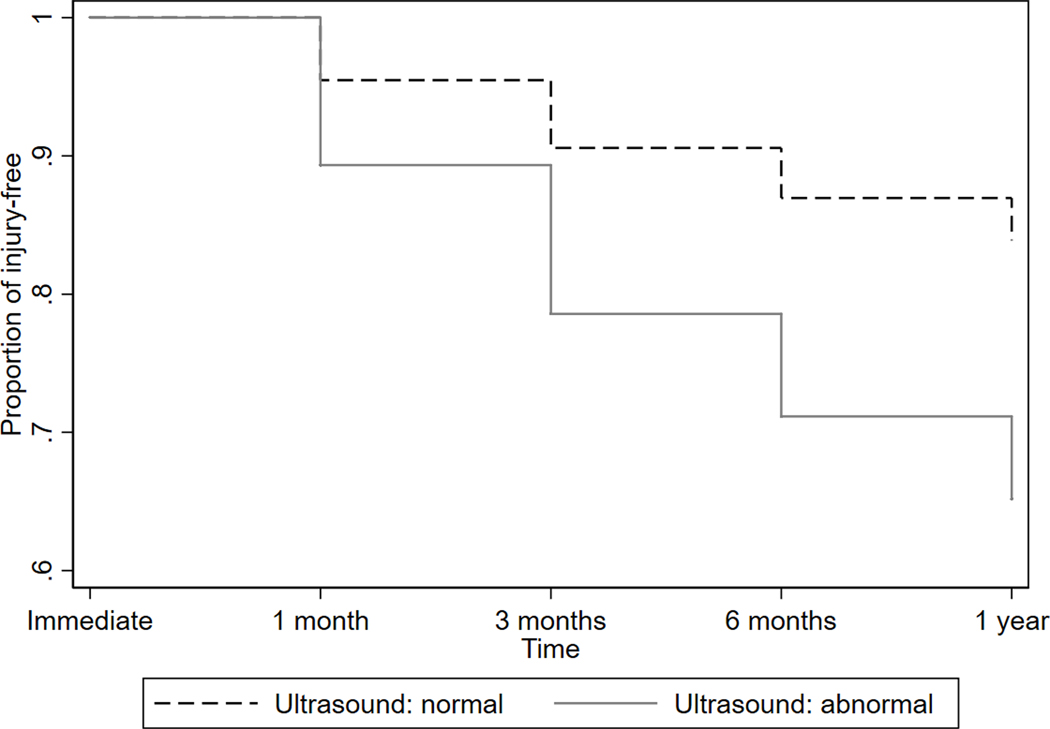
Survival estimates (pain-free period) for the Achilles and patellar tendons by ultrasound findings derived from the Cox proportional hazards models are shown in Figures 2a and 2b. After adjusting for gender, age, years of running, average miles per week of running over a year, and pre-race pain, runners with ultrasound abnormality in the Achilles tendon were about 3 times more likely to develop pain in the Achilles tendon after the race (hazard ratio = 2.89; 95% CI = 1.05, 7.93; p = 0.039). Likewise, there was a significant association between ultrasound abnormality and post-race pain in the patellar tendon (hazard ratio = 2.73; 95% CI = 11.11, 6.78; p = 0.30), after adjusting for the same covariates above.
Figure 2a -.
Cox proportional hazards regression model for development of pain in the Achilles tendon.
Figure 2b -.
Cox proportional hazards regression model for development of pain in the patellar tendon.
DISCUSSION
The most important finding of this study is that runners with structurally abnormal tendons have an increased risk of developing pain within one year and that ultrasonographic evaluation may be associated with tendons being at higher risk of subsequently developing pain. Prior prospective studies have found conflicting results on the clinical importance of asymptomatic structural tendon findings. These sonographic findings are thought to either represent a disease spectrum identifying at-risk tendons, a form of active adaptation to loading, or a benign asymptomatic finding which does not contribute to later pain. Our study specifically found an approximately 3-fold increased relative risk of developing Achilles and patellar pain in the presence of asymptomatic sonographic tendon abnormality in our cohort of recreational half-marathon and marathon runners.
Specific to runners, studies on incidence of symptomatic tendinopathy lack robust prospective studies with high power, however meta-analysis reveals rates of Achilles tendinopathy in general runners with an incidence ranging from 9.1% to 10.9% and prevalence ranging from 6.2% to 9.5%[66] which are similar to incidences in other running and jumping sports.[67] High rates of lifetime cumulative incidence have been described in specific cohorts, for example a 52% lifetime cumulative incidence of Achilles tendinopathy in former elite male endurance runners.[68] If the overall incidence of symptomatic tendinopathy in recreational runners is estimated to be low, or around 5–7% as previously published[69,70] then predictions from ultrasonographic evaluations may not be clinically sufficient to consider screening an general population. Meanwhile, ultrasound could be a clinically useful tool for predicting Achilles and patellar tendinopathy in populations with a high risk, such as marathon runners in this study in which the incidence rate could be as high as 25.0%.
Meta-analysis of other prospective studies using ultrasonographically abnormal tendons to predict injury risk found an average relative risk of 7.33 for the Achilles tendon and 4.35 for the patellar tendon[46], which is higher than that found in our study with Achilles tendon relative risk of 2.52 and relative risk of 3.14 for the patellar tendon looking at all follow-up time points. The studies included in the meta-analysis included a heterogenous group of athletes from various sports, as well as various time-points which differ from our study design. Only two studies looking at runners were included in the meta-analysis, and both exclusively included the Achilles tendon.[59,61] Ooi et al. examined the predictive value of ultrasound abnormalities on development of pain within 3 days after a marathon race.[61] They followed up any abnormal tendons with imaging within 4–6 weeks after the race, finding that certain ultrasonographic tendon abnormalities, in their case tendon stiffness found on sonoelastography, were significantly correlated with increased risk of pain after the race.[61] Hirschmüller et al. examined the predictive value of Achilles abnormalities on development of subsequent pain in a cohort of 427 runners at 6 or 12 months after a marathon race.[59] The study found a 6.9 fold increased risk of developing symptoms in participants with presence of neovascularization in the tendon at time of ultrasound evaluation. One limiting factor in interpreting these results is that acute bouts of exercise may transiently influence the appearance of tendons,[22,60,71–73] and unfortunately it is not clear from the study report whether the ultrasonographic evaluation was performed before the race in each case, although it was noted to have been performed after at least a two hour rest period. It has been shown that Achilles tendon stiffness is unchanged after a marathon,[74] which may suggest that alternative mechanisms are responsible for the development of tendinopathic change.
Our study offers additional evidence that asymptomatic abnormal tendon structure increases risk of subsequent development of Achilles and patellar tendon pain in runners. This association was strongest for a finding of delamination in our study in both the Achilles and patellar tendon, as well as for presence of paratenon blurring and calcification, which were statistically significant in the patellar tendon and approached significance for the Achilles tendon. Ultrasonographic delamination was similarly found to be associated with increased risk of development of Achilles tendon pain in previously asymptomatic Australian rules football players.[39] Similar studies looking at the predictive value of ultrasonographic evaluation of asymptomatic tendons found highest association with presence of neovascularization,[44,59] tendon thickening[30,31,38], and hypoechoic tendon changes.[29,31,75] In our study, however, areas of hypoechoic tendon changes and tendon thickening were not predictive of subsequent pain. Other prospective studies, looking primarily at elite jumping sport athletes such as basketball players, likewise found no increased risk of clinical injury on long-term follow up in athletes with hypoechoic patellar tendon changes.[45,76] Tendon changes, including changes in echogenicity and thickening, have been found to change asymptomatically with loading in runners[48,77–79] and thicker tendons have not been found to have statistically significant correlation with increased risk of pain on the limited prospective studies on runners available.[59,61] This suggests that thickening and hypoechoic tendon changes may be part of normal tissue adaptation to running load, or that they appear early or transiently in the continuum of pathological load-tendon adaptation. Furthermore, thickening may be a positive adaptation, and not necessarily pathologic.[79,80]
No correlation was found between demographic characteristics including mass, height, smoking history, training history and development of pain. Male sex was associated with an increased risk of development of patellar tendon symptoms. Other studies looking at Achilles and patellar tendinopathy in marathon runners have similarly not identified any statistically significant association between sex, mass, height and number of marathons run, but did identify an association with age.[81] Additional studies looking at Achilles and patellar tendinopathy in masters track and field runners found no influence of age, gender, mass.[82,83] Similarly, in athletes from other sports, demographic factors predictive of injury in these tendons have not been identified.[84] The cohort of runners who had symptoms had mean VISA-A scores in the range of 46.2 to 53.5 and VISA-P scores from 54.9 to 61.6, depending on the time point. Though a severity grading system has not been developed for the VISA-A or VISA-P, these scores correlate with poor to fair Achilles tendinopathy [62]and coincide with normal scores for patellar tendinopathy.[85,86] This suggests that these are not just mild irritation to the tendons, and are consistent with tendinopathic pain.
Our study has a number of limitations, which limits the generalizability of the study. The first limitation is the self-reported nature of the primary outcome, or development of tendon pain. Objective evaluation of tendons was only performed prior to the race, and no objective physical examination or imaging of tendon pathology was performed at follow-up. Since no physical examination was performed for the participants reporting pain on follow-up, a possibility exists that pain in the reported region was due to abnormalities in adjacent structures. Furthermore, many runners may have peaked their training for this race, and thus decreased their running mileage thereafter; this could conceivably decrease the number of post-race injuries. Additionally, although participants were not told of the results of the scan, there is a possibility of participant suggestion bias for reporting pain in the previously scanned area due to participation in the study. The second limitation is the loss to follow-up, which was most prominent at 6 months after the race. Despite the loss of follow up, the subjects’ demographic variables were comparable between pre-race and any time point at post-race. A third limitation is that ultrasonographic evaluation of tendon neovascularization was not performed. Although tendon neovascularization assessment has been shown to be a reliable additional method for evaluating tendon pathology[46,87], the trade-off for time to perform neovascularization assessments was considered against the decrease in number of subjects able to be recruited and scanned at the time of the race. A single experienced physician ultrasonographer performed the studies and a single experienced ultrasonographer blindly interpreted the studies, which could limit generalizability, but did allow for consistency. In general, intra-rater and inter-rater reliability of tendon ultrasonographic evaluation has been shown to be high.[88,89] The dynamic nature of ultrasound does limit the ability to accurately assess static (saved) ultrasound images, and may introduce additional bias. Future studies can help address some of these concerns by having in-person follow-ups and consideration of multiple ultrasonographers.
Nevertheless, this study suggests that asymptomatic Achilles and patellar tendons in distance runners with a pathological appearance are more likely to develop pain within the following year than tendons with normal ultrasonographic appearance. No prior studies have evaluated the risk to the patellar and Achilles tendons in a large cohort over a full year. This suggests that ultrasonography may be used to screen for runners at increased risk for tendon pathology. Consequently, these ‘high-risk’ runners could be screened for biomechanical and training risk factors and recommended a training plan intervention or preventative exercise interventions to decrease the risk of pain, and/or earlier rehabilitation should these patients develop symptoms. Currently, the effectiveness of preventative exercises based on these abnormalities is scant.[31,90] Likewise, further research identifying biomechanical and training risk factors for development of tendon pathology in runners is the logical next step.
CONCLUSION
A brief ultrasonographic evaluation of asymptomatic tendons in runners may be used to identify asymptomatic structural changes which are associated with increased risk of development of Achilles and patellar tendon pain. Further research into potential primary and secondary prevention interventions for runners with presence of abnormalities is needed to decrease the high-prevalence of tendon injury in the running community and the general public.
ACKNOWLEDGEMENTS
The authors would like to thank the coordinators of the Salt Lake City Marathon.
Preliminary results of the study at 3-month follow-up were presented at the 2018 AMSSM annual conference in Orlando, Florida, but the study has not been previously published.
Internal departmental research funding was obtained from the University of Utah Division of Physical Medicine & Rehabilitation. No additional funding was obtained for this study.
The authors do not have any competing interests or other financial conflicts for this case.
Footnotes
Author disclosures: The authors declare no competing interests or financial benefit in this original research.
REFERENCES
- [1].de Jonge S, van den Berg C, de Vos RJ, et al. Incidence of midportion Achilles tendinopathy in the general population. Br J Sport Med. 2011/09/20. 2011;45:1026–1028. [DOI] [PubMed] [Google Scholar]
- [2].Hagglund M, Zwerver J, Ekstrand J. Epidemiology of patellar tendinopathy in elite male soccer players. Am J Sport Med. 2011/06/07. 2011;39:1906–1911. [DOI] [PubMed] [Google Scholar]
- [3].Jarvinen TA, Kannus P, Maffulli N, et al. Achilles tendon disorders: etiology and epidemiology. Foot Ankle Clin. 2005;10:255–266. [DOI] [PubMed] [Google Scholar]
- [4].Shaikh Z, Perry M, Morrissey D, et al. Achilles tendinopathy in club runners. Int J Sport Med. 2012/03/02. 2012;33:390–394. [DOI] [PubMed] [Google Scholar]
- [5].Sobhani S, Dekker R, Postema K, et al. Epidemiology of ankle and foot overuse injuries in sports: A systematic review. Scand J Med Sci Sport. 2012/08/01. 2013;23:669–686. [DOI] [PubMed] [Google Scholar]
- [6].Zwerver J, Bredeweg SW, van den Akker-Scheek I. Prevalence of Jumper’s knee among nonelite athletes from different sports: a cross-sectional survey. Am J Sport Med. 2011/07/09. 2011;39:1984–1988. [DOI] [PubMed] [Google Scholar]
- [7].Roos KG, Marshall SW, Kerr ZY, et al. Epidemiology of Overuse Injuries in Collegiate and High School Athletics in the United States. Am J Sport Med. 2015/05/02. 2015;43:1790–1797. [DOI] [PubMed] [Google Scholar]
- [8].Cassel M, Baur H, Hirschmuller A, et al. Prevalence of Achilles and patellar tendinopathy and their association to intratendinous changes in adolescent athletes. Scand J Med Sci Sport. 2014/09/13. 2015;25:e310–8. [DOI] [PubMed] [Google Scholar]
- [9].Hess GW. Achilles tendon rupture: a review of etiology, population, anatomy, risk factors, and injury prevention. Foot Ankle Spec. 2010/04/20. 2010;3:29–32. [DOI] [PubMed] [Google Scholar]
- [10].van der Worp H, van Ark M, Roerink S, et al. Risk factors for patellar tendinopathy: a systematic review of the literature. Br J Sport Med. 2011/03/04. 2011;45:446–452. [DOI] [PubMed] [Google Scholar]
- [11].Magnan B, Bondi M, Pierantoni S, et al. The pathogenesis of Achilles tendinopathy: a systematic review. Foot Ankle Surg. 2014;20:154–159. [DOI] [PubMed] [Google Scholar]
- [12].Alfredson H, Pietila T, Jonsson P, et al. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sport Med. 1998/06/09. 1998;26:360–366. [DOI] [PubMed] [Google Scholar]
- [13].Jonsson P, Alfredson H, Sunding K, et al. New regimen for eccentric calf-muscle training in patients with chronic insertional Achilles tendinopathy: results of a pilot study. Br J Sport Med. 2008;42:746–749. [DOI] [PubMed] [Google Scholar]
- [14].Gajhede-Knudsen M, Ekstrand J, Magnusson H, et al. Recurrence of Achilles tendon injuries in elite male football players is more common after early return to play: an 11-year follow-up of the UEFA Champions League injury study. Br J Sports Med. 2013;47:763–768. [DOI] [PubMed] [Google Scholar]
- [15].van der Plas A, de Jonge S, de Vos RJ, et al. A 5-year follow-up study of Alfredson’s heel-drop exercise programme in chronic midportion Achilles tendinopathy. Br J Sports Med. 2012;46:214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gaida JE, Cook J. Treatment options for patellar tendinopathy: critical review. Curr Sports Med Rep. 2011;10:255–270. [DOI] [PubMed] [Google Scholar]
- [17].Thorpe CT, Riley GP, Birch HL, et al. Fascicles from energy-storing tendons show an age-specific response to cyclic fatigue loading. J R Soc Interface. 2014/01/10. 2014;11:20131058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Andarawis-Puri N, Philip A, Laudier D, et al. Temporal effect of in vivo tendon fatigue loading on the apoptotic response explained in the context of number of fatigue loading cycles and initial damage parameters. J Orthop Res. 2014/05/20. 2014;32:1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cook JL, Khan KM, Harcourt PR, et al. Patellar tendon ultrasonography in asymptomatic active athletes reveals hypoechoic regions: a study of 320 tendons. Victorian Institute of Sport Tendon Study Group. Clin J Sport Med. 1998/06/26. 1998;8:73–77. [DOI] [PubMed] [Google Scholar]
- [20].Cook JL, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med. 2009;43:409–416. [DOI] [PubMed] [Google Scholar]
- [21].van Ark M, Docking SI, van den Akker-Scheek I, et al. Does the adolescent patellar tendon respond to 5 days of cumulative load during a volleyball tournament? Scand J Med Sci Sport. 2015/02/20. 2016;26:189–196. [DOI] [PubMed] [Google Scholar]
- [22].Tardioli A. Structural changes in the achilles tendon in response to a marathon: ultrasonographically detectable changes immediately and at 2 weeks postmarathon. Br J Sports Med. 2011;45:e1–e1. [Google Scholar]
- [23].Rabello LM, Albers IS, van Ark M, et al. Running a Marathon—Its Influence on Achilles Tendon Structure. J Athl Train. 2020;55:176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fritschy D, Wallensten R. Surgical treatment of patellar tendinitis. Knee Surg Sport Traumatol Arthrosc. 1993/01/01. 1993;1:131–133. [DOI] [PubMed] [Google Scholar]
- [25].Jozsa L, Reffy A, Kannus P, et al. Pathological alterations in human tendons. Arch Orthop Trauma Surg. 1990/01/01. 1990;110:15–21. [DOI] [PubMed] [Google Scholar]
- [26].Khan KM, Bonar F, Desmond PM, et al. Patellar tendinosis (jumper’s knee): findings at histopathologic examination, US, and MR imaging. Victorian Institute of Sport Tendon Study Group. Radiology. 1996/09/01. 1996;200:821–827. [DOI] [PubMed] [Google Scholar]
- [27].Matthews W, Ellis R, Furness J, et al. Classification of Tendon Matrix Change Using Ultrasound Imaging: A Systematic Review and Meta-analysis. Ultrasound Med Biol. 2018;44:2059–2080. [DOI] [PubMed] [Google Scholar]
- [28].Astrom M, Gentz CF, Nilsson P, et al. Imaging in chronic achilles tendinopathy: a comparison of ultrasonography, magnetic resonance imaging and surgical findings in 27 histologically verified cases. Skelet Radiol. 1996/10/01. 1996;25:615–620. [DOI] [PubMed] [Google Scholar]
- [29].Comin J, Cook JL, Malliaras P, et al. The prevalence and clinical significance of sonographic tendon abnormalities in asymptomatic ballet dancers: a 24-month longitudinal study. Br J Sport Med. 2012/10/16. 2013;47:89–92. [DOI] [PubMed] [Google Scholar]
- [30].Fredberg U, Bolvig L. Significance of ultrasonographically detected asymptomatic tendinosis in the patellar and achilles tendons of elite soccer players: a longitudinal study. Am J Sport Med. 2002/07/20. 2002;30:488–491. [DOI] [PubMed] [Google Scholar]
- [31].Fredberg U, Bolvig L, Andersen NT. Prophylactic training in asymptomatic soccer players with ultrasonographic abnormalities in Achilles and patellar tendons: the Danish Super League Study. Am J Sport Med. 2007/12/15. 2008;36:451–460. [DOI] [PubMed] [Google Scholar]
- [32].Giombini A, Dragoni S, Di Cesare A, et al. Asymptomatic Achilles, patellar, and quadriceps tendinopathy: a longitudinal clinical and ultrasonographic study in elite fencers. Scand J Med Sci Sport. 2011/11/19. 2013;23:311–316. [DOI] [PubMed] [Google Scholar]
- [33].Ooi CC, Richards PJ, Maffulli N, et al. A soft patellar tendon on ultrasound elastography is associated with pain and functional deficit in volleyball players. J Sci Med Sport. 2015/06/23. 2016;19:373–378. [DOI] [PubMed] [Google Scholar]
- [34].Robinson P. Sonography of common tendon injuries. AJR Am J Roentgenol. 2009;193:607–618. [DOI] [PubMed] [Google Scholar]
- [35].Gisslèn K, Gyulai C, Söderman K, et al. High prevalence of jumper’s knee and sonographic changes in Swedish elite junior volleyball players compared to matched controls. Br J Sports Med. 2005;39:298–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Warden SJ, Kiss ZS, Malara FA, et al. Comparative accuracy of magnetic resonance imaging and ultrasonography in confirming clinically diagnosed patellar tendinopathy. Am J Sport Med. 2007;35:427–436. [DOI] [PubMed] [Google Scholar]
- [37].Splittgerber LE, Ihm JM. Significance of Asymptomatic Tendon Pathology in Athletes. Curr Sports Med Rep. 2019;18:192–200. [DOI] [PubMed] [Google Scholar]
- [38].Jhingan S, Perry M, O’Driscoll G, et al. Thicker Achilles tendons are a risk factor to develop Achilles tendinopathy in elite professional soccer players. Muscles Ligaments Tendons J. 2011/04/01. 2011;1:51–56. [PMC free article] [PubMed] [Google Scholar]
- [39].Ooi CC, Schneider ME, Malliaras P, et al. Sonoelastography of the Achilles Tendon: Prevalence and Prognostic Value Among Asymptomatic Elite Australian Rules Football Players. Clin J Sport Med. 2016;26:299–306. [DOI] [PubMed] [Google Scholar]
- [40].Docking SI, Rosengarten SD, Daffy J, et al. Structural integrity is decreased in both Achilles tendons in people with unilateral Achilles tendinopathy. J Sci Med Sport. 2014/07/16. 2015;18:383–387. [DOI] [PubMed] [Google Scholar]
- [41].Boesen AP, Boesen MI, Torp-Pedersen S, et al. Associations between abnormal ultrasound color Doppler measures and tendon pain symptoms in badminton players during a season: a prospective cohort study. Am J Sport Med. 2012/02/14. 2012;40:548–555. [DOI] [PubMed] [Google Scholar]
- [42].Kulig K, Landel R, Chang YJ, et al. Patellar tendon morphology in volleyball athletes with and without patellar tendinopathy. Scand J Med Sci Sport. 2012/12/21. 2013;23:e81–8. [DOI] [PubMed] [Google Scholar]
- [43].Balaban M, Idilman IS, Ipek A, et al. Elastographic Findings of Achilles Tendons in Asymptomatic Professional Male Volleyball Players. J Ultrasound Med. 2016/11/23. 2016;35:2623–2628. [DOI] [PubMed] [Google Scholar]
- [44].Gisslen K, Alfredson H. Neovascularisation and pain in jumper’s knee: a prospective clinical and sonographic study in elite junior volleyball players. Br J Sport Med. 2005/06/25. 2005;39:423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Khan KM, Cook JL, Kiss ZS, et al. Patellar tendon ultrasonography and jumper’s knee in female basketball players: a longitudinal study. Clin J Sport Med. 1997/07/01. 1997;7:199–206. [DOI] [PubMed] [Google Scholar]
- [46].McAuliffe S, McCreesh K, Culloty F, et al. Can ultrasound imaging predict the development of Achilles and patellar tendinopathy? A systematic review and meta-analysis. Br J Sport Med. 2016/09/17. 2016; [DOI] [PubMed] [Google Scholar]
- [47].Giacchino M, Caresio C, Gorji NE, et al. Quantitative analysis of patellar tendon size and structure in asymptomatic professional players: sonographic study. Muscle Ligaments Tendons J. 2019;07:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hullfish TJ, Hagan KL, Casey E, et al. Achilles tendon structure differs between competitive distance runners and non-runners despite no clinical signs or symptoms of mid-substance tendinopathy. J Appl Physiol. 2018; [DOI] [PubMed] [Google Scholar]
- [49].Hirschmuller A, Frey V, Deibert P, et al. [Achilles tendon power Doppler sonography in 953 long distance runners - a cross sectional study]. Ultraschall Med. 2010/03/18. 2010;31:387–393. [DOI] [PubMed] [Google Scholar]
- [50].Tillander B, Gauffin H, Lyth J, et al. Symptomatic Achilles Tendons are Thicker than Asymptomatic Tendons on Ultrasound Examination in Recreational Long-Distance Runners. Sports. 2019;7:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sponbeck JK, Perkins CL, Berg MJ, et al. Achilles Tendon Cross Sectional Area Changes Over a Division I NCAA Cross Country Season. Int J Exerc Sci. 10:1226–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Stanley LE, Lucero A, Mauntel TC, et al. Achilles tendon adaptation in cross-country runners across a competitive season. Scand J Med Sci Sports. 2018;28:303–310. [DOI] [PubMed] [Google Scholar]
- [53].Hagan KL, Hullfish T, Casey E, et al. Tendon structure quantified using ultrasound imaging differs based on location and training type. J Appl Physiol. 2018;125:1743–1748. [DOI] [PubMed] [Google Scholar]
- [54].Viljoen JT, Viviers PL, Kirby JH. Continuum of Change in the Achilles and Patella Tendons of Asymptomatic Track and Field Athletes. Med Sci Sport Exerc. 2015;47:506–507. [Google Scholar]
- [55].Cassel M, Risch L, Intziegianni K, et al. Incidence of Achilles and Patellar Tendinopathy in Adolescent Elite Athletes. Int J Sports Med. 2018;39:726–732. [DOI] [PubMed] [Google Scholar]
- [56].Taunton JE, Ryan MB, Clement DB, et al. A retrospective case-control analysis of 2002 running injuries. Br J Sports Med. 2002;36:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Jacobs SJ, Berson BL. Injuries to runners: a study of entrants to a 10,000 meter race. Am J Sports Med. 14:151–155. [DOI] [PubMed] [Google Scholar]
- [58].Nicol AM, McCurdie I, Etherington J. Use of ultrasound to identify chronic Achilles tendinosis in an active asymptomatic population. J R Army Med Corps. 2006;152:212–216. [DOI] [PubMed] [Google Scholar]
- [59].Hirschmuller A, Frey V, Konstantinidis L, et al. Prognostic value of Achilles tendon Doppler sonography in asymptomatic runners. Med Sci Sport Exerc. 2011/07/02. 2012;44:199–205. [DOI] [PubMed] [Google Scholar]
- [60].Proft F, Grunke M, Reindl C, et al. The influence of long distance running on sonographic joint and tendon pathology: results from a prospective study with marathon runners. BMC Musculoskelet Disord. 2016/07/13. 2016;17:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ooi CC, Schneider ME, Malliaras P, et al. Prevalence of morphological and mechanical stiffness alterations of mid Achilles tendons in asymptomatic marathon runners before and after a competition. Skelet Radiol. 2015/03/20. 2015;44:1119–1127. [DOI] [PubMed] [Google Scholar]
- [62].Robinson JM, Cook JL, Purdam C, et al. The VISA-A questionnaire: a valid and reliable index of the clinical severity of Achilles tendinopathy. Br J Sport Med. 2001/10/02. 2001;35:335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Visentini PJ, Khan KM, Cook JL, et al. The VISA score: an index of severity of symptoms in patients with jumper’s knee (patellar tendinosis). Victorian Institute of Sport Tendon Study Group. J Sci Med Sport. 1998/09/10. 1998;1:22–28. [DOI] [PubMed] [Google Scholar]
- [64].Fu S, Cui L, He X, et al. Elastic Characteristics of the Normal Achilles Tendon Assessed by Virtual Touch Imaging Quantification Shear Wave Elastography. J Ultrasound Med. 2016/07/03. 2016;35:1881–1887. [DOI] [PubMed] [Google Scholar]
- [65].GRAMBSCH PM, THERNEAU TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- [66].Lopes AD, Hespanhol LC, Yeung SS, et al. What are the Main Running-Related Musculoskeletal Injuries? Sport Med. 2012;42:891–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Florit D, Pedret C, Casals M, et al. Incidence of tendinopathy in team sports in a multidisciplinary sports club over 8 seasons. J Sport Sci Med. 2019;18:780–788. [PMC free article] [PubMed] [Google Scholar]
- [68].Kujala UM, Sarna S, Kaprio J. Cumulative Incidence of Achilles Tendon Rupture and Tendinopathy in Male Former Elite Athletes. Clin J Sport Med. 2005;15:133–135. [DOI] [PubMed] [Google Scholar]
- [69].Lagas IF, Fokkema T, Verhaar JAN, et al. Incidence of Achilles tendinopathy and associated risk factors in recreational runners: A large prospective cohort study. J Sci Med Sport. 2020;23:448–452. [DOI] [PubMed] [Google Scholar]
- [70].Lysholm J, Wiklander J. Injuries in runners. Am J Sport Med. 1987/03/01. 1987;15:168–171. [DOI] [PubMed] [Google Scholar]
- [71].Thygesen MM, Jordt I, Kristensen MS, et al. High-Intensity Resistance Training Does Not Produce Immediate Ultrasonographic Changes in Muscle Tendons. Orthop J Sport Med. 2019;7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Fisker FY, Kildegaard S, Thygesen M, et al. Acute tendon changes in intense CrossFit workout: an observational cohort study. Scand J Med Sci Sports. 2017;27:1258–1262. [DOI] [PubMed] [Google Scholar]
- [73].Roesch HJ, Milanese S, Osborne B, et al. The acute effects of exercise on tendon dimensions and vascularity. An exploratory study using diagnostic ultrasound of the male Achilles tendon. J Sci Med Sport. 2018;21:982–987. [DOI] [PubMed] [Google Scholar]
- [74].Peltonen J, Cronin NJ, Stenroth L, et al. Achilles tendon stiffness is unchanged one hour after a marathon. J Exp Biol. 2012;215:3665–3671. [DOI] [PubMed] [Google Scholar]
- [75].Visnes H, Tegnander A, Bahr R. Ultrasound characteristics of the patellar and quadriceps tendons among young elite athletes. Scand J Med Sci Sport. 2014/03/13. 2015;25:205–215. [DOI] [PubMed] [Google Scholar]
- [76].Cook JL, Khan KM, Kiss ZS, et al. Asymptomatic hypoechoic regions on patellar tendon ultrasound: A 4-year clinical and ultrasound followup of 46 tendons. Scand J Med Sci Sports. 2002/01/10. 2001;11:321–327. [DOI] [PubMed] [Google Scholar]
- [77].Sponbeck JK, Perkins CL, Berg MJ, et al. Achilles Tendon Cross Sectional Area Changes Over a Division I NCAA Cross Country Season. Int J Exerc Sci. 2017;10:1226–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Hagan KL, Hullfish T, Casey E, et al. Tendon structure quantified using ultrasound imaging differs based on location and training type. J Appl Physiol. 2018;125:1743–1748. [DOI] [PubMed] [Google Scholar]
- [79].Abate M, Oliva F, Schiavone C, et al. Achilles tendinopathy in amateur runners: role of adiposity (Tendinopathies and obesity). Muscles Ligaments Tendons J. 2012;2:44–48. [PMC free article] [PubMed] [Google Scholar]
- [80].Bissas A, Havenetidis K, Walker J, et al. Muscle-tendon morphology and function following long-term exposure to repeated and strenuous mechanical loading. Scand J Med Sci Sports. 2020;30:1151–1162. [DOI] [PubMed] [Google Scholar]
- [81].Longo UG, Berton A, Stelitano G, et al. 2017 Marathon of Rome: Anthropometry and Sport Profile in 350 Runners and Association With Achilles and Patellar Tendinopathy. Clin J Sport Med. 2018;Publish Ah. [DOI] [PubMed] [Google Scholar]
- [82].Longo UG, Rittweger J, Garau G, et al. No influence of age, gender, weight, height, and impact profile in achilles tendinopathy in masters track and field athletes. Am J Sports Med. 2009;37:1400–1405. [DOI] [PubMed] [Google Scholar]
- [83].Longo UG, Rittweger J, Garau G, et al. Patellar tendinopathy in master track and field athletes: influence of impact profile, weight, height, age and gender. Knee Surg Sports Traumatol Arthrosc. 2011;19:508–512. [DOI] [PubMed] [Google Scholar]
- [84].Sprague AL, Smith AH, Knox P, et al. Modifiable risk factors for patellar tendinopathy in athletes: a systematic review and meta-analysis. Br J Sports Med. 2018;52:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Hernandez-Sanchez S, Abat F, Hidalgo MD, et al. Confirmatory factor analysis of VISA-P scale and measurement invariance across sexes in athletes with patellar tendinopathy. J Sport Heal Sci. 2017;6:365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Zwerver J, Kramer T, Van Den Akker-Scheek I. Validity and reliability of the Dutch translation of the VISA-P questionnaire for patellar tendinopathy. BMC Musculoskelet Disord. 2009;10:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Risch L, Wochatz M, Messerschmidt J, et al. Reliability of evaluating achilles tendon vascularization assessed with doppler ultrasound advanced dynamic flow. J Ultrasound Med. 2018;37:737–744. [DOI] [PubMed] [Google Scholar]
- [88].Matthews W, Ellis R, Furness JW, et al. Staging achilles tendinopathy using ultrasound imaging: The development and investigation of a new ultrasound imaging criteria based on the continuum model of tendon pathology. BMJ Open Sport Exerc Med. 2020;6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Del Baño-Aledo ME, Martínez-Payá JJ, Ríos-Díaz J, et al. Ultrasound measures of tendon thickness: Intra-rater, Inter-rater and Inter-machine reliability. Muscles Ligaments Tendons J. 2017;7:192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Peters JA, Zwerver J, Diercks RL, et al. Preventive interventions for tendinopathy: A systematic review. J Sci Med Sport. 2016;19:205–211. [DOI] [PubMed] [Google Scholar]