Abstract
Background: Several bleeding risk assessment models have been developed in atrial fibrillation (AF) patients with oral anticoagulants, but the most appropriate tool for predicting bleeding remains uncertain. Therefore, we aimed to assess the diagnostic accuracy of the Hypertension, Abnormal liver/renal function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly, Drugs/alcohol concomitantly (HAS-BLED) score compared with other risk scores in anticoagulated patients with AF.
Methods: We comprehensively searched the PubMed and Embase databases until July 2021 to identify relevant pieces of literature. The predictive abilities of risk scores were fully assessed by the C-statistic, net reclassification improvement (NRI) and integrated discrimination improvement (IDI) values, calibration data, and decision curve analyses.
Results: A total of 39 studies met the inclusion criteria. The C-statistic of the HAS-BLED score for predicting major bleeding was 0.63 (0.61–0.65) in anticoagulated patients regardless of vitamin k antagonists [0.63 (0.61–0.65)] and direct oral anticoagulants [0.63 (0.59–0.67)]. The HAS-BLED had the similar C-statistic to the Hepatic or renal disease, Ethanol abuse, Malignancy, Older, Reduced platelet count or function, Re-bleeding risk, Hypertension (uncontrolled), Anemia, Genetic factors, Excessive fall risk, Stroke (HEMORR2HAGES), the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA), the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT), the Global Anticoagulant Registry in the FIELD-Atrial Fibrillation (GARFIELD-AF), or the Age, Biomarkers, Clinical History (ABC) scores, but significantly higher C-statistic than the Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Stroke/transient ischemic attack history (CHADS2) or the Congestive heart failure/left ventricular ejection fraction ≤ 40%, Hypertension, Age ≥75 years, Diabetes mellitus, Stroke/transient ischemic attack/thromboembolism history, Vascular disease, Age 65–74 years, Sex (female) (CHA2DS2-VASc) scores. NRI and IDI values suggested that the HAS-BLED score performed better than the CHADS2 or the CHA2DS2-VASc scores and had similar or superior predictive ability compared with the HEMORR2HAGES, the ATRIA, the ORBIT, or the GARFIELD-AF scores. Calibration and decision curve analyses of the HAS-BLED score compared with other scores required further assessment due to the limited evidence.
Conclusion: The HAS-BLED score has moderate predictive abilities for bleeding risks in patients with AF regardless of type of oral anticoagulants. Current evidence support that the HAS-BLED score is at least non-inferior to the HEMORR2HAGES, the ATRIA, the ORBIT, the GARFIELD-AF, the CHADS2, the CHA2DS2-VASc, or the ABC scores.
Keywords: HAS-BLED, major bleeding, risk prediction, atrial fibrillation, meta-analysis
Introduction
Atrial fibrillation (AF), the most common cardiac arrhythmia in clinical practice, is associated with a 5-fold risk of ischemic stroke. Oral anticoagulation (OAC) is recommended to reduce the risk of thromboembolism among AF patients with the Congestive heart failure/left ventricular ejection fraction ≤ 40%, Hypertension, Age ≥75 years, Diabetes mellitus, Stroke/transient ischemic attack/thromboembolism history, Vascular disease, Age 65–74 years, Sex (female) (CHA2DS2-VASc) score of ≥2 in males or ≥3 in females (1). However, the use of OAC could increase the bleeding risks, especially major bleeding and intracranial bleeding, which are associated with increased rates of cardiovascular adverse events and death (2). Therefore, the risk assessment of bleeding after the initiation of OAC should be taken into consideration. The Hypertension, Abnormal liver/renal function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly, Drugs/alcohol concomitantly (HAS-BLED) score is originated from the European Heart Survey database in 2010 (3), mainly focusing on the modifiable bleeding risk factors. The HAS-BLED score has been routinely recommended for predicting the bleeding risks in patients with AF who are taking anticoagulation (1).
In addition to the HAS-BLED score, several other bleeding risk assessment models have been developed in patients with AF (4–6). However, the differences in the predictive ability of the HAS-BLED score compared with other risk scores remain uncertain. Chang et al. (5) performed a network meta-analysis of 18 studies involving 321,888 patients and found that the HAS-BLED score was the most balanced bleeding risk prediction tool regarding sensitivity and specificity. Nevertheless, the sensitivity and specificity have limited guidance to clinicians when considering the probability of bleeding events in patients with AF (7). Zhu et al. (4) performed a meta-analysis including more critical measures (i.e., the C-statistic, reclassification, and calibration data), suggesting that the HAS-BLED score performed better for predicting major bleeding than other risk scores including the Hepatic or renal disease, Ethanol abuse, Malignancy, Older, Reduced platelet count or function, Re-bleeding risk, Hypertension (uncontrolled), Anemia, Genetic factors, Excessive fall risk, Stroke (HEMORR2HAGES), the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA), the Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Stroke/transient ischemic attack history (CHADS2), and the CHA2DS2-VASc scores. However, in this study, they assessed the HAS-BLED score only in patients with AF with the use of vitamin k antagonists (VKAs). Since direct oral anticoagulants (DOACs) including dabigatran, rivaroxaban, apixaban, and edoxaban are recommended as the preferred drugs for stroke prevention among patients with non-valvular AF (1, 8), whether the application of the HAS-BLED score could be extended to DOAC-treated patients is still unclear. Therefore, this systematic review and meta-analysis aimed to: (1) assess the diagnostic accuracy of the HAS-BLED score in anticoagulated (VKAs or DOACs) patients with AF and (2) compared the performances of the HAS-BLED score with other risk scores to determine the most appropriate tool for predicting bleeding risks.
Methods
This meta-analysis and systematic review were carried out based on the Cochrane Handbook for systemic reviews. The results were presented according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement. Ethical approval was not necessary because the published studies of electronic databases were included.
Literature Search
We comprehensively conducted a search of the PubMed and Embase electronic databases until July 2021 to identify relevant pieces of literature reporting the HAS-BLED score in anticoagulated patients with AF. The following keywords in the search strategies were used: (1) AF, (2) VKAs or warfarin or coumadin or phenprocoumon or acenocoumarol or indandione or fluindione or phenindione or anisindione or non-VKAs or DOACs or dabigatran or rivaroxaban or apixaban or edoxaban, and (3) the HAS-BLED score. To obtain the qualified studies comprehensively, we also performed the cross-reference retrieval by screening the reference lists of included studies and prior meta-analyses. English language restrictions in the literature research were applied in this study.
Eligibility Criteria
Studies were included if they met the following inclusion criteria: (a) patients with non-valvular AF (aged ≥ 18 years) with anticoagulants (VKAs or DOACs); (b) post-hoc analyses of randomized controlled trials (RCTs) or observational (prospective or retrospective cohorts) studies reported the diagnostic performance of the HAS-BLED score or focused on the predictive ability of the HAS-BLED score compared with any of other risk scores including the HEMORR2HAGES, the ATRIA, the ORBIT, the CHADS2, the CHA2DS2-VASc, the Global Anticoagulant Registry in the FIELD-Atrial Fibrillation (GARFIELD-AF), or Age, Biomarkers, Clinical History (ABC) (Supplementary Table 1); (c) the primary outcome was major bleeding and other bleeding events included any clinically relevant bleeding, any bleeding, intracranial bleeding, and gastrointestinal bleeding; and (d) at least one of the following data should be available: discrimination analysis (sensitivity/specificity or the C-statistic), net reclassification improvement (NRI) and integrated discrimination improvement (IDI) analyses, calibration data, and decision curve analyses. The sensitivity and specificity of the risk models have been analyzed by Chang et al. (5) in 2020 and, thus, were not included in this study.
We excluded studies that focused on the non-AF population or patients with AF with specific diseases (e.g., myocardial infarction, dialysis, ischemic stroke). In addition, we also excluded studies limiting to patients with AF with certain interventions (e.g., percutaneous coronary intervention, ablation). The bleeding risk prediction tools [e.g., the Rutherford score (9), mOBRI@@ (10), Adam score (11)] compared with the HAS-BLED score were not included, if they were analyzed for one bleeding endpoint in less than two independent studies. We also excluded studies reporting the modified HAS-BLED score version by adding additional factors (e.g., biomarkers, gene polymorphisms) into the original HAS-BLED score. Certain publication types with insufficient data such as reviews, case reports, editorials, or meeting abstracts were excluded.
Study Selection and Data Extraction
Potentially relevant studies were selected by two reviewers independently based on the predetermined criteria. Qualified articles were included after the title/abstract screenings and the full-text screenings. At this step, if two or more studies had the same data source, we selected the study that was more designed to meet our inclusion criteria. If both the studies meet the inclusion criteria, we selected the newly published study or the study with the longest follow-up or highest sample size. Disagreements were resolved through discussion between the two researchers or consultation with a third reviewer.
Data from included studies were extracted by two researchers independently. We abstracted the following baseline information including the authors, year of publication, study type, data source, demographic data, baseline characteristics of the patient [age, sex ratio, sample size, type of anticoagulants, concomitant antiplatelet drugs, or non-steroidal anti-inflammatory drugs (NSAIDs)], study endpoints and their definitions, and the follow-up time.
Quality Assessment
We applied the Prediction Model Risk of Bias Assessment Tool (PROBAST) (www.probast.org) to assess the risk prediction models (12). The PROBAST consists of four domains, namely participants, predictors, outcomes, and analysis. Risk assessment was rated as low risk, high risk, or unclear.
Consistency Test and Publication Bias
The consistency of the included studies was assessed through the Cochrane Q test and I2 index. Significant heterogeneity was considered if the p-value of the Cochrane Q test < 0.1 or if the I2 value of >50%. We used the funnel plots to examine the publication bias and a visual inspection of asymmetry indicated a bias.
Statistical Analysis
All the statistical analyses were carried out by using the Review Manager 5.3 software (The Nordic Cochrane Center, The Cochrane Collaboration, 2014, Copenhagen, Denmark, UK) (https://community.cochrane.org/). p < 0.05 was considered as statistically significant.
The C-statistic and their 95% CIs were abstracted from the included studies for the discrimination analysis. The C-statistic of ≤ 0.5 indicated that discrimination was no better than chance. The pooled analyses were performed if at least two studies reported the C-statistic for the HAS-BLED score. A random-effects model with an inverse variance method was chosen in the pooled analysis due to the observed significant heterogeneity. For the primary major bleeding events, the subgroup analyses were conducted on the basis of study design, the OAC type, and the follow-up time. We also assessed the predictive ability of the HAS-BLED based on available vs. unavailable labile international normalized ratio (INR) in the score.
The Z-statistic was calculated to compare the two C-statistic of the HAS-BLED score vs. other risk prediction models (the HEMORR2HAGES, the ATRIA, the ORBIT, the CHADS2, the CHA2DS2-VASc, the GARFIELD-AF, or the ABC scores) (4). In addition, we assessed the improvement in predictive accuracy by the reclassification analyses including the NRI and IDI parameters. The probability of correctly predicting bleeding events by using the HAS-BLED score was reflected in the percentage of events correctly reclassified. Calibration data represented the extent to which predicted risks correspond to observed risks. The net benefits of the HAS-BLED vs. other risk scores were assessed by using the decision curve analyses. Narrative analyses were performed with respect to reclassification, calibration, and decision curve analyses due to the lack of numerical data.
Results
Study Selection
The flowchart of document retrieval and screening process in this meta-analysis is shown in Supplementary Figure 1. We initially retrieved 1,601 studies through the electronic search of the PubMed and the Embase databases. After the screenings of the titles and abstracts, 97 studies were assessed for more detail. Furthermore, 58 of these studies were excluded because: (1) patients with OACs were not analyzed separately (n = 5); (2) duplicate data (n = 9); (3) the anticoagulated drugs were not VKAs or DOACs or unknown OAC data (n = 8); (4) studies did not report the C-statistic and/or their CIs (n = 8); (5) non-AF population or AF patients with coexisting specific diseases (n = 25); and (6) the outcome of bleeding was not analyzed separately (n = 3). Finally, a total of 39 studies published from 2010 to 2021 met the inclusion criteria and were included in this study (Supplementary Table 2).
The baseline characteristics of patient of the included studies are summarized in Table 1. The variables of the HAS-BLED score in the included studies are presented in Supplementary Table 3. The component of “labile INR” in the HAS-BLED score was available in 10 included studies. “Labile INR” was not applicable in three studies (13–15) because they only included DOAC-treated patients for analysis. As shown in Supplementary Table 4, all the included studies had high (n = 20, 51%) or unclear (n = 19, 49%) risk of bias according to the PROBAST tool.
Table 1.
Baseline characteristics of patient of the included studies.
| Study (author-year) | Data source | Study design | Sample size* | Type of anticoagulants analyzed | Concomitant antiplatelet or NSAIDS | Study endpoints | Bleeding scales | Comparisons of HAS-BLED vs. others | Follow-up time (y) |
|---|---|---|---|---|---|---|---|---|---|
| Pisters-2010 | Euro Heart Survey on AF; 2003–2004 | Retrospective cohort | 1,722 | VKAs | NA | Major bleeding | ISTH | HEMORR2HAGES | 1.0 |
| Olesen-2011 | Danish National Patient Registry; 1997–2006 | Retrospective cohort | 44,771 | VKAs (99.8%), heparins |
33% | Major bleeding | ICD codes | HEMORR2HAGES | 1.0 |
| Friberg-2012 | Swedish National Hospital Discharge Registry; 2005–2008 | Retrospective cohort | 48,599 | Warfarin | NA | Major bleeding; Intracranial bleeding | ICD codes | HEMORR2HAGES | 1.50 |
| Apostolakis-2012 | The AMADEUS trial | Post-hoc analysis of RCT | 2,293 | Warfarin | 18% | Major bleeding; Any clinically relevant bleeding | ISTH | HEMORR2HAGES; ATRIA | 1.18 |
| Apostolakis-2013 | The AMADEUS trial | Post-hoc analysis of RCT | 2,293 | Warfarin | 18% | Any clinically relevant bleeding | ISTH | CHADS2; CHA2DS2-VASc | 1.18 |
| Senoo-2016 | The AMADEUS trial | Post-hoc analysis of RCT | 2,293 | Warfarin | 16.5% | Major bleeding; Any clinically relevant bleeding | ISTH | ORBIT | 1.18 |
| Proietti-2016 | The SPORTIF III and V clinical trials | Post-hoc analysis of RCT | 3,551 | Warfarin | 19.9% | Major bleeding | ISTH | ORBIT; ATRIA; HEMORRAGES | 1.6 |
| Proietti-2018a | The SPORTIF III clinical trial | Post-hoc analysis of RCT | 3,550 | Warfarin | 19.9% | Major bleeding; Any clinically relevant bleeding; Any bleeding | ISTH | GARFIELD-AF | 1.56 |
| Roldan-2013a | Outpatient anticoagulation clinic; City Hospital, Birmingham, UK; 2007.03–2008.11 | Prospective cohort | 937 | Acenocoumarol | 17% | Major bleeding | ISTH | ATRIA | 2.61 |
| Roldan-2013b | Outpatient anticoagulation clinic; City Hospital, Birmingham, UK; 2007–2008 | Prospective cohort | 1,370 | Acenocoumarol | 18% | Major bleeding | ISTH | CHADS2; CHA2DS2-VASc | 2.73 |
| Barnes-2014 | Michigan Anticoagulation Quality Improvement Initiative (MAQI2); 2009–2012 | Prospective cohort | 2,600 | Warfarin | NA | Major bleeding | ISTH | HEMORR2HAGES; ATRIA; CHADS2; CHA2DS2-VASc | 1.0 |
| Esteve-Pastor-2016 | The FANTASIIA registry; Spanish; 2013–2014 | Prospective cohort | 1,276 | DOACs; VKAs | 10.9% | Major bleeding | ISTH | ORBIT | 1.0 |
| Hijazi-2016 | The ARISTOTLE derivation cohort | Post-hoc analysis of RCT | 14,537 | Apixaban; warfarin | 39% | Major bleeding | ISTH | ABC; ORBIT | 1.9 |
| The RE-LY validation cohort | Post-hoc analysis of RCT | 8,468 | Dabigatran; warfarin | 44% | Major bleeding | ISTH | ABC; ORBIT | 1.9 | |
| Proietti-2018b | The RE-LY trial, whole cohort | Post-hoc analysis of RCT | 18,113 | Dabigatran; warfarin | 40% | Major bleeding; Intracranial bleeding | ISTH | HEMORR2HAGES; ATRIA; ORBIT | 2.0 |
| Berg-2019 | The ENGAGE AF-TIMI 48 trial | Post-hoc analysis of RCT | 8,705 | Edoxaban; Warfarin | NA | Major bleeding | ISTH | ABC | 2.8 |
| Jaspers Focks-2016 | The anticoagulation clinic in the region Arnhem/Nijmegen, the Netherlands | Prospective cohort | 1,157 | VKAs (acenocoumarol 90%) | 4.1% | Major bleeding; Any clinically relevant bleeding; Any bleeding | ISTH | HEMORR2HAGES; ATRIA | 2.5 |
| Steinberg-2016 | The ORBIT-AF registry; US outpatients | Prospective cohort | 7,420 | Dabigatran; Warfarin | NA | Major bleeding | ISTH | ATRIA | NA |
| Poli-2017 | The START register, multicenter in Italy | Prospective cohort | 4,579 | DOACs; VKAs | 16.5% | Major bleeding | ISTH | CHADS2; CHA2DS2-VASc | 1.4 |
| Caro Martínez-2017 | Three Spanish hospitals; 2013–2014 | Retrospective cohort | 973 | DOACs | NA | Major bleeding; Gastrointestinal bleeding | ISTH | ATRIA; ORBIT | 1.77 |
| Elvira-Ruiz-2020 | Two hospitals in Spain; 2013–2016 | Retrospective cohort | 2,880 | DOACs; VKAs | 17.7% | Major bleeding | ISTH | ATRIA; ORBIT | 1.5 |
| Esteve-Pastor-2017 | Single anticoagulation centre in a tertiary hospital in Murcia, Spain; 2007 | Prospective cohort | 1,120 | Acenocoumarol | NA | Major bleeding; Intracranial bleeding; Gastrointestinal bleeding | ISTH | ABC | 6.5 |
| Rivera-Caravaca-2017 | Single anticoagulation centre in a tertiary hospital in Murcia, Spain; 2007 | Retrospective cohort | 1,361 | Acenocoumarol | 18% | Major bleeding | ISTH | HEMORR2HAGES; ATRIA; ORBIT | 6.5 |
| Fox-2021 | The GARFIELD-AF registry from 35 countries; 2010–2016 | Retrospective cohort | 52,032 | DOACs; VKAs | >12% | Major bleeding | ISTH | GARFIELD-AF | 2.0 |
| Beshir-2018 | University of Malaya Medical Centre and Institut Jantung Negara or the National Heart Institute of Malaysia | Retrospective cohort | 1,017 | Warfarin, rivaroxaban, dabigatran | 35% | Major bleeding; Clinically relevant non-major bleeding | ISTH | HEMORR 2HAGES, ATRIA; ORBIT | 1.0 |
| Chao-2018 | National Health Insurance Research Database, Taiwan; 1998–2011 | Retrospective cohort | 40,450 | Warfarin | 22.7% | Major bleeding; Intracranial bleeding | NA | HEMORR2HAGES; ATRIA; ORBIT | 4.6 |
| Dalgaard-2019 | Danish nationwide databases | Retrospective cohort | 51,180 | DOACs; VKAs | NA | Major bleeding | ICD codes | GARFIELD-AF | 1.0 |
| Lip-2018 | Danish nationwide databases | Retrospective cohort | 57,930 | DOACs | 39.1% | Any bleeding | ICD codes | ATRIA; ORBIT | 2.5 |
| Mori-2019 | The DIRECT registry; single-center in Japan | Prospective cohort | 2,216 | DOACs | 21.5% | Major bleeding | ISTH | ORBIT | 0.86 |
| O'Brien-2015 | The ORBIT-AF registry; 176 sites in the USA | Prospective cohort | 7,411 | Dabigatran; Warfarin | 37.9% | Major bleeding | ISTH | ATRIA; ORBIT | 2.0 |
| The ROCKET-AF validation cohort | Post-hoc analysis of RCT | 14,264 | Warfarin, rivaroxaban | NA | Major bleeding | ISTH | ATRIA; ORBIT | 1.9 | |
| Quinn-2016 | The ATRIA Study; California; 1996–1997 | Retrospective cohort | 13,559 | Warfarin | NA | Major bleeding | ISTH | CHADS2; CHA2DS2-VASc; ATRIA | NA |
| Yao-2017 | OptumLabs Data Warehouse; US; 2010–2015 | Retrospective cohort | 39,539 | DOACs | 7% | Major bleeding; Intracranial bleeding | NA | CHADS2; CHA2DS2-VASc | 0.6 |
| Claxton-2018 | The derivation (MarketScan, 2007–2014) and validation (Optum Clinformatics, 2009–2015) cohorts | Prospective cohort | 81,285 | DOACs; Warfarin | NA | Major bleeding | ISTH | HEMORR2HAGES; ATRIA; ORBIT | 1.0 |
| Rutherford-2018 | Norwegian Patient Registry and Norwegian Prescription Database; 2013–2015 | Retrospective cohort | 21,248 | DOACs | 52.8% | Any clinically relevant bleeding | ICD codes | ATRIA; ORBIT | 0.5 |
| Adam-2021 | Multicenter cohort study in Switzerland (SWISS-AF) | Prospective cohort | 2,147 | DOACs; VKAs | 18% | Any clinically relevant bleeding | ISTH | ATRIA; ORBIT | 4.4 |
| Siu-2014 | Queen Mary Hospital, Hong Kong; 1997–2011 | Retrospective cohort | 1,912 | Warfarin | NA | Intracranial bleeding | NA | NA | 3.19 |
| Suzuki-2014 | Kameda Medical Center; Japan; 2005 | Prospective cohort | 231 | Warfarin | 36.9–50% | Major bleeding | ISTH | NA | 7.1 |
| Prochaska-2018 | The thrombEVAL cohort. Denmark | Prospective cohort | 1,089 | Phenprocoumon | 37.9% | Any clinically relevant bleeding | NA | NA | 3.0 |
| Schwartz-2019 | Northwestern Healthcare system's Enterprise Database Warehouse; US; 2011–2017 | Retrospective cohort | 9,819 | DOACs; VKAs | NA | Major bleeding | ISTH | NA | 1.84 |
| Ravvaz-2021 | Longitudinal electronic health records in eastern Wisconsin and northern Illinois | Retrospective cohort | 7,274 | Warfarin | NA | Any bleeding | ICD codes | NA | 0.93 |
Number of anticoagulated patients.
HAS-BLED, Hypertension, Abnormal liver/renal function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly, Drugs/alcohol concomitantly; HEMORR2HAGES, Hepatic or renal disease, Ethanol abuse, Malignancy, Older, Reduced platelet count or function, Re-bleeding risk, Hypertension (uncontrolled), Anemia, Genetic factors, Excessive fall risk, Stroke; ATRIA, Anticoagulation and Risk Factors in Atrial Fibrillation; ORBIT, Outcomes Registry for Better Informed Treatment of Atrial Fibrillation; GARFIELD-AF, Global Anticoagulant Registry in the FIELD-Atrial Fibrillation; ABC, Age, Biomarkers, Clinical History; CHADS2, Congestive heart failure, Hypertension, Age ≥ 75 years, Diabetes mellitus, Stroke/transient ischemic attack history; CHA2DS2-VASc, Congestive heart failure/left ventricular ejection fraction ≤ 40%, Hypertension, Age ≥ 75 years, Diabetes mellitus, Stroke/transient ischemic attack/thromboembolism history, Vascular disease, Age 65–74 years, Sex (female); ICD, International Classification of Diseases; ISTH, International Society of Thrombosis and Haemostasis; NSAIDS, non-steroidal anti-inflammatory drugs; NA, not available.
Diagnostic Accuracy of the HAS-BLED Score
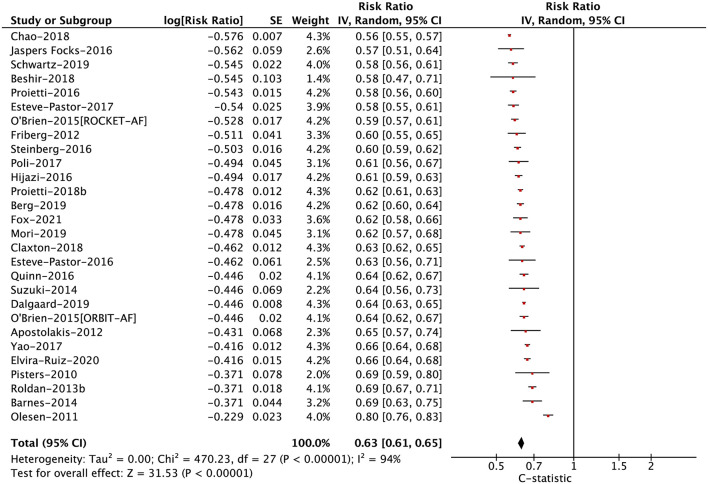
In anticoagulated patients with AF, the C-statistic for the HAS-BLED score ranged from 0.56 to 0.80 for major bleeding (median 0.62), 0.53 to 0.62 for any clinically relevant bleeding (median 0.58), 0.51 to 0.64 for any bleeding (median 0.57), 0.53 to 0.64 for intracranial bleeding (median 0.57), and 0.61 to 0.74 for gastrointestinal bleeding (median 0.68). In the pooled analysis, the C-statistic for major bleeding, any clinically relevant bleeding, any bleeding, intracranial bleeding, and gastrointestinal bleeding were 0.63 (0.61–0.65), 0.58 (0.56–0.61), 0.57 (0.53–0.61), 0.58 (0.53–0.62), and 0.67 (0.55–0.82), respectively (Figure 1; Supplementary Figure 2). For this part, there were no potential publication biases when inspecting the funnel plots (Supplementary Figure 3).
Figure 1.
The pooled analysis of the C-statistic for major bleeding in anticoagulated patients with atrial fibrillation.
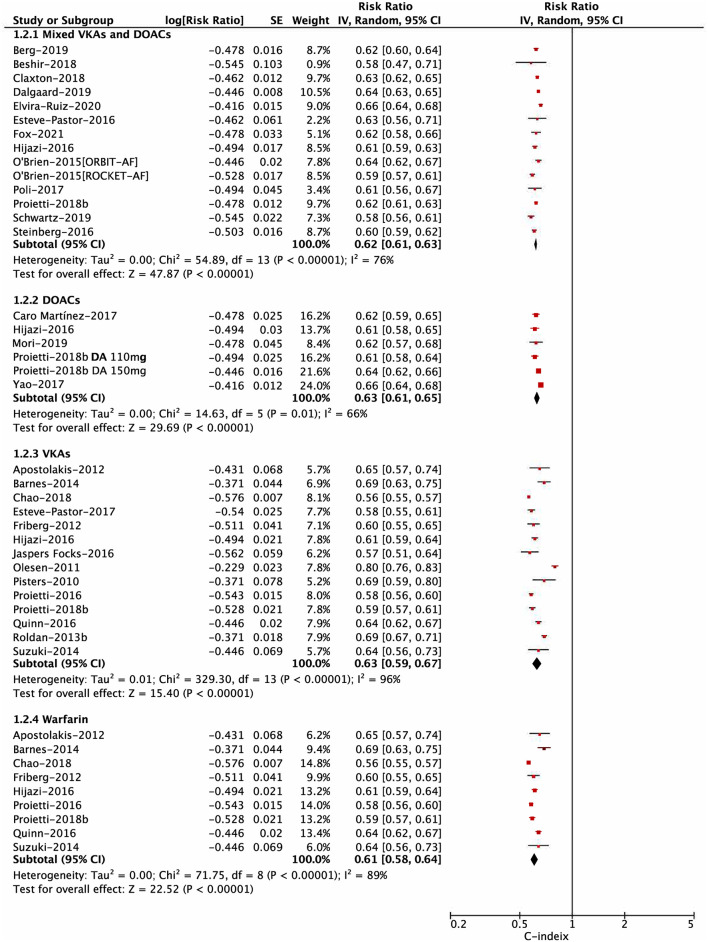
For the risk of major bleeding, in the subgroup analysis based on the OAC type shown in Figure 2, the C-statistic for major bleeding in four subgroups of mixed DOACs or VKAs, DOACs, VKAs, and warfarin were 0.62 (0.61–0.63), 0.63 (0.61–0.65), 0.63 (0.59–0.67), and 0.61 (0.58–0.64), respectively (Pinteraction = 0.58). The subgroup analysis based on study design (post-hoc analysis of RCT, prospective cohort, and retrospective cohort) indicated no interaction between them (Pinteraction = 0.14; Supplementary Figure 4). In addition, the predictive ability for major bleeding was also similar between available vs. unavailable labile INR in the HAS-BLED score (Pinteraction = 0.19; Supplementary Figure 5). However, there was a difference in the subgroup analysis based on the follow-up time, suggesting that the HAS-BLED score performed better in the group of ≤ 12 months compared with that of >12 months (Pinteraction = 0.01; Supplementary Figure 6).
Figure 2.
Subgroup analysis for pooling the C-statistic of the Hypertension, Abnormal liver/renal function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly, Drugs/alcohol concomitantly (HAS-BLED) score based on the types of oral anticoagulation.
Performances of the HAS-BLED Score With Other Risk Scores
Discrimination Analysis
As shown in Table 2, for comparisons of the C-statistic between two different risk scores, there were no statistically significant differences between the HAS-BLED vs. the HEMORR2HAGES scores (major bleeding: Z-statistic = 0.396; any clinically relevant bleeding: 0.321; intracranial bleeding: −0.408); vs. the ORBIT (major bleeding: −0.911; any clinically relevant bleeding: 0; intracranial bleeding: −0.158); vs. the ATRIA (major bleeding −0.502; any clinically relevant bleeding: 0.257; intracranial bleeding: 0); vs. the GARFIELD-AF (major bleeding: −0.448); or vs. the ABC scores (major bleeding: −1.09) (p > 0.05), suggesting similar discrimination performances. However, the HAS-BLED score had significantly higher C-statistic for predicting major bleeding than the CHADS2 (Z-statistic = 2.19, p < 0.05) or the CHA2DS2-VASc scores (Z-statistic = 1.99, p < 0.05), suggesting that the HAS-BLED score performed better than the CHADS2 or CHA2DS2-VASc scores.
Table 2.
Summary of the C-statistic and 95% CIs of the included studies.
| Major bleeding | Any clinically relevant bleeding | Intracranial bleeding | |
|---|---|---|---|
| Overall | |||
| No. of studies | 28 | 7 | 6 |
| C-statistic: HAS-BLED | 0.63 [0.61, 0.65] | 0.58 [0.56, 0.61] | 0.58 [0.53, 0.62] |
| HAS-BLED vs. HEMORR 2 HAGES | |||
| No. of studies | 12 | 3 | 3 |
| C-statistic: HAS-BLED | 0.63 [0.60, 0.67] | 0.57 [0.54, 0.61] | 0.56 [0.51, 0.63] |
| C-statistic: HEMORR2HAGES | 0.62 [0.58, 0.65] | 0.56 [0.51, 0.61] | 0.58 [0.51, 0.66] |
| Z-statistic | 0.396# | 0.321# | −0.408# |
| HAS-BLED vs. ORBIT | |||
| No. of studies | 12 | 4 | 2 |
| C-statistic: HAS-BLED | 0.61 [0.59, 0.64] | 0.59 [0.56, 0.63] | 0.54 [0.51, 0.57] |
| C-statistic: ORBIT | 0.63 [0.60, 0.67] | 0.59 [0.53, 0.66] | 0.55 [0.45, 0.69] |
| Z-statistic | −0.911# | 0# | −0.158# |
| HAS-BLED vs. ATRIA | |||
| No. of studies | 15 | 5 | 2 |
| C-statistic: HAS-BLED | 0.62 [0.60, 0.65] | 0.59 [0.56, 0.62] | 0.54 [0.51, 0.57] |
| C-statistic: ATRIA | 0.63 [0.60, 0.66] | 0.58 [0.51, 0.65] | 0.54 [0.47, 0.62] |
| Z-statistic | −0.502# | 0.257# | 0# |
| HAS-BLED vs. CHADS2 | |||
| No. of studies | 5 | - | - |
| C-statistic: HAS-BLED | 0.66 [0.64, 0.68] | - | - |
| C-statistic: CHADS2 | 0.61 [0.57, 0.65] | - | - |
| Z-statistic | 2.19* | - | - |
| HAS-BLED vs. CHA2DS2-VASc | |||
| No. of studies | 5 | - | - |
| C-statistic: HAS-BLED | 0.66 [0.64, 0.68] | - | - |
| C-statistic: CHA2DS2-VASc | 0.61 [0.57, 0.66] | - | - |
| Z-statistic | 1.99* | - | - |
| HAS-BLED vs. GARFIELD-AF | |||
| No. of studies | 3 | - | - |
| C-statistic: HAS-BLED | 0.61 [0.57, 0.66] | - | - |
| C-statistic: GARFIELD-AF | 0.63 [0.56, 0.71] | - | - |
| Z-statistic | −0.448# | - | - |
| HAS-BLED vs. ABC | |||
| No. of studies | 4 | - | - |
| C-statistic: HAS-BLED | 0.61 [0.60, 0.63] | - | - |
| C-statistic: ABC | 0.65 [0.58, 0.72] | - | - |
| Z-statistic | −1.09# | - | - |
The absolute value of Z-statistic more than 1.96 indicated a p-value of < 0.05, suggesting a significant difference in the discrimination between the two risk scores.
p < 0.05;
p > 0.05.
HAS-BLED, Hypertension, Abnormal liver/renal function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly, Drugs/alcohol concomitantly; HEMORR2HAGES, Hepatic or renal disease, Ethanol abuse, Malignancy, Older, Reduced platelet count or function, Re-bleeding risk, Hypertension (uncontrolled), Anemia, Genetic factors, Excessive fall risk, Stroke; ATRIA, Anticoagulation and Risk Factors in Atrial Fibrillation; ORBIT, Outcomes Registry for Better Informed Treatment of Atrial Fibrillation; GARFIELD-AF, Global Anticoagulant Registry in the FIELD-Atrial Fibrillation; ABC, Age, Biomarkers, Clinical History; CHADS2, Congestive heart failure, Hypertension, Age ≥ 75 years, Diabetes mellitus, Stroke/transient ischemic attack history; CHA2DS2-VASc, Congestive heart failure/left ventricular ejection fraction ≤ 40%, Hypertension, Age ≥ 75 years, Diabetes mellitus, Stroke/transient ischemic attack/thromboembolism history, Vascular disease, Age 65–74 years, Sex (female).
Reclassification Analysis
As presented in Table 3, the HAS-BLED score for predicting major bleeding had both the significantly positive NRI and IDI values compared with the CHADS2 (16–18) or the CHA2DS2-VASc scores (13, 16, 17), suggesting that the predictive ability of the HAS-BLED score was more dominant than the CHADS2 or the CHA2DS2-VASc scores. The HAS-BLED score compared with the HEMORR2HAGES (19–22), the ATRIA (16, 19–23), or the ORBIT score (20–22) had positive NRI and IDI values, although non-significant in some studies (19, 21). Only one study of Jaspers Focks et al. (24) reported non-significant negative NRI values between the HAS-BLED vs. the HEMORR2HAGES or the ATRIA scores. In the study of Proietti et al. (25), the GARFIELD-AF compared with the HAS-BLED scores had both non-significant negative NRI and IDI values. Overall, the HAS-BLED score had at least non-inferior predictive ability for predicting major bleeding compared with the HEMORR2HAGES, the ATRIA, the ORBIT, or the GARFIELD-AF scores.
Table 3.
NRI and IDI analysis for predicting the bleeding risks in anticoagulated patients with AF.
| Study (author-year) | NRI analysis | IDI analysis |
|---|---|---|
| Major bleeding | ||
| Apostolakis-2012 | HAS-BLED vs. HEMORR2HAGES (+6.8%, P = 0.42); HAS-BLED vs. ATRIA(+9.0%, P = 0.33) |
Not available |
| Roldan-2013a | Continuous: HAS-BLED vs. ATRIA(+13.6%, P = 0.43) |
Continuous: HAS-BLED vs. ATRIA(+6.9%, P = 0.033) |
| Categorical: HAS-BLED vs. ATRIA(+19.6%, P = 0.019) |
Categorical: HAS-BLED vs. ATRIA(+7.0%, P = 0.001) |
|
| Roldan-2013b | HAS-BLED vs. CHADS2 (+38.62%, P < 0.001); HAS-BLED vs. CHA2DS2-VASc (+37.6%, P <0.001) |
HAS-BLED vs. CHADS2 (+10.0%, P < 0.001);HAS-BLED vs. CHA2DS2-VASc (+12.0%, P < 0.001) |
| Barnes-2014 | HAS-BLED vs. HEMORR2HAGES (+26.0%, P = 0.006); HAS-BLED vs. ATRIA (+31.0%, P = 0.001); HAS-BLED vs. CHADS2 (+58.0%, P < 0.001); HAS-BLED vs. CHA2DS2-VASc (+36.0%, P < 0.001) |
Not available |
| Berg-2019 | ABC vs. HAS-BLED +13.8% (8.0–22.8%) | Not available |
| Chao-2018 | HAS-BLED vs. HEMORR2HAGES (+4.3%, P < 0.001); HAS-BLED vs. ATRIA (+4.9%, P < 0.001); HAS-BLED vs. ORBIT (+5.5%, P < 0.001) |
Not available |
| Esteve-Pastor-2017 | ABC vs. HAS-BLED (−13.74%, P = 0.005) | ABC vs. HAS-BLED (−13.14%, P = 0.002) |
| Jaspers Focks-2016 | HAS-BLED vs. HEMORR2HAGES (−3.60%, P = 0.460); HAS-BLED vs. ATRIA (−6.32%, P = 0.894) |
Not available |
| Proietti-2016 | ORBIT vs. HAS-BLED (-0.77%, P = 0.392); ATRIA vs. HAS-BLED (-8.83%, P = 0.323); HEMORR2HAGES vs. HAS-BLED (−13.66%, P = 0.119) |
ORBIT vs. HAS-BLED (0%, P = 0.646);ATRIA vs. HAS-BLED (0%, P = 0.611);HEMORR2HAGES vs. HAS-BLED (−0.18%, P = 0.039) |
| Proietti-2018a | GARFIELD vs. HAS-BLED (−4.2%, P = 0.448) | GARFIELD vs. HAS-BLED (−0.2%, P = 0.318) |
| Rivera-Caravaca-2017 | HAS-BLED vs. HEMORR2HAGES (+15.74%, P < 0.001); HAS-BLED vs. ATRIA (+15.98%, P < 0.001); HAS-BLED vs. ORBIT (+12.12%, P = 0.007) |
HAS-BLED vs. HEMORR2HAGES (+3.11%, P = 0.347);HAS-BLED vs. ATRIA (+3.09%, P = 0.142);HAS-BLED vs. ORBIT (+2.4%, P = 0.067) |
| Quinn-2016 | HAS-BLED vs. CHADS2 (+0.4%) | Not available |
| Yao-2017 | HAS-BLED vs. CHA2DS2-VASc (+2.0%, P < 0.001) | Not available |
| Any clinically relevant bleeding | ||
| Apostolakis-2012 | HAS-BLED vs. HEMORR2HAGES (+10.3%, P < 0.001); HAS-BLED vs. ATRIA(+13.0%, P < 0.001) |
|
| Apostolakis-2013 | Continuous: HAS-BLED vs. CHADS2 (+16.0%, P = 0.017); HAS-BLED vs. CHA2DS2-VASc (+29.0%, P < 0.001) |
Not available |
| Categorical: HAS-BLED vs. CHADS2 (+13.0%, P = 0.001); HAS-BLED vs. CHA2DS2-VASc (+10.0%, P = 0.04) |
||
| Jaspers Focks-2016 | HAS-BLED vs. HEMORR2HAGES (−5.61%, P = 0.194); HAS-BLED vs. ATRIA (−3.6%, P = 0.119) |
Not available |
| Proietti-2018a | GARFIELD vs. HAS-BLED (+3.3%, P = 0.756); | GARFIELD vs. HAS-BLED (−0.1%, P = 0.746); |
| Intracranial bleeding | ||
| Chao-2018 | HAS-BLED vs. HEMORR2HAGES (+3.0%, P = 0.056); HAS-BLED vs. ATRIA (+6.0%, P < 0.001); HAS-BLED vs. ORBIT (+4.8%, P < 0.001) |
Not available |
| Esteve-Pastor-2017 | ABC vs. HAS-BLED (−13.96%, P = 0.075) | ABC vs. HAS-BLED (−0.11%, P = 0.536) |
| Yao-2017 | HAS-BLED vs. CHA2DS2-VASc (+7.0%, P < 0.001) | Not available |
| Gastrointestinal bleeding | ||
| Esteve-Pastor-2017 | ABC vs. HAS-BLED (−8.17%, P = 0.362) | ABC vs. HAS-BLED (−5.55%, P = 0.164) |
| Any bleeding | ||
| Jaspers Focks-2016 | HAS-BLED vs. HEMORR2HAGES (−3.72%, P = 0.334); HAS-BLED vs. ATRIA (−8.51%, P = 0.009) |
Not available |
| Proietti-2018a | GARFIELD vs. HAS-BLED (−8.7%, P < 0.001) | GARFIELD vs. HAS-BLED (−1.1%, P < 0.001) |
HAS-BLED, Hypertension, Abnormal liver/renal function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly, Drugs/alcohol concomitantly; HEMORR2HAGES, Hepatic or renal disease, Ethanol abuse, Malignancy, Older, Reduced platelet count or function, Re-bleeding risk, Hypertension (uncontrolled), Anemia, Genetic factors, Excessive fall risk, Stroke; ATRIA, Anticoagulation and Risk Factors in Atrial Fibrillation; ORBIT, Outcomes Registry for Better Informed Treatment of Atrial Fibrillation; GARFIELD-AF, Global Anticoagulant Registry in the FIELD-Atrial Fibrillation; ABC, Age, Biomarkers, Clinical History; CHADS2, Congestive heart failure, Hypertension, Age ≥ 75 years, Diabetes mellitus, Stroke/transient ischemic attack history; CHA2DS2-VASc, Congestive heart failure/left ventricular ejection fraction ≤ 40%, Hypertension, Age ≥ 75 years, Diabetes mellitus, Stroke/transient ischemic attack/thromboembolism history, Vascular disease, Age 65–74 years, Sex (female); NRI, net reclassification improvement; IDI, integrated discrimination improvement; AF, atrial fibrillation.
The NRI values of the ABC score compared with the HAS-BLED score had the reverse effects in two included studies [+13.8% in Berg et al. (26) and −13.74% in Esteve-Pastor et al. (27)]. In the study of Esteve-Pastor et al. (27), the ABC score showed significant negative IDI values compared with the HAS-BLED score (−13.14%, p = 0.002). Further study should confirm the improvement in the predictive accuracy of ABC vs. HAS-BLED scores in anticoagulated patients with AF.
The values from the NRI and IDI analyses assessing the improvement in predictive accuracy for any clinically relevant bleeding, any bleeding, intracranial bleeding, and gastrointestinal bleeding are presented in Table 3. The results of these sections should be interpreted cautiously due to the limiting number of included studies.
Calibration and Decision Curve Analysis
A total of seven included studies provided the calibration analysis of the HAS-BLED score, but the findings were inconsistent (Supplementary Table 5). Jaspers Focks et al. (24) and Beshir et al. (10) found that the HAS-BLED score had an adequate calibration. Two studies demonstrated that compared with the rates in the derivation cohort, the HAS-BLED score overestimated (28) or underestimated (29) the risk of bleeding. The HAS-BLED score had a better calibration than the ATRIA score (13, 30), but showed a similar or lower calibration compared with the ORBIT score (14, 30).
The net benefits of the HAS-BLED score vs. other risk scores were assessed by using the decision curve analysis (Supplementary Table 6). The HAS-BLED score might have larger net benefits than the HEMORR2HAGES, the CHADS2, the CHA2DS2-VASc, or the GARFIELD-AF scores (19, 25, 31). Two studies reported the net benefits between the HAS-BLED and the ABC scores, but reached the opposite conclusion (27, 32). The net benefits between the HAS-BLED and the ATRIA or the ORBIT scores might be related to the intervention thresholds (29).
Discussion
In this study, our results suggested that the HAS-BLED score had moderate predictive abilities for bleeding risks in anticoagulated patients with AF regardless of the OAC type. We also observed the suitable application of the HAS-BLED score in patients with AF when the labile INR was unavailable. The discrimination performance of the HAS-BLED score assessed by the C-statistic was comparable to the HEMORR2HAGES, the ATRIA, the ORBIT, the GARFIELD-AF, or the ABC scores, but performed better than the CHADS2 or the CHA2DS2-VASc scores. The NRI and IDI data suggested that the HAS-BLED score performed better than the CHADS2 or the CHA2DS2-VASc scores and had similar or superior predictive ability compared with the HEMORR2HAGES, the ATRIA, the ORBIT, or the GARFIELD-AF scores. Calibration and decision curve analyses of the HAS-BLED score compared with other risk models required further evidence-based assessment due to the different findings among the included studies.
The use of OAC effectively reduces the embolic risks but at cost of an increased risk of bleeding. Over the past few decades, VKAs such as warfarin have been confirmed to be effective for preventing stroke in patients with AF. Since the effectiveness and safety of DOACs are superior or non-inferior to warfarin in patients with AF, DOACs are increasingly used with time. However, the bleeding events and their related cardiovascular outcomes are not negligible. The optimal use of VKAs or DOACs in the management of AF should be based on a balanced risk-to-benefit assessment during anticoagulation. For this situation, it is vital that the potentially preventable risk factors of bleeding events should be monitored sufficiently and addressed appropriately. The HAS-BLED score has been currently recommended by current AF guidelines, where a score of ≥3 points indicates high-risk bleeding. Note that the HAS-BLED score is previously derived and validated mainly in VKA-treated patients with AF, whether it could be used in DOAC-treated patients remains unclear. In this study, we found that the HAS-BLED score had moderate predictive values for bleeding risks in anticoagulated patients with AF and the findings were consistent in the subgroups of mixed anticoagulated drugs, DOACs, VKAs, and warfarin. The variable of labile INR in the HAS-BLED score was not available in all the included studies. Nevertheless, the predictive ability for major bleeding was not significantly changed, if we only included the studies with labile INR in the pooled analysis.
Although several other bleeding risk prediction models have been proposed in published studies, whether the predictive ability of these models is parallel to the guideline recommended HAS-BLED score remains unclear. The HAS-BLED score has been previously assessed as the most balanced bleeding risk prediction tool in terms of sensitivity and specificity by using a meta-analytic approach (5, 33). However, discrimination outcome data (sensitivity/specificity or the C-statistic) are less critical than other predictive accuracy outcome measures. In combination with NRI and IDI values, calibration, and decision curve analyses, our current meta-analysis comprehensively assessed the predictive abilities of the HAS-BLED vs. the HEMORR2HAGES, the ATRIA, the ORBIT, the GARFIELD-AF, or the ABC bleeding scores. Overall, as reflected by these multiple methods, the HAS-BLED score showed at least non-inferior abilities for bleeding risk prediction than the HEMORR2HAGES, the ATRIA, the ORBIT, or the GARFIELD-AF scores in VKA- or DOAC-treated patients with AF. Although there was no significant difference in the C-statistic between the HAS-BLED and the ABC scores, data of reclassification, calibration, and decision curve analyses between them were still controversial and needed further clarifications. In addition, there is an overlap of risk factors such as age, hypertension, previous stroke, and diabetes between stroke and bleeding risk scores. As such, the CHADS2 and the CHA2DS2-VASc stroke scores are also closely associated with the increased bleeding risks. Nevertheless, we found that the HAS-BLED score performed better than the CHADS2 or the CHA2DS2-VASc scores in anticoagulated patients with AF.
Several published studies have modified the HAS-BLED score by revising the original variables or including additional factors. As shown in Supplementary Table 7, integration of additional factors (e.g., biomarkers, gene polymorphisms, aortic stenosis, area deprivation index) on the basis of the original HAS-BLED score has been taken into account. The modified HAS-BLED score might improve the predictive ability, but certainly at the expense of additional complexity, increased cost, and reduced practicality. The number and definition of variables may vary from study to study, potentially affecting the diagnostic performance of the HAS-BLED score.
The dynamic changes of bleeding risks should be assessed in the management of AF (34). Chao et al. (35) found that the prediction values of the follow-up or the delta HAS-BLED score were better compared with the baseline HAS-BLED score. The HAS-BLED score has been tested prospectively in the mobile atrial fibrillation application-II (mAFA-II) randomized trial, suggesting that dynamic monitoring management could reduce major bleeding and increase OAC uptake at 1 year (36). Therefore, the dynamic changes in modifiable risk factors for bleeding outcomes during the follow-up should be monitored and corrected timely to improve AF patient care (36). Current evidence supports the HAS-BLED score regularly used in patients with AF to identify patients at high risk of bleeding as early as possible. However, the HAS-BLED score is sometimes inappropriately used in clinical practice as an excuse to preclude the use of oral anticoagulants. For the majority of patients with AF, the benefits of OAC in reducing the stroke risk outweigh the bleeding risk. Clinicians should flag up high bleeding-risk patients (e.g., the HAS-BLED score of ≥3) for the early review and follow-up. Appropriate monitoring services and more efforts are necessary to be taken to correct modifiable bleeding risk factors such as uncontrolled hypertension, poor control of INR (VKA users), concomitant use of medications such as aspirin or NSAIDs, and alcohol abuse.
The 2021 UK National Institute for Health and Care Excellence (NICE) guidelines tend to recommend the use of the ORBIT score in the bleeding risk prediction for patients with AF (especially for DOAC users) (37). This recommendation is mainly supported by the better calibration evidence than the HAS-BLED score although the low quality data. As the committee pointed out by using new risk models of the ORBIT score in current clinical practice, it remains a challenge potentially due to the unknown cost-effectiveness and extra resources. Clinicians are not familiar with the ORBIT score and learning and training may take time and cost. More importantly, the ORBIT bleeding score mainly consists of non-modifiable risk factors. In this context, a newly published study by Proietti et al. compared the abilities of the HAS-BLED vs. the ORBIT scores in contemporary patients with AF with DOACs based on the data from the European Society of Cardiology-European Heart Rhythm Association (ESC-EHRA) and the EURObservational Research Programme AF (EORP-AF) General Long-Term Registry (38). The authors found that the ORBIT score identified less patients at high bleeding risk, showed no improvements in predictive accuracy for major bleeding assessed by the NRI and IDI values, and had a poorer calibration compared with the HAS-BLED score (38). These findings seemingly do not support the use of the ORBIT over the HAS-BLED scores for bleeding risk prediction in patients with AF. The simple and practical use of the HAS-BLED score is still appropriate for assessing VKA- or DOAC- related bleeding risks and helps clinicians to make informed decisions in clinical practice.
Limitations
There were still several limitations in this study. First, due to the high heterogeneity observed across the included studies, the discrimination performances of bleeding prediction models evaluated by the C-statistic should be interpreted cautiously. More studies focusing on the improvement in predictive accuracy by the NRI and IDI analyses, calibration, or net benefits by decision curve analysis could be taken to fully assess the performances of risk scores. Second, compared to the primary major bleeding, our results suggested a relatively lower predictive value of the HAS-BLED score for any clinically relevant bleeding, any bleeding, or intracranial bleeding. Only two studies reported the C-statistic for gastrointestinal bleeding. Therefore, more studies should further assess the value of the HAS-BLED score for these other bleeding outcomes. Third, the bleeding risk prediction tools of interest were derived and validated in studies with different study types ranging from observational cohorts to clinical trials, potentially complicating the synthesis of the C-statistic. Nevertheless, we observed no significant interaction in the subgroup analysis based on the study design. Fourth, we provided the data of concomitant antiplatelet drugs, but the effect of these drugs on the predictive value of the HAS-BLED score could not be analyzed due to the unclear cutoff points. Finally, this study was performed based on most included studies with low-quality data, but we comprehensively assessed the role of the HAS-BLED score vs. other risk models in predicting bleeding events in patients with AF, which had implications for clinical application and future research development.
Conclusion
The HAS-BLED score had moderate predictive abilities for bleeding risks in VKA- or DOAC-treated patients with AF. The HAS-BLED score was at least non-inferior to the HEMORR2HAGES, the ATRIA, the ORBIT, the GARFIELD-AF, or the ABC scores, but performed better than the CHADS2 or the CHA2DS2-VASc scores.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study was funded by the National Natural Science Foundation of China (82100273), the China Postdoctoral Science Foundation (2020M673016), and the China National Postdoctoral Program for Innovative Talents (BX20200400).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.757087/full#supplementary-material
References
- 1.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2021) 42:373–498. 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 2.Held C, Hylek EM, Alexander JH, Hanna M, Lopes RD, Wojdyla DM, et al. Clinical outcomes and management associated with major bleeding in patients with atrial fibrillation treated with apixaban or warfarin: insights from the ARISTOTLE trial. Eur Heart J. (2015) 36:1264–72. 10.1093/eurheartj/ehu463 [DOI] [PubMed] [Google Scholar]
- 3.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY, et al. novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. (2010) 138:1093–100. 10.1378/chest.10-0134 [DOI] [PubMed] [Google Scholar]
- 4.Zhu W, He W, Guo L, Wang X, Hong K. The HAS-BLED score for predicting major bleeding risk in anticoagulated patients with atrial fibrillation: a systematic review and meta-analysis. Clin Cardiol. (2015) 38:555–61. 10.1002/clc.22435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang G, Xie Q, Ma L, Hu K, Zhang Z, Mu G, et al. Accuracy of HAS-BLED and other bleeding risk assessment tools in predicting major bleeding events in atrial fibrillation: a network meta-analysis. J Thromb Haemost. (2020) 18:791–801. 10.1111/jth.14692 [DOI] [PubMed] [Google Scholar]
- 6.Borre E, Goode A, Raitz G, Shah B, Lowenstern A, Chatterjee R, et al. Predicting thromboembolic and bleeding event risk in patients with non-valvular atrial fibrillation: a systematic review. Thromb Haemostasis. (2018) 118:2171–87. 10.1055/s-0038-1675400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding WY, Harrison SL, Lane DA, Lip GYH. Considerations when choosing an appropriate bleeding risk assessment tool for patients with atrial fibrillation. J Thromb Haemost. (2020) 18:788–90. 10.1111/jth.14738 [DOI] [PubMed] [Google Scholar]
- 8.Zhu W, Ye Z, Chen S, Wu D, He J, Dong Y, et al. Comparative effectiveness and safety of non–vitamin K antagonist oral anticoagulants in atrial fibrillation patients. Stroke. (2021) 52:1225–33. 10.1161/STROKEAHA.120.031007 [DOI] [PubMed] [Google Scholar]
- 9.Rutherford OW, Jonasson C, Ghanima W, Holst R, Halvorsen S. New score for assessing bleeding risk in patients with atrial fibrillation treated with NOACs. Open Heart. (2018) 5:e931. 10.1136/openhrt-2018-000931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beshir SA, Aziz Z, Yap LB, Chee KH, Lo YL. Evaluation of the predictive performance of bleeding risk scores in patients with non-valvular atrial fibrillation on oral anticoagulants. J Clin Pharm Ther. (2018) 43:209–19. 10.1111/jcpt.12634 [DOI] [PubMed] [Google Scholar]
- 11.Adam L, Feller M, Syrogiannouli L, Del Giovane C, Donzé J, Baumgartner C, et al. Novel bleeding risk score for patients with atrial fibrillation on oral anticoagulants, including direct oral anticoagulants. J Thromb Haemost. (2021) 19:931–40. 10.1111/jth.15251 [DOI] [PubMed] [Google Scholar]
- 12.Moons K, Wolff RF, Riley RD, Whiting PF, Westwood M, Collins GS, et al. A tool to assess risk of bias and applicability of prediction model studies: explanation and elaboration. Ann Intern Med. (2019) 170:W1–33. 10.7326/M18-1377 [DOI] [PubMed] [Google Scholar]
- 13.Yao X, Gersh BJ, Sangaralingham LR, Kent DM, Shah ND, Abraham NS, et al. Comparison of the CHA2DS2-VASc, CHADS2, HAS-BLED, ORBIT, and ATRIA risk scores in predicting non-vitamin k antagonist oral anticoagulants-associated bleeding in patients with atrial fibrillation. Am J Cardiol. (2017) 120:1549–56. 10.1016/j.amjcard.2017.07.051 [DOI] [PubMed] [Google Scholar]
- 14.Mori N, Sotomi Y, Hirata A, Hirayama A, Sakata Y, Higuchi Y. External validation of the ORBIT bleeding score and the HAS-BLED score in non-valvular atrial fibrillation patients using direct oral anticoagulants (Asian Data from the DIRECT Registry). Am J Cardiol. (2019) 124:1044–8. 10.1016/j.amjcard.2019.07.005 [DOI] [PubMed] [Google Scholar]
- 15.Caro Martínez C, Andreu Cayuelas JM, Flores Blanco PJ, Valdés M, Bailén Lorenzo JL, Manzano Fernández S. Comparison of bleeding risk scores in patients with nonvalvular atrial fibrillation starting direct oral anticoagulants. Rev Esp Cardiol. (2017) 70:878–80. 10.1016/j.rec.2017.01.021 [DOI] [PubMed] [Google Scholar]
- 16.Barnes GD, Gu X, Haymart B, Kline-Rogers E, Almany S, Kozlowski J, et al. The predictive ability of the CHADS2 and CHA2DS2-VASc scores for bleeding risk in atrial fibrillation: the MAQI(2) experience. Thromb res. (2014) 134:294–9. 10.1016/j.thromres.2014.05.034 [DOI] [PubMed] [Google Scholar]
- 17.Roldan V, Marin F, Manzano-Fernandez S, Gallego P, Vilchez JA, Valdes M, et al. The HAS-BLED score has better prediction accuracy for major bleeding than CHADS2 or CHA2DS2-VASc scores in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. (2013) 62:2199–204. 10.1016/j.jacc.2013.08.1623 [DOI] [PubMed] [Google Scholar]
- 18.Quinn GR, Singer DE, Chang Y, Go AS, Borowsky LH, Fang MC. How well do stroke risk scores predict hemorrhage in patients with atrial fibrillation? Am J Cardiol. (2016) 118:697–9. 10.1016/j.amjcard.2016.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apostolakis S, Lane DA, Guo Y, Buller H, Lip GY. Performance of the HEMORR(2)HAGES, ATRIA, and HAS-BLED bleeding risk-prediction scores in patients with atrial fibrillation undergoing anticoagulation: the AMADEUS (evaluating the use of SR34006 compared to warfarin or acenocoumarol in patients with atrial fibrillation) study. J Am Coll Cardiol. (2012) 60:861–7. 10.1016/j.jacc.2012.06.019 [DOI] [PubMed] [Google Scholar]
- 20.Chao T, Lip GYH, Lin Y, Chang S, Lo L, Hu Y, et al. Major bleeding and intracranial hemorrhage risk prediction in patients with atrial fibrillation: attention to modifiable bleeding risk factors or use of a bleeding risk stratification score? a nationwide cohort study. Int J Cardiol. (2018) 254:157–61. 10.1016/j.ijcard.2017.11.025 [DOI] [PubMed] [Google Scholar]
- 21.Proietti M, Senoo K, Lane DA, Lip GYH. Major bleeding in patients with non-valvular atrial fibrillation: impact of time in therapeutic range on contemporary bleeding risk scores. Sci Rep. (2016) 6:24376. 10.1038/srep24376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rivera-Caravaca JM, Roldán V, Esteve-Pastor MA, Valdés M, Vicente V, Lip GYH, et al. Importance of time in therapeutic range on bleeding risk prediction using clinical risk scores in patients with atrial fibrillation. Sci Rep. (2017) 7:12066. 10.1038/s41598-017-11683-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roldan V, Marin F, Fernandez H, Manzano-Fernandez S, Gallego P, Valdes M, et al. Predictive value of the HAS-BLED and ATRIA bleeding scores for the risk of serious bleeding in a “real-world” population with atrial fibrillation receiving anticoagulant therapy. Chest. (2013) 143:179–84. 10.1378/chest.12-0608 [DOI] [PubMed] [Google Scholar]
- 24.Jaspers Focks J, van Vugt SPG, Albers-Akkers MTH, Lamfers EJP. Bloem-de Vries LM, Verheugt FWA, Brouwer MA. Low performance of bleeding risk models in the very elderly with atrial fibrillation using vitamin K antagonists. J Thromb Haemost. (2016) 14:1715–24. 10.1111/jth.13361 [DOI] [PubMed] [Google Scholar]
- 25.Proietti M, Rivera Caravaca JM, Esteve Pastor MA, Romiti GF, Marin F, Lip GYH. Predicting bleeding events in anticoagulated patients with atrial fibrillation: a comparison between the HAS-BLED and GARFIELD-AF bleeding scores. J Am Heart Assoc. (2018) 7:e009766. 10.1161/JAHA.118.009766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berg DD, Ruff CT, Jarolim P, Giugliano RP, Nordio F, Lanz HJ, et al. Performance of the ABC scores for assessing the risk of stroke or systemic embolism and bleeding in patients with atrial fibrillation in ENGAGE AF-TIMI 48. Circulation. (2019) 139:760–71. 10.1161/CIRCULATIONAHA.118.038312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esteve-Pastor MA, Rivera-Caravaca JM, Roldan V, Vicente V, Valdes M, Marin F, et al. Long-term bleeding risk prediction in 'real world' patients with atrial fibrillation: comparison of the HAS-BLED and ABC-Bleeding risk scores. The Murcia Atrial Fibrillation Project. Thromb Haemost. (2017) 117:1848–58. 10.1160/TH17-07-0478 [DOI] [PubMed] [Google Scholar]
- 28.Proietti M, Hijazi Z, Andersson U, Connolly SJ, Eikelboom JW, Ezekowitz MD, et al. Comparison of bleeding risk scores in patients with atrial fibrillation: insights from the RE-LY trial. J Intern Med. (2018) 283:282–92. 10.1111/joim.12702 [DOI] [PubMed] [Google Scholar]
- 29.Lip GYH, Skjøth F, Nielsen PB, Kjældgaard JN, Larsen TB. The HAS-BLED, ATRIA, and ORBIT bleeding scores in atrial fibrillation patients using non-vitamin K antagonist oral anticoagulants. Am J Med. (2018) 131:513–74. 10.1016/j.amjmed.2017.11.046 [DOI] [PubMed] [Google Scholar]
- 30.O'Brien EC, Simon DN, Thomas LE, Hylek EM, Gersh BJ, Ansell JE, et al. The ORBIT bleeding score: a simple bedside score to assess bleeding risk in atrial fibrillation. Eur Heart J. (2015) 36:3258–64. 10.1093/eurheartj/ehv476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Apostolakis S, Lane DA, Buller H, Lip GY. Comparison of the CHADS2, CHA2DS2-VASc and HAS-BLED scores for the prediction of clinically relevant bleeding in anticoagulated patients with atrial fibrillation: the AMADEUS trial. Thromb Haemost. (2013) 110:1074–9. 10.1160/TH13-07-0552 [DOI] [PubMed] [Google Scholar]
- 32.Hijazi Z, Oldgren J, Lindbäck J, Alexander JH, Connolly SJ, Eikelboom JW, et al. The novel biomarker-based ABC (age, biomarkers, clinical history)-bleeding risk score for patients with atrial fibrillation: a derivation and validation study. Lancet. (2016) 387:2302–11. 10.1016/S0140-6736(16)00741-8 [DOI] [PubMed] [Google Scholar]
- 33.Caldeira D, Costa J, Fernandes RM, Pinto FJ, Ferreira JJ. Performance of the HAS-BLED high bleeding-risk category, compared to ATRIA and HEMORR2HAGES in patients with atrial fibrillation: a systematic review and meta-analysis. J Interv Card Electrophysiol. (2014) 40:277–84. 10.1007/s10840-014-9930-y [DOI] [PubMed] [Google Scholar]
- 34.Chang T, Lip GYH, Chen S, Chao T. Importance of risk reassessment in patients with atrial fibrillation in guidelines: assessing risk as a dynamic process. Can J Cardiol. (2019) 35:611–8. 10.1016/j.cjca.2019.01.018 [DOI] [PubMed] [Google Scholar]
- 35.Chao T, Lip G, Lin Y, Chang S, Lo L, Hu Y, et al. Incident risk factors and major bleeding in patients with atrial fibrillation treated with oral anticoagulants: a comparison of baseline, follow-up and delta HAS-BLED scores with an approach focused on modifiable bleeding risk factors. Thromb Haemostasis. (2018) 47:768–77. 10.1055/s-0038-1636534 [DOI] [PubMed] [Google Scholar]
- 36.Guo Y, Lane DA, Chen Y, Lip GYH. Regular bleeding risk assessment associated with reduction in bleeding outcomes: the mAFA-II randomized trial. Am J Med. (2020) 133:1195–202. 10.1016/j.amjmed.2020.03.019 [DOI] [PubMed] [Google Scholar]
- 37.Perry M, Kemmis BS, Downes N, Andrews N, Mackenzie S. Atrial fibrillation: diagnosis and management-summary of NICE guidance. BMJ. (2021) 373:n1150. 10.1136/bmj.n1150 [DOI] [PubMed] [Google Scholar]
- 38.Proietti M, Romiti GF, Vitolo M, Potpara TS, Boriani G, Lip GYH. Comparison of HAS-BLED and ORBIT bleeding risk scores in af patients treated with NOACs: a report from the ESC-EHRA EORP-AF general long-term registry. Eur Heart J Qual Care Clin Outcomes. (2021). Online ahead of print. 10.1093/ehjqcco/qcab069. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.