Abstract
Purpose
The retinal circulation regulates blood flow through various internal and external factors; however, it is unclear how locally these factors act within the retinal microcirculation. We measured the temporal and spatial variability of blood velocity in small retinal vessels using a dual-beam adaptive optics scanning laser ophthalmoscope.
Methods
In young healthy subjects (n = 3), temporal blood velocity variability was measured in a local vascular region consisting of an arteriole, capillary, and venule repeatedly over 2 days. Data consisted of 10 imaging periods separated into two sessions: (1) five 6-minute image acquisition periods with 30-minute breaks, and (2) five 6-minute image acquisition periods with 10-minute breaks. In another group of young healthy subjects (n = 5), spatial distribution of velocity variability was measured by imaging three capillary segments during three 2-minute conditions: (1) baseline imaging condition (no flicker), (2) full-field flicker, and (3) no flicker condition again.
Results
Blood velocities were measurable in all subjects with a reliability of about 2%. The coefficient of variation (CV) was used as an estimate of the physiological variability of each vessel. Over 2 days, the average CV in arterioles was 7% (±2%); in capillaries, it was 19% (±6%); and, in venules, it was 8% (±2%). During flicker stimulation, the average capillary CV was 16% during baseline, 15% during flicker stimulation, and 18% after flicker stimulation.
Conclusions
Capillaries in the human retina exhibit spatial and temporal variations in blood velocity. This inherent variation in blood velocity places limits on studying the vascular regulation of individual capillaries, and the study presented here serves as a foundation for future endeavors.
Keywords: blood flow, retinal imaging, neurovascular coupling
Because the retina is one of the most active metabolic tissues in the human body,1,2 it requires a continuous supply of oxygen and nutrients in order to maintain healthy metabolic activity.2,3 The retina is supplied by a dual-circulatory system (choroidal and retinal).4 In the current study, we were interested in the retinal circulation that delivers oxygen carried by red blood cells (RBCs) to the inner layers of the retina. The delivery of blood to the inner retina is regulated by numerous mechanisms. These regulatory mechanisms are critical to maintaining the appropriate amount of blood flow in the face of variations in the metabolic demands of retinal tissue. Factors influencing retinal blood flow regulation include intraocular pressure,5,6 hypoxia,7 and hyperoxia,7–9 as well as changes in neural activity.10,11 Changes in the demand of the retina arising from neural or other activity results in changes to RBC velocity and/or vessel diameter, and, consequently, blood flow. This process, termed neurovascular coupling, is utilized in functional magnetic resonance imaging to detect changes in blood-oxygen levels in the central nervous system12 under varying regional demands.
In the human retina, techniques such as the retinal vessel analyzer,13 laser Doppler velocimetry,14 laser Doppler flowmetry,15 and color Doppler optical coherence tomography16,17 can measure vascular hemodynamics in large vessels with high signal-to-noise ratios. However, these tools are limited in their spatial resolution and are typically used to target relatively large vessels. These techniques have shown that variations in blood velocity measured in arterioles and venules averages less than 10% over time in the human retina.18,19 However, little is known about the variability or the regulation of blood flow in capillaries, including how velocity varies over time and across space and whether responses to changes in tissue demand are controlled at the level of individual capillaries or more globally by controlling flow at the feeding arteriole.
Recently, adaptive optics (AO) imaging has allowed the development of techniques to measure RBC and white blood cell motion in small retinal vessels (<100 µm) in the microcirculation and can do so noninvasively.20–22 Using the dual-beam approach,23 we found an upregulation in blood velocity and ultimately flow in retinal arterioles and venules (<50 µm) during increases in metabolic demand (due to flicker stimulation) across nine subjects.24 This increase in blood flow was similar in capillaries, yet the measured velocities had larger variations in responses. This led us to question if there is an additional source of variability present in capillaries, perhaps similar to what has been recorded for cortical capillaries.25–27 We hypothesized that the regulation of blood velocity in capillaries varies over time and across space, such that flow within capillaries is more variable than their feeding arterioles and draining venules. In this study, we measured the temporal and spatial variability in RBC velocity in small parafoveal vessels in the human retina using dual-beam adaptive optics scanning laser ophthalmoscopy (AOSLO).
Methods
Subjects
For the first experiment, we measured the temporal variability in RBC velocity in the right eye of young healthy subjects (n = 3; average age, 29.9 ± 2.5 years). On the first day, prior to the start of the AOSLO imaging, informed consent was obtained from each subject after a thorough explanation of the procedures and goals of the experiments was provided. A medical history was performed for each subject to confirm that there were no systemic conditions, and each subject had a retinal examination prior to participating in the experiment. A best-corrected visual acuity test was performed, and subjects were then dilated with 1% tropicamide. A 30° fundus image and an optical coherence tomography angiography (OCTA) image of the superior parafoveal retina was obtained (Spectralis; Heidelberg Engineering, Heidelberg, Germany). For the second experiment, which measured spatial variability in capillary RBC velocity response to flicker stimulation, the right eye of young healthy subjects (n = 5; average age, 28.8 ± 3.7 years) was imaged following a similar screening procedure. This research adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board at Indiana University.
AOSLO System
The dual-beam AOSLO at Indiana University uses two deformable mirrors: a Mirao mirror (Imagine Optics, Orsay, France) with 52 actuators to correct for low-order aberrations and a Multi-DM mirror (Boston Micromachines Corporation, Cambridge, MA, USA) with 144 actuators to correct for high-order aberrations. The mirrors are utilized in a woofer–tweeter configuration.28 In this study, we used two galvanometers to produce a 1.3° × 1.1° raster scan of the retina, with the horizontal scanner oscillating at a frequency of 15.1 kHz and vertically at a frequency of 29 Hz. Light is collected using two avalanche photodiodes (C30659-90 series APD; Excelitas Technologies, Mississauga, Ontario, Canada) that are positioned behind a retinal conjugate plane containing a 10-Airy disk diameter (ADD) aperture offset at least 6 ADD from the center of beam to produce multiply scattered light images.29 The light source is a filtered supercontinuum laser. The imaging beams are separated using a dichroic mirror and have center wavelengths of 842 nm and 769 nm.
To achieve the temporal resolution necessary to quantify RBC motion, we used the dual-beam technique23 and displaced the shorter wavelength beam approximately 50 lines (33 µm) on the retina from the longer wavelength beam. The two beams image the same retinal region with a temporal offset of 3.32 ms within each video frame (50 lines × 15,100−1 s/line). Both wavelengths are at safe power levels according to the American National Standards Institute (ANSI Z136). During image acquisition periods, a 30° scanning laser ophthalmoscope (SLO) image of the subject's fundus is displayed to the experimenter along with the location of the AOSLO imaging field superimposed onto the image for navigation of the retina during experimentation30 with flicker stimulus parameters replicated from previous studies.24
Image Acquisition and Processing
Images were recorded in multiple 3.5-second (100-frame) videos with individual frames collected at 29 Hz. Image distortions in the videos generated from the sinusoidal horizontal scan were corrected prior to image registration and frame alignment, and all measurements were performed using custom MATLAB software (MathWorks, Natick, MA, USA). For processing, a template frame was automatically selected by the computer program based on parameters such as brightness and cross-correlation values compared with previous frames. Other frames within the 3.5-second video were then aligned to the template frame by custom software. Frames containing large eye movements and blinks were automatically rejected, and the remaining frames were aligned to the template using strip alignment.
AO Imaging Procedures
Prior to the experiments, a preliminary AO imaging session was performed on a separate day. For vascular images, a 2° × 1.75° imaging field was moved in 1° steps to generate an 8° × 8° montage centered on the macula, as well as a 3° × 10° montage temporal to the macula. The AOSLO was focused on the retinal microvasculature. From these vascular montages a local vascular region of interest superior to the macula, which included an arteriole, capillaries, and venule where blood arriving from the arteriole supplied capillaries which in turn supplied the venule, was selected for measurements in the main experiments. Custom MATLAB software was then used to steer the scanner to these regions of interest during the experiments to position the scanner to sample the retina at the desired locations.30 The criterion for selecting arterioles and venules was based on the identification of the vessel being terminal (last branching vessel) to identify relative vessel size and the ability to make successful simultaneous measurements of more than one capillary in a region between the arteriole and venule.
Experiment 1. Temporal Variability of RBC Velocity
To evaluate how blood velocity varied over time we performed repeated testing without a background illumination. AO imaging was separated into two sessions: (1) five 6-minute image acquisition periods separated by 30-minute breaks, and (2) five 6-minute image acquisition periods separated by 10-minute breaks. Prior to each acquisition period, a 1-minute baseline period was provided where subjects sat in the system to adapt to the system luminance. This baseline period was followed by three 2-minute image acquisition periods for the selected arteriole, capillary, and venule. To help negate the impact of tear film instability on image quality, artificial tears were given prior to baseline imaging. The imaging procedure was replicated a second day.
Experiment 2. Spatial Variability of RBC Response to Flicker Stimulation in Multiple Capillaries
To measure RBC velocity differences among capillaries supplied by the same arteriole, three capillary segments (at least 50 µm in length) were imaged simultaneously in three 2-minute conditions: (1) no flicker (baseline), (2) full-field flicker stimulus (at 10 Hz), and (3) no flicker again. As in Experiment 1, subjects sat in the system during a 1-minute baseline adaptation period. Figure 1 illustrates the regions imaged in one subject, including the selected capillaries and RBC velocity measurements from an individual acquisition of 100 video frames (3.5 seconds) for the three selected capillaries (Figs. 1C–1E).
Figure 1.
A representation of the targeted vascular region in a subject. (A) An OCTA image of the superficial vascular plexus superimposed on a subject's SLO image taken prior to AO imaging. The red square indicates the region within which blood velocity was measured. (B) A vascular flow image obtained by calculating the variation in pixel intensity across all frames throughout the video. Color-coded arrows mark the selected capillaries measured for Experiment 2. Labels A and V correspond to the terminal arteriole and venule visible in the image. Each capillary segment selected was supplied by the same arteriole. (C–E) The RBCs in the capillary segments indicated by the red arrows (C) and green arrows (D) are traveling to the venule above the arteriole. The RBCs in the capillary segment indicated by the blue arrow (E) are traveling to the venule to the right of the arteriole. Three-second velocity measurements of the three capillary segments were acquired in the same video. Individual capillaries can have missing data because small eye movements could move one capillary, but not another, out of the frame (compare D and E). If we could not detect RBCs in a capillary at any time, the data were not plotted, and the missing data are not included in the CV computation. Scale bar: 50 µm.
Statistical Analysis
Statistical analysis was performed using StatView software (SAS Institute, Cary, NC, USA) with a significance level set at P < 0.05. The coefficient of variation (CV) was used to measure RBC velocity variability in each vessel type for all acquisition periods for both experiments.
Control Experiment
In this study, we measured the RBC velocity in different capillaries, as well as the variability in velocity. Because capillary flow is relatively slow and the measurements are based on the motion of a relatively small number of blood cells, we tested whether we could reliably measure velocity. We selected a region of the retina in one subject where there was a relatively long capillary segment without branches. This capillary was broken into two segments, each about the length of the capillary segments used in the rest of this paper. We then measured RBC velocity separately in the two segments (Fig. 2). For this capillary, the CV of blood velocity measurements was 17% in capillary segment 1 and 14% in capillary segment 2 across all conditions. However, when comparing the velocity measured in the two segments of the same capillary for the same time intervals, the measurements agreed to within 2% (±1%). Because these were segments of the same capillary, the velocities should have been identical at any given time. The results confirm that capillary blood velocity can be measured reproducibly with a reliability of about 2%.
Figure 2.
A control experiment verifying the reproducibility of this technique for measuring RBC velocity in an individual capillary by making measurements over two non-overlapping sections of an unbranched capillary. (A) A flow map identifying the region measured. The two dashed red lines indicate the capillary segments selected for measurements. (B) The average of five RBC velocity measurements for two segments of the selected capillary under different flicker conditions, as in Experiment 2 (before, during, and after exposure to 10-Hz full-field flicker). The RBC velocities of the capillary segments differed on average 2% (±1%) from each other across conditions. Scale bar: 50 µm.
Results
Experiment 1. RBC Velocity Variability Over Time
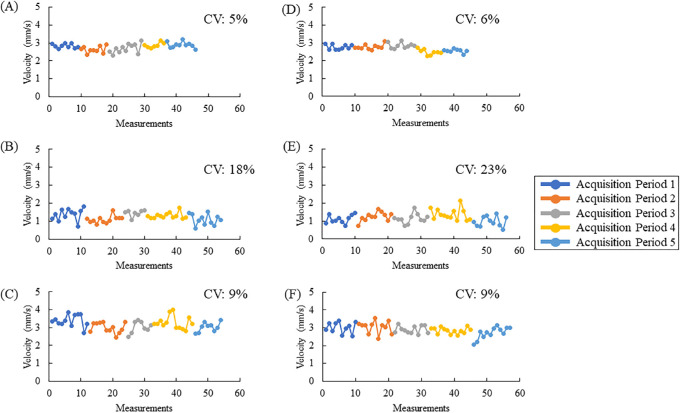
Arteriole, capillary, and venule RBC velocities were measurable in all subjects. The average CV for both days in arterioles was 7% (±2%); in capillaries, it was 19% (±6%); and, in venules, it was 8% (±2%). A two-way ANOVA (vessel type and subject) indicated that only the CV of the vessel type was significant (F2,118 = 199.70; P < 0.001). A Bonferroni post hoc t-test that was performed indicated that capillaries have significantly different CVs compared to arterioles and venules. In separate analyses, there was not a significant difference between the variability measured across the two imaging sequences (10 minutes vs. 30 minutes), nor was there between days. Figure 3 shows the velocity measurements acquired in one subject over the course of the imaging protocol for 1 day, and Figure 4 illustrates the average box plot.
Figure 3.
Repeated RBC velocity measurements of an arteriole (A, D), capillary (B, E), and venule (C, F), as well as corresponding CV for one subject plotted for each session on day 2 of imaging. RBC velocity measurements in an arteriole (A), capillary (B), and venule (C) with 30-minute breaks in between acquisition periods (A–C) and 10-minute breaks (D–F). The number of measurements made varied somewhat between acquisitions due to eye blinks or eye motion. The x-axis indicates the sequence number of the 3-second videos taken during each acquisition period, and the y-axis indicates the average RBC velocities measured. The CV is also shown for each acquisition period separated by the appropriate break.
Figure 4.
Box plots of the CV of blood velocity for arterioles, capillaries, and venules across all subjects for each imaging session in Experiment 1. The median CV is indicated by the line within the box and the mean by an “×.” The filled vertical dots indicate outliers. The filled portions show the 25% to 75% interquartile range.
Experiment 2. Capillary RBC Velocity Response to Flicker Stimulation
We were able to measure the velocity of RBCs in three capillary segments in all subjects. For each condition, the average RBC velocity of all 15 capillaries (three capillaries in each of five subjects) was 1.70 mm/s (maximum, 3.32 mm/s; minimum, 0.93 mm/s) during baseline; 1.72 mm/s (maximum, 3.57 mm/s; minimum, 0.84 mm/s) during flicker stimulation; and 1.80 mm/s (maximum, 3.49 mm/s; minimum, 1.05 mm/s) after flicker stimulation. The Table shows the average RBC velocity of the three capillary segments for each subject during each condition.
Table.
Average RBC Velocity and RBC Velocity Variability of Individual Capillary Segments of Five Subjects Under the Stimulus Conditions in Experiment 2
| Average RBC Velocity (mm/s) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject 1 | Subject 2 | Subject 3 | Subject 4 | Subject 5 | |||||||||||
| Capillary Segment | B | D | A | B | D | A | B | D | A | B | D | A | B | D | A |
| 1 | 2.24 | 1.80 | 3.50 | 2.41 | 3.00 | 2.75 | 0.93 | 0.84 | 1.08 | 1.34 | 1.17 | 1.14 | 1.06 | 1.22 | 1.05 |
| 2 | 1.76 | 1.95 | 1.59 | 1.21 | 1.36 | 1.47 | 1.08 | 1.07 | 1.06 | 1.21 | 1.16 | 1.33 | 2.30 | 2.77 | 2.80 |
| 3 | 1.46 | 1.08 | 1.13 | 2.24 | 1.84 | 1.77 | 1.68 | 1.95 | 1.89 | 1.18 | 1.07 | 1.10 | 3.32 | 3.57 | 3.15 |
| RBC Velocity Variability per Condition (CV, %) | |||||||||||||||
| 1 | 9 | 18 | 19 | 11 | 10 | 14 | 22 | 36 | 14 | 14 | 11 | 20 | 17 | 11 | 24 |
| 2 | 28 | 23 | 13 | 17 | 15 | 16 | 21 | 17 | 21 | 14 | 10 | 24 | 18 | 13 | 15 |
| 3 | 15 | 13 | 16 | 17 | 11 | 15 | 21 | 18 | 28 | 12 | 11 | 17 | 11 | 7 | 13 |
| Average | 17 | 18 | 16 | 15 | 12 | 15 | 21 | 24 | 21 | 13 | 11 | 20 | 15 | 10 | 17 |
Variations are defined as variation of RBC velocity relative to the mean. Before (B), during (D), and after (A) are the three 2-minute imaging conditions. Variation across all subjects was 16% during the baseline condition, 15% during the full-field flicker stimulation, and 18% after the flicker stimulus was off.
The CVs of the velocity measurements were 16% during baseline, 15% during flicker stimulation, and 18% after flicker stimulation. The Table shows the CV of each capillary segment for each subject during the different imaging conditions. To measure capillary response to flicker stimulation, each capillary segment was normalized by dividing the average RBC velocity relative to the average RBC velocity during the baseline condition. There was no significant change in capillary blood velocity during flicker stimulation as in our previous study.24 Within the 2-minute measurements, the individual capillaries did not respond uniformly to the stimulus, even though all were supplied by the same arteriole. The sizes of the variations in RBC velocity were consistent with the results of Experiment 1. Even though flicker stimulation has been shown to cause an overall increase in capillary blood velocity of about 15%,24 the larger ongoing variability of 17% to 20% in the individual capillaries is consistent with some increasing blood velocity and others decreasing during the flicker stimulation.
Discussion
Autoregulation in the retinal vasculature is essential to maintaining healthy metabolic function. Blood velocity regulation in the retina has been shown to respond to changes in metabolic states that extend over large regions, such as increases in metabolic demand,10,31 changes in oxygen tension,8,32 or drops in perfusion pressure.5 In addition, there are variations in blood velocity that seem to arise from more local changes in demand.
In this study, we have shown that in the absence of external stimuli, RBC velocities vary over time by up to 8% in arterioles and venules and up to 20% in capillaries. This variation was observed over both 50-minute and 2.5-hour imaging windows in Experiment 1 and was present on multiple days. The variation in arterioles and venules fall within measurement ranges of previous studies,18,19 suggesting that RBC velocity variability remains consistent from larger vessels down to the smaller arterioles and venules (<100 µm in diameter). Capillaries, on the other hand, exhibited much larger variations in RBC velocity, with an average CV of 19%. This may arise from numerous factors influencing capillary RBC velocity such as vasomotion,33–36 local neuronal demand distinct from stimulus responses (i.e., retinal oscillatory potentials37), or varying local metabolic states of small regions. The smaller size of capillaries makes the velocity of flow within them susceptible to changes in local hematocrit levels, thus increasing viscosity,38,39 which in turn could contribute to variable velocity and a cycling of flow from high to low values.40 Retinal neuronal activity is modulated apart from the visual response as evidenced by retinal oscillatory potentials.37 As the neuronal activity is modulated, the local metabolic requirements must also be. Finally, if each retinal area is locally regulating its blood velocity according to metabolic demand and there are delays in the control system, as there must be due to diffusion, various areas supplied by distinct capillaries interacting both by metabolite diffusion and via upstream pressure effects on the arteriole system would induce instability (i.e., variability). When looking at the velocity at two different time points within a 2-minute acquisition period, a capillary was shown to exhibit increased velocity during one acquisition and decreased velocity in another acquisition, confirming that the variability in velocity occurs, but there was still a normal pulsatile shape, thus this change did not arise from brief interruptions in flow (stasis) arising from temporary leukocyte or rouleaux blockage of flow (Supplementary Fig. S1). The current data cannot disentangle these various possible factors, as the suggestions are speculative based on the literature.
In Experiment 2, despite the introduction of a full-field flicker stimulus, which on average increases blood velocity, we still observed a CV in capillary blood velocities of 15%. Because these capillaries are fed by a common arteriole the implication is reasonable that control occurs on a local level, perhaps at the level of a single capillary. This variability over time and space cannot be attributed solely to measurement noise, as the control experiment indicated that we can make measurements with a reproducibility of about 2%, much smaller than the variability present in these capillaries. The data support the suggestion that blood velocity within the capillaries is not solely controlled by arteriole flow, as individual capillaries vary in flow separately, even when supplied by a single arteriole. Certainly, although increases in perfusion pressure in arterioles would lead to increases in perfusion pressure at the capillary level, our data deviate from the notion that this upstream variation in perfusion pressure solely controls flow in individual capillaries. For arterioles and venules, when measuring RBC velocity responses to flicker stimulation, we have shown that individual capillary RBC velocities vary across capillaries (either increasing or decreasing), differing from arterioles and venules, which show a consistent response.10,24,41 Unlike other studies that have found increases in RBC velocity in larger retinal vessels during flicker stimulation,42–44 our capillary measurements in this study showed an increase in RBC velocity in only three out of the five subjects, although from a larger sample we found on average that capillary flow increased by about the same amount as arteriole flow, but the change was not statistically significant, in part due to the variability in capillary measurements.24
There are limitations to the study, however. One limitation is based on the need for high-quality images. Fatigue can increase voluntary or involuntary eye movements, making it difficult to record 3-second videos of small vessels over time. To account for this, rest periods were given between imaging sessions, and the image acquisition periods were condensed to 2 minutes per vessel to give the subjects ample time to rest while having enough time to acquire sufficient image data. The length of the study also limits the criteria for subject selection. Subjects were required to maintain focus and be willing to participate over a course of 4.5 hours for each day in Experiment 1. Due to the high signal-to-noise image quality required to identify RBC motion in capillaries over the course of 9 hours, recruiting elderly participants, or even many younger subjects, was not an option, resulting in a small sample size. The size of the imaging field is another limitation to the study. Having a small imaging field makes imaging of complete sequences of data difficult, as it is desirable to measure the data across several cardiac cycles. Small eye movements or blinks also negatively impact the image quality of the vessel region and make capturing multiple capillaries within the small imaging field for the entire time period difficult. Finally, all measurements reported were made on capillaries in the superficial vascular plexus in the macula. It is possible that capillaries within the deep vascular plexus will behave differently.
Vascular diseases can alter the healthy metabolic state of the retina and affect blood velocity regulation. In diabetes, this could cause changes in variability by decreasing the ability to control flow. Such losses could occur either due to a loss of pericytes33,34 or through altered signaling pathways that control the vasomotive properties of blood vessels.1,26,27 These changes could impact the change in blood velocity in patients with different stages of diabetic retinopathy.45 In glaucoma, increases in intraocular pressure lead to decreases in perfusion pressure and result in decreases in retinal blood flow,3 and the decrease in ganglion cell numbers could change demand.
We have been able to measure RBC capillary velocity in multiple superficial retinal capillaries and demonstrate significant ongoing variation in blood velocity in individual capillaries and in their response to the local metabolic state and hemodynamic factors. Although this study alone cannot separate out the specific mechanism of control, it is clear that blood velocity varies at the individual capillary level. The data are merely speculative and serve as a foundation to explore these possible factors. It is not clear how this regulation is beneficial to the neural tissue, but it was a reliable feature in our normal control subjects. This in turn suggests that the data presented could serve as the groundwork for further studies measuring physiological regulation in the microcirculation of young healthy subjects. Such research could provide useful insights into interpreting changes that occur in diseased states such as diabetes and hypertension, where the regulatory control of vessels and especially capillaries may change.27,46–48
Supplementary Material
Acknowledgments
Supported by a grant from the National Eye Institutes, National Institutes of Health (R01-EY024315 to SAB).
Disclosure: R.L. Warner, None; T.J. Gast, None; K.A. Sapoznik, None; A. Carmichael-Martins, None; S.A. Burns, None
References
- 1. Newman EA. Functional hyperemia and mechanisms of neurovascular coupling in the retinal vasculature. J Cereb Blood Flow Metab. 2013; 33: 1685–1695, 10.1038/jcbfm.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roy CS, Sherrington CS.. On the regulation of the blood-supply of the brain. J Physiol. 1890; 11: 85–108, 158-7–158-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Riva CE, Logean E, Falsini B.. Visually evoked hemodynamical response and assessment of neurovascular coupling in the optic nerve and retina. Prog Retin Eye Res. 2005; 24: 183–215, 10.1016/j.preteyeres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 4. Delaey C, Van De Voorde J.. Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic Res. 2000; 32: 249–256, 10.1159/000055622. [DOI] [PubMed] [Google Scholar]
- 5. Riva CE, Sinclair SH, Grunwald JE.. Autoregulation of retinal circulation in response to decrease of perfusion pressure. Invest Ophthalmol Vis Sci. 1981; 21: 34–38. [PubMed] [Google Scholar]
- 6. Riehm E, Podesta HH, Bartsch C.. [Blood flow in retinal capillaries in the presence of increased intraocular pressure]. Ophthalmologica. 1972; 164: 249–251, 10.1159/000306759. [DOI] [PubMed] [Google Scholar]
- 7. Luksch A, Garhöfer G, Imhof A, et al.. Effect of inhalation of different mixtures of O(2) and CO(2) on retinal blood flow. Br J Ophthalmol. 2002; 86: 1143–1147, 10.1136/bjo.86.10.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Riva CE, Pournaras CJ, Tsacopoulos M.. Regulation of local oxygen tension and blood flow in the inner retina during hyperoxia. J Appl Physiol (1985). 1986; 61: 592–598, 10.1152/jappl.1986.61.2.592. [DOI] [PubMed] [Google Scholar]
- 9. Duan A, Bedggood PA, Metha AB, Bui BV.. Reactivity in the human retinal microvasculature measured during acute gas breathing provocations. Sci Rep. 2017; 7: 2113, 10.1038/s41598-017-02344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Michelson G, Patzelt A, Harazny J.. Flickering light increases retinal blood flow. Retina. 2002; 22: 336–343, 10.1097/00006982-200206000-00013. [DOI] [PubMed] [Google Scholar]
- 11. Falsini B, Riva CE, Logean E.. Flicker-evoked changes in human optic nerve blood flow: relationship with retinal neural activity. Invest Ophthalmol Vis Sci. 2002; 43: 2309–2316. [PubMed] [Google Scholar]
- 12. Huneau C, Benali H, Chabriat H.. Investigating human neurovascular coupling using functional neuroimaging: a critical review of dynamic models. Front Neurosci. 2015; 9: 467, 10.3389/fnins.2015.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Polak K, Schmetterer L, Riva CE.. Influence of flicker frequency on flicker-induced changes of retinal vessel diameter. Invest Ophthalmol Vis Sci. 2002; 43: 2721–2726. [PubMed] [Google Scholar]
- 14. Grunwald JE, Riva CE, Sinclair SH, Brucker AJ, Petrig BL.. Laser Doppler velocimetry study of retinal circulation in diabetes mellitus. Arch Ophthalmol. 1986; 104: 991–996. [DOI] [PubMed] [Google Scholar]
- 15. Riva CE, Harino S, Petrig BL, Shonat RD.. Laser Doppler flowmetry in the optic nerve. Exp Eye Res. 1992; 55: 499–506. [DOI] [PubMed] [Google Scholar]
- 16. Lieb WE, Cohen SM, Merton DA, Shields JA, Mitchell DG, Goldberg BB. Color Doppler imaging of the eye and orbit. Technique and normal vascular anatomy. Arch Ophthalmol. 1991; 109: 527–531, 10.1001/archopht.1991.01080040095036. [DOI] [PubMed] [Google Scholar]
- 17. Yazdanfar S, Rollins AM, Izatt JA.. In vivo imaging of human retinal flow dynamics by color Doppler optical coherence tomography. Arch Ophthalmol. 2003; 121: 235–239, 10.1001/archopht.121.2.235. [DOI] [PubMed] [Google Scholar]
- 18. Burgansky-Eliash Z, Bartov E, Barak A, Grinvald A, Gaton D.. Blood-flow velocity in glaucoma patients measured with the retinal function imager. Curr Eye Res. 2016; 41: 965–970, 10.3109/02713683.2015.1080278. [DOI] [PubMed] [Google Scholar]
- 19. Riva CE, Grunwald JE, Sinclair SH, Petrig BL.. Blood velocity and volumetric flow rate in human retinal vessels. Invest Ophthalmol Vis Sci. 1985; 26: 1124–1132. [PubMed] [Google Scholar]
- 20. Martin JA, Roorda A.. Direct and noninvasive assessment of parafoveal capillary leukocyte velocity. Ophthalmology. 2005; 112: 2219–2224, 10.1016/j.ophtha.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 21. Burns SA, Elsner AE, Sapoznik KA, Warner RL, Gast TJ.. Adaptive optics imaging of the human retina. Prog Retin Eye Res. 2019; 68: 1–30, 10.1016/j.preteyeres.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhong Z, Petrig BL, Qi X, Burns SA.. In vivo measurement of erythrocyte velocity and retinal blood flow using adaptive optics scanning laser ophthalmoscopy. Opt Express. 2008; 16: 12746–12756, 10.1364/oe.16.012746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Castro A, Huang G, Sawides L, Luo T, Burns SA.. Rapid high resolution imaging with a dual-channel scanning technique. Opt Lett. 2016; 41: 1881–1884, 10.1364/OL.41.001881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Warner RL, de Castro A, Sawides L, et al.. Full-field flicker evoked changes in parafoveal retinal blood flow. Sci Rep. 2020; 10: 16051, 10.1038/s41598-020-73032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kleinfeld D, Mitra PP, Helmchen F, Denk W.. Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex. Proc Natl Acad Sci USA. 1998; 95: 15741–15746, 10.1073/pnas.95.26.15741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang T, Wu DM, Xu GZ, Puro DG.. The electrotonic architecture of the retinal microvasculature: modulation by angiotensin II. J Physiol. 2011; 589: 2383–2399, 10.1113/jphysiol.2010.202937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shibata M, Nakaizumi A, Puro DG.. Electrotonic transmission in the retinal vasculature: inhibitory role of the diabetes/VEGF/aPKC pathway. Physiol Rep. 2019; 7: e14095, 10.14814/phy2.14095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zou W, Qi X, Burns SA.. Woofer-tweeter adaptive optics scanning laser ophthalmoscopic imaging based on Lagrange-multiplier damped least-squares algorithm. Biomed Opt Express. 2011; 2: 1986–2004, 10.1364/BOE.2.001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chui TY, Vannasdale DA, Burns SA.. The use of forward scatter to improve retinal vascular imaging with an adaptive optics scanning laser ophthalmoscope. Biomed Opt Express. 2012; 3: 2537–2549, 10.1364/BOE.3.002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang G, Qi X, Chui TY, Zhong Z, Burns SA.. A clinical planning module for adaptive optics SLO imaging. Optom Vis Sci. 2012; 89: 593–601, 10.1097/OPX.0b013e318253e081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhong Z, Huang G, Chui TY, Petrig BL, Burns SA.. Local flicker stimulation evokes local retinal blood velocity changes. J Vis. 2012; 12: 3, 10.1167/12.6.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palkovits S, Told R, Boltz A, et al.. Effect of increased oxygen tension on flicker-induced vasodilatation in the human retina. J Cereb Blood Flow Metab. 2014; 34: 1914–1918, 10.1038/jcbfm.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hirschi KK, D'Amore PA.. Pericytes in the microvasculature. Cardiovasc Res. 1996; 32: 687–698. [PubMed] [Google Scholar]
- 34. Hamilton NB, Attwell D, Hall CN.. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics. 2010; 2: 5, 10.3389/fnene.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hall CN, Reynell C, Gesslein B, et al.. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014; 508: 55–60, 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hill RA, Tong L, Yuan P, Murikinati S, Gupta S, Grutzendler J. . Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron. 2015; 87: 95–110, 10.1016/j.neuron.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koepsell K, Wang X, Vaingankar V, et al.. Retinal oscillations carry visual information to cortex. Front Syst Neurosci. 2009; 3: 4, 10.3389/neuro.06.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Agrawal R, Sherwood J, Chhablani J, et al.. Red blood cells in retinal vascular disorders. Blood Cells Mol Dis. 2016; 56: 53–61, 10.1016/j.bcmd.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 39. Baskurt OK, Meiselman HJ.. Blood rheology and hemodynamics. Semin Thromb Hemost. 2003; 29: 435–450, 10.1055/s-2003-44551. [DOI] [PubMed] [Google Scholar]
- 40. Hu D, Cai D, Rangan AV.. Blood vessel adaptation with fluctuations in capillary flow distribution. PLoS One. 2012; 7: e45444, 10.1371/journal.pone.0045444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Garhofer G, Huemer KH, Zawinka C, Schmetterer L, Dorner GT.. Influence of diffuse luminance flicker on choroidal and optic nerve head blood flow. Curr Eye Res. 2002; 24: 109–113, 10.1076/ceyr.24.2.109.8164. [DOI] [PubMed] [Google Scholar]
- 42. Aschinger GC, Schmetterer L, Fondi K, et al.. Effect of diffuse luminance flicker light stimulation on total retinal blood flow assessed with dual-beam bidirectional Doppler OCT. Invest Ophthalmol Vis Sci. 2017; 58: 1167–1178, 10.1167/iovs.16-20598. [DOI] [PubMed] [Google Scholar]
- 43. Riva CE, Falsini B, Logean E.. Flicker-evoked responses of human optic nerve head blood flow: luminance versus chromatic modulation. Invest Ophthalmol Vis Sci. 2001; 42: 756–762. [PubMed] [Google Scholar]
- 44. Son T, Wang B, Lu Y, Chen Y, Cao D, Yao X. . Concurrent OCT imaging of stimulus evoked retinal neural activation and hemodynamic responses. Proc SPIE Int Soc Opt Eng. 2017; 10045: 1004522, 10.1117/12.2252480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Palochak CMA, Lee HE, Song J, et al.. Retinal blood velocity and flow in early diabetes and diabetic retinopathy using adaptive optics scanning laser ophthalmoscopy. J Clin Med. 2019; 8: 1165, 10.3390/jcm8081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gugleta K, Kochkorov A, Waldmann N, et al.. Dynamics of retinal vessel response to flicker light in glaucoma patients and ocular hypertensives. Graefes Arch Clin Exp Ophthalmol. 2012; 250: 589–594, 10.1007/s00417-011-1842-2. [DOI] [PubMed] [Google Scholar]
- 47. Riva CE, Salgarello T, Logean E, Colotto A, Galan EM, Falsini B. Flicker-evoked response measured at the optic disc rim is reduced in ocular hypertension and early glaucoma. Invest Ophthalmol Vis Sci. 2004; 45: 3662–3668, 10.1167/iovs.04-0100. [DOI] [PubMed] [Google Scholar]
- 48. Arend O, Wolf S, Jung F, et al.. Retinal microcirculation in patients with diabetes mellitus: dynamic and morphological analysis of perifoveal capillary network. Br J Ophthalmol. 1991; 75: 514–518, 10.1136/bjo.75.9.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.