Abstract
Aims
Fractional flow reserve (FFRCT) using computed tomography coronary angiography (CTCA) determines both the presence of coronary artery disease and vessel-specific ischaemia. We tested whether an evaluation strategy based on FFRCT would improve economic and clinical outcomes compared with standard care.
Methods and results
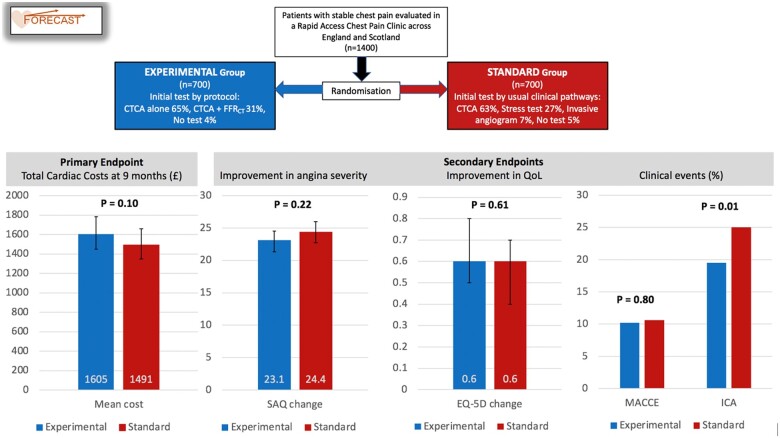
Overall, 1400 patients with stable chest pain in 11 centres were randomized to initial testing with CTCA with selective FFRCT (experimental group) or standard clinical care pathways (standard group). The primary endpoint was total cardiac costs at 9 months. Secondary endpoints were angina status, quality of life, major adverse cardiac and cerebrovascular events, and use of invasive coronary angiography. Randomized groups were similar at baseline. Most patients had an initial CTCA: 439 (63%) in the standard group vs. 674 (96%) in the experimental group, 254 of whom (38%) underwent FFRCT. Mean total cardiac costs were higher by £114 (+8%) in the experimental group, with a 95% confidence interval from −£112 (−8%) to +£337 (+23%), though the difference was not significant (P = 0.10). Major adverse cardiac and cerebrovascular events did not differ significantly (10.2% in the experimental group vs. 10.6% in the standard group) and angina and quality of life improved to a similar degree over follow-up in both randomized groups. Invasive angiography was reduced significantly in the experimental group (19% vs. 25%, P = 0.01).
Conclusion
A strategy of CTCA with selective FFRCT in patients with stable angina did not differ significantly from standard clinical care pathways in cost or clinical outcomes, but did reduce the use of invasive coronary angiography.
Keywords: Computed tomography coronary angiography, Cost analysis, Fractional flow reserve (FFRCT), Myocardial, Randomized controlled trial, Stable angina, Quality of life
Graphical Abstract
Summarising trial design and main results.
See page 3853 for the editorial comment on this article (doi:10.1093/eurheartj/ehab538)
Introduction
The optimal approach to investigating patients who present with stable chest pain remains controversial. The majority of such patients in the UK are referred to a Rapid Access Chest Pain Clinic, which offers clinical assessment in a secondary care setting within 2 weeks of referral. Options for further testing have traditionally either assessed the coronary arteries for evidence of atheroma or used stress techniques to reveal reversible myocardial ischaemia.1 The role of invasive assessment and treatment in patients with stable chest pain is controversial, especially after the recent ISCHEMIA trial2 found that coronary revascularization did not improve prognosis when added to optimal medical therapy, despite its effectiveness in alleviating anginal symptoms. The addition of intracoronary pressure wire data, such as fractional flow reserve (FFR), to angiographic assessment has improved the management of patients with stable chest pain in both observational3–8 and randomized9–12 studies by identifying coronary lesions that are physiologically significant, which is poorly predicted by their angiographic appearance.13
The ideal test to assess patients with new onset chest pain might therefore simultaneously provide information about the extent of both coronary atheroma and myocardial ischaemia. Fractional flow reserve derived from computed tomography coronary angiography (FFRCT) is a well validated test14–18 that provides information about both the coronary atheroma burden, from a computed tomography coronary angiogram (CTCA), and assesses their functional importance using a computerized model of fluid dynamics based on the CTCA dataset.14 FFRCT alters decision-making and patient management compared with CTCA data alone,19 and observational clinical studies, such as PLATFORM20 and ADVANCE,21 have demonstrated that the use of FFRCT can reduce the requirement for invasive coronary angiography, without increasing ischaemic clinical events. Furthermore, the PLATFORM study suggested that the use of CTCA with FFRCT reduces costs in the patients who would have undergone invasive coronary angiography, and is cost neutral in patients who would have had a non-invasive test.22 The UK National Institute for Health and Care Excellence (NICE) in 201723 recommended that CTCA with FFRCT be considered as a frontline test in patients with chest pain with the expectation of large cost savings.
The FORECAST trial was designed to test the hypothesis that, in a population of patients presenting to a Rapid Access Chest Pain Clinic, a strategy of using CTCA with selective FFRCT would reduce total cardiac resource utilization and costs at 9 months, when compared with the standard clinical pathways based on NICE guidance.24 The secondary aims were to assess the effect of the experimental strategy on quality of life, angina status, subsequent clinical events, and the rate of invasive coronary angiography.
Methods
Trial design and oversight
FORECAST was an open-label, multicentre, randomized, controlled clinical trial. The rationale and design have previously been described,25 and the trial protocol is available in Supplementary material online, Appendix SA. The trial complies with the Declaration of Helsinki, was approved by the South Central Berkshire B Research Ethics Service Committee (REC Reference 18/SC/0490, IRAS Project ID: 231037) and is registered at ClinicalTrials.gov (NCT03187639). The trial was investigator-initiated and funded by an unrestricted research grant from HeartFlow®. The company had no role in the design or conduct of the trial, or in the data collection, analysis, or reporting. The trial steering committee oversaw the conduct of the trial, ensuring that: (i) it was conducted in a manner consistent with the protocol, (ii) the data were complete, and (iii) the analyses were performed according to the Statistical Analysis Plan.
Patient population
All screened patients were at least 18 years old and were attending a Rapid Access Chest Pain Clinic for assessment of stable chest pain. A full list of exclusion criteria is available in the trial protocol (Supplementary material online, Appendix SA). In brief, patients were excluded if they had a history consistent with acute coronary syndrome, were deemed not to require a test to investigate their symptoms, were ineligible to undergo a CTCA, had a history of previous coronary revascularization, or had a life expectancy of <12 months.
Randomization groups
Patients were randomized, using an independent computerized system with block sizes of two and four, to either the usual care strategy based on clinical pathways (standard group) or a strategy of CTCA with selective FFRCT (experimental group). In the standard group, patients were assessed according to usual clinical care pathways at the Rapid Access Chest Pain Clinic, based upon the local implementation of the NICE CG95 Guidance for Chest Pain of Recent Onset.24 In these pathways, patients with a high pre-test likelihood of having important coronary disease could be referred for invasive coronary angiography, while patients with intermediate pre-test likelihood were referred for non-invasive evaluation, which could include stress testing (i.e. stress echocardiography, stress cardiac magnetic resonance, nuclear medicine perfusion imaging, and exercise electrocardiography), and CTCA (without FFRCT). In the experimental group, all patients were referred for CTCA as the initial test and selectively referred for FFRCT if the CTCA demonstrated a stenosis of ≥40% in a coronary artery segment of diameter suitable for revascularization by either a coronary stent or coronary artery bypass graft surgery. Prior to randomization, the clinical team declared which initial test would be used in the event the patient was randomized to standard care. Subsequent clinical management was determined by the supervising physician based on the results of initial testing and clinical judgement.
Trial endpoints
The primary endpoint was cardiovascular costs over 9 months of follow-up, calculated from the use of all cardiac-related invasive and non-invasive tests, revascularization procedures, hospital admissions and outpatient attendances due to a cardiovascular cause [including myocardial infarction (MI), arrhythmia, heart failure, revascularization], and cardiac medications. Data were collected using direct patient contact by research staff at each centre, as well as from local healthcare records. The total costs were calculated for each patient as the sum, over all specified resources, of the numbers of each resource used multiplied by a standardized cost weight (the UK tariffs, listed in the Supplementary material online, Appendix SB).
The two principal secondary endpoints were the changes in (i) quality of life, as assessed using the EQ-5D-5L questionnaire26 and (ii) angina status, as assessed using the Seattle Angina Questionnaire,27 which were completed at baseline and 9 months of follow-up. The other pre-specified secondary endpoints at 9 months of follow-up included major adverse cardiac and cerebrovascular events (MACCE), a composite of all-cause death, non-fatal MI, stroke, and cardiovascular hospitalization; the rate of invasive coronary angiography; and the rate of invasive angiography showing unobstructed coronaries (no stenosis of ≥50%).
Statistical analysis
The sample size calculation and statistical analysis plan have been described in detail previously.25 Cost differences of 20% between the randomized groups were taken to be plausible and of importance for policy setting, since the PLATFORM economic substudy reported a 32% change in per-patient costs within the invasive stratum and 25% change within the non-invasive stratum.22 Based on the cost distributions in PLATFORM, we calculated that a sample size of 700 patients per group would provide 90% power to detect a 20% difference in costs between groups if there was no loss to follow-up, and 85% power with a loss to follow-up of up to 12%.
The Statistical Analysis Plan for the trial data was determined in advance (Supplementary material online, Appendix SC), conforms to the International Conference on Harmonization E9 guidelines, and is reported using the Consolidated Standards of Reporting Trials (CONSORT) guidelines. Categorical data are presented as counts and percentages, and continuous variables are presented as means and standard deviations, and medians and interquartile ranges. The analysis of the binary clinical outcomes was based on the frequency of the events and conducted using χ2 tests. The primary endpoint was compared using a two-sample t-test after a log transformation due to skew in the cost data. Confidence limits on mean costs were calculated by bootstrapping. A two-sided P-value of 0.05 or less was considered to constitute statistical significance for all analyses. All analyses of outcome data were conducted using an intention-to-treat framework.
Results
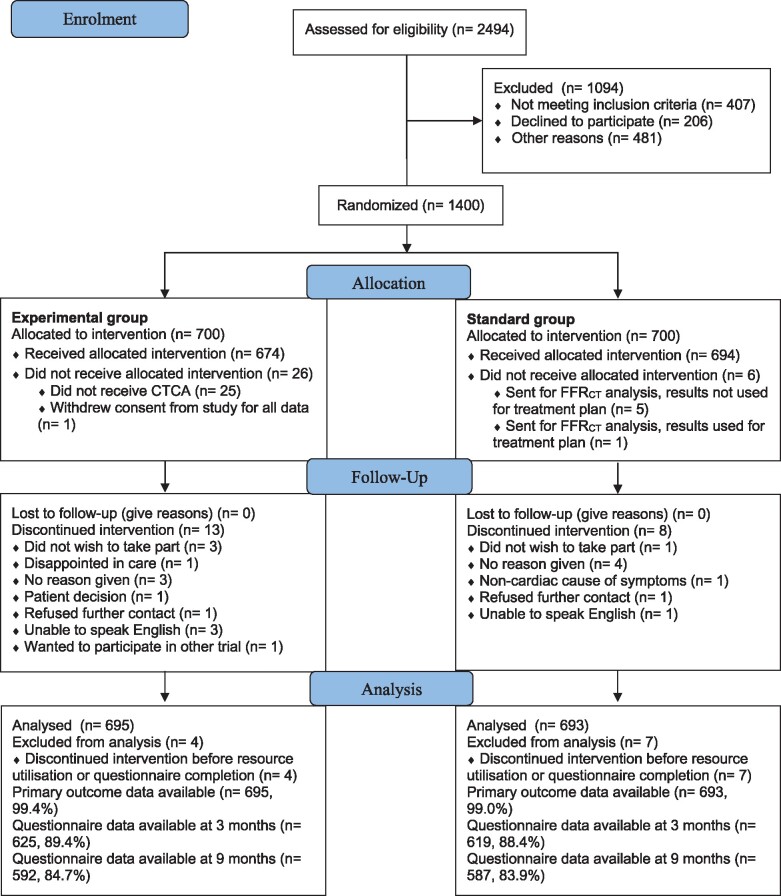
Between December 2017 and July 2019, 2494 patients with stable chest pain attending one of the 11 participating Rapid Access Chest Pain Clinics were screened for study entry, and 1400 patients were randomized (Figure 1). Baseline characteristics were well balanced between the arms (Table 1).
Figure 1.
CONSORT diagram: flow of patients in the study, from screening to randomization and follow-up.
Table 1.
Baseline characteristics
| Baseline characteristics | Standard group | Experimental group |
|---|---|---|
| (n = 700) | (n = 699) | |
| Age, years, mean (SD) | 59.6 (10.8) | 60.0 (10.9) |
| Sex | ||
| Male | 364 (52.0) | 359 (51.4) |
| Female | 336 (48.0) | 340 (48.6) |
| Ethnicity | ||
| White | 641 (91.6) | 635 (90.8) |
| Black or Black British | 11 (1.6) | 10 (1.4) |
| Mixed | 5 (0.7) | 1 (0.1) |
| Asian or Asian British | 32 (4.6) | 47 (6.7) |
| Chinese or other ethnic group | 11 (1.6) | 4 (0.6) |
| Prefer not to answer | 0 (0.0) | 2 (0.3) |
| Smoking | ||
| Never | 319 (45.6) | 348 (49.8) |
| Former | 276 (39.4) | 259 (37.1) |
| Current | 104 (14.9) | 92 (13.2) |
| Diabetes | 86 (12.4) | 91 (13.0) |
| Hypertension | 234 (33.4) | 266 (38.1) |
| Treated hyperlipidaemia | 198 (28.3) | 231 (33.1) |
| Family history of IHD | 426 (60.9) | 416 (59.5) |
| Previous MI | 3 (0.4) | 5 (0.7) |
Values are n (%) unless otherwise specified.
IHD, ischaemic heart disease; MI, myocardial infarction; SD, standard deviation.
Initial tests
Among the 700 patients randomized to the standard group, 439 (63%) had CTCA as the initial test, 187 (27%) had an initial stress test, and 47 (7%) had direct invasive coronary angiography (Table 2). Nine patients in the standard group were erroneously referred for FFRCT analysis, but the test results were not used in clinical management.
Table 2.
Initial tests undertaken
| Initial tests requested | Standard group | Experimental group |
|---|---|---|
| (n = 700) | (n = 699) | |
| Non-invasive tests | ||
| CTCA alone | 430 (61.4) | 454 (64.9) |
| FFRCT | 9 (1.2) | 220 (31.5) |
| Stress echo | 103 (14.7) | 0 (0.0) |
| Perfusion scan | 13 (1.8) | 0 (0.0) |
| Stress MRI | 1 (0.1) | 0 (0.0) |
| Exercise ECG | 70 (10.0) | 0 (0.0) |
| Invasive tests | ||
| Coronary angiogram | 47 (6.7) | 0 (0.0) |
| No initial test done | 36 (5.1) | 25 (3.6) |
Values are n (%).
CTCA, computed tomography coronary angiography; ECG, electrocardiogram; FFRCT, fractional flow reserve derived from computed tomography coronary angiography; MRI, magnetic resonance imaging.
In the experimental group, 674 (96%) patients underwent CTCA and 254 (38%) were selected for FFRCT analysis by protocol because a lesion of ≥40% was seen in an epicardial coronary artery; five additional patients were also referred for FFRCT who did not meet protocol criteria. Of the 259 patients referred for FFRCT, 39 (15%) scans could not be analysed due to technical issues. In the 220 patients who had FFRCT performed, 126 patients (59%) had at least one epicardial vessel with an FFRCT ≤ 0.8, which led to requests for invasive angiography in 98 patients, a non-invasive stress test in 16 patients, and no further testing in 12 patients. Invasive angiography was performed more often in patients with lower levels of FFRCT: in 26 of 29 patients (90%) with an FFRCT between 0.50 and 0.60, in 23 of 23 patients (100%) with an FFRCT between 0.61 and 0.70, in 39 of 56 patients (70%) with an FFRCT between 0.71 and 0.80, and in 4 of 94 patients (4%) with an FFRCT >0.80. The FFRCT value was not recorded in 18 patients (although it is known that the value was <0.80), invasive angiography was performed in 14 (78%) of these patients.
Tests and revascularization procedure at 9 months
Over 9 months of follow-up, fewer stress tests were performed at the discretion of the supervising physician in the experimental group than in the standard group (60 vs. 95, Table 3). The use of invasive coronary angiography was 22% lower in the experimental group (Table 3): 136 patients vs. 175 patients in standard care strategy (P = 0.01). The number of invasive angiograms showing no obstructive epicardial lesion was 52% lower in the experimental group: 30 patients vs. 62 patients in the standard care strategy. The use of invasive pressure wire assessment was also lower in the experimental group: 18 patients vs. 28 patients (P = 0.18).
Table 3.
Components of the primary outcome: total cardiac costs at 9 months
| Resource | Standard group | Experimental group | P-valuea |
|---|---|---|---|
| (n = 700) | (n = 699) | ||
| Non-invasive tests | |||
| CTCA | 462 (460) | 690 (674) | <0.001 |
| FFRCT | 9 (9) | 220 (220) | <0.001 |
| Stress echo | 124 (124) | 13 (13) | <0.001 |
| Perfusion scan | 34 (34) | 4 (4) | <0.001 |
| Stress MRI | 20 (20) | 15 (15) | 0.494 |
| Exercise ECG | 104 (99) | 28 (27) | <0.001 |
| Invasive procedures | |||
| Coronary angiogram | 182 (175) | 156 (136) | 0.014 |
| FFR/iFR (invasive)b | 28 (28) | 18 (18) | 0.177 |
| PCI | 75 (69) | 88 (74) | 0.660 |
| CABG | 28 (28) | 28 (28) | 1.000 |
| Hospitalizations | |||
| Myocardial infarction | 3 (3) | 10 (9) | 0.091 |
| Stroke | 1 (1) | 1 (1) | 1.000 |
| Transient ischaemic attack | 2 (2) | 2 (2) | 1.000 |
| Other | 50 (46) | 43 (35) | 0.252 |
| Emergency department visits | 30 (27) | 21 (20) | 0.374 |
| Cardiac outpatient visits | 241 (156) | 239 (139) | 0.294 |
| Total hospitalizations | 327 (182) | 316 (165) | 0.322 |
|
Medications— months of prescription (patients) | |||
| Statin | 3279 (405) | 3312 (410) | 0.622 |
| Aspirin | 2379 (315) | 2532 (331) | 0.331 |
| Antiplateletc | 771 (104) | 744 (103) | 1.000 |
| Beta-blocker | 1740 (238) | 1728 (233) | 0.910 |
| Calcium blocker | 1080 (152) | 1422 (190) | 0.015 |
| Oral nitrate | 360 (64) | 486 (76) | 0.285 |
| ACE inhibitor | 1245 (160) | 1275 (169) | 0.488 |
| ARB | 549 (75) | 492 (73) | 1.000 |
| Alpha-blocker | 120 (18) | 192 (28) | 0.135 |
Values are numbers of tests or events (numbers of patients) unless otherwise specified.
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CABG, coronary artery bypass graft; CTCA, computed tomography coronary angiography; ECG, electrocardiogram; FFR, fractional flow reserve; FFRCT, fractional flow reserve derived from computed tomography coronary angiography; iFR, instantaneous wave-free ratio; MRI, magnetic resonance imaging; PCI, percutaneous coronary intervention.
Fisher’s exact test for the number of patients with one or more tests by group.
Of the 28 invasive FFRs in the reference group, 16 were conducted as part of invasive coronary angiography and 12 were conducted as part of PCI. Of the 18 invasive FFRs in the test group, 12 were conducted as part of invasive coronary angiography and 6 were conducted as part of PCI. The counts of invasive coronary angiography and PCI are inclusive of these procedures, extra care was taken to ensure related costs were not double-counted.
Clopidogrel, Ticagrelor, or Prasugrel.
The overall rate of coronary revascularization did not differ significantly between the groups: 15% in the experimental group vs. 14% in the standard group (P = 0.69). A total of 88 percutaneous coronary intervention (PCI) procedures were undertaken in 74 patients (11%) in the experimental group, compared with 75 PCIs in 69 patients (10%) in the standard group, with 28 patients in each group undergoing CABG surgery (Table 3). In the experimental group, 90 of the 102 patients who underwent coronary revascularization had a functional study (a stress test or FFR), compared with 49 of the 97 patients who underwent coronary revascularization in the standard group (P < 0.001).
Primary endpoint: total cardiac costs at 9 months
The mean total cardiac costs at 9 months were slightly higher in the experimental group (£1605) than in the standard group (£1491) [mean difference £114 (8%), 95% confidence interval of −£112 (−8%) to +£337 (+23%)], though the difference in mean costs was not significant (P = 0.10). The distribution of costs (Figure 2) was skewed upward by a minority of patients with high costs, such that the median costs were £70 lower in the experimental group than the standard group (£600 vs. £670).
Figure 2.
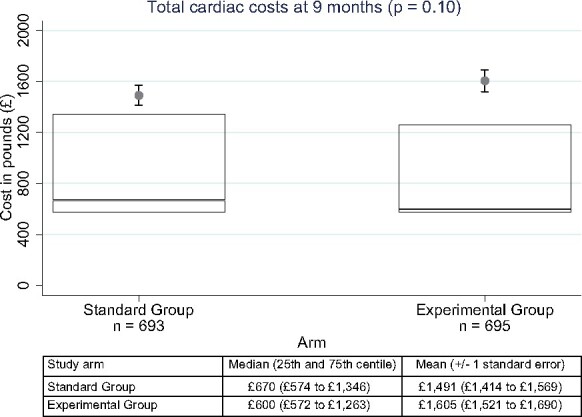
Primary endpoint: total cardiovascular costs at 9 months. Distribution of 9-month costs in UK pounds by randomized assignment. The top line of each box is the 75th percentile, the bottom line is the 25th percentile, and the line inside the box is the median (50th percentile). The mean cost is indicated by the filled circle, and one standard error of the mean is indicated by the error bars around the mean. The P-value (0.10) represents the result of the two-sample t-test applied to a log transformation of costs.
The pattern of non-invasive test use varied significantly (by design) between the two randomized groups (Table 3), and while there was significantly lower use of invasive coronary angiography in the experimental group, the number of hospitalizations, visits to outpatient clinics and emergency departments, and medication use did not differ significantly (Table 3).
Major adverse cardiac and cerebrovascular events at 9 months
The overall rate of MACCE (including death, non-fatal MI, non-fatal stroke, cardiovascular hospitalization) was 71 (10.2%) in the experimental group vs. 74 (10.6%) in the standard group (P = 0.80). Individual components of MACCE did not differ significantly between groups (Table 4). There were two deaths in the experimental group due to non-cardiac causes (metastatic cancer and progressive lung fibrosis).
Table 4.
Major adverse cardiac and cerebrovascular events
| Major adverse cardiac events | Standard group | Experimental group | P-value a |
|---|---|---|---|
| (n = 700) | (n = 699) | ||
| At least one major adverse cardiac event | 74 (10.6) | 71 (10.2) | 0.799 |
| Died from any cause | 0 (0.0) | 2 (0.3) | 0.157 |
| At least one hospitalization | 74 (10.6) | 69 (9.9) | 0.666 |
| At least one non-fatal MI | 3 (0.4) | 9 (1.3) | 0.082 |
| At least one non-fatal CVA | 1 (0.1) | 0 (0.0) | 0.317 |
Values are n (%).
CVA, cerebrovascular accident; MI, myocardial infarction.
The χ2 test for the number of patients with one or more tests by group.
Quality of life and angina status
Seattle Angina Questionnaire scores showed impairment at baseline (median score of 65 on a scale from 0 to 100 in both randomized groups) that improved significantly over 9 months of follow-up (to a median of 95.8 in both randomized groups). Scores improved to a similar degree in the experimental group (mean change 23.1, median change 23.3) and the standard group (mean change 25.0, median change 22.8), with no significant difference in the change in scores from baseline to 9 months (P = 0.22, Figure 3). The same pattern was evident in the EQ-5D scores over follow-up: both groups showed the reduced quality of life at baseline (median score 0.7 on a scale from 0 to 1 in both groups) that improved over follow-up (to a median score of 0.8 at 9 months in both groups), with no significant difference in the change in scores (0.1 in both groups, P = 0.61).
Figure 3.
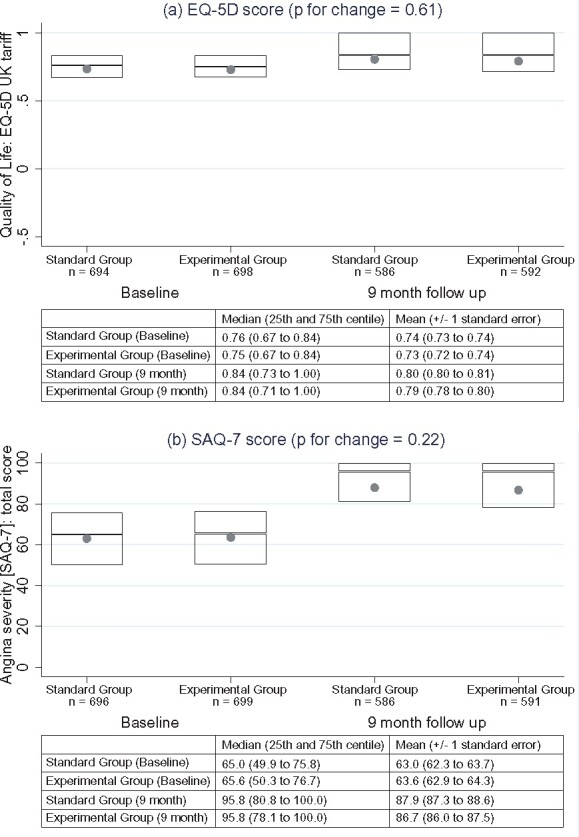
Principal secondary endpoints. Distribution of quality of life (A) and Seattle Angina Questionnaire scores (B) at baseline and 9 months. The boxes indicate the 75th percentile (top line), 25th percentile (bottom line), and 50th percentile (line within the box). The P-values, for changes in scores from baseline to 90 days, are based on the t-test. Note: higher Seattle Angina Questionnaire scores indicate lower angina severity.
Strata of planned initial test
Prior to randomization, the supervising clinician identified the test that would be performed in the event the patient was randomized to the standard care strategy. The pattern of costs varied depending on whether the planned test was an invasive angiogram, a stress test, or a CTCA (Table 5). The experimental group had 6.5% lower costs in the 94 patients with planned invasive angiography, 6.8% lower costs in the 393 patients with planned stress testing, but 20% higher costs in the 912 patients with planned CTCA (Table 5). The rates of MACCE did not differ between groups in any stratum, and the changes in Seattle Angina Questionnaire and EQ-5D scores were similar in all three strata. The effect of the experimental strategy on the use of invasive angiography was significantly greater (interaction P = 0.042) in the planned invasive angiography stratum than in the strata of planned CTCA and planned stress testing (Table 5).
Table 5.
Trial endpoints by group and planned initial testing route
|
Invasive angiography
|
Stress test planned
|
CTCA planned
|
|||||
|---|---|---|---|---|---|---|---|
| Experimental group | Standard group | Experimental group | Standard group | Experimental group | Standard group | Interaction | |
| (n = 46) | (n = 48) | (n = 200) | (n = 193) | (n = 453) | (n = 459) | P-value a | |
| Cost (UK pounds), mean (SD) | 3702 (3246) | 3958 (3313) | 1297 (1592) | 1392 (1812) | 1527 (2220) | 1272 (1777) | 0.087 |
| MACCE | 15 (31) | 15 (33) | 15 (8) | 12 (6) | 44 (10) | 44 (10) | 0.663 |
| Invasive angiography | 29 (63) | 46 (96) | 29 (15) | 43 (22) | 78 (17) | 86 (19) | 0.042 |
| SAQ change, mean (SD) | 23 (21) | 22 (20) | 25 (19) | 24 (19) | 22 (20) | 25 (21) | 0.157 |
| EQ-5D change, mean (SD) | 0.03 (0.18) | 0.08 (0.18) | 0.04 (0.20) | 0.07 (0.18) | 0.07 (0.19) | 0.06 (0.21) | 0.064 |
Values are numbers of tests or events (numbers of patients) unless otherwise specified.
CTCA, computed tomography coronary angiography; EQ-5D, EuroQol five dimensions questionnaire; MACCE, major adverse cardiac and cerebrovascular event; SAQ, Seattle Angina Questionnaire; SD, standard deviation.
Comparing between groups within patients in the CTCA planned strata vs. all other strata.
Discussion
FORECAST is the first randomized trial to assess the strategy of CTCA with selective FFRCT for the initial evaluation of patients presenting with stable chest pain. The main finding of the trial was that, in a low-risk population attending a Rapid Access Chest Pain Clinic, there was no significant difference in cost over 9 months between the experimental strategy and the standard strategy. Furthermore, there were no significant differences in symptoms, quality of life, MACCE, or use of coronary revascularization between the randomized groups. However, the experimental strategy led to a significant, 22% reduction in invasive coronary angiography, with 52% fewer patients having no significant obstructive coronary artery disease on invasive angiography .
FORECAST was designed using cost as the primary endpoint because we anticipated, based upon previous observational studies, that clinical outcomes would be similar in a well-managed population of stable patients with chest pain, irrespective of the initial testing strategy. We hypothesized that a strategy based on initial CTCA with selective FFRCT would be more efficient, with lower resource use and cost. In 2017, the UK Medical Technologies Guidance on FFRCT 23 predicted substantial cost savings for the National Health Service with the adoption of CTCA with FFRCT. Economic analysis of the observational PLATFORM study had shown cost savings from the use of FFRCT when an invasive approach was planned, and cost neutrality when a non-invasive approach was planned.22 In FORECAST, we formally tested the hypothesis that there would be a meaningful cost saving from the experimental strategy based on FFRCT, but found no significant difference overall in costs compared with the standard care strategy. This negative result for the primary outcome might be due to the low prevalence of planned initial invasive angiography (7% of the trial population), and the high prevalence of CTCA as the planned initial test (65% of the trial population). This shift in standard practice in the UK towards routine CTCA, which was stimualted by the NICE CG95 Guidance on Chest Pain of Recent Onset, may have limited the cost savings potential of the experimental strategy based on initial CTCA and selective FFRCT.
Previous studies have consistently shown that the major benefit of FFRCT has been to reduce the use of invasive coronary angiography, particularly angiograms showing no obstructed coronary arteries. In the observational PLATFORM study, invasive angiography was reduced by 61% in the FFRCT cohort, and the clinical event rates at 1 year were equally low in both groups.28 The ADVANCE Registry of patients having CTCA and FFRCT in routine clinical practice found unobstructed coronaries at invasive angiography in 14% of patients with FFRCT ≤0.8, compared with 44% of patients with FFRCT >0.8.21 In addition, there were no deaths or MIs within 90 days in the 1529 patients with FFRCT >0.80, vs. 14 (0.3%) in subjects with an FFRCT ≤0.80 (P = 0.039). We therefore anticipated that the experimental strategy in FORECAST would result in less invasive angiography, and no difference in clinical event rates, compared with standard clinical pathways. The results of FORECAST have confirmed these expectations, with equivalent rates of clinical events, 22% fewer invasive angiograms, and half the rate of unobstructed coronary arteries at invasive angiography in the experimental group, and are consistent with the previous observational studies.
In FORECAST, quality of life and angina status improved to a similar degree in both groups by 9 months of follow-up. This result is consistent with the 1-year data from PLATFORM, in which the five-item EuroQOL score did not differ significantly between the groups overall.28 The improvements seen in both groups are likely due to clinicians actively treating all subjects to relieve anginal symptoms, resulting in the similar use of anti-anginal medications and similar rates of coronary revascularization (Table 3). From a patient’s perspective, achieving similar quality of life and angina outcomes with fewer invasive procedures represents a potential advantage for the experimental strategy based on FFRCT.
There are some limitations of FORECAST. First, and most important, is that we could not anticipate the precise rate of use of CTCA in the standard group. The national guidelines were revised during planning of the trial, and while they recommended that CTCA become the default test for most patients attending Rapid Access Chest Pain Clinics, the infrastructure in many areas of the National Health Service at that time could not provide the test. The subsequent major expansion in CT facilities greatly improved access to CTCA in the last few years. The FORECAST trial, however, was based upon a pragmatic design: the experimental strategy (CTCA with selective FFRCT) vs. standard clinical care pathways, whatever tests that should include. With almost two-thirds of patients in the standard group having planned initial CTCA, the contrast between the randomized groups in FORECAST was diminished, along with the potential for cost savings with the experimental strategy based on the use of CTCA with selective FFRCT. A recent individual-based Markov microsimulation model for patients with low-risk stable chest pain, based upon the PROMISE population, suggested that an anatomical approach using CTCA was cost-effective compared with functional testing.29
A second limitation of the trial is that the costs in this study were based on UK National Health Service cost tariffs, and may not be generalizable to other countries with different cost structures in their health delivery systems. In an attempt to address this, one pre-specified sensitivity analysis for this trial is to apply US-specific cost tariffs to the FORECAST data, and this is the subject of ongoing analysis. Third, we used cardiac costs, rather than total medical costs, as the primary endpoint. Cardiac costs are more likely to be affected by the alternative strategies and were simpler for the local research teams to document. While it seems unlikely that non-cardiac costs would be affected by the management strategies tested, we cannot exclude the possibility that total medical costs differed, even though the cardiac costs did not.
The significant reduction in death from coronary heart disease and non-fatal MI seen at 5 years in the SCOT-HEART trial30 in the cohort undergoing CTCA, compared with routine care alone, indicates that there is considerable prognostic benefit from identifying coronary atheroma and initiating optimal medical therapy based on CTCA findings. Indeed, the results of FORECAST raise an important question that the trial cannot answer: namely, what is the optimal use of FFRCT in routine clinical practice when CTCA is the default approach? In light of the findings from SCOT-HEART30 and ISCHEMIA,2 one could speculate that, rather than using FFRCT based on the burden of atheroma found on CTCA, FFRCT analysis could be reserved only for patients with insufficient symptomatic response to optimal medical therapy in whom revascularization is therefore being considered. This approach would be consistent with a sub-analysis of the PROMISE trial31 that demonstrated the value of describing degrees of coronary atheroma by CTCA in patients presenting with suspected angina, even in the absence of any functional testing for ischaemia, for predicting the primary endpoint of death, MI, and hospitalization for unstable angina. This suggests that the optimal application of FFRCT in the setting of stable symptoms may be after optimal medical therapy fails to control angina adequately, at which time FFRCT could be performed using the previously collected CTCA dataset, and thereby assess the need for revascularization as part of a shared decision-making process with the patient.
In conclusion, the experimental strategy of initial CTCA with selective FFRCT in patients presenting with stable angina did not significantly reduce costs compared with standard clinical evaluation pathways, and led to similar clinical outcomes, including major adverse cardiovascular events, anginal symptoms, and quality of life. The experimental strategy based on FFRCT did, however, reduce the use of invasive coronary angiography, without reducing the use of coronary revascularization.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
FORECAST is an investigator-initiated trial and was supported by an unrestricted research grant from HeartFlow. The authors do hereby declare that all illustrations and figures in the manuscript are entirely original and do not require reprint permission. The authors would like to acknowledge the support received from the Research & Development Department of University Hospital Southampton NHS Foundation Trust.
Recruiting sites: University Hospital Southampton (367 patients); Sandwell Hospital, Birmingham (33 patients); Dorset Heart Centre, University Hospitals Dorset, Bournemouth (227 patients); Queen Alexandra Hospital, Portsmouth (73 patients); Royal Victoria Hospital, Blackpool (154 patients); Royal Infirmary, Edinburgh (93 patients); Royal Infirmary, Glasgow (27 patients); University Hospital of North Tees, Stockton on Tees (120 patients); Derriford Hospital, Plymouth (74 patients); and Royal Stoke University Hospital, Stoke-on-Trent (101 patients).
Funding
G.P.M. is supported by an NIHR Research Professorship (2017-08-ST2-007) and by the NIHR Leicester Biomedical Research Centre and the NIHR Leicester Clinical Research Facility.
Conflict of interest: FORECAST was funded by an unrestricted research grant from HeartFlow. The company had no role in writing the protocol, prosecution of the study or its analysis or drafting of the manuscript. N.C. reports unrestricted research grants from HeartFlow, Boston Scientific, Beckmann Coulter; consultancy/speaker fees from Abbott, HeartFlow, Boston Scientific, Phillips; travel sponsorship from Haemonetics, Abbott, Medtronic, Biosensors, and Edwards. C.B. reports grants from HeartFlow, Abbott, Astra Zeneca, Boehringer I, GSK, Novartis, Siemens; consulting fees/honoraria from HeartFlow, Abbott, Astra Zeneca, Boehringer I, GSK, Novartis, Siemens, and Menarini. G.P.M. reports grants from HeartFlow & Bayer. K.F. reports consulting fees from Servier and Astra Zeneca; travel sponsorship from Servier, Astra, UCB, and Broadview Ventures. M.H. reports a grant for FORECAST from HeartFLow. P.D. reports grants from HeartFlow.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Contributor Information
Nick Curzen, Faculty of Medicine, University of Southampton; Coronary Research Group, University Hospital Southampton.
Zoe Nicholas, Coronary Research Group, University Hospital Southampton.
Beth Stuart, Clinical Trials Unit, University of Southampton.
Sam Wilding, Clinical Trials Unit, University of Southampton.
Kayleigh Hill, Clinical Trials Unit, University of Southampton.
James Shambrook, Cardiothoracic Radiology, University Hospital Southampton.
Zina Eminton, Clinical Trials Unit, University of Southampton.
Darran Ball, Clinical Trials Unit, University of Southampton.
Camilla Barrett, Clinical Trials Unit, University of Southampton.
Lucy Johnson, Clinical Trials Unit, University of Southampton.
Jacqui Nuttall, Clinical Trials Unit, University of Southampton.
Kim Fox, Imperial College, London, UK.
Derek Connolly, Sandwell Hospital, Birmingham, UK.
Peter O’Kane, Dorset Heart Centre, University Hospitals Dorset, Bournemouth.
Alex Hobson, Queen Alexandra Hospital, Portsmouth.
Anoop Chauhan, Royal Victoria Hospital, Blackpool.
Neal Uren, Royal Infirmary, Edinburgh.
Gerry Mccann, Department of Cardiovascular Sciences, University of Leicester & NIHR Biomedical Research Centre, Glenfield Hospital, Leicester, UK.
Colin Berry, British Heart Foundation Glasgow Cardiovascular Research Centre, University of Glasgow.
Justin Carter, University Hospital of North Tees, Stockton on Tees.
Carl Roobottom, Derriford Hospital, Plymouth.
Mamas Mamas, Royal Stoke University Hospital, Stoke-on-Trent.
Ronak Rajani, Guy’s & St Thomas’ Hospital, London.
Ian Ford, Robertson Centre for Biostatistics, University of Glasgow, Glasgow.
Pamela Douglas, Duke University, Durham, NC, USA.
Mark Hlatky, Stanford University, Stanford, CA, USA.
References
- 3. Curzen N, Rana O, Nicholas Z, Golledge P, Zaman A, Oldroyd K, Hanratty C, Banning A, Wheatcroft S, Hobson A, Chitkara K, Hildick-Smith D, McKenzie D, Calver A, Dimitrov BD, Corbett S. Does routine pressure wire assessment influence management strategy at coronary angiography for diagnosis of chest pain? The RIPCORD Study. Circ Cardiovasc Interv 2014;7:248–255. [DOI] [PubMed] [Google Scholar]
- 4. Frohlich GM, Redwood S, Rakhit R, MacCarthy PA, Lim P, Crake T, White SK, Knight CJ, Kustosz C, Knapp G, Dalby MC, Mali IS, Archbold A, Wragg A, Timmis AD, Meier P. Long-term survival in patients undergoing percutaneous interventions with or without intracoronary pressure wire guidance or intracoronary ultrasonographic imaging: a large cohort study. JAMA Intern Med 2014;174:1360–1366. [DOI] [PubMed] [Google Scholar]
- 5. Baptista SB, Raposo L, Santos L, Ramos R, Calé R, Machado JE, Costa C, Infante de Oliveira M, Costa E, Pipa J, Fonseca J, Guardado N, Silva J, Sousa B, Silva MJ, Rodrigues JC, Seca A, Fernandes LR. Impact of routine fractional flow reserve evaluation during coronary angiography on management strategy and clinical outcome: one-year results of the POST-IT. Circ Cardiovasc Interv 2016;9:e003288. [DOI] [PubMed] [Google Scholar]
- 6. Nakamura M, Yamagishi M, Ueno T, Hara K, Ishiwata S, Itoh T, Hamanaka I, Wakatsuki T, Sugano T, Kawai K, Akasaka T, Tanaka N, Kimura T. Modification of treatment strategy after FFR measurement: CVIT-DEFER registry. Cardiovasc Interv Ther 2015;30:12–21. [DOI] [PubMed] [Google Scholar]
- 7. Van Belle E, Rioufol G, Pouillot C, Cuisset T, Bougrini K, Teiger E, Champagne S, Belle L, Barreau D, Hanssen M, Besnard C, Dauphin R, Dallongeville J, El Hahi Y, Sideris G, Bretelle C, Lhoest N, Barnay P, Leborgne L, Dupouy P; Investigators of the Registre Français de la FFR–R3F. Outcome impact of coronary revascularization strategy reclassification with fractional flow reserve at time of diagnostic angiography: insights from a large French multicenter fractional flow reserve registry. Circulation 2014;129:173–185. [DOI] [PubMed] [Google Scholar]
- 8. Sud M, Han L, Koh M, Austin PC, Farkouh ME, Ly HQ, Madan M, Natarajan MK, So DY, Wijeysundera HC, Fang J, Ko DT. Association between adherence to fractional flow reserve treatment thresholds and major adverse cardiac events in patients with coronary artery disease. JAMA 2020;324:2406–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pijls NHJ, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, van’t Veer M, Bar F, Hoorntje J, Koolen J, Wijns W, de Bruyne B. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol 2007;49:2105–2111. [DOI] [PubMed] [Google Scholar]
- 10. Tonino PAL, De Bruyne B, Pijls NHJ, Siebert U, Ikeno F, van `T Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF; FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213–224. [DOI] [PubMed] [Google Scholar]
- 11. Xaplanteris P, Fournier S, Pijls NHJ, Fearon WF, Barbato E, Tonino PAL, Engstrøm T, Kääb S, Dambrink J-H, Rioufol G, Toth GG, Piroth Z, Witt N, Fröbert O, Kala P, Linke A, Jagic N, Mates M, Mavromatis K, Samady H, Irimpen A, Oldroyd K, Campo G, Rothenbühler M, Jüni P, De Bruyne B; FAME 2 Investigators. Five-year outcomes with PCI guided by fractional flow reserve. N Engl J Med 2018;379:250–259. [DOI] [PubMed] [Google Scholar]
- 12. De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Möbius-Winkler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engström T, Oldroyd KG, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Jüni P, Fearon WF; FAME 2 Trial Investigators. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012;367:991–1001. [DOI] [PubMed] [Google Scholar]
- 13. Toth G, Hamilos M, Pyxaras S, Mangiacapra F, Nelis O, De Vroey F, Di Serafino L, Muller O, Van Mieghem C, Wyffels E, Heyndrickx GR, Bartunek J, Vanderheyden M, Barbato E, Wijns W, De Bruyne B. Evolving concepts of angiogram: fractional flow reserve discordances in 4000 coronary stenoses. Eur Heart J 2014;35:2831–2838. [DOI] [PubMed] [Google Scholar]
- 14. Taylor CA, Fonte TA, Min JK. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol 2013;61:2233–2241. [DOI] [PubMed] [Google Scholar]
- 15. Kueh SH, Mooney J, Ohana M, Kim U, Blanke P, Grover R, Sellers S, Ellis J, Murphy D, Hague C, Bax JJ, Norgaard BL, Rabbat M, Leipsic JA. Fractional flow reserve derived from coronary computed tomography angiography reclassification rate using value distal to lesion compared to lowest value. J Cardiovasc Comput Tomogr 2017;11:462–467. [DOI] [PubMed] [Google Scholar]
- 16. Cami E, Tagami T, Raff G, Gallagher MJ, Fan A, Hafeez A, Willner SJ, Arce PS, George J, Bilolikar A, Chinnaiyan K, Safian RD. Importance of measurement site on assessment of lesion-specific ischemia and diagnostic performance by coronary computed tomography angiography-derived fractional flow reserve. J Cardiovasc Comput Tomogr 2021;15:114–120. [DOI] [PubMed] [Google Scholar]
- 17. Nørgaard BL, Leipsic J, Gaur S, Seneviratne S, Ko BS, Ito H, Jensen JM, Mauri L, De Bruyne B, Bezerra H, Osawa K, Marwan M, Naber C, Erglis A, Park SJ, Christiansen EH, Kaltoft A, Lassen JF, Bøtker HE, Achenbach S; NXT Trial Study Group. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J Am Coll Cardiol 2014;63:1145–1155. [DOI] [PubMed] [Google Scholar]
- 18. Driessen RS, Danad I, Stuijfzand WJ, Raijmakers PG, Schumacher SP, van Diemen PA, Leipsic JA, Knuuti J, Underwood SR, van de Ven PM, van Rossum AC, Taylor CA, Knaapen P. Comparison of coronary computed tomography angiography, fractional flow reserve, and perfusion imaging for ischemia diagnosis. J Am Coll Cardiol 2019;73:161–173. [DOI] [PubMed] [Google Scholar]
- 19. Curzen N, Nolan J, Zaman A, Norgaard B, Rajani R. Does the routine availability of CT-derived FFR influence management of patients with stable chest pain compared to CT angiography alone?: the FFR CT RIPCORD Study. JACC Cardiovasc Imaging 2016;9:1188–1194. [DOI] [PubMed] [Google Scholar]
- 20. Douglas PS, Pontone G, Hlatky MA, Patel MR, Norgaard BL, Byrne RA, Curzen N, Purcell I, Gutberlet M, Rioufol G, Hink U, Schuchlenz HW, Feuchtner G, Gilard M, Andreini D, Jensen JM, Hadamitzky M, Chiswell K, Cyr D, Wilk A, Wang F, Rogers C, De Bruyne B; PLATFORM Investigators. Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: the prospective longitudinal trial of FFRCT: outcome and resource impacts study. Eur Heart J 2015;36:3359–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patel MR, Nørgaard BL, Fairbairn TA, Nieman K, Akasaka T, Berman DS, Raff GL, Hurwitz Koweek LM, Pontone G, Kawasaki T, Sand NPR, Jensen JM, Amano T, Poon M, Øvrehus KA, Sonck J, Rabbat MG, Mullen S, De Bruyne B, Rogers C, Matsuo H, Bax JJ, Leipsic J. 1-year impact on medical practice and clinical outcomes of FFRCT: the ADVANCE registry. JACC Cardiovasc Imaging 2020;13:97–110. [DOI] [PubMed] [Google Scholar]
- 22. Hlatky MA, De Bruyne B, Pontone G, Patel MR, Norgaard BL, Byrne RA, Curzen N, Purcell I, Gutberlet M, Rioufol G, Hink U, Schuchlenz HW, Feuchtner G, Gilard M, Andreini D, Jensen JM, Hadamitzky M, Wilk A, Wang F, Rogers C, Douglas PS; PLATFORM Investigators. Quality-of-life and economic outcomes of assessing fractional flow reserve with computed tomography angiography: PLATFORM. J Am Coll Cardiol 2015;66:2315–2323. [DOI] [PubMed] [Google Scholar]
- 23.National Institute for Health and Care Excellence. HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography. Medical Technologies Guidance [MTG32]. https://www.nice.org.uk/guidance/mtg32 (13 February 2017).
- 24.National Institute for Health and Clinical Excellence. Recent-onset chest pain of suspected cardiac origin: assessment and diagnosis. Clinical guideline [CG95]. https://www.nice.org.uk/guidance/cg95 (24 March 2010). [PubMed]
- 25. Mahmoudi M, Nicholas Z, Nuttall J, Bresser M, Maishman M, Berry C, Hlatky M, Douglas P, Rajani R, Fox K, Curzen N. Fractional flow reserve derived from computed tomography coronary angiography in the assessment and management of stable chest pain: rationale and design of the FORECAST trial. Cardiovas Revasc Med 2020;21:890–896. [DOI] [PubMed] [Google Scholar]
- 26.EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 27. Spertus J, Winder J, Dewhurst T, Deyo R, Prodzinski J, McDonnell M, Fihn S. Development and evaluation of the Seattle Angina questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol 1995;25:333–341. [DOI] [PubMed] [Google Scholar]
- 28. Douglas PS, De Bruyne B, Pontone G, Patel MR, Norgaard BL, Byrne RA, Curzen N, Purcell I, Gutberlet M, Rioufol G, Hink U, Schuchlenz HW, Feuchtner G, Gilard M, Andreini D, Jensen JM, Hadamitzky M, Chiswell K, Cyr D, Wilk A, Wang F, Rogers C, Hlatky MA; PLATFORM Investigators. 1-year outcomes of FFRCT-guided care in patients with suspected coronary disease: the PLATFORM study. J Am Coll Cardiol 2016;68:435–445. [DOI] [PubMed] [Google Scholar]
- 29. Karády J, Mayrhofer T, Ivanov A, Foldyna B, Lu MT, Ferencik M, Pursnani A, Salerno M, Udelson JE, Mark DB, Douglas PS, Hoffmann U. Cost-effectiveness analysis of anatomic vs functional index testing in patients with low-risk stable chest pain. JAMA Netw Open 2020;3:e2028312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Newby DE, Adamson PD, Berry C, Boon NA, Dweck MR, Flather M, Forbes J, Hunter A, Lewis S, MacLean S, Mills NL, Norrie J, Roditi G, Shah ASV, Timmis AD, van Beek EJR, Williams MC; SCOT-HEART Investigators. Coronary CT angiography and 5 year risk of myocardial infarction. N Engl J Med 2018;379:924–933. [DOI] [PubMed] [Google Scholar]
- 31. Hoffmann U, Ferencik M, Udelson JE, Picard MH, Truong QA, Patel MR, Huang M, Pencina M, Mark DB, Heitner JF, Fordyce CB, Pellikka PA, Tardif JC, Budoff M, Nahhas G, Chow B, Kosinski AS, Lee KL, Douglas PS; PROMISE Investigators. Prognostic value of noninvasive cardiovascular testing in patients with stable chest pain: insights from the PROMISE trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017;135:2320–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.