Abstract
Natural biopolymers, polymeric organic molecules produced by living organisms and/or renewable resources, are considered greener, sustainable, and eco-friendly materials. Natural polysaccharides comprising cellulose, chitin/chitosan, starch, gum, alginate, and pectin are sustainable materials owing to their outstanding structural features, abundant availability, and nontoxicity, ease of modification, biocompatibility, and promissing potentials. Plentiful polysaccharides have been utilized for making assorted (nano)catalysts in recent years; fabrication of polysaccharides-supported metal/metal oxide (nano)materials is one of the effective strategies in nanotechnology. Water is one of the world’s foremost environmental stress concerns. Nanomaterial-adorned polysaccharides-based entities have functioned as novel and more efficient (nano)catalysts or sorbents in eliminating an array of aqueous pollutants and contaminants, including ionic metals and organic/inorganic pollutants from wastewater. This review encompasses recent advancements, trends and challenges for natural biopolymers assembled from renewable resources for exploitation in the production of starch, cellulose, pectin, gum, alginate, chitin and chitosan-derived (nano)materials.
Keywords: Polysaccharides, Sustainable nanomaterials, Wastewater treatment, Degradation, Organic dyes, Heavy metals, Pollutants
1. Introduction
Water is one of the world’s foremost environmental stress concerns; the supply of safe, affordable drinking and/or clean water is a massively challenging proposition throughout the world. Rapidly escalating environmental contamination of natural resources is an emerging issue in recent years that needs to be tackled on priority basis for sustaining the earth and its inhabitants for future generations. Indeed, resources of freshwater are limited and they are deteriorating fast due to the discharge of untreated or inadequately-treated wastewaters. Traditionally, coagulation/flocculation, ion exchange, floatation, reverse osmosis, oxidation, adsorption, membrane separation, ultra-filtration, sedimentation, electro-precipitation, and advanced oxidation processes are mainly resorted to as the accessible technologies in treating waste-water. Conventional methods of wastewater treatment and purification cannot possibly yield the desired extent of purification to attain accurate or cost-effective discharge standards (Khoramzadeh, Nasernejad, & Halladj, 2013; Nasrollahzadeh, Sajjadi, Dasmeh, & Sajadi, 2018; Yargıç, Şahin, Özbay, & Önal, 2015).
Beyond the stoichiometric use of reagents, catalysis is one of the most important foundation of “green chemistry” with novel processing systems and deployment of assorted novel catalysts with many benefits in terms of product selectivity, process utilization, energy reduction as well as the utilization of safer materials and alternative reaction media/conditions. Novel wastewater treatment approaches such as UV photolysis/photocatalysis, activated carbon adsorption, ozonation, and perovskite adsorption, among others can be utilized for the degradation of pharmaceutically active compounds, metal ions, and toxic dyes (Cai et al., 2018; Garba, Xiao et al., 2019; Garba, Zhou, Zhang, & Yuan, 2020; Karnib, Kabbani, Holail, & Olama, 2014; Xu, Nasrollahzadeh, Sajjadi et al., 2019). Interestingly, the expensive energy-intensive commercially-activated carbons applied for this goal can be effectively replaced by renewable alternatives and low-cost biosorbents that are based on natural biopolymers (Crini, 2006; Xu, Nasrollahzadeh, Sajjadi et al., 2019). In general, the development of ‘greener’ and eco-friendly treatment technologies must be perceived as a key element for the industries dealing with toxic, hazardous and chemically laden wastewater (Atarod, Nasrollahzadeh, & Sajadi, 2015; Hatamifard, Nasrollahzadeh, & Lipkowski, 2015; Hatamifard, Nasrollahzadeh, & Sajadi, 2016; Iravani & Varma, 2020a; Khodadadi, Bordbar, & Nasrollahzadeh, 2017; Khodadadi, Bordbar, & Nasrollahzadeh, 2017; Maryami, Nasrollahzadeh, Mehdipour, & Sajadi, 2016; Nasrollahzadeh, Bagherzadeh, & Karimi, 2016; Nasrollahzadeh, Sajadi, & Maham, 2016; Omidvar, Jaleh, & Nasrollahzadeh, 2017; Sharma, Zboril, & Varma, 2015; Sivan et al., 2019; Varma, 2014; Zhang, Yu et al., 2018; Zhang, Hou et al., 2018).
Among the greener technologies, the synthesis of natural (nano) catalysts with lower costs, and enhanced efficiency has been a focus area for the treatment of wastewater pollutants as exemplified by nanoscale filtration procedures and the adsorption of pollutants by metal/metal oxide based nanoparticles (NPs) (Ai, Yue, & Jiang, 2012; Crini, 2006; Meng, Zhu, Choi, Park, & Oh, 2011; Nasrollahzadeh, Baran et al., 2020; Nasrollahzadeh, Sajjadi, Dasmeh et al., 2018; Xu, Nasrollahzadeh, Sajjadi et al., 2019; Zhang, Sèbe, Rentsch, Zimmermann, & Tingaut, 2014). The utilization of nanostructures with unique features namely large surface area, significant chemical reactivity, cost-effectiveness, and lower power consumption, can meaningfully exploit multifunctional nanosystems that enable particle retention and removal/elimination of pollutants.
Over the decades, the accessibility of synthetic polymers derived from gas, petroleum, and nonrenewable carbon sources is diminishing as researchers are exploring more readily available and sustainable alternatives, namely, natural biopolymer-derived materials from renewable resources. Natural biopolymers are polymeric organic molecules acquired from renewable resources (alga, plants, microbial biomass and animals) comprising monomeric parts that are bonded covalently to form larger molecules (Baran & Nasrollahzadeh, 2020; Den, Sharma, Lee, Nadadur, & Varma, 2018; Hebbalalu, Lalley, Nadagouda, & Varma, 2013; Iravani & Varma, 2020b; Mohazzab et al., 2020; Motahharifar, Nasrollahzadeh, Taheri-Kafrani, Varma, & Shokouhimehr, 2020; Nasrollahzadeh, Shafiei, Nezafat, & Bidgoli, 2020; Nasrollahzadeh, Issaabadi, & Varma, 2019). They represent a highly promising option for the generation of sustainable materials owing to their extraordinary structural and physical features, safety, availability, and economics; biocompatibility and the biodegradability of these natural resources can enhance their utilization as nanocatalysts and nanosorbents. Low-cost biopolymers such as polysaccharides are diverse in size, structure, and molecular chains, making them attractive candidates for stabilization and immobilization and the reduction of NPs; biopolymer-based (nano) catalysts can be immobilized on their uniquely featured reactive groups to enhance (nano)catalytic efficiency and stability (Crini, 2005; Kumar, 2000; Oladoja, Adelagun, Ahmad, Unuabonah, & Bello, 2014; Xu, Nasrollahzadeh, Sajjadi et al., 2019; Xu, Nasrollahzadeh, Selva, Issaabadi, & Luque, 2019). The salient advantageous features for the utilization of polysaccharide supports include the release of nanostructures during operations, the prevention of aggregation, the ease of recovery of catalytic materials, and importantly, improving the photocatalytic efficiency compared to the conventional slurry systems.
Pollution generated by dyes, heavy metals, nitroarenes, and pesticides in water/wastewater is a global major problem; in particular, the dye effluents are identified as largest class of industrial colorants and significant threat to aquatic environments as the discarded dyes seriously affect humans and flora/fauna (Albukhari, Ismail, Akhtar, & Danish, 2019; Khan et al., 2016; Sajjadi et al., 2020). Toxic dyes/nitroarenes are hazardous, biologically and chemically stable, non-biodegradable and water soluble organic contaminants that are responsible for a diversity of human diseases e.g. kidney failure, skin irritation, nervous system damage, liver disease, etc. (Chamjangali, Bagherian, Javid, Boroumand, & Farzaneh, 2015; Dai et al., 2009; Mazaheri, Ghaedi, Azqhandi, & Asfaram, 2017; Nasrollahzadeh, Nezafat, Gorab, & Sajjadi, 2020). The main sources of contamination from dyes have their origin in diverse industries, namely coloration of textiles, inks, paints, paper, plastics, dye sensitized solar cells, energy transfer cascades, display devices, light emitting diodes, laser welding processes, as well as food and/or cosmetic dyes which are particularly derived from azo dyes (Sannino, Vaiano, Sacco, & Ciambelli, 2013; Singh & Arora, 2011). Textile dye effluents are generally present as a mixture of several dyes in diverse percentages depending on the factory schedule, and the extent of dye fixation on a fabric (Yaseen & Scholz, 2018). Consequently, the removal of such admixed dyes (in diverse concentration levels and mixing ratios) in wastewaters is extremely vital. The development/improvement of novel eco-friendly treatment approaches should be considered as a critical element for the industries generating toxic and hazardous chemical-laden wastewater.
Generally, polysaccharide derivatives showed high removal efficiency of both inorganic (e.g. heavy metal ions) and organic (e.g. dyes, nitroarenes, and pesticide formulations) pollutants via adsorption, reduction/degradation and coagulation/flocculation methods (Kanmani, Aravind, Kamaraj, Sureshbabu, & Karthikeyan, 2017; Sajjadi, Nasrollahzadeh, & Tahsili, 2019; Sivan et al., 2019). Among these, adsorption can be considered as a good option for the removal of the hazardous organic contaminants, since it can be simply deployed thus avoiding the formation of various toxic intermediates, which gets generated while treating novel organic contaminants in aqueous solutions (Ertas & Uyar, 2017; Ghaedi et al., 2012; Liu et al., 2020). The utilization of biopolymer-based adsorbents is not only restricted to the removal of heavy metals, dyes and nitro compounds, but extends to a range of toxic contaminants including pharmaceuticals (Amouzgar & Salamatinia, 2015), hydrocarbons (Xu, Yong, Lim, & Obbard, 2005), pesticides (Sahithya, Das, & Das, 2015), phosphates (An, Jung, Zhao, Lee, & Choi, 2014), nitrates (Rajeswari, Amalraj, & Pius, 2016), fluo-rides (Jagtap, Yenkie, Labhsetwar, & Rayalu, 2011), perchlorates (Sayed & Jardine, 2015), and radioactive ions (Lu et al., 2016), etc.
Polysaccharides are sustainable and environmental-friendly organic biopolymers naturally engineered by living organisms and comprise the repeat unit of monosaccharides (Cn(H2O)n). In this respect, chitin/chitosan, cellulose (Ahmad, Ahmed, Swami, & Ikram, 2015; Olivera et al., 2016; Xu, Nasrollahzadeh, Sajjadi et al., 2019), starch (Yusof & Kadir, 2016), pectin (Sharma, Naushad, Pathania, & Kumar, 2016), alginate (Swain, Patnaik, & Dey, 2013; Xu, Nasrollahzadeh, Sajjadi et al., 2019), guar gum (Kee, Mukherjee, & Pariatamby, 2015) and xanthan gum (Kee et al., 2015; Pi et al., 2016) are important examples of sustainable and environmental-friendly organic biopolymers (Fig. 1). Among the polysaccharides, after cellulose (most abundant biopolymer on earth), chitosan, the second most abundant biopolymers have been preferred for broad ranging environmental appliances (Ahmad et al., 2015; Olivera et al., 2016; Xu, Nasrollahzadeh, Sajjadi et al., 2019). Herein, this paper sums up the recent advances in the remediation and elimination of aqueous pollutants and noxious contaminants using some of the most abundant natural biopolymer resources, namely chitin/chitosan, starch, gum, alginate, pectin, and cellulosic (nano)materials.
Fig. 1.
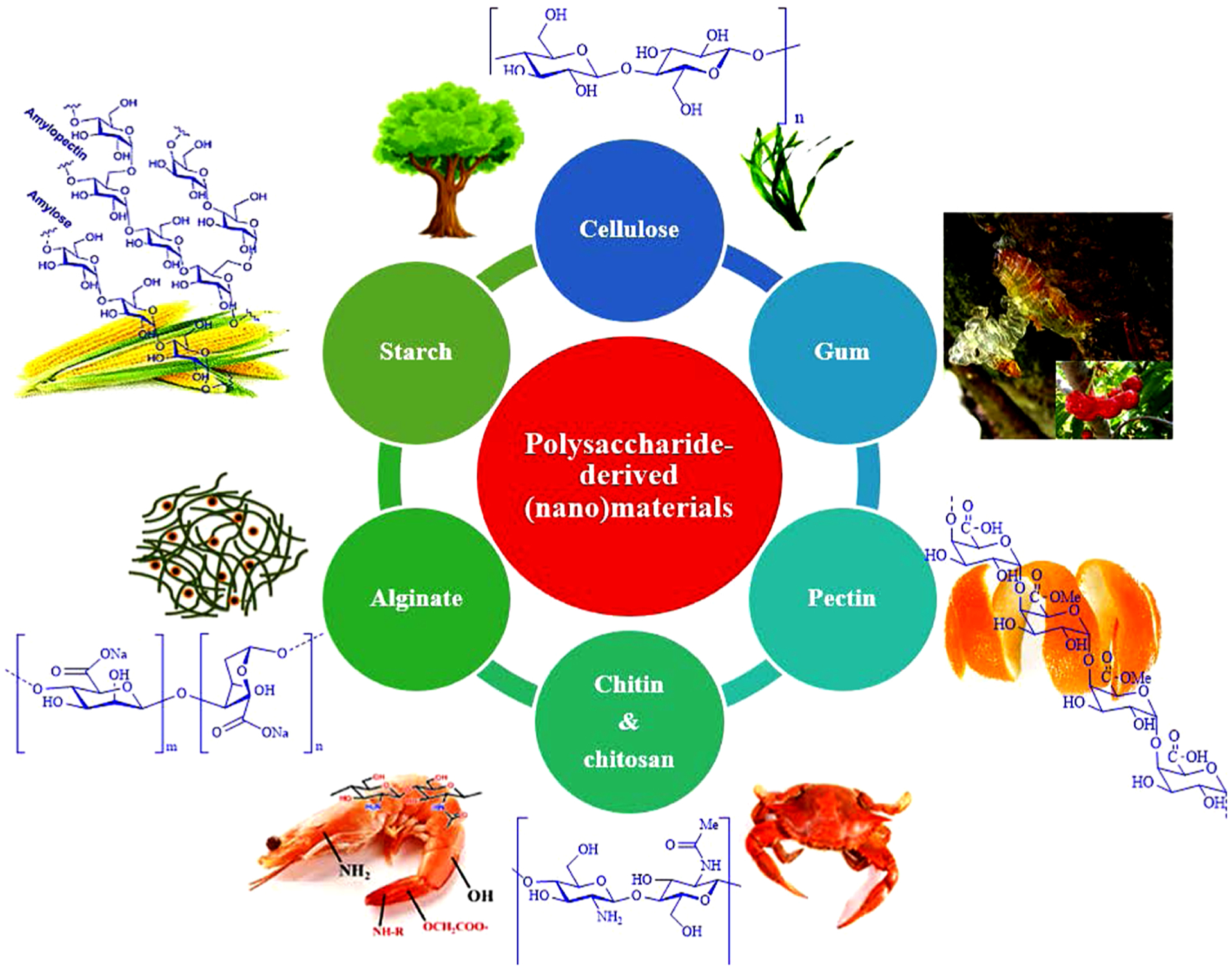
Sustainable and environmental-friendly organic polysaccharides.
2. Polysaccharide derived (nano)catalysts for water treatment
Recently, polysaccharide derived (nano)catalysts have been investigated as heterogeneous and novel catalysts with excellent catalytic prowess for the water treatment. Different applications of polysaccharide derived (nano)catalysts in water treatment are summarized in this section.
2.1. Cellulose-based nanomaterials
2.1.1. Chemistry and properties
Cellulose-based materials for example, cellulose nanofibrils (CNFs) and nanocrystals (CNCs) have found numerous applications in medicine, bioplastics, barrier films, biomedicine, pharmaceutics, electronics, nanocomposites, membranes, supercapacitors, and cosmetic products. These nanomaterials have garnered substantial interest for deployment as (nano)sorbents because of their unique properties (Fig. 2) (Moon, Martini, Nairn, Simonsen, & Youngblood, 2011; Ray & Shipley, 2015; Trache, Hussin, Haafiz, & Thakur, 2017); cellulose-based adsorbents and their use in water and wastewater treatment have been reviewed (Table 1) (Mohammed, Grishkewich, & Tam, 2018).
Fig. 2.
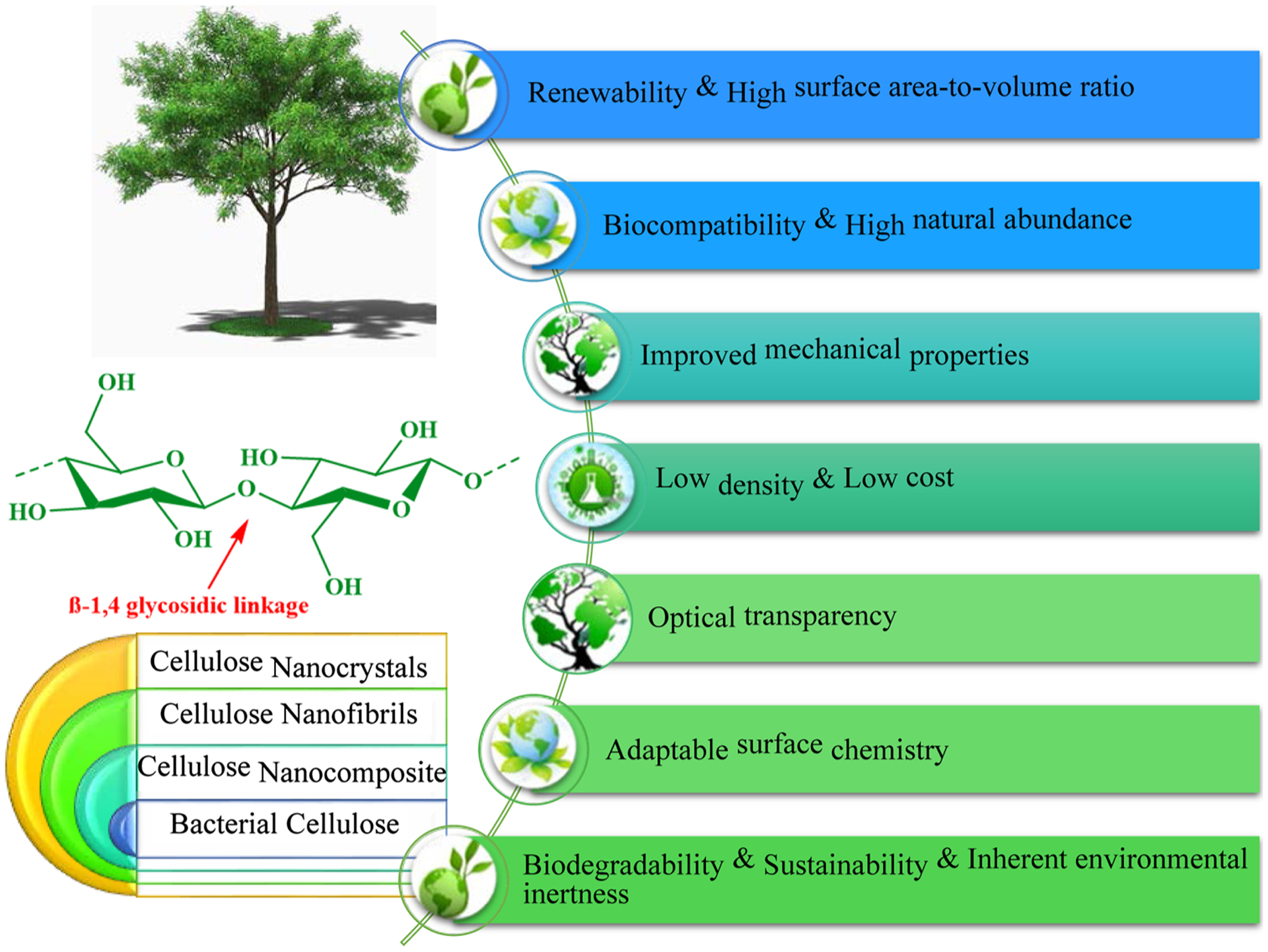
Significant properties and attributes of cellulosic nanomaterials.
Table 1.
Cellulosic nanomaterial-based adsorbents for the removal of pollutants in water.
| Cellulosic nanomaterial-based adsorbents | Contaminants | Ref. |
|---|---|---|
| β-Cyclodextrin modified CNCs@Fe3O4@SiO2 superparamagnetic nanorods | Procaine, imipramine | (Chen, Berry, & Tam, 2014) |
| PEG modified CNCs | Acetaminophen, sulfamethoxazole, N, N-diethyl-meta-toluamide | (Herrera-Morales et al., 2017) |
| Pristine CNCs | Chlorpyrifos | (Moradeeya et al., 2017) |
| Dialdehyde functionalized CNCs | Creatinine | (Huang, Liu, Sun, & Fatehi, 2016) |
| Poly(acrylic acid) modified poly (glycidylmethacrylate) grafted CNCs | Trypsin | (Anirudhan & Rejeena, 2012) |
| Poly(methacrylic acid-co-vinyl sulfonic acid) grafted magnetic CNCs | Hemoglobin, immunoglobulin G | (Anirudhan & Rejeena, 2013) |
| Aminopropyltriethoxysilane modified, hydroxyl-carbonated apatite modified and Epoxy modified CNFs | Hydrogen sulphide | (Hokkanen, Repo, Bhatnagar, Tang, & Sillanpää, 2014) |
| Carbonated hydroxyapatite modified CNFs | Phosphate, nitrate | (Hokkanen, Repo, Westholm et al., 2014) |
| UiO-66/polydopamine/bacterial cellulose | Aspirin, tetracycline hydrochloride | (Cui et al., 2020) |
| Carboxy methyl cellulose/citric acid aerogel | Nitrate, nitrite, phosphate | (Darabitabar, Yavari, Hedayati, Zakeri, & Yousefi, 2020) |
While the materials have shown demonstrative effectiveness in separating and eliminating various contaminating materials, the environmental influences of modified cellulose nanomaterials should be assessed; their non-toxicity and biodegradability attributes, though suitable for wastewater treatment, need stability evaluation. Sizeable amounts of cellulose nanomaterials are needed for remedial applications, thus their cost, feasibility of access, and life cycle considerations for the large scale manufacturing of these materials must be considered (Shatkin, Wegner, & Neih, 2013) although cellulosic nanomaterials have environmental advantages over activated carbon derived from charcoal. Biochar (obtainable from plant biomass) with its similarity to activated carbon has less functionality than cellulose nanomaterial. Cellulosic nano- and microfibers with suitable dimensions and strength have been used to generate membranes for water management. Membranes have been prepared as pristine cellulose nanomaterial mats as well as from cellulose nanomaterials incorporated into assorted polymer matrices such as cellulose triacetate, polypyrrole, poly(vinylidene fluoride), poly (ethylene oxide), poly(ether sulfone), poly(vinyl alcohol), poly(acrylonitrile), and poly(3-hydroxybutyrate) (Carpenter, de Lannoy, & Wiesner, 2015); they can be applied in membrane distillation, nanofiltration, hemodialysis, microfiltration, and ultrafiltration. The presence of cellulose nanomaterials within polymer matrices can noticeably change the membrane characteristics. For instance, better membrane tensile strength, surface hydrophilicity, superior permeability, resistance to biofouling, and enhanced selectivity can be attained by adding cellulose nanomaterials which are highly biocompatible and eco-friendly and ideally suited for pharmaceutical, biomedical and environmental applications of these nanocomposite membranes (Carpenter et al., 2015; Yin & Deng, 2015); challenge being the application of polymer-cellulose nanocomposites realizing uniform and homogeneous dispersing within polymer matrices (Xie, Mai, & Zhou, 2005). Homo-aggregating cellulose nanomaterials destructively influence the amorphous and semi-crystalline polymers by upsetting the polymer solutions homogeneity (Varma, 2016).
2.1.2. Applications for water treatment
2.1.2.1. Removal of oil and organic solvents.
Cellulose is an ideal adsorbent due to its low-cost and abundance relative to commercial ion exchange sorbents. Although unmodified cellulose lacks certain properties to be applied as an effective adsorbent, namely variable physical stabilities and low heavy metal adsorption capacities (O’Connell, Birkinshaw, & O’Dwyer, 2008), among others; thus, surface engineering via chemical modification have been studied in recent years. Modified cellulose nanomaterials matrices can be applied for the sorption of organic contaminants; inherently hydrophilic cellulose nanomaterials are modifiable to enhance their affinity for hydrophobic materials. The surface modification of cellulosic nanomaterials can be attained by insertion of both, the organic and inorganic groups as has been exemplified via the atomic layer placement of titanium dioxide (TiO2) NPs onto cellulose nanomaterial aerogels (Korhonen, Kettunen, Ras, & Ikkala, 2011); TiO2 veneer formed a low-energy surface on the fibers to generate nanocellulose-based material which has both, the oleophilic and hydrophobic properties and they could absorb oil and diverse organic solvents from the water’s surface with 20–40 g/g and 80–90 % vol/vol capacity. Jiang and Hseih achieved better sorption capabilities of model organic solvents ranging from 139–345 g/g by vapor phase deposition of triethoxyl(octyl)silane on CNFs aerogels (Jiang & Hsieh, 2014). The addition of hydrophobic silanes to cellulose nanomaterials transformed them into water-repellant and oleophilic materials which could eliminate oil from the top or below the surface of water; heterogeneous catalyst could be reused 6 times. Wang, Yadav et al. (2014) and Wang, Zhang et al. (2014) prepared hydrogels with graphene oxide embedded in the nanocellulose matrix. After the H2 gas reduction, the graphene oxide-cellulose nanomaterials composites could sorb cyclohexane and dimethylformamide (DMF).
Other hazardous atmospheric volatile organic compounds (VOCs), namely, phenol, toluene and xylenes are of health concern owing to their low solubility and volatility that adversely effects the environment and human health (Al Momani, 2007); toluene, an ingredient utilized in adhesives, paints, detergents, and inks, is a typical VOC in water (Zeng et al., 2009). Rezaee, Pourtagi, Hossini, and Loloi (2016) reported that TiO2 NPs impregnated on the microbial cellulose (MC) surface (MC/TiO2) could degrade toluene in air at ambient temperature under UV-irradiation conditions with maximum photodegradation abilities towards toluene pollutants being 87.79 % and 76.87 % after 40 min irradiation with UVC and UVA, respectively.
2.1.2.2. Removal of pesticides.
Pesticides are major organic pollutants in water bodies that are generally treated using assorted techniques such as photocatalytic degradation, aerobic degradation, ozonation, ultrasound combined with (photo)Fenton treatment, advanced oxidation processes, electrodialysis, reverse osmosis, adsorption, etc. (Ahmad et al., 2010; Hladik, Roberts, & Bouwer, 2005; Salman, Njoku, & Hameed, 2011). Based on target organisms, the main classes of pesticides are herbicides, fungicides, and insecticides, with herbicides accounting for approximately 46 % of the total pesticide (4.1 million tons) use worldwide (Mojiri et al., 2020). 2,4-Dichlorophenoxyacetic acid (2,4-D), one of the oldest herbicides and a common type of pesticide utilized broadly in the agricultural industry, is an organochlorine compound commercially accessible since 1945 (Kanmani et al., 2017). Nevertheless, pesticides are widely used in agriculture, industry and households, and they pose a risk to ecosystems and human health. Salman et al. (2011) reported that the maximum allowable concentration of 2,4-D in affordable drinking water is 100 μg L−1. Due to its endocrine disrupting and plant hormone activities, 2,4-D (well-known plant growth regulator) has been listed as one of the top 10 bestselling pesticides (Wang, Ge et al., 2013). The 2, 4-D accumulation in agricultural products and natural environment not only can cause serious contamination to the environment/ecosystem, but also jeopardizes public safety, human health, and economic advancement; indeed, it is associated with the occurrence of human cancer, endocrine disruption, etc. (Smith, Smith, La Merrill, Liaw, & Steinmaus, 2017). In yet another attempt, Zhang, Zhao et al. (2019) and Zhang, Ma et al. (2019) described a facile and novel method to prepare fluorescent microfluidic paper chips (paper@QDs@MIPs) by depositing fluorescence signal material, CdTe quantum dots (QDs) onto cellulose paper as a base material, and studied the ability of the resulting paper@QDs@MIPs in rapid detection of pesticide 2,4-D.
2,4-Bis(isopropyl amino)-6-(methylthio)-s-triazine or prometryn (Pr), a colorless crystal, nonionic and hydrophobic herbicides pose a serious threat to the environment as well as human/animal’s health (Plakas & Karabelas, 2009). In this context, Garba, Zhou, Lawan, Zhang, and Yuan (2019) synthesized a copper modified microcrystalline cellulose (Cu@MCC) by a facile synthesis and deployed it as an effective composite adsorbent for Pr herbicide adsorption from synthetic waste-water; good adsorption capacity of 97.80 mg g−1 at ambient temperature was discerned with sufficient stability for 6 sequential adsorption-desorption runs. In another study, a facile and novel process was developed for embedding triolein into cellulose acetate sphere as a sustainable and efficient composite adsorbent for removing lipophilic pollutants (Liu, Dai, Qu, & Ru, 2005); adsorbent could be used for the effective removal of two organochlorinated pesticides (OCPs) of low concentrations from water. In addition, a novel class of recoverable CdS@x%SCNF (x = 5, 10, 15, 20, 50) bionanocomposites was attained by depositing cadmium sulfide NPs on a matrix of biomass-derived silanized cellulose nanofibers via a solvothermal methodological route (Gupta, Kumar, Tikoo, Kaushik, & Singhal, 2020), and used for the adsorptive detoxification of pesticide (organophosphate insecticide chlorpyrifos) and textile dye (MB and safranin O) contaminants from wastewater. The as-synthesized CdS@10 %SCNF bionanocomposite exhibited maximum adsorption capacities for all the contaminants and could be reused up to six adsorptive runs.
Cellulose composites and metal organic frameworks (MOFs) are promising adsorption candidates as they combine the high adsorption capacities of MOFs and the sustainability of cellulose-based (nano)materials. Abdelhameed, Abdel-Gawad, Elshahat, and Emam (2016) selected Cu-BTC MOFs for the adsorption of 14C-ethion as an organo-phosphorus insecticide pollutant. Cu-BTC@cotton was fabricated via a facile method by an interaction of Cu in MOF and cellulose functional groups; indeed, the ethion molecule can bind with adsorbent by forming chemical bonds with Cu-BTC (copper-benzene-1,3,5-tricarboxylic acid) and cotton fabrics (chemisorption). As a result, the maximum sorption capacity of as-prepared composite reached 182 mg g−1 and the ethion removal percent exceeded 97 %. After recovering five times, the Cu-BTC@cotton adsorption efficiency was still retained and surpassed 85 %. Interestingly, Gan et al. (2019) extracted carbon nanofiber (CCNF) from abundant cellulosic source via electrospinning and pyrolysis treatment strategy and coated CoFe2O4 on it via a hydrothermal method to generate a novel catalyst for the activation of peroxymonosulfate (PMS). The appliance of CoFe2O4/CCNF nanocomposite activated PMS has been illustrated in the degradation of dimethyl phthalate (DMP), a classical organic pesticide pollutant, in wastewater. Besides, the catalyst could be easily reused in catalytic degradation reactions for 5 cycles with an insignificant loss in catalytic performance.
2.1.2.3. Removal of heavy metal ions.
As aforementioned, there has been a growing ecological and global public health concern associated with wastewater contamination by heavy metals. Heavy metals represent any naturally occurring element that has a high atomic weight or a high density (5 times higher than that of water) and are toxic/hazardous even at very low concentration (Huang, Liu, Zhang, Wu, & Tang, 2017; Sajjadi et al., 2019; Varghese, Paul, & Latha, 2019; Yadav & Xu, 2013); numerous heavy metals are summarized in Table S1. One of the common heavy metals is chromium [Cr(VI)] that finds diverse applications in leather tanning, pigment production, stainless steel manufacturing, and is detrimental to the human health. Simultaneous exposure to several heavy metals can generate a toxic effect, which is either additive/synergistic or antagonistic. Yu, Tong, Ge, Wu et al. (2013) and Yu, Tong, Ge, Zuo et al. (2013) reported that applying succinic acid groups onto CNCs considerably accelerated the binding efficiency to Pb2+ and Cd2+ in water; transformation of the carboxylic acid groups to sodiated carboxylates improved their ability to eliminate toxic metal ions from solutions. Researchers have established the capability of COO-amended CNFs to sorb Ni2+, Cd2+, Pb2+ and Cr3+, with competences 3–10 % greater than original CNFs (Srivastava, Kardam, & Raj, 2012). Besides, the catalyst could be easily separated and reused for 5 times. On the other hand, cysteine usage offered appended thiol functionalities to effectively bind Cr(VI) and Pb(II) (Yang et al., 2014). The amine group mobilization on the surface of CNCs enabled more than 98 % elimination of anionic chromate comprising Cr6+ in the concentration range of 12.5 mg g−1 (Singh, Arora, Sinha, & Srivastava, 2014). Moreover, the catalyst could be recycled and reused at least five times without any noticeable decrease in catalytic activity.
Bacterial cellulose (BC) possesses numerous advantages such as high purity and crystallinity, favorable biocompatibility, low density, high porosity, durable mechanical properties, high absorption capacity, low-cost, and three dimensional interconnected structures with ultrafine nanofibers (Brandes, Carminatti, Mikowski, Al-Qureshi, & Recouvreux, 2017; Campano, Balea, Blanco, & Negro, 2016; Chawla, Bajaj, Survase, & Singhal, 2009; Qiu & Netravali, 2014; Shao, Liu, Liu, Wang, & Zhang, 2015). The high crystallinity of BC endows it with excellent physico-chemical stabilities (Fang, Zhou, Deng, Zheng, & Liu, 2016), and its hydrophilicity originating from its abundant hydroxyl groups makes it suitable catalyst support for deployment in water bodies (Costa, Gonçalves, Zaguete, Mazon, & Nogueira, 2013; Thiruvengadam & Vitta, 2013). Bacterial nanocellulose (BNC) is another family member of natural biopolymers and renewable raw materials, similar to nanoscale forms of cellulose, i.e. CNCs and CNFs that display tremendous potential for environmental and water treatment as highlighted recently (Mahfoudhi & Boufi, 2017; Voisin, Bergström, Liu, & Mathew, 2017; Wang, 2019). For the water purification application, Ma, Lou, Chen, Shi, and Xu (2019) reported a BC@zeolitic imidazolate framework-8 (ZIF-8) composite aerogel with low density below (<0.03 g cm−3), large surface area, and hierarchical porosity, which displayed prominent heavy metal adsorption performance and recyclability superior to original ZIF-8 NPs (1.2 times).
Magnetite NPs have been simply incorporated into the cellulose nanomaterial structure for their controlled retrieval through magnetic separation (Zhou, Wu, Lei, & Negulescu, 2014; Zhou, Fu, Zhang, Zhan, & Levit, 2014). Zhu et al. (2011) prepared CNFs encompassing magnetite NPs trapped inside the fibers via the growth media of CNFs making bacteria during their generation; a low energy pathway for altering cellulose nanomaterials is a major advantage although extended time for growth and up-scaling challenges may hamper the commercialization process. Bacteria-originated CNFs has been applied for removing the heavy metals (e.g. Pb2+, Mn2+, and Cr3+) and the fabricated catalyst could be recovered and reused for three cycles without noticeable drop in catalytic activity.
2.1.2.4. Removal of dyes.
Organic dyes are complex organic pollutants exemplified by cationic, anionic and/or nonionic properties that originate from several industrial sources e.g. textile, printing, pulp and paper, rubber leather tanning and cosmetic industries for coloring various products; toxic pigments and dyes are notable environmental afflictions in various parts of the world that need to be eliminated (Crini, 2006; Nasrollahzadeh, Sajjadi, Maham, Sajadi, & Barzinjy, 2018; Rafatullah, Sulaiman, Hashim, & Ahmad, 2010). It has been estimated that ~1.6 million tons of toxic dyes are generated each year and ~10–15% of aforesaid volume is released as wastewater. Various cationic dyes are eliminated deploying CNCs and CNFs modified with anionic moieties as adsorbent materials or catalysts. Carboxylation of cellulose-based nanomaterials is one of the most investigated procedures for enhancing their sorption capacity. He et al. (2013) investigated the adsorption characteristics of carboxylated nanocelluloses fabricated via a single-extraction step hydrolysis process. Carboxylated or COO-modified CNCs are prepared through an ammonium persulfate (APS) hydrolysis of microcrystalline cellulose, in which carboxyl groups could be introduced on their surface during the cellulose hydrolysis. Adsorption studies conducted for a cationic dye e.g. methylene blue (MB) confirmed that the carboxylate groups bind to positively charged dyes; the adsorption capacity of MB onto CNCs approached a balance (0.32 mmol g−1) at 22 °C after 10 min. The MB desorption from CNCs by ethanol was vastly efficient with more than 90 % dye removal up to seven desorption cycles. Similarly, Yu, Zhang, Lu, and Yao (2016) described a facile and single-step method to prepare carboxylated CNCs by deploying HCl/citric acid hydrolysis of the microcrystalline cellulose which has been utilized for the adsorption of MB; nearly complete UV degradation of methyl blue by the COO-modified CNCs, was observed after 4 h, and with an increased rate compared to other types of CNCs prepared using acids like sulfuric acid and formic acid. This result is attributed to the surface modification of carboxylated CNCs wherein additional carboxyl groups could effectively serve as a binding site for the dyes.
Zhou et al. prepared porous hydrolyzed polyacrylamide (HPAM)/CNC nanocomposite hydrogels via facile thermal treatment, and studied their activities for MB dye adsorption in aqueous solutions (Zhou, Wu et al., 2014); synergy between CNCs and HPAM can effectively increase the removal of toxic MB via the improvement in swelling properties wherein enhanced adsorption capacity for toxic dye can be attained by increasing CNCs content (~20 wt.%), raising HPAM and decreasing the pH of the prepared solution. Generally, porosity and the availability of the hydroxyl groups on surfaces of CNCs and/or CNFs can provide an outstanding mechanical template and support for nanocatalysts, thus ensuring good dispersion of NPs and stability of nanocatalysts (Mohammed et al., 2018; Xu, Nasrollahzadeh, Sajjadi et al., 2019; Zeng, Liu, Cai, & Zhang, 2010).
In another study, the fabrication of a novel CoPc@BC by covalent immobilization and decoration of amino cobalt phthalocyanine (CoPc) onto BC nanofibers has been described (Chen & Huang, 2015); ensuing nanocatalyst could be successfully deployed in the ≥90 % destruction of Rhodamine B (RhB) dye in water/wastewater using H2O2 as an oxidant within 3 h. An effective approach was disclosed by Yang et al. (2011) for growing cadmium sulfide NPs and stabilizing them through coordination effects with the bacterial CNFs via hydrothermal reaction; CdS/BCNFs hybrid composites were affirmed as robust recoverable photocatalysts for the 82 % MO degradation after 90 min exposure to visible light irradiation. The nanocomposite could be reused 5 times with no remarkable decrease of catalytic activity/efficiency. In another development, Zhang, Yu et al. (2018) and Zhang, Hou et al. (2018) prepared BC@TiO2 nanocomposite by immersing well-preserved 3D interconnected porous BC blocks into a solution of titanium source that exhibited tremendous potential as MO adsorbent. Furthermore, a novel class of robust and highly scalable polydopamine (PDA)/BNC hybrid membrane has been prepared by Derami et al. (2019) which could be effectively used for the adsorptive removal of organic dyes (rhodamine 6 G, MB, and MO) from contaminated water.; the membrane catalyst was separated and reused 10 times with no detectable decrease of the catalytic performance.
After the capture of toxic pollutants, the ability to isolate and separate them from the nanosorbent or nanocatalyst and especially the reuse of such nanomaterials are some key issues in producing and designing sustainable treatment systems. The development of novel and recoverable CNCs is of paramount importance in numerous research areas such as remediation of toxic pollutants via adsorption and degradation process, however, the recovery of nanosorbents (or nanocatalysts) can limit their practical utilization on large-scale remediation processes. One of the straightforward procedures for the fabrication of reusable adsorbents is the incorporation of pristine nanocellulose (CNCs and/or CNFs) into various nanocomposite hydrogels or polymers that can be easily recycled using sieves, filtration, and magnets, etc. These materials could also be packed within columns and applied in wastewater remediation operations. In this context, a novel class of recoverable microgel comprising pristine CNCs and amphoteric poly(vinyl amine) (PVAm) has been reported by Jin and co-workers (Jin, Sun, Xu, & Xu, 2015), which can be utilized for the adsorptive removal of anionic dyes. The protonation of amine functionalities on the microgel particle’s surface organized positively charged microgels under an acidic pH medium wherein the electrostatic attraction among negatively charged sulfate groups and protonated amine promote the adsorption of anionic dyes into/onto microgels surface.
Besides, active NPs for photocatalytic destruction of pollutants has been introduced by placing TiO2 particles on CNFs via controlled surface hydrolysis strategy (Sun, Yang, & Wang, 2010); significant ultra violet degradation of methyl orange (MO) could be detected within 20 min, that was 20 % faster compared to the rate attained with TiO2 alone. Likewise, Snyder, Bo, Moon, Rochet, and Stanciu (2013) fabricated hybrid gold/TiO2- and silver/TiO2-cellulose CNF composites which eliminated MB ~75 % and 70 %, respectively, after one hour via photocatalytic degradation and adsorption. Notably, the mechanical strength of the CNF films can be augmented by addition of metallic NPs thus rendering them recyclable and imparting them additional durability.
A variety of cellulose-based nanosorbents deployed for the elimination of dyes and heavy metals are presented in Table 2.
Table 2.
Organic dyes and heavy metal ions adsorption onto assorted cellulose-related nanosorbents.
| Cellulose-based nanosorbent | Contaminant | Max. adsorption capacity (mg g−1) or removal percent | T. (K)/pH | Ref. |
|---|---|---|---|---|
| Pristine CNFs | Ag(I) | 34.35 mg g−1 | 298/5.45 | (Liu et al., 2014) |
| Pristine CNCs | 15.45 mg g−1 | 298/6.39 | ||
| Pristine CNCs from sludge | Cu(II), Fe(III) | 25.5, 1.85 mg g−1 | 298/3.5–4.5 | (Liu, Borrell et al., 2015) |
| Pristine CNCs from bioethanol | 30.15, 4.35 mg g−1 | |||
| Pristine CNCs | MB | 118 mg g−1 | 298/9.0 | (Batmaz et al., 2014) |
| TEMPOa oxidized carboxylated CNCs | 769 mg g−1 | |||
| Pristine CNCs | Crystal violet (CV) | 185.19 mg g−1 | 303/6.0 | (Qiao et al., 2015) |
| Maleic anhydride grafted CNCs | 243.90 mg g−1 | |||
| Pristine CNFs | MB | 122.2 mg g−1 | 293/9.0 | (Chan, Chia, Zakaria, Sajab, & Chin, 2015) |
| Phosphorylated CNFs from sludge | Cu(II) | 72.75 mg g−1 | 298/3.5–4.5 | (Liu, Borrell et al., 2015) |
| Phosphorylated CNCs from sludge | 72.8 mg g−1 | |||
| Sodium substituted succinic anhydride-amended CNCs | Pb(II), Cd(II) | 465.1, 344.8 mg g−1 | 298/5.5, 6.5 | (Yu, Tong, Ge, Wu et al., 2013) |
| Succinic anhydride-modified CNCs | 367.6, 259.7 mg g−1 | |||
| Succinic anhydride modified CNCs | Cr(III) | 2.88 mg g−1 | 298/6.5 | (Singh et al., 2014) |
| Acrylamide modified CNCs | Cr(VI) | 2.77 mg g−1 | 298/2.5 | |
| Carboxylated CNFs | Cu(II) | 135 mg g−1 | 298/6.2 | (Sehaqui et al., 2014) |
| Carboxylated CNFs | 167 mg g−1 | 298/6.5 | (Ma et al., 2012) | |
| Fe-Cu@CNC | Pb(II) | 93.98 % | – | (Chen, Yu, Deutschman, Yang, & Tam, 2020) |
| Sulfonated CNFs | Au(III) | 57 and 60 mg g−1 | 295/3.26 | (Dwivedi, Dubey, Hokkanen, & Sillanpää, 2014) |
| Electrosterically stabilized CNCs | Cu(II) | 185 mg g−1 | 298/4.0 | (Sheikhi, Safari, Yang, & Van De Ven, 2015) |
| CHAb modified CNFs | Ni(II), Cd(II) | 99.33 %, 99.30 % | 298/5.0 | (Hokkanen, Repo, Westholm et al., 2014) |
| 2-Mercaptobenzamide altered itaconic acid attached magnetite CNCs | Hg(II) | 240.0 mg g−1 | 303/8.0 | (Anirudhan & Shainy, 2015) |
| Magnetic CNC@Zn-benzene-1,3,5-tricarboxylic acid | Pb(II) | 558.66 mg g−1 | 298.2/- | (Wang, Ouyang, Yang, & Omer, 2017) |
| TEMPO oxidized CNFs modified by PEI | Cu(II) | 52.32 mg g−1 | 303/5.0 | (Zhang, Zang, Shi, Yu, & Sheng, 2016) |
| Amino functionalized CNCs | Acid red GR | 555.6 mg g−1 | 298/4.7 | (Jin, Li, Xu, & Sun, 2015) |
| Au@BSA NCs loaded CNC-ALG hydrogel beads | Hg(II) | 26 mg g−1 | 298/7.0 | (Mohammed, Baidya et al., 2016) |
| Poly(itaconic acid/methacrylic acid) embedded CNCs/bentonite nanocomposite | Co(II) | 350.8 mg g−1 | 303/6.0 | (Anirudhan, Deepa, & Christa, 2016) |
| FeNP modified CNFs | As(V) | 241.8 mg g−1 | 293/2.0 | (Hokkanen, Repo, Lou, & Sillanpää, 2015) |
| Carboxylated CNFs/PVA hybrid aerogels | Hg(II), Pb(II), Cu(II), Ag(I), | 157.5, 110.6, 151.3, 114.3 mg g−1 | – | (Zheng, Cai, & Gong, 2014) |
| Carboxylated CNFs/magnetic chitosan hydrogel beads | Pb(II) | 171.0 mg g−1 | 298/4.5 | (Zhou, Fu et al., 2014) |
| CNCs/HPAMc nano-hydrogels (by casting) | MB | 326.08 mg g−1 | 298/5.0 | (Zhou, Wu et al., 2014; Zhou, Fu et al., 2014) |
| CNCs/HPAM nano-hydrogels (by electrospinning) | MB | – | 298/6.5 | (Zhou, Lee, Dooley, & Wu, 2013) |
| MnO2 coated CNFs | MB | 99.8 % | 298/9.6 | (Wang, Yadav et al., 2014; Wang, Zhang et al., 2014) |
| D-CNCs/PVAmd microgels | Congo red 4BS, reactive light-yellow K-4G, acid red GR | 869.1, 1250.9, 1469.7 mg g−1 | 298/3.5 | (Jin, Sun et al., 2015) |
| CNC@polydopamine | MB | 2066.72 mg g−1 | 298/10.0 | (Wang, Zhang et al., 2020) |
| Cellulose nanocrystals | Victoria Blue 2B, methyl Violet 2B, rhodamine 6 G | 98 %, 90 %, 78 % | r.t./5.01 | (Karim, Mathew, Grahn, Mouzon, & Oksman, 2014) |
| CMC/GOCOOHe microbeads | MB | 180.32 mg g−1 | 298/10.0 | (Eltaweil, Elgarhy, El-Subruiti, & Omer, 2020) |
| Ag, Au, Pt NPs- and Ag@Au, Ag@Pt NPs-CNF | 4-nitrophenol (4-NP) | – | r.t./- | (Esquivel-Pena et al., 2020) |
| CTABf modified CNC | CR | 448.43 mg g−1 | 298/7.5 | (Ranjbar, Raeiszadeh, Lewis, MacLachlan, & Hatzikiriakos, 2020) |
| CNC-ALG hydrogel bead | MB | 255.5 mg g−1 | – | (Mohammed, Grishkewich, Waeijen, Berry, & Tam, 2016) |
| CNF-GnPg aerogel | MB, CR | 1178.5, 585.3 mg g−1 | – | (Yu, Hu, Dichiara, Jiang, & Gu, 2020) |
| CMC/g-C3N4/ZnO | Methyl violet | 96.43 mg g−1 | 298/8.0 | (Sharma et al., 2020) |
| G-C3N4/CNCs-H hydrogel | MB | 232.558 mg g−1 | r.t./7.0 | (Wang, Li et al., 2020) |
| M3D-PAA-CCNh | MB | 332 mg g−1 | – | (Samadder et al., 2020) |
| Carboxylated cellulose fabric filter | MB, Pb(II) | 76.92, 81.30 mg g−1 | 298/5.0 | (Li, Ma, Venkateswaran, & Hsiao, 2020) |
| PAETMACi-g-CNC | Neutral reactive blue 19 (RB 19) | >80 % | 298/7.0 | (Jiang, Lou, Hua, Deng, & Tian, 2020) |
| Cellulose-modified La0.9Sr0.1FeO3 | CR | 38.46 mg g−1 | r.t./4.0 | (Ali & Al-Oufi, 2020) |
| MBCNF/GOPAj | Malachite green (MG) | 270.27 mg g−1 | 298/7.0 | (Arabkhani & Asfaram, 2020) |
| BCk@CdS nanocomposite | MB | 10.92 mg g−1 | 293/- | (Qian, Xu, Yue, Wang, & Liu, 2020) |
| CNF-Fe(0)@FeS | MB, CR | 200.0, 111.1 mg g−1 | 298/7.0, 5.0 | (Sankararamakrishnan, Singh, & Srivastava, 2020) |
| CNF/PEIl/Ag NPs aerogel membrane | 4-NP, MB, CR | 96 %, 99.2 %, 96.4 % | – | (Zhang et al., 2020) |
| TOCN/CGGm hydrogel | Cu(II), MO, thioflavin T | 498.5, 134.3, 430.2 mg g−1 | – | (Dai et al., 2020) |
| D-ZSMn/CNF, Cu- and Fe- ZSM/CNF | Rhodamine 6B, Reactive blue 4 | 34.36, 9.22 and 16.55 mg g−1 | r.t./≥ &≤7.0 | (Lakhane, Mahabole, Bogle, Khairnar, & Kokol, 2019) |
| CA-PANI/β-CDo nanofiber | MB | 49.51 mg g−1 | 298/8.0 | (Ali, El-Aassar, Hashem, & Moussa, 2019) |
| IDA@CMC-PBQp microbeads | CV | 107.52 mg g−1 | 298/8.0 | (Omer, Elgarhy, El-Subruiti, Khalifa, & Eltaweil, 2020) |
| PEI-Pt@BCq membrane | Acid black ATT | 1157.9 mg g−1 | 298/5.2 | (Huang et al., 2020) |
| α-Fe2O3 nanodisk/bacterial cellulose membrane | Orange II (OII), MO, Rhodamine B (RhB), MB, CV, MG | – | – | (Zhu et al., 2018) |
| PMPC/BNCr nanocomposite | MB, MO | 4.44 ± 0.32, 4.56 ± 0.43 mg g−1 | – | (Vilela, Moreirinha, Almeida, Silvestre, & Freire, 2019) |
| MoS2-graphene-CFPs | MB | 485.4 mg g−1 | 298/7.0 | (Gopalakrishnan, Singh, & Badhulika, 2020) |
| hPEIt modified cellulose-based bioadsorbent | Cationic bright yellow M-7 G, anionic reactive yellow X-RG, nonionic disperse brown S-3RL | 571.43, 970.87, 581.40 mg g−1 | r.t./- | (Chen et al., 2018) |
| PANi/BC mat | Cr(VI) | – | 298 ± 2/1–5 | (Jahan, Kumar, & Verma, 2018) |
| CMC-Ni-BC | MB, 2-NP | >95 %, >90 % | – | (Kamal, Ahmad, Khan, & Asiri, 2019) |
| BC/PDA/TiO2 | RhB, MB, MO | 100 %, 99.5 %, 95.1 % | – | (Yang et al., 2020) |
| BC/NiHCFu membrane | Cs(I) | 175.44 mg g−1 | 298/6.0 | (Zhuang & Wang, 2019) |
| BC-ACv | MB | 505.8 mg g−1 | – | (Khamkeaw, Jongsomjit, Robison, & Phisalaphong, 2019) |
| ZIF-67/BC/CHw aerogel | Cu(II), Cr(VI), active red X-3B | 200.6, 152.1 mg g−1, 100% | 298/6.0 | (Li, Tian et al., 2020) |
| Cellulose acetate-ceria/zirconia@Cu° NPs | Mixture of 4-NP-MB, 4-NP-RhB & 4-NP-MB-RhB | – | – | (Khan et al., 2020) |
| Cellulose/Ag3PO4 | Mixture of industrial fertilizer effluent + RhB dye | 52 % and 86 % | – | (Tavker, Gaur, & Sharma, 2020) |
2,2,6,6-Tetramethyl-1-piperidinyloxy.
Carbonated hydroxyapatite.
Hydrolyzed polyacrylamide.
Polyvinylamine.
Carboxymethyl cellulose/carboxylated graphene oxide.
Cetyltrimethylammonium bromide.
Cellulose nanofibrils-graphene nanoplates.
Polyacrylic acid-magnetic 3D crosslinkers-carboxylated cellulose nanocrystal.
Poly acryloyloxyethyltrimethyl ammonium chloride.
Magnetic bacterial cellulose nanofiber/graphene oxide polymer aerogel.
Bacterial cellulose/cadmium sulphide.
Poly(ethylene imine).
TEMPO-oxidized cellulose nanofibers/cationic guar gum.
De-aluminated ZSM-5 zeolite.
Cellulose acetate-polyaniline/β-cyclodextrin.
Iminodiacetic acid@carboxymethyl cellulose-p-benzoquinone.
Polyethylenimine caged platinum nanomaterials@bacterial cellulose.
Poly(2-methacryloyloxyethyl phosphorylcholine)/bacterial nanocellulose.
Cellulose filter paper.
Hyperbranched polyethyleneimine.
Nickel hexacyanoferrates loaded bacterial cellulose.
Activated carbon.
Zeolitic imidazolate framework-67/bacterial cellulose/chitosan.
2.1.2.5. Removal of other pollutants.
Non-biodegradable nitrophenols are extremely hazardous, and carcinogenic and are responsible for several ailments in humans; their removal from water/wastewater is of utmost importance. They have numerous applications in diverse industries e.g. synthesis of anilines as a crucial feedstock to produce pharmaceuticals, dyes, explosives, resins, agrochemicals and synthetic polymers, among others (Crini, 2006; Nasrollahzadeh, Sajjadi, Dasmeh et al., 2018; Rafatullah et al., 2010) and are on the list of regulated materials. Shi et al. synthesized CNCs-supported gold NPs with a quick swelling rate and superior catalytic prowess to catalyze the aqueous sodium borohydride (NaBH4)-mediated reduction of pollutant, 4-nitrophenol (4-NP) (Scheme S1) with turnover frequency value and maximum rate constant of 641 h−1 and 0.0147 s−1, respectively (Yan et al., 2016); synthesis was accomplished via electrospinning and thermal treatment of polyethylene glycol, CNCs and HAuCl4 at 80 °C for 60 min wherein CNCs and polyethylene glycol act as support and reductant, respectively.
Additionally, cellulosic nanomaterials can be applied for the passive nanoremediation of reactive NPs where they act as scaffolds or particle-stabilizers. These NPs are often altered by using polymers to prevent aggregation; though, these surface-bound stabilizers cover the surface of reactive particle and may hinder the degradation and sorption of targeted materials (Yu, Tong, Ge, Zuo et al., 2013). Nata, Sureshkumar, and Lee (2011) reported one-pot simple solvothermal approach by incorporating the aminated iron oxide particles in bacterial cellulose nanofibers (BCNFs) for the remediation of arsenic; hardy CNF template prohibited the aggregation of particles and simplified the amine modification of magnetite particles. Therefore, ensuing materials demonstrated extremely superior arsenic elimination capacity (36.49 mg g−1) than the bare iron oxide-based adsorbents and the nanocomposite could be reused 5 times with a minimal catalytic activity impairment. In one of the studies, Ma, Hsiao, and Chu (2012) demonstrated the capability of CNFs for eliminating radioactive uranyl ions () from solutions; ions coordinated to the carboxylate groups of TEMPO-oxidized CNFs removed more than 167 mg/g, which was 2–3 times more than traditional adsorbents including hydrogels, montmorillonite, silica and polymer particles.
Polyethylene glycol (PEG) modified CNCs composites have been prepared for adsorbing pharmaceutical compounds, including sulfa-methoxazole, acetaminophen, and N,N-diethyl-meta-toluamide (DEET) in aqueous media. Such cellulose-based nanocomposite can be applied for eliminating the hydrophobic drugs from water; PEG-functionalized CNCs could facilitate the interaction between the cellulose nano-crystals and drugs. Such composite have been prepared via carboxylation of CNCs surface via 2,2,6,6-tetramethyl-1-piperidinyloxy oxidation, tailed by covalent appendage of hydrophilic polyether diamine of molecular weight of 600 g mol−1 using sodium salt of N-(3-dimethylaminopropyl)-N′-ethyl-carbodiimide/N-hydroxysulfosuccinimide (Herrera-Morales et al., 2017).
2.2. Chitin/chitosan-based nanomaterials
2.2.1. Chemistry and properties
After cellulose, chitin and chitosan are the next most abundant bio-polymers in nature. Chitosan is a renewable and biodegradable carbohydrate and is essentially, N-deacetylated chitin, the main constituent of insect cuticles and crustaceous shells, built up from linear aminopolysaccharide of glucosamine (Fig. 3). Bioconversion of chitin into chitosan via enzymatic N-deacetylation could be achieved with chitindeacetylase. The hydrophilic functional groups, including hydroxyl and amino groups, present in chitosan cannot change its hydrophobic nature enough to allow its use for adsorption and modification (Wang & Zhuang, 2017). In general, chitin/chitosan as a biogenic raw material is of specific significance because of its abundance, high adsorption capacity and ease of modification; chitosan has been deployed in biocatalysis, wastewater treatment, drug/gene delivery, agricultural/industrial use, and cell/enzyme immobilization (Bagheri, Roostaie, & Baktash, 2014; Dotto & Pinto, 2017; Rangel-Mendez, Monroy-Zepeda, Leyva-Ramos, Diaz-Flores, & Shirai, 2009; Xu, Nasrollahzadeh, Sajjadi et al., 2019).
Fig. 3.
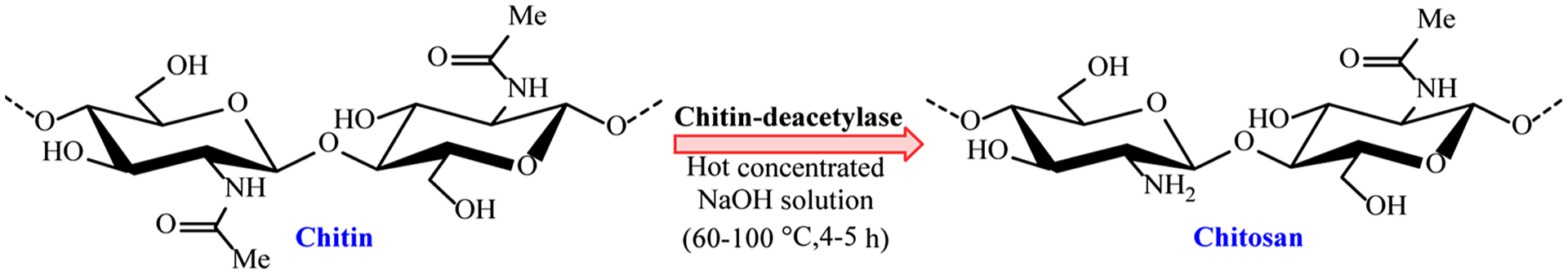
Deacetylation of chitin for the preparation of chitosan.
Chitosan-based nanomaterials have garnered significant interest as they are utilized as nanosorbents and nanocatalysts because of their distinctive physicochemical properties (Fig. 4). The presence of N-acetamido functionality is responsible for the formation of various inter/intra-molecular hydrogen bond between linear structures of chitin. Indeed, the extended hydrogen bonded chitin chains does limit their solubility in solvents, and therefore, their processing and novel appliances have been under scrutiny for the preparation of sustainable nanomaterials. Their application as eco-friendly, low-cost, sustainable and renewable resources for the synthesis of chitin/chitosan-based nanocatalysts and for the assembly of potable and safe water systems are under rigorous investigation (Crini, Morin-Crini, Fatin-Rouge, Deon, & Fievet, 2017; Krajewska, 2001; Xu, Nasrollahzadeh, Sajjadi et al., 2019).
Fig. 4.
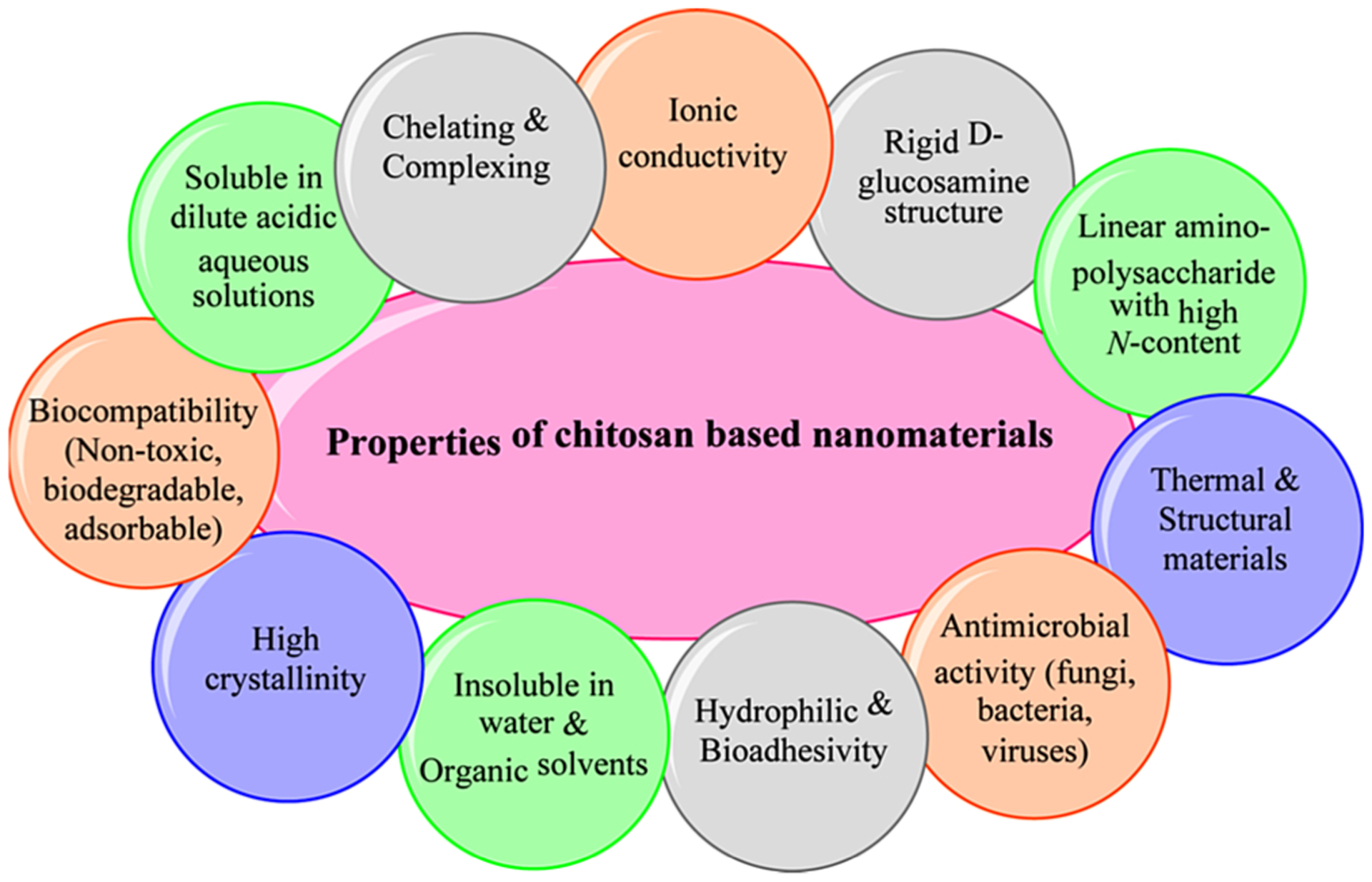
Essential physicochemical properties of chitosan.
2.2.2. Applications for water treatment
2.2.2.1. Removal of heavy metal ions.
Coagulation and flocculation of ionic substances and charged particles in wastewater has efficiently been performed with the aid of natural polymers, while diminishing the dependability on synthetic polyelectrolytes (Zemmouri, Drouiche, Sayeh, Lounici, & Mameri, 2013). Chitin, chitosan and their derived molecules have been exploited as natural and eco-friendly coagulants/flocculants to eliminate various charged particles such as dyes and metal ions from wastewater (Kanmani et al., 2017; Sami, Khalid, Iqbal, Afzal, & Shakoori, 2017). Chitosan and its derivatives present a cationic character in acidic media that facilitates their dissolution and enable ion-exchange interactions or electrostatic attraction with various anionic characters, while, the non-protonated amino groups in neutral media expedite complexation of metal ions and organic molecules. Polyvinyl alcohol-chitosan and PEG-chitosan composites have been investigated by Rajeswari et al. (2016) to remove aqueous nitrate ions with a adsorption capacity of > 35.03 and 50.68 mg g−1, respectively, while the preparation of carboxymethyl chitosan (Borsagli, Mansur, Chagas, Oliveira, & Mansur, 2015) and goethite/chitosan nano-composites (goethite NPs 10~60 nm) (Rahimi, Moattari, Rajabi, & Derakhshan, 2015) were selective towards the complexation of Cd2+/Cr6+ and Pb2+, respectively. Wang, Chen, Yuan, Sheng, and Yu (2009) reported a highly water-soluble chitosan-based flocculant by grafting it with (2-methacryloyloxyethyl) trimethyl ammonium chloride (grafting percentage >236.4 %) for the treatment of pulp mill waste-water; as-prepared flocculant revealed a more notable flocculation capacity and performance than that of polyacrylamide.
Considerable endeavors have been explored to ameliorate the experimental processes towards capacitive deionization (CDI) using a diversity of conducting polymers (e.g. polypyrrole) in view of their ion-exchange property and doped process (Abdi, Nasiri, Mesbahi, & Khani, 2017; Fang, Jiang, Luo, & Geng, 2018). Besides, the synergistic effect of polypyrrole and chitin/chitosan was described to improve the stability of polypyrrole material because of its −COOH and –NH2 (Huang et al., 2013). For example, Zhang, Xue, Li, Dai, and Zhang (2019) reported a polypyrrole(PPy)/CS/CNT nanoelectrode (34.57 nm) with various mass ratios of PPy and CS via in-situ polymerization for the adsorption of copper ion from water/wastewater by CDI process (Fig. S1); high adsorption competence of 16.83 mg g−1 for the Cu2+ removal was attained by this nanocomposite. The result of 100 cycles CV test shows that the specific capacitance of PPy/CS/CNT composite electrode decreased 13.1 % after 100 cycles, but only 3.4 % after the last 50 cycles, which indicated that this composite electrode has a good stability after 50 cycles.
The stabilization of various NPs has been achieved via their impregnation on organic renewable supports by preparation of nano-composite as shown by Chen, Cao, Quinlan, Berry, and Tam (2015) where the amino functionalization on surface of organic supports under mild conditions greatly diminished the agglomeration of metal/metal oxide NPs. The adsorption capacity of chitin/chitosan-supported NPs as nanocatalysts or nanosorbents could be further improved by a fusion of NPs and natural polymers culminating in promising natural polymer-based nanocomposites (Qiu, Ma, & Hu, 2014). Low-cost natural polymers, such as chitin/chitosan, has largely been applied as highly effective catalytic support in the heterogeneous catalysis field owing to its inherent functional groups (Murugadoss & Chattopadhyay, 2007; Wang, Zhu et al., 2017); reactive amino group-bearing chitosan has become an ideal support compared to other biopolymers. Environmental applications of bio(nano)composites of chitin/chitosan formed by combining them with diverse nanostructures, namely iron oxide (Fe3O4), titanium dioxide (TiO2), etc. have recently been reported (Anaya-Esparza et al., 2020; Li, Xiao, & Qin, 2010). For example, a novel nano-TiO2-enabled CS beads crosslinked with copper were prepared that could achieve the (photo)oxidation of toxic arsenite (As(III)) to a less-toxic and more easily adsorbed arsenate (As(V)) in UV light as well as the selective adsorption of As(III) and As(V) in presence of phosphate (Pincus, Melnikov, Yamani, & Zimmerman, 2018), which serves as a strong adsorption competitor and inhibitor of As removal in such protocols.
2.2.2.2. Removal of dyes.
Chitosan-based nanomaterials have been examined to adsorb or remove dye molecules wherein hydroxyl groups are effectively exploited in the dye adsorption, while the amine groups profoundly remain as a most active group and influences other biopolymer activities. Gibbs, Tobin, and Guibal (2003) have shown that by diminishing the acetylation degree of chitosan increases the relative proportions of amine groups available for protonation, thus favoring the adsorption of dyes like Acid Green 25. However, the alteration in these adsorption features is not proportional to the deacetylation or acetylation degree, but it varies with the nature of dyes (Saha, Ichikawa, & Fukumori, 2006) and also the repartitioning of acetyl groups in the macromolecular chains (Rinaudo, 2006), depending largely on the preparative process. Gopi and co-workers have reported the efficient wastewater treatment by using multifunctional bio-hybrid aerogels based on CNFs decorated with chitin nanocrystal (CNC) (Fig. S2a) (Gopi et al., 2017). A rare semi-square CNCs (20–100 nm) and wire-like CNFs (60–120 nm) were initially extracted from shrimp shells and corn husks, respectively (Fig. S2b). Hybrid bio-aerogels (neat AR, AR1 or AR2) were prepared with varying percentages of CNCs (0, 1% or 2%) and decorated on the CNFs via an eco-friendly freeze-drying procedure. This mixture comprised CNFs aqueous solution with well-dispersed CNCs that was frozen at about −70 °C in the dry ice-isopropanol and finally freeze dried at −88 °C under vacuum for at least 4 days. The higher amount of CNCs can lead to better alignment, organization and morphology of nanofibers in AR2, and higher crystallinity, in addition, the nanofibers orientation of AR2 mimicked the multilayer maple seed structure (Fig. S2b). The AR2 aerogel showed considerable adsorption capability for the remediation of dyes (MB and rhodamine 6 G) from aqueous solutions (Fig. S2c); the electrostatic interactions among positively charged dyes and negatively charged CNCs decorated AR2 with acetamide-enriched groups favored the dye adsorption. Besides, the hybrid bio-aerogels could be reused 5 times with no noticeable loss in activity/efficiency.
Marrakchi, Khanday, Asif, and Hameed (2016) developed a reinforced chitosan with sepiolite as an additive and epichlorohydrin as a crosslinker to fabricate crosslinked chitosan/sepiolite composites for the removal of reactive orange 16 and MB from aqueous solutions; attained maximum adsorption capacity of modified chitosan for MB and reactive orange 16, being 40.99 mg g−1 and 190.97 mg g−1, respectively, at initial dye concentration of 100 mg L−1 and adsorbent dosage of 1 g L−1 at 30 °C for 30 h. The adsorption processes were best explained using pseudo-second-order kinetics and Freundlich model that provided a finer explanation of the adsorption process for both the organic dyes. The functioning of this crosslinked chitosan composite was superior to what has been reported in earlier studies (37.04 mg g−1) (Xie, Li, Chi, & Wu, 2013), (11.94 mg g−1) (Yao et al., 2014) and (24.690 mg g−1) (Zeng et al., 2015) for the removal of MB. In addition, the elimination of Acid Red-2 from textile wastewater by a glutaraldehyde crosslinked magnetic chitosan nanocomposite has been investigated (Kadam & Lee, 2015); it could effectively adsorb 91.6 % textile pollutant, while iron oxide could adsorb only 16.4 %. This improved performance of magnetic chitosan nanocomposite in view of the available free amino and hydroxyl groups and 96 % pollutant removal with 100 % recovery bodes well for its practical uses.
Moreover, the combination of Fe3O4 and chitin/chitosan can afford fascinating magnetic support towards hassle-free separation of nano-catalysts. Chang and Chen (2005) designed carboxymethylated chitosan-conjugated magnetic nanosorbents (~13.5 nm) for the elimination of anionic dyes from aqueous solutions; excellent adsorption efficiencies were attained (1471 and 1883 mg g−1) for acid green 25 and crocein orange G, respectively.
2.2.2.3. Removal of other pollutants.
The adsorption of pesticides onto low-cost materials could help effectively remediate contaminated waters; particularly, nanomaterials, nanosorbents and polysaccharide-based adsorbents display high performance in eliminating pesticides from water bodies as exemplified by chitin and chitosan towards the biosorption of pesticides. Indeed, the presence of hydroxyl groups in chitin/chitosan determined its conformation and also the stereochemistry of chemical transformations and kinetics. In this respect, chitosan removed more than 90 % of oxadiazon (herbicide) from aqueous solutions (Arvand et al., 2009) wherein strong binding of oxadiazon to the chitosan was observed (chemisorption). Moreover, 76.2 % of atrazine (herbicide) could be removed (with a maximum adsorption capacity of 17.92 mg g−1) from aqueous solutions by chitosan/modified sepiolite (Liu, Chen, Cui, & Liu, 2015).
Modified chitosan nanomaterial matrixes can be applied for the biosorption of organic/inorganic contaminants; sustainability of chitosan and its derivatives can be boosted by mixing with reinforcement and/or supporting matrixes like crosslinkers and polymers. This entails the presence of at least two reactive sites or functional groups in linkers to suitably transform the chitosan by producing bridges amongst their polymeric chains and/or neighboring molecules (Xing, Ju, Yang, Xu, & Qian, 2013). The used crosslinkers for buttressing chitosan namely glutaraldehyde, silicate, tripolyphosphate, starch, polyvinyl alcohol and cellulose must enable the flow of organic/inorganic contaminants towards the charged particles of the supported chitosan and concomitantly ensuring the physicochemical stability of chitosan (Xing et al., 2013). To ensure the adsorption competence close to the parent chitosan, the use of various additives like alginate (Nadavala, Swayampakula, Boddu, & Abburi, 2009), polyethylenimine, chloroacetic acid and Fe3O4 is deemed necessary. The adsorption efficiency depends on numerous factors such as the adsorbent characteristics, the degree of crosslinking, crystallinity and the stiffness of the chitosan linkages, and the chemistry of pollutant (Alaba et al., 2018). Mi, Shyu, Chen, and Lai (2002) reported a novel protocol for the synthesis of bundle-like porous chitosan beads via a phase inversion wet process; this chemically modified chitosan could absorb anti-inflammatory drug such as indomethacin.
Chitin/chitosan-supported metal/metal oxide NPs have displayed high performances for removing pollutants from water/wastewater; indeed, the synergistic effects of chitin/chitosan and nanostructures can improve (nano)materials in terms of antimicrobial, UV blocking, and magnetic properties. In this context, Mujeeb Rahman et al. (Mujeeb Rahman, Muraleedaran, & Mujeeb, 2015) studied the antimicrobial properties of ZnO NP reinforced chitosan nanocomposites which could be utilized as photocatalytic and/or natural antimicrobial agents; superior antimicrobial activities against gram-negative and gram-positive bacteria (E. coli and S. aureus) was observed compared to chitosan itself. Utilizing the well-developed preparative procedures for chitin/chitosan-based (nano)materials, multifunctional chitosan/TiO2 (nano)composite photocatalysts could be easily attained owing to the miscibility between CS and hydrophilic TiO2 NPs (Wiaącek, Gozdecka, & Jurak, 2018).
In one study, TiO2 NPs (1% w/v) were embedded in two different matrixes, viz. chitosan and polyvinyl alcohol-chitosan blend (PVA-CS), through a precipitation procedure using an alkali/solvent medium (Neghi, Kumar, & Burkhalov, 2019) where the nanocomposites/blends exhibited high metronidazole (MNZ) removal efficiency (76.1 % and 63.7 % in PVA-CS-TiO2 and CS-TiO2 systems, respectively) from aqueous solutions under UV irradiation as compared to the TiO2 NPs suspension alone. As a result, PVA-CS-TiO2 showed enhanced stability than CS-TiO2 in an aqueous solution at different pH values and could be reused under UV exposure up to 15 cycles as an effective photocatalyst without significant loss of catalytic performance. In a recent study, Shoueir et al. (Shoueir, Kandil, El-hosainy, & El-Kemary, 2019) reported an efficient visible-light photocatalyst by stacking a layer of nano-structured Au@TiO2 on a chitosan fiber substrate; the as-prepared plasmonic fiber displayed catalytic activities in visible light for the degradation of various water pollutants (MB, MNZ, and carbofuran (CBN)) and for the Cr(VI) reduction (~98.9 %) in presence of citric acid (pH = 1) within 21 min. The plasmonic fiber photocatalyzed the degradation of MB within 12 min under visible irradiation using a low catalyst dosage of 1 × 10−3 g L−1, with an efficiency of 98.8 %. In the case of MNZ and CBN, the degradation reaction times were longer, namely 260 min and 130 min, respectively, which could be improved by deploying H2O2 in these systems, when the photocatalytic degradation degree of MNZ reached 96 % and 98.3 % for CBN.
Additionally, by designing the core/shell structures, the surface functionalities of the natural polymers could be made easily available to metal/metal oxide NPs (Ghosh Chaudhuri & Paria, 2012). In this regard, Antony et al. (Antony, Marimuthu, & Murugavel, 2019) developed a facile and novel approach for anchoring bimetallic AgNi NPs (20~25 nm) on Fe3O4@chitosan core/shell support (Fe3O4@CS_AgNi) as a heterogeneous retrievable nanocatalyst (Fig. 5); magnetic nanocomposite has been applied for the rapid and nearly quantitative reduction of 4-nitrophenol (4-NP) using NaBH4 within 10 min under ambient conditions. Furthermore, the heterogeneous nanocatalyst could be simply recovered/separated using an external magnet and reused seven times.
Fig. 5.
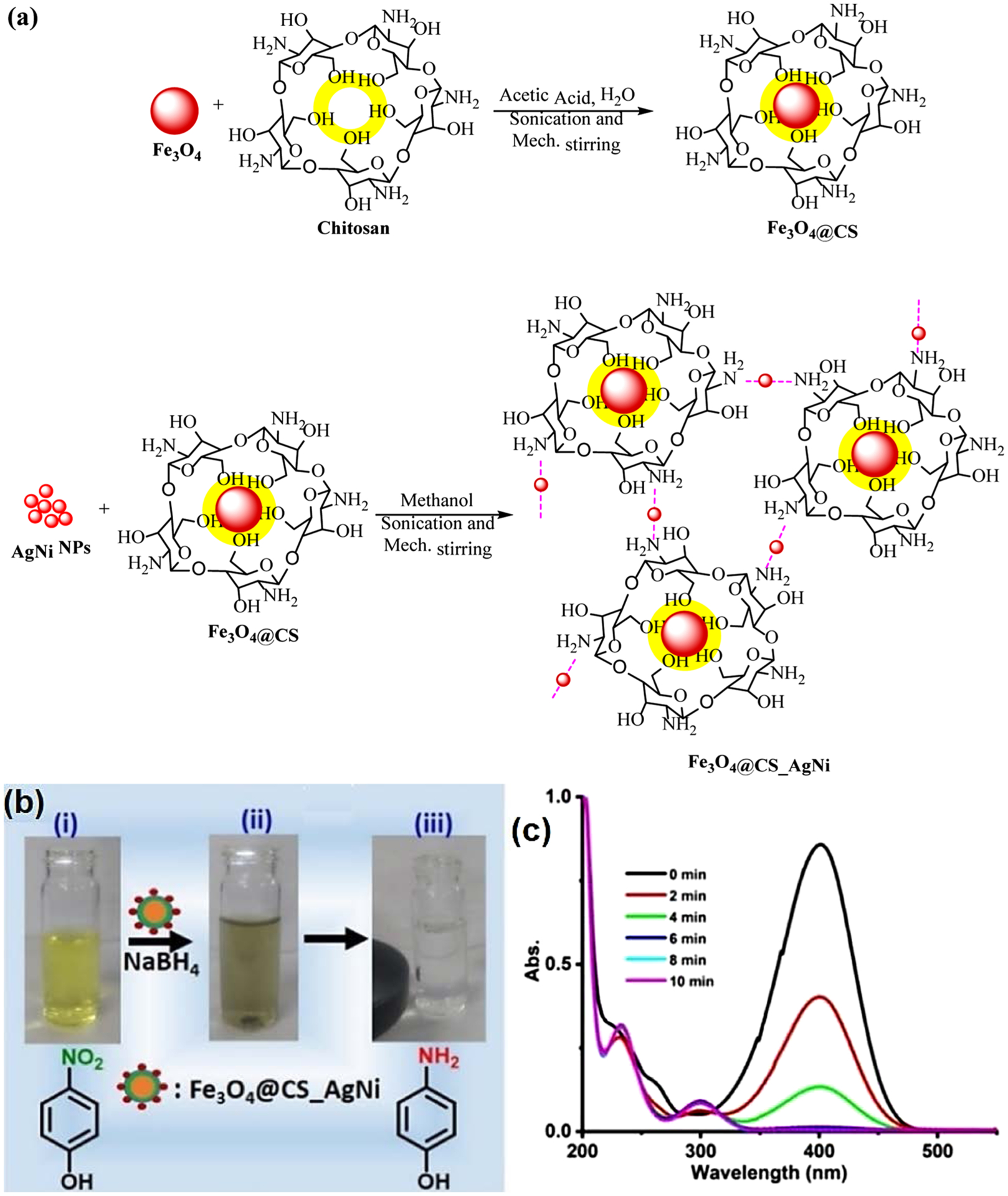
Preparation of Fe3O4@CS_AgNi nanocomposite, (b) Color changes observed during 4-NP reduction; (i) bare 4-NP, (ii) 4-NP + magnetic nanocomposite + NaBH4, (iii) easy separation of magnetic nanocomposite via a magnet after the complete 4-NP reduction, (c) time dependent evolution illustrating the 4-NP reduction to 4-AP catalyzed by Fe3O4@CS_AgNi nanocomposite. Reproduced with permission from Ref. (Antony et al., 2019).
Applications of wide ranging chitin/chitosan-based nanomaterials for the removal of water contaminants and toxic pollutants such as various dyes, heavy metals and pharmaceutical materials are summarized in Table 3.
Table 3.
Various chitin and chitosan-based nanomaterials used for water/wastewater treatment.
| Chitin and chitosan-based nanomaterials | Contaminants | Ref. |
|---|---|---|
| Chitosan films containing urea modified TiO2 | MG | (Pérez-Obando et al., 2019) |
| CS/MCM-41/nano-γ alumina | MB | (Teimouri, Ghased, Nasab, & Habibollahi, 2019) |
| Chitosan clay nanocomposites | Cu(II) | (Azzam et al., 2016) |
| Chitosan-polyethyleneimine-graphene oxide nanocomposite membrane coating | Cr(VI) and Cu(II) | (Bandara, Nadres, & Rodrigues, 2019) |
| Carboxymethyl chitosan coated nano zerovalent iron beads | Cr(VI) | (Xie et al., 2018) |
| Chitosan modified with polyhexamethylene biguanide | Cr(VI) | (Aslani, Kosari, Naseri, Nabizadeh, & Khazaei, 2018) |
| Chitosan/organic rectorite-Fe3O4 composite microspheres | Cu(II) and Cd(II) | (Xie et al., 2015) |
| Nano-ZnO/chitosan microspheres | MO | (Zhong, Zhong, Huo, Yang, & Li, 2020) |
| Attapulgite/CoFe2O4@SiO2-chitosan/EDTA | Cd(II) | (Wang, Ran et al., 2020; Wang, Zhou et al., 2020) |
| Polymaleic acid-chitosan microspheres | Cd(II) | (Yu et al., 2017) |
| Cu@chitosan-silica nanoparticles | 1,1-Dimethylhydrazine | (Wang et al., 2019) |
| Chitosan-coated fly ash | Cr(VI) | (Wen, Tang, Chen, & Gu, 2011) |
| Polyvinylidene fluoride/chitosan/dopamine | MB and orange G | (Zhang, Li et al., 2019) |
| Bilayer amino-functionalized cellulose nanocrystals/chitosan composite | Diclofenac sodium | (Hu et al., 2019) |
| Zinc ferrite-CS, Nickel ferrite-CS, Cobalt ferrite-CS | Fluoride | (Kumar, Dosanjh, & Singh, 2019) |
| Chitosan-magnetite nanocomposite | Cr(VI) | (Bavasso, Vuppala, & Cianfrini, 2019) |
| Chitosan-4-nitroacetophenone/CuO-CeO2-Al2O3 and Chitosan-4-nitroacetophenon/CuO-CeO2-Fe2O3 | Red 60 | (Mohamed et al., 2019) |
| Fe3O4-chitosan micro- and nanoparticles | Bromothymol Blue | (Akın Sahbaz, Yakar, & Gündüz, 2019) |
| MIL-100(Fe) and nano-Fe3O4 onto the chitosan | Sb(III) | (Xiong et al., 2020) |
| Nano titanium oxide/chitosan/nano-bentonite | Levofloxacin and Ceftriaxone | (Mahmoud, El-Ghanam, Mohamed, & Saad, 2020) |
| Chitosan/cerium oxide/iron oxide nano-composite | Cr(VI) and Co(II) | (Farokhia, Parvareha, & Moravejia, 2019) |
| Chitosan supported ZnO and Ce-ZnO nano-flowers | Malachite green | (Saad et al., 2020) |
| Chitosan nano zerovalent iron activated carbon composite beads | Cu(II) | (Sikdera et al., 2019) |
| Salicylaldehyde functionalized chitosan NPs | Cu(II), Cd(II) and Pb(II) | (Hussain, Musharraf, Bhanger, & Malik, 2020) |
| 2D Ag-TiO2/γ-Al2O3/Chitosan nano-composite | Nitrate | (Zarei et al., 2020) |
| Chitosan/silver nanoparticle/copper nanoparticle/carbon nanotube multifunctional nano-composite | Cu(II), Cd(II) and Pb(II) | (Alsabagh, Fathy, & Morsi, 2015) |
| Zirconium chitosan composite | Cr(VI) | (Zhang, Xia, Teng, Liu, & Zhang, 2013) |
| Wet-spun nanoTiO2/chitosan nanocomposite fibers | Free fatty acids | (Bao et al., 2019) |
| MnFe2O4 impregnated chitosan-microspheres | MB | (Jyothi et al., 2019) |
| Humic acid modified magnetic chitosan NPs | Uranium | (Basu, Saha, Pimple, & Singhal, 2019) |
| Pd NPs@Fe3O4/CS-AG microcapsules | 4-NP | (Baran & Nasrollahzadeh, 2019) |
| Chitosan-zirconium phosphate nanostructures | Reactive blue-21, Reactive Red 141, Rhodamine-6 G | (Bhatt, Ageetha, Rathod, & Padmaja, 2019) |
| Chitosan grafted thin film nanohydrogel | Cr(VI) | (Sethy, Pradhan, & Sahoo, 2019) |
| Nanoscale zero-valent iron loaded chitosan | U(VI) | (Zhang, Zhao et al., 2019; Zhang, Ma et al., 2019) |
| Functionalized chitosan clinoptilolite nanocomposites | Nitrate | (Yazdi, Anbia, & Salehi, 2019) |
| Chitosan coated magnetic NPs | Cu(II) | (Zhou, Nie, Branford-White, He, & Zhu, 2009) |
| Nickel oxide/chitosan nano-composite | Zn(II) | (Abdolmohammad-Zadeh, Ayazi, & Naghdi, 2019) |
| Magnetic β-cyclodextrin-chitosan/graphene oxide | MB | (Fan, Luo, Sun, Qiu, & Li, 2013) |
| Magnetic graphene/chitosan | Acid Orange 7 | (Sheshmani, Ashori, & Hasanzadeh, 2014) |
| Carboxymethyl chitosan coated Fe3O4 NPs | Direct Red 16 | (Zinadini et al., 2014) |
| CS/β-CD/Nano-ZnO composite | CR | (Yan, Zhang, & Li, 2018) |
| Ag2O/TiO2 modified chitosan | MO | (Zhao, Tao, Xiao, & Su, 2017) |
| NiFe2O4 nanocomposite grafted chitosan | Cr(VI) | (Zhang, Wu, & Fan, 2019) |
| Thiocarbohydrazide chitosan gel | Cr(VI) | (Li et al., 2017) |
| 3,5-Dinitrosalicylic acid/chitosan/MnFe2O4 | MB | (Shoueir et al., 2018) |
| Chitosan zinc oxide nano-beads | MB and safranin | (Roshitha, Mithra, Saravanan, Sadasivam, & Gnanadesigan, 2019) |
| Chitin/chitosan nano hydroxyapatite composite | Cu(II) | (Gandhi, Kousalya, & Meenakshi, 2011) |
| Chitin powder | Ag(I) | (Songkroah, Nakbanpote, & Thiravetyan, 2004) |
| Chitosan gel beads | Ag(I) | (Zhang, Helleur, & Zhang, 2015) |
| Chitosan/triethanolamine composite | Ag(I) | (Zhang et al., 2012) |
| Thiol-functionalized chitin nanofibers | As | (Yang et al., 2015) |
| Chitosan-coated biosorbent | As | (Boddu, Abburi, Talbott, Smith, & Haasch, 2008) |
| Fly ash coated by chitosan | As | (Adamczuk & Kołodyńska, 2015) |
| Chitin | Au | (Côrtes, Tanabe, Bertuol, & Dotto, 2015) |
| Chemically modified chitosan | Au | (Donia, Atia, & Elwakeel, 2007) |
| Chitosan derivative | Au | (Wang et al., 2012) |
| Glycine crosslinked chitosan resin | Au | (Wang et al., 2012) |
| Chitosan films | V | (Cadaval, Dotto, Seus, Mirlean, & de Almeida Pinto, 2016) |
| Ti-doped chitosan bead | V | (Liu & Zhang, 2015) |
| Protonated chitosan flakes | V | (Padilla-Rodríguez et al., 2015) |
| N-citryl chitosan | V | (Mujeeb, Alikutty, & Muraleedharan, 2014) |
| Chitin networks | U | (Schleuter et al., 2013) |
| Amidoximated chitosan polyacrylonitrile | U | (Xu et al., 2015) |
| Chitosan/bentonite composite | U | (Anirudhan & Rijith, 2012) |
| Magnetic chitosan resin | U | (Zhou, Shang, Liu, Huang, & Adesina, 2012) |
| Chitosan saturated montmorillonite | Pb(II) | (Hu, Zhu, Cai, Hu, & Fu, 2017) |
| Modified chitosan/CoFe2O4 particles | Pb(II) | (Fan et al., 2017) |
| Thiosemicarbazide-modified chitosan | Pb(II) | (Li, Zhang, Li, Wang, & Ali, 2016) |
| Magnetic chitosan/clinoptilolite/magnetite | Pb(II) | (Javanbakht, Ghoreishi, Habibi, & Javanbakht, 2016) |
| Magnetic chitosan | Hg(II) | (Kyzas & Deliyanni, 2013) |
| Coarse chitin | Hg(II) | (Barriada, Herrero, Prada-Rodríguez, & de Vicente, 2008) |
| Raw chitin and surface-modified chitin | Co(II) | (Dotto, Cunha, Calgaro, Tanabe, & Bertuol, 2015) |
| Chitosan polymethacrylate nanoparticles | Co(II) | (Shaker, 2015) |
| Modified chitosan resin | Co(II) | (Monier, Ayad, Wei, & Sarhan, 2010) |
| Cellulose acetate/chitosan/single walled carbon nanotubes/Fe3O4/TiO2 | As(V), Cr(VI), MB, CR | (ZabihiSahebi et al., 2019) |
| Titanium dioxide/chitosan/poly(lactide-co-caprolactone) composite membrane | Cu(II) | (He et al., 2019) |
| Molecularly imprinted polymer (MIP) chitosan-TiO2 nanocomposite | Rose Bengal | (Ahmed, Abdelbar, & Mohamed, 2018) |
| TiO2(KH-570)-g-(chitosan-glycidyl methacrylate) | Toluene, Pb(II) | (Chen, Song, Huang, & Wang, 2019) |
| TiO2 doped chitosan microspheres supported on cellulose acetate | MO | (Shi, Zhang, Ma, Xiang, & Li, 2019) |
| Chitosan tripolyphosphate/TiO2 nanocomposite | Reactive orange 16 | (Abdulhameed, Mohammad, & Jawad, 2019) |
| Schiff’s base cross linked chitosan-glutaraldehyde TiO2 nanoparticles | Reactive red 120 | (Jawad, Mubarak, & Abdulhameed, 2020) |
| Hybrid crosslinked chitosan-epichlorohydrin/TiO2 nanocomposite | Reactive red 120 | (Jawad, Mubarak, & Abdulhameed, 2020) |
| Chitosan ethylene glycol diglycidyl ether/TiO2 nanoparticles | Reactive orange 16 | (Abdulhameed, Jawad, & Mohammad, 2019) |
| TiO2 supported 3D printed chitosan scaffolds | Amoxicillin | (Bergamonti et al., 2019) |
| Hybrid chitosan-TiO2/ZnS | Aromatic amines, carboxylic acids | (Jbeli, Ferraria, do Rego, Boufi, & Bouattour, 2018) |
| Crosslinked magnetic EDTA/chitosan/TiO2 | Phenol, Cd(II) | (Alizadeh, Delnavaz, & Shakeri, 2018) |
| 2D Ag-TiO2/γ-Al2O3/chitosan | Nitrate | (Zarei et al., 2020) |
| Chitosan-AgCl/Ag/TiO2 | Toluidine, salicylic acid, 4-aminomethyl benzoic acid | (Jbeli, Hamden et al., 2018) |
| Chitosan beads | Tartrazine, amido black, CR | (Mincea, Patrulea, Negrulescu, Szabo, & Ostafe, 2013) |
| Chitosan films | Mixture of Diclofenac and Ketoprofen | (Rizzi et al., 2019) |
2.3. Starch-based (nano)materials
2.3.1. Chemistry and properties
Starch, a natural, abundant, renewable, biocompatible and biode gradable biopolymer is present in sundry plants as a reserve carbohydrate and is commonly found in many parts of plants such as stalks, roots, and crop seeds; main sources being cassava, wheat, rice, maize or corn, and potatoes, among others. Starch granules have a 3D architecture with crystallinity in the range of 15–45 % and comprise d-glucose units with bio-macromolecules including amylopectin, branched (1→6) α-d-glucan, amylose, and linear (1→4)-linked α-d-glucan (Visakh, Mathew, Oksman, & Thomas, 2012; Zobel, 1988). Microcrystalline starch, starch nanocrystal, starch crystallite, and hydrolyzed starch all embody the crystalline part of the starch generated via hydrolysis (Le Corre, Bras, & Dufresne, 2010); modes for preparing crystalline and amorphous starch nanostructures with varying morphologies and crystallinities are shown in Fig. S3.
The mixing of fibrous clays with natural polymers is an appealing alternative for removing pollutants from water. Biopolymers e.g. guar gum or starch can be combined with polyacrylic acid (PAA) and polyacrylamide (PAAm) to develop eco-friendly and biodegradability superabsorbents (Li, Liu, & Wang, 2005). Generally, the incorporation of clays has boosted water absorption rate and water absorbency of these catalytic systems as noted for the starch/g-PAAm and guar gum-g-PAA (Ruiz-Hitzky et al., 2013). For example, the effect of sepiolite modification with cationic starch on the physical properties such as increased Young’s modulus of the composites obtained as a reinforced starch film was investigated (Chivrac, Pollet, Schmutz, & Avérous, 2010).
Among the naturally occurring polymers, starch polysaccharides; especially starch nanocrystals, have been increasingly utilized as ideal supports to afford environmentally benign and practical catalyst systems due to their high surface area, high abundance, non-toxicity, low cost, renewability, and biocompatibility (Ghaderi, Gholinejad, & Firouzabadi, 2016; Gholinejad, Saadati, Shaybanizadeh, & Pullithadathil, 2016; Herreros-López et al., 2016). Nanocrystalline starch is ideal for the reinforcement of a biopolymer compared to amorphous or amorphous starch (Angellier, Molina-Boisseau, & Dufresne, 2005; Jenkins, Cameron, & Donald, 1993). Functionalized natural starches can generally be attractive supports for various colloidal metal NP-based catalysts owing to their abundance, biocompatibility, and biodegradability.
2.3.2. Applications for water treatment
2.3.2.1. Removal of organic pollutants.
The modified starch had significantly greater water binding capacities than native starch and their amylase digestibility decreased as their degree of crosslinking increased (Jyothi, Moorthy, & Rajasekharan, 2006); for example, epichlorohydrin is the most familiar crosslinking agent that can be utilized for natural polysaccharides. Guo, Li, Liu, Meng, and Tang (2013) synthesized a crosslinked porous starch by crosslinking corn starch with epichlorohydrin and then hydrolyzing it with α-amylase, ensuing catalyst could be successfully utilized in MB adsorption from water with a maximum adsorption capacity from Langmuir isotherm model being 9.46 mg g−1 at 293 k.
(Photo)catalysts, often deployed for (photo)degradation of environmental contaminants, are commonly metal oxide/metal acid salts of n-type semiconductors, including TiO2, Fe2O3, ZnO, etc; (Rostami--Vartooni, Nasrollahzadeh, Salavati-Niasari, & Atarod, 2016; Wang, Wang et al., 2018; Wang, Li et al., 2018) relatively inexpensive TiO2 NPs have been extensively studied as environmentally friendly (photo) nanocatalysts owing to their nontoxicity, and stability (Al-Harbi, Kosa, Abd El Maksod, & Hegazy, 2015; Hassan, Chen, Liu, Zhu, & Cai, 2014; Rostami-Vartooni et al., 2016). Starch as a biodegradable and renewable raw material, offers additional sustainability/stability to the nano-particles similar to graphene (Doustkhah & Rostamnia, 2016; Ye, Hao, Liu, Li, & Xu, 2017) and carbon nanotubes (Ihsanullah, 2019). The modification of starch can be accomplished easily because of its hydroxyl groups which have strong bonding with diverse functional groups and are amenable to easy chemical transformation. Guo, Wang, Zheng, and Jiang (2019) investigated the photodegradation and adsorption of cationic golden yellow X-GL/cationic yellow 28 dye from water/wastewater by TiO2 NPs (~10 nm) loaded onto the crosslinked carboxymethyl starch (CCMS) surface (TiO2 NPs/CCMS) as a novel biosorbent by the sol-gel technique (Fig. S4); TiO2 NPs/CCMS was reused for four successive cycles.
Negatively charged starch can efficiently adsorb various cationic dyes (Huang, Chang, Lin, & Dufresne, 2014; Pourjavadi, Abedin-Moghanaki, & Tavakoli, 2016). In a related study, Guo, Wang, Zheng, and Jiang (2019) prepared crosslinked cationic starch from corn starch and 3-chloro-2-hydroxypropyl trimethylammonium chloride and epichlorohydrin as cationic etherification and crosslinked agents, respectively. Crosslinked cationic starch was applied to eliminate reactive golden yellow SNE dye from aqueous solutions and its maximum adsorption capacity was found to be 208.77 mg g−1 at 308.15 K. Besides, Pourjavadi et al. (2016) functionalized magnetic crosslinked starch materials with PVA modified by chlorosulfonic acid based vinyl acetate copolymerization onto crude starch (sulfation of its hydroxyl groups) to generate MNPs@Starch-g-poly(vinyl sulfate) nanocomposite (Fig. 6). It can effectively eliminate cationic dyes such as MG and MB from water; excellent adsorption capacities of 567 and 621 mg g−1, respectively, were demonstrated and up to 90 % of MB and MG dyes could be removed from the solution by the regenerated adsorbent even after five cycles of adsorption-desorption.
Fig. 6.
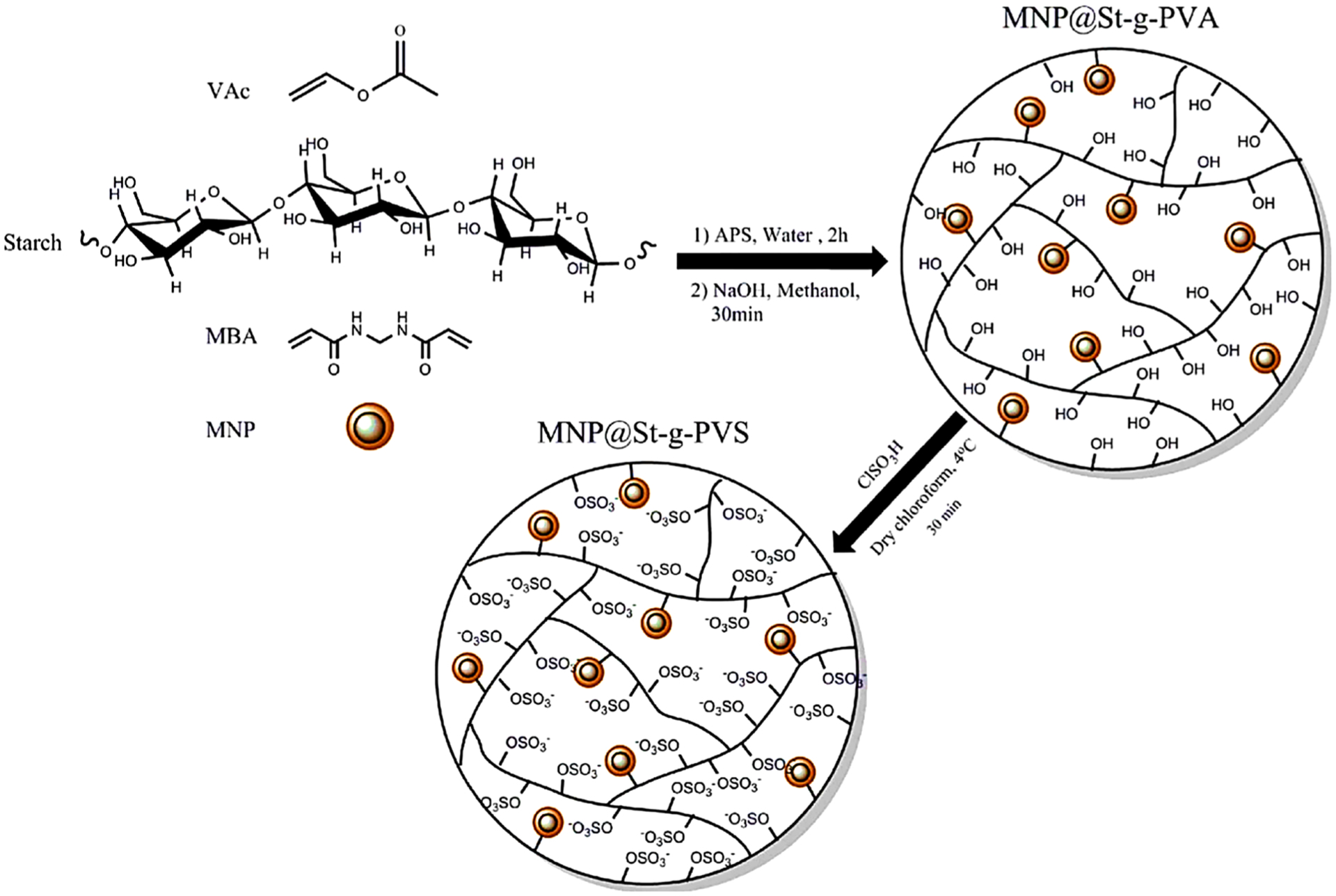
The MNP@St-g-PVS preparation. Reproduced with permission from Ref. (Pourjavadi et al., 2016).
The utilization of metal/metal oxide NPs on surface of biopolymers for the development of easily recoverable magnetic nanocatalysts has led to a dramatic expansion of their potential applications; in this regard, a variety of surface modified magnetic NPs with biodegradable polymers have been utilized for environmental remediation. Generally, starch nanocrystals help conceive more sustainable solutions to current technological challenges. Very recently, Sharma, Bhardwaj, Kour, and Paul (2017) fabricated a series of versatile magnetic Pd NP catalysts functionalized with starch and amine (Pd NPs@Fe3O4-NH2/Starch); they accomplished the reductive amination of nitroarenes in EtOH:H2O at ambient temperature with reuse for five successive cycles. Nevertheless, the adsorption capacity of easily retrievable magnetic nano-composites/nanosorbents functionalized with crude biopolymers is low which could be ameliorated by chemical modification. For instance, magnetic CMS/poly(vinyl alcohol) hydrogel (mCMS/PVA) was fabricated for MB removal from wastewater (Gong, Zhang, Cheng, & Zhou, 2015) and the catalyst was reused for eight successive cycles.
2.3.2.2. Removal of inorganic pollutants.
Other researchers (Sekhavat Pour & Ghaemy, 2015) have studied the adsorption of Cu2+, Pb2+, and Cd2+ contaminants from drinking water by versatile magnetic nano-composite hydrogel bead (mCVP) based on PVA/CMS-g-poly(vinyl imidazole) as is illustrated in Fig. S5. The adsorption capacities of mCVP beads for Cu2+, Pb2+, and Cd2+ were found to be 83.6, 65, and 53.2 mg g−1, respectively with reuse of nanocomposite for four successive cycles.
Several studies on the documented exploitation of starch-based (nano)materials for handling of organic/inorganic contaminants in wastewater has been summarized in Table 4.
Table 4.
Various starch-based nanomaterials used for water/wastewater treatment.
| Chitin, chitosan and starch-based nanomaterials | Contaminants | Ref. |
|---|---|---|
| Starch stabilized Fe° NPs | Cr(VI) | (Alidokht, Khataee, Reyhanitabar, & Oustan, 2011) |
| Monodisperse functional magnetic dialdehyde starch nanocomposite | Hg(II) | (Wang et al., 2015) |
| Ag NPs base starch/PEG-polyacrylic acid hydrogel | Hg(II) | (Saberi, Sadeghi, & Alipour, 2020) |
| Fe3O4 based starch-poly (acrylic acid) nanocomposite hydrogel | Cu(II), Pb(II), Methylene Violet and Congo Red | (Saberi, Alipour, & Sadeghi, 2019) |
| Oxidized starch NPs | Urea | (Abidin et al., 2018) |
| Starch stabilized nanoscale zero-valent iron | Cr(VI) | (Chen, Xie et al., 2019) |
| Starch modified nano zero-valent iron | Cr(VI) | (Dong et al., 2016) |
| Starch, carboxymethyl cellulose CMC-stabilized nano zero-valent iron | Sulfamethazine | (Dong et al., 2020) |
| Starch modified nanoscale zero-valent iron | Acid Blue-25 | (Elkady, Shokry, El-Sharkawy, El-Subruiti, & Hamad, 2019) |
| Starch stabilized nanoscale zero-valent iron | Nitrate | (Zhou, Sun, Chen, Wang, & Yang, 2017) |
| Na-montmorillonite NPs/P (acrylic acid-acrylamide)-g-starch | Safranin | (Zarei, Sadeghi, & Bardajee, 2018) |
| Starch-modified magnetic Fe° NPs | Naphthalene | (Malekzadeh, Nejaei, Baneshi, Kokhdan, & Bardania, 2018) |
| Starch- stabilized Fe° NPs | Nitrate | (Rajab Beigy, Rasekh, Yazdian, Aminzadeh, & Shekarriz, 2018) |
| Superparamagnetic starch functionalized maghemite NPs | Cr(VI) | (Singh, Tiwary, & Sinha, 2015) |
| Starch coated Fe3O4 magnetic NPs | Optilan Blue | (Stan et al., 2019) |
| Mungbean starch/PVA/ZnS bionanocomposite | Bisphenol A & MO | (Yun, Kim, Shim, & Yoon, 2018) |
| γ-Fe2O3@starch | Arsenic | (Siddiqui et al., 2020) |
| AgNPs-base starch/PEG-poly (acrylic acid) hydrogel | Hg(II) | (Saberi et al., 2020) |
2.4. Gum-based (nano)materials
2.4.1. Chemistry and properties
Gums are an outstanding representative of eco-friendly, green bio-polymers and these natural polysaccharides and their derivatives have been used to produce a large number of materials for varied applications. Guar gum (GG), a natural water soluble, biodegradable, mucoadhesive, polysaccharide, is obtained from the endosperm of guar beans, comprising linear chains of D-mannopyranosyl with d-galactopyranosyl units. Gum arabic (GA) or acacia gum is a branched heteropolysaccharide and a complex exudate of Acacia seyal and Acacia senegal trees composed of d-galactopyranosyl units and is one of the oldest amongst all known gums with broad applications in pharmaceutical and food industries (Padil, Senan, & Černík, 2019; Patel & Goyal, 2015; Yadav, Igartuburu, Yan, & Nothnagel, 2007).
GG and GA have been extensively used as a binder, ion exchange resin and dispersing agent, thickening agent due to their good capability to alter rheological properties (Iqbal & Hussain, 2013; Miao et al., 2018; Patel & Goyal, 2015; Soumya, Ghosh, & Abraham, 2010). They have also been applied for stabilization or the reduction of nanomaterials and ordained to effectively stabilize magnetic NPs (Kattumuri et al., 2007; Padil, Wacławek, Černík, & Varma, 2018; Williams, Gold, Holoman, Ehrman, & Wilson, 2006) besides being used as additive, emulsifying and reducing agent, and potential stabilizer in preparation of various nanocatalysts (Devi et al., 2011; Padil et al., 2018), and as biosorbent in various applications (Fig. 7) (Sharma et al., 2018).
Fig. 7.
Applications of guar gum. Reproduced with permission from Ref. (Sharma et al., 2018).
One of the straightforward biological methods for the assembly of inorganic materials is the biomimetic synthesis of various metal NPs on the surfaces of branched natural polysaccharides which serve as reducing, supporting, additive, emulsifying, and stabilizing agents; metal NPs have been stabilized on gum acacia (Chasteen & Harrison, 1999; Devi et al., 2011; Xie, Lee, Wang, & Ting, 2007). Along this theme, Supriya, Srinivas, Chowdeswari, Naidu, and Sreedhar (2018) have reported a series of Pd-based nanomaterials supported on modified ZnO/TiO2 NPs built by gum acacia (GA) support (GA-Pd/ZnO and GA-Pd/TiO2), and evaluated their catalytic prowess in the selective hydrogenation of highly toxic nitroarenes under very mild conditions. The remarkable activity of as prepared nanocomposite ensued from inherently biocompatible and nontoxic properties of GA, which could be applied as a modifier of crystal growth, stabilizer, and reductant in the preparation of nanostructures. The nanocomposite was reused for five successive cycles.
2.4.2. Applications for water treatment
2.4.2.1. Removal of heavy metals.
Gum kondagogu (GK) is a natural, partially acetylated, harmless polymer and in view of its various functional groups, the native gum itself could be deployed as a potential biosorbent for elimination of toxic metal pollutants (Vinod, Sashidhar, & Sreedhar, 2010; Vinod, Sashidhar, & Sukumar, 2010; Vinod et al., 2009). In this context, Saravanan et al. (Saravanan, Vinod, Sreedhar, & Sashidhar, 2012) described a magnetic nanosorbent by surface modification of Fe3O4 NPs (8~15 nm) with gum kondagogu (GK) for the adsorption/removal of highly toxic metal cations from water. The removal efficiencies of the GK grafted magnetic NPs (GK-MNPs) as a magnetic nanosorbent was investigated for the removal of diverse toxic metals namely Cd2+, Cu2+, Zn2+, Ni2+, Hg2+ and Pb2+; a lowest of 35.07 mg g−1 and a highest of 106.8 mg g−1 adsorption competences were reported for the Hg2+ and Cd2+ cations, respectively.
In another work, a novel class of easily retrievable magnetic Fe3O4 nanocomposite comprising poly(methyl methacrylate) and Tragacanth gum [P(MMA)-g-TG-MNs] were reported by Sadeghi, Rad, and Moghaddam (2014), for highly selective elimination of Cr6+ from waste-water in presence of Cr(III). Notably, the excellent selectivity of the nanosorbent for Cr6+ ions in presence of Cr3+ (at pH 5.5), lower adsorbent dosage (3 g L−1), high percent removal (~95 %), and ease of separation from solution, make this nanosorbent more effective and valuable compared to others (Hu, Chen, & Lo, 2005; Singh, Kumari, Pandey, & Narayan, 2009).
2.4.2.2. Removal of dyes.
One of the most premier adsorbents, 3D hydrogels, are suitable for the treatment of complex wastewater owing to their excellent concentration or adsorption capacities and high (photo)catalytic degradation. The 3D biopolymer-based hydrogels could be prepared from natural GG through self-assembly or using a cross-linking agent. Recently, GG based hydrogel nanocomposites have displayed potential in drug delivery, biosorption and separation owing to their intrinsic superior properties e.g. biocompatibility, biodegrad-ability, high active-site availabilities, large surface areas, and low-mass transfer limitations (Dai, Liu, Hu, & Si, 2017; Sharma, Kalia et al., 2015). Duan et al. (2020) prepared a versatile 3D hybrid nanocomposite hydrogel based on MIL-100(Fe) and Ag NPs via simple blending and self-crosslinking, and studied the activities of the resulting Ag NPs@MIL-100(Fe)/GG hybrid hydrogels in adsorption/(photo)catalytic degradation of dye pollutants, antibacterial property, and oily waste-water purification (Fig. 8).
Fig. 8.
Adsorption and photodegradation mechanism of nanohybrid hydrogel. Reproduced with permission from Ref. (Duan et al., 2020).
Other documented studies on the use of gum-derived (nano)materials for the removal of heavy metals/dyes are summarized in Table 5.
Table 5.
Gum-based photocatalysts/catalysts in removal of organic/inorganic contaminants.
| Gum-based photocatalysts/catalysts | Contaminants | Highlights | Ref. |
|---|---|---|---|
| L-Methionine montmorillonite encapsulated guar gum-g-polyacrylonitrile copolymer hybrid nanocomposite | Heavy metal ions | Adsorption capacities (mg g−1): 125.00 for Pb(II) and 90.91 for Cu (II) | (Ahmad & Hasan, 2017) |
| Gum arabic modified magnetic nano | Copper ions | Adsorption capacity (mg g−1): 38.5 | (Banerjee & Chen, 2007) |
| Cellulose/carbon tubes hybrid adsorbent anchored with welan gum polysaccharide | MB | Adsorption capacity (mg g−1): 302.1 | (Deng et al., 2012) |
| Magnetic gel beads composed of Fe3O4 nanoparticles and gellan gum | Heavy metal ions | High adsorption capacities for Pb2+, Mn2+ and Cr3+ | (Wang, Chen et al., 2009; Wang, Zhao et al., 2009) |
| Xanthan gum stabilized nano Pd/Fe | Polychlorinated biphenyls (PCBs) | Remediation of PCBs contaminated soils | (Fan, Cang et al., 2013) |
| Gum karaya-grafted poly(acrylamide-co-acrylic acid) incorporated Fe3O4 NPs hydrogel nanocomposite | Metal ions (Pb(II), Cr(VI) & Ni(II)) | 100 % removal in 5 min | (Fosso-Kankeu, Mittal, Waanders, Ntwampe, & Ray, 2016) |
| Xanthan gum-g-polyacrylamide/SiO2 | Pb(II) | Adsorption capacity (mg g−1): 537.634 | (Ghorai, Sinhamahpatra, Sarkar, Panda, & Pal, 2012) |
| Chitosan-guar gum blend silver nanoparticle bionanocomposite | Reactive blue-21 (RB-21), Reactive red-141, Rhodamine-6 G (RH-6 G) and 4-NP | High degradation and reduction | (Vanaamudan, Sadhu, & Pamidimukkala, 2018) |
| Titania/gum tragacanth nanohydrogel | MB | Efficiency of 88.86 % | (Rahimdokht, Pajootan, & Ranjbar-Mohammadi, 2019) |
| Gum Tragacanth-based carbon dots-nano zero-valent iron composite | Amoxicillin and ciprofloxacin | 90 (amoxicillin) and 51 % (ciprofloxacin) removal | (Pirsaheb, Moradi, Shahlaei, Wang, & Farhadian, 2019) |
| Pt NPs stabilized by guar gum | 4-NP | Efficiency of 97 % | (Pandey & Mishra, 2014) |
| Co-polymer-grafted gum karaya and silica hybrid organic-inorganic hydrogel nanocomposite | MB | 96 % removal | (Mittal, Maity, & Ray, 2015) |
| Fe3O4 NPs incorporated gum ghatti based nanocomposite | MB | Adsorption capacity (mg g−1): 671.14 | (Mittal, Ballav, & Mishra, 2014) |
| Gum ghatti and Fe3O4 magnetic NPs based nanocomposites | RhB | Adsorption capacity (mg g−1): 654.87 | (Mittal & Mishra, 2014) |
| Composite hydrogels prepared by in situ incorporation of guar gum and nano sized bentonite clay in an acrylic network | Cr(VI) | 97.8 % removal | (Maity & Ray, 2016) |
| ZnSe-WO3 nano-hetero-assembly stacked on gum ghatti | Bisphenol A | 99.5 % removal | (Kumar et al., 2017) |
| Guar gum-nano ZnO biocomposite | Cr(VI) | Adsorption capacity (mg g−1): 55.56 | (Khan, Nazir, Ali, & Kumar, 2017) |
| CoFe2O4@silica-shell@tragacanth gum-grafted-poly (methacrylic acid) | MO, methyl red | Adsorption capacities (mg g−1): 336 and 387 for MO and methyl red | (Moghaddam, Jazi, Allahrasani, Ganjali, & Badiei, 2020) |
| Fe°@guar gum-crosslinked-soya lecithin nano hydrogel | Methyl violet | 81 % removal | (Sharma et al., 2019) |
| PdNPs/guar gum | Mixture of MO, MB & CR | – | (Anjum, Gul, Khan, & Khan, 2019) |
2.5. Alginate-based (nano)materials
Among the low cost biomaterials, alginates are useful linear block copolymers comprising two uronic acid residues, e.g. β-D-mannuronic and α-l-guluronic acid linked by β−1,4-glycosidic bonds. This biopolymer has been extensively utilized in wastewater remediation due to its stability, high water permeability, biodegradability, and nontoxic nature (Ahmed, Moustafa, El-Masry, & Hassan, 2014; Xu, Nasrollahzadeh, Sajjadi et al., 2019) for the adsorption of contaminants, particularly, heavy metal cations (An, Lee, Lee, Lee, & Choi, 2015; Idris, Ismail, Hassan, Misran, & Ngomsik, 2012; Kuang et al., 2015; Li et al., 2013).
Alginate-based adsorbents have recently garnered attention as they could form stable biohydrogel beads and can be applied as a catalytic support material with numerous benefits, e.g. high surface area, network structure, and their rich surface functionalities (Li, Mo et al., 2016; Wang, Vincent, Roux, Faur, & Guibal, 2017). For example, mesoporous calcium-alginate/titania hybrid beads were prepared as a biosorbent for treating aqueous solutions comprising Cr6+, Co2+, Cr3+, Cd2+, and Cu2+ ions (Wu, Wei, & Zhang, 2012); adsorption capacity of 8.4 mg g−1 for Cr6+was reported with reusable capacity for six successive cycles. Besides, Gupta et al. (Gupta et al., 2014) fabricated bimetallic and core/shell Fe@Ag NPs (15~20 nm) involving modified aminothiophenol-calcium alginate (Fe@Ag-ATP-CA) beads for the reduction of 2-NP and 4-NP in wastewater using NaBH4. A heterogeneous core/shell Fe3O4@alginate-Fe magnetic nanocomposite has been fabricated, via an oxidation-precipitation technique, for the degradation of bisphenol A from aqueous media in the catalytic ozonation (Ahmadi, Rahmani, Takdastan, Jaafarzadeh, & Mostoufi, 2016); degradation or mineralization of bisphenol A is influenced by concentration of pollutant (10 ppm)/H2O2 (30 mmol L−1)/nanocatalyst (0.7 g L−1), and O3 dosage (0.1 g h−1).
In addition to alginate-derived biohydrogel beads, carbon beads can provide an outstanding catalytic support, ensuring good dispersion of NPs and stability of nanocatalysts, and thus attracted interest due to their elevated porosity, large surface area, excellent mechanical stability, and adjustable surface chemistry (Teng, Wu, Fan, Zhang, & Zhao, 2015). Zheng et al. (2016) presented the fabrication of a novel alginate-obtained carbon beads (Alg/CB) with an advanced nano-network via a facile combinational carbothermal reduction and acid treatment of Ca2+ gelled sodium alginate biohydrogel beads (Fig. S6); they were successfully used/reused in the decoloration and removal of Cr6+ from wastewater in six successive runs.
One interesting property of alginate-based biosorbents is their selective cationic interactions with multivalent ions such as Ca2+, Fe3+, Ba2+, Ag+, Al3+, etc. to transform them into macromolecular hydrogels, creating a 3D network structure (Cheng, Luo, Payne, & Rubloff, 2012; Topuz, Henke, Richtering, & Groll, 2012; Yang et al., 2013). In this context, Yang et al. (2013) successfully used a variety of multivalent cations to prepare a crosslinked alginate/polyacrylamide hydrogel, thereby, improving the mechanical features of the biopolyanionic network. Furthermore, Ai et al. (2012) fabricated Ag NPs in situ grown on a magnetic alginate/magnetite hybrid (Ag@AMH) biohydrogel via an eco-friendly light-driven technique (Fig. S7). Ag+ ions could be simply fixed and uniformly dispersed onto the AMH biohydrogel, thereby ensuring the next in situ photochemical preparation of Ag NPs (~72 nm) with good distribution; ensuing bio-hydrogel efficiently accomplished the catalytic reduction of toxic 4-NP in aqueous solutions and the catalyst could be reused for successive three cycles.
As mentioned earlier in Section 3.3, the integration or surface modification of fibrous clay with starch, guar gum or alginate has boosted water absorbency or water absorption rate of the catalytic systems. Along this theme, Olad and Farshi Azhar (2014) reported a facile method to synthesize an effective alginate/montmorillonite/polyaniline (Alg/MMT/PANI) hybrid nanocomposite by a chemical oxidative polymerization for the removal of Cr6+. Additionally, Ahmad and Mirza (2015) synthesized Meth-bent/Alg nano-composite by integrating methionine-modified bentonite onto sodium alginate that lead to the enhanced performance of the as prepared nanosorbent; biosorption capacities of 217.39 and 30.86 mg g−1 for Cd2+ and Pb2+, respectively, was discerned. This nanocomposite could be reused for five successive cycles.
The related works on the application of alginate-based (nano)materials in the wastewater remediation are summarized in Table 6.
Table 6.
Various alginate-based nanomaterials used for water/wastewater treatment.
| Alginate-based nanomaterials | Contaminants | Ref. |
|---|---|---|
| Polyvinyl alcohol/alginate/zeolite nanohybrid | Ni(II) and Co(II) metal ions | (Tabatabaeefar, Keshtkar, Talebi, & Abolghasemi, 2020) |
| Nano zerovalent iron immobilized in alginate beads | Cr(VI) | (Ravikumar, Sudakaran, Pulimi, Natarajan, & Mukherjee, 2018) |
| Nano silver chloride and alginate incorporated composite copolymer | Brilliant cresyl blue | (Bhangi & Ray, 2020) |
| Glycine functionalized magnetic nanoparticle entrapped calcium alginate beads | Cu(II) | (Asthana, Verma, Singh, & Susan, 2016) |
| Activated carbon/nano zerovalent copper/hydroxyapatite-alginate | As(III) | (Iqbal et al., 2019) |
| Polyaniline nanofibers assembled on alginate microsphere | Cu(II) and Pb(II) | (Jiang, Xu, Dai, Luo, & Dai, 2012) |
| Three dimensional silver/polyethyleneimine/alginate hydrogel beads | 4-NP | (Gao et al., 2018) |
| Sodium alginate dispersed nano zero-valent iron | Cr(VI) | (Iqbal et al., 2019) |
| Calcium alginate beads impregnated with nano zerovalent iron, magnetite NPs and powdered activated carbon | Nitrate | (Bahrami, Yu, Zou, Sun, & Sun, 2020) |
| PVA alginate maghemite and titania beads | Pb(II) | (Majidnia & Idris, 2016) |
| Maghemite and titania nanoparticles in PVA alginate beads | Iodine | (Majidnia & Idris, 2015) |
| Chitin/alginate magnetic nano gel beads | MO | (Li, Du et al., 2010) |
| CdS quantum dots immobilized on calcium alginate microbeads | Hg(II) | (Kumar & Dutta, 2015) |
| Fe@graphene oxide alginate beads | Cr(VI) | (Lv et al., 2017) |
| GOx/MnFe2O4/Calcium alginate nano-composite | MB | (Zolfaghari, Shojaat, Karimi, & Saadatjoo, 2018) |
| Bimetallic zerovalent iron silver nanoparticles immobilized in calcium alginate beads | 4-Chlorophenol | (Barreto-Rodrigues, Silveira, García-Muñoz, & Rodriguez, 2017) |
| Oleic acid coated nanoscale palladium/zero-valent iron alginate beads | Trichlorophenol | (Chang et al., 2015) |
| TiO2/ZnO calcium alginate beads | Cu(II) | (Kanakaraju, Ravichandar, & Lim, 2017) |
| Nanohydroxyapatite-alginate composite | Pb(II) | (Googerdchian, Moheb, & Emadi, 2012) |
| Nanozerovalent iron immobilized alginate beads | Cr(VI) | (Ravikumar et al., 2016) |
| Fe3O4@nano-hydroxyapatite alginate beads | Cr(VI) | (Periyasamy, Gopalakannan, & Viswanathan, 2018) |
| Poly(acrylic acid)-sodium alginate nanofibrous hydrogel | Cu(II) | (Wang, Wang et al., 2018; Wang, Li et al., 2018) |
| Nano zero-valent iron/carbon/alginate composite gel | Cr(VI) | (Wen et al., 2020) |
| Co(II)-doped Fe3O4 NPs immobilized in PVA alginate | Cu(II) | (Wong, Chan, & Idris, 2015) |
| Nanoporous hydrogel based on vinyl functionalized alginate | Pb(II) | (Wang, Zong, & Wang, 2013) |
| TiO2/calcium alginate hydrogel | MO | (Zhao et al., 2014) |
| Acrylic acid grafted sodium alginate-based TiO2 hydrogel nanocomposite | Methyl violet | (Thakur & Arotiba, 2018) |
| Polyvinyl alcohol alginate entrapped nanoscale zero-valent iron | Cu(II), Cr(VI), Zn(II), and As(V) | (Sun et al., 2018) |
| Cellulose nanocrystal alginate hydrogel beads | MB | (Mohammed, Grishkewich, Berry, & Tam, 2015) |
| Nano iron oxide loaded alginate microspheres | Malachite green | (Soni, Tiwari, & Bajpai, 2014) |
| Magnetic sodium alginate beads | Reactive Blue 222 | (Shokoohi et al., 2019) |
| MnFe2O4/calcium alginate nano-composites coupled with GOx and laccase | MB, indigo and acid red 14 | (Shojaat, Saadatjoo, Karimi, & Aber, 2016) |
| Nanocomposite bead based on biopolymer alginate caged magnetic graphene oxide | Pb(II) and Cu(II) | (Sharif, Khorasani, & Shemirani, 2018) |
| Fe3O4@nano hydroxyapatite/alginate | Fluoride | (Pandi & Viswanathan, 2015) |
| Nano hydroxyapatite/alginate composite beads | Ni(II) and rhodamine B | (Oladipo & Gazi, 2016) |
| Alginate/Fe@Fe3O4 core/shell structured nanoparticles | Norfloxacin | (Niu, Meng, & Cai, 2012) |
| Nano Zn-Al-Fe3O4 blended alginate/Ca beads | Rhodamin B | (Kumar, Vijayakumar, & Tamilarasan, 2019) |
| Nano-sized carbon immobilized alginate beads | Co(II) and Ni(II) | (Jung et al., 2015) |
| Nano-sized montmorillonite (MMT)/calcium alginate | Basic red 46 | (Hassani, Soltani, Karaca, & Khataee, 2015) |
| Fe3O4/activated charcoal/β-cyclodextrin/sodium alginate | MB | (Yadav et al., 2020) |
| Pd nanocatalyst supported on cationic nanocellulose-alginate hydrogel | MB | (Wang, Ran et al., 2020; Wang, Zhou et al., 2020) |
| Alginate/CMC/ZnO Nanocomposite | CR | (Ramadhani & Helmiyati, 2020) |
| Calcium alginate activated carbon fiber beads | Tetrahydrofuran (THF) | (Chen et al., 2013) |
| Calcium alginate beads | Cu(II) removal from the tetra metallic [Cu(II), Cd(II), Ni(II) and Zn(II)] mixture | (Yang et al., 2019) |
2.6. Pectin-based (nano)materials
Pectin, as biocompatible, flexible, nontoxic, high-molecular weight and anionic naturally occurring polysaccharide, is extractable from the higher plant cell walls. Pectin is a linear polysaccharide as most plants contain it in intercellular layer between primary cell walls of adjoining cells. In recent years, pectin as a class of complex polysaccharides has gained increasing importance with applications in pharmaceutical, biotechnology and a number of other industries. Similar to most other polysaccharides, pectin’s composition differs with source and conditions deployed during isolation (Mualikrishna & Tharanathan, 1994; Ridley, O’Neill, & Mohnen, 2001). Pectin mainly consists of d-galacturonic acid molecules that are joined in chains by α-(1–4) glycosidic linkage in which a number of carboxyl/hydroxyl groups are distributed along backbone, in addition to a certain amount of neutral sugars present as side chains (Mukhiddinov, Khalikov, Abdusamiev, & Avloev, 2000; Sundar Raj, Rubila, Jayabalan, & Ranganathan, 2012). Some of the carboxyl groups are naturally occurring as methyl esters while others can be treated with ammonia to prepare carboxamide groups; these functional groups can form complexes with metal ions in solution and reduce them to metal NPs without using any toxic reducing/stabilizing agents.
One of the simplest greener methods for the preparation of naturally recoverable Pd nanocatalysts is the direct immobilization of Pd NPs on the surface of carbohydrate-based materials; in this regard, Pd NPs were stabilized on polysaccharides such as Pd/starch, Pd/gelatin and Pd/chitosan (Balanta, Godard, & Claver, 2011; Budarin, Clark, Luque, Macquarrie, & White, 2008; Primo, Liebel, & Quignard, 2009; Sun et al., 2005). Hybrid organic/inorganic nanocomposites are of tremendous interest owing to their multifunctionality via combined incorporation of diverse compounds. Fe-pectin (Rakhshaee & Panahandeh, 2011), Fe°-PO-CHA (pectin derived orange skin-carboxylic acid) and Fe°-PO-IPA (isopropylglutaricacid) (Rakhshaee, 2011), FeNPs/Fe3O4NPs-CPA (crosslinked pectin adipic acid) (Rakhshaee, 2014), Fe3O4-pectin and Fe3O4-humic acid nanocomposites (Liu, Zhao, & Jiang, 2008) have been used for the adsorptional elimination of heavy metals and/or dyes. The combination of unique properties of NPs and carbohydrate-based materials leads to a novel nanomaterial with high mechanical/thermal stability, large surface area and sorption properties. Baran (2018) prepared Pd NPs (34~54 nm) immobilized on the natural agar/pectin composite (PdNPs@APC) and evaluated their catalytic abilities in the reduction of o-nitroaniline reduction using aqueous NaBH4 at room temperature. The agar/pectin composite with highly active surface, high thermal stability (up to 239 °C), and strong covalent bonds was designed as a stabilizer. Subsequently, Pd NPs were synthesized through in situ reduction Pd ions and immobilized on the surface of APC without any hazardous reducing agents under greener conditions. Catalytic activity in reduction reactions showed that biopolymer composites could be efficiently used as stabilizers for various noble metallic NPs. Recycling studies of the nanocomposite were also conducted for o-nitroaniline reduction to 1,2-benzenediamine, wherein the reaction yield decreased approximately from 100 % to 83 % after eight cycles.
Pectin-based bio(nano)sorbents have been utilized for the heavy metal selective elimination from aqueous media with affinity sequence of Pb2+> Cu2+> Co2+> Ni2+> Zn2+> Cd2+ (Kartel, Kupchik, & Veisov, 1999). The cumbersome separation, lack of stability, or low recovery post desorption are the main constraints for the broad utilization of pectin-derived biosorbents. Mata, Blázquez, Ballester, González, and Muñoz (2009) reported the adsorption capacities of multimetals like Cu2+, Cd2+, Pb2+ using a sugar-beet pulp pectin biosorbent. In another study, Gong et al. (2012) studied the adsorption capability of magnetically recoverable pectin-Fe3O4 nanocomposite for the Cu2+ removal (48.99 mg g−1) from wastewater in 5 successive cycles. Additionally, the pectin-CuS nanocomposite (~50 nm) was fabricated via a facile co-precipitation route (Gupta, Pathania, Agarwal, & Singh, 2012), which was successfully utilized to photodegrade and adsorb MB after 10 h under solar-light conditions at 30 °C and could be reused for successive 10 cycles. Bok-Badura, Jakóbik-Koloń, Karon, and Mitko (2018) produced hybrid pectin/titanium oxide nanobeads as an efficient nanosorbent for the elimination of ionic heavy multimetals; adsorption capacities of hybrid nanobeads were 0.83, 1.37, 0.51, and 0.68, mmol g−1 for Pb2+, Cu2+, Zn2+, and Cd2+, respectively.
Table 7 summarizes other known examples concerning the applications of pectin-based (nano)materials in water/wastewater remediation.
Table 7.
Pectin-based photocatalysts/catalysts in removal of organic/inorganic contaminants.
| Pectin-based photocatalysts/catalysts | Contaminants | Highlights | Ref. |
|---|---|---|---|
| Ethylenediamine modified pectins | Pb(II) | High adsorption capacity | (Liang et al., 2020) |
| (PPA3)a-Cu and (PPA3/Fe3O4)-Cu nanocomposite hydrogels | 2-Nitrophenol | High reduction | (El Fadl, Mahmoud, & Mohamed, 2019) (El Fadl et al., 2019) |
| Modified Fe3O4 NPs with the extracted pectin of Azolla filicoloides | Methyl orange | Maximum uptake capacity at 5 °C: 0.533 | (Rakhshaee, Giahi, & Pourahmad, 2011) |
| Magnetite/silica/pectin NPs | Fluoroquinolones (Ciprofloxacin & Moxifloxacin) | Removal percent: 89 | (Attallah, Al-Ghobashy, Nebsen, & Salem, 2017) |
| Pectin stabilized nanoscale zerovalent iron | Cr(VI) | Removal from water | (Chen, Yang, Wang, Zhou, & Zhang, 2015) |
| Pectin stabilized magnetic graphene oxide Prussian blue nanocomposites | Cesium | Adsorption capacity (mg g−1): 1.230 | (Kadam, Jang, & Lee, 2016) |
PPA: Crosslinked pectin-(polyvinyl alcohol-co-acrylamide) hydrogel.
3. Summary and discussion
There are several reports in the literature on the water treatment using different (nano)catalysts including natural biopolymer-based nanomaterials. The polysaccharide-based (nano)materials utilized in the adsorption and reduction/degradation reactions are summarized in Table 8. Having reviewed these publications, it became clear that there are no special rules or general ways to theoretically predict the outcomes of a catalytic reduction/adsorption process. Mostly, the differences in the catalytic prowess of these (nano)materials depend on their amount and type, surface area, particle size, morphology and porosity, chemical composition, loading of metal/metal oxide on the catalyst surface, reaction temperature, solvent, reducing agents, etc. In other words, this comparison depends on the preparative method and/or reaction conditions and follows no predictive rules.
Table 8.
Summary of different kinds of natural polysaccharide-based nanomaterials used for the removal of MB in water.
| Biopolymer-based nanomaterials | Preparation method | Reaction conditions | Ref. |
|---|---|---|---|
| Pristine CNFs | Acidified chlorite bleaching method | Adsorbent (0.1 g), MB (100 mL, 100 mg/L), pH 9, 20 °C, maximum adsorption capacity (122.2 mg g−1) | (Chan et al., 2015) |
| CNCs/hydrolyzed polyacrylamide nano-hydrogels (by casting) | Thermal method | Adsorbent (2 mg), MB (20 mL, 5 mg/L), r.t., adsorption efficiency >90 %, maximum adsorption capacity (326.08 mg g−1) | (Zhou, Wu et al., 2014; Zhou, Fu et al., 2014) |
| CNCs/hydrolyzed polyacrylamide nano-hydrogels (by electrospinning) | Electrospinning and thermal treatment | Adsorbent (2 mg), MB (20 mL, 5 mg/L), pH 6.5, 25 °C | (Zhou et al., 2013) |
| MnO2 coated CNFs hybrid | A one-step in situ method using bamboo CNFs under alkaline conditions | Hybrid (25 mg), MB (25 mL, 80 mg/L), pH 9.6, r. t., 2 min, decolorization efficiency (99.8 %) | (Wang, Yadav et al., 2014; Wang, Zhang et al., 2014) |
| CMC/GOCOOH microbeads | Reaction of GOCOOH and CMC mixture with AlCl3 solution | Adsorbent (0.1 g), MB (50 mL, 250 mg/L), pH 10, 25 °C, 2 h, maximum adsorption capacity (180.32 mg g−1) | (Eltaweil et al., 2020) |
| CNC-ALG hydrogel bead | Mixing the CNCs with alginate solution and then dispensing small droplets into a gelation bath containing CaCl2 | Adsorbent, MB (4.4 mL, 50 mg/L), 25 °C, 90 min, maximum adsorption capacity (255.5 mg g−1) | (Mohammed, Grishkewich et al., 2016) |
| CNF-graphene nanoplates aerogel | Incorporation of GnPs into CNFs | Adsorbent (5 mg), MB (20 mL, 100 mg/L), 25 °C, 16 h, maximum adsorption capacity (1178.5 mg g−1) | (Yu et al., 2020) |
| G-C3N4/CNCs-H hydrogel | Chemical method | Adsorbent (10 mg), MB (50 mL, 10 mg/L), pH 7, r.t., maximum adsorption capacity (> 198.6 mg g−1) | (Wang, Li et al., 2020) |
| Polyacrylic acid-magnetic 3D crosslinkers-carboxylated cellulose nanocrystal | In situ polymerization in water | Adsorbent (8 mg), MB (50 mL, 20 mg/L), pH 7.2, r.t., maximum adsorption capacity (332 mg g−1) | (Samadder et al., 2020) |
| Carboxylated cellulose fabric filter | 2,2,6,6-Tetramethylpiperidine-1-oxyl (TEMPO)-mediated oxidation | Adsorbent (0.01 g), MB (10 mL, 50 mg/L), r.t., maximum adsorption capacity (76.92 mg g−1) | (Li, Ma et al., 2020) |
| BC@CdS nanocomposite | A “anchoring-reacting-forming” pathway | Adsorbent (1.1 g), MB (20 mL, 20 mg/L), r.t., visible light irradiation, 180 min, 77.39 % removal, maximum adsorption capacity (12.68 mg g−1) | (Qian et al., 2020) |
| CNF-Fe(0)@FeS | Chemical method | Adsorbent (0.5 g/L), MB (20 mL, 50 mg/L), r.t., pH 7, 15 min, maximum adsorption capacity (200 mg g−1) | (Sankararamakrishnan et al., 2020) |
| CNF/poly(ethylene imine)/Ag NPs aerogel membrane | A green method | Catalyst (diameter: 24 mm; height: 18 mm), MB (40 mL, 10 mg/L), NaBH4 (10 mL, 50 mM), r.t., 4 min | (Zhang et al., 2020) |
| MoS2-graphene-cellulose filter paper | Simple hydrothermal method | Adsorbent (30 mg), MB (30 mL, 100 mg/L), r.t., pH 7, 2 min, maximum adsorption capacity (485.4 mg g−1) | (Gopalakrishnan et al., 2020) |
| Bacterial cellulose activated carbon | Phosphoric acid activation at a carbonization temperature of 500 °C | Adsorbent (0.02 g), MB (40 mL, 50–100 mg/L), 30 °C, pH 7, 10 min, maximum adsorption capacity (505.8 mg g−1) | (Khamkeaw et al., 2019) |
| BC/polydopamine/TiO2 | Using BC nanofibers, coated with the polydopamine and a protective agent for immobilization of TiO2 NPs | Photocatalyst (30 mg), MB (50 mL, 20 mg/L), 25 °C, 20 min, dark conditions, ultraviolet light, 99.5 % removal, maximum adsorption capacity (38.7 mg g−1) | (Yang et al., 2020) |
| CS/MCM-41/nano-γ alumina | Chemical method | Adsorbent (0.088 mg), MB (15 mg/L), pH 7.5, 45 min, maximum removal efficiency (90.23%) | (Teimouri et al., 2019) |
| Polyvinylidene fluoride/chitosan/dopamine | A facile one-step codeposition strategy | Adsorbent (100 mg), MB (500 mL, 5 mg/L), high adsorption efficiency (96.8 %) | (Zhang, Li et al., 2019) |
| Magnetic β-cyclodextrin-chitosan/graphene oxide | Chemical method | Adsorbent (0.01 g), MB (25 mL), pH 11, 25 °C, maximum adsorption capacity (84.32 mg g−1) | (Fan, Luo et al., 2013) |
| Chitosan zinc oxide nanobeads | A green method using the Musa X paradisiaca | Photocatalyst, MB (10 mL, 10 ppm), sunlight, 10 h, efficiency of the photocatalytic degradation (97.47 %) | (Roshitha et al., 2019) |
| Polymer coated ZnO with chitosan | Microwave hydrothermal method | Photocatalyst (0.75 g), MB (500 mL, 50 ppm), visible-light, pH 6, 90 min, decolorization adsorption efficiency (98.01 %) | (Sani, Aliyu, & Tukur, 2015) |
| Chitosan-Fe | Chemical method | CS-Fe (10000 mg L−1), H2O2 (1200 mg L−1), MB (100 mL, 120 mg L−1), 30 °C, pH 6–7, 30 min, >99% removal | (Gao et al., 2016) |
| Chitosan/polyaniline hybrid | In situ polymerization of aniline in the presence of chitosan | Adsorbent (50 mg), MB (100 mL, 0.003 M), r.t., pH 11, 60 min, maximum adsorption capacity (81.3 mg g−1) | (Minisy, Salahuddin, & Ayad, 2019) |
| Iron oxide/chitosan magnetic nanocomposite immobilized manganese peroxidase | A green method | Nanocomposite (10 mg), MB (50 mL, 50 mg L−1), 27 °C, pH 7, 50 min, 96% ± 2% removal | (Siddeeg, Tahoon, Mnif, & Ben Rebah, 2020) |
| Chitosan/zeolite composite | Chemical method | Adsorbent (2.5 g L−1), MB (100 mL, 43.75 mg L−1), r.t., pH 9, 138.65 min, 84.85% removal, adsorption capacity (24.5 mg g−1) | (Dehghani et al., 2017) |
| Base-catalyzed hydrolysis and water condensation reactions of tetraethylorthosilicate in an alcohol | (Mittal et al., 2015) | ||
| Co-polymer-grafted gum karaya and silica hybrid organic-inorganic hydrogel nanocomposite | medium containing a dispersion of GK-cl-P(AA-co-AAM) | Adsorbent (0.2 g L−1), MB (50 mL, 200 mg/L), 25 °C, pH 7, 96% removal, maximum adsorption capacity (1408.67 mg g−1) | |
| Fe3O4 NPs incorporated gum ghatti based nanocomposite | Graft co-polymerization of acrylic acid onto gum ghatti and incorporation of Fe3O4 MNPs within the crosslinked network | Adsorbent (0.6 g L−1), MB (50 mL, 100 mg/L), 25 °C, pH 7, maximum adsorption capacity (671.14 mg g−1) | (Mittal et al., 2014) |
| GOx/MnFe2O4/Calcium alginate nano-composite | Mixing the GOx-immobilized MnFe2O4 NPs with sodium alginate solution and then CaCl2 | Adsorbent (16 g), MB (200 mL), 30 °C, pH 10.68, 2 h, 73.68 % removal | (Zolfaghari et al., 2018) |
| MnFe2O4/calcium alginate nano-composites coupled with GOx and laccase | Chemical method | Adsorbent, MB (50 mL), 30 °C, pH 9, 3 h, 93.46 % removal | (Shojaat et al., 2016) |
| Fe3O4/activated charcoal/β-cyclodextrin/sodium alginate | Direct mixing of the polymer matrix with the nanofillers | Adsorbent (0.2 g), MB (10 mL, 5 ppm), r.t., pH 6, 90 min, maximum adsorption capacity (2.079 mg g−1) | (Yadav et al., 2020) |
| Pd nano-catalyst supported on cationic nanocellulose-alginate hydrogel | Drop-by-drop addition of the CNCC and sodium alginate mixture solution into CaCl2 solution | Catalyst (0.1 g), MB (2 mL, 30 mg/L), NaBH4 (1 mL, 60 mg/L), 30 °C, 5 min | (Wang, Zhou et al., 2020) |
| Pectin-thorium(IV) tungstomolybdate nanocomposite | Sol-gel method | Photocatalyst (0.5 mg/mL,100 mg), MB (1.5 × 10−5 M), solar light, 5 h, 76% removal | (Gupta, Agarwal, Pathania, Kothiyal, & Sharma, 2013) |
| Starch-derived activated carbon | Carbonization of starch at 500 °C | Adsorbent (0.1 g, 5 g/L), MB (20 mL, 200 mg/L), 30 °C, pH 10.5, 90 min, 99.86 % removal | (Benhachem, Attar, & Bouabdallah, 2019) |
4. Conclusion and future perspectives
Natural biopolymer-based resources (particularly polysaccharides) derived from diverse derivatives are unquestionably the future wave of abundant resources that need to be exploited. Natural biopolymers are especially preferred because such they have unique structural, biological, physicochemical and biomechanical features and biodegradable properties; indeed, abundant availability, nontoxicity, renewalability, ease of modification, biocompatibility, and application potential have steered research in their direction. Importantly, aforementioned polysaccharides are considered alternative renewable (bio)resources and effective supports for the (nano)catalysts fabrication such as biopolymer supported Pd, Ag, Cu, TiO2, ZnO, Fe3O4, Au NPs; it is one of the more effective strategies in nanotechnologies.
Additional explorations need to be conducted to optimize the production of natural/biopolymeric-based (nano)materials to render them viable and sustainable towards the industrial application e.g. the traditional adsorbents. Coagulation/flocculation of solid substances in wastewater has been accomplished with the assistance of natural/biopolymeric-based (nano)materials. Among polysaccharides, cellulose and chitosan are promising natural/biopolymers because of their sustainability, reactivity, chemical stability, excellent physicochemical attributes, and considerable selectivity towards toxic metals, dyes and aromatic compounds that makes them competitive to the conventional activated carbon.
Treatment of water/wastewater (especially industrial water) is very critical and essential for safeguarding the environmental quality and maintaining human health, and it has been a main trepidation for public health. As is clear from this review, emerging nanotechnologies have the potential to make industrial water remediation more effective where the pollutants could be removed using relatively greener nanotechnology, but this field still needs extensive explorations; major technical hurdle being their non-adaptability for industrial/large-scale systems and competitiveness to the traditionally deployed existing treatment options. Nonetheless, recent efforts have identified great potential for many appliances of the biopolymer-based (nano)materials across diverse research domains; the preparation of polysaccharide-based (nano)materials via conventional approaches is quite convenient, facile and harmless to the environment. Thus, they have the potential to emerge as an effectual, cost-effective, and environment-friendly substitute for predominant treatment supplies, from the standpoints of both environmental remediation and resource conservation.
In spite of notable advances in the synthetic and catalytic utilizations of natural biopolymer-based (nano)materials, particular consideration should be paid to the following aspects in future investigations:
Scalable fabrication of polysaccharide-based (nano)materials, at a relatively lower cost.
Application of diverse biowaste (nano)materials as renewable alternative feedstocks for fabrication of biopolymers and their wastewater treatment application.
Exploitation of natural supports e.g. clays, zeolites, and montmorillonite as cost-effective, nontoxic and abundantly available resources for the synthesis of (nano)catalysts and application in water/waste-water treatment.
Manipulation of agricultural and animal residues e.g. bone, bristles, or eggshell for the synthesis of biopolymer-based (nano)materials and application in water remediation.
Greener biological processes to reduce the cost of the biopolymer-based (nano)materials production under mild conditions and improve their (nano)catalytic prowess.
Improvement of polysaccharide-based magnetic nanomaterials for water/wastewater treatment.
Supplementary Material
Acknowledgements
We gratefully acknowledge the University of Qom, Isfahan University of Medical Sciences and the Iranian Nano Council for the support of this work. The participation and generous contribution of all our collaborators over the years is appreciated.
Footnotes
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The research presented was not performed or funded by EPA and was not subject to EPA’s quality system requirements. The views expressed in this article are those of the author(s) and do not necessarily represent the views or the policies of the U.S. Environmental Protection Agency.
Declaration of Competing Interest
The authors report no declarations of interest.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.carbpol.2020.116986.
References
- Abdelhameed RM, Abdel-Gawad H, Elshahat M, & Emam HE (2016). Cu-BTC@ cotton composite: Design and removal of ethion insecticide from water. RSC Advances, 6(48), 42324–42333. [Google Scholar]
- Abdi S, Nasiri M, Mesbahi A, & Khani MH (2017). Investigation of uranium(VI) adsorption by polypyrrole. Journal of Hazardous Materials, 332, 132–139. [DOI] [PubMed] [Google Scholar]
- Abdolmohammad-Zadeh H, Ayazi Z, & Naghdi Z (2019). Nickel oxide/chitosan nano-composite as a magnetic adsorbent for pre-concentration of Zn (II) ions. Journal of Magnetism and Magnetic Materials, 488, Article 165311. [Google Scholar]
- Abdulhameed AS, Jawad AH, & Mohammad A-T (2019). Synthesis of chitosan-ethylene glycol diglycidyl ether/TiO2 nanoparticles for adsorption of reactive orange 16 dye using a response surface methodology approach. Bioresource Technology, 293, Article 122071. [DOI] [PubMed] [Google Scholar]
- Abdulhameed AS, Mohammad A-T, & Jawad AH (2019). Application of response surface methodology for enhanced synthesis of chitosan tripolyphosphate/TiO2 nanocomposite and adsorption of reactive orange 16 dye. Journal of Cleaner Production, 232, 43–56. [Google Scholar]
- Abidin MNZ, Goh PS, Ismail AF, Said N, Othman MHD, Hasbullah H, et al. (2018). Highly adsorptive oxidized starch nanoparticles for efficient urea removal. Carbohydrate Polymers, 201, 257–263. [DOI] [PubMed] [Google Scholar]
- Adamczuk A, & Kołodyńska D (2015). Equilibrium, thermodynamic and kinetic studies on removal of chromium, copper, zinc and arsenic from aqueous solutions onto fly ash coated by chitosan. Chemical Engineering Journal, 274, 200–212. [Google Scholar]
- Ahmad R, & Hasan I (2017). L-methionine montmorillonite encapsulated guar gum-gpolyacrylonitrile copolymer hybrid nanocomposite for removal of heavy metals. Groundwater for Sustainable Development, 5, 75–84. [Google Scholar]
- Ahmad R, & Mirza A (2015). Sequestration of heavy metal ions by Methionine modified bentonite/Alginate (Meth-bent/Alg): A bionanocomposite. Groundwater for Sustainable Development, 1(1–2), 50–58. [Google Scholar]
- Ahmad M, Ahmed S, Swami BL, & Ikram S (2015). Adsorption of heavy metal ions: Role of chitosan and cellulose for water treatment. Langmuir, 79, 109–155. [Google Scholar]
- Ahmad T, Rafatullah M, Ghazali A, Sulaiman O, Hashim R, & Ahmad A (2010). Removal of pesticides from water and wastewater by different adsorbents: A review. Journal of Environmental Science and Health, Part C, 28(4), 231–271. [DOI] [PubMed] [Google Scholar]
- Ahmadi M, Rahmani H, Takdastan A, Jaafarzadeh N, & Mostoufi A (2016). A novel catalytic process for degradation of bisphenol A from aqueous solutions: A synergistic effect of nano-Fe3O4@Alg-Fe on O3/H2O2. Process Safety and Environmental Protection, 104, 413–421. [Google Scholar]
- Ahmed AESI, Moustafa HY, El-Masry AM, & Hassan SA (2014). Natural and synthetic polymers for water treatment against dissolved pharmaceuticals. Journal of Applied Polymer Science, 131(13). [Google Scholar]
- Ahmed MA, Abdelbar NM, & Mohamed AA (2018). Molecular imprinted chitosan-TiO2 nanocomposite for the selective removal of Rose Bengal from wastewater. International Journal of Biological Macromolecules, 107, 1046–1053. [DOI] [PubMed] [Google Scholar]
- Ai L, Yue H, & Jiang J (2012). Environmentally friendly light-driven synthesis of Ag nanoparticles in situ grown on magnetically separable biohydrogels as highly active and recyclable catalysts for 4-nitrophenol reduction. Journal of Materials Chemistry, 22(44), 23447–23453. [Google Scholar]
- Akın Sahbaz D, Yakar A, & Gündüz U (2019). Magnetic Fe3O4-chitosan micro-and nanoparticles for wastewater treatment. Particulate Science and Technology, 37(6), 732–740. [Google Scholar]
- Al Momani F (2007). Treatment of air containing volatile organic carbon: Elimination and post treatment. Environmental Engineering Science, 24(8), 1038–1047. [Google Scholar]
- Alaba PA, Oladoja NA, Sani YM, Ayodele OB, Mohammed IY, Olupinla SF, et al. (2018). Insight into wastewater decontamination using polymeric adsorbents. Journal of Environmental Chemical Engineering, 6(2), 1651–1672. [Google Scholar]
- Albukhari SM, Ismail M, Akhtar K, & Danish EY (2019). Catalytic reduction of nitrophenols and dyes using silver nanoparticles@cellulose polymer paper for the resolution of waste water treatment challenges. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 577, 548–561. [Google Scholar]
- Al-Harbi LM, Kosa SA, Abd El Maksod IH, & Hegazy EZ (2015). The photocatalytic activity of TiO2-Zeolite composite for degradation of dye using synthetic UV and Jeddah sunlight. Journal of Nanomaterials, 2015, Article 565849. [Google Scholar]
- Ali SM, & Al-Oufi B (2020). Synergistic sorption performance of cellulose-modified La0.9Sr0.1FeO3 for organic pollutants. Cellulose, 27(1), 429–440. [Google Scholar]
- Ali ASM, El-Aassar MR, Hashem FS, & Moussa NA (2019). Surface modified of cellulose acetate electrospun nanofibers by polyaniline/β-cyclodextrin composite for removal of cationic dye from aqueous medium. Fibers and Polymers, 20(10), 2057–2069. [Google Scholar]
- Alidokht L, Khataee AR, Reyhanitabar A, & Oustan S (2011). Reductive removal of Cr(VI) by starch-stabilized Fe° nanoparticles in aqueous solution. Desalination, 270 (1–3), 105–110. [Google Scholar]
- Alizadeh B, Delnavaz M, & Shakeri A (2018). Removal of Cd(ӀӀ) and phenol using novel cross-linked magnetic EDTA/chitosan/TiO2 nanocomposite. Carbohydrate Polymers, 181, 675–683. [DOI] [PubMed] [Google Scholar]
- Alsabagh AM, Fathy M, & Morsi RE (2015). Preparation and characterization of chitosan/silver nanoparticle/copper nanoparticle/carbon nanotube multifunctional nano-composite for water treatment: Heavy metals removal; kinetics, isotherms and competitive studies. RSC Advances, 5(69), 55774–55783. [Google Scholar]
- Amouzgar P, & Salamatinia B (2015). A short review on presence of pharmaceuticals in water bodies and the potential of chitosan and chitosan derivatives for elimination of pharmaceuticals. Journal of Molecular and Genetic J Medicine, S4, 001. [Google Scholar]
- An B, Jung K-Y, Zhao D, Lee S-H, & Choi J-W (2014). Preparation and characterization of polymeric ligand exchanger based on chitosan hydrogel for selective removal of phosphate. Reactive and Functional Polymers, 85, 45–53. [Google Scholar]
- An B, Lee H, Lee S, Lee S-H, & Choi J-W (2015). Determining the selectivity of divalent metal cations for the carboxyl group of alginate hydrogel beads during competitive sorption. Journal of Hazardous Materials, 298, 11–18. [DOI] [PubMed] [Google Scholar]
- Anaya-Esparza LM, Ruvalcaba-Gómez JM, Maytorena-Verdugo CI, González-Silva N, Romero-Toledo R, Aguilera-Aguirre S, et al. (2020). Chitosan-TiO2: A versatile hybrid composite. Materials, 13(4), 811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angellier H, Molina-Boisseau S, & Dufresne A (2005). Mechanical properties of waxy maize starch nanocrystal reinforced natural rubber. Macromolecules, 38(22), 9161–9170. [Google Scholar]
- Anirudhan TS, & Rejeena SR (2012). Adsorption and hydrolytic activity of trypsin on a carboxylate-functionalized cation exchanger prepared from nanocellulose. Journal of Colloid and Interface Science, 381(1), 125–136. [DOI] [PubMed] [Google Scholar]
- Anirudhan TS, & Rejeena SR (2013). Selective adsorption of hemoglobin using polymer-grafted-magnetite nanocellulose composite. Carbohydrate Polymers, 93(2), 518–527. [DOI] [PubMed] [Google Scholar]
- Anirudhan TS, & Rijith S (2012). Synthesis and characterization of carboxyl terminated poly(methacrylic acid) grafted chitosan/bentonite composite and its application for the recovery of uranium(VI) from aqueous media. Journal of Environmental Radioactivity, 106, 8–19. [DOI] [PubMed] [Google Scholar]
- Anirudhan TS, & Shainy F (2015). Effective removal of mercury(II) ions from chlor-alkali industrial wastewater using 2-mercaptobenzamide modified itaconic acid-grafted-magnetite nanocellulose composite. Journal of Colloid and Interface Science, 456, 22–31. [DOI] [PubMed] [Google Scholar]
- Anirudhan TS, Deepa JR, & Christa J (2016). Nanocellulose/nanobentonite composite anchored with multi-carboxyl functional groups as an adsorbent for the effective removal of Cobalt(II) from nuclear industry wastewater samples. Journal of Colloid and Interface Science, 467, 307–320. [DOI] [PubMed] [Google Scholar]
- Anjum F, Gul S, Khan MI, & Khan MA (2019). Efficient synthesis of palladium nanoparticles using guar gum as stabilizer and their applications as catalyst in reduction reactions and degradation of azo dyes. Green Processing and Synthesis, 9(1), 63–76. [Google Scholar]
- Antony R, Marimuthu R, & Murugavel R (2019). Bimetallic nanoparticles anchored on core-shell support as an easily recoverable and reusable catalytic system for efficient nitroarene reduction. ACS Omega, 4(5), 9241–9250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabkhani P, & Asfaram A (2020). Development of a novel three-dimensional magnetic polymer aerogel as an efficient adsorbent for malachite green removal. Journal of Hazardous Materials, 384, Article 121394. [DOI] [PubMed] [Google Scholar]
- Arvand M, Latify L, Tajmehri H, Yagubov AI, Nuriyev AN, Pourhabib A, et al. (2009). Comparative study for the removal of oxadiazon from aqueous solutions by adsorption on chitosan and activated carbon. Analytical Letters, 42(6), 856–869. [Google Scholar]
- Aslani H, Kosari TE, Naseri S, Nabizadeh R, & Khazaei M (2018). Hexavalent chromium removal from aqueous solution using functionalized chitosan as a novel nano-adsorbent: Modeling and optimization, kinetic, isotherm, and thermodynamic studies, and toxicity testing. Environmental Science and Pollution Research, 25(20), 20154–20168. [DOI] [PubMed] [Google Scholar]
- Asthana A, Verma R, Singh AK, & Susan MABH (2016). Glycine functionalized magnetic nanoparticle entrapped calcium alginate beads: A promising adsorbent for removal of Cu(II) ions. Journal of Environmental Chemical Engineering, 4(2), 1985–1995. [Google Scholar]
- Atarod M, Nasrollahzadeh M, & Sajadi SM (2015). Green synthesis of a Cu/reduced graphene oxide/Fe3O4 nanocomposite using Euphorbia wallichii leaf extract and its application as a recyclable and heterogeneous catalyst for the reduction of 4-nitrophenol and rhodamine B. RSC Advances, 5(111), 91532–91543. [Google Scholar]
- Attallah OA, Al-Ghobashy MA, Nebsen M, & Salem MY (2017). Adsorptive removal of fluoroquinolones from water by pectin-functionalized magnetic nanoparticles: Process optimization using a spectrofluorimetric assay. ACS Sustainable Chemistry & Engineering, 5(1), 133–145. [Google Scholar]
- Azzam EMS, Eshaq GH, Rabie AM, Bakr AA, Abd-Elaal AA, El Metwally AE, et al. (2016). Preparation and characterization of chitosan-clay nanocomposites for the removal of Cu(II) from aqueous solution. International Journal of Biological Macromolecules, 89, 507–517. [DOI] [PubMed] [Google Scholar]
- Bagheri H, Roostaie A, & Baktash MY (2014). A chitosan-polypyrrole magnetic nanocomposite as μ-sorbent for isolation of naproxen. Analytica Chimica Acta, 816, 1–7. [DOI] [PubMed] [Google Scholar]
- Bahrami F, Yu X, Zou Y, Sun Y, & Sun G (2020). Impregnated calcium-alginate beads as floating reactors for the remediation of nitrate-contaminated groundwater. Chemical Engineering Journal, 382, Article 122774. [Google Scholar]
- Balanta A, Godard C, & Claver C (2011). Pd nanoparticles for C-C coupling reactions. Chemical Society Reviews, 40(10), 4973–4985. [DOI] [PubMed] [Google Scholar]
- Bandara PC, Nadres ET, & Rodrigues DF (2019). Use of response surface methodology to develop and optimize the composition of a chitosanpolyethyleneimine-graphene oxide nanocomposite membrane coating to more effectively remove Cr(VI) and Cu(II) from water. ACS Applied Materials & Interfaces, 11(19), 17784–17795. [DOI] [PubMed] [Google Scholar]
- Banerjee SS, & Chen D-H (2007). Fast removal of copper ions by gum arabic modified magnetic nano-adsorbent. Journal of Hazardous Materials, 147(3), 792–799. [DOI] [PubMed] [Google Scholar]
- Bao Y, Zhou Q, Zhang M, Zhang H, Luan Q, Zhou W, et al. (2019). Wet-spun nanoTiO2/chitosan nanocomposite fibers as efficient and retrievable absorbent for the removal of free fatty acids from edible oil. Carbohydrate Polymers, 210, 119–126. [DOI] [PubMed] [Google Scholar]
- Baran T (2018). Pd(0) nanocatalyst stabilized on a novel agar/pectin composite and its catalytic activity in the synthesis of biphenyl compounds by Suzuki-Miyaura cross coupling reaction and reduction of o-nitroaniline. Carbohydrate Polymers, 195, 45–52. [DOI] [PubMed] [Google Scholar]
- Baran T, & Nasrollahzadeh M (2019). Facile synthesis of palladium nanoparticles immobilized on magnetic biodegradable microcapsules used as effective and recyclable catalyst in Suzuki-Miyaura reaction and p-nitrophenol reduction. Carbohydrate Polymers, 222, Article 115029. [DOI] [PubMed] [Google Scholar]
- Baran T, & Nasrollahzadeh M (2020). Cyanation of aryl halides and Suzuki-Miyaura coupling reaction using palladium nanoparticles anchored on developed biodegradable microbeads. International Journal of Biological Macromolecules, 148, 565–573. [DOI] [PubMed] [Google Scholar]
- Barreto-Rodrigues M, Silveira J, García-Muñoz P, & Rodriguez JJ (2017). Dechlorination and oxidative degradation of 4-chlorophenol with nanostructured iron-silver alginate beads. Journal of Environmental Chemical Engineering, 5(1), 838–842. [Google Scholar]
- Barriada JL, Herrero R, Prada-Rodríguez D, & de Vicente MES (2008). Interaction of mercury with chitin: A physicochemical study of metal binding by a natural biopolymer. Reactive & Functional Polymers, 68(12), 1609–1618. [Google Scholar]
- Basu H, Saha S, Pimple MV, & Singhal RK (2019). Novel hybrid material humic acid impregnated magnetic chitosan nano particles for decontamination of uranium from aquatic environment. Journal of Environmental Chemical Engineering, 7(3), Article 103110. [Google Scholar]
- Batmaz R, Mohammed N, Zaman M, Minhas G, Berry RM, & Tam KC (2014). Cellulose nanocrystals as promising adsorbents for the removal of cationic dyes. Cellulose, 21(3), 1655–1665. [Google Scholar]
- Bavasso I, Vuppala S, & Cianfrini C (2019). Cr (vi) removal by chitosan-magnetite nano-composite in aqueous solution. Chemical Engineering Transactions, 73, 163–168. [Google Scholar]
- Benhachem FZ, Attar T, & Bouabdallah F (2019). Kinetic study of adsorption methylene blue dye from aqueous solutions using activated carbon. Chemical Review and Letters, 2(1), 33–39. [Google Scholar]
- Bergamonti L, Bergonzi C, Graiff C, Lottici PP, Bettini R, & Elviri L (2019). 3D printed chitosan scaffolds: A new TiO2 support for the photocatalytic degradation of amoxicillin in water. Water Research, 163, Article 114841. [DOI] [PubMed] [Google Scholar]
- Bhangi BK, & Ray SK (2020). Nano silver chloride and alginate incorporated composite copolymer adsorbent for adsorption of a synthetic dye from water in a fixed bed column and its photocatalytic reduction. International Journal of Biological Macromolecules, 144, 801–812. [DOI] [PubMed] [Google Scholar]
- Bhatt R, Ageetha V, Rathod SB, & Padmaja P (2019). Self-assembled chitosanzirconium phosphate nanostructures for adsorption of chromium and degradation of dyes. Carbohydrate Polymers, 208, 441–450. [DOI] [PubMed] [Google Scholar]
- Boddu VM, Abburi K, Talbott JL, Smith ED, & Haasch R (2008). Removal of arsenic(III) and arsenic(V) from aqueous medium using chitosan-coated biosorbent. Water Research, 42(3), 633–642. [DOI] [PubMed] [Google Scholar]
- Bok-Badura J, Jakóbik-Kolon A, Karoń K, & Mitko K (2018). Sorption studies of heavy metal ions on pectin-nano-titanium dioxide composite adsorbent. Separation Science and Technology, 53(7), 1034–1044. [Google Scholar]
- Borsagli FGLM, Mansur AAP, Chagas P, Oliveira LCA, & Mansur HS (2015). O-carboxymethyl functionalization of chitosan: Complexation and adsorption of Cd(II) and Cr(VI) as heavy metal pollutant ions. Reactive and Functional Polymers, 97, 37–47. [Google Scholar]
- Brandes R, Carminatti C, Mikowski A, Al-Qureshi H, & Recouvreux D (2017). A mini-review on the progress of spherical bacterial cellulose production. Journal of Nano Research, 45, 142–154. [Google Scholar]
- Budarin VL, Clark JH, Luque R, Macquarrie DJ, & White RJ (2008). Palladium nanoparticles on polysaccharide-derived mesoporous materials and their catalytic performance in C–C coupling reactions. Green Chemistry, 10(4), 382–387. [Google Scholar]
- Cadaval TRS, Dotto GL, Seus ER, Mirlean N, & de Almeida Pinto LA (2016). Vanadium removal from aqueous solutions onto chitosan films. Desalination and Water Treatment, 57(35), 16583–16591. [Google Scholar]
- Cai Z, Dwivedi AD, Lee W-N, Zhao X, Liu W, Sillanpää M, et al. (2018). Application of nanotechnologies for removing pharmaceutically active compounds from water: Development and future trends. Environmental Science: Nano, 5(1), 27–47. [Google Scholar]
- Campano C, Balea A, Blanco A, & Negro C (2016). Enhancement of the fermentation process and properties of bacterial cellulose: A review. Cellulose, 23(1), 57–91. [Google Scholar]
- Carpenter AW, de Lannoy C-F, & Wiesner MR (2015). Cellulose nanomaterials in water treatment technologies. Environmental Science & Technology, 49(9), 5277–5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamjangali MA, Bagherian G, Javid A, Boroumand S, & Farzaneh N (2015). Synthesis of Ag-ZnO with multiple rods (multipods) morphology and its application in the simultaneous photo-catalytic degradation of methyl orange and methylene blue. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 150, 230–237. [DOI] [PubMed] [Google Scholar]
- Chan CH, Chia CH, Zakaria S, Sajab MS, & Chin SX (2015). Cellulose nanofibrils: A rapid adsorbent for the removal of methylene blue. RSC Advances, 5 (24), 18204–18212. [Google Scholar]
- Chang Y-C, & Chen D-H (2005). Adsorption kinetics and thermodynamics of acid dyes on a carboxymethylated chitosan-conjugated magnetic nano-adsorbent. Macromolecular Bioscience, 5(3), 254–261. [DOI] [PubMed] [Google Scholar]
- Chang J, Woo H, Ko M-S, Lee J, Lee S, Yun S-T, et al. (2015). Targeted removal of trichlorophenol in water by oleic acid-coated nanoscale palladium/zero-valent iron alginate beads. Journal of Hazardous Materials, 293, 30–36. [DOI] [PubMed] [Google Scholar]
- Chasteen ND, & Harrison PM (1999). Mineralization in ferritin: An efficient means of iron storage. Journal of Structural Biology, 126(3), 182–194. [DOI] [PubMed] [Google Scholar]
- Chawla PR, Bajaj IB, Survase SA, & Singhal RS (2009). Microbial cellulose: Fermentative production and applications. Food Technology and Biotechnology, 47(2), 107–124. [Google Scholar]
- Chen S, & Huang Y (2015). Bacterial cellulose nanofibers decorated with phthalocyanine: Preparation, characterization and dye removal performance. Materials Letters, 142, 235–237. [Google Scholar]
- Chen D-Z, Fang J-Y, Shao Q, Ye J-X, Ouyang D-J, & Chen J-M (2013). Biodegradation of tetrahydrofuran by Pseudomonas oleovorans DT4 immobilized in calcium alginate beads impregnated with activated carbon fiber: mass transfer effect and continuous treatment. Bioresource Technology, 139, 87–93. [DOI] [PubMed] [Google Scholar]
- Chen L, Berry RM, & Tam KC (2014). Synthesis of β-cyclodextrin-modified cellulose nanocrystals (CNCs)@Fe3O4@SiO2 superparamagnetic nanorods. ACS Sustainable Chemistry & Engineering, 2(4), 951–958. [Google Scholar]
- Chen L, Yu H, Deutschman C, Yang T, & Tam KC (2020). Novel design of Fe-Cu alloy coated cellulose nanocrystals with strong antibacterial ability and efficient Pb2+ removal. Carbohydrate Polymers, 234, Article 115889. [DOI] [PubMed] [Google Scholar]
- Chen X, Liu L, Luo Z, Shen J, Ni Q, & Yao J (2018). Facile preparation of a cellulose-based bioadsorbent modified by hPEI in heterogeneous system for high-efficiency removal of multiple types of dyes. Reactive and Functional Polymers, 125, 77–83. [Google Scholar]
- Chen L, Cao W, Quinlan PJ, Berry RM, & Tam KC (2015). Sustainable catalysts from gold-loaded polyamidoamine dendrimer-cellulose nanocrystals. ACS Sustainable Chemistry & Engineering, 3(5), 978–985. [Google Scholar]
- Chen G, Song K, Huang X, & Wang W (2019). Removal of toluene and Pb(II) using a novel adsorbent modified by titanium dioxide and chitosan. Journal of Molecular Liquids, 295, Article 111683. [Google Scholar]
- Chen H, Xie H, Zhou J, Tao Y, Zhang Y, Zheng Q, et al. (2019). Removal efficiency of hexavalent chromium from wastewater using starch-stabilized nanoscale zero-valent iron. Water Science and Technology, 80(6), 1076–1084. [DOI] [PubMed] [Google Scholar]
- Chen D, Yang K, Wang H, Zhou J, & Zhang H (2015). Cr(VI) removal by combined redox reactions and adsorption using pectin-stabilized nanoscale zero-valent iron for simulated chromium contaminated water. RSC Advances, 5(80), 65068–65073. [Google Scholar]
- Cheng Y, Luo X, Payne GF, & Rubloff GW (2012). Biofabrication: Programmable assembly of polysaccharide hydrogels in microfluidics as biocompatible scaffolds. Journal of Materials Chemistry, 22(16), 7659–7666. [Google Scholar]
- Chivrac F, Pollet E, Schmutz M, & Avérous L (2010). Starch nano-biocomposites based on needle-like sepiolite clays. Carbohydrate Polymers, 80(1), 145–153. [Google Scholar]
- Côrtes LN, Tanabe EH, Bertuol DA, & Dotto GL (2015). Biosorption of gold from computer microprocessor leachate solutions using chitin. Waste Management, 45, 272–279. [DOI] [PubMed] [Google Scholar]
- Costa SV, Gonçalves AS, Zaguete MA, Mazon T, & Nogueira AF (2013). ZnO nanostructures directly grown on paper and bacterial cellulose substrates without any surface modification layer. Chemical Communications, 49(73), 8096–8098. [DOI] [PubMed] [Google Scholar]
- Crini G (2005). Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Progress in Polymer Science, 30(1), 38–70. [Google Scholar]
- Crini G (2006). Non-conventional low-cost adsorbents for dye removal: A review. Bioresource Technology, 97(9), 1061–1085. [DOI] [PubMed] [Google Scholar]
- Crini G, Morin-Crini N, Fatin-Rouge N, Deon S, & Fievet P (2017). Metal removal from aqueous media by polymer-assisted ultrafiltration with chitosan. Arabian Journal of Chemistry, 10, S3826–S3839. [Google Scholar]
- Cui J, Xu X, Yang L, Chen C, Qian J, Chen X, et al. (2020). Soft foam-like UiO-66/polydopamine/bacterial cellulose composite for the removal of aspirin and tetracycline hydrochloride. Chemical Engineering Journal, 395, Article 125174. [Google Scholar]
- Dai L, Cheng T, Xi X, Nie S, Ke H, Liu Y, et al. (2020). A versatile TOCN/CGG self-assembling hydrogel for integrated wastewater treatment. Cellulose, 27(2), 915–925. [Google Scholar]
- Dai L, Liu R, Hu L-Q, & Si C-L (2017). Simple and green fabrication of AgCl/Ag-cellulose paper with antibacterial and photocatalytic activity. Carbohydrate Polymers, 174, 450–455. [DOI] [PubMed] [Google Scholar]
- Dai R, Chen J, Lin J, Xiao S, Chen S, & Deng Y (2009). Reduction of nitro phenols using nitroreductase from E. coli in the presence of NADH. Journal of Hazardous Materials, 170(1), 141–143. [DOI] [PubMed] [Google Scholar]
- Darabitabar F, Yavari V, Hedayati A, Zakeri M, & Yousefi H (2020). Novel cellulose nanofiber aerogel for aquaculture wastewater treatment. Environmental Technology & Innovation, 18, Article 100786. [Google Scholar]
- Dehghani MH, Dehghan A, Alidadi H, Dolatabadi M, Mehrabpour M, & Converti A (2017). Removal of methylene blue dye from aqueous solutions by a new chitosan/zeolite composite from shrimp waste: Kinetic and equilibrium study. The Korean Journal of Chemical Engineering, 34(6), 1699–1707. [Google Scholar]
- Den W, Sharma VK, Lee M, Nadadur G, & Varma RS (2018). Lignocellulosic biomass transformations via greener oxidative pretreatment processes: Access to energy and value-added chemicals. Frontiers in Chemistry, 6, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C, Liu J, Zhou W, Zhang Y-K, Du K-F, & Zhao Z-M (2012). Fabrication of spherical cellulose/carbon tubes hybrid adsorbent anchored with welan gum polysaccharide and its potential in adsorbing methylene blue. Chemical Engineering Journal, 200, 452–458. [Google Scholar]
- Derami HG, Jiang Q, Ghim D, Cao S, Chandar YJ, Morrissey JJ, et al. (2019). A robust and scalable polydopamine/bacterial nanocellulose hybrid membrane for efficient wastewater treatment. ACS Applied Nano Materials, 2(2), 1092–1101. [Google Scholar]
- Devi DK, Pratap SV, Haritha R, Sivudu KS, Radhika P, & Sreedhar B (2011). Gum acacia as a facile reducing, stabilizing, and templating agent for palladium nanoparticles. Journal of Applied Polymer Science, 121(3), 1765–1773. [Google Scholar]
- Dong H, He Q, Zeng G, Tang L, Zhang C, Xie Y, et al. (2016). Chromate removal by surface-modified nanoscale zero-valent iron: Effect of different surface coatings and water chemistry. Journal of Colloid and Interface Science, 471, 7–13. [DOI] [PubMed] [Google Scholar]
- Dong H, Ning Q, Li L, Wang Y, Wang B, Zhang L, et al. (2020). A comparative study on the activation of persulfate by bare and surface-stabilized nanoscale zero-valent iron for the removal of sulfamethazine. Separation and Purification Technology, 230, Article 115869. [Google Scholar]
- Donia AM, Atia AA, & Elwakeel KZ (2007). Recovery of gold(III) and silver(I) on a chemically modified chitosan with magnetic properties. Hydrometallurgy, 87(3–4), 197–206. [Google Scholar]
- Dotto GL, & Pinto L (2017). General considerations about Chitosan. Materials and its applications (pp. 3–33). Sharjah: Bentham Science Publishers. [Google Scholar]
- Dotto GL, Cunha JM, Calgaro CO, Tanabe EH, & Bertuol DA (2015). Surface modification of chitin using ultrasound-assisted and supercritical CO2 technologies for cobalt adsorption. Journal of Hazardous Materials, 295, 29–36. [DOI] [PubMed] [Google Scholar]
- Doustkhah E, & Rostamnia S (2016). Covalently bonded sulfonic acid magnetic graphene oxide: Fe3O4@GO-Pr-SO3H as a powerful hybrid catalyst for synthesis of indazolophthalazinetriones. Journal of Colloid and Interface Science, 478, 280–287. [DOI] [PubMed] [Google Scholar]
- Duan C, Liu C, Meng X, Gao K, Lu W, Zhang Y, et al. (2020). Facile synthesis of Ag NPs@MIL-100 (Fe)/guar gum hybrid hydrogel as a versatile photocatalyst for wastewater remediation: Photocatalytic degradation, water/oil separation and bacterial inactivation. Carbohydrate Polymers, 230, Article 115642. [DOI] [PubMed] [Google Scholar]
- Dwivedi AD, Dubey SP, Hokkanen S, & Sillanpää M (2014). Mechanistic investigation on the green recovery of ionic, nanocrystalline, and metallic gold by two anionic nanocelluloses. Chemical Engineering Journal, 253, 316–324. [Google Scholar]
- El Fadl FIA, Mahmoud GA, & Mohamed AA (2019). Effect of metal nanoparticles on the catalytic activity of pectin (poly vinyl alcohol-co-polyacrylamide) nanocomposite hydrogels. Journal of Inorganic and Organometallic Polymers and Materials, 29(2), 332–339. [Google Scholar]
- Elkady M, Shokry H, El-Sharkawy A, El-Subruiti G, & Hamad H (2019). New insights into the activity of green supported nanoscale zero-valent iron composites for enhanced acid blue-25 dye synergistic decolorization from aqueous medium. Journal of Molecular Liquids, 294, Article 111628. [Google Scholar]
- Eltaweil AS, Elgarhy GS, El-Subruiti GM, & Omer AM (2020). Novel carboxymethyl cellulose/carboxylated graphene oxide composite microbeads for efficient adsorption of cationic methylene blue dye. International Journal of Biological Macromolecules, 154, 307–318. [DOI] [PubMed] [Google Scholar]
- Ertas Y, & Uyar T (2017). Fabrication of cellulose acetate/polybenzoxazine cross-linked electrospun nanofibrous membrane for water treatment. Carbohydrate Polymers, 177, 378–387. [DOI] [PubMed] [Google Scholar]
- Esquivel-Pena V, Guccini V, Kumar S, Salazar-Alvarez G, de San Miguel ER, & de Gyves J (2020). Hybrids based on borate-functionalized cellulose nanofibers and noble-metal nanoparticles as sustainable catalysts for environmental applications. RSC Advances, 10(21), 12460–12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Li K, Li J, Ying D, Wang Y, & Jia J (2017). Comparative and competitive adsorption of Pb(II) and Cu(II) using tetraethylenepentamine modified chitosan/CoFe2O4 particles. Journal of Hazardous Materials, 326, 211–220. [DOI] [PubMed] [Google Scholar]
- Fan G, Cang L, Qin W, Zhou C, Gomes HI, & Zhou D (2013). Surfactants-enhanced electrokinetic transport of xanthan gum stabilized nanoPd/Fe for the remediation of PCBs contaminated soils. Separation and Purification Technology, 114, 64–72. [Google Scholar]
- Fan L, Luo C, Sun M, Qiu H, & Li X (2013). Synthesis of magnetic β-cyclodextrinchitosan/graphene oxide as nanoadsorbent and its application in dye adsorption and removal. Colloids and Surfaces B: Biointerfaces, 103, 601–607. [DOI] [PubMed] [Google Scholar]
- Fang Q, Zhou X, Deng W, Zheng Z, & Liu Z (2016). Freestanding bacterial cellulose-graphene oxide composite membranes with high mechanical strength for selective ion permeation. Scientific Reports, 6(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W, Jiang X, Luo H, & Geng J (2018). Synthesis of graphene/SiO2@polypyrrole nanocomposites and their application for Cr (VI) removal in aqueous solution. Chemosphere, 197, 594–602. [DOI] [PubMed] [Google Scholar]
- Farokhia M, Parvareha A, & Moravejia MK (2019). Adsorption optimization of Cr (VI) and Co(II) onto the synthesized chitosan/cerium oxide/iron oxide nano-composite in water system using RSM according to CCD method. Desalination and Water Treatment, 143, 240–255. [Google Scholar]
- Fosso-Kankeu E, Mittal H, Waanders F, Ntwampe IO, & Ray SS (2016). Preparation and characterization of gum karaya hydrogel nanocomposite flocculant for metal ions removal from mine effluents. International Journal of Environmental Science and Technology, 13(2), 711–724. [Google Scholar]
- Gan L, Zhong Q, Geng A, Wang L, Song C, Han S, et al. (2019). Cellulose derived carbon nanofiber: A promising biochar support to enhance the catalytic performance of CoFe2O4 in activating peroxymonosulfate for recycled dimethyl phthalate degradation. Science of the Total eEnvironment, 694, Article 133705. [DOI] [PubMed] [Google Scholar]
- Gandhi MR, Kousalya GN, & Meenakshi S (2011). Removal of copper(II) using chitin/chitosan nano-hydroxyapatite composite. International Journal of Biological Macromolecules, 48(1), 119–124. [DOI] [PubMed] [Google Scholar]
- Gao C, An Q, Xiao Z, Zhai S, Zhai B, & Shi Z (2018). Alginate and polyethyleneimine dually mediated synthesis of nanosilver-containing composites for efficient p-nitrophenol reduction. Carbohydrate Polymers, 181, 744–751. [DOI] [PubMed] [Google Scholar]
- Gao M, Zhang D, Li W, Chang J, Lin Q, Xu D, et al. (2016). Degradation of methylene blue in a heterogeneous Fenton reaction catalyzed by chitosan crosslinked ferrous complex. Journal of the Taiwan Institute of Chemical Engineers, 67, 355–361. [Google Scholar]
- Garba ZN, Zhou W, Zhang M, & Yuan Z (2020). A review on the preparation, characterization and potential application of perovskites as adsorbents for wastewater treatment. Chemosphere, 244, Article 125474. [DOI] [PubMed] [Google Scholar]
- Garba ZN, Xiao W, Zhou W, Lawan I, Jiang Y, Zhang M, et al. (2019). Process optimization and synthesis of lanthanum-cobalt perovskite type nanoparticles (LaCoO3) prepared by modified proteic method: Application of response surface methodology. The Korean Journal of Chemical Engineering, 36(11), 1826–1838. [Google Scholar]
- Garba ZN, Zhou W, Lawan I, Zhang M, & Yuan Z (2019). Enhanced removal of prometryn using copper modified microcrystalline cellulose (Cu-MCC): Optimization, isotherm, kinetics and regeneration studies. Cellulose, 26(10), 6241–6258. [Google Scholar]
- Ghaderi A, Gholinejad M, & Firouzabadi H (2016). Palladium deposited on naturally occurring supports as a powerful catalyst for carbon-carbon bond formation reactions. Current Organic Chemistry, 20(4), 327–348. [Google Scholar]
- Ghaedi M, Sadeghian B, Pebdani AA, Sahraei R, Daneshfar A, & Duran C (2012). Kinetics, thermodynamics and equilibrium evaluation of direct yellow 12 removal by adsorption onto silver nanoparticles loaded activated carbon. Chemical Engineering Journal, 187, 133–141. [Google Scholar]
- Gholinejad M, Saadati F, Shaybanizadeh S, & Pullithadathil B (2016). Copper nanoparticles supported on starch micro particles as a degradable heterogeneous catalyst for three-component coupling synthesis of propargylamines. RSC Advances, 6(6), 4983–4991. [Google Scholar]
- Ghorai S, Sinhamahpatra A, Sarkar A, Panda AB, & Pal S (2012). Novel biodegradable nanocomposite based on XG-g-PAM/SiO2: Application of an efficient adsorbent for Pb2+ ions from aqueous solution. Bioresource Technology, 119, 181–190. [DOI] [PubMed] [Google Scholar]
- Ghosh Chaudhuri R, & Paria S (2012). Core/shell nanoparticles: Classes, properties, synthesis mechanisms, characterization, and applications. Chemical Reviews, 112(4), 2373–2433. [DOI] [PubMed] [Google Scholar]
- Gibbs G, Tobin JM, & Guibal E (2003). Sorption of Acid Green 25 on chitosan: Influence of experimental parameters on uptake kinetics and sorption isotherms. Journal of Applied Polymer Science, 90(4), 1073–1080. [Google Scholar]
- Gong G, Zhang F, Cheng Z, & Zhou L (2015). Facile fabrication of magnetic carboxymethyl starch/poly(vinyl alcohol) composite gel for methylene blue removal. International Journal of Biological Macromolecules, 81, 205–211. [DOI] [PubMed] [Google Scholar]
- Gong J-L, Wang X-Y, Zeng G-M, Chen L, Deng J-H, Zhang X-R, et al. (2012). Copper(II) removal by pectin–iron oxide magnetic nanocomposite adsorbent. Chemical Engineering Journal, 185–186, 100–107. [Google Scholar]
- Googerdchian F, Moheb A, & Emadi R (2012). Lead sorption properties of nanohydroxyapatite–alginate composite adsorbents. Chemical Engineering Journal, 200, 471–479. [Google Scholar]
- Gopalakrishnan A, Singh SP, & Badhulika S (2020). Reusable, few-layered-MoS2 nanosheets/graphene hybrid on cellulose paper for superior adsorption of methylene blue dye. New Journal of Chemistry, 44(14), 5489–5500. [Google Scholar]
- Gopi S, Balakrishnan P, Divya C, Valic S, Bajsic EG, Pius A, et al. (2017). Facile synthesis of chitin nanocrystals decorated on 3D cellulose aerogels as a new multi-functional material for waste water treatment with enhanced anti-bacterial and anti-oxidant properties. New Journal of Chemistry, 41(21), 12746–12755. [Google Scholar]
- Guo L, Li G, Liu J, Meng Y, & Tang Y (2013). Adsorptive decolorization of methylene blue by crosslinked porous starch. Carbohydrate Polymers, 93(2), 374–379. [DOI] [PubMed] [Google Scholar]
- Guo J, Wang J, Zheng G, & Jiang X (2019a). Optimization of the removal of reactive golden yellow SNE dye by cross-linked cationic starch and its adsorption properties. Journal of Engineered Fibers and Fabrics, 14, 1–13. [Google Scholar]
- Guo J, Wang J, Zheng G, & Jiang X (2019b). A TiO2/crosslinked carboxymethyl starch composite for high-efficiency adsorption and photodegradation of cationic golden yellow X-GL dye. Environmental Science and Pollution Research, 26(24), 24395–24406. [DOI] [PubMed] [Google Scholar]
- Gupta K, Kumar V, Tikoo KB, Kaushik A, & Singhal S (2020). Encrustation of cadmium sulfide nanoparticles into the matrix of biomass derived silanized cellulose nanofibers for adsorptive detoxification of pesticide and textile waste. Chemical Engineering Journal, 385, Article 123700. [Google Scholar]
- Gupta VK, Agarwal S, Pathania D, Kothiyal NC, & Sharma G (2013). Use of pectin-thorium(IV) tungstomolybdate nanocomposite for photocatalytic degradation of methylene blue. Carbohydrate Polymers, 96(1), 277–283. [DOI] [PubMed] [Google Scholar]
- Gupta VK, Pathania D, Agarwal S, & Singh P (2012). Adsorptional photocatalytic degradation of methylene blue onto pectin–CuS nanocomposite under solar light. Journal of Hazardous Materials, 243, 179–186. [DOI] [PubMed] [Google Scholar]
- Gupta VK, Yola ML, Eren T, Kartal F, Çağlayan MO, & Atar N (2014). Catalytic activity of Fe@Ag nanoparticle involved calcium alginate beads for the reduction of nitrophenols. Journal of Molecular Liquids, 190, 133–138. [Google Scholar]
- Hassan ME, Chen J, Liu G, Zhu D, & Cai J (2014). Enhanced photocatalytic degradation of methyl orange dye under the daylight irradiation over CN-TiO2 modified with OMS-2. Materials, 7(12), 8024–8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani A, Soltani RDC, Karaca S, & Khataee A (2015). Preparation of montmorillonite-alginate nanobiocomposite for adsorption of a textile dye in aqueous phase: isotherm, kinetic and experimental design approaches. Journal of Industrial and Engineering Chemistry, 21, 1197–1207. [Google Scholar]
- Hatamifard A, Nasrollahzadeh M, & Lipkowski J (2015). Green synthesis of a natrolite zeolite/palladium nanocomposite and its application as a reusable catalyst for the reduction of organic dyes in a very short time. RSC Advances, 5(111), 91372–91381. [Google Scholar]
- Hatamifard A, Nasrollahzadeh M, & Sajadi SM (2016). Biosynthesis, characterization and catalytic activity of an Ag/zeolite nanocomposite for base-and ligand-free oxidative hydroxylation of phenylboronic acid and reduction of a variety of dyes at room temperature. New Journal of Chemistry, 40(3), 2501–2513. [Google Scholar]
- He N, Li L, Wang P, Zhang J, Chen J, & Zhao J (2019). Dioxide/chitosan/poly (lactide-co-caprolactone) composite membrane with efficient Cu(II) adsorption. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 580, Article 123687. [Google Scholar]
- He X, Male KB, Nesterenko PN, Brabazon D, Paull B, & Luong JHT (2013). Adsorption and desorption of methylene blue on porous carbon monoliths and nanocrystalline cellulose. ACS Applied Materials & Interfaces, 5(17), 8796–8804. [DOI] [PubMed] [Google Scholar]
- Hebbalalu D, Lalley J, Nadagouda MN, & Varma RS (2013). Greener techniques for the synthesis of silver nanoparticles using plant extracts, enzymes, bacteria, biodegradable polymers, and microwaves. ACS Sustainable Chemistry & Engineering, 1 (7), 703–712. [Google Scholar]
- Herrera-Morales J, Morales K, Ramos D, Ortiz-Quiles EO, LopezEncarnacion JM, & Nicolau E (2017). Examining the use of nanocellulose composites for the sorption of contaminants of emerging concern: An experimental and computational study. ACS Omega, 2(11), 7714–7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herreros-López A, Hadad C, Yate L, Alshatwi AA, Vicentini N, Carofiglio T, et al. (2016). Synthesis and catalytic activity of gold nanoparticles supported on dendrimeric nanocellulose hybrids. European Journal of Organic Chemistry, 2016(19), 3186–3192. [Google Scholar]
- Hladik ML, Roberts AL, & Bouwer EJ (2005). Removal of neutral chloroacetamide herbicide degradates during simulated unit processes for drinking water treatment. Water Research, 39(20), 5033–5044. [DOI] [PubMed] [Google Scholar]
- Hokkanen S, Repo E, Lou S, & Sillanpää M (2015). Removal of arsenic(V) by magnetic nanoparticle activated microfibrillated cellulose. Chemical Engineering Journal, 260, 886–894. [Google Scholar]
- Hokkanen S, Repo E, Bhatnagar A, Tang WZ, & Sillanpää M (2014). Adsorption of hydrogen sulphide from aqueous solutions using modified nano/micro fibrillated cellulose. Environmental Technology, 35(18), 2334–2346. [DOI] [PubMed] [Google Scholar]
- Hokkanen S, Repo E, Westholm LJ, Lou S, Sainio T, & Sillanpää M (2014). Adsorption of Ni2+, Cd2+, and from aqueous solutions by nanostructured microfibrillated cellulose modified with carbonated hydroxyapatite. Chemical Engineering Journal, 252, 64–74. [Google Scholar]
- Hu C, Zhu P, Cai M, Hu H, & Fu Q (2017). Comparative adsorption of Pb(II), Cu(II) and Cd(II) on chitosan saturated montmorillonite: Kinetic, thermodynamic and equilibrium studies. Applied Clay Science, 143, 320–326. [Google Scholar]
- Hu D, Jiang R, Wang N, Xu H, Wang Y-G, & Ouyang X. k. (2019). Adsorption of diclofenac sodium on bilayer amino-functionalized cellulose nanocrystals/chitosan composite. Journal of Hazardous Materials, 369, 483–493. [DOI] [PubMed] [Google Scholar]
- Hu J, Chen G, & Lo IMC (2005). Removal and recovery of Cr(VI) from wastewater by maghemite nanoparticles. Water Research, 39(18), 4528–4536. [DOI] [PubMed] [Google Scholar]
- Huang H, Wu J, Lin X, Li L, Shang S, Yuen M. C. w., et al. (2013). Self-assembly of polypyrrole/chitosan composite hydrogels. Carbohydrate Polymers, 95(1), 72–76. [DOI] [PubMed] [Google Scholar]
- Huang J, Chang PR, Lin N, & Dufresne A (2014). Polysaccharide-based nanocrystals: Chemistry and applications (p. 328). John Wiley & Sons. [Google Scholar]
- Huang R, Liu Z, Sun B, & Fatehi P (2016). Preparation of dialdehyde cellulose nanocrystal as an adsorbent for creatinine. The Canadian Journal of Chemical Engineering, 94(8), 1435–1441. [Google Scholar]
- Huang W, Liu N, Zhang X, Wu M, & Tang L (2017). Metal organic framework gC3N4/MIL-53 (Fe) heterojunctions with enhanced photocatalytic activity for Cr(VI) reduction under visible light. Applied Surface Science, 425, 107–116. [Google Scholar]
- Huang X, Li B, Wang S, Yue X, Zhengguo Y, Deng X, et al. (2020). Facile in-situ synthesis of PEI-Pt modified bacterial cellulose bio-adsorbent and its distinctly selective adsorption of anionic dyes. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 586, Article 124163. [Google Scholar]
- Hussain MS, Musharraf SG, Bhanger MI, & Malik MI (2020). Salicylaldehyde derivative of nano-chitosan as an efficient adsorbent for lead(II), copper(II), and cadmium(II) ions. International Journal of Biological Macromolecules, 147, 643–652. [DOI] [PubMed] [Google Scholar]
- Idris A, Ismail NSM, Hassan N, Misran E, & Ngomsik A-F (2012). Synthesis of magnetic alginate beads based on maghemite nanoparticles for Pb(II) removal in aqueous solution. Journal of Industrial and Engineering Chemistry, 18(5), 1582–1589. [Google Scholar]
- Ihsanullah. (2019). Carbon nanotube membranes for water purification: Developments, challenges, and prospects for the future. Separation and Purification Technology, 209, 307–337. [Google Scholar]
- Iqbal DN, & Hussain EA (2013). Green biopolymer guar gum and its derivatives. International Journal of Pharma and Bio Sciences, 4(3), 423–435. [Google Scholar]
- Iqbal J, Shah NS, Sayed M, Imran M, Muhammad N, Howari FM, et al. (2019). Synergistic effects of activated carbon and nano-zerovalent copper on the performance of hydroxyapatite-alginate beads for the removal of As3+ from aqueous solution. Journal of Cleaner Production, 235, 875–886. [Google Scholar]
- Iravani S, & Varma RS (2020a). Bacteria in heavy metal remediation and nanoparticle biosynthesis. ACS Sustainable Chemistry & Engineering, 8(14), 5395–5409. [Google Scholar]
- Iravani S, & Varma RS (2020b). Greener synthesis of lignin nanoparticles and their applications. Green Chemistry, 22(3), 612–636. [Google Scholar]
- Jagtap S, Yenkie MKN, Labhsetwar N, & Rayalu S (2011). Defluoridation of drinking water using chitosan based mesoporous alumina. Microporous and Mesoporous Materials, 142(2–3), 454–463. [Google Scholar]
- Jahan K, Kumar N, & Verma V (2018). Removal of hexavalent chromium from potable drinking using a polyaniline-coated bacterial cellulose mat. Environmental Science: Water Research & Technology, 4(10), 1589–1603. [Google Scholar]
- Javanbakht V, Ghoreishi SM, Habibi N, & Javanbakht M (2016). A novel magnetic chitosan/clinoptilolite/magnetite nanocomposite for highly efficient removal of Pb (II) ions from aqueous solution. Powder Technology, 302, 372–383. [Google Scholar]
- Jawad AH, Mubarak NSA, & Abdulhameed AS (2020a). Hybrid crosslinked chitosan-epichlorohydrin/TiO2 nanocomposite for reactive red 120 dye adsorption: kinetic, isotherm, thermodynamic, and mechanism study. Journal of Polymers and the Environment, 28(2), 624–637. [Google Scholar]
- Jawad AH, Mubarak NSA, & Abdulhameed AS (2020b). Tunable Schiff’s base-cross-linked chitosan composite for the removal of reactive red 120 dye: Adsorption and mechanism study. International Journal of Biological Macromolecules, 142, 732–741. [DOI] [PubMed] [Google Scholar]
- Jbeli A, Ferraria AM, do Rego AMB, Boufi S, & Bouattour S (2018). Hybrid chitosan-TiO2/ZnS prepared under mild conditions with visible-light driven photocatalytic activity. International Journal of Biological Macromolecules, 116, 1098–1104. [DOI] [PubMed] [Google Scholar]
- Jbeli A, Hamden Z, Bouattour S, Ferraria AM, Conceição DS, Ferreira LFV, et al. (2018). Chitosan-Ag-TiO2 films: An effective photocatalyst under visible light. Carbohydrate Polymers, 199, 31–40. [DOI] [PubMed] [Google Scholar]
- Jenkins PJ, Cameron RE, & Donald AM (1993). A universal feature in the structure of starch granules from different botanical sources. Starch-Stärke, 45(12), 417–420. [Google Scholar]
- Jiang F, & Hsieh Y-L (2014). Amphiphilic superabsorbent cellulose nanofibril aerogels. Journal of Materials Chemistry A, 2(18), 6337–6342. [Google Scholar]
- Jiang N, Xu Y, Dai Y, Luo W, & Dai L (2012). Polyaniline nanofibers assembled on alginate microsphere for Cu2+ and Pb2+ uptake. Journal of Hazardous Materials, 215, 17–24. [DOI] [PubMed] [Google Scholar]
- Jiang X, Lou C, Hua F, Deng H, & Tian X (2020). Cellulose nanocrystals-based flocculants for high-speed and high-efficiency decolorization of colored effluents. Journal of Cleaner Production, 251, Article 119749. [Google Scholar]
- Jin L, Li W, Xu Q, & Sun Q (2015). Amino-functionalized nanocrystalline cellulose as an adsorbent for anionic dyes. Cellulose, 22(4), 2443–2456. [Google Scholar]
- Jin L, Sun Q, Xu Q, & Xu Y (2015). Adsorptive removal of anionic dyes from aqueous solutions using microgel based on nanocellulose and polyvinylamine. Bioresource Technology, 197, 348–355. [DOI] [PubMed] [Google Scholar]
- Jung W, Jeon B-H, Cho D-W, Roh H-S, Cho Y, Kim S-J, et al. (2015). Sorptive removal of heavy metals with nano-sized carbon immobilized alginate beads. Journal of Industrial and Engineering Chemistry, 26, 364–369. [Google Scholar]
- Jyothi AN, Moorthy SN, & Rajasekharan KN (2006). Effect of cross-linking with epichlorohydrin on the properties of cassava (Manihot esculenta Crantz) starch. Starch-Stärke, 58(6), 292–299. [Google Scholar]
- Jyothi MS, Angadi VJ, Kanakalakshmi TV, Padaki M, Geetha BR, & Soontarapa K (2019). Magnetic nanoparticles impregnated, cross-linked, porous chitosan microspheres for efficient adsorption of methylene blue from pharmaceutical waste water. Journal of Polymers and the Environment, 27(11), 2408–2418. [Google Scholar]
- Kadam AA, & Lee DS (2015). Glutaraldehyde cross-linked magnetic chitosan nanocomposites: Reduction precipitation synthesis, characterization, and application for removal of hazardous textile dyes. Bioresource Technology, 193, 563–567. [DOI] [PubMed] [Google Scholar]
- Kadam AA, Jang J, & Lee DS (2016). Facile synthesis of pectin-stabilized magnetic graphene oxide Prussian blue nanocomposites for selective cesium removal from aqueous solution. Bioresource Technology, 216, 391–398. [DOI] [PubMed] [Google Scholar]
- Kamal T, Ahmad I, Khan SB, & Asiri AM (2019). Anionic polysaccharide stabilized nickel nanoparticles-coated bacterial cellulose as a highly efficient dip-catalyst for pollutants reduction. Reactive and Functional Polymers, 145, Article 104395. [Google Scholar]
- Kanakaraju D, Ravichandar S, & Lim YC (2017). Combined effects of adsorption and photocatalysis by hybrid TiO2/ZnO-calcium alginate beads for the removal of copper. Journal of the Environmental Sciences, 55, 214–223. [DOI] [PubMed] [Google Scholar]
- Kanmani P, Aravind J, Kamaraj M, Sureshbabu P, & Karthikeyan S (2017). Environmental applications of chitosan and cellulosic biopolymers: A comprehensive outlook. Bioresource Technology, 242, 295–303. [DOI] [PubMed] [Google Scholar]
- Karim Z, Mathew AP, Grahn M, Mouzon J, & Oksman K (2014). Nanoporous membranes with cellulose nanocrystals as functional entity in chitosan: Removal of dyes from water. Carbohydrate Polymers, 112, 668–676. [DOI] [PubMed] [Google Scholar]
- Karnib M, Kabbani A, Holail H, & Olama Z (2014). Heavy metals removal using activated carbon, silica and silica activated carbon composite. Energy Procedia, 50, 113–120. [Google Scholar]
- Kartel MT, Kupchik LA, & Veisov BK (1999). Evaluation of pectin binding of heavy metal ions in aqueous solutions. Chemosphere, 38(11), 2591–2596. [DOI] [PubMed] [Google Scholar]
- Kattumuri V, Katti K, Bhaskaran S, Boote EJ, Casteel SW, Fent GM, et al. (2007). Gum arabic as a phytochemical construct for the stabilization of gold nanoparticles: In vivo pharmacokinetics and X-ray-contrast-imaging studies. Small, 3 (2), 333–341. [DOI] [PubMed] [Google Scholar]
- Kee YL, Mukherjee S, & Pariatamby A (2015). Effective remediation of phenol, 2,4-bis(1,1-dimethylethyl) and bis(2-ethylhexyl)phthalate in farm effluent using guar gum-A plant based biopolymer. Chemosphere, 136, 111–117. [DOI] [PubMed] [Google Scholar]
- Khamkeaw A, Jongsomjit B, Robison J, & Phisalaphong M (2019). Activated carbon from bacterial cellulose as an effective adsorbent for removing dye from aqueous solution. Separation Science and Technology, 54(14), 2180–2193. [Google Scholar]
- Khan SA, Khan N, Irum U, Farooq A, Asiri AM, Bakhsh EM, et al. (2020). Cellulose acetate-Ce/Zr@Cu° catalyst for the degradation of organic pollutant. International Journal of Biological Macromolecules, 153, 806–816. [DOI] [PubMed] [Google Scholar]
- Khan SB, Ali F, Kamal T, Anwar Y, Asiri AM, & Seo J (2016). CuO embedded chitosan spheres as antibacterial adsorbent for dyes. International Journal of Biological Macromolecules, 88, 113–119. [DOI] [PubMed] [Google Scholar]
- Khan TA, Nazir M, Ali I, & Kumar A (2017). Removal of chromium(VI) from aqueous solution using guar gum-nano zinc oxide biocomposite adsorbent. Arabian Journal of Chemistry, 10, S2388–S2398. [Google Scholar]
- Khodadadi B, Bordbar M, & Nasrollahzadeh M (2017a). Achillea millefolium L. extract mediated green synthesis of waste peach kernel shell supported silver nanoparticles: Application of the nanoparticles for catalytic reduction of a variety of dyes in water. Journal of Colloid and Interface Science, 493, 85–93. [DOI] [PubMed] [Google Scholar]
- Khodadadi B, Bordbar M, & Nasrollahzadeh M (2017b). Green synthesis of Pd nanoparticles at Apricot kernel shell substrate using Salvia hydrangea extract: Catalytic activity for reduction of organic dyes. Journal of Colloid and Interface Science, 490, 1–10. [DOI] [PubMed] [Google Scholar]
- Khoramzadeh E, Nasernejad B, & Halladj R (2013). Mercury biosorption from aqueous solutions by sugarcane bagasse. Journal of the Taiwan Institute of Chemical Engineers, 44(2), 266–269. [Google Scholar]
- Korhonen JT, Kettunen M, Ras RHA, & Ikkala O (2011). Hydrophobic nanocellulose aerogels as floating, sustainable, reusable, and recyclable oil absorbents. ACS Applied Materials & Interfaces, 3(6), 1813–1816. [DOI] [PubMed] [Google Scholar]
- Krajewska B (2001). Diffusion of metal ions through gel chitosan membranes. Reactive and Functional Polymers, 47(1), 37–47. [Google Scholar]
- Kuang Y, Du J, Zhou R, Chen Z, Megharaj M, & Naidu R (2015). Calcium alginate encapsulated Ni/Fe nanoparticles beads for simultaneous removal of Cu(II) and monochlorobenzene. Journal of Colloid and Interface Science, 447, 85–91. [DOI] [PubMed] [Google Scholar]
- Kumar MNVR (2000). A review of chitin and chitosan applications. Reactive and Functional Polymers, 46(1), 1–27. [Google Scholar]
- Kumar A, & Dutta RK (2015). CdS quantum dots immobilized on calcium alginate microbeads for rapid and selective detection of Hg2+ ions. RSC Advances, 5(93), 76275–76284. [Google Scholar]
- Kumar A, Naushad M, Rana A, Sharma G, Ghfar AA, Stadler FJ, et al. (2017). ZnSe-WO3 nano-hetero-assembly stacked on Gum ghatti for photo-degradative removal of Bisphenol A: Symbiose of adsorption and photocatalysis. International Journal of Biological Macromolecules, 104, 1172–1184. [DOI] [PubMed] [Google Scholar]
- Kumar M, Dosanjh HS, & Singh H (2019). Biopolymer modified transition metal spinel ferrites for removal of fluoride ions from water. Environmental Nanotechnology Monitoring & Management, 12, Article 100237. [Google Scholar]
- Kumar M, Vijayakumar G, & Tamilarasan R (2019). Synthesis, characterization and experimental studies of nano Zn–Al–Fe3O4 blended alginate/Ca beads for the adsorption of rhodamin B. Journal of Polymers and the Environment, 27(1), 106–117. [Google Scholar]
- Kyzas GZ, & Deliyanni EA (2013). Mercury(II) removal with modified magnetic chitosan adsorbents. Molecules, 18(6), 6193–6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhane M, Mahabole M, Bogle K, Khairnar R, & Kokol V (2019). Nanocomposite films prepared from differently modified ZSM-5 zeolite and cellulose nanofibrils for cationic and anionic dyes removal. Fibers and Polymers, 20(10), 2127–2139. [Google Scholar]
- Le Corre D, Bras J, & Dufresne A (2010). Starch nanoparticles: A review. Biomacromolecules, 11(5), 1139–1153. [DOI] [PubMed] [Google Scholar]
- Li A, Liu R, & Wang A (2005). Preparation of starch-graft-poly(acrylamide)/attapulgite superabsorbent composite. Journal of Applied Polymer Science, 98(3), 1351–1357. [Google Scholar]
- Li R, Liang W, Li M, Jiang S, Huang H, Zhang Z, et al. (2017). Removal of Cd(II) and Cr(VI) ions by highly cross-linked Thiocarbohydrazide-chitosan gel. International Journal of Biological Macromolecules, 104, 1072–1081. [DOI] [PubMed] [Google Scholar]
- Li X, Qi Y, Li Y, Zhang Y, He X, & Wang Y (2013). Novel magnetic beads based on sodium alginate gel crosslinked by zirconium (IV) and their effective removal for Pb2+ in aqueous solutions by using a batch and continuous systems. Bioresource Technology, 142, 611–619. [DOI] [PubMed] [Google Scholar]
- Li G, Du Y, Tao Y, Deng H, Luo X, & Yang J (2010). Iron (II) cross-linked chitin-based gel beads: Preparation, magnetic property and adsorption of methyl orange. Carbohydrate Polymers, 82(3), 706–713. [Google Scholar]
- Li C, Ma H, Venkateswaran S, & Hsiao BS (2020). Highly efficient and sustainable carboxylated cellulose filters for removal of cationic dyes/heavy metals ions. Chemical Engineering Journal, 389, Article 123458. [Google Scholar]
- Li J, Mo L, Lu C-H, Fu T, Yang H-H, & Tan W (2016). Functional nucleic acid-based hydrogels for bioanalytical and biomedical applications. Chemical Society Reviews, 45(5), 1410–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Tian X, Wang Z, Guan Z, Li X, Qiao H, et al. (2020). Multifunctional adsorbent based on metal-organic framework modified bacterial cellulose/chitosan composite aerogel for high efficient removal of heavy metal ion and organic pollutant. Chemical Engineering Journal, 383, Article 123127. [Google Scholar]
- Li W, Xiao L, & Qin C (2010). The characterization and thermal investigation of chitosan-Fe3O4 nanoparticles synthesized via a novel one-step modifying process. Journal of Macromolecular Science, Part A, 48(1), 57–64. [Google Scholar]
- Li M, Zhang Z, Li R, Wang JJ, & Ali A (2016). Removal of Pb(II) and Cd(II) ions from aqueous solution by thiosemicarbazide modified chitosan. International Journal of Biological Macromolecules, 86, 876–884. [DOI] [PubMed] [Google Scholar]
- Liang R. h., Li Y, Huang L, Wang X. d., Hu X. x., Liu C-M, et al. (2020). Pb2+ adsorption by ethylenediamine-modified pectins and their adsorption mechanisms. Carbohydrate Polymers, 234, Article 115911. [DOI] [PubMed] [Google Scholar]
- Liu H, Dai R, Qu J, & Ru J (2005). Preparation and characterization of a novel adsorbent for removing lipophilic organic from water. Science in China Series B: Chemistry, 48(6), 600–604. [Google Scholar]
- Liu J-F, Zhao Z-S, & Jiang G-B (2008). Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water. Environmental Science & Technology, 42(18), 6949–6954. [DOI] [PubMed] [Google Scholar]
- Liu K, Huang Z, Dai J, Jiang Y, Yang G, Liu Y, et al. (2020). Fabrication of amino-modified electrospun nanofibrous cellulose membrane and adsorption for typical organoarsenic contaminants: Behavior and mechanism. Chemical Engineering Journal, 382, Article 122775. [Google Scholar]
- Liu P, Sehaqui H, Tingaut P, Wichser A, Oksman K, & Mathew AP (2014). Cellulose and chitin nanomaterials for capturing silver ions (Ag+) from water via surface adsorption. Cellulose, 21(1), 449–461. [Google Scholar]
- Liu X, & Zhang L (2015). Insight into the adsorption mechanisms of vanadium(V) on a high-efficiency biosorbent (Ti-doped chitosan bead). International Journal of Biological Macromolecules, 79, 110–117. [DOI] [PubMed] [Google Scholar]
- Liu P, Borrell PF, Božič M, Kokol V, Oksman K, & Mathew AP (2015). Nanocelluloses and their phosphorylated derivatives for selective adsorption of Ag+, Cu2+ and Fe3+ from industrial effluents. Journal of Hazardous Materials, 294, 177–185. [DOI] [PubMed] [Google Scholar]
- Liu H. c., Chen W, Cui B, & Liu C (2015). Enhanced atrazine adsorption from aqueous solution using chitosan-modified sepiolite. Journal of Central South University, 22(11), 4168–4176. [Google Scholar]
- Lu Y, Liu H, Gao R, Xiao S, Zhang M, Yin Y, et al. (2016). Coherent-interface-assembled Ag2O-anchored nanofibrillated cellulose porous aerogels for radioactive iodine capture. ACS Applied Materials & Interfaces, 8(42), 29179–29185. [DOI] [PubMed] [Google Scholar]
- Lv X, Zhang Y, Fu W, Cao J, Zhang J, Ma H, et al. (2017). Zero-valent iron nanoparticles embedded into reduced graphene oxide-alginate beads for efficient chromium(VI) removal. Journal of Colloid and Interface Science, 506, 633–643. [DOI] [PubMed] [Google Scholar]
- Ma H, Hsiao BS, & Chu B (2012). Ultrafine cellulose nanofibers as efficient adsorbents for removal of in water. ACS Macro Letters, 1(1), 213–216. [DOI] [PubMed] [Google Scholar]
- Ma X, Lou Y, Chen X-B, Shi Z, & Xu Y (2019). Multifunctional flexible composite aerogels constructed through in-situ growth of metal-organic framework nanoparticles on bacterial cellulose. Chemical Engineering Journal, 356, 227–235. [Google Scholar]
- Mahfoudhi N, & Boufi S (2017). Nanocellulose as a novel nanostructured adsorbent for environmental remediation: A review. Cellulose, 24(3), 1171–1197. [Google Scholar]
- Mahmoud ME, El-Ghanam AM, Mohamed RHA, & Saad SR (2020). Enhanced adsorption of Levofloxacin and Ceftriaxone antibiotics from water by assembled composite of nanotitanium oxide/chitosan/nano-bentonite. Materials Science and Engineering: C, 108, Article 110199. [DOI] [PubMed] [Google Scholar]
- Maity J, & Ray SK (2016). Enhanced adsorption of Cr(VI) from water by guar gum based composite hydrogels. International Journal of Biological Macromolecules, 89, 246–255. [DOI] [PubMed] [Google Scholar]
- Majidnia Z, & Idris A (2015). Photocatalytic reduction of iodine in radioactive waste water using maghemite and titania nanoparticles in PVA-alginate beads. Journal of the Taiwan Institute of Chemical Engineers, 54, 137–144. [Google Scholar]
- Majidnia Z, & Idris A (2016). Synergistic effect of maghemite and titania nanoparticles in PVA-alginate encapsulated beads for photocatalytic reduction of Pb(II). Chemical Engineering Communications, 203(4), 425–434. [Google Scholar]
- Malekzadeh M, Nejaei A, Baneshi MM, Kokhdan EP, & Bardania H (2018). The use of starch-modified magnetic Fe0 nanoparticles for naphthalene adsorption from water samples: Adsorption isotherm, kinetic and thermodynamic studies. Applied Organometallic Chemistry, 32(8), e4434. [Google Scholar]
- Marrakchi F, Khanday WA, Asif M, & Hameed BH (2016). Cross-linked chitosan/sepiolite composite for the adsorption of methylene blue and reactive orange 16. International Journal of Biological Macromolecules, 93, 1231–1239. [DOI] [PubMed] [Google Scholar]
- Maryami M, Nasrollahzadeh M, Mehdipour E, & Sajadi SM (2016). Preparation of the Ag/RGO nanocomposite by use of Abutilon hirtum leaf extract: A recoverable catalyst for the reduction of organic dyes in aqueous medium at room temperature. International Journal of Hydrogen Energy, 41(46), 21236–21245. [Google Scholar]
- Mata YN, Blázquez ML, Ballester A, Gonźalez F, & Muñoz JA (2009). Sugar-beet pulp pectin gels as biosorbent for heavy metals: Preparation and determination of biosorption and desorption characteristics. Chemical Engineering Journal, 150 (2–3), 289–301. [Google Scholar]
- Mazaheri H, Ghaedi M, Azqhandi MHA, & Asfaram A (2017). Application of machine/statistical learning, artificial intelligence and statistical experimental design for the modeling and optimization of methylene blue and Cd(II) removal from a binary aqueous solution by natural walnut carbon. Physical Chemistry Chemical Physics, 19(18), 11299–11317. [DOI] [PubMed] [Google Scholar]
- Meng Z-D, Zhu L, Choi J-G, Park C-Y, & Oh W-C (2011). Preparation, characterization and photocatalytic behavior of WO3-fullerene/TiO2 catalysts under visible light. Nanoscale Research Letters, 6(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi F-L, Shyu S-S, Chen C-T, & Lai J-Y (2002). Adsorption of indomethacin onto chemically modified chitosan beads. Polymer, 43(3), 757–765. [Google Scholar]
- Miao Q, Jiang H, Gao L, Cheng Y, Xu J, Fu X, et al. (2018). Rheological properties of five plant gums. American Journal of Analytical Chemistry, 9(04), 210. [Google Scholar]
- Mincea M, Patrulea V, Negrulescu A, Szabo R, & Ostafe V (2013). Adsorption of three commercial dyes onto chitosan beads using spectrophotometric determination and a multivariate calibration method. Journal of Water Resource and Protection, 5 (04), 446. [Google Scholar]
- Minisy IM, Salahuddin NA, & Ayad MM (2019). Chitosan/polyaniline hybrid for the removal of cationic and anionic dyes from aqueous solutions. Journal of Applied Polymer Science, 136(6), 47056. [Google Scholar]
- Mittal H, & Mishra SB (2014). Gum ghatti and Fe3O4 magnetic nanoparticles based nanocomposites for the effective adsorption of rhodamine B. Carbohydrate Polymers, 101, 1255–1264. [DOI] [PubMed] [Google Scholar]
- Mittal H, Ballav N, & Mishra SB (2014). Gum ghatti and Fe3O4 magnetic nanoparticles based nanocomposites for the effective adsorption of methylene blue from aqueous solution. Journal of Industrial and Engineering Chemistry, 20(4), 2184–2192. [Google Scholar]
- Mittal H, Maity A, & Ray SS (2015). Synthesis of co-polymer-grafted gum karaya and silica hybrid organic-inorganic hydrogel nanocomposite for the highly effective removal of methylene blue. Chemical Engineering Journal, 279, 166–179. [Google Scholar]
- Moghaddam AZ, Jazi ME, Allahrasani A, Ganjali MR, & Badiei A (2020). Removal of acid dyes from aqueous solutions using a new eco-friendly nanocomposite of CoFe2O4 modified with tragacanth gum. Journal of Applied Polymer Science, 137(17), 48605. [Google Scholar]
- Mohamed HS, Soliman NK, Moustafa AF, Abdel-Gawad OF, Taha RR, & Ahmed SA (2019). Nano metal oxide impregnated chitosan-4-nitroacetophenone for industrial dye removal. International Journal of Environmental Analytical Chemistry, 1–28. [Google Scholar]
- Mohammed N, Grishkewich N, Berry RM, & Tam KC (2015). Cellulose nanocrystal-alginate hydrogel beads as novel adsorbents for organic dyes in aqueous solutions. Cellulose, 22(6), 3725–3738. [Google Scholar]
- Mohammed N, Grishkewich N, & Tam KC (2018). Cellulose nanomaterials: Promising sustainable nanomaterials for application in water/wastewater treatment processes. Environmental Science Nano, 5(3), 623–658. [Google Scholar]
- Mohammed N, Baidya A, Murugesan V, Kumar AA, Ganayee MA, Mohanty JS, et al. (2016). Diffusion-controlled simultaneous sensing and scavenging of heavy metal ions in water using atomically precise cluster-cellulose nanocrystal composites. ACS Sustainable Chemistry & Engineering, 4(11), 6167–6176. [Google Scholar]
- Mohammed N, Grishkewich N, Waeijen HA, Berry RM, & Tam KC (2016). Continuous flow adsorption of methylene blue by cellulose nanocrystal-alginate hydrogel beads in fixed bed columns. Carbohydrate Polymers, 136, 1194–1202. [DOI] [PubMed] [Google Scholar]
- Mohazzab BF, Jaleh B, Nasrollahzadeh M, Khazalpour S, Sajjadi M, & Varma RS (2020). Upgraded valorization of biowaste: Laser-assisted synthesis of Pd/calcium lignosulfonate nanocomposite for hydrogen storage and environmental remediation. ACS Omega, 5(11), 5888–5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojiri A, Zhou JL, Robinson B, Ohashi A, Ozaki N, Kindaichi T, et al. (2020). Pesticides in aquatic environments and their removal by adsorption methods. Chemosphere, Article 126646. [DOI] [PubMed] [Google Scholar]
- Monier M, Ayad DM, Wei Y, & Sarhan AA (2010). Adsorption of Cu(II), Co(II), and Ni(II) ions by modified magnetic chitosan chelating resin. Journal of Hazardous Materials, 177(1–3), 962–970. [DOI] [PubMed] [Google Scholar]
- Moon RJ, Martini A, Nairn J, Simonsen J, & Youngblood J (2011). Cellulose nanomaterials review: Structure, properties and nanocomposites. Chemical Society Reviews, 40(7), 3941–3994. [DOI] [PubMed] [Google Scholar]
- Moradeeya PG, Kumar MA, Thorat RB, Rathod M, Khambhaty Y, & Basha S (2017). Nanocellulose for biosorption of chlorpyrifos from water: Chemometric optimization, kinetics and equilibrium. Cellulose, 24(3), 1319–1332. [Google Scholar]
- Motahharifar N, Nasrollahzadeh M, Taheri-Kafrani A, Varma RS, & Shokouhimehr M (2020). Magnetic chitosan-copper nanocomposite: A plant assembled catalyst for the synthesis of amino-and N-sulfonyl tetrazoles in eco-friendly media. Carbohydrate Polymers, 232, Article 115819. [DOI] [PubMed] [Google Scholar]
- Mualikrishna G, & Tharanathan RN (1994). Characterization of pectic polysaccharides from pulse husks. Food Chemistry, 50(1), 87–89. [Google Scholar]
- Mujeeb VMA, Alikutty P, & Muraleedharan K (2014). Synthesis, characterization and vanadium(V) sorption studies on some chitosan derivatives. Journal of Water Process Engineering, 4, 143–148. [Google Scholar]
- Mujeeb Rahman P, Muraleedaran K, & Mujeeb VMA (2015). Applications of chitosan powder with in situ synthesized nano ZnO particles as an antimicrobial agent. International Journal of Biological Macromolecules, 77, 266–272. [DOI] [PubMed] [Google Scholar]
- Mukhiddinov ZK, Khalikov DK, Abdusamiev FT, & Avloev CC (2000). Isolation and structural characterization of a pectin homo and ramnogalacturonan. Talanta, 53(1), 171–176. [DOI] [PubMed] [Google Scholar]
- Murugadoss A, & Chattopadhyay A (2007). A ‘green’chitosan-silver nanoparticle composite as a heterogeneous as well as micro-heterogeneous catalyst. Nanotechnology, 19(1), Article 015603. [DOI] [PubMed] [Google Scholar]
- Nadavala SK, Swayampakula K, Boddu VM, & Abburi K (2009). Biosorption of phenol and o-chlorophenol from aqueous solutions on to chitosan-calcium alginate blended beads. Journal of Hazardous Materials, 162(1), 482–489. [DOI] [PubMed] [Google Scholar]
- Nasrollahzadeh M, Issaabadi Z, & Varma RS (2019). Magnetic lignosulfonate-supported Pd complex: Renewable resource-derived catalyst for aqueous Suzuki-Miyaura reaction. ACS Omega, 4(10), 14234–14241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrollahzadeh M, Bagherzadeh M, & Karimi H (2016). Preparation, characterization and catalytic activity of CoFe2O4 nanoparticles as a magnetically recoverable catalyst for selective oxidation of benzyl alcohol to benzaldehyde and reduction of organic dyes. Journal of Colloid and Interface Science, 465, 271–278. [DOI] [PubMed] [Google Scholar]
- Nasrollahzadeh M, Baran T, Baran NY, Sajjadi M, Tahsili MR, & Shokouhimehr M (2020). Pd nanocatalyst stabilized on amine-modified zeolite: antibacterial and catalytic activities for environmental pollution remediation in aqueous medium. Separation and Purification Technology, 239, Article 116542. [Google Scholar]
- Nasrollahzadeh M, Nezafat Z, Gorab MG, & Sajjadi M (2020). Recent progresses in graphene-based (photo)catalysts for reduction of nitro compounds. Molecular Catalysis, 484, Article 110758. [Google Scholar]
- Nasrollahzadeh M, Sajadi SM, & Maham M (2016). Aqueous extract from seeds of Silybum marianum L. as a green material for preparation of the Cu/Fe3O4 nanoparticles: a magnetically recoverable and reusable catalyst for the reduction of nitroarenes. Journal of Colloid and Interface Science, 469, 93–98. [DOI] [PubMed] [Google Scholar]
- Nasrollahzadeh M, Sajjadi M, Dasmeh HR, & Sajadi SM (2018). Green synthesis of the Cu/sodium borosilicate nanocomposite and investigation of its catalytic activity. Journal of Alloys and Compounds, 763, 1024–1034. [Google Scholar]
- Nasrollahzadeh M, Sajjadi M, Maham M, Sajadi SM, & Barzinjy AA (2018). Biosynthesis of the palladium/sodium borosilicate nanocomposite using Euphorbia milii extract and evaluation of its catalytic activity in the reduction of chromium(VI), nitro compounds and organic dyes. Materials Research Bulletin, 102, 24–35. [Google Scholar]
- Nasrollahzadeh M, Shafiei N, Nezafat Z, & Bidgoli NSS (2020). Recent progresses in the application of lignin derived (nano) catalysts in oxidation reactions. Molecular Catalysis, 489, Article 110942. [DOI] [PubMed] [Google Scholar]
- Nata IF, Sureshkumar M, & Lee C-K (2011). One-pot preparation of amine-rich magnetite/bacterial cellulose nanocomposite and its application for arsenate removal. RSC Advances, 1(4), 625–631. [Google Scholar]
- Neghi N, Kumar M, & Burkhalov D (2019). Synthesis and application of stable, reusable TiO2 polymeric composites for photocatalytic removal of metronidazole: Removal kinetics and density functional analysis. Chemical Engineering Journal, 359, 963–975. [Google Scholar]
- Niu H, Meng Z, & Cai Y (2012). Fast defluorination and removal of norfloxacin by alginate/Fe@Fe3O4 core/shell structured nanoparticles. Journal of Hazardous Materials, 227, 195–203. [DOI] [PubMed] [Google Scholar]
- O’Connell DW, Birkinshaw C, & O’Dwyer TF (2008). Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresource Technology, 99 (15), 6709–6724. [DOI] [PubMed] [Google Scholar]
- Olad A, & Farshi Azhar F (2014). A study on the adsorption of chromium(VI) from aqueous solutions on the alginate-montmorillonite/polyaniline nanocomposite. Desalination and Water Treatment, 52(13–15), 2548–2559. [Google Scholar]
- Oladipo AA, & Gazi M (2016). Uptake of Ni2+ and rhodamine B by nanohydroxyapatite/alginate composite beads: Batch and continuous-flow systems. Toxicological and Environmental Chemistry, 98(2), 189–203. [Google Scholar]
- Oladoja NA, Adelagun ROA, Ahmad AL, Unuabonah EI, & Bello HA (2014). Preparation of magnetic, macro-reticulated cross-linked chitosan for tetracycline removal from aquatic systems. Colloids and Surfaces B: Biointerfaces, 117, 51–59. [DOI] [PubMed] [Google Scholar]
- Olivera S, Muralidhara HB, Venkatesh K, Guna VK, Gopalakrishna K, & Kumar Y (2016). Potential applications of cellulose and chitosan nanoparticles/composites in wastewater treatment: A review. Carbohydrate Polymers, 153, 600–618. [DOI] [PubMed] [Google Scholar]
- Omer AM, Elgarhy GS, El-Subruiti GM, Khalifa RE, & Eltaweil AS (2020). Fabrication of novel iminodiacetic acid-functionalized carboxymethyl cellulose microbeads for efficient removal of cationic crystal violet dye from aqueous solutions. International Journal of Biological Macromolecules, 148, 1072–1083. [DOI] [PubMed] [Google Scholar]
- Omidvar A, Jaleh B, & Nasrollahzadeh M (2017). Preparation of the GO/Pd nanocomposite and its application for the degradation of organic dyes in water. Journal of Colloid and Interface Science, 496, 44–50. [DOI] [PubMed] [Google Scholar]
- Padil VVT, Senan C, & Černík M (2019). Green. Materials for biomedical engineering (pp. 127–172). Elsevier. [Google Scholar]
- Padil VVT, Wacławek S, Černík M, & Varma RS (2018). Tree gum-based renewable materials: Sustainable applications in nanotechnology, biomedical and environmental fields. Biotechnology Advances, 36(7), 1984–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla-Rodríguez A, Hernández-Viezcas JA, Peralta-Videa JR, Gardea Torresdey JL, Perales-Pérez O, & Román-Velázquez FR (2015). Synthesis of protonated chitosan flakes for the removal of vanadium(III, IV and V) oxyanions from aqueous solutions. Microchemical Journal, 118, 1–11. [Google Scholar]
- Pandey S, & Mishra SB (2014). Catalytic reduction of p-nitrophenol by using platinum nanoparticles stabilised by guar gum. Carbohydrate Polymers, 113, 525–531. [DOI] [PubMed] [Google Scholar]
- Pandi K, & Viswanathan N (2015). Enhanced defluoridation and facile separation of magnetic nano-hydroxyapatite/alginate composite. International Journal of Biological Macromolecules, 80, 341–349. [DOI] [PubMed] [Google Scholar]
- Patel S, & Goyal A (2015). Applications of natural polymer gum arabic: A review. International Journal of Food Properties, 18(5), 986–998. [Google Scholar]
- Pérez-Obando J, Marín-Silva DA, Pinotti AN, Pizzio LR, Osorio-Vargas P, & Rengifo-Herrera JA (2019). Degradation study of malachite green on chitosan films containing heterojunctions of melon/TiO2 absorbing visible-light in solid-gas interfaces. Applied Catalysis B: Environmental, 244, 773–785. [Google Scholar]
- Periyasamy S, Gopalakannan V, & Viswanathan N (2018). Hydrothermal assisted magnetic nano-hydroxyapatite encapsulated alginate beads for efficient Cr(VI) uptake from water. Journal of Environmental Chemical Engineering, 6(1), 1443–1454. [Google Scholar]
- Pi G, Li Y, Bao M, Mao L, Gong H, & Wang Z (2016). Novel and environmentally friendly oil spill dispersant based on the synergy of biopolymer xanthan gum and silica nanoparticles. ACS Sustainable Chemistry & Engineering, 4(6), 3095–3102. [Google Scholar]
- Pincus LN, Melnikov F, Yamani JS, & Zimmerman JB (2018). Multifunctional photoactive and selective adsorbent for arsenite and arsenate: Evaluation of nano titanium dioxide-enabled chitosan cross-linked with copper. Journal of Hazardous Materials, 358, 145–154. [DOI] [PubMed] [Google Scholar]
- Pirsaheb M, Moradi S, Shahlaei M, Wang X, & Farhadian N (2019). A new composite of nano zero-valent iron encapsulated in carbon dots for oxidative removal of bio-refractory antibiotics from water. Journal of Cleaner Production, 209, 1523–1532. [Google Scholar]
- Plakas KV, & Karabelas AJ (2009). Triazine retention by nanofiltration in the presence of organic matter: The role of humic substance characteristics. Journal of Membrane Science, 336(1–2), 86–100. [Google Scholar]
- Pourjavadi A, Abedin-Moghanaki A, & Tavakoli A (2016). Efficient removal of cationic dyes using a new magnetic nanocomposite based on starch-g-poly (vinylalcohol) and functionalized with sulfate groups. RSC Advances, 6(44), 38042–38051. [Google Scholar]
- Primo A, Liebel M, & Quignard F (2009). Palladium coordination biopolymer: A versatile access to highly porous dispersed catalyst for suzuki reaction. Chemistry of Materials, 21(4), 621–627. [Google Scholar]
- Qian X, Xu Y, Yue X, Wang C, Liu M, et al. (2020). Microwave-assisted solvothermal in-situ synthesis of CdS nanoparticles on bacterial cellulose matrix for photocatalytic application. Cellulose, 27, 5939–5954. [Google Scholar]
- Qiao H, Zhou Y, Yu F, Wang E, Min Y, Huang Q, et al. (2015). Effective removal of cationic dyes using carboxylate-functionalized cellulose nanocrystals. Chemosphere, 141, 297–303. [DOI] [PubMed] [Google Scholar]
- Qiu K, & Netravali AN (2014). A review of fabrication and applications of bacterial cellulose based nanocomposites. Polymer Reviews, 54(4), 598–626. [Google Scholar]
- Qiu Y, Ma Z, & Hu P (2014). Environmentally benign magnetic chitosan/Fe3O4 composites as reductant and stabilizer for anchoring Au NPs and their catalytic reduction of 4-nitrophenol. Journal of Materials Chemistry A, 2(33), 13471–13478. [Google Scholar]
- Rafatullah M, Sulaiman O, Hashim R, & Ahmad A (2010). Adsorption of methylene blue on low-cost adsorbents: A review. Journal of Hazardous Materials, 177(1–3), 70–80. [DOI] [PubMed] [Google Scholar]
- Rahimdokht M, Pajootan E, & Ranjbar-Mohammadi M (2019). Titania/gum tragacanth nanohydrogel for methylene blue dye removal from textile wastewater using response surface methodology. Polymer International, 68(1), 134–140. [Google Scholar]
- Rahimi S, Moattari RM, Rajabi L, & Derakhshan AA (2015). Optimization of lead removal from aqueous solution using goethite/chitosan nanocomposite by response surface methodology. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 484, 216–225. [Google Scholar]
- Rajab Beigy M, Rasekh B, Yazdian F, Aminzadeh B, & Shekarriz M (2018). High nitrate removal by starch-stabilized Fe° nanoparticles in aqueous solution in a controlled system. Engineering in Life Sciences, 18(3), 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajeswari A, Amalraj A, & Pius A (2016). Adsorption studies for the removal of nitrate using chitosan/PEG and chitosan/PVA polymer composites. Journal of Water Process Engineering, 9, 123–134. [Google Scholar]
- Rakhshaee R (2011). Rule of Fe° nano-particles and biopolymer structures in kinds of the connected pairs to remove Acid Yellow 17 from aqueous solution: Simultaneous removal of dye in two paths and by four mechanisms. Journal of Hazardous Materials, 197, 144–152. [DOI] [PubMed] [Google Scholar]
- Rakhshaee R (2014). Decreasing Fe° and Fe3O4 nano particle size by simultaneous synthesis on a bio-polymeric structure: Kinetic study to remove Amaranth from aqueous solution. Powder Technology, 254, 494–499. [Google Scholar]
- Rakhshaee R, & Panahandeh M (2011). Stabilization of a magnetic nano-adsorbent by extracted pectin to remove methylene blue from aqueous solution: A comparative studying between two kinds of cross-likened pectin. Journal of Hazardous Materials, 189(1–2), 158–166. [DOI] [PubMed] [Google Scholar]
- Rakhshaee R, Giahi M, & Pourahmad A (2011). Removal of methyl orange from aqueous solution by Azolla filicoloides: synthesis of Fe3O4 nano-particles and its surface modification by the extracted pectin of Azolla. Chinese Chemical Letters, 22 (4), 501–504. [Google Scholar]
- Ramadhani S, & Helmiyati H (2020). Alginate/CMC/ZnO nanocomposite for photocatalytic degradation of Congo red dye (1 ed., Vol. 2242, p. 040026). AIP Publishing LLC. [Google Scholar]
- Rangel-Mendez JR, Monroy-Zepeda R, Leyva-Ramos E, Diaz-Flores PE, & Shirai K (2009). Chitosan selectivity for removing cadmium(II), copper(II), and lead(II) from aqueous phase: pH and organic matter effect. Journal of Hazardous Materials, 162(1), 503–511. [DOI] [PubMed] [Google Scholar]
- Ranjbar D, Raeiszadeh M, Lewis L, MacLachlan MJ, & Hatzikiriakos SG (2020). Adsorptive removal of Congo red by surfactant modified cellulose nanocrystals: A kinetic, equilibrium, and mechanistic investigation. Cellulose, 1–22. [Google Scholar]
- Ravikumar KVG, Kumar D, Kumar G, Mrudula P, Natarajan C, & Mukherjee A (2016). Enhanced Cr(VI) removal by nanozerovalent iron-immobilized alginate beads in the presence of a biofilm in a continuous-flow reactor. Industrial & Engineering Chemistry Research, 55(20), 5973–5982. [Google Scholar]
- Ravikumar KVG, Sudakaran SV, Pulimi M, Natarajan C, & Mukherjee A (2018). Removal of hexavalent chromium using nano zero valent iron and bacterial consortium immobilized alginate beads in a continuous flow reactor. Environmental Technology & Innovation, 12, 104–114. [Google Scholar]
- Ray PZ, & Shipley HJ (2015). Inorganic nano-adsorbents for the removal of heavy metals and arsenic: A review. RSC Advances, 5(38), 29885–29907. [Google Scholar]
- Rezaee A, Pourtagi G, Hossini H, & Loloi M (2016). Microbial cellulose as a support for photocatalytic oxidation of toluene using TiO2 nanoparticles. Journal of Applied Polymer Science, 133(8), 43051. [Google Scholar]
- Ridley BL, O’Neill MA, & Mohnen D (2001). Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry, 57(6), 929–967. [DOI] [PubMed] [Google Scholar]
- Rinaudo M (2006). Chitin and chitosan: Properties and applications. Progress in Polymer Science, 31(7), 603–632. [Google Scholar]
- Rizzi V, Romanazzi F, Gubitosa J, Fini P, Romita R, Agostiano A, et al. (2019). Chitosan film as eco-friendly and recyclable bio-adsorbent to remove/recover diclofenac, ketoprofen, and their mixture from wastewater. Biomolecules, 9(10), 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshitha SS, Mithra V, Saravanan V, Sadasivam SK, & Gnanadesigan M (2019). Photocatalytic degradation of methylene blue and safranin dyes using chitosan zinc oxide nano-beads with Musa×paradisiaca L. pseudo stem. Bioresource Technology Reports, 5, 339–342. [Google Scholar]
- Rostami-Vartooni A, Nasrollahzadeh M, Salavati-Niasari M, & Atarod M (2016). Photocatalytic degradation of azo dyes by titanium dioxide supported silver nanoparticles prepared by a green method using Carpobrotus acinaciformis extract. Journal of Alloys and Compounds, 689, 15–20. [Google Scholar]
- Ruiz-Hitzky E, Darder M, Fernandes FM, Wicklein B, Alcântara ACS, & Aranda P (2013). Fibrous clays based bionanocomposites. Progress in Polymer Science, 38(10–11), 1392–1414. [Google Scholar]
- Saad AM, Abukhadra MR, Ahmed SA-K, Elzanaty AM, Mady AH, Betiha MA, et al. (2020). Photocatalytic degradation of malachite green dye using chitosan supported ZnO and Ce-ZnO nano-flowers under visible light. Journal of Environmental Management, 258, Article 110043. [DOI] [PubMed] [Google Scholar]
- Saberi A, Alipour E, & Sadeghi M (2019). Superabsorbent magnetic Fe3O4-based starch-poly(acrylic acid) nanocomposite hydrogel for efficient removal of dyes and heavy metal ions from water. Journal of Polymer Research, 26(12), 271. [Google Scholar]
- Saberi A, Sadeghi M, & Alipour E (2020). Design of AgNPs-base starch/PEG-poly (acrylic acid) hydrogel for removal of mercury(II). Journal of Polymers and the Environment, 28(3), 906–917. [Google Scholar]
- Sadeghi S, Rad FA, & Moghaddam AZ (2014). A highly selective sorbent for removal of Cr(VI) from aqueous solutions based on Fe3O4/poly(methyl methacrylate) grafted Tragacanth gum nanocomposite: Optimization by experimental design. Materials Science and Engineering: C, 45, 136–145. [DOI] [PubMed] [Google Scholar]
- Saha TK, Ichikawa H, & Fukumori Y (2006). Gadolinium diethylenetriaminopentaacetic acid-loaded chitosan microspheres for gadolinium neutron-capture therapy. Carbohydrate Research, 341(17), 2835–2841. [DOI] [PubMed] [Google Scholar]
- Sahithya K, Das D, & Das N (2015). Effective removal of dichlorvos from aqueous solution using biopolymer modified MMT-CuO composites: Equilibrium, kinetic and thermodynamic studies. Journal of Molecular Liquids, 211, 821–830. [Google Scholar]
- Sajjadi M, Baran NY, Baran T, Nasrollahzadeh M, Tahsili MR, & Shokouhimehr M (2020). Palladium nanoparticles stabilized on a novel Schiff base modified Unye bentonite: Highly stable, reusable and efficient nanocatalyst for treating wastewater contaminants and inactivating pathogenic microbes. Separation and Purification Technology, 237, Article 116383. [Google Scholar]
- Sajjadi M, Nasrollahzadeh M, & Tahsili MR (2019). Catalytic and antimicrobial activities of magnetic nanoparticles supported N-heterocyclic palladium(II) complex: A magnetically recyclable catalyst for the treatment of environmental contaminants in aqueous media. Separation and Purification Technology, 227, Article 115716. [Google Scholar]
- Salman JM, Njoku VO, & Hameed BH (2011). Adsorption of pesticides from aqueous solution onto banana stalk activated carbon. Chemical Engineering Journal, 174(1), 41–48. [Google Scholar]
- Samadder R, Akter N, Roy AC, Uddin MM, Hossen MJ, & Azam MS (2020). Magnetic nanocomposite based on polyacrylic acid and carboxylated cellulose nanocrystal for the removal of cationic dye. RSC Advances, 10(20), 11945–11956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sami AJ, Khalid M, Iqbal S, Afzal M, & Shakoori AR (2017). Synthesis and application of chitosan-starch based nanocomposite in wastewater treatment for the removal of anionic commercial dyes. Pakistan Journal of Zoology, 49(1), 21–26. [Google Scholar]
- Sani HA, Aliyu HS, & Tukur SA (2015). Methylene blue dye adsorption using a polymer coated ZnO with chitosan nano-catalyst. Journal of Applied Chemistry, 8(8), 34–38. [Google Scholar]
- Sankararamakrishnan N, Singh N, & Srivastava I (2020). Hierarchical nano Fe(0) @FeS doped cellulose nanofibres derived from agrowaste-potential bionanocomposite for treatment of organic dyes. International Journal of Biological Macromolecules, 151, 713–722. [DOI] [PubMed] [Google Scholar]
- Sannino D, Vaiano V, Sacco O, & Ciambelli P (2013). Mathematical modelling of photocatalytic degradation of methylene blue under visible light irradiation. Journal of Environmental Chemical Engineering, 1(1–2), 56–60. [Google Scholar]
- Saravanan P, Vinod VTP, Sreedhar B, & Sashidhar RB (2012). Gum kondagogu modified magnetic nano-adsorbent: An efficient protocol for removal of various toxic metal ions. Materials Science and Engineering: C, 32(3), 581–586. [Google Scholar]
- Sayed S, & Jardine A (2015). Chitosan derivatives as important biorefinery intermediates. Quaternary tetraalkylammonium chitosan derivatives utilized in anion exchange chromatography for perchlorate removal. International Journal of Molecular Sciences, 16(5), 9064–9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleuter D, Günther A, Paasch S, Ehrlich H, Kljajíc Z, Hanke T, et al. (2013). Chitin-based renewable materials from marine sponges for uranium adsorption. Carbohydrate Polymers, 92(1), 712–718. [DOI] [PubMed] [Google Scholar]
- Sehaqui H, de Larraya UP, Liu P, Pfenninger N, Mathew AP, Zimmermann T, et al. (2014). Enhancing adsorption of heavy metal ions onto biobased nanofibers from waste pulp residues for application in wastewater treatment. Cellulose, 21(4), 2831–2844. [Google Scholar]
- Sekhavat Pour Z, & Ghaemy M (2015). Removal of dyes and heavy metal ions from water by magnetic hydrogel beads based on poly (vinyl alcohol)/carboxymethyl starch-g-poly(vinyl imidazole). RSC Advances, 5(79), 64106–64118. [Google Scholar]
- Sethy TR, Pradhan AK, & Sahoo PK (2019). Simultaneous studies on kinetics, bio-adsorption behaviour of chitosan grafted thin film nanohydrogel for removal of hazardous metal ion from water. Environmental Nanotechnology Monitoring & Management, 12, Article 100262. [Google Scholar]
- Shaker MA (2015). Adsorption of Co(II), Ni(II) and Cu(II) ions onto chitosan-modified poly (methacrylate) nanoparticles: Dynamics, equilibrium and thermodynamics studies. Journal of the Taiwan Institute of Chemical Engineers, 57, 111–122. [Google Scholar]
- Shao W, Liu H, Liu X, Wang S, & Zhang R (2015). Anti-bacterial performances and biocompatibility of bacterial cellulose/graphene oxide composites. RSC Advances, 5 (7), 4795–4803. [Google Scholar]
- Sharif A, Khorasani M, & Shemirani F (2018). Nanocomposite bead (NCB) based on bio-polymer alginate caged magnetic graphene oxide synthesized for adsorption and preconcentration of lead(II) and copper(II) ions from urine, saliva and water samples. Journal of Inorganic and Organometallic Polymers and Materials, 28(6), 2375–2387. [Google Scholar]
- Sharma G, Kumar A, Sharma S, Ala’a H, Naushad M, Ghfar AA, et al. (2019). Fabrication and characterization of novel Fe°@Guar gum-crosslinked-soya lecithin nanocomposite hydrogel for photocatalytic degradation of methyl violet dye. Separation and Purification Technology, 211, 895–908. [Google Scholar]
- Sharma G, Kumar A, Sharma S, Naushad M, Ghfar AA, Ala’a H, et al. (2020). Carboxymethyl cellulose structured nano-adsorbent for removal of methyl violet from aqueous solution: Isotherm and kinetic analyses. Cellulose, 1–15. [Google Scholar]
- Sharma G, Naushad M, Pathania D, & Kumar A (2016). A multifunctional nanocomposite pectin thorium(IV) tungstomolybdate for heavy metal separation and photoremediation of malachite green. Desalination and Water Treatment, 57(41), 19443–19455. [Google Scholar]
- Sharma G, Sharma S, Kumar A, Ala’a H, Naushad M, Ghfar AA, et al. (2018). Guar gum and its composites as potential materials for diverse applications: A review. Carbohydrate Polymers, 199, 534–545. [DOI] [PubMed] [Google Scholar]
- Sharma H, Bhardwaj M, Kour M, & Paul S (2017). Highly efficient magnetic Pd(0) nanoparticles stabilized by amine functionalized starch for organic transformations under mild conditions. Molecular Catalysis, 435, 58–68. [Google Scholar]
- Sharma R, Kalia S, Kaith BS, Pathania D, Kumar A, & Thakur P (2015). Guaran-based biodegradable and conducting interpenetrating polymer network composite hydrogels for adsorptive removal of methylene blue dye. Polymer Degradation and Stability, 122, 52–65. [Google Scholar]
- Sharma VK, Zboril R, & Varma RS (2015). Ferrates: Greener oxidants with multimodal action in water treatment technologies. Accounts of Chemical Research, 48(2), 182–191. [DOI] [PubMed] [Google Scholar]
- Shatkin JA, Wegner T, & Neih W (2013). Incorporating life cycle thinking into risk assessment for nanoscale materials: Case study of nanocellulose. Chapter 1.2. Health safety and environment. In Postek MT, Moon RJ, Rudie AW, & Bilodeau MA (Eds.), “production and applications of cellulose nanomaterials”. TAPPI international conference on nanotechnology for renewable materials. [Google Scholar]
- Sheikhi A, Safari S, Yang H, & Van De Ven TGM (2015). Copper removal using electrosterically stabilized nanocrystalline cellulose. ACS Applied Materials & Interfaces, 7(21), 11301–11308. [DOI] [PubMed] [Google Scholar]
- Sheshmani S, Ashori A, & Hasanzadeh S (2014). Removal of acid orange 7 from aqueous solution using magnetic graphene/chitosan: A promising nano-adsorbent. International Journal of Biological Macromolecules, 68, 218–224. [DOI] [PubMed] [Google Scholar]
- Shi X, Zhang X, Ma L, Xiang C, & Li L (2019). TiO2-doped chitosan microspheres supported on cellulose acetate fibers for adsorption and photocatalytic degradation of methyl orange. Polymers, 11(8), 1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaat R, Saadatjoo N, Karimi A, & Aber S (2016). Simultaneous adsorption-degradation of organic dyes using MnFe2O4/calcium alginate nano-composites coupled with GOx and laccase. Journal of Environmental Chemical Engineering, 4(2), 1722–1730. [Google Scholar]
- Shokoohi R, Torkshavand Z, Mahmoudi MM, Behgoo AM, Ghaedrahmati E, & Hosseini FM (2019). Effective removal of azo dye reactive blue 222 from aqueous solutions using modified magnetic nanoparticles with sodium alginate/hydrogen peroxide. Environmental Progress & Sustainable Energy, 38(s1), S205–S213. [Google Scholar]
- Shoueir K, El-Sheshtawy H, Misbah M, El-Hosainy H, El-Mehasseb I, & El Kemary M (2018). Fenton-like nanocatalyst for photodegradation of methylene blue under visible light activated by hybrid green DNSA@Chitosan@MnFe2O4. Carbohydrate Polymers, 197, 17–28. [DOI] [PubMed] [Google Scholar]
- Shoueir K, Kandil S, El-hosainy H, & El-Kemary M (2019). Tailoring the surface reactivity of plasmonic Au@TiO2 photocatalyst bio-based chitosan fiber towards cleaner of harmful water pollutants under visible-light irradiation. Journal of Cleaner Production, 230, 383–393. [Google Scholar]
- Siddeeg SM, Tahoon MA, Mnif W, & Ben Rebah F (2020). Iron oxide/chitosan magnetic nanocomposite immobilized manganese peroxidase for decolorization of textile wastewater. Processes, 8(1), 5. [Google Scholar]
- Siddiqui SI, Singh PN, Tara N, Pal S, Chaudhry SA, & Sinha I (2020). Arsenic removal from water by starch functionalized maghemite nano-adsorbents: Thermodynamics and kinetics investigations. Colloid and Interface Science Communications, 36, Article 100263. [Google Scholar]
- Sikdera MT, Kubotad R, Akterd M, Rahmand MM, Hossaind KFB, Rahamand MS, et al. (2019). Adsorption mechanism of Cu(II) in water environment using chitosan-nano zero valent iron-activated carbon composite beads. Desalination and Water Treatment, 145, 202–210. [Google Scholar]
- Singh K, & Arora S (2011). Removal of synthetic textile dyes from wastewaters: A critical review on present treatment technologies. Critical Reviews in Environmental Science and Technology, 41(9), 807–878. [Google Scholar]
- Singh K, Arora JK, Sinha TJM, & Srivastava S (2014). Functionalization of nanocrystalline cellulose for decontamination of Cr(III) and Cr(VI) from aqueous system: Computational modeling approach. Clean Technologies and Environmental Policy, 16(6), 1179–1191. [Google Scholar]
- Singh PN, Tiwary D, & Sinha I (2015). Chromium removal from aqueous media by superparamagnetic starch functionalized maghemite nanoparticles. Journal of Chemical Sciences, 127(11), 1967–1976. [Google Scholar]
- Singh V, Kumari P, Pandey S, & Narayan T (2009). Removal of chromium(VI) using poly(methylacrylate) functionalized guar gum. Bioresource Technology, 100(6), 1977–1982. [DOI] [PubMed] [Google Scholar]
- Sivan SK, Padinjareveetil AKK, Padil VVT, Pilankatta R, George B, Senan C, et al. (2019). Greener assembling of MoO3 nanoparticles supported on gum arabic: Cytotoxic effects and catalytic efficacy towards reduction of p-nitrophenol. Clean Technologies and Environmental Policy, 21(8), 1549–1561. [Google Scholar]
- Smith AM, Smith MT, La Merrill MA, Liaw J, & Steinmaus C (2017). 2,4 Dichlorophenoxyacetic acid (2,4-D) and risk of non-Hodgkin lymphoma: A meta-analysis accounting for exposure levels. Annals of Epidemiology, 27(4), 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder A, Bo Z, Moon R, Rochet J-C, & Stanciu L (2013). Reusable photocatalytic titanium dioxide–cellulose nanofiber films. Journal of Colloid and Interface Science, 399, 92–98. [DOI] [PubMed] [Google Scholar]
- Songkroah C, Nakbanpote W, & Thiravetyan P (2004). Recovery of silver-thiosulphate complexes with chitin. Process Biochemistry, 39(11), 1553–1559. [Google Scholar]
- Soni A, Tiwari A, & Bajpai AK (2014). Removal of malachite green from aqueous solution using nano-iron oxide-loaded alginate microspheres: Batch and column studies. Research on Chemical Intermediates, 40(3), 913–930. [Google Scholar]
- Soumya RS, Ghosh S, & Abraham ET (2010). Preparation and characterization of guar gum nanoparticles. International Journal of Biological Macromolecules, 46(2), 267–269. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Kardam A, & Raj KR (2012). Nanotech reinforcement onto cellulosic fibers: Green remediation of toxic metals. International Journal of Green Nanotechnology, 4(1), 46–53. [Google Scholar]
- Stan M, Lung I, Soran M-L, Opris O, Leostean C, Popa A, et al. (2019). Starch-coated green synthesized magnetite nanoparticles for removal of textile dye Optilan Blue from aqueous media. Journal of the Taiwan Institute of Chemical Engineers, 100, 65–73. [Google Scholar]
- Sun D, Yang J, & Wang X (2010). Bacterial cellulose/TiO2 hybrid nanofibers prepared by the surface hydrolysis method with molecular precision. Nanoscale, 2 (2), 287–292. [DOI] [PubMed] [Google Scholar]
- Sun Y, Guo Y, Lu Q, Meng X, Xiaohua W, Guo Y, et al. (2005). Highly selective asymmetry transfer hydrogenation of prochiral acetophenone catalyzed by palladium-chitosan on silica. Catalysis Letters, 100(3–4), 213–217. [Google Scholar]
- Sun Y, Lei C, Khan E, Chen SS, Tsang DCW, Ok YS, et al. (2018). Aging effects on chemical transformation and metal (loid) removal by entrapped nanoscale zero-valent iron for hydraulic fracturing wastewater treatment. The Science of the Total Environment, 615, 498–507. [DOI] [PubMed] [Google Scholar]
- Sundar Raj AA, Rubila S, Jayabalan R, & Ranganathan TV (2012). A review on pectin: Chemistry due to general properties of pectin and its pharmaceutical uses. Scientific Reports, 1(12), 550. [Google Scholar]
- Supriya P, Srinivas BTV, Chowdeswari K, Naidu NVS, & Sreedhar B (2018). Biomimetic synthesis of gum acacia mediated Pd-ZnO and Pd-TiO2–Promising nanocatalysts for selective hydrogenation of nitroarenes. Materials Chemistry and Physics, 204, 27–36. [Google Scholar]
- Swain SK, Patnaik T, & Dey RK (2013). Efficient removal of fluoride using new composite material of biopolymer alginate entrapped mixed metal oxide nanomaterials. Desalination and Water Treatment, 51(22–24), 4368–4378. [Google Scholar]
- Tabatabaeefar A, Keshtkar AR, Talebi M, & Abolghasemi H (2020). Polyvinyl Alcohol/alginate/zeolite nanohybrid for removal of metals. Chemical Engineering & Technology, 43(2), 343–354. [Google Scholar]
- Tavker N, Gaur UK, & Sharma M (2020). Agro-waste extracted cellulose supported silver phosphate nanostructures as a green photocatalyst for improved photodegradation of RhB dye and industrial fertilizer effluents. Nanoscale Advances, 2, 2870–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teimouri A, Ghased N, Nasab SG, & Habibollahi S (2019). Statistical design of experiment as a tool for optimization of methylene blue sorption on CS/MCM-41/nano-gamma alumina as a novel and environmentally friendly adsorbent: Isotherm and kinetic studies. Desalination and Water Treatment, 139, 327–341. [Google Scholar]
- Teng W, Wu Z, Fan J, Zhang W. x., & Zhao D (2015). Amino-functionalized ordered mesoporous carbon for the separation of toxic microcystin-LR. Journal of Materials Chemistry A, 3(37), 19168–19176. [Google Scholar]
- Thakur S, & Arotiba O (2018). Synthesis, characterization and adsorption studies of an acrylic acid-grafted sodium alginate-based TiO2 hydrogel nanocomposite. Adsorption Science and Technology, 36(1–2), 458–477. [Google Scholar]
- Thiruvengadam V, & Vitta S (2013). Ni-bacterial cellulose nanocomposite; a magnetically active inorganic–organic hybrid gel. RSC Advances, 3(31), 12765–12773. [Google Scholar]
- Topuz F, Henke A, Richtering W, & Groll J (2012). Magnesium ions and alginate do form hydrogels: A rheological study. Soft Matter, 8(18), 4877–4881. [Google Scholar]
- Trache D, Hussin MH, Haafiz MKM, & Thakur VK (2017). Recent progress in cellulose nanocrystals: Sources and production. Nanoscale, 9(5), 1763–1786. [DOI] [PubMed] [Google Scholar]
- Vanaamudan A, Sadhu M, & Pamidimukkala P (2018). Chitosan-Guar gum blend silver nanoparticle bionanocomposite with potential for catalytic degradation of dyes and catalytic reduction of nitrophenol. Journal of Molecular Liquids, 271, 202–208. [Google Scholar]
- Varghese AG, Paul SA, & Latha MS (2019). Remediation of heavy metals and dyes from wastewater using cellulose-based adsorbents. Environmental Chemistry Letters, 17(2), 867–877. [Google Scholar]
- Varma RS (2014). Journey on greener pathways: From the use of alternate energy inputs and benign reaction media to sustainable applications of nano-catalysts in synthesis and environmental remediation. Green Chemistry, 16(4), 2027–2041. [Google Scholar]
- Varma RS (2016). Greener and sustainable trends in synthesis of organics and nanomaterials. ACS Sustainable Chemistry and Engineering, 4, 5866–5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilela C, Moreirinha C, Almeida A, Silvestre AJD, & Freire CSR (2019). Zwitterionic nanocellulose-based membranes for organic dye removal. Materials, 12 (9), 1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod VTP, Sashidhar RB, Sreedhar B, Rao BR, Rao TN, & Abraham JT (2009). Interaction of Pb2+ and Cd2+ with gum kondagogu (Cochlospermum gossypium): A natural carbohydrate polymer with biosorbent properties. Carbohydrate Polymers, 78(4), 894–901. [Google Scholar]
- Vinod VTP, Sashidhar RB, & Sreedhar B (2010). Biosorption of nickel and total chromium from aqueous solution by gum kondagogu (Cochlospermum gossypium): A carbohydrate biopolymer. Journal of Hazardous Materials, 178(1–3), 851–860. [DOI] [PubMed] [Google Scholar]
- Vinod VTP, Sashidhar RB, & Sukumar AA (2010). Competitive adsorption of toxic heavy metal contaminants by gum kondagogu (Cochlospermum gossypium): A natural hydrocolloid. Colloids and Surfaces B: Biointerfaces, 75(2), 490–495. [DOI] [PubMed] [Google Scholar]
- Visakh PM, Mathew AP, Oksman K, & Thomas S (2012). Starch-based bionanocomposites: Processing and properties. Polysaccharide building blocks: A sustainable approach to the development of renewable biomaterials (pp. 287–306). [Google Scholar]
- Voisin H, Bergström L, Liu P, & Mathew AP (2017). Nanocellulose-based materials for water purification. Nanomaterials, 7(3), 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D (2019). A critical review of cellulose-based nanomaterials for water purification in industrial processes. Cellulose, 26(2), 687–701. [Google Scholar]
- Wang B, Ran M, Fang G, Wu T, Tian Q, Zheng L, et al. (2020). Palladium nano-catalyst supported on cationic nanocellulose-alginate hydrogel for effective catalytic reactions. Cellulose, 1–14. [Google Scholar]
- Wang G, Zhang J, Lin S, Xiao H, Yang Q, Chen S, et al. (2020). Environmentally friendly nanocomposites based on cellulose nanocrystals and polydopamine for rapid removal of organic dyes in aqueous solution. Cellulose, 27, 2085–2097. [Google Scholar]
- Wang H, Li J, Ding N, Zeng X, Tang X, Sun Y, et al. (2020). Eco-friendly polymer nanocomposite hydrogel enhanced by cellulose nanocrystal and graphitic-like carbon nitride nanosheet. Chemical Engineering Journal, 386, Article 124021. [Google Scholar]
- Wang J, Zhou Y, Shao Y, He F, Wu M, Ni H, et al. (2019). Chitosan-silica nanoparticles catalyst (M@CS-SiO2) for the degradation of 1,1-dimethylhydrazine. Research on Chemical Intermediates, 45(4), 1721–1735. [Google Scholar]
- Wang L, Peng H, Liu S, Yu H, Li P, & Xing R (2012). Adsorption properties of gold onto a chitosan derivative. International Journal of Biological Macromolecules, 51(5), 701–704. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang Y, Hou C, Qi Z, He X, & Li Y (2015). Facile synthesis of monodisperse functional magnetic dialdehyde starch nano-composite and used for highly effective recovery of Hg(II). Chemosphere, 141, 26–33. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhou R, Wang C, Zhou G, Hua C, Cao Y, et al. (2020). Novel environmental-friendly nano-composite magnetic attapulgite functionalized by chitosan and EDTA for cadmium(II) removal. Journal of Alloys and Compounds, 817, Article 153286. [Google Scholar]
- Wang J, & Zhuang S (2017). Removal of various pollutants from water and wastewater by modified chitosan adsorbents. Critical Reviews in Environmental Science and Technology, 47(23), 2331–2386. [Google Scholar]
- Wang J-P, Chen Y-Z, Yuan S-J, Sheng G-P, & Yu H-Q (2009). Synthesis and characterization of a novel cationic chitosan-based flocculant with a high water-solubility for pulp mill wastewater treatment. Water Research, 43(20), 5267–5275. [DOI] [PubMed] [Google Scholar]
- Wang S, Ge L, Li L, Yan M, Ge S, & Yu J (2013). Molecularly imprinted polymer grafted paper-based multi-disk micro-disk plate for chemiluminescence detection of pesticide. Biosensors and Bioelectronics, 50, 262–268. [DOI] [PubMed] [Google Scholar]
- Wang M, Li X, Zhang T, Deng L, Li P, Wang X, et al. (2018). Eco-friendly poly (acrylic acid)-sodium alginate nanofibrous hydrogel: A multifunctional platform for superior removal of Cu(II) and sustainable catalytic applications. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 558, 228–241. [Google Scholar]
- Wang N, Ouyang XK, Yang LY, & Omer AM (2017). Fabrication of a magnetic cellulose nanocrystal/metal-organic framework composite for removal of Pb(II) from water. ACS Sustainable Chemistry & Engineering, 5, 10447–10458. [Google Scholar]
- Wang S, Vincent T, Roux J-C, Faur C, & Guibal E (2017). Pd(II) and Pt(IV) sorption using alginate and algal-based beads. Chemical Engineering Journal, 313, 567–579. [Google Scholar]
- Wang H, Wang C, Cui X, Qin L, Ding R, Wang L, et al. (2018). Design and facile one-step synthesis of FeWO4/Fe2O3 di-modified WO3 with super high photocatalytic activity toward degradation of quasi-phenothiazine dyes. Applied Catalysis B: Environmental, 221, 169–178. [Google Scholar]
- Wang Y, Yadav S, Heinlein T, Konjik V, Breitzke H, Buntkowsky G, et al. (2014). Ultra-light nanocomposite aerogels of bacterial cellulose and reduced graphene oxide for specific absorption and separation of organic liquids. RSC Advances, 4(41), 21553–21558. [Google Scholar]
- Wang Y, Zhang X, He X, Zhang W, Zhang X, & Lu C (2014). In situ synthesis of MnO2 coated cellulose nanofibers hybrid for effective removal of methylene blue. Carbohydrate Polymers, 110, 302–308. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhao C, Zhao P, Dou P, Ding Y, & Xu P (2009). Gellan gel beads containing magnetic nanoparticles: An effective biosorbent for the removal of heavy metals from aqueous system. Bioresource Technology, 100(7), 2301–2304. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhu L, You J, Chen F, Zong L, Yan X, et al. (2017). Catecholic coating and silver hybridization of chitin nanocrystals for ultrafiltration membrane with continuous flow catalysis and gold recovery. ACS Sustainable Chemistry & Engineering, 5(11), 10673–10681. [Google Scholar]
- Wang W, Zong L, & Wang A (2013). A nanoporous hydrogel based on vinylfunctionalized alginate for efficient absorption and removal of Pb2+ ions. International Journal of Biological Macromolecules, 62, 225–231. [DOI] [PubMed] [Google Scholar]
- Wen R, Tu B, Guo X, Hao X, Wu X, & Tao H (2020). An ion release controlled Cr (VI) treatment agent: Nano zero-valent iron/carbon/alginate composite gel. International Journal of Biological Macromolecules, 146, 692–704. [DOI] [PubMed] [Google Scholar]
- Wen Y, Tang Z, Chen Y, & Gu Y (2011). Adsorption of Cr(VI) from aqueous solutions using chitosan-coated fly ash composite as biosorbent. Chemical Engineering Journal, 175, 110–116. [Google Scholar]
- Wiaącek AE, Gozdecka A, & Jurak M (2018). Physicochemical characteristics of chitosan-TiO2 biomaterial. 1. Stability and swelling properties. Industrial & Engineering Chemistry Research, 57(6), 1859–1870. [Google Scholar]
- Williams DN, Gold KA, Holoman TRP, Ehrman SH, & Wilson OC (2006). Surface modification of magnetic nanoparticles using gum arabic. Journal of Nanoparticle Research, 8(5), 749–753. [Google Scholar]
- Wong ET, Chan KH, & Idris A (2015). Kinetic and equilibrium investigation of Cu (II) removal by Co(II)-doped iron oxide nanoparticle-immobilized in PVA-alginate recyclable adsorbent under dark and photo condition. Chemical Engineering Journal, 268, 311–324. [Google Scholar]
- Wu N, Wei H, & Zhang L (2012). Efficient removal of heavy metal ions with biopolymer template synthesized mesoporous titania beads of hundreds of micrometers size. Environmental Science & Technology, 46(1), 419–425. [DOI] [PubMed] [Google Scholar]
- Xie J, Lee JY, Wang DIC, & Ting YP (2007). Silver nanoplates: From biological to biomimetic synthesis. ACS Nano, 1(5), 429–439. [DOI] [PubMed] [Google Scholar]
- Xie J, Li C, Chi L, & Wu D (2013). Chitosan modified zeolite as a versatile adsorbent for the removal of different pollutants from water. Fuel, 103, 480–485. [Google Scholar]
- Xie M, Zeng L, Zhang Q, Kang Y, Xiao H, Peng Y, et al. (2015). Synthesis and adsorption behavior of magnetic microspheres based on chitosan/organic rectorite for low-concentration heavy metal removal. Journal of Alloys and Compounds, 647, 892–905. [Google Scholar]
- Xie X-L, Mai Y-W, & Zhou X-P (2005). Dispersion and alignment of carbon nanotubes in polymer matrix: A review. Materials Science and Engineering: R: Reports, 49(4), 89–112. [Google Scholar]
- Xie Y, Zhang L, Ren L, Yang J, Zhu X, Yi Y, et al. (2018). Enhancement of Cr(VI) removal from aqueous solution by carboxymethyl chitosan coated nano-zero-valent iron beads. Desalination and Water Treatment, 108, 268–278. [Google Scholar]
- Xing Z, Ju Z, Yang J, Xu H, & Qian Y (2013). One-step solid state reaction to selectively fabricate cubic and tetragonal CuFe2O4 anode material for high power lithium ion batteries. Electrochimica Acta, 102, 51–57. [Google Scholar]
- Xiong N, Wan P, Zhu G, Xie F, Xu S, Zhu C, et al. (2020). Sb(III) removal from aqueous solution by a novel nano-modified chitosan (NMCS). Separation and Purification Technology, 236, Article 116266. [Google Scholar]
- Xu C, Wang J, Yang T, Chen X, Liu X, & Ding X (2015). Adsorption of uranium by amidoximated chitosan-grafted polyacrylonitrile, using response surface methodology. Carbohydrate Polymers, 121, 79–85. [DOI] [PubMed] [Google Scholar]
- Xu R, Yong LC, Lim YG, & Obbard JP (2005). Use of slow-release fertilizer and biopolymers for stimulating hydrocarbon biodegradation in oil-contaminated beach sediments. Marine Pollution Bulletin, 51(8–12), 1101–1110. [DOI] [PubMed] [Google Scholar]
- Xu C, Nasrollahzadeh M, Sajjadi M, Maham M, Luque R, & Puente-Santiago AR (2019). Benign-by-design nature-inspired nanosystems in biofuels production and catalytic applications. Renewable and Sustainable Energy Reviews, 112, 195–252. [Google Scholar]
- Xu C, Nasrollahzadeh M, Selva M, Issaabadi Z, & Luque R (2019). Waste-to-wealth: Biowaste valorization into valuable bio (nano) materials. Chemical Society Reviews, 48(18), 4791–4822. [DOI] [PubMed] [Google Scholar]
- Yadav M, & Xu Q (2013). Catalytic chromium reduction using formic acid and metal nanoparticles immobilized in a metal–organic framework. Chemical Communications, 49(32), 3327–3329. [DOI] [PubMed] [Google Scholar]
- Yadav MP, Igartuburu JM, Yan Y, & Nothnagel EA (2007). Chemical investigation of the structural basis of the emulsifying activity of gum arabic. Food Hydrocolloids, 21(2), 297–308. [Google Scholar]
- Yadav S, Asthana A, Chakraborty R, Jain B, Singh AK, Carabineiro SAC, et al. (2020). Cationic dye removal using novel magnetic/activated charcoal/β-cyclodextrin/alginate polymer nanocomposite. Nanomaterials, 10(1), 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Chen C, Wang L, Zhang D, Li A-J, Yao Z, et al. (2016). Facile and green synthesis of cellulose nanocrystal-supported gold nanoparticles with superior catalytic activity. Carbohydrate Polymers, 140, 66–73. [DOI] [PubMed] [Google Scholar]
- Yan X, Zhang X, & Li Q (2018). Preparation and characterization of CS/β-CD/NanoZnO composite porous membrane optimized by Box-Behnken for the adsorption of Congo red. Environmental Science and Pollution Research, 25(22), 22244–22258. [DOI] [PubMed] [Google Scholar]
- Yang CH, Wang MX, Haider H, Yang JH, Sun J-Y, Chen YM, et al. (2013). Strengthening alginate/polyacrylamide hydrogels using various multivalent cations. ACS Applied Materials & Interfaces, 5(21), 10418–10422. [DOI] [PubMed] [Google Scholar]
- Yang J, Yu J, Fan J, Sun D, Tang W, & Yang X (2011). Biotemplated preparation of CdS nanoparticles/bacterial cellulose hybrid nanofibers for photocatalysis application. Journal of Hazardous Materials, 189(1–2), 377–383. [DOI] [PubMed] [Google Scholar]
- Yang L, Chen C, Hu Y, Wei F, Cui J, Zhao Y, et al. (2020). Three-dimensional bacterial cellulose/polydopamine/TiO2 nanocomposite membrane with enhanced adsorption and photocatalytic degradation for dyes under ultraviolet-visible irradiation. Journal of Colloid and Interface Science, 562, 21–28. [DOI] [PubMed] [Google Scholar]
- Yang N, Wang R, Rao P, Yan L, Zhang W, Wang J, et al. (2019). The fabrication of calcium Alginate beads as a green sorbent for selective recovery of Cu(II) from metal mixtures. Crystals, 9(5), 255. [Google Scholar]
- Yang R, Aubrecht KB, Ma H, Wang R, Grubbs RB, Hsiao BS, et al. (2014). Thiol-modified cellulose nanofibrous composite membranes for chromium(VI) and lead(II) adsorption. Polymer, 55(5), 1167–1176. [Google Scholar]
- Yang R, Su Y, Aubrecht KB, Wang X, Ma H, Grubbs RB, et al. (2015). Thiolfunctionalized chitin nanofibers for As(III) adsorption. Polymer, 60, 9–17. [Google Scholar]
- Yao Y, Gao B, Fang J, Zhang M, Chen H, Zhou Y, et al. (2014). Characterization and environmental applications of clay-biochar composites. Chemical Engineering Journal, 242, 136–143. [Google Scholar]
- Yargıç A, Şahin RZY, Özbay N, & Önal E (2015). Assessment of toxic copper(II) biosorption from aqueous solution by chemically-treated tomato waste. Journal of Cleaner Production, 88, 152–159. [Google Scholar]
- Yaseen DA, & Scholz M (2018). Treatment of synthetic textile wastewater containing dye mixtures with microcosms. Environmental Science and Pollution Research, 25(2), 1980–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdi F, Anbia M, & Salehi S (2019). Characterization of functionalized chitosanclinoptilolite nanocomposites for nitrate removal from aqueous media. International Journal of Biological Macromolecules, 130, 545–555. [DOI] [PubMed] [Google Scholar]
- Ye J, Hao Q, Liu B, Li Y, & Xu C (2017). Facile preparation of graphene nanosheets encapsulated Fe3O4 octahedra composite and its high lithium storage performances. Chemical Engineering Journal, 315, 115–123. [Google Scholar]
- Yin J, & Deng B (2015). Polymer-matrix nanocomposite membranes for water treatment. Journal of Membrane Science, 479, 256–275. [Google Scholar]
- Yu H-Y, Zhang D-Z, Lu F-F, & Yao J (2016). New approach for single-step extraction of carboxylated cellulose nanocrystals for their use as adsorbents and flocculants. ACS Sustainable Chemistry & Engineering, 4(5), 2632–2643. [Google Scholar]
- Yu Z, Dang Q, Liu C, Cha D, Zhang H, Zhu W, et al. (2017). Preparation and characterization of poly (maleic acid)-grafted cross-linked chitosan microspheres for Cd(II) adsorption. Carbohydrate Polymers, 172, 28–39. [DOI] [PubMed] [Google Scholar]
- Yu Z, Hu C, Dichiara AB, Jiang W, & Gu J (2020). Cellulose nanofibril/carbon nanomaterial hybrid aerogels for adsorption removal of cationic and anionic organic dyes. Nanomaterials, 10(1), 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Tong S, Ge M, Wu L, Zuo J, Cao C, et al. (2013). Adsorption of heavy metal ions from aqueous solution by carboxylated cellulose nanocrystals. Journal of the Environmental Sciences, 25(5), 933–943. [DOI] [PubMed] [Google Scholar]
- Yu X, Tong S, Ge M, Zuo J, Cao C, & Song W (2013). One-step synthesis of magnetic composites of cellulose@iron oxide nanoparticles for arsenic removal. Journal of Materials Chemistry A, 1(3), 959–965. [Google Scholar]
- Yun Y-H, Kim E-S, Shim W-G, & Yoon S-D (2018). Physical properties of mungbean starch/PVA bionanocomposites added nano-ZnS particles and its photocatalytic activity. Journal of Industrial and Engineering Chemistry, 68, 57–68. [Google Scholar]
- Yusof YM, & Kadir MFZ (2016). Electrochemical characterizations and the effect of glycerol in biopolymer electrolytes based on methylcellulose-potato starch blend. Molecular Crystals and Liquid Crystals, 627(1), 220–233. [Google Scholar]
- ZabihiSahebi A, Koushkbaghi S, Pishnamazi M, Askari A, Khosravi R, & Irani M (2019). Synthesis of cellulose acetate/chitosan/SWCNT/Fe3O4/TiO2 composite nanofibers for the removal of Cr(VI), As(V), Methylene blue and Congo red from aqueous solutions. International Journal of Biological Macromolecules, 140, 1296–1304. [DOI] [PubMed] [Google Scholar]
- Zarei S, Farhadian N, Akbarzadeh R, Pirsaheb M, Asadi A, & Safaei Z (2020). Fabrication of novel 2D Ag-TiO2/γ-Al2O3/Chitosan nano-composite photocatalyst toward enhanced photocatalytic reduction of nitrate. International Journal of Biological Macromolecules, 145, 926–935. [DOI] [PubMed] [Google Scholar]
- Zarei S, Sadeghi M, & Bardajee GR (2018). Dye removal from aqueous solutions using novel nanocomposite hydrogel derived from sodium montmorillonite nanoclay and modified starch. International Journal of Environmental Science and Technology, 15 (11), 2303–2316. [Google Scholar]
- Zemmouri H, Drouiche M, Sayeh A, Lounici H, & Mameri N (2013). Chitosan application for treatment of Beni-Amrane’s water dam. Energy Procedia, 36, 558–564. [Google Scholar]
- Zeng J, Liu S, Cai J, & Zhang L (2010). TiO2 immobilized in cellulose matrix for photocatalytic degradation of phenol under weak UV light irradiation. The Journal of Physical Chemistry C, 114(17), 7806–7811. [Google Scholar]
- Zeng L, Xie M, Zhang Q, Kang Y, Guo X, Xiao H, et al. (2015). Chitosan/organic rectorite composite for the magnetic uptake of methylene blue and methyl orange. Carbohydrate Polymers, 123, 89–98. [DOI] [PubMed] [Google Scholar]
- Zeng X, Wu J, Zhang D, Li G, Zhang S, Zhao H, et al. (2009). Degradation of toluene gas at the surface of ZnO/SnO2 photocatalysts in a baffled bed reactor. Research on Chemical Intermediates, 35(6–7), 827–838. [Google Scholar]
- Zhang B, Wu Y, & Fan Y (2019). Synthesis of novel magnetic NiFe2O4 nanocomposite grafted chitosan and the adsorption mechanism of Cr(VI). Journal of Inorganic and Organometallic Polymers and Materials, 29(1), 290–301. [Google Scholar]
- Zhang G, Li Y, Gao A, Zhang Q, Cui J, Zhao S, et al. (2019). Bio-inspired underwater superoleophobic PVDF membranes for highly-efficient simultaneous removal of insoluble emulsified oils and soluble anionic dyes. Chemical Engineering Journal, 369, 576–587. [Google Scholar]
- Zhang L, Xia W, Teng B, Liu X, & Zhang W (2013). Zirconium cross-linked chitosan composite: Preparation, characterization and application in adsorption of Cr(VI). Chemical Engineering Journal, 229, 1–8. [Google Scholar]
- Zhang L, Yang S, Han T, Zhong L, Ma C, Zhou Y, et al. (2012). Improvement of Ag (I) adsorption onto chitosan/triethanolamine composite sorbent by an ion-imprinted technology. Applied Surface Science, 263, 696–703. [Google Scholar]
- Zhang M, Helleur R, & Zhang Y (2015). Ion-imprinted chitosan gel beads for selective adsorption of Ag+ from aqueous solutions. Carbohydrate Polymers, 130, 206–212. [DOI] [PubMed] [Google Scholar]
- Zhang N, Zang G-L, Shi C, Yu H-Q, & Sheng G-P (2016). A novel adsorbent TEMPO-mediated oxidized cellulose nanofibrils modified with PEI: Preparation, characterization, and application for Cu(II) removal. Journal of Hazardous Materials, 316, 11–18. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zhao D, Feng S, Wang Y, Jin J, Alsaedi A, et al. (2019). Synthesis of nanoscale zero-valent iron loaded chitosan for synergistically enhanced removal of U (VI) based on adsorption and reduction. Journal of Colloid and Interface Science, 552, 735–743. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang X, Zhang Y, Seppälä J, Xu W, Willför S, et al. (2020). Robust shape-memory nanocellulose-based aerogels decorated with silver nanoparticles for fast continuous catalytic discoloration of organic dyes. Separation and Purification Technology, 242, Article 116523. [Google Scholar]
- Zhang Y-J, Xue J-Q, Li F, Dai JI-Z, & Zhang X-Z-Y (2019). Preparation of polypyrrole/chitosan/carbon nanotube composite nano-electrode and application to capacitive deionization process for removing Cu2+. Chemical Engineering and Processing-Process Intensification, 139, 121–129. [Google Scholar]
- Zhang Z, Ma X, Jia M, Li B, Rong J, & Yang X (2019). Deposition of CdTe quantum dots on microfluidic paper chips for rapid fluorescence detection of pesticide 2,4-D. Analyst, 144(4), 1282–1291. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Sèbe G, Rentsch D, Zimmermann T, & Tingaut P (2014). Ultralightweight and flexible silylated nanocellulose sponges for the selective removal of oil from water. Chemistry of Materials, 26(8), 2659–2668. [Google Scholar]
- Zhang P, Hou D, O’Connor D, Li X, Pehkonen S, Varma RS, et al. (2018). Green and size-specific synthesis of stable Fe-Cu oxides as earth-abundant adsorbents for malachite green removal. ACS Sustainable Chemistry & Engineering, 6(7), 9229–9236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B.-x., Yu H, Zhang Y, Luo Z, Han W, Qiu W, et al. (2018). Bacterial cellulose derived monolithic titania aerogel consisting of 3D reticulate titania nanofibers. Cellulose, 25(12), 7189–7196. [Google Scholar]
- Zhao K, Feng L, Lin H, Fu Y, Lin B, Cui W, et al. (2014). Adsorption and photocatalytic degradation of methyl orange imprinted composite membranes using TiO2/calcium alginate hydrogel as matrix. Catalysis Today, 236, 127–134. [Google Scholar]
- Zhao Y, Tao C, Xiao G, & Su H (2017). Controlled synthesis and wastewater treatment of Ag2O/TiO2 modified chitosan-based photocatalytic film. RSC Advances, 7(18), 11211–11221. [Google Scholar]
- Zheng Q, Cai Z, & Gong S (2014). Green synthesis of polyvinyl alcohol (PVA)-cellulose nanofibril (CNF) hybrid aerogels and their use as superabsorbents. Journal of Materials Chemistry A, 2(9), 3110–3118. [Google Scholar]
- Zheng W, An Q, Lei Z, Xiao Z, Zhai S, & Liu Q (2016). Efficient batch and column removal of Cr(VI) by carbon beads with developed nano-network. RSC Advances, 6 (106), 104897–104910. [Google Scholar]
- Zhong R, Zhong Q, Huo M, Yang B, & Li H (2020). Preparation of biocompatible nano-ZnO/chitosan microspheres with multi-functions of antibacterial, UV-shielding and dye photodegradation. International Journal of Biological Macromolecules, 146, 939–945. [DOI] [PubMed] [Google Scholar]
- Zhou C, Lee S, Dooley K, & Wu Q (2013). A facile approach to fabricate porous nanocomposite gels based on partially hydrolyzed polyacrylamide and cellulose nanocrystals for adsorbing methylene blue at low concentrations. Journal of Hazardous Materials, 263, 334–341. [DOI] [PubMed] [Google Scholar]
- Zhou J, Sun Q, Chen D, Wang H, & Yang K (2017). Ochrobactrum anthropi used to control ammonium for nitrate removal by starch-stabilized nanoscale zero valent iron. Water Science and Technology, 76(7), 1827–1832. [DOI] [PubMed] [Google Scholar]
- Zhou L, Shang C, Liu Z, Huang G, & Adesina AA (2012). Selective adsorption of uranium(VI) from aqueous solutions using the ion-imprinted magnetic chitosan resins. Journal of Colloid and Interface Science, 366(1), 165–172. [DOI] [PubMed] [Google Scholar]
- Zhou Y-T, Nie H-L, Branford-White C, He Z-Y, & Zhu L-M (2009). Removal of Cu2+ from aqueous solution by chitosan-coated magnetic nanoparticles modified with α-ketoglutaric acid. Journal of Colloid and Interface Science, 330(1), 29–37. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Fu S, Zhang L, Zhan H, & Levit MV (2014). Use of carboxylated cellulose nanofibrils-filled magnetic chitosan hydrogel beads as adsorbents for Pb(II). Carbohydrate Polymers, 101, 75–82. [DOI] [PubMed] [Google Scholar]
- Zhou C, Wu Q, Lei T, & Negulescu II (2014). Adsorption kinetic and equilibrium studies for methylene blue dye by partially hydrolyzed polyacrylamide/cellulose nanocrystal nanocomposite hydrogels. Chemical Engineering Journal, 251, 17–24. [Google Scholar]
- Zhu H, Jia S, Wan T, Jia Y, Yang H, Li J, et al. (2011). Biosynthesis of spherical Fe3O4/bacterial cellulose nanocomposites as adsorbents for heavy metal ions. Carbohydrate Polymers, 86(4), 1558–1564. [Google Scholar]
- Zhu Z-S, Qu J, Hao S-M, Han S, Jia K-L, & Yu Z-Z (2018). α-Fe2O3 nanodisk/bacterial cellulose hybrid membranes as high-performance sulfate-radical-based visible light photocatalysts under stirring/flowing states. ACS Applied Materials & Interfaces, 10(36), 30670–30679. [DOI] [PubMed] [Google Scholar]
- Zhuang S, & Wang J (2019). Removal of cesium ions using nickel hexacyanoferrates-loaded bacterial cellulose membrane as an effective adsorbent. Journal of Molecular Liquids, 294, Article 111682. [Google Scholar]
- Zinadini S, Zinatizadeh AA, Rahimi M, Vatanpour V, Zangeneh H, & Beygzadeh M (2014). Novel high flux antifouling nanofiltration membranes for dye removal containing carboxymethyl chitosan coated Fe3O4 nanoparticles. Desalination, 349, 145–154. [Google Scholar]
- Zobel HF (1988). Molecules to granules: A comprehensive starch review. Starch-Stärke, 40(2), 44–50. [Google Scholar]
- Zolfaghari P, Shojaat R, Karimi A, & Saadatjoo N (2018). Application of fluidized bed reactor containing GOx/MnFe2O4/calcium alginate nano-composite in degradation of a model pollutant. Journal of Environmental Chemical Engineering, 6 (5), 6414–6420. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


