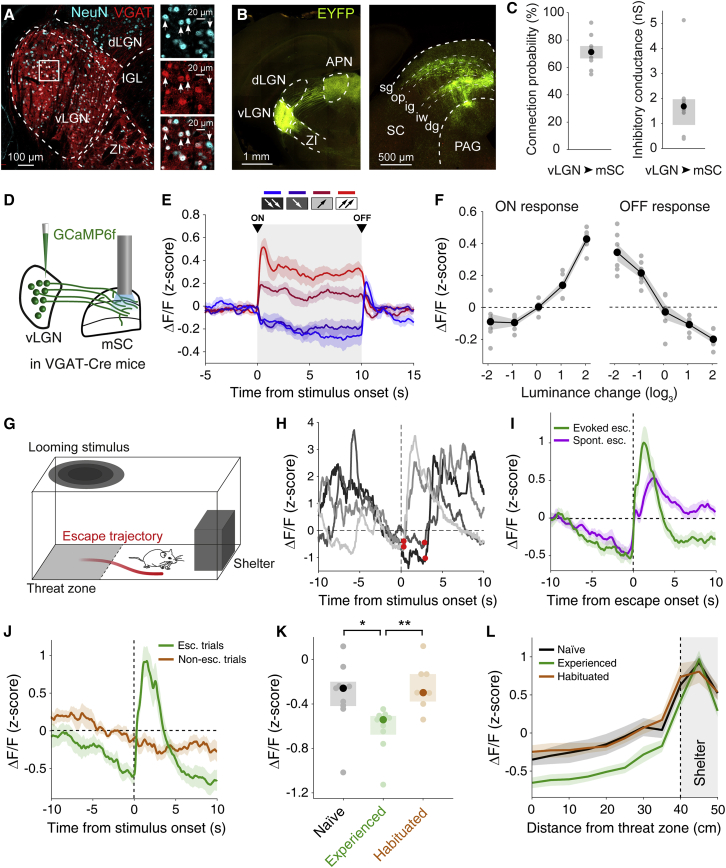
Figure 1.
Activity of vLGN axons in mSC reflects previous experience of threat
(A) Example images of tdTomato expression in VGAT+ neurons in the vLGN (red), combined with NeuN staining (cyan). Images on the right are from the inset in the left image, showing only NeuN staining (top), tdTomato expression (middle), and both combined (bottom). Arrows indicate examples of NeuN+ neurons.
(B) Left, expression of EYFP in the vLGN after injection of AAV-flex-EYFP in VGAT-Cre mice. Right, GABAergic vLGN axons in the SC and PAG. APN, anterior pretectal nucleus; dLGN, dorsal lateral geniculate nucleus; IGL, intergeniculate leaflet; PAG, periaqueductal gray; SC, superior colliculus; vLGN, ventral lateral geniculate nucleus; ZI, zona incerta. SC layers: sg, superficial gray layer; op, optical layer; ig, intermediate gray layer; iw, intermediate white layer; dg, deep gray layer.
(C) Left, mean connection probability between GABAergic vLGN axons and cells in the medial SC (mSC), observed using ChR2-assisted circuit mapping in vitro. Error bars represent standard errors of the mean (SEM) across mice. Right, median inhibitory conductance in mSC cells in response to stimulation of GABAergic vLGN axons. Error bars represent the interquartile range (IQR) across mice. Grey dots represent data from single animals; n = 8 mice, 81 cells.
(D) Experimental paradigm for fiber photometry recordings of calcium signals from GABAergic vLGN axons in the mSC.
(E) Mean calcium activity of vLGN axons in the mSC in response to increases (27 cd × m−2, magenta; 81 cd × m−2, red) and decreases (3 cd × m−2, indigo; 1 cd × m−2, blue) in luminance from baseline levels (9 cd × m−2). Stimulus duration is indicated by gray shading. Error-bar shading represents SEM across mice; n = 6 mice.
(F) Mean change in calcium activity due to the onset (ON response, left; see Method details) and the offset (OFF response, right) of the change in luminance. Luminance change values are the base-3 logarithm of the ratio between the stimulus and baseline luminance. Grey dots represent data points from individual mice. Shading shows SEM across mice; n = 6 mice.
(G) Schematic of the experimental approach. Red line denotes escape trajectory.
(H) Four representative single-trial calcium traces of vLGN axons in the mSC during threat-evoked escape aligned to stimulus onset. Red dots, escape onset.
(I) Mean calcium activity of vLGN axons in the mSC during threat-evoked (green) and spontaneous (purple) escapes aligned to escape onset. Shading shows SEM across mice; n = 9 mice.
(J) Mean calcium activity of vLGN axons recorded in the mSC in escape trials early in the recording session, before the start of the habituation protocol (green, n = 9 mice), and during non-escape trials in habituated animals (orange, n = 7 mice), aligned to stimulus onset. Shading shows SEM across mice.
(K) Median calcium activity of vLGN axons in mice approaching the threat zone before presentation of the first looming stimulus (black, naive, n = 9 mice), after presentation of the first looming stimulus (green, experienced, n = 9 mice), and after habituation (orange, n = 7 mice). Pale dots represent data from single animals. Error bars represent the IQR across mice. Naive-experienced: p = 0.0130, experienced-habituated: p = 8.31 × 10−3, naive-habituated: p = 0.983, Dunn’s multiple comparison test, preceded by Kruskal-Wallis one-way analysis of variance, p = 2.96 × 10−3.
(L) Mean calcium activity of vLGN axons binned by distance during the 30 s before reaching the threat zone in naive mice (black, n = 9 mice), after presentation of the first looming stimulus (green, experienced, n = 9 mice), and after habituation (orange, n = 7 mice). Shading shows SEM across mice. Dashed line and gray shading indicate the location of the shelter.