1. Introduction
Among the most prominent hallmarks of biological development is its remarkable plasticity over the entire span of individual lives (Bateson et al., 2004; Belsky and Pluess, 2009). Rather than a lockstep succession of phenotypic change, development is marked, especially in the early years, by a striking tractability in the adaptive demands and signals emanating from the individual’s environment. Such phenotypic plasticity derives from the transcription regulatory capacities of the epigenome and is the centerpiece of a “developmental synthesis” describing how changes in developmental trajectories drive and constrain evolution (Gilbert and Epel, 2009). A core assertion of that evolutionary-developmental synthesis is that differences in where, when, and how genes are activated and expressed are the sources of variation in phenotypes (Muller, 2007) and that such variation is a key element in the developmental origins of human morbidities (Gluckman et al., 2009). Developmental outcomes thus serve as the primary focus for studies of phenotypic plasticity. An important but understudied dimension of such plasticity is the parameter of pace: alterations in the timing and tempo of developmental change.
Two unanticipated findings from our research group at the University of California, San Francisco and the University of British Columbia, Vancouver prompted a more intensive consideration of developmental pace within accessible data sets with measures of developmental timing. First, in the Peers and Wellness Study (PAWS), a prospective investigation of classroom social hierarchies and health among kindergarten children attending Berkeley public schools (see, for example: Bush et al., 2018; Obradovic and Boyce, 2009; Obradovic et al., 2010), dental exams conducted in a sub-sample of children during first grade yielded an unexplained, incidental finding: that children with higher basal salivary cortisol expression over three sequential school days had fewer primary teeth than children with lower basal cortisol measures (r = −.40, p < .01).1 That is, children with evidence of greater stress-related, chronic hypothalamic-pituitary-adrenocortical (HPA) activation had exfoliated, by age 6, more of their primary dentition than had peers with more normative HPA basal activity, an association not attributable to either dental caries or tooth extractions. Second, another study by Moore and colleagues (Moore et al., 2017) examined epigenetic age data among children from a community sample of mothers and babies in whom maternal-infant contact was recorded as either unusually low or unusually high. Temperamentally fussy babies with low-contact mothers had significantly slower epigenetic “clocks,” reflecting a developmentally earlier pattern of DNA methylation. Both studies thus revealed evidence of alterations in the pace of developmental change, one with dental exfoliation data demonstrating developmental acceleration and the other with epigenetic clock data suggesting developmental deceleration.
Human behavioral development is the sequential, integrative acquisition of motoric, socioemotional, cognitive, and linguistic skills, which begin prenatally, continue over the lifespan, and proceed in a time-dependent, predictable ordering. Like many biological processes, development shows extensive, normative variation and plasticity, with pathological, atypical states emerging only at the extremes of such diversity. Increasingly well-documented are the joint, interactive roles of genetic variation and environmental exposures in setting the course and endpoints of development, with epigenetic events (e.g., DNA methylation (DNAm), post-translational modifications of nucleosome proteins, and production of non-coding-, micro-RNAs) guiding the differential expression of developmentally formative genes (Czamara et al., 2019; Teh et al., 2014). Less recognized than such gene-environment (GxE) interactions and only more recently examined, time and timing constitute an essential third factor in developmental biology, comprising a previously missing vertex in triadic gene-environment-time (GxExT) interactions (Boyce et al., 2020). Differential transcription of specific gene networks by time is, of course, a fundamental driver of human embryogenesis, in which at least 200 different histological cell types are sequentially generated. Even beyond fetal embryonic development, however, time and timing may also play an essential, but not yet fully explored, role in metabolic, adaptive, and developmental processes (Boyce et al, 2021). Triadic, GxExT interactions are the basis for a set of now well-recognized, temporal events, including: critical and sensitive periods2 (Takesian and Hensch, 2013); timed, experience-expectant processes (McLaughlin et al., 2017); optimal timing of interventions (Marin, 2016); the most injurious developmental windows of adversity exposure (Dunn et al., 2020; Riem and Karreman, 2019); and the timed emergence of developmental psychopathology (Maughan and Collishaw, 2015). Molecular events determining the timing of critical periods and the perturbations of development attending various exposures span multiple temporal scales, ranging from milliseconds in the case of neuronal oscillations to generational or even intergenerational continuities in the case of epigenetic processes (Cameron et al., 2017; Reh et al., 2019).
An enigmatic, temporal phenomenon only recently discerned and partially explained is the acceleration or deceleration of early development observed to occur under certain environmental conditions and in specific developmental periods. Such observable changes in developmental pace appear analogous to how contemporary quantum mechanics views the plasticity of time: never singular or absolute, but rather expanding or contracting as a consequence of perspective and its ontological coupling to a space-time continuum (Rovelli, 2018). Thus, in the perspective of the physical sciences, time runs measurably slower for an observer that is closer to the earth or traveling at high velocity, relative to a stationary observer located some distance from earth’s surface. While the Newtonian idea that time is absolute and uniform—independent of things, their locations, and movements—is far more intuitive, the observable, objective realities of time are less instinctive, even at the macro level of organisms and planetary bodies.
In certain circumstances, it seems that developmental time also figuratively expands or contracts, resulting in a slower or more rapid pace of developmental change, with outcomes that are predictable and often consequential to the individual’s well-being and health. To date, developmental acceleration and deceleration have largely been explained through the lens of medical models of disease (i.e., adversity “wears down” biological systems, resulting in dysfunction/death) or evolutionary-developmental theories (i.e., adversity accelerates maturation to optimize an organism’s reproductive fitness). This paper advances a broadened, more explicit theoretical account for how and why variations in early environments govern alterations in the pace of neurodevelopment. More specifically, we propose a framework with five main tenets that diverge from prior explanatory theories of developmental pace changes.
First, we suggest that the parent-child dyad, rather than the individual child, is the focal unit that governs developmental pace alterations. Second, we advance an explanatory account for why adversity-induced changes in developmental pace occur by suggesting that accelerations and decelerations are enacted to obviate the shortfalls that emerge from children’s unmet physiological and safety needs. Third, specificity in the direction of developmental pace alterations (i.e., acceleration or deceleration) is proposed to emerge from the uniquely-shaped trajectories of physiological needs versus safety needs during early childhood. Fourth, we hypothesize that developmental pace alterations emerge not only to optimize long-term reproductive fitness, but also as a response to unmet developmental needs in the short-term. Finally, we propose that linkages between early adversity exposures and developmental acceleration or deceleration involve neurobiological mechanisms operating along specific causal pathways. In order to develop this explanatory framework (see Section 4), we draw upon theoretical/conventional views of developmental needs in early childhood, as well as empirical literature on the effects of adverse exposures on aging, another instantiation of differences in developmental pace.
2. Prior Evidence of Developmental Acceleration and Deceleration
2.1. Animal models
The non-human animal literature comprises many examples of developmental acceleration and deceleration, often illustrating rapid calibrations between an organism’s current energetic state and capricious environmental conditions. This homologous evidence across animal models in different species provides support for a unifying theoretical framework. For example, Western spadefoot tadpoles exhibit an accelerated larval period in response to adversely dry pond conditions, one of the greatest risks to their survival (Crespi and Denver, 2005; Denver and Crespi, 2006; Gomez-Mestre et al., 2013). This adaptive developmental plasticity is so finely attuned to the local ecology that an improvement in environmental conditions can induce a subsequent deceleration in physiological and cellular development (Sadeh et al., 2011). Evidence of developmental pace alterations has also been observed in sheep, where exposure to chronic prenatal hypoxia induces accelerated fetal relaxation of pulmonary vasculature, allowing accommodation to high altitude, low oxygen births (Blum-Johnston et al., 2016). In juvenile Coho salmon, maturational events (e.g., seaward migration) may be delayed when environments are unfavorable in order to wait for more propitious conditions (Grand, 1999).
The rodent literature provides evidence of developmental pace accelerations induced by differences in early life stress exposure and maternal care. For example, infant rats reared under stressful conditions (e.g., low maternal licking and grooming, limited bedding environment, maternal separation) exhibit earlier odor aversion learning (Moriceau, Shionoya, Jakubs & Sullivan, 2008) and fear retention (Callaghan and Richardson, 2012a), as well as earlier pubertal onset (Cameron, 2004) and accelerated synaptic maturity (Guadagno, Verlezza, Long, Wong, & Walker, 2020) compared to infant rats reared under normal conditions. Elevated cortisol levels have also been observed among rat pups raised in stressful environments (Moriceau et al., 2008) and exposure to the stress hormone cortisol early in life has been shown to prompt the same pattern of developmental acceleration that emerges in the context of stressful rearing conditions (Callaghan and Richardson, 2012a, 2012b). Among male mice, early weaning prompts accelerated myelin formation in the anterior part of the basolateral amygdala and more frequent anxious behaviors (Ono et al., 2008). Interestingly, there is also evidence that enriched environments (e.g., larger cages and groups, running wheels, toys) accelerate the development of the visual system in mice, potentially via higher levels of maternal licking and physical contact (Cancedda et al., 2004; Sale et al., 2004).
Developmental acceleration and deceleration are also evident in parallel, cross-species maturational processes. Among many species with a larval life cycle phase (e.g., C. elegans), a “developmentally arrested” dauer stage is observed, induced by adverse environmental conditions, including unfavorably high temperatures, humidity, and food scarcity (Karp, 2018). Dauer larvae may survive for months in disadvantageous conditions, with a relatively swift recovery and recommencement of development once environmental circumstances improve (Nika et al., 2016). In over 130 species of mammals, non-optimal environments may induce embryonic diapause, a temporary cessation of embryo development that prolongs gestation until the prevailing context will better support offspring survival (Deng et al., 2018). Finally, both endotherms and ectotherms also enter states of metabolic suppression—topor and estivation, respectively—when environmental conditions are energetically insufficient to facilitate growth and reproduction (Staples, 2011).
2.2. Human studies
In addition to such controlled models, evidence from numerous human research domains has documented developmental acceleration and deceleration at different stages across the life course. Here, we highlight three particularly notable works that lay the foundation for the present model of developmental acceleration and deceleration. First and most notably, the recent and comprehensive meta-analytic and systematic review of literature by Colich, Rosen, Williams, and McLaughlin (2020) found that threat-related adverse exposures (e.g., childhood abuse, witness to domestic violence) were associated with earlier pubertal timing, cellular aging, and cortical thinning, especially in the ventro-medial prefrontal cortex (vmPFC) involved with emotion processing. By contrast, meta-analysis did not find childhood experiences of deprivation (e.g. neglect, institutionalization) nor low socioeconomic status to relate to metrics of developmental acceleration. Their systematic review of the literature also suggested that the area of the brain in which adversity-induced accelerations in cortical thinning occur depend on the type of adversity. This paper stands as the most comprehensive, data-centered review of studies examining developmental acceleration and deceleration in response to early life adversity.
In a second seminal contribution to recent human studies of adversity-related changes in developmental pace, the Neuro-Environmental Loop for Plasticity model advanced by Callaghan & Tottenham (2016a) suggests that the tempo of development within emotion-processing circuitry is guided by the intersection of caregiver dependence and child independence during sensitive periods. Under normative conditions in early childhood, the authors propose that a state of “semi-independence” of offspring from caregivers opens a sensitive period for the vmPFC circuitry to acquire greater regulatory capacities in response to parental inputs. This period of plasticity begins to close as the child achieves greater independence and is no longer as strongly reliant on the caregiver. The early independence from one’s caregiver that emerges under non-normative conditions (e.g., in settings of institutional rearing) operates as a signal to accelerate the closure of the sensitive period, contributing to early maturation of vmPFC development.
Finally, Tooley, Bassett, & Mackey (2021) focus on the pervasive effects of childhood socioeconomic status (SES). Lower childhood socioeconomic status (SES) has been associated with accelerated cortical thinning and an earlier peak in cortical surface area development, though the authors caution that the evidence is far from unequivocal and limited by largely cross-sectional study designs. Drawing upon this extant literature, they propose a framework whereby the valence and frequency of early experiences predict changes in the pace of brain maturation. More specifically, childhood experiences that are negative and chronic are predicted to accelerate brain development via repetitive use of regulatory circuitry, allostatic load, and (aligned with life history theory), increasing perceptions of environmental threat that suggest the need for faster maturation to facilitate reproductive success. In contrast, positive and rare experiences are expected to slow brain development, as they signal greater variability in the environment that would benefit from a more prolonged period of plasticity.
Despite this strong foundation of theoretical and empirical studies reviewed by Colich et al (2020), Callaghan & Tottenham (2016a), and Tooley et al. (2021), other work has observed more variable effects of early adversity on biological and developmental endpoints, e.g., the delayed development of amygdala-PFC connectivity among children exposed to aversive environments. Such findings suggest that the overall direction and magnitude of adversity-related effects on the pacing of neurobiological development remain equivocal and depend upon the types of adversities encountered, the maturational parameters assessed, and the developmental age of children studied. Such variation in findings is well-represented in the assembly of other human studies summarized below.
2.2.1. Stress-induced premature birth.
Large-scale, prospective studies provide strong evidence for the influence of gestational stressors on prematurity, with findings that have been replicated across diverse types of adversity, populations, and geographic regions (Dunkel Schetter, 2011; Dunkel Schetter and Glynn, 2011; Glynn et al., 2008; Wadhwa et al., 1993). Preterm birth is frequently conceptualized using a fetal programming or fetal origins hypothesis, which describes how adverse intrauterine environments may influence fetal development, as well as offspring health and disease susceptibility (Barker, 2007; Bateson et al., 2004). Although the concept of fetal programming provides an organizational framework for research on prenatal stress exposure, premature birth can also be viewed through the lens of adaptive developmental acceleration. Early adversity may prompt accelerated fetal development, ultimately leading to delivery as an evasion of conditions threatening survival. Maternal uterine infection, for example, with its accompanying exposure to pro-inflammatory cytokines has been advanced as an explanation for prematurity (Gilman-Sachs et al., 2018; Goldenberg et al., 2008).
2.2.2. Sexual maturation.
The onset and timing of puberty have also been examined as biological processes linking early adversity and accelerated development. An extensive body of literature has observed accelerated pubertal development, resulting in earlier menarche, among girls exposed to early parental separation (Quinlan, 2003), father absence (Chisholm et al., 2005; Deardorff et al., 2011), and childhood sexual abuse (Boynton-Jarrett and Harville, 2012; Boynton-Jarrett et al., 2013; Li et al., 2014; Romans et al., 2003). Less adequate parental support and maternal depression have been associated with earlier adrenarche among children of both sexes (Belsky et al., 2015; Ellis and Essex, 2007) and maternal stress during pregnancy predicts a more advanced age at menarche among female offspring (Duchesne et al., 2017). Life history theory, rooted in an evolutionary-developmental framework, accounts for the hastening of pubertal maturation in terms of efforts to maximize reproductive fitness and avoid extinction of the lineage in harsh or unpredictable environments (Coall and Chisholm, 2003). Metabolic resources, it is argued, are preferentially allocated to pubertal maturation at the expense of other developmental processes in order to increase the probability of reproducing in environmental conditions ill-suited to reproductive efforts (Belsky et al., 2012; Ellis, 2004).
Delayed pubertal maturation, on the other hand, can be conceptualized as developmental deceleration, though the preconditions in which late maturation may occur are still unclear. Consistent research finds that serious and sustained nutritional deprivation delays puberty (Wells, 2018); beyond resource scarcity, evidence of the association between psychosocial stressors and late pubertal maturation has been somewhat more limited (Ellis, 2004). A criticism of this research has been the limited inclusion of racially and ethnically diverse samples, but in a recent study of minority children, exposure to maltreatment, violence, and other forms of trauma was associated with delays in the timing of pubertal development (Suglia et al., 2020).
2.2.3. Accelerated aging.
Successful adaptation of the brain and body to environmental stress occurs through allostasis, a process through which varied and interacting physiological systems maintain stability of function (homeostasis) through change (McEwen, 2004). However, psychosocial stressors that are severe or prolonged may lead to chronically activated or insufficient biological responses to stress; such physiologic “wear and tear” on the body, also known as allostatic load, promulgate accelerated biological aging (Juster et al., 2010). Illustratively, studies of biological “weathering” report relations among psychosocial stress exposure, heightened physiological burden (e.g., greater allostatic load), and age-related declines in health across the lifespan (Das, 2013; Geronimus et al., 2006). Compared to age-matched controls, youth reared in an international orphanage during childhood exhibited cardiometabolic health problems suggestive of advanced cardiovascular aging, even after adoption into a stable family environment (Reid et al., 2018).
Research has also identified associations between adversity and shorter telomere length, a marker of cellular aging (Epel and Prather, 2018). Telomeres are the protective ends of chromosomes, which shorten progressively with each cell division and, can lead to cellular death or senescence (Frenck et al., 1998). Telomere erosion has been observed in relation to varied types of early life stressors (for review, see Price, Kao, Burgers, Carpenter, & Tyrka, 2013), and across numerous developmental periods, including infancy (Entringer et al., 2013), childhood (Drury et al., 2014), and adolescence (Humphreys et al., 2016; Theall et al., 2013). Beyond discrete indicators of aging, a novel, algorithmically-derived “pace of aging” measure based on eighteen biomarkers revealed an accelerated rate of aging among young adults with exposures to adversity in childhood (Belsky et al., 2017). In the accelerated group, 75% of individuals were exposed to at least one childhood risk factor, and aging occurred at a pace more than 1.4 years faster than average. Risk exposure was markedly lower among individuals with an average (50%) or below average (43%) pace of aging (Belsky et al., 2017). Greater exposure to threat-related adverse childhood has also been associated with accelerated aging across studies of telomere length and DNA methylation age (Colich et al., 2020)
Recently, novel methods have yielded an intriguing measure of biological aging in children. Based on normative patterns of age-related changes in DNAm, research has identified epigenetic “clocks” serving as highly accurate predictors of chronological age (Horvath and Raj, 2018). An epigenetic clock-based prediction of age that exceeds actual age is believed to reflect developmental acceleration, which has been associated with maternal risk factors during pregnancy (Girchenko et al., 2017), maternal prenatal anxiety (McGill et al., under review), and children’s exposure to neighborhood violence (Jovanovic et al., 2017).
2.2.4. Accelerated maturation of brain circuitry and structure.
A recent study by Gur and colleagues (Gur et al., 2019) revealed a compelling illustration of adversity-induced, developmental acceleration by directly comparing effects associated with low SES with those linked to traumatic life events—two different, albeit inter-correlated, aspects of adverse early environments. The study replicated the effects of early life adversity on the timing of puberty, with more widespread effects in females. The authors then analyzed MRI data from individuals 8 – 21 years of age to test for accelerated development. The age range permitted the use of machine learning approaches to classify adult vs non-adult brain structural and connectivity profiles using a range of parameters. A significantly higher proportion of the low SES or traumatized individuals in the younger ages were (mis)classified as adults compared to controls.
Maternal prenatal depression associates with alterations in microstructure and functional connectivity of the amygdala at birth (Hay et al., 2020; Rifkin-Graboi et al., 2013; Scheinost et al., 2016), greater functional connectivity of the amygdala in 6-month-old infants (Qiu et al., 2015) and 4-years-old children (Soe et al., 2018), a larger right amygdala volume in young girls at age of 4 and 7 years (Buss et al., 2012; Wen et al., 2017), and alterations of the amygdala-prefrontal structural circuit from birth to early childhood (Lee et al., 2019). The effects are largely specific to the right hemisphere where there is a specialization for the processing of threat (Fox and Davidson, 1986). Importantly, these effects are apparent in neuroimaging studies performed with neonates, thus confirming prenatal influences that persist beyond birth. Studies from Tottenham and colleagues (Gee et al., 2013a; Gee et al., 2013b) provide what may be the most compelling evidence for adversity-induced developmental acceleration. Under typical rearing conditions, children exhibit positive coupling of amygdala-mPFC activation when viewing fearful faces, while adolescents and adults exhibit negative coupling. However, children exposed to institutionalized rearing have been shown to exhibit negative amygdala-mPFC connectivity when viewing fearful faces, demonstrating a pattern characteristic of normally-developing adolescents (Gee et al. 2013a). This pattern suggests accelerated maturation of amygdala-PFC circuits following exposure to the severe adversities associated with institutional care.
2.2.5. Psychosocial short stature.
Empirical studies of developmental deceleration are more limited than those providing evidence of accelerated developmental pace. Some of the most compelling examples of developmental deceleration may be observed from research on psychosocial short stature, a phenomenon first documented over 70 years ago, in which reduced growth is found among children reared within emotionally deprived environments (Gohlke et al., 2004; Talbot et al., 1947). A variety of stress-sensitive biological mechanisms have been advanced to explain the growth effects of neglectful conditions, which appear to emerge independent of adequate nutritional supply (Johnson et al., 1992; Muñoz-Hoyos et al., 2011). In humans, fetal growth retardation in response to nutrient deprivation or placental insufficiency, followed by postnatal catch-up growth, may represent another such phenomenon (Baschat, 2014).
2.2.6. Decelerated aging.
Research on the epigenetic clock suggests that negative epigenetic-to-chronological age deviations (i.e., age deceleration) may be associated with risk in a manner similar to the previously reviewed positive deviations (i.e., age acceleration). Prenatal risk factors, for example, have been associated with both epigenetic age acceleration and deceleration. Offspring born to mothers who experienced heightened prenatal depressive symptoms showed lower epigenetic gestational age (age deceleration based on DNAm of fetal cord blood DNA), compared to women without depression (Suarez et al., 2018). Further, evidence of lower epigenetic age has also been observed in highly distressed infants who experience lower maternal physical contact, compared to those with more extensive maternal-infant contact (Moore et al., 2017).
3. Deficiencies in existing explanatory accounts of changes in developmental pace
Existing explanatory accounts for observable shifts in the pace of development include the psychosomatic medicine or social determinants of health models, as well as evolutionary developmental theory. These frameworks are largely descriptive in character, and description has become conflated with explanation.
3.1. Psychosomatic medicine and social determinants of health models
The psychosomatic medicine model views cellular aging and bodily “weathering” as the biological consequences of chronic exposures to adversity, and a social determinants orientation has pointed to poverty and disadvantage as impediments to normative acquisition of developmental skills. Primarily used to account for developmental acceleration, these frameworks are difficult to reconcile with empirical evidence of developmental deceleration. Moreover, they suggest disparate types of adversities compromise development through a similar, singular pathway of stress exposure and do not consider the nuanced implications of different dimensions of adverse exposures. Recent empirical studies find variable implications of environmental adversity on developmental pace, depending upon whether adverse exposures represent sources of threat (e.g., physical abuse, interpersonal violence) or sources of deprivation (physical neglect, institutionalization, low family socioeconomic status (Sumner et al., 2019)). As elucidated by McLaughlin and colleagues, the dimensional model of adversity and psychopathology (DMAP) argues that experiences of threat and deprivation differentially influence mechanisms of developmental plasticity that link adverse exposures to health and developmental outcomes (McLaughlin and Sheridan, 2016; McLaughlin et al., 2014; Sheridan and McLaughlin, 2014, 2016). In a meta-analysis and systematic review of the literature, adversity type moderated the direction of adversity-induced developmental pace changes: early experiences of threat were associated with accelerated development across indicators of puberty and cellular aging, but there was no association of these metrics with childhood deprivation or SES (Colich et al., 2020; see Section 2.2 for more information). Although a comprehensive discussion of the varied neurodevelopment pathways that link threat/deprivation to health is outside the scope of the current paper (for review, see Sheridan & McLaughlin, 2020), we underscore the importance of this dimensional perspective on adverse exposures: not all adversities are comparable in the demands they place on the developing organism nor, necessarily, in their outcomes.
3.2. Evolutionary developmental theory
Evolutionary developmental theory has been proposed to explain both acceleration and deceleration in developmental pace—each framed as means for optimizing reproductive fitness. In a seminal 1991 paper, Belsky and colleagues (Belsky et al., 1991) advanced an evolutionary interpretation for why girls from harsh, insensitive families appeared to follow an accelerated pace of pubertal development, relative to those from more supportive, securely attached family environments. Invoking life history theory (e.g., Coall and Chisholm, 2003), which views developmental resource allocations as a preconscious optimization of fitness, the authors proposed that girls reared in adverse family conditions utilized a “reproductive strategy” of early pubertal development and precocious sexuality as means of promoting reproductive success. A similar evolutionary interpretive lens is used to describe delays in pubertal timing: In a context of deprivation and scarce bioenergetic resources, late maturation is posited to conserve resources until the emergence of environmental conditions that are more conducive to reproduction (Ellis et al., 2012).
The nearly exclusive focus of life history theory on reproductive benchmarks, such as menarche or sexual debut, does not address broader maturational markers, such as exfoliation of deciduous teeth, the acquisition of executive functioning, or shifts in the pace of an epigenetic clock. Although well-grounded in evolutionary biology, life history theory places principal focus on eventual, future outcomes, narrowing its utility for understanding the implications of adversity across the full range of development and into adulthood. Also common across extant frameworks is a predominant focus on the individual: how adversity operates to inhibit individual functioning or constrain individual patterns of resource allocation in service of reproductive goals. Notwithstanding such person-driven processes, these accounts largely ignore the essential dyadic nature of early rearing experiences, namely, the ways in which children’s development is powerfully shaped by primary caregiver relationships. Particularly during infancy and childhood, children’s most basic needs for physiologic resources and safety are fulfilled within the context of transactions with primary caregivers (Roubinov et al., 2021). Thus, it is reasonable to consider ways in which the insufficient fulfillment of needs within this dyadic context may be instrumental in determining developmental pace changes. Without explicitly conceptualizing a central role of the parent-child dyad, extant frameworks may omit one of the most central influences on developmental pace.
Life history theory also contends that developmental pace adjustments are adaptive because they reliably predict future environmental conditions. Evolutionary strategies, termed “predictive adaptive responses (PAR),” operate through developmental plasticity to modify an organism so that the resulting physiology “matches” (or is advantageous within) the environment that is expected later in life (Gluckman et al., 2005). Within such a framework, environmental matches (i.e., a harsh early environment and a harsh future environment) should be more advantageous than environmental mismatches (i.e., a supportive early environment and a harsh future environment), even when early life conditions are favorable (Rickard et al., 2014). Although often relied upon as a guiding theoretical framework, there are limited empirical tests of PAR, and those that have been conducted yield inconclusive results. For example, an experimentally-manipulated mismatch of prenatal and postnatal nutrient environments induced cardiovascular dysfunction in a sample of sheep that was not observed under matched prenatal-postnatal environmental conditions (Cleal et al., 2007). Yet in an analysis of a well-documented famine in preindustrial Finland, Hayward, Rickard, and Lummaa (2013) found that individuals born during “lean years” did not experience better survival or fertility rates despite the “match” of early and later life conditions. Rather, it was those individuals who experienced more favorable, nutrient-rich early life conditions (“mismatches”) who fared better during the famine. Overall, research into PAR bears the same weakness of the life history approach: it predominantly focus upon the relation of exposures to outcomes, rather than the developmental processes that lie between.
Notably, under each of the aforementioned extant theoretical accounts, adversity-induced health consequences may be promulgated as “trade-offs” or costs of survival in non-optimal conditions. None of the existing accounts of plasticity in developmental pace have offered a clear, heuristic explanation for: a) why development can be either accelerated or decelerated or b) what common underlying mechanism(s) might allow pace shifts in either direction.
4. A novel explanatory framework of developmental pacing
Although the presented theory rests on a foundation of evolutionary principles, there are a number of ways in which the ideas presented here distinguish themselves from past views. Our objective is not to challenge or supplant life history theory, but to provide a possibly more nuanced and mechanistic explanatory framework that places developmental time in the foreground of studies examining maturational pace. Below, we outline the features of this novel framework, highlighting the fundamental ways in which the present theory diverges from life history theory.
4.1. The parent-child dyad is the focal unit of influence
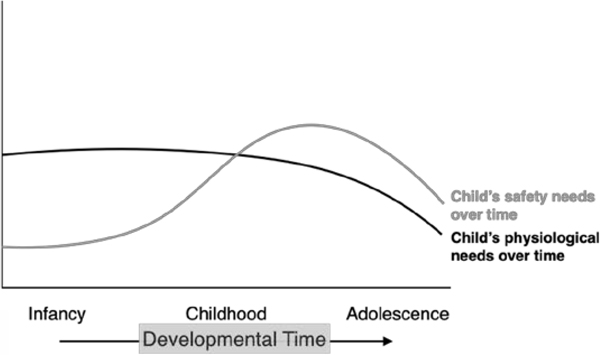
An alternative account for environmentally driven shifts in the pace of early development might center upon parent and caregiver provision for the needs of young children. The canonical 1943 paper by psychologist Abraham Maslow advanced a theory about such needs that became deeply rooted within developmental science (Maslow, 1943). Developmental needs, Maslow argued, are arrayed in a pyramidal hierarchy in which the earliest, most fundamental necessities (physiological and safety needs) must be first addressed in order for the child to progress to the fulfillment of higher-order psychological needs (love and self-esteem) and ultimately to self-actualization (see Figure 1). Others have suggested that the intensity of early needs follows specific developmental trajectories but is not necessarily hierarchical; rather, physiological needs (for sustenance, hydration, warmth, human contact) begin high and steady and decrease through middle childhood, and safety needs (for protection, security) remain relatively low in the first several years but peak in adolescence (Figure 2).
Figure 1.
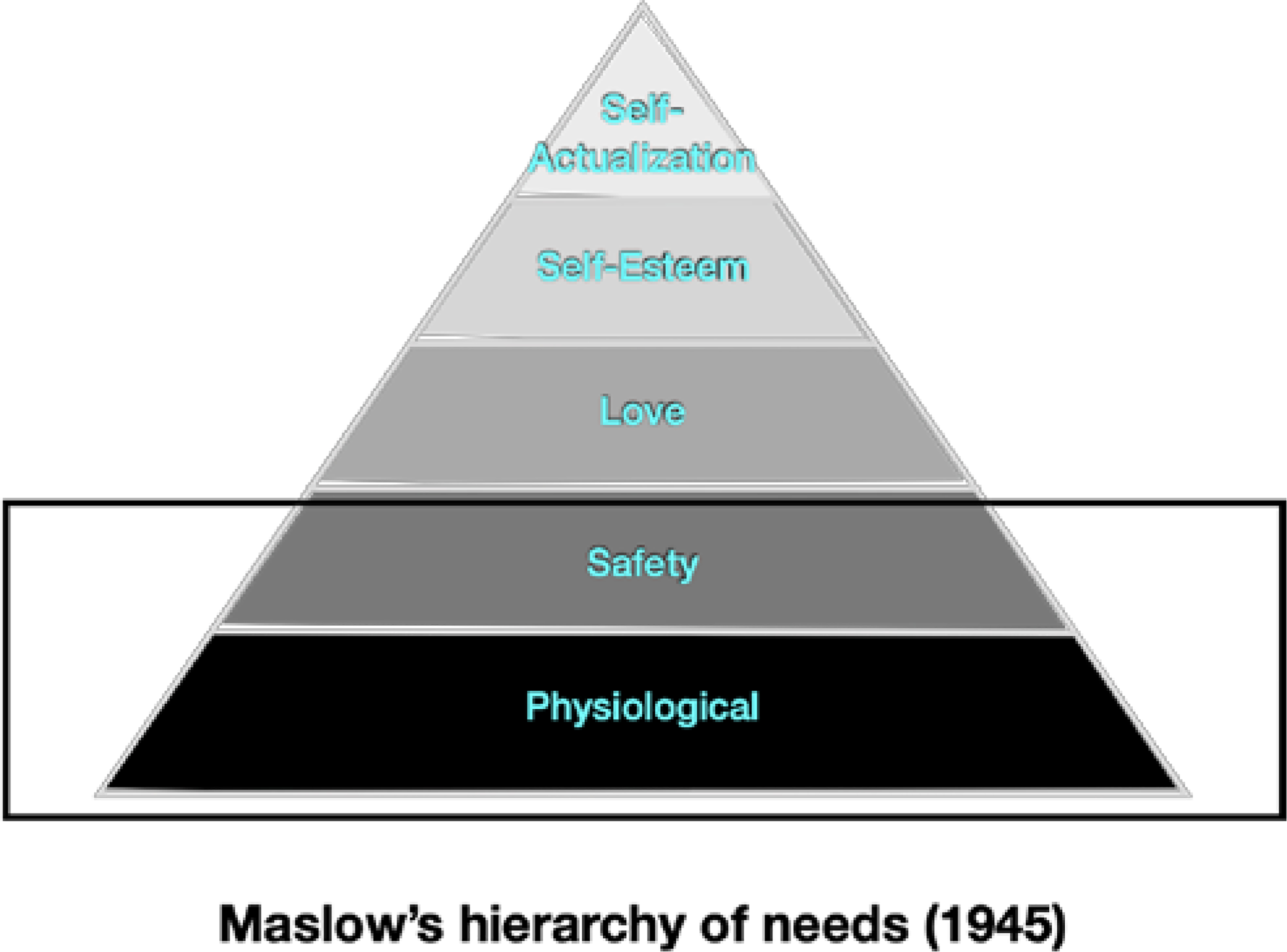
Maslow’s hierarchy of needs
Abraham Maslow’s hierarchy of needs. An ordered ranking of needs, ranging from the earliest and most fundamental (e.g., physiological and safety needs) to the later and most complex (e.g., self-esteem and self-actualization). Early physiological needs compromised by food insecurity and other forms of deprivation. Early safety needs compromised by threat or adversity (Maslow, 1943).
Figure 2.
Trajectories of early, physiological and safety needs over developmental time.
Physiological needs are high and sustained early in development, diminishing as a child matures into adolescence. By contrast, safety needs are relatively low and easily met early in development but increase later, peaking in adolescence (Krech, Crutchfield, Ballachey, 1962).
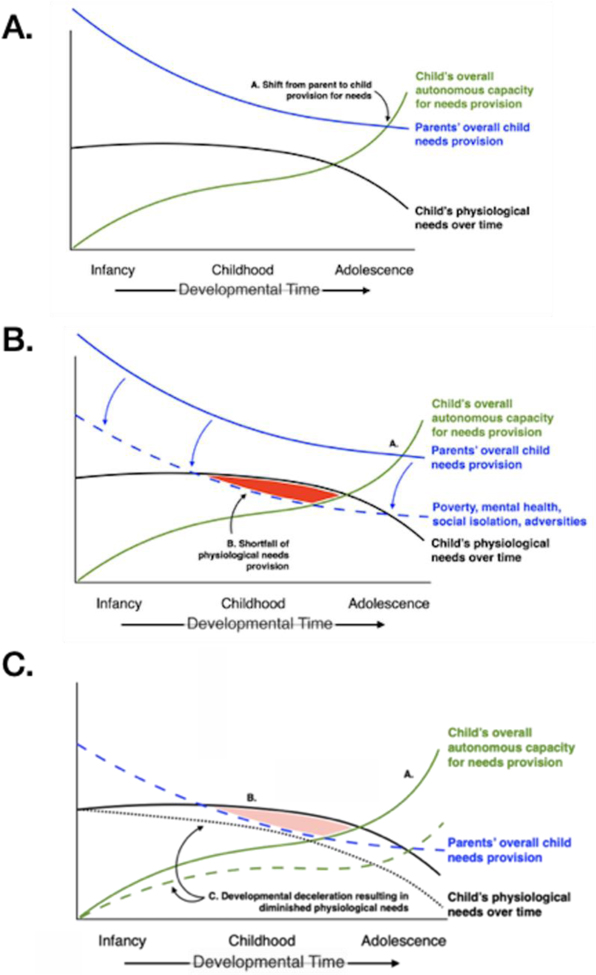
The first two decades of life are also characterized by changes in the balance between caregiver needs provision and offspring autonomy: a gradually diminishing of parental provision for children’s needs, as the child’s own ability to meet needs increases over time, ultimately exceeding parental contributions (see point A in Figure 3A). The displayed models in Figures 3, 3A are based not on data describing measured needs provision, because, to our knowledge, no such data presently exist. Rather, the dual trajectories of needs provision and needs fulfillment within the dyad have been drawn based on conventional understandings of the shape of their developmental courses (see, for example, Krech et al, 1962). Empirical data robustly demonstrate that parents’ capacities for meeting early childhood needs can be compromised by the presence of poverty, mental disorders or addictions, social isolation, or other forms of family adversity, resulting in a systematic down-shift in parents’ abilities to provide for children’s needs (e.g., Madigan et al., 2019; Stein et al., 2014). As shown in Figure 3B, the modeled trajectory of parents’ capacities for such provision is depressed over the full range of children’s early development, reflecting the obstacles encountered by parents experiencing economic disadvantage, substance dependence, psychiatric impairments, or other forms of adversity (Reiss, 2013; McLaughlin et al., 2011). As we outline below, the parent-child dyad occupies a more central operational role in change of pace theory than has been the case within traditional evolutionary perspectives. Change of pace places the dyadic relationship (and needs deficits therein) squarely at the heart of adaptive change, rather than more conventionally regarding the individual child as the unit of conditional adaptation and the child’s developmental traits as the target of natural selection.
Figure 3.
Developmental deceleration as a response to unmet physiological needs.
A. Trajectories of parental and child provisions of needs. Parents’ provisions gradually diminish over time as the child’s capacity for meeting needs increases in adolescence. Point A depicts the position in developmental time when needs provision shifts from mostly parental to mostly child.
B. Under conditions of poverty, disordered mental health, social isolation, or adversity, parents’ capacities for meeting a child’s needs become systematically lower, resulting in (Point B) a shortfall of physiological needs provision.
C. Such deprivation is countered by an evolutionarily conserved developmental deceleration strategy, in which slowed developmental maturation results in diminished physiological needs over time (Paths C), obviating the needs provision shortfall. Such a developmental strategy is comparable to the diminution in metabolic needs that attends cold water immersion.
4.2. Need shortfalls are obviated via alterations in developmental pace
In this theoretical account, compromised parents’ diminished capacity for needs provision results in a shortfall of needs satisfaction for both physiological and safety needs over early development (Figures 3 and 4, respectively). Due to the distinctive trajectories of these two categories of needs over early development, the shape and timing of the two areas of red denoting provision shortfalls are also distinctive in shape and timing. As a consequence and as shown in Figures 3 and 4, two different developmental strategies are employed for overcoming the problem of unmet needs. In the first (Figure 3B and 3C), unmet physiological necessities—resulting, for example, from parental or caregiver neglect of a child’s nutritional or interpersonal needs—could be surmounted by a strategy of developmental deceleration. Such slowing of physiological requirements over time would result in both delayed maturation of a child’s autonomous needs provision (dashed green line) and a diminution in physiological needs themselves (dotted black line). Together, these two alterations in pace would result in an ablation of the needs provision shortfall caused by diminished parental capacities (Figure 3C). The result of a deceleration strategy might be viewed as analogous to survival by the physiological slowing that attends cold water immersion (Daanen and Van Marken Lichtenbelt, 2016; Tipton et al., 2017). In a meta-analysis of studies across 21 mammalian species, prenatal maternal stress induced deceleration in offspring growth (Berghänel et al., 2017). Notably, Bergähnel et al. found that adversity-induced deceleration was less pronounced at higher levels of offspring autonomy, a process captured by the current framework: Figures 3 and 4 illustrate a lessening of the effect of adversity later in development, as the nature of dyadic transactional exchanges shifts from reliance on primary caregivers toward children’s autonomous needs provision. Similarly, some studies of low childhood SES and accelerations in cortical thinning have failed to replicate in adolescent samples (Tooley et al., 2021). While this may be explained by the rate of cortical thinning (which slows in adolescence), it may also relate to changes in the balance of needs provision between caregivers and offspring across development.
Figure 4.
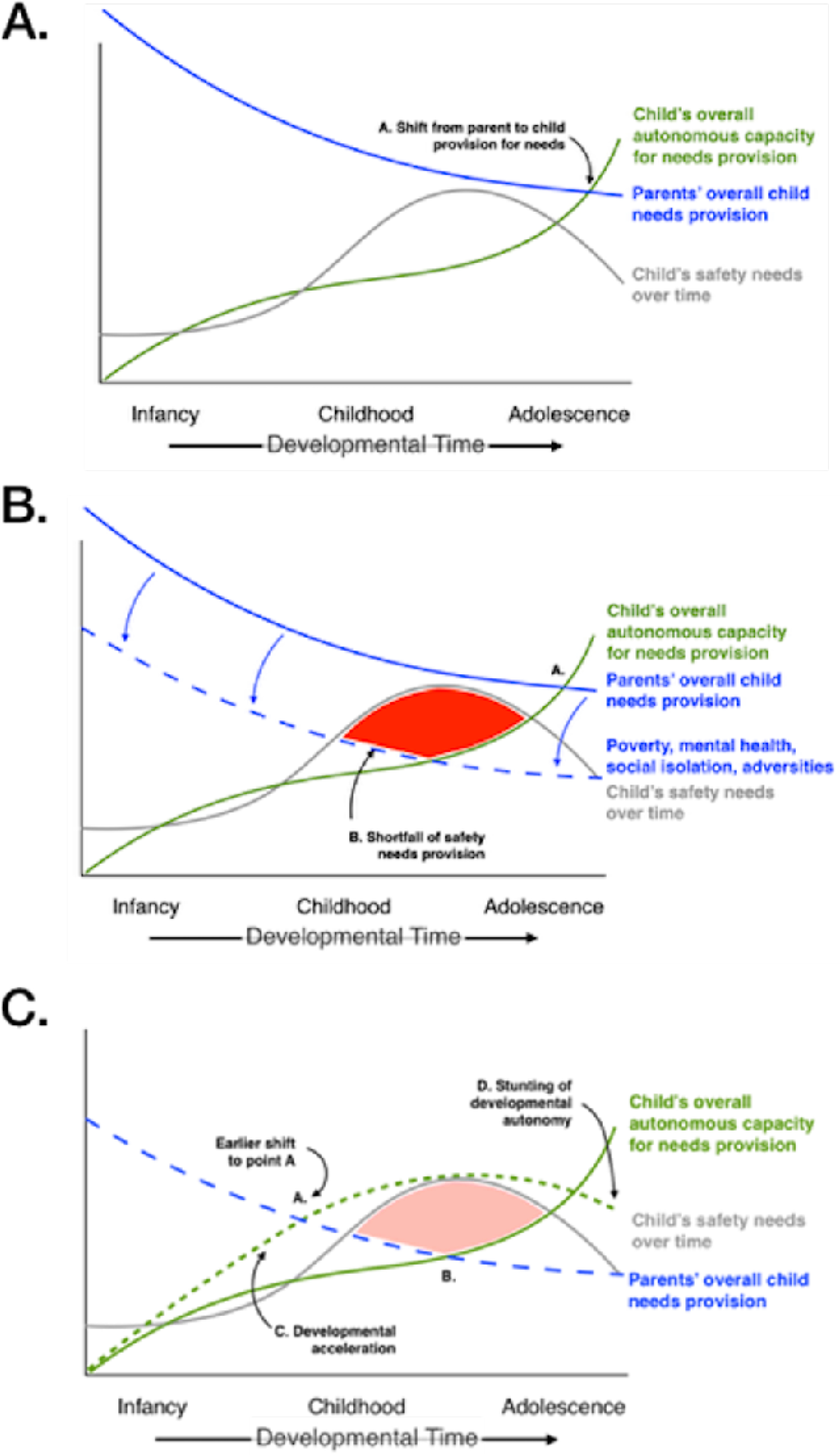
Developmental acceleration as a response to unmet safety needs.
A. Trajectories of parental and child provisions of needs. Parents’ provisions gradually diminish over time as the child’s capacity for meeting needs increases in adolescence. Point A depicts the position in developmental time when needs provision shifts from mostly parental to mostly child.
B. Under conditions of poverty, disordered mental health, social isolation, or adversity, parents’ capacities for meeting a child’s needs become systematically lower, resulting in (Point B) a shortfall of safety needs provision.
C. Such a safety needs provision shortfall under threat is countered by an evolutionarily conserved developmental acceleration strategy, in which more rapid maturation (Path C) results in an earlier shift to predominantly child-derived needs provision (Point A), a meeting of developmental safety needs, and an eventual stunting of developmental autonomy (Point D). Such a developmental strategy entails a life history tradeoff between earlier safety needs provision and longer term autonomy.
In the second developmental strategy, unmet safety needs—due to insufficient parental protection or support in the face of threat or adversity—might be overcome by an engagement in developmental acceleration. Consistent with the basic, unperturbed trajectories of early physiological needs, the graphical representations of children’s safety needs, autonomy, and needs fulfillment (Figure 4A) draw upon conventional wisdom as opposed to empirical data. As shown in Figure 4, such accelerated change in the pace of a child’s autonomous needs provision would result in an obviation of the needs fulfillment shortfall (dashed green line) and a shift to an earlier temporal point at which a child’s capacity would override parental contributions (point A in Figure 4C). Acceleration might be expected to result in a later stunting of developmental autonomy (i.e., the downturned segment D on the child’s developmental curve in Figure 4C). Accelerated development to meet early, immediate environmental demands or threats has been observed in prior studies to have longer-term effects on cardiometabolic risk (Reid et al., 2018), the integrity and functioning of limbic circuitry (Callaghan and Tottenham, 2016b), and physical growth (Berghänel et al., 2017), in both humans and non-human models (Ludewig et al., 2017; Metcalfe and Monaghan, 2001). In describing developmental pace changes (Figures 3C and 4C, respectively), we distinguish between the existing empirical data that documents the occurrence of these purposefully slowed and more rapid maturational patterns (e.g., Colich et al., 2020; Sumner et al., 2019) and what remains more theoretical in nature, which are the rationale, function, and mechanistic underpinnings of such strategies that are proposed by the present framework.
4.3. Unique trajectories of early physiological versus safety needs underlie the directionality of pace changes
This “change of pace” model provides a rationale for the discrete responses that emerge in the context of unmet physiological needs (i.e., neglect, deprivation) versus unmet safety needs (i.e., protection from threat). The distinctiveness of these responses is commensurate with reports that neglect and physical abuse are only moderately linked in studies of child maltreatment (e.g., Castro et al., 2017) and is aligned with the dimensional model of adversity and psychopathology (McLaughlin & Sheridan, 2016). This model stipulation also builds upon prior theory of the importance of early parental care (Callaghan & Tottenham, 2016a); here, we indicate that specificity in the direction of developmental pace changes depends upon the domain of children’s needs in which caregiving deficits emerge. The current model postulates that the direction of developmental pace changes are determined, at least in part, by the unique trajectories of physiological versus safety needs. Physiological needs begin high and steady early in life and decrease during middle childhood; thus developmental deceleration averts the deficiencies that would appear under a normative developmental trajectory (Figure 3C). In contrast, unmet safety needs begin lower in the early years of life and rise as offspring mature; thus an acceleration strategy precludes the shortfall that would emerge if developmental pace were unperturbed (Figure 4C). Although threat and neglect can co-occur within individual family units, the predominance of one over the other may be reflected in directional changes in developmental tempo of children. Most importantly, the model’s predictions match previous findings that threat-related early life adversity is associated with accelerated epigenetic age (DNA methylation within an epigenetic “clock” array) and advanced pubertal development, while deprivation is linked to slowed pubertal maturation (Sumner et al., 2019), and also extend this work by proposing a unified account that describes why directional differences in pace changes may emerge under varied conditions. A model based on the trajectories of early needs provision may also more easily account for the striking individual differences in susceptibility to early life adversity by positing that some individuals may be more sensitive to aversive stimuli in both the external environment (i.e., threat) and the internal milieu (i.e., physiological deprivation; Boyce, 2016; Ellis et al., 2011; Rickard et al., 2014).
4.4. The short- and long-term functionality of pace alterations
The change of pace theory focuses more intensively upon the implications of immediate, unmet physiological and safety needs on developmental processes, rather than on the longer term, eventual outcomes that are central to life history theory. We contend that failures to fulfill key early needs figure prominently in developmental accelerations and decelerations, and the character of the unmet needs, as explicated in Figures 3 and 4, determine the direction of change in the pace of development. As such, the presented theory offers a more explicit account of the temporal mechanisms at work within changes of developmental pace.
5. Neurobiologic Mechanisms
A “change of pace” or temporal plasticity framework rests inherently on an understanding that all complex forms of life express the capacity to alter phenotypic development in response to environmental conditions. Studies reviewed above illustrate the impact of environmental circumstances on the timing of developmental processes as a specific form of such phenotypic plasticity. A key question concerns the biological signals by which environmental conditions might influence the pace of development—mechanisms that would have widespread, coordinated effects across multiple systems. The hypothalamic-pituitary-adrenal (HPA) axis may operate as one such illustrative mechanism for determining developmental pacing across a range biological systems and phenomena.
An extensive literature in non-mammalian species has identified neuroendocrine systems affecting the timing of life history transitions (Denver, 2009b; Wada, 2008). These studies illustrate the critical and evolutionarily conserved role of the HPA axis, a classic example being the advanced timing of metamorphosis apparent in tadpoles in response to environmental adversity (Denver, 2009a). Tadpole development occurs in aquatic environments, such as ponds, which contain the nutrients required for normal development. Evaporation of the pond and dwindling nutrient resources trigger the release of corticotrophin-releasing factor (CRF), a hypothalamic peptide that ultimately stimulates the release of both adrenal glucocorticoids and thyroid hormones. These hormones act in synergy to accelerate the timing of tadpole metamorphosis and to stimulate the transition to a developmentally mature stage, permitting independence from the pond and promoting survival through the alternative foraging capabilities. These findings are consistent with those from a remarkable range of vertebrates, revealing the importance of glucocorticoids in pacing the major life course transitions from the earliest stages of development to the end of the life cycle (Wada, 2008).
5.1. Parturition and fetal development
While findings with tadpoles might seem remote to those focused on human development, the processes are remarkably similar to those governing parturition in humans, as well as the effects of environmental adversity on the timing of parturition (Liggins et al., 1977; Smith and Nicholson, 2007). CRF and CRF-induced levels of glucocorticoids normally rise over the course of human pregnancy. The associated glucocorticoid action on conceptual tissues provides the basis for parturition timing. Glucocorticoids facilitate the cellular effects of pro-inflammatory cytokines on prostaglandins, among other mediators (Challis et al., 1999; Challis and Smith, 2001), and prepare the fetus for ‘life on the outside’ through advancing maturational effects on lung, liver, kidney and other organs (Liggins et al., 1977). Adverse conditions such as maternal infections, malnutrition and stress can all drive increased release of CRF, and the resulting glucocorticoid action serves as a basis for premature birth by enhancing the actions of pro-inflammatory uterine signaling. Importantly, the placenta is the source of CRF, and the timing of parturition is governed by a fetal signal that uterine conditions no longer support growth and/or threaten survival. The fetal signal is transmitted to conceptual tissues to promote the early exit and the transition to postnatal conditions.
The increased levels of glucocorticoids triggered by adversity in utero influence the timing of birth, as well as fetal growth and birth weight. The highly catabolic glucocorticoids constrain fetal growth (Meaney et al., 2007). Low birth weight in humans associates with increased cord blood levels of both CRF and cortisol (Goland et al., 1993). Prenatal administration of glucocorticoid receptor agonists, such as dexamethasone or betamethasone, produce fetal growth retardation in humans and other species (Price et al., 1992). An exhaustive meta-analysis of the effects of prenatal maternal stress across 21 nonhuman mammalian species (Berghänel et al., 2017) revealed evidence for both an initial dampening of fetal growth, a developmental constraint associated with limited maternal investment, followed by an adaptive calibration of a developmental trajectory marked by acceleration (catch-up) of growth and advanced reproductive maturation. Here, two points are critical. First, this profile was a common outcome across multiple forms of prenatal maternal adversity. Second, both the initial deceleration of fetal growth and the later acceleration (i.e., ‘catch-up growth’) were replicated in studies using only fetal glucocorticoid exposure without exposure to external maternal stressors. Fetal exposure to increased glucocorticoid levels restrains fetal growth and produces sustained hyperinsulinemia— a function of insulin resistance in selected tissues—which then promotes postnatal, accelerated somatic growth. Hence fetal glucocorticoids are positioned to establish both an initial growth retardation as well as a later acceleration of growth. The glucocorticoid-mediated hyperinsulinemia also associates with accelerated pubertal development (Dunger et al., 2005; Morton et al., 2001).
5.2. Maternal care and postnatal development
While the level of HPA activity during the prenatal period is linked to maternal signals associated with the quality of the uterine environment, the maternal role extends into the postnatal period. The mammalian mother provides thermoregulation, nutrients, protection from predators, and even regulates cardio-metabolic function (Hofer, 1970, 1975). The mother thus spares the rodent pup the need for independent HPA activity. This period of HPA quiescence associated with maternal ‘buffering’ ensures that growth proceeds without interruption of the highly catabolic glucocorticoids, a period referred to as the ‘stress hyporesponsive period’ (Sapolsky and Meaney, 1986). Under optimal conditions, activity of the HPA axis and its capacity to respond to stressors emerge as the developing animal advances towards weaning, a stage of independence that requires physiological and behavioral self-sufficiency. However, pups deprived of maternal care for abnormally extended periods of time are obligated to physiologically ‘fend for themselves’, in part by advancing the maturation of the HPA axis and enhancing the response of the adrenal cortex to CRF-induced pituitary adrenocorticotropic hormone (ACTH). Maternal separation accelerates adrenal maturation of the steroidogenic response to ACTH, enabling the synthesis and release of glucocorticoids. The HPA axis is thus able to meet the demands of adversity with an increase in circulating glucocorticoid levels.
5.3. Developmental timing of fear behavior
Maternal pacing of the development of defensive responses includes neural circuits implicated in the activation of ‘innate’ behavioral fear responses activated during periods of stress, avoidance of cues associated with pain as well as fear conditioning (Callaghan et al., 2014). These responses comprise a highly adaptive set of defensive behaviors that allow for the avoidance of conditions (e.g., predation) that imperil survival. These responses function in the form of stable differences in fearfulness that enhance the avoidance of novel, uncertain conditions and in the capacity for avoidance learning. The capacity for the expression of such defensive responses is critical for the successful transition to independence from the parent.
This maternal regulation of HPA maturation timing is produced in part by downstream effects on amygdala-mediated fear behaviors. Such behavior in the developing rat pup emerges towards the beginning of the third week of life, thus mapping nicely onto the timing of weaning and, not coincidentally, HPA development. This timing of development anticipates the transition of weaning that requires independence of the offspring well served by the coincident development of HPA responsivity and fear behaviors. The temporal coordination of these effects on defensive systems is reflected in studies showing accelerated onset of fear behaviors in pups treated with glucocorticoids during the period of normal HPA quiescence; blocking glucocorticoid signaling prolongs the immaturity of the fear behavior system (Moriceau et al., 2006; Takahashi et al., 2005). Infusion of glucocorticoids directly into the amygdala accelerates the onset of fear behaviors, and glucocorticoid receptor antagonism produces the opposite effect. Further, genomic analyses of neuroimaging data obtained from human newborns implicates glucocorticoid signaling as a mechanism for inter-individual variation in amygdala structure (Ong et al., 2019). These findings are consistent with those of Buss and colleagues (Buss et al., 2012) showing that maternal cortisol levels during pregnancy associate with fetal amygdala development. A more recent report from this group reveals a comparable influence of pro-inflammatory cytokines, thus once again reflecting the glucocorticoid-inflammation pathways (Gyllenhammer et al., 2020).
Recent studies provide insights into cellular mechanisms underlying variation in developmental pacing of the onset of fear-related behaviors. Bath, Walker and their colleagues (Guadagno et al. 2020; Nieves et al., 2020) used a mouse model of early life adversity that involves limiting the bedding material provided to the mother for nesting. The limited bedding delayed the ability of peri-weanling mice to express an auditory conditioned fear memory, which would normally be apparent by this stage of development. Importantly, the effect was apparent in the timing, but not the ultimate developmental outcome: by 50 days of age there was no longer any effect of the resource limitation. The limited bedding condition accelerated the developmental emergence of parvalbumin (PV)-positive cells, a marker for inhibitory neurons, in the BLA. The delay in the expression of the condition fear response was reversed through optogenetic inactivation of PV-positive cells in the BLA in the Nieves et al. (2020) study.
The focus on the PV-positive cells in these models provides an important bridging to the developmental pacing. Hensch (2005) have provided compelling evidence for the importance the GABAergic PV-positive cells in the timing of critical periods in neurodevelopment. Benzodiazepines prematurely open critical periods of plasticity in the visual system by acting through GABA-receptors (GABARs) containing the subunit α1 (Fagiolini et al., 2004), which is a primary target of PV-positive interneurons. Genetic manipulations directed towards glutamic acid decarboxylase 67 (GAD67), a GABA synthesizing enzyme, delay the onset of this critical period of plasticity (Chattopadhyaya et al., 2007). Thus, GABAergic signaling affecting excitatory/inhibitory balances provides a molecular mechanism for developmental pacing.
Batista and Hensch (2019) provide evidence for a distal signaling mechanism that targets the timing of critical periods through the PV-interneurons. Interestingly, this proposed mechanism, thyroid hormone signaling, brings us full circle back to the amphibian models noted at the outset of this section (Denver, 2009b; Wada, 2008). While these models emphasized the role of CRF in morphogenesis, thyroid hormones serve as a critical trigger for CRF. Likewise, thyroid hormones are regulators of the pacing of the critical periods for imprinting in chicks (e.g., Yamaguchi et al., 2012) as well as neural circuitry in rodents (Gould et al., 1990; Koibuchi, 2008). Batista and Hensch (2019) mobilized compelling evidence in support of the idea that thyroid hormone regulation of PV neurons and thus GABAerigc signaling can determine the timing of critical periods and thus developmental pacing: 1) thyroid hormones regulates GAD expression in the brain, an effect that is particularly pronounced in early life (Wiens &Trudeau, 2006); 2) thyroid hormones shape the morphology and connectivity of GABAergic cells during development, an influence mediated by the mTOR signaling pathway (Westerholz et al., 2013). The mTOR pathway also underlies the plasticity during imprinting in chickens (Batista et al., 2018). Thyroid hormones control a switch in GABAa/GABAb receptors to open the critical period for imprinting in chicks (Aoki et al., 2018). Finally, in rodents, thyroid hormones regulate neural PV expression in multiple brain regions (e.g., Gilbert et al., 2007; Royland et al., 2008; Sawano et al., 2013; Harder et al., 2018).
The thyroid hormones also emerge as regulators of the effects of maternal care in the rat. Hellstrom et al (2012) showed that the influence of maternal licking on glucocorticoid receptor expression in hippocampal neurons is initiated by activation of the thyroid hormones receptors in the raphe with subsequent serotonergic activation of a signaling cascade that involves the transcription factors CBP and NGFI-A. Importantly, Hellstrom et al. showed that the tactile stimulation derived from pup licking was the critical signal for the maternally-regulated increase in circulating thyroid hormones. An intriguing, but as yet unanswered question, is whether maternal licking might regulate the ontogeny of PV-interneurons and thus, influence developmental pacing. Such studies might mechanistically link the human research on parental care or deprivation thereof to developmental pacing (see Hostinar et al., 2014; Tottenham et al,. 2019; Sullivan & Opendak, 2021).
5.4. Pubertal development
We previously noted the influence of parental care on the timing of pubertal development in human females (Coall and Chisholm, 2003). Conditions of parental neglect and/or abuse, for example, are linked to deviations in the timing of pubertal development in human females. There are comparable effects observed in the same rodent model of maternal care that features effects on HPA development. Female offspring of rat mothers that exhibit low levels of pup licking and grooming enter puberty significantly earlier than do those reared by high licking mothers (Cameron, 2004). The effect is driven by increased hypothalamic expression of the gene for the alpha subtype of the estrogen receptor, which serves as a basis for the activation of gonadotropin releasing hormone (GnRH) neurons that drive the onset of the hypothalamic-pituitary-ovarian function defining the onset of puberty. The increased hypothalamic expression of estrogen receptor alpha associated with low maternal investment emerges early in postnatal development as a function of the differential methylation of the estrogen receptor alpha gene promoter region. This region is hypomethylated in the female offspring of low-licking mothers (Champagne et al., 2003), leading to increased estrogen sensitivity and an earlier onset of puberty. The same effect produces increased sexual behavior and fecundity in the female offspring of low licking mothers (Cameron, 2004), an effect that is also supported by the greater acute stress-induced HPA activity in the offspring of low licking mothers.
Active maternal care ‘buffers’ the young from adversity and promotes the physiological conditions allowing growth. Levine and Gunnar (Gunnar et al., 1981) anticipated the framework presented above by positioning the mother and maternal care as the relevant environmental signal that determined the timing for HPA maturation, reflected in the capacity for mobilizing an adrenal corticosteroid response to stress. Hostinar and colleagues (Hostinar et al., 2014) provide a compelling review of the evidence for maternal “social buffering” of the young across multiple species, including humans. The critical role for the mother in modifying the development of ‘defensive responses to threat was first elucidated in studies dating back almost seven decades, where Levine (Levine, 2002), Denenberg (Zarrow et al., 1968) and their colleagues documented what, at the time, was an unexpected level of phenotypic plasticity in the HPA axis in rodents. These studies revealed that brief periods of postnatal handling in rodents increase mother – pup interactions and, in doing so, regulate the pace of HPA maturation, as well as HPA function over the life course (Liu et al., 1997). The enhanced tactile stimulation derived from handling-induced increases in mother–pup interactions in the rat is critical for sustaining the stress -hyporesponsive period. Thus, greater maternal investment ensures the availability of energy to sustain growth, as well as protection from threat. Offspring so indulged can afford to prolong HPA immaturity and invest in growth supported by maternal contact-induced release of growth hormone (Schanberg et al., 1984; Schanberg et al., 2003).
The increased maternal stimulation, in the form of licking, induced with brief periods of postnatal handling, has long term consequences for HPA function, which ultimately determine the later pace of brain aging (Meaney et al., 1988). Maternal licking alters intracellular signaling processes in hippocampal cells in the pup that results in a reduced methylation of glucocorticoid receptor gene promoters and increased glucocorticoid receptor expression. In adulthood, the increased hippocampal glucocorticoid receptor signaling provides more effective feedback regulation of HPA function. With age, the rat shows increasing basal levels of HPA activity, increased exposure to glucocorticoids, and glucocorticoid-induced atrophy of hippocampal neurons (Meaney et al., 2000). Postnatal handling spares animals the exposure to increased glucocorticoid levels and hippocampal atrophy. Thus even in the later stage of the lifecycle animals that were handled in early life show little evidence of hippocampal neuron loss nor of aged-related impairments in hippocampal-dependent forms of learning and memory, all of which become increasingly apparent in non-handled animals by mid-life (Meaney et al., 1988). Maternal care in early life is thus registered on HPA function to alter the timing of biological aging.
5.5. Summary and future directions
Work described above on the HPA axis and glucocorticoid influences on developmental pace emphasizes resource availability, or metabolic demands, and safety/threat, which implicates biological defenses. The HPA axis lies at the fulcrum of these systems. A wealth of HPA hormones not only regulate the activation of stress responses, the body’s primary line of defense, but the adrenal glucocorticoids are also major regulators of cardio-metabolic function resulting in altered resource availability and, in synergy with catecholamines, the circulation of energy substrates. This system, proposed by Meaney, Szyf, and Seckl (2007) as a mechanism for fetal programming of developmental outcomes, is thus ideally positioned as a biological mediator of the trade-offs ultimately defining developmental pace. Interestingly, low birth weight, which reflects glucocorticoid-mediated effects on somatic growth, is also associated with advanced pubertal development, increased HPA responses to stress (Phillips and Jones, 2006), as well as the later risk for stress-related psychopathology.
There is compelling evidence for the importance of glucocorticoid signaling in regulating the pace of development in multiple biological systems, stemming from a wealth of science in multiple disciplines. Nevertheless, there is considerable reason to assume that glucocorticoids account for only a portion of linkage between exposure and developmental outcomes (O’Donnell and Meaney, 2017). For example, studies suggest importance of inflammatory signals as a mechanism for the pacing of development within the systems described above (Danese et al., 2009; Kuhlman et al., 2017). A further consideration is the topic of gender and gender-specific effects. The associations between maternal adversity and the development of amygdala – PFC circuitry, for example, is largely apparent only in girls by the time of childhood (Wen et al., 2017). This finding is consistent with the association between maternal depression and risk for depression in the offspring, which is likewise mostly apparent in daughters. Studies of the developmental outcomes associated with birth weight and prematurity are similarly replete with examples of gender differences. These findings seem to suggest that effects on the pacing of development would, likewise, be gender dependent. Future research is well-poised to explicate the varied biological pathways through which adversity determines developmental pace alterations. Studies of gender dependency will add a fascinating dimension to these analyses.
6. Conclusions
Developmental time, in a manner analogous to the plasticity of physical time, might be usefully viewed as expanding or contracting adaptively in response to early life environmental signaling. Such temporal elasticity, if present, would reveal itself in observable decelerations or accelerations among maturational events—changes in developmental pace that adaptively accommodate the child to the conditions of early life. Evolutionary biology (e.g., Gilbert and Epel, 2009), life history theory (e.g., Ellis and Del Giudice, 2019), and developmental origins research (e.g., Fleming et al., 2018) have all attributed such alterations in the pace of development to “tradeoffs” in the allocation of limited metabolic and energetic resources—tradeoffs that are responsive to the assets and liabilities, supports and adversities that together characterize early rearing environments. These tradeoffs, it is argued, are enacted to maximize reproductive fitness, but result in shifts in the timing of events, such as birth and sexual maturation, that are consequential to both health and development. Here, a heuristic, mechanistic extension of this theoretic account is advanced, centering upon how unmet, early developmental needs operate as key determinants of the direction and timing of changes in developmental pace. Further, we summarize emerging evidence describing how neurobiological processes may guide developmental pace.
This “change of pace” theory of developmental acceleration and deceleration takes as its point of departure several extensions or annotations of classical evolutionary, life history, and developmental origins frameworks. First, the focal unit of influence on developmental pace is deemed to be the parent-child dyad, rather than the individual child in isolation. Whereas the life history perspective centers upon an individual’s expenditures of effort and energy in the service of reproductive fitness, the present theory regards the transactional relationship between parent and child as a key determinant of developmental pace. Second, change of pace theory offers an explanatory account for why poverty, social isolation, and other adversities contribute to the tempo of maturational change by interceding in families’ responsiveness to early developmental needs. Poor, disadvantaged parents sustaining multiple adversities and/or symptoms of mental illness are simply unable to provide the resources and supports that young children require, especially in the early childhood years. Alterations in developmental pace operate to obviate such shortfalls, either via a decelerated pace that reduces children’s overall physiological needs or via an accelerated pace that increases children’s overall capacity for autonomous safety needs provision. Third, an accelerated or decelerated pace—that is, the direction of the change in developmental maturation—is proposed to occur by virtue of whether physiological needs (i.e., nutrition, metabolic stability, and parental care) or safety needs (i.e., protection, harm reduction, and shelter from threat) are slighted or neglected within early family environments. As suggested by recent evidence (Sumner et al., 2019), exposures to physiological deprivation can result in developmental slowing and delays, while exposures to threat and adversity may bias development toward acceleration and hastened maturation. This specificity in how different categories of early environmental experiences affect developmental acceleration or deceleration has been extensively reviewed in prior literature (Colich et al., 2020); we draw upon this supportive information but importantly, do not assert it as a novel component of our proposed framework. Rather, key determinants of developmental pace, we argue, are the differentially-shaped trajectories of physiological needs versus safety needs during early life and parental capacities for sustained fulfillment of those needs. It is not enough to consider only the balance of caregiver dependence-child independence early in life (i.e., Callaghan & Tottenham, 2016a); rather, we must address whether premature independence from caregivers occurs in the domain of physiological or safety needs, as this differentially affects the pace of development. Fourth, while life history theory attends principally to the optimization of long-term outcomes (i.e., reproductive fitness), the present amendment also attributes differences in pace to the sufficiency of the family’s efforts to meet immediate, short-term early developmental needs (i.e., Maslovian needs for physiological stability and safety from threat). Fifth and finally, the theory promulgated here is closely tied to increasingly known neurobiological mechanisms by which accelerations or decelerations in developmental pace could be induced. The effects of early environments signaling insufficient resources for meeting physiological and safety needs are demonstrably mediated by systematic changes in developmental brain circuitry, prompted most likely by epigenetic processes guiding direction and pace. Related to this point is the concept of the “experience expectant” brain, which relies upon the environment to provide key pieces of information required for optimal development. Over time, evolutionary processes are assumed to have “canalized” brain development, producing phenotypes that are optimized for regularly encountered environmental conditions (McGrath, Hannon, & Gibson, 2011). When particular genotypes are exposed to environments that deviate from the expected, the brain may “decanalize” away from normative trajectories of development (Burrows & Hannon, 2013). It has been proposed that GxE interactions operate via decanalization to influence the development of neurodevelopmental disorders (Burrows & Hannon, 2013). Decanalization that occurs in response to non-normative, adverse environmental conditions may be akin to processes of developmental acceleration or deceleration (deviations from normative pacing), which we propose emerge when children’s fundamental needs are not met.
This novel framework accounting for shifts in developmental pace rests upon tenets supported by research across a variety of disciplines, including epidemiology, neuroscience, psychiatry, pediatrics, and epigenetics, among others. The presented evidence, while compelling, is drawn from discrete studies that generally address only singular aspects of the model. Collaborative, team science efforts are ideally suited for studies that comprehensively explore the calibration of developmental pace on the basis of unmet needs. The features of such studies, while ambitious, are achievable with current, methodologically accessible approaches. For example, future research must thoughtfully measure diverse qualities of early adverse exposures and the manner in which they variably inhibit parents’ provisions for children’s early needs. There is compelling evidence of non-uniformity in the consequences of different types of adverse conditions, with some that may inhibit the fulfillment of physiological resources and others that may hinder the procurement of safety needs. Summative measures of adversity exposure cannot offer the nuance to disentangle these effects. Similar careful assessment is required of developmental outcomes; such measures should be inclusive of (but not limited to) reproductive milestones and span multiple temporal scales across the full life course. This may also necessitate the development of novel measures of accelerated or decelerated development, beyond traditional measures of precocious maturation or aging. To achieve this, longitudinal designs are imperative.
Future studies should also adopt a reconceptualization of changes in pace as context-dependent. In other words, the implications of developmental acceleration and deceleration should not be deemed advantageous nor deleterious per se, but rather must be evaluated by considering their function within the early rearing conditions in which they emerge. As evidence of developmental acceleration and deceleration continues to accumulate, studies may advance the field not by simply continuing to document these main effects, but by pursuing evidence of processes that explain, exacerbate, diminish or reverse developmental changes of pace. We have reviewed the potential explanatory power of neurobiological mechanisms, but urge consideration of other mediators and moderators that span biological, psychological and social domains relevant to the pace of children’s development.
Finally, this change of pace theory offers, as well, a richer framework for conceptualizing the content and timing of preventive interventions. Such interventions might, for example, utilize the signaling inherent in developmental pace alterations or might identify novel means of addressing unmet early needs. The change of pace perspective suggests potential ways of incorporating sensitive or critical period biology into explanations for developmental acceleration and deceleration. Variations in pace may occur predominantly in the early years of life, for example, due to the unique, developmentally-influenced shapes of curves describing early needs (Figure 2) and child versus parent capacities for provision for such needs (Figure 3 and 4).
Developmental science has conventionally viewed children’s acquisitions of competencies over time as a linear, ordinal progression varying only modestly from child to child, in the absence of neurodevelopmental pathology. However, like the process of human growth (Caino et al., 2006; Hermanussen et al., 1988), evidence now suggests that trajectories of development can be non-linear and uneven in configuration, with differences in timing and pace from one child to another. Further, findings reviewed here indicate that such temporal differences can be systematically linked to the environmental conditions of early life. Specifically, the pace of development over time may reveal the proximal social environment’s capacity or incapacity for fulfilling a child’s the most basic, early Maslovian needs: those for physiological resources and protection. In their most fundamental forms, developmental acceleration and deceleration may constitute not only a salient and useful bioassay for the adequacy of parental care but also an evolutionarily conserved mechanism for mitigating shortfalls of parental provision for needs. Careful attention to inter-individual changes of pace in early development might thus offer new clinical and research windows onto the character and efficacy of early parent-child relationships.
Funding:
This publication was made possible by the National Institute of Mental Health (NIMH) grant K23MH113709 (Roubinov); Jacobs Research Prize from the Jacob’s Foundation, the JPB Foundation, and the Webster Foundation (Meaney); Canadian Institute for Advanced Research (CIFAR; Boyce).
Footnotes
Boyce, Bush, and Roubinov (2020), unpublished data. See Boyce et al. (2010) for description of the dental component of the PAWS project.
Both critical and sensitive periods modify the plasticity or malleability of development. Critical periods have more sharply defined beginning and end points, and plasticity is not graded over time. Sensitive periods have less well-defined onsets and endings and show a plasticity gradient over time (Columbo et al., 2019).
Declarations of interest: None
References
- Aoki N, Yamaguchi S, Fujita T, Mori C, Fujita E, Matsushima T, et al. (2018). GABA-A and GABA-B receptors in filial imprinting linked with opening and closing of the sensitive period in domestic chicks (Gallus gallus domesticus). Frontiers in Physiology 9:1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, 2007. The origins of the developmental origins theory. Journal of Internal Medicine 261, 412–417. [DOI] [PubMed] [Google Scholar]
- Baschat AA, 2014. Neurodevelopment after fetal growth restriction. Fetal Diagnosis and Therapy 36, 136–142. [DOI] [PubMed] [Google Scholar]
- Bateson P, Barker D, Clutton-Brock T, Deb D, D’Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Lahr MM, 2004. Developmental plasticity and human health. Nature 430, 419–421. [DOI] [PubMed] [Google Scholar]
- Batista G, & Hensch TK (2019). Critical period regulation by thyroid hormones: potential mechanisms and sex-specific aspects. Frontiers in Molecular Neuroscience, 12, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Caspi A, Cohen HJ, Kraus WE, Ramrakha S, Poulton R, Moffitt TE, 2017. Impact of early personal-history characteristics on the Pace of Aging: implications for clinical trials of therapies to slow aging and extend healthspan. Aging Cell 16, 644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M, 2009. The nature (and nurture?) of plasticity in early human development. Perspectives on Psychological Science 4, 345–351. [DOI] [PubMed] [Google Scholar]
- Belsky J, Ruttle PL, Boyce WT, Armstrong JM, Essex MJ, 2015. Early adversity, elevated stress physiology, accelerated sexual maturation, and poor health in females. Developmental Psychology 51, 816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Schlomer GL, Ellis BJ, 2012. Beyond cumulative risk: distinguishing harshness and unpredictability as determinants of parenting and early life history strategy. Developmental Psychology 48, 662–673. [DOI] [PubMed] [Google Scholar]
- Belsky J, Steinberg L, Draper P, 1991. Childhood experience, interpersonal development, and reproductive strategy: An evolutionary theory of socialization. Child Development 62, 647–670. [DOI] [PubMed] [Google Scholar]
- Berghänel A, Heistermann M, Schülke O, Ostner J, 2017. Prenatal stress accelerates offspring growth to compensate for reduced maternal investment across mammals. Proceedings of the National Academy of Sciences 114, E10658–E10666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum-Johnston C, Thorpe RB, Wee C, Romero M, Brunelle A, Blood Q, Wilson R, Blood AB, Francis M, Taylor MS, 2016. Developmental acceleration of bradykinin-dependent relaxation by prenatal chronic hypoxia impedes normal development after birth. American Journal of Physiology-Lung Cellular and Molecular Physiology 310, L271–L286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT, 2016. Differential Susceptibility of the Developing Brain to Contextual Adversity and Stress. Neuropsychopharmacology 41, 142–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT, Bush NR, Roubinov DS, 2020. Chronic HPA activation and dental exfoliation among kindergarten children. Unpublished data. [Google Scholar]
- Boyce WT, Den Besten PK, Stamperdahl J, Zhan L, Jiang Y, Adler NE, Featherstone JD, 2010. Social inequalities in childhood dental caries: the convergent roles of stress, bacteria and disadvantage. Social Science and Medicine 71, 1644–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT, Levitt P, Martinez FD, McEwen BS and Shonkoff JP, 2021. Genes, environments, and time: the biology of adversity and resilience. Pediatrics, 147(2). [DOI] [PubMed] [Google Scholar]
- Boyce WT, Robinson G, Sokolowski MB, 2020. Genes and environments, development and time. Proceedings of the National Academy of Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton-Jarrett R, Harville EW, 2012. A prospective study of childhood social hardships and age at menarche. Annals of Epidemiology 22, 731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton-Jarrett R, Wright RJ, Putnam FW, Hibert EL, Michels KB, Forman MR, Rich-Edwards J, 2013. Childhood abuse and age at menarche. Journal of Adolescent Health 52, 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows EL, & Hannan AJ 2013. Decanalization mediating gene-environment interactions in schizophrenia and other psychiatric disorders with neurodevelopmental etiology. Frontiers in Behavioral Neuroscience, 7, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush NR, Edgar RD, Park M, MacIsaac JL, McEwen LM, Adler NE, Essex MJ, Kobor MS, Boyce WT, 2018. The biological embedding of early-life socioeconomic status and family adversity in children’s genome-wide DNA methylation. Epigenomics 10, 1445–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA, 2012. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proceedings of the National Academy of Science 109, E1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caino S, Kelmansky D, Adamo P, Lejarraga H, 2006. Short-term growth in healthy infants, schoolchildren and adolescent girls. Annals of Human Biology 33, 213–226. [DOI] [PubMed] [Google Scholar]
- Callaghan BL, Richardson R 2012a. The effect of adverse rearing environments on persistent memories in young rats: removing the brakes on infant fear memories. Translational Psychiatry, 2, e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, Richardson R 2012b. Early emergence of adult-like fear renewal in the developing rat after chronic corticosterone treatment of the dam or the pups. Behavioral Neuroscience, 128, 594–602. [DOI] [PubMed] [Google Scholar]
- Callaghan BL, Sullivan RM, Howell B, Tottenham N, 2014. The international society for developmental psychobiology Sackler symposium: early adversity and the maturation of emotion circuits--a cross-species analysis. Developmental Psychobiology 56, 1635–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL and Tottenham N, 2016. The neuro-environmental loop of plasticity: A cross-species analysis of parental effects on emotion circuitry development following typical and adverse caregiving. Neuropsychopharmacology, 41(1), pp.163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, Tottenham N, 2016. The Stress Acceleration Hypothesis: Effects of early-life adversity on emotion circuits and behavior. Current Opinion in Behavioral Science 7, 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron JL, 2004. Interrelationships between hormones, behavior, and affect during adolescence: understanding hormonal, physical, and brain changes occurring in association with pubertal activation of the reproductive axis. Introduction to part III. Annals of the New York Academy of Science 1021, 110–123. [DOI] [PubMed] [Google Scholar]
- Cameron JL, Eagleson KL, Fox NA, Hensch TK, Levitt P, 2017. Social Origins of Developmental Risk for Mental and Physical Illness. J Neurosci 37, 10783–10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancedda L, Putignano E, Sale A, Viegi A, Berardi N, Maffei L 2004. Acceleration of visual system development by environmental enrichment. Journal of Neuroscience, 24(20), 4840–4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro M, Alcantara-Lopez M, Martinez A, Fernandez V, Sanchez-Meca J, Lopez-Soler C, 2017. Mother’s IPV, Child Maltreatment Type and the Presence of PTSD in Children and Adolescents. International Journal of Environmental Research and Public Health 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis JR, Patel FA, Pomini F, 1999. Prostaglandin dehydrogenase and the initiation of labor. Journal of Perinatal Medicine 27, 26–34. [DOI] [PubMed] [Google Scholar]
- Challis JR, Smith SK, 2001. Fetal endocrine signals and preterm labor. Biology of the Neonate 79, 163–167. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Weaver IC, Diorio J, Sharma S, Meaney MJ, 2003. Natural variations in maternal care are associated with estrogen receptor alpha expression and estrogen sensitivity in the medial preoptic area. Endocrinology 144, 4720–4724. [DOI] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Wu CZ, Knott G, Kuhlman S, Fu Y, ... & Huang ZJ (2007). GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron, 54(6), 889–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm JS, Quinlivan JA, Petersen RW, Coall DA, 2005. Early stress predicts age at menarche and first birth, adult attachment, and expected lifespan. Human Nature 16, 233–265. [DOI] [PubMed] [Google Scholar]
- Cleal JK, Poore KR, Boullin JP, Khan O, Chau R, Hambidge O, Torrens C, Newman JP, Poston L, Noakes DE, 2007. Mismatched pre-and postnatal nutrition leads to cardiovascular dysfunction and altered renal function in adulthood. Proceedings of the National Academy of Sciences 104, 9529–9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coall DA, Chisholm JS, 2003. Evolutionary perspectives on pregnancy: maternal age at menarche and infant birth weight. Social Science and Medicine 57, 1771–1781. [DOI] [PubMed] [Google Scholar]
- Colich NL, Rosen ML, Williams ES, McLaughlin KA, 2020. Biological aging in childhood and adolescence following experiences of threat and deprivation: A systematic review and meta-analysis. Psychological Bulletin, 146(9), 721–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collishaw S, Pickles A, Messer J, Rutter M, Shearer C, Maughan B, 2007. Resilience to adult psychopathology following childhood maltreatment: Evidence from a community sample. Child Abuse & Neglect 31, 211–229. [DOI] [PubMed] [Google Scholar]
- Colombo J, Gustafson KM, Carlson SE, 2019. Critical and Sensitive Periods in Development and Nutrition. Annals of Nutrition and Metabolism 75 Suppl 1, 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi EJ, Denver RJ, 2005. Ancient origins of human developmental plasticity. American Journal of Human Biology 17, 44–54. [DOI] [PubMed] [Google Scholar]
- Czamara D, Eraslan G, Page CM, Lahti J, Lahti-Pulkkinen M, Hamalainen E, Kajantie E, Laivuori H, Villa PM, Reynolds RM, Nystad W, Haberg SE, London SJ, O’Donnell KJ, Garg E, Meaney MJ, Entringer S, Wadhwa PD, Buss C, Jones MJ, Lin DTS, MacIsaac JL, Kobor MS, Koen N, Zar HJ, Koenen KC, Dalvie S, Stein DJ, Kondofersky I, Muller NS, Theis FJ, Major Depressive Disorder Working Group of the Psychiatric Genomics, C., Raikkonen K, Binder EB, 2019. Integrated analysis of environmental and genetic influences on cord blood DNA methylation in new-borns. Nature Communications 10, 2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daanen HA, Van Marken Lichtenbelt WD, 2016. Human whole body cold adaptation. Temperature, 3, 104–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, ... & Caspi A (2009). Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Archives of Pediatrics & Adolescent Medicine, 163(12), 1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, 2013. How does race get “under the skin”?: Inflammation, weathering, and metabolic problems in late life. Social Science & Medicine 77, 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deardorff J, Ekwaru JP, Kushi LH, Ellis BJ, Greenspan LC, Mirabedi A, Landaverde EG, Hiatt RA, 2011. Father absence, body mass index, and pubertal timing in girls: differential effects by family income and ethnicity. Journal of Adolescent Health 48, 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Li C, Chen L, Liu Y, Hou R, Zhou X, 2018. Research advances on embryonic diapause in mammals. Animal Reproduction Science 198, 1–10. [DOI] [PubMed] [Google Scholar]
- Denver RJ, 2009a. Stress hormones mediate environment-genotype interactions during amphibian development. General and Comparative Endocrinology 164, 20–31. [DOI] [PubMed] [Google Scholar]
- Denver RJ, 2009b. Structural and functional evolution of vertebrate neuroendocrine stress systems. Annals of the New York Academy of Science 1163, 1–16. [DOI] [PubMed] [Google Scholar]
- Denver RJ, Crespi EJ, 2006. Stress hormones and human developmental plasticity: lessons from tadpoles. NeoReviews 7, e183–e188. [Google Scholar]
- Drury SS, Mabile E, Brett ZH, Esteves K, Jones E, Shirtcliff EA, Theall KP, 2014. The association of telomere length with family violence and disruption. Pediatrics 134, e128–e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchesne A, Liu A, Jones S, Laplante D, King S, 2017. Childhood body mass index at 5.5 years mediates the effect of prenatal maternal stress on daughters’ age at menarche: Project Ice Storm. Journal of Developmental Origins of Health and Disease 8, 168–177. [DOI] [PubMed] [Google Scholar]
- Dunger DB, Ahmed ML, Ong KK, 2005. Effects of obesity on growth and puberty. Best Practices and Research: Clinical Endocrinology and Metabolism 19, 375–390. [DOI] [PubMed] [Google Scholar]
- Dunkel Schetter C, 2011. Psychological science on pregnancy: stress processes, biopsychosocial models, and emerging research issues. Annual Review of Psychology 62, 531–558. [DOI] [PubMed] [Google Scholar]
- Dunkel Schetter C, & Glynn LM (2011). Stress in pregnancy: Empirical evidence and theoretical issues to guide interdisciplinary research. In Contrada RJ & Baum A (Eds.), The handbook of stress science: Biology, psychology, and health (pp. 321–347). Springer Publishing Company. [Google Scholar]
- Dunn EC, Nishimi K, Neumann A, Renaud A, Cecil CAM, Susser ES, Tiemeier H, 2020. Time-Dependent Effects of Exposure to Physical and Sexual Violence on Psychopathology Symptoms in Late Childhood: In Search of Sensitive Periods in Development. Journal of the American Academy of Child and Adolescent Psychiatry 59, 283–295 e284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, 2004. Timing of pubertal maturation in girls: an integrated life history approach. Psychological Bulletin 130, 920–958. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH, 2011. Differential susceptibility to the environment: an evolutionary--neurodevelopmental theory. Development and Psychopathology 23, 7–28. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Del Giudice M, 2019. Developmental Adaptation to Stress: An Evolutionary Perspective. Annual Review of Psychology 70, 111–139. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Del Giudice M, Dishion TJ, Figueredo AJ, Gray P, Griskevicius V, Hawley PH, Jacobs WJ, James J, Volk AA, 2012. The evolutionary basis of risky adolescent behavior: implications for science, policy, and practice. Developmental psychology 48, 598. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Essex MJ, 2007. Family environments, adrenarche, and sexual maturation: a longitudinal test of a life history model. Child Development 78, 1799–1817. [DOI] [PubMed] [Google Scholar]
- Entringer S, Epel ES, Lin J, Buss C, Shahbaba B, Blackburn EH, Simhan HN, Wadhwa PD, 2013. Maternal psychosocial stress during pregnancy is associated with newborn leukocyte telomere length. American Journal of Obstetrics and Gynecology 208, 134. e131–134. e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Prather AA, 2018. Stress, telomeres, and psychopathology: toward a deeper understanding of a triad of early aging. Annual Review of Clinical Psychology 14, 371–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Fritschy J-M, Löw K, Möhler H, Rudolph U, and Hensch TK (2004). Specific GABAA circuits for visual cortical plasticity. Science 303, 1681–1683. [DOI] [PubMed] [Google Scholar]
- Fleming TP, Watkins AJ, Velazquez MA, Mathers JC, Prentice AM, Stephenson J, Barker M, Saffery R, Yajnik CS, Eckert JJ, Hanson MA, Forrester T, Gluckman PD, Godfrey KM, 2018. Origins of lifetime health around the time of conception: causes and consequences. Lancet 391, 1842–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Davidson RJ, 1986. Taste-elicited changes in facial signs of emotion and the asymmetry of brain electrical activity in human newborns. Neuropsychologia 24, 417–422. [DOI] [PubMed] [Google Scholar]
- Frenck RW, Blackburn EH, Shannon KM, 1998. The rate of telomere sequence loss in human leukocytes varies with age. Proceedings of the National Academy of Sciences 95, 5607–5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, Tottenham N, 2013a. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences 110, 15638–15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Hare TA, Bookheimer SY, Tottenham N, 2013b. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci 33, 4584–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, Bound J, 2006. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. American Journal of Public Health 96, 826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SF, Epel D, 2009. Ecological Developmental Biology: Integrating Epigenetics, Medicine, and Evolution. Sinauer Associates, Sunderland, MA. [Google Scholar]
- Gilbert ME, Sui L, Walker MJ, Anderson W, Thomas S, Smoller SN, et al. (2007). Thyroid hormone insufficiency during brain development reduces parvalbumin immunoreactivity and inhibitory function in the hippocampus. Endocrinology 148, 92–102. [DOI] [PubMed] [Google Scholar]
- Gilman-Sachs A, Dambaeva S, Garcia MDS, Hussein Y, Kwak-Kim J, Beaman K, 2018. Inflammation induced preterm labor and birth. Journal of Reproductive Immunology 129, 53–58. [DOI] [PubMed] [Google Scholar]
- Girchenko P, Lahti J, Czamara D, Knight AK, Jones MJ, Suarez A, Hämäläinen E, Kajantie E, Laivuori H, Villa PM, 2017. Associations between maternal risk factors of adverse pregnancy and birth outcomes and the offspring epigenetic clock of gestational age at birth. Clinical Epigenetics 9, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Bateson P, Beedle AS, Law CM, Bhutta ZA, Anokhin KV, Bougneres P, Chandak GR, Dasgupta P, Smith GD, Ellison PT, Forrester TE, Gilbert SF, Jablonka E, Kaplan H, Prentice AM, Simpson SJ, Uauy R, West-Eberhard MJ, 2009. Towards a new developmental synthesis: adaptive developmental plasticity and human disease. Lancet 373, 1654–1657. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Spencer HG, 2005. Predictive adaptive responses and human evolution. Trends in Ecology & Evolution 20, 527–533. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Schetter CD, Hobel CJ, Sandman CA, 2008. Pattern of perceived stress and anxiety in pregnancy predicts preterm birth. Health Psychology 27, 43. [DOI] [PubMed] [Google Scholar]
- Gohlke B, Frazer F, Stanhope R, 2004. Growth hormone secretion and long-term growth data in children with psychosocial short stature treated by different changes in environment. Journal of Pediatric Endocrinology and Metabolism 17, 637–644. [DOI] [PubMed] [Google Scholar]
- Goland RS, Jozak S, Warren WB, Conwell IM, Stark RI, Tropper PJ, 1993. Elevated levels of umbilical cord plasma corticotropin-releasing hormone in growth-retarded fetuses. The Journal of Clinical Endocrinology and Metabolism 77, 1174–1179. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R, 2008. Epidemiology and causes of preterm birth. The Lancet 371, 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Mestre I, Kulkarni S, Buchholz DR, 2013. Mechanisms and consequences of developmental acceleration in tadpoles responding to pond drying. PLoS One 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Allan MD, & McEwen BS (1990). Dendritic spine density of adult hippocampal pyramidal cells is sensitive to thyroid hormone. Brain Research, 525(2), 327–329. [DOI] [PubMed] [Google Scholar]
- Grand TC, 1999. Risk-taking behaviour and the timing of life history events: consequences of body size and season. Oikos, 467–480. [Google Scholar]
- Guadagno A, Verlezza S, Long H, Wong TP, & Walker CD (2020). It is all in the right amygdala: increased synaptic plasticity and perineuronal nets in male, but not female, juvenile rat pups after exposure to early-life stress. Journal of Neuroscience, 40(43), 8276–8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Gonzalez CA, Goodlin BL, Levine S, 1981. Behavioral and pituitary--adrenal responses during a prolonged separation period in infant rhesus macaques. Psychoneuroendocrinology 6, 65–75. [DOI] [PubMed] [Google Scholar]
- Gur RE, Moore TM, Rosen AFG, Barzilay R, Roalf DR, Calkins ME, Ruparel K, Scott JC, Almasy L, Satterthwaite TD, Shinohara RT, Gur RC, 2019. Burden of Environmental Adversity Associated With Psychopathology, Maturation, and Brain Behavior Parameters in Youths. JAMA Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllenhammer LE, Entringer S, Buss C, Simhan HN, Grobman WA, Adam EK, Keenan-Devlin L, Borders AE, Wadhwa PD, 2020. Prospective association of maternal immune proinflammatory responsivity and regulation in pregnancy with length of gestation. American Journal of Reproductive Immunology, e13366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder L, Dudazy-Gralla S, Müller-Fielitz H, Hjerling Leffler J, Vennström B, Heuer H, et al. (2018). Maternal thyroid hormone is required for parvalbumin neurone development in the anterior hypothalamic area. Journal of Neuroendocrinology 30:e12573. [DOI] [PubMed] [Google Scholar]
- Hay RE, Reynolds JE, Grohs MN, Paniukov D, Giesbrecht GF, Letourneau N, Dewey D, Lebel C, 2020. Amygdala-Prefrontal Structural Connectivity Mediates the Relationship between Prenatal Depression and Behavior in Preschool Boys. Journal of Neuroscience 40, 6969–6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward AD, Rickard IJ, & Lummaa V (2013). Influence of early-life nutrition on mortality and reproductive success during a subsequent famine in a preindustrial population. Proceedings of the National Academy of Sciences, 110(34), 13886–13891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom IC, Dhir S, Diorio J, Zhang TY, Meaney MJ. (2012) Maternal licking regulates hippocampal glucocorticoid receptor transcription through a thyroid hormone, serotonin, NGFI-A signalling cascade. Philosophical Transactions of the Royal Society B (Biological Sciences) 367:2495–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK (2005). Critical period plasticity in local cortical circuits. Nature Reviews Neuroscience, 6(11), 877–888. [DOI] [PubMed] [Google Scholar]
- Hermanussen M, Geiger-Benoit K, Burmeister J, Sippell WG, 1988. Periodical changes of short term growth velocity (‘mini growth spurts’) in human growth. Annals of Human Biology 15, 103–109. [DOI] [PubMed] [Google Scholar]
- Hofer MA, 1970. Physiological responses of infant rats to separation from their mothers. Science 168, 871–873. [DOI] [PubMed] [Google Scholar]
- Hofer MA, 1975. Survival and recovery of physiologic functions after early maternal separation in rats. Physiology and Behavior 15, 475–480. [DOI] [PubMed] [Google Scholar]
- Horvath S, Raj K, 2018. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nature Reviews Genetics 19, 371. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR, 2014. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development. Psychological Bulletin 140, 256–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys KL, Esteves K, Zeanah CH, Fox NA, Nelson CA III, Drury SS, 2016. Accelerated telomere shortening: Tracking the lasting impact of early institutional care at the cellular level. Psychiatry Research 246, 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EO, Kamilaris TC, Chrousos GP, Gold PW, 1992. Mechanisms of stress: a dynamic overview of hormonal and behavioral homeostasis. Neuroscience and Biobehavioral Reviews 16, 115–130. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Vance LA, Cross D, Knight AK, Kilaru V, Michopoulos V, Klengel T, Smith AK, 2017. Exposure to violence accelerates epigenetic aging in children. Scientific Reports 7, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ, 2010. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and Biobehavioral Reviews 35, 2–16. [DOI] [PubMed] [Google Scholar]
- Karp X, 2018. Working with dauer larvae. WormBook: the online review of C. elegans biology 2018, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koibuchi N (2008). The role of thyroid hormone on cerebellar development. Cerebellum 7, 530–533. [DOI] [PubMed] [Google Scholar]
- Krech D, Crutchfield RS, Ballachey EL, 1962. Individual in Society: A Textbook of Social Psychology. American Psychological Association. [Google Scholar]
- Kuhlman KR, Chiang JJ, Horn S, & Bower JE (2017). Developmental psychoneuroendocrine and psychoneuroimmune pathways from childhood adversity to disease. Neuroscience and Biobehavioral Reviews, 80, 166–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Poh JS, Wen DJ, Guillaume B, Chong YS, Shek LP, Fortier MV, Qiu A, 2019. Long-term Influences of Prenatal Maternal Depressive Symptoms on the Amygdala-Prefrontal Circuitry of the Offspring From Birth to Early Childhood. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 4, 940–947. [DOI] [PubMed] [Google Scholar]
- Levine S, 2002. Regulation of the hypothalamic-pituitary-adrenal axis in the neonatal rat: the role of maternal behavior. Neurotoxicty Research 4, 557–564. [DOI] [PubMed] [Google Scholar]
- Li L, Denholm R, Power C, 2014. Child maltreatment and household dysfunction: associations with pubertal development in a British birth cohort. International Journal of Epidemiology 43, 1163–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liggins GC, Forster CS, Grieves SA, Schwartz AL, 1977. Control of parturition in man. Biology of Reproduction 16, 39–56. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ, 1997. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 277, 1659–1662. [DOI] [PubMed] [Google Scholar]
- Ludewig AH, Gimond C, Judkins JC, Thornton S, Pulido DC, Micikas RJ, Doring F, Antebi A, Braendle C, Schroeder FC, 2017. Larval crowding accelerates C. elegans development and reduces lifespan. PLoS Genet 13, e1006717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan S, Prime H, Graham SA, Rodrigues M, Anderson N, Khoury J, Jenkins JM, 2019. Parenting Behavior and Child Language: A Meta-analysis. Pediatrics 144. [DOI] [PubMed] [Google Scholar]
- Marin O, 2016. Developmental timing and critical windows for the treatment of psychiatric disorders. Nature Medicine 22, 1229–1238. [DOI] [PubMed] [Google Scholar]
- Maslow AH, 1943. A theory of human motivation. Psychol Rev. [Google Scholar]
- Maughan B, Collishaw S, 2015. Development and psychopathology: A life course perspective, in: Thapar A, Pine DS, Leckman JF, Scott S, Snowling MJ, taylor E (Eds.), Rutter’s Child and Adolescent Psychiatry, Sixth ed. John Wiley & Sons, West Sussez, UK, pp. 5–16. [Google Scholar]
- McEwen BS, 2004. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Annals of the New York Academy of Science 1032, 1–7. [DOI] [PubMed] [Google Scholar]
- McGill MG, McEwen L, Pokhbisneva I, Beijers R, Tollenaar M, Kee M, Garg E, Karnani N, Silveira PP, Kobor MS, de Weerth C, Meaney MJ, O’Donnell KJ under review. Maternal prenatal anxiety and the fetal origins of pediatric epigenetic age acceleration. [DOI] [PubMed]
- McGrath JJ, Hannan AJ, & Gibson G 2011. Decanalization, brain development and risk of schizophrenia. Translational Psychiatry, 1(6), e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Fox NA, Zeanah CH, & Nelson CA 2011. Adverse rearing environments and neural development in children: The development of frontal electroencephalogram asymmetry. Biological Psychiatry, 70(11), 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, 2016. Beyond cumulative risk: A dimensional approach to childhood adversity. Current Directions in Psychological Science 25, 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Lambert HK, 2014. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neuroscience and Biobehavioral Reviews 47, 578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Nelson CA, 2017. Neglect as a Violation of Species-Expectant Experience: Neurodevelopmental Consequences. Biological Psychiatry 82, 462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, van Berkel C, Bhatnagar S, Sapolsky RM, 1988. Effect of neonatal handling on age-related impairments associated with the hippocampus. Science 239, 766–768. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Weaver S, Yau J, Chapman K, Seckl JR, 2000. Postnatal handling increases the expression of cAMP-inducible transcription factors in the rat hippocampus: the effects of thyroid hormones and serotonin. Journal of Neuroscience 20, 3926–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M, Seckl JR, 2007. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends Mol Med 13, 269–277. [DOI] [PubMed] [Google Scholar]
- Metcalfe NB, Monaghan P, 2001. Compensation for a bad start: grow now, pay later? Trends in Ecology and Evolution 16, 254–260. [DOI] [PubMed] [Google Scholar]
- Moore SR, McEwen LM, Quirt J, Morin A, Mah SM, Barr RG, Boyce WT, Kobor MS, 2017. Epigenetic correlates of neonatal contact in humans. Development and Psychopathology 29, 1517–1538. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Shionoya K, Jakubs K, Sullivan R 2009. Early-life stress disrupts attachment learning: The role of amygdala corticosterone, locus ceruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. The Journal of Neuroscience, 29(50, 15745–15755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Wilson DA, Levine S, & Sullivan RM 2006. Dual circuitry for odor–shock conditioning during infancy: corticosterone switches between fear and attraction via amygdala. The Journal of Neuroscience, 26(25), 6737–6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton NM, Holmes MC, Fievet C, Staels B, Tailleux A, Mullins JJ, Seckl JR, 2001. Improved lipid and lipoprotein profile, hepatic insulin sensitivity, and glucose tolerance in 11beta-hydroxysteroid dehydrogenase type 1 null mice. Journal of Biological Chemistry 276, 41293–41300. [DOI] [PubMed] [Google Scholar]
- Muller GB, 2007. Evo-devo: extending the evolutionary synthesis. Nat Rev Genet 8, 943–949. [DOI] [PubMed] [Google Scholar]
- Muñoz-Hoyos A, Molina-Carballo A, Augustin-Morales M, Contreras-Chova F, Naranjo-Gómez A, Justicia-Martínez F, Uberos J, 2011. Psychosocial dwarfism: Psychopathological aspects and putative neuroendocrine markers. Psychiatry Research 188, 96–101. [DOI] [PubMed] [Google Scholar]
- Nieves GM, Bravo M, Baskoylu S, & Bath KG (2020). Early life adversity decreases pre-adolescent fear expression by accelerating amygdala PV cell development. Elife, 9, e55263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nika L, Gibson T, Konkus R, Karp X, 2016. Fluorescent beads are a versatile tool for staging Caenorhabditis elegans in different life histories. G3: Genes, Genomes, Genetics 6, 1923–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell KJ, Meaney MJ, 2017. Fetal Origins of Mental Health: The Developmental Origins of Health and Disease Hypothesis. American Journal of Psychiatry 174, 319–328. [DOI] [PubMed] [Google Scholar]
- Obradovic J, Boyce WT, 2009. Individual differences in behavioral, physiological, and genetic sensitivities to contexts: implications for development and adaptation. Developmental Neuroscience 31, 300–308. [DOI] [PubMed] [Google Scholar]
- Obradovic J, Bush NR, Stamperdahl J, Adler NE, Boyce WT, 2010. Biological sensitivity to context: The interactive effects of stress reactivity and family adversity on socio-emotional behavior and school readiness. Child Development 81, 270–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong ML, Tuan TA, Poh J, Teh AL, Chen L, Pan H, MacIsaac JL, Kobor MS, Chong YS, Kwek K, Saw SM, Godfrey KM, Gluckman PD, Fortier MV, Karnani N, Meaney MJ, Qiu A, Holbrook JD, 2019. Neonatal amygdalae and hippocampi are influenced by genotype and prenatal environment, and reflected in the neonatal DNA methylome. Genes, Brain Behavior 18, e12576. [DOI] [PubMed] [Google Scholar]
- Ono M, Kikusui T, Sasaki N, Ichikawa M, Mori Y, Murakami-Murofushi K 2008. Early weaning induces anxiety and precocious myelination in the anterior part of the basolateral amygdala of male balb/c mice. Neuroscience, 156, 1103–1110. [DOI] [PubMed] [Google Scholar]
- Phillips DI, Jones A, 2006. Fetal programming of autonomic and HPA function: do people who were small babies have enhanced stress responses? Journal of Physiology 572, 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price WA, Stiles AD, Moats-Staats BM, D’Ercole AJ, 1992. Gene expression of insulin-like growth factors (IGFs), the type 1 IGF receptor, and IGF-binding proteins in dexamethasone-induced fetal growth retardation. Endocrinology, 130, 1424–1432. [DOI] [PubMed] [Google Scholar]
- Qiu A, Anh TT, Li Y, Chen H, Rifkin-Graboi A, Broekman BF, Kwek K, Saw SM, Chong YS, Gluckman PD, Fortier MV, Meaney MJ, 2015. Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Translational Psychiatry 5, e508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan RJ, 2003. Father absence, parental care, and female reproductive development. Evolution and Human Behavior 24, 376–390. [Google Scholar]
- Reh RK, Dias BG, Nelson CA, Kaufer D, Werker JF, Kolb B, Levine JD, Hensch TK, 2019. Critical period regulation across multiple timescales. Proceedings of the National Academy of Sciences, 117(38), 23242–23251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid BM, Harbin MM, Arend JL, Kelly AS, Dengel DR, Gunnar MR, 2018. Early life adversity with height stunting is associated with cardiometabolic risk in adolescents independent of body mass index. The Journal of Pediatrics 202, 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss F (2013). Socioeconomic inequalities and mental health problems in children and adolescents: a systematic review. Social Science & Medicine, 90, 24–31. [DOI] [PubMed] [Google Scholar]
- Rickard IJ, Frankenhuis WE, Nettle D, 2014. Why are childhood family factors associated with timing of maturation? A role for internal prediction. Perspectives on Psychological Science 9, 3–15. [DOI] [PubMed] [Google Scholar]
- Riem MME, Karreman A, 2019. Childhood Adversity and Adult Health: The Role of Developmental Timing and Associations With Accelerated Aging. Child Maltreatment 24, 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin-Graboi A, Bai J, Chen H, Hameed WB, Sim LW, Tint MT, Leutscher-Broekman B, Chong YS, Gluckman PD, Fortier MV, Meaney MJ, Qiu A, 2013. Prenatal maternal depression associates with microstructure of right amygdala in neonates at birth. Biological Psychiatry 74, 837–844. [DOI] [PubMed] [Google Scholar]
- Romans S, Martin J, Gendall K, Herbison G, 2003. Age of menarche: The role of some psychosocial factors. Psychological Medicine 33, 933–939. [DOI] [PubMed] [Google Scholar]
- Roubinov DS, Luecken LJ, Curci S, Somers J, Winstone L, 2021. A prenatal programming perspective on the intergenerational transmission of maternal ACEs to offspring health problems. American Psychologist, 76, 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovelli C, 2018. The Order of Time. Riverhead Books, New York. [Google Scholar]
- Royland JE, Parker JS, and Gilbert ME (2008). A genomic analysis of subclinical hypothyroidism in hippocampus and neocortex of the developing rat brain. Journal of Neuroendocrinology 20, 1319–1338. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Truskanov N, Mangel M, Blaustein L, 2011. Compensatory development and costs of plasticity: larval responses to desiccated conspecifics. PloS One 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale A, Putignano E, Cancedda L, Landi S, Cirulli F, Berardi N, Maffei L (2004). Enriched environment and acceleration of visual system development. Neuropharmacology, 47(5), 649–660. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ, 1986. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Research 396, 64–76. [DOI] [PubMed] [Google Scholar]
- Sawano E, Takahashi M, Negishi T, and Tashiro T (2013). Thyroid hormone-dependent development of the GABAergic pre- and post-synaptic components in the rat hippocampus. International Journal of Developmental Neuroscience 31, 751–761. [DOI] [PubMed] [Google Scholar]
- Schanberg SM, Evoniuk G, Kuhn CM, 1984. Tactile and nutritional aspects of maternal care: specific regulators of neuroendocrine function and cellular development. Proceedings of the Society for Experimental Biology and Medicine 175, 135–146. [DOI] [PubMed] [Google Scholar]
- Schanberg SM, Ingledue VF, Lee JY, Hannun YA, Bartolome JV, 2003. PKC alpha mediates maternal touch regulation of growth-related gene expression in infant rats. Neuropsychopharmacology 28, 1026–1030. [DOI] [PubMed] [Google Scholar]
- Scheinost D, Kwon SH, Lacadie C, Sze G, Sinha R, Constable RT, Ment LR, 2016. Prenatal stress alters amygdala functional connectivity in preterm neonates. NeuroImage Clinical 12, 381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, McLaughlin KA, 2014. Dimensions of early experience and neural development: deprivation and threat. Trends in Cognitive Sciences 18, 580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, McLaughlin KA, 2016. Neurobiological models of the impact of adversity on education. Current Opinion in Behavioral Sciences 10, 108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, & McLaughlin KA (2020). Neurodevelopmental mechanisms linking ACEs with psychopathology. In Adverse Childhood Experiences (pp. 265–285). Academic Press. [Google Scholar]
- Smith R, Nicholson RC, 2007. Corticotrophin releasing hormone and the timing of birth. Frontiers in Bioscience 12, 912–918. [DOI] [PubMed] [Google Scholar]
- Soe NN, Wen DJ, Poh JS, Chong YS, Broekman BF, Chen H, Shek LP, Tan KH, Gluckman PD, Fortier MV, Meaney MJ, Qiu A, 2018. Perinatal maternal depressive symptoms alter amygdala functional connectivity in girls. Human Brain Mapping 39, 680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples JF, 2011. Metabolic flexibility: hibernation, torpor, and estivation. Comprehensive Physiology 6, 737–771. [DOI] [PubMed] [Google Scholar]
- Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, Howard LM, Pariante CM, 2014. Effects of perinatal mental disorders on the fetus and child. Lancet 384, 1800–1819. [DOI] [PubMed] [Google Scholar]
- Suarez A, Lahti J, Czamara D, Lahti-Pulkkinen M, Knight AK, Girchenko P, Hämäläinen E, Kajantie E, Lipsanen J, Laivuori H, 2018. The epigenetic clock at birth: associations with maternal antenatal depression and child psychiatric problems. Journal of the American Academy of Child & Adolescent Psychiatry 57, 321–328. e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suglia SF, Chen C, Wang S, Cammack AL, April-Sanders AK, McGlinchey EL, Kubo A, Bird H, Canino G, Duarte CS, 2020. Childhood Adversity and Pubertal Development Among Puerto Rican Boys and Girls. Psychosomatic Medicine 82, 487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Opendak M (2021) Neurobiology of Infant Fear and Anxiety: Impacts of Delayed Amygdala Development and Attachment Figure Quality. Biological Psychiatry. 89:641–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Colich NL, Uddin M, Armstrong D, McLaughlin KA, 2019. Early Experiences of Threat, but Not Deprivation, Are Associated With Accelerated Biological Aging in Children and Adolescents. Biological Psychiatry 85, 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi LK, Nakashima BR, Hong H, Watanabe K, 2005. The smell of danger: a behavioral and neural analysis of predator odor-induced fear. Neuroscience and Biobehavioral Reviews 29, 1157–1167. [DOI] [PubMed] [Google Scholar]
- Takesian AE, Hensch TK, 2013. Balancing plasticity/stability across brain development. Progress in Brain Research 207, 3–34. [DOI] [PubMed] [Google Scholar]
- Talbot NB, Sobel EH, Burke BS, Lindemann E, Kaufman SB, 1947. Dwarfism in healthy children: Its possible relation to emotional, nutritional and endocrine disturbances. New England Journal of Medicine 236, 783–793. [DOI] [PubMed] [Google Scholar]
- Teh AL, Pan H, Chen L, Ong ML, Dogra S, Wong J, MacIsaac JL, Mah SM, McEwen LM, Saw SM, Godfrey KM, Chong YS, Kwek K, Kwoh CK, Soh SE, Chong MF, Barton S, Karnani N, Cheong CY, Buschdorf JP, Stunkel W, Kobor MS, Meaney MJ, Gluckman PD, Holbrook JD, 2014. The effect of genotype and in utero environment on interindividual variation in neonate DNA methylomes. Genome Research 24, 1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theall KP, Brett ZH, Shirtcliff EA, Dunn EC, Drury SS, 2013. Neighborhood disorder and telomeres: Connecting children’s exposure to community level stress and cellular response. Social Science and Medicine 85, 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipton MJ, Collier N, Massey H, Corbett J, Harper M, 2017. Cold water immersion: kill or cure? Experimental Physiology 102, 1335–1355. [DOI] [PubMed] [Google Scholar]
- Tooley UA, Bassett DS and Mackey AP, 2021. Environmental influences on the pace of brain development. Nature Reviews Neuroscience, 22(6), pp.372–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Shapiro M, Flannery J, Caldera C, Sullivan RM (2019): Parental presence switches avoidance to attraction learning in children. Nature Human Behavior 3:1070–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H, 2008. Glucocorticoids: mediators of vertebrate ontogenetic transitions. General and Comparative Endocrinology 156, 441–453. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Sandman CA, Porto M, Dunkel-Schetter C, Garite TJ, 1993. The association between prenatal stress and infant birth weight and gestational age at birth: a prospective investigation. American Journal of Obstetrics and Gynecology 169, 858–865. [DOI] [PubMed] [Google Scholar]
- Wells JCK, 2018. Life history trade-offs and the partitioning of maternal investment: Implications for health of mothers and offspring. Evolution, Medicine, and Public Health 2018, 153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen DJ, Poh JS, Ni SN, Chong YS, Chen H, Kwek K, Shek LP, Gluckman PD, Fortier MV, Meaney MJ, Qiu A, 2017. Influences of prenatal and postnatal maternal depression on amygdala volume and microstructure in young children. Translational Psychiatry 7, e1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerholz S, de Lima AD, & Voigt T (2013). Thyroid hormone-dependent development of early cortical networks: temporal specificity and the contribution of trkB and mTOR pathways. Frontiers in Cellular Neuroscience, 7, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens SC, and Trudeau VL (2006). Thyroid hormone and γ-aminobutyric acid (GABA) interactions in neuroendocrine systems. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 144, 332–344. [DOI] [PubMed] [Google Scholar]
- Zarrow MX, Philpott JE, Denenberg VH, 1968. Postnatal changes in the pituitary-adrenal axis of the rat. Proceedings of the Society for Experimental Biology and Medicine 128, 269–272 [DOI] [PubMed] [Google Scholar]