Summary
Vitamin D3 is well-known as a major regulator of calcium and phosphorus homeostasis. A growing body of evidence highlights its crucial role in the regulation of reproductive processes in females. The role of vitamin D3 in the female reproductive tract has been extensively investigated because its receptor is abundant in reproductive organs, including ovary. Importantly, besides expression of vitamin D3 receptor, the ovary is an extrarenal site of vitamin D3 metabolism. The influence of vitamin D3 on follicular development and ovarian steroidogenesis has been investigated. Furthermore, vitamin D3 deficiency has also been associated with polycystic ovary syndrome, premature ovarian failure and ovarian cancer. The objective of this review is to summarize our knowledge about the contribution of vitamin D3 to physiological and pathological processes within the ovary.
Keywords: Vitamin D3, Vitamin D3 receptor, Ovary, Polycystic Ovary Syndrome, Premature Ovarian Failure
Introduction
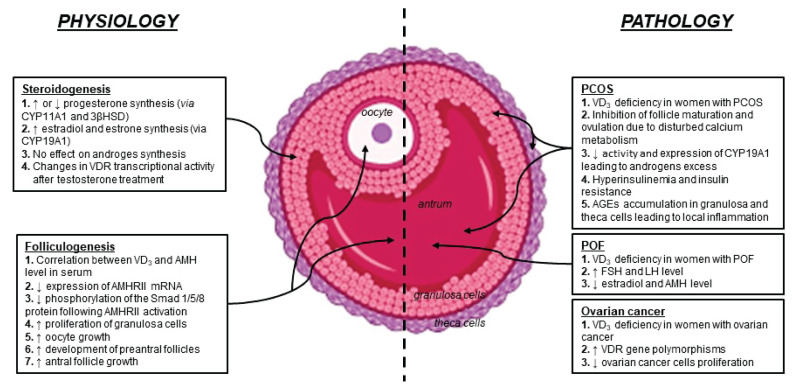
Vitamin D3 (VD3) deficiency is recognized as a global problem, which increases the risk of many chronic diseases. The status of VD3 in the organism depends on sun exposure, diet, intake of VD3 supple-ments, lifestyle and genetic factors (DeLuca 2004). It is well-known that VD3 is predominantly involved in the regulation of calcium and phosphorus homeostasis and crucial for bone mineralization. However, a growing body of literature indicates its pleiotropic actions within the organism including an influence on various physiological and pathological processes. The classical VD3 target tissues are the intestine, kidneys and bones. Importantly, among the non-classical sites of VD3 action are tissues of the female reproductive tract. VD3 receptor (VDR) and VD3 metabolic enzymes have been found in the ovary, uterus, fallopian tube, vagina and placenta of both human and animals, confirming the direct role of VD3 in these organs (Lerchbaum and Obermayer-Pietsch 2012). In recent years there have been an increasing number of scientific papers suggesting a correlation between low VD3 level and reduced fertility, metabolic and endocrine disorders, polycystic ovary syndrome (PCOS), premature ovarian failure (POF) and ovarian cancer (Muscogiuri et al. 2017). This review focuses on the influence of VD3 on physiological processes within the ovary as well as its contribution to ovarian pathologies that is summarized in Figure 1.
Fig. 1.
Vitamin D3 contribution to physiological and pathological processes within the ovary. AGEs: advanced glycation end-products; AMH: anti-Müllerian hormone; AMHRII: AMH receptor type II; 3β-HSD: 3β-hydroxysteroid dehydrogenase; CYP11A1: cholesterol side-chain cleavage enzyme; CYP19A1: cytochrome P450 aromatase; FSH: follicle-stimulating hormone; GCs: granulosa cells; LH: luteinizing hormone; PCOS: polycystic ovary syndrome; POF: premature ovarian failure; TCs: theca cells; VD3: vitamin D3; VDR: vitamin D3 receptor.
VD3 metabolism and mechanism of action
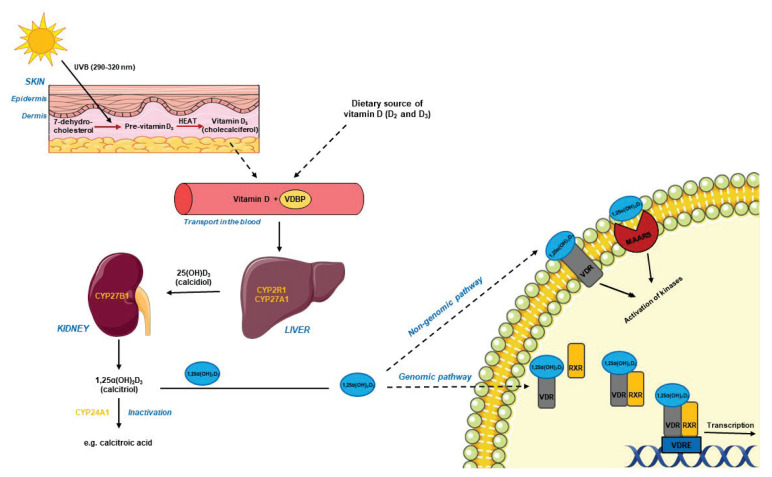
The main source of circulating VD3 is endogenous synthesis in the skin following ultraviolet-B irradiation (UVB). Only small amount of VD3 is derived from the diet (fatty fish, cod-liver oil, milk, eggs) or supplements (Bikle 2014). In keratinocytes, 7-dehydro-cholesterol is converted to previtamin D3 under UVB. Next, previtamin D3 undergoes isomerization under the influence of the body’s thermal energy and forms biologically inactive VD3 (cholecalciferol). VD3 is released from the keratinocyte membranes into the extracellular space and hence into the blood, where it is transported with vitamin D binding proteins (VDBP). In the liver, hydroxylation to 25(OH)D3 (25-hydroxycalci-ferol, calcidiol) takes place in the presence of 25-hydro-xylases (e.g. CYP2R1, CYP27A1). A second hydroxy-lation in the kidneys involves 1α-hydroxylase (CYP27B1) action, resulting in biologically active VD3, i.e. 1α,25(OH)2D3 (1α,25-dihydroxycholecalciferol, calcitriol). Both calcidiol and calcitriol may be degraded as a result of further hydroxylation by CYP24A1 (Christakos et al. 2016) (Fig. 2). The concentration of calcitriol circulating in the blood is not a reliable indicator of the VD3 level in the body because its content and metabolism are controlled by parathormone and depend on the concentration of calcium and phosphorus. Thus, calcidiol is considered the best indicator due to its long half-life and lack of mechanisms regulating its level (DeLuca 2004).
Fig. 2.
Overview of vitamin D3 metabolism and intracellular action. CYP2R1: 25-hydroxylase present in the endoplasmic reticulum; CYP24A1: 24-hydroxylase; CYP27A1: 25-hydroxylase present in the mitochondria; CYP27B1: 1α-hydroxylase; MAARS: membrane-associated rapid response steroid protein; RXR: 9-cis-retinoic acid receptor; UVB: ultraviolet-B irradiation; VDBP: vitamin D3 binding protein; VDR: vitamin D3 receptor; VDRE: vitamin D response element.
The biological effect of 1α,25(OH)2D3 on target cells is mediated by VDR that belongs to the superfamily of ligand-activated steroids receptor and acts as a transcriptional factor. VDR is composed of a short N-terminal domain, a highly conserved DNA binding domain, a hinge region and a α-helical C-terminal ligand binding domain (Christakos et al. 2016). Calcitriol binding to the ligand binding domain induces heterodimerization of VDR with the 9-cis-retinoic acid receptor (RXR). The VDR-RXR complex is translocated to the nucleus and binds to the VD3 response element (VDRE) regulating the expression of target genes. The activation/inhibition of transcription requires also the recruitment of wide range of co-regulators (Keane et al. 2017). Studies on the structure of VDR have shown the presence of two overlapping ligand binding sites in the C-terminal domain. They were defined as the genomic pocket (VDR-GP) and the alternative pocket (VDR-AP). The first of these initiates the genomic response, while the second one can cause both genomic and non-genomic effects (Mizwicki et al. 2004). The final signaling pathway triggered after ligand (calcitriol or its synthetic analogues) binding to VDR also depends on VDR localization in the cell. The receptor has been found in the cytoplasm/nucleus and mitochondria as well as in cell membrane cavities, i.e. caveolae. VDR located in caveolae triggers a rapid cell response by activating receptors associated with G proteins, phosphatases, kinases and ion channels (Keane et al. 2017). Recently it has been demonstrated that 1α,25(OH)2D3 can act by interaction with the MARRS (Membrane-Associated Rapid Response Steroid) protein that occurs in caveolae together with VDR. This type of VD3 receptor is also known as GRP58 (Glucose Responsive Protein, 58 kDa), ERp57 or ERp60 (Endoplasmic Reticulum Protein 57/60 kDa) and Pdia3 (Protein Disulfide Isomerase Family A, Member 3) (Hii and Ferrante 2016) (Fig. 2).
The role of VD3 in the regulation of folliculogenesis
A growing body of literature suggests that VD3 plays an important role in the regulation of ovarian processes that determine female fertility. Female reproductive potential is expressed as the number of primary follicles in the ovary at birth, known as the ovarian reserve. It decreases during postnatal life as a result of the recruitment of primary follicles to the growth (Monniaux et al. 2014). This process is controlled by growth factors and hormones, among which the most important is anti-Müllerian hormone (AMH). It is produced by granulosa cells of preantral and early antral follicles and inhibits initial recruitment of follicles, maintaining the ovarian reserve (Visser et al. 2006). Recently the influence of VD3 on AMH concentration, and thereby on the ovarian reserve, has been extensively discussed. Studies conducted on a group of premeno-pausal women with regular menstrual cycles showed a positive correlation between the plasma concentration of 25(OH)D3 and AMH. In addition, a decrease in the level of both hormones was observed in the winter and this effect was reversed after VD3 administration (Merhi et al. 2012). The effect of VD3 on AMH level is probably due to the presence of the VDRE sequence in the AMH gene promoter as found in prostate cells (Malloy et al. 2009). Furthermore, Merhi et al. (2014) has observed that 25(OH)D3 deficiency in follicular fluid correlated with an increased expression of the transcript for AMH type II receptor (AMHR-II) in human granulosa cells. VD3 has also been shown to reduce the phosphorylation of the Smad 1/5/8 protein that contributes to signal transduction from AMHR-II. Thus, VD3 may increase the synthesis of AMH but also modulate its effect on follicular cells by regulating intracellular signaling pathways (Irani and Merhi 2014). Despite the lack of literature data indicating the direct role of VD3 in maintaining ovarian reserve, the effect exerted on AMH suggests a synergistic action between both hormones.
The effect of VD3 on folliculogenesis has been demonstrated for the first time in studies conducted on Vdr- and Cyp27b1-knockout mice. They displayed increased ovarian interstitial tissue, weakened follicular development and lack of corpus luteum suggesting ovulatory disorders (Kinuta et al. 2000, Panda et al. 2001). The influence of VD3 on follicular development in vitro has been studied on primates by Xu et al. (2016). They isolated preantral follicles and cultured them to the antral stage with addition of a low (25 pg/ml) or high (100 pg/ml) concentration of 1α,25(OH)2D3. The low dose had a positive effect on oocyte growth, survival and development of preantral follicles, suggested by the authors as being due to an increased sensitivity to follicle-stimulating hormone (FSH). However, after reaching the antral stage, the higher dose of 1α,25(OH)2D3 was more effective and promoted follicular growth (Xu et al. 2016). These results show that VD3 affects both the early and late stages of folliculogenesis, and that its effect is dose-dependent.
The growth and development of ovarian follicles are associated with the proliferation and differentiation of granulosa cells. Yao et al. (2017) demonstrated an effect of VD3 on the proliferation of goat granulosa cells by regulation of oxidative stress and changes in the expression of genes regulated cell cycle. The influence of VD3 on granulosa cell proliferation has also been observed in hens (Wojtusik and Johnson 2012).
The role of VD3 in the regulation of steroidogenesis
Besides production of germ cells, the ovary synthesizes steroid hormones including progesterone, androgens and estrogens. Studies so far indicate that VD3 regulates the expression and activity of steroidogenic enzymes and that the effect is tissue specific (Lundquist 2014). In human granulosa cells, there is an augmented expression and activity of 3β-hydroxysteroid dehydrogenase (3β-HSD) as well as an increase in progesterone production (Merhi et al. 2014), consistent with the studies of Parikh et al. (2010). Studies on porcine granulosa cells in vitro revealed no effect of 1α,25(OH)2D3 on basal progesterone production, but noted its increase after insulin and FSH stimulation (Smolikova et al. 2013). In contrast, other studies on porcine granulosa cells showed a reduced progesterone synthesis following 1α,25(OH)2D3 treatment that was associated with decreased cholesterol side-chain cleavage enzyme (CYP11A1) mRNA and protein expression and increased 3β-HSD mRNA and protein expression (Hong et al. 2016). Results from experiments on the effect of VD3 on ovarian estrogen synthesis are clearer. Studies on human (Parikh et al. 2010), porcine (Hong et al. 2017) and goat (Yao et al. 2017) granulosa cells revealed a stimulatory effect of 1α,25(OH)2D3 on estradiol and estrone production and on the expression of aromatase (CYP19A1), which converts androgens to estrogens. These results may be explained by the fact that the VDRE element is present in the promoter of the gene encoding CYP19A1 in human placental cells (Sun et al. 1998). The role of VD3 in ovarian androgen synthesis has not yet been intensively studied. Parikh et al. (2010) found no effect of VD3 on androgen production in humans. However, it has been observed that testosterone affects the transcriptional activity of VDR in porcine granulosa cells by inhibiting the formation of VDR-RXR complexes (Herian et al. 2018). This information suggests that VD3 is an important modulator of steroidogenesis in the human and mammalian ovary.
VD3 and PCOS
PCOS is one of the most common endocrinopathies of women of reproductive age. It is characterized by ovulation disorders, irregular cycles, the presence of ovarian cysts, hyperandrogenism, abnormal level of gonadotropins and metabolic disturbances (hyperinsulinemia, insulin resistance, dyslipidemia) resulting in infertility. Recent studies show a reduced calcidiol level in women with PCOS suggesting a relationship between VD3 deficiency and the occurrence of many PCOS symptoms (Dravecka et al. 2016, Shahrokhi et al. 2016). VD3 deficiency is often associated with a disturbed calcium metabolism, which in women with PCOS may inhibit follicle maturation and ovulation. The diminished level of circulating VD3 also reduces the activity and expression of CYP19A1, which disturbs conversion of androgens to estrogens. An increase in androgen concentration blocks follicular maturation before ovulation and leads to ovarian cyst appearance (Lorenzen et al. 2017).
One of the metabolic symptoms occurring in 60–80 % of women with PCOS is insulin resistance. VD3 has been shown to increase insulin synthesis and secretion, and expression of its receptor. In addition, it increases cell sensitivity to insulin by inhibiting the production of pro-inflammatory cytokines (Sung et al. 2012). The direct effect of VD3 on insulin secretion and consequently on glucose metabolism is mediated via VDR present in β cells of the pancreas. Importantly, VDRE sequence was found in the promoter of the gene coding insulin (Sung et al. 2012). It is also believed that the indirect effect of VD3 on insulin sensitivity depends on the regulation of intracellular calcium level, which is necessary for proper cell signaling in the insulin-dependent tissues (muscle and fat) (Pittas et al. 2007). Insulin resistance results in elevated glucose concentrations, which may in turn modify proteins, lipids and nucleic acids in a non-enzymatic way leading to the formation of Advanced Glycation End-products (AGEs). An increased plasma concentration of AGEs as well as their accumulation in granulosa and theca cells of ovarian follicles has been observed in PCOS. These compounds bind to their soluble receptor (sRAGE) and induce the formation of reactive oxygen species and cytokines with pro-inflammatory properties. The involvement of AGEs and their receptors in the pathogenesis of PCOS is mainly associated with the disturbance of follicular growth. This effect can be attenuated by 1α,25(OH)2D3, which has anti-inflammatory properties (Merhi 2019).
VD3 and POF
POF is defined as the loss of ovarian function before the age of 40 years. It is characterized by premature depletion of the ovarian reserve due to autoimmune damage or genetic predisposition. Typical POF symptoms include amenorrhea, high gonadotropins and low estradiol levels, as well as decreased AMH concentration in the plasma (Knauff et al. 2009). Keeping in mind the effect of VD3 on the synthesis of AMH as an ovarian reserve marker, VD3 contribution to the POF etiology seems to be possible. Research conducted on a population of women with POF showed the negative correlation between VD3 deficiency and FSH level (Kebapcilar et al. 2013). On the other hand, study that confirmed the characteristic hormonal profile (high FSH and LH level, low estradiol level) in patients with POF, has also shown no changes in 25(OH)D3 concentration (Ersoy et al. 2015). The above mentioned results do not allow to unequivocally confirm the role of VD3 in the pathogenesis of POF. However, it is suggested that VD3 deficiency may decrease the AMH level, which in turn leads to increased FSH concentration and the occurrence of POF as a consequence (Ersoy et al. 2015).
VD3 and ovarian cancer
Epidemiological studies have shown that the occurrence of ovarian cancer is inversely correlated with exposure to UVB radiation, which is necessary for the synthesis of VD3 in the skin, thus suggesting its involvement in the pathogenesis of ovarian cancer (Guo et al. 2018). Analysis of plasma calcidiol concentrations among ovarian cancer patients showed that this was significantly lower (under 20 ng/ml) than in the control group. In addition, a group of patients with calcidiol level below 10 ng/ml had a statistically lower survival rate. From the results, it was noticed that VD3 deficiency may have greater impact on patients with more aggressive cancers (Colonese et al. 2015).
It is believed that VDR gene polymorphisms increase the risk of ovarian cancer. The most common is the single FokI nucleotide polymorphism located at the 5′ end, which leads to the synthesis of VDR protein with a longer amino acid sequence. Further identified polymorphisms – BsmI, ApaI and TaqI – are located at the 3′ end and do not affect the synthesis of functional VDR protein but regulate the stability of VDR mRNA (Guo et al. 2018).
The anti-tumor mechanism of VD3 action involves the inhibition of cell proliferation by affecting the cell cycle regulatory proteins (p21, p27, cyclins). In addition, cell cycle inhibition in the G2/M phases and induction of ovarian cancer cell death by increasing mRNA and GADD45α protein expression were reported. Further research indicates that VD3 inhibits cancer angiogenesis and metastasis. It has also been reported that it affects glucose and fatty acids metabolism in cancer cells (Guo et al. 2018). Previous studies confirm that administration of VD3 or its analogues is not an effective method of ovarian cancer treatment. On the other hand, proper VD3 supplementation may reduce the risk of illness (Guo et al. 2018).
Conclusions
Numerous epidemiological data and results of animal studies confirm that VD3 plays a key role in supporting ovarian function. It has a positive effect on folliculogenesis and maintenance of the ovarian reserve, and also stimulates steroidogenesis. There is growing concern that the global problem with VD3 deficiency among women contributes to reproductive complications. Therefore, VD3 supplementation seems to be a great opportunity for the treatment and insertion of ovarian pathologies. In conclusion, monitoring of plasma calcidiol level should become a preventive diagnostic for ensuring female health.
Acknowledgements
The websites https://biorender.com (BioRender) and https://smart.servier.com (SMART Sevier Medical Art) and were applied to prepare Figure 1 and Figure 2, respectively.
Footnotes
Conflict of interest
There is no conflict of interest.
References
- BIKLE DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21:319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTAKOS S, DHAWAN P, VERSTUYF A, VERLINDEN L, CARMELIET G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96:365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLONESE F, LAGANÀ AS, COLONESE E, SOFO V, SALMERI FM, GRANESE R, TRIOLO O. The pleiotropic effects of vitamin D in gynaecological and obstetric diseases: an overview on a hot topic. Biomed Res Int. 2015;2015;986281 doi: 10.1155/2015/986281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELUCA HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689–1696. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- DRAVECKÁ I, FIGUROVÁ J, JAVORSKÝ M, PETRÍKOVÁ J, VAL’KOVÁ M, LAZÚROVÁ I. The effect of alfacalcidiol and metformin on phenotype manifestations in women with polycystic ovary syndrome - a preliminary study. Physiol Res. 2016;65:815–822. doi: 10.33549/physiolres.933266. [DOI] [PubMed] [Google Scholar]
- ERSOY E, ERSOY AO, YILDIMIR G, BUYUKKAGNICI U, TOKMAK A, YILMAZ N. Vitamin D levels in patients with premature ovarian failure. Ginekol Pol. 2016;87:32–36. doi: 10.17772/gp/57839. [DOI] [PubMed] [Google Scholar]
- GUO H, GUO J, XIE W, YUAN L, SHENG X. The role of vitamin D in ovarian cancer: epidemiology, molecular mechanism and prevention. J Ovarian Res. 2018;11:71. doi: 10.1186/s13048-018-0443-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERIAN M, LUCK MR, GRZESIAK M. The influence of testosterone on the expression and function of vitamin D3 receptor (VDR) protein in the porcine ovarian follicle. Physiol Res. 2018;67:515–519. doi: 10.33549/physiolres.933762. [DOI] [PubMed] [Google Scholar]
- HII CS, FERRANTE A. The non-genomic actions of vitamin D. Nutrients. 2016;8:135. doi: 10.3390/nu8030135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HONG SH, LEE JE, KIM HS, JUNG YJ, HWANG D, LEE JH, YANG SY, KIM SC, CHO SK, AN BS. Effect of vitamin D3 on production of progesterone in porcine granulosa cells by regulation of steroidogenic enzymes. J Biomed Res. 2016;30:203–208. doi: 10.7555/JBR.30.2016K0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HONG SH, LEE JE, AN SM, SHIN YY, HWANG DY, YANG SY, CHO SK, AN BS. Effect of vitamin D3 on biosynthesis of estrogen in porcine granulosa cells via modulation of steroidogenic enzymes. Toxicol Res. 2017;33:49–54. doi: 10.5487/TR.2017.33.1.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRANI M, MERHI Z. Role of vitamin D in ovarian physiology and its implication in reproduction: a systematic review. Fertil Steril. 2014;102:460–468. doi: 10.1016/j.fertnstert.2014.04.046. [DOI] [PubMed] [Google Scholar]
- KEANE KN, CRUZAT VF, CALTON EK, HART PH, SOARES MJ, NEWSHOLME P, YOVICH JL. Molecular actions of vitamin D in reproductive cell biology. Reproduction. 2017;153:R29–R42. doi: 10.1530/REP-16-0386. [DOI] [PubMed] [Google Scholar]
- KEBAPCILAR AG, KULAKSIZOGLU M, KEBAPCILAR L, GONEN MS, UNLÜ A, TOPCU A, DEMIRCI F, TANER CE. Is there a link between premature ovarian failure and serum concentrations of vitamin D, zinc, and copper? Menopause. 2013;20:94–99. doi: 10.1097/gme.0b013e31826015ca. [DOI] [PubMed] [Google Scholar]
- KINUTA K, TANAKA H, MORIWAKE T, AYA K, KATO S, SEINO Y. Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology. 2000;141:1317–1324. doi: 10.1210/endo.141.4.7403. [DOI] [PubMed] [Google Scholar]
- KNAUFF EA, EIJKEMANS MJ, LAMBALK CB, TEN KATE-BOOIJ MJ, HOEK A, BEERENDONK CC, LAVEN JS, GOVERDE AJ, BROEKMANS FJ, THEMMEN AP, De JONG FH, FAUSER BC. Anti-Mullerian hormone, inhibin B, and antral follicle count in young women with ovarian failure. J Clin Endocrinol Metab. 2009;94:786–792. doi: 10.1210/jc.2008-1818. [DOI] [PubMed] [Google Scholar]
- LERCHBAUM E, OBERMAYER-PIETSCH B. Vitamin D and fertility: a systematic review. Eur J Endocrinol. 2012;166:765–778. doi: 10.1530/EJE-11-0984. [DOI] [PubMed] [Google Scholar]
- LORENZEN M, BOISEN IM, MORTENSEN LJ, LANSKE B, JUUL A, BLOMBERG JENSEN M. Reproductive endocrinology of vitamin D. Mol Cell Endocrinol. 2017;453:103–112. doi: 10.1016/j.mce.2017.03.023. [DOI] [PubMed] [Google Scholar]
- LUNDQVIST J. Vitamin D as a regulator of steroidogenic enzymes. F1000Research. 2014;3:155. doi: 10.12688/f1000research.4714.1. [DOI] [Google Scholar]
- MALLOY PJ, PENG L, WANG J, FELDMAN D. Interaction of the vitamin D receptor with a vitamin D response element in the mullerian-inhibiting substance (MIS) promoter: regulation of MIS expression by calcitriol in prostate cancer cells. Endocrinology. 2009;150:1580–1587. doi: 10.1210/en.2008-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERHI Z. Crosstalk between advanced glycation end products and vitamin D: A compelling paradigm for the treatment of ovarian dysfunction in PCOS. Mol Cell Endocrinol. 2019;479:20–26. doi: 10.1016/j.mce.2018.08.010. [DOI] [PubMed] [Google Scholar]
- MERHI ZO, SEIFER DB, WEEDON J, ADEYEMI O, HOLMAN S, ANASTOS K, GOLUB ET, YOUNG M, KARIM R, GREENBLATT R, MINKOFF H. Circulating vitamin D correlates with serum antimüllerian hormone levels in late-reproductive-aged women: Women’s Interagency HIV Study. Fertil Steril. 2012;98:228–234. doi: 10.1016/j.fertnstert.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERHI Z, DOSWELL A, KREBS K, CIPOLLA M. Vitamin D alters genes involved in follicular development and steroidogenesis in human cumulus granulosa cells. J Clin Endocrinol Metab. 2014;99:E1137–E1145. doi: 10.1210/jc.2013-4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIZWICKI MT, KEIDEL D, BULA CM, BISHOP JE, ZANELLO LP, WURTZ JM, MORAS D, NORMAN AW. Identification of an alternative ligand-binding pocket in the nuclear vitamin D receptor and its functional importance in 1α,25(OH)2-vitamin D3 signaling. Proc Natl Acad Sci U S A. 2004;101:12876–12881. doi: 10.1073/pnas.0403606101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONNIAUX D, CLÉMENT F, DALBIÈS-TRAN R, ESTIENNE A, FABRE S, MANSANET C, MONGET P. The ovarian reserve of primordial follicles and the dynamic reserve of antral growing follicles: what is the link? Biol Reprod. 2014;90:85. doi: 10.1095/biolreprod.113.117077. [DOI] [PubMed] [Google Scholar]
- MUSCOGIURI G, ALTIERI B, De ANGELIS C, PALOMBA S, PIVONELLO R, COLAO A, ORIO F. Shedding new light on female fertility: The role of vitamin D. Rev Endocr Metab Disord. 2017;18:273–283. doi: 10.1007/s11154-017-9407-2. [DOI] [PubMed] [Google Scholar]
- PANDA DK, MIAO D, TREMBLAY ML, SIROIS J, FAROOKHI R, HENDY GN, GOLTZMAN D. Targeted ablation of the 25-hydroxyvitamin D 1alpha-hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci U S A. 2001;98:7498–7503. doi: 10.1073/pnas.131029498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARIKH G, VARADINOVA M, SUWANDHI P, ARAKI T, ROSENWAKS Z, PORETSKY L, SETO-YOUNG D. Vitamin D regulates steroidogenesis and insulin-like growth factor binding protein-1 (IGFBP-1) production in human ovarian cells. Horm Metab Res. 2010;42:754–757. doi: 10.1055/s-0030-1262837. [DOI] [PubMed] [Google Scholar]
- PITTAS AG, LAU J, HU FB, DAWSON-HUGHES B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOLIKOVA K, MLYNARCIKOVA A, SCSUKOVA S. Effect of 1α,25-dihydroxyvitamin D3 on progesterone secretion by porcine ovarian granulosa cells. Endocr Regul. 2013;47:123–131. doi: 10.4149/endo_2013_03_123. [DOI] [PubMed] [Google Scholar]
- SHAHROKHI SZ, GHAFFARI F, KAZEROUNI F. Role of vitamin D in female reproduction. Clin Chim Acta. 2016;455:33–38. doi: 10.1016/j.cca.2015.12.040. [DOI] [PubMed] [Google Scholar]
- SUN T, ZHAO Y, MANGELSDORF DJ, SIMPSON ER. Characterization of a region upstream of exon I.1 of the human CYP19 (aromatase) gene that mediates regulation by retinoids in human choriocarcinoma cells. Endocrinology. 1998;139:1684–1691. doi: 10.1210/endo.139.4.5959. [DOI] [PubMed] [Google Scholar]
- SUNG CC, LIAO MT, LU KC, WU CC. Role of vitamin D in insulin resistance. J Biomed Biotechnol. 2012;2012;634195 doi: 10.1155/2012/634195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VISSER JA, De JONG FH, LAVEN JS, THEMMEN AP. Anti-Müllerian hormone: a new marker for ovarian function. Reproduction. 2006;131:1–9. doi: 10.1530/rep.1.00529. [DOI] [PubMed] [Google Scholar]
- WOJTUSIK J, JOHNSON PA. Vitamin D regulates anti-Mullerian hormone expression in granulosa cells of the hen. Biol Reprod. 2012;86:91. doi: 10.1095/biolreprod.111.094110. [DOI] [PubMed] [Google Scholar]
- XU J, HENNEBOLD JD, SEIFER DB. Direct vitamin D3 actions on rhesus macaque follicles in three-dimensional culture: assessment of follicle survival, growth, steroid, and anti-müllerian hormone production. Fertil Steril. 2016;106:1815–1820. doi: 10.1016/j.fertnstert.2016.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAO X, ZHANG G, GUO Y, EI-SAMAHY M, WANG S, WAN Y, HAN L, LIU Z, WANG F, ZHANG Y. Vitamin D receptor expression and potential role of vitamin D on cell proliferation and steroidogenesis in goat ovarian granulosa cells. Theriogenology. 2017;102:162–173. doi: 10.1016/j.theriogenology.2017.08.002. [DOI] [PubMed] [Google Scholar]