Summary
Obesity is a disease that affects about 13 % of the world population (2016) (Who 2018). This condition generates a process of systemic inflammation that may contribute to the release of cell-free DNA (cfDNA) into the bloodstream. cfDNA has been considered a potential biomarker to monitor several physiological and pathological conditions, such as tumors, exercise intensity and obesity. Therefore, the objective of this study was to evaluate the association of cfDNA levels with the amount of weight and fat mass lost six months after bariatric surgery. Thirty-eight subjects classified as obese (BMI, 43.5±6.2; BFP, 46.6±4.8) were evaluated anthropometrically and underwent bariatric surgery. Weight, BMI, body fat percentage (BFP), waist circumference, C-Reactive Protein (CRP) and cfDNA levels were evaluated before and six months after surgery; furthermore, a correlation was performed between cfDNA levels and BFP and CRP. Decrease in total body weight and CRP were observed after bariatric surgery; however, the cfDNA levels remained unchanged. There was a weak correlation between cfDNA levels and BFP before the bariatric surgery, and a moderate correlation between cfDNA and CRP. Obese subjects who underwent bariatric surgery, the decrease in body fat percentage did not result in changes in cfDNA levels six months after surgery.
Keywords: cfDNA, Obesity, Fat Mass
Obesity is a disease that affects about 13 % of the world population (Who 2018). It is characterized by excess body fat due to caloric imbalance (greater intake x less energy expenditure), as well as, by other factors including: genetic, epigenetic, dietary, economic, psychosocial, endocrine disruptor, urban environment temperatures, maternal age, smoking cessation, and pharmacologic factors (Hubácek 2009, McAllister et al. 2009, Dhurandhar et al. 2014). Obesity can be classified by body mass index (BMI) or body fat percentage (BFP) (Pi-Sunyer 2000, De Lorenzo et al. 2016). According to BMI, obesity may be classified as degree 1 (BMI>30), degree 2 (BMI>35), and degree 3 (BMI>40) (Pi-Sunyer 2000). Another method of classification is the BFP, where values >25 % in men and >33 % in women are classified as obese (Bray 1999).
Increased body fat may increase the levels of proinflammatory cytokines, resulting in a state of chronic inflammation leading to the process of apoptosis and cellular necrosis. (Schmidt et al. 2005, Alkhouri et al. 2010, Haghiac et al. 2012). This process can negatively influence the health of the individual and impart a high risk in the development of diverse diseases (Schmidt et al. 2005). Active lifestyle and healthy eating are strategies used to combat this growing epidemic; however, they have modest success rates (Lagerros and Rössner 2013). Thus, patients undergo to bariatric surgery as an effective, both at short- and long-term strategy, for the control of severe obesity.
Cell-free DNA (cfDNA) has received much attention as a potential biomarker for monitoring both physiological and pathological conditions. Physiologically low levels of cfDNA are detected in the plasma of healthy individuals (Haghiac et al. 2012, Andreatta et al. 2017, Ferrandi et al. 2018). However, the increase in cfDNA occurs due to a variety of acute and chronic pathological conditions such as cancer, autoimmune diseases, sepsis, stroke, myocardial infarction, and trauma, as reported in the literature (Sandquist and Wong 2014, Volckman et al. 2018, Ferrandi et al. 2018, Xu et al. 2018). cfDNA is released into plasma by several cells including neutrophils, monocytes, eosinophils and tumor cells and may originate from apoptosis and necrosis (thereby reflecting the degree of cellular damage) or be deliberately released (Leite et al. 2017, Ferrandi et al. 2018).
An association between cfDNA levels in obese individuals and BMI and visceral fat has been suggested in the literature (Nishimoto et al., 2016). The process of systemic inflammation associated with metabolic dysfunction that occurs in obese subjects may contribute to the release of cfDNA into the bloodstream (Haghiac et al. 2012, Nishimoto et al. 2016). Thus, to further understand this association, we monitored cfDNA levels and fat loss for 6 months in patients that underwent bariatric surgery.
Thirty-eight obese adult volunteers from the Bariatric and Metabolic Surgery Program of the University Hospital Cassiano Antônio Moraes (HUCAM) were included in this study. Twenty-five volunteers received the gastric bypass surgery and thirteen volunteers received the sleeve gastrectomy surgery. No significant differences were observed between the volunteers characteristics from each surgery before the procedure (PRE) for age, weight, BMI, % lean mass, BFP, and waist circumference. All volunteers were able to receive either gastric bypass or sleeve gastrectomy surgery, according to the criteria of the program (BMI>35, age 18–60 years). All the procedures performed in this study followed the Brazilian National Health Council guidelines for studies with humans in accordance with resolution 466/2012. Informed consent was obtained from all individual participants included in the study. The following exclusion criteria were adopted: a) pregnant women, b) patients with pacemakers, c) indwelling metallic structures and d) silicone prostheses (contraindicated for performing the electrical bioimpedance).
Participants were assessed before undergoing the bariatric surgery (PRE) and 6 months after the surgery (6 MO). The body mass index (BMI) was calculated by the formula weight/height2 (weight in kg; height in meters) and the BMI classification was established according to the World Health Organization (WHO). Body composition was evaluated by means of the electrical bioimpedance, using the Biodynamics Equipment Model 450 according to the European Society for Clinical Nutrition and Metabolism Guidelines (Kyle et al. 2004). The values obtained were used to calculate the % lean and BFP of each participant. Prior to the evaluation, participants were instructed to maintain an eight-hour fast and not to engage in physical activity, and women should not be in their menstrual period.
Serum samples were collected before (PRE) and 6 months (6 MO) after the bariatric surgery. Approximately 4 ml of blood was collected in tubes without anticoagulants. The blood samples were centrifuged at 10 000× g for 10 min at 4 °C and the serum was stored at −80 °C. Analysis of cfDNA was assessed fluorometrically through the Synergy Hybrid Multi-Mode Reader (BioTek Instruments) using SYBR® Gold Nucleic Acid Stain (catalog number: S11494, Thermo Fisher Scientific) as previously published by our group (Andreatta et al. 2017). Values were expressed as ng/μl. Serum ultrasensitive CRP was measured by a trained technician by an immunoturbidimetric method.
Values are expressed as the mean ± standard deviation (mean ± SD) for all variables. Characteristics of participants were compared by the unpaired t-test and 95 % confidence intervals (95 % CI) were calculated. The paired t-test was performed to evaluate the effect of surgery on BMI, BFP, and waist circumference after 6 months. A Shapiro-Wilk normality test was performed to evaluate whether the variables assumed a Gaussian distribution. Then, a Spearman’s correlation was performed for the non-parametric variables. The value of p≤0.05 was considered statistically significant.
Characteristics of the participants included in the study are shown in Table 1. As we evaluated patients who underwent either gastric bypass or sleeve gastrectomy surgeries, we first compared the characteristics between groups. No significant differences were observed between groups before the surgery for age, weight, BMI, % lean mass, BFP, waist circumference and CRP. Next, we evaluated the effectiveness of both gastric bypass and sleeve gastrectomy surgeries to reduce obesity. Table 2 shows the effect of both surgeries on the parameters of obesity before surgery and 6 months after surgery. After 6 months, both bariatric surgeries were able to reduce total weight levels compared to PRE values (gastric bypass, 26.5 %; sleeve gastrectomy, 22.2 %), BMI (gastric bypass, 27 %; sleeve gastrectomy, 22.2 %;), BFP (gastric bypass, 15.3 %; sleeve gastrectomy, 13.3 %), waist circumference values (gastric bypass, 18.2 %; sleeve gastrectomy, 18.2 %) and C-Reactive Protein levels (gastric bypass, 64.5 %; sleeve gastrectomy, 71.7 %). Since similar results were observed in both surgeries, they were considered as one group for further analysis.
Table 1.
Characteristic of the participants.
| Gastric bypass | Sleeve gastrectomy | p value | |||
|---|---|---|---|---|---|
| n=25 | 95% CI | n=13 | 95% CI | ||
| Sex, % | |||||
| Women | 80 % | 84.6 % | |||
| Men | 20 % | 15.4 % | |||
| Age, years | 41.2 ± 7.8 | 38 – 44.4 | 43.2 ± 5.3 | 40.0 – 46.4 | 0.404 |
| Weight, kg | 114.7 ± 19.2 | 106.8 – 122.6 | 107.8 ± 17.3 | 97.3 – 118.2 | 0.279 |
| BMI | 43.7 ± 5.9 | 41.2 – 46.1 | 41.0 ± 4.9 | 38.0 – 43.9 | 0.167 |
| % Lean mass | 54.0 ± 4.9 | 51.9 – 56.1 | 53.4 ± 5.1 | 50.4 – 56.5 | 0.724 |
| BFP | 45.9 ± 4.9 | 43.9 – 48.0 | 46.5 ± 5.0 | 43.4 – 49.5 | 0.766 |
| Waist circumference, cm | 121.6 ± 14.8 | 115.4 – 127.9 | 122.5 ± 10.5 | 115.4 – 129.6 | 0.581 |
| C-reactive protein, mg/l | 11.0 ± 8.8 | 7.4 – 14.6 | 9.2 ± 7.4 | 4.2 – 14.1 | 0.423 |
Characteristic of the participants at baseline (PRE) of Gastric bypass and Sleeve gastrectomy surgeries. BMI, body mass index. Data are expressed as mean ± SD. A Shapiro-Wilk normality test was performed to evaluate whether the variables assumed a Gaussian distribution. Characteristics of participants were compared by the unpaired t-test and 95 % confidence intervals (95 % CI) were calculated. p<0.05 indicates statistical difference.
Table 2.
Effect of bariatric surgery on obesity parameters.
| Gastric bypass | Sleeve gastrectomy | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PRE | 95 % CI | 6 MO | 95 % CI | p value | PRE | 95 % CI | 6 MO | 95 % CI | p value | |
| Weight, kg | 114.7±19.2 | 106.8–122.6 | 84.3±16.5 | 77.5–91.1 | <0.005 | 107.8±17.3 | 97.3–118.2 | 83.9±14.9 | 74.9–92.9 | <0.005 |
| BMI | 43.7±5.9 | 41.2 – 46.1 | 31.9±5.4 | 29.7–34.2 | <0.005 | 41.0±4.9 | 38.0–43.9 | 31.9±4.0 | 29.5–34.3 | <0.005 |
| BFP | 45.9±4.9 | 43.9–48.0 | 38.9±5.7 | 36.5–41.2 | <0.005 | 46.5±5.0 | 43.4–49.5 | 40.3±6.4 | 36.4–44.2 | <0.005 |
| Waist circumference, cm | 121.6±14.8 | 115.4–127.9 | 99.6±15.4 | 93.1–106.1 | <0.005 | 122.5±10.5 | 115.4–129.6 | 100.2±13.2 | 91.4–109.1 | <0.005 |
| C-reactive protein, mg/l | 11.0±8.8 | 7.4–14.6 | 3.9±5.7 | 1.6–6.3 | <0.005 | 9.2±7.4 | 4.2–14.1 | 2.6±2.1 | 1.7–4.0 | <0.005 |
Effect of Gastric bypass (n=25) and Sleeve gastrectomy (n=13) surgery on obesity parameters after 6 months. BMI, body mass index. Data are expressed as mean ± SD. A Shapiro-Wilk normality test was performed to evaluate whether the variables assumed a Gaussian distribution. The paired t-test and 95 % confidence intervals (95 % CI) was performed to evaluate the effect of surgery on BMI, BFP, waist circumference and C-Reactive Protein after 6 months. p<0.05 indicates the difference between PRE and 6 MO surgical.
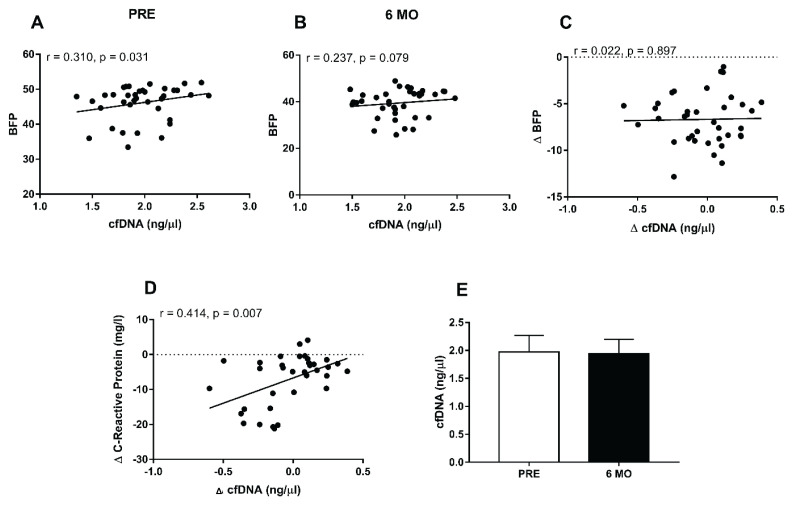
Next, we studied the correlation between cfDNA levels and BFP. Spearman’s correlation was performed with the cfDNA levels and BFP before bariatric surgery (Fig. 1A) and 6 months after the bariatric surgery (Fig. 1B). Weak correlation for cfDNA levels and BFP was observed before the surgery (Fig. 1A, r=0.310, p=0.031) while non-significant correlation was observed after 6 months (Fig. 1B, r=0.237, p=0.079). We next evaluated whether the changes in BFP correlate with changes in cfDNA levels. Figure 1C shows negligible and non-significant correlation (r=0.022, p=0.897). However, when evaluated whether the changes in CRP correlate with changes in cfDNA levels, a moderate correlation was observed (Fig. 1D, r=0.414, p=0.007). Furthermore, although bariatric surgeries were able to reduce body weight, BFP and CRP, cfDNA levels remained unchanged 6 months after bariatric surgery when compared to PRE. (Fig. 1E, p=0.578).
Fig. 1.
cfDNA levels and correlation with BFP and C-Reactive Protein. Correlation between cfDNA levels and (A) BFP at the time PRE bariatric surgery, (B) BFP 6 months after bariatric surgery, (C) Δ BFP, (D) Δ C-Reactive Protein and (E) cfDNA levels at PRE vs. 6 MO after bariatric surgery. Gastric bypass (n=25) and Sleeve gastrectomy surgeries (n=13). PRE, before the bariatric surgery; POS, after 6 months of bariatric surgery. Data are expressed as mean ± SD. A Shapiro-Wilk normality test was performed to evaluate whether the variables assumed a Gaussian distribution. Then, a Spearman’s correlation was performed for the non-parametric variables. p<0.05 indicates a statistical difference.
There are few data in the literature related to cfDNA levels and obesity. To our knowledge, we are the first group to evaluate obese subjects who underwent bariatric surgery, as well as to monitor the cfDNA levels 6 months after the surgical procedure. We showed that bariatric surgery was able to decrease the body weight, BMI, BFP, and CRP but the cfDNA levels were not altered.
It is reported in the literature that obesity causes an imbalance in adipocyte proliferation and hypertrophy. Systemic inflammation is the main factor in the pathology of obesity and complications related to obesity. The adipose tissue of obese subjects may suffer necrosis or apoptosis depending on the stimulus (Haghiac et al. 2012). Both mechanisms have been suggested as the main factors in the release of cfDNA in obese subjects. Nishimoto et al. (2016) have shown that obesity-related adipocyte degeneration causes release of cfDNA, which promotes the accumulation of macrophages in adipose tissue via Toll-like receptor 9 (TLR9), originally known as a sensor of exogenous DNA fragments. However, the cfDNA released in obese subjects may play, at least partially, a causal role in the development of inflammation of adipose tissue via TLR9 (Nishimoto et al. 2016).
Haghiac et al. (2012) showed that obese women who had an increase in body weight during pregnancy presented hyperleptinemia, hyperinsulinemia, and increased plasma concentrations of IL-6, which are characteristic of metabolic inflammation. In addition, the morphological changes associated with necrosis and apoptosis in the adipose tissue were higher in obese women. In this study a positive correlation between the cfDNA levels, BMI, and gestational weight gain was also observed. The authors suggest that such results indicate an association between increased fat mass of obese women and total cfDNA released into the circulation (Haghiac et al. 2012).
Another study developed by Nishimoto et al. (2016) also found an increase in cfDNA levels in mice administered a diet to induce obesity as in obese humans (visceral fat area ≥100 cm2). Elevated cfDNA levels in the circulating blood of obese subjects were positively associated with visceral adipose tissue weight in mice and visceral fat area in humans. We also found a weak positive correlation between cfDNA levels and BFP, however, cfDNA levels did not change 6 months after bariatric surgery.
In summary, our results confirm previous data in the literature showing weak correlation between cfDNA levels and BFP. In addition we shoed for the first time a moderate correlation between cfDNA levels and CRP and also that decrease in BFP or CRP does not result in changes in cfDNA levels.
Acknowledgements
This study was supported by the Fundação de Amparo a Pesquisa do Espirito Santo (grant #151/2019 – Universal/FAPES and grant #85198560) and Universdade Federal do Espírito Santo (UFES). Valerio G. Barauna was supported by Conselho Nacional de Pesquisa e Tecnologia (CNPq fellowship).
Footnotes
Conflict of interest
There is no conflict of interest.
References
- ALKHOURI N, GORNICKA A, BERK MP, THAPALIYA S, DIXON LJ, KASHYAP S, SCHAUER PR, FELDSTEIN AE. Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. J Biol Chem. 2010;285:3428–3438. doi: 10.1074/jbc.m109.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDREATTA MV, CURTY VM, COUTINHO JVS, SANTOS MÂA, VASSALLO PF, De SOUSA NF, BARAUNA VG. cfDNA as an earlier predictor of exercise-induced performance decrement related to muscle damage. Int J Sports Physiol Perform. 2017. pp. 1–14. [DOI] [PubMed]
- BLÜHER M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288–298. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- BRAY GA. Etiology and pathogenesis of obesity. Clin Cornerstone. 1999;2:1–15. doi: 10.1016/s1098-3597(99)90001-7. [DOI] [PubMed] [Google Scholar]
- DHURANDHAR EJ, KEITH SW. The etiology of obesity beyond eating more and exercising less. Best Pract Res Clin Gastroenterol. 2014;28:533–544. doi: 10.1016/j.bpg.2014.07.001. [DOI] [PubMed] [Google Scholar]
- FERRANDI PJ, FICO BG, WHITEHURST M, ZOURDOS MC, BAO F, DODGE KM, RODRIGUEZ AL, PENA G, HUANG CJ. Acute high-intensity interval exercise induces comparable levels of circulating cell-free DNA and Interleukin-6 in obese and normal-weight individuals. Life Sci. 2018;202:161–166. doi: 10.1016/j.lfs.2018.04.007. [DOI] [PubMed] [Google Scholar]
- HAGHIAC M, VORA NL, BASU S, JOHNSON KL, PRESLEY L, BIANCHI DW, HAUGUEL-De MOUZON S. Increased death of adipose cells, a path to release cell-free DNA into systemic circulation of obese women. Obesity (Silver Spring) 2012;20:2213–2219. doi: 10.1038/oby.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBÁCEK JA. Eat less and exercise more - is it really enough to knock down the obesity pandemia? Physiol Res. 2009;58(Suppl 1):S1–S6. doi: 10.33549/physiolres.931855. [DOI] [PubMed] [Google Scholar]
- KYLE UG, BOSAEUS I, De LORENZO AD, DEURENBERG P, ELIA M, MANUEL GÓMEZ J, LILIENTHAL HEITMANN B, KENT-SMITH L, MELCHIOR J-C, PIRLICH M, SCHARFETTER H, SCHOLS MWJA, PICHARD C, ESPEN Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr. 2004;23:1430–1453. doi: 10.1016/j.clnu.2004.09.012. [DOI] [PubMed] [Google Scholar]
- LAGERROS YT, RÖSSNER S. Obesity management: what brings success? Therap Adv Gastroenterol. 2013;6:77–88. doi: 10.1177/1756283x12459413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEITE F, LEITE Â, SANTOS A, LIMA M, BARBOSA J, COSENTINO M, RIBEIRO L. Predictors of subclinical inflammatory obesity: plasma levels of leptin, very low-density lipoprotein cholesterol and CD14 expression of CD16+ monocytes. Obes Facts. 2017;10:308–322. doi: 10.1159/000464294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De LORENZO A, SOLDATI L, SARLO F, CALVANI M, Di LORENZO N, Di RENZO L. New obesity classification criteria as a tool for bariatric surgery indication. World J Gastroenterol. 2016;22:681–703. doi: 10.3748/wjg.v22.i2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McALLISTER EJ, DHURANDHAR NV, KEITH SW, ARONNE LJ, BARGER J, BASKIN M, BENCA RM, BIGGIO J, BOGGIANO MM, EISENMANN JC, ELOBEID M, FONTAINE KR, GLUCKMAN P, HANLON EC, KATZMARZYK P, PIETROBELLI A, REDDEN DT, RUDEN DM, WANG C, WATERLAND RA, WRIGHT SM, ALLISON DB. Ten putative contributors to the obesity epidemic. Crit Rev Food Sci Nutr. 2009;49:868–913. doi: 10.1080/10408390903372599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIMOTO S, FUKUDA D, HIGASHIKUNI Y, TANAKA K, HIRATA Y, MURATA C, KIM-KANEYAMA J, SATO F, BANDO M, YAGI S, SOEKI T, HAYASHI T, IMOTO I, SAKAUE H, SHIMABUKURO M, SATA M. Obesity-induced DNA released from adipocytes stimulates chronic adipose tissue inflammation and insulin resistance. Sci Adv. 2016;2:1–11. doi: 10.1126/sciadv.1501332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PI-SUNYER FX. Obesity: criteria and classification. Proc Nutr Soc. 2000;59:505–509. doi: 10.1017/s0029665100000732. [DOI] [PubMed] [Google Scholar]
- SANDQUIST M, WONG HR. Biomarkers of sepsis and their potential value in diagnosis, prognosis and treatment. Expert Rev Clin Immunol. 2014;10:1349–1356. doi: 10.1586/1744666X.2014.949675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMIDT MI, SAAD MJA, DUNCAN BB. Subclinical inflammation and obesity, diabetes and related disorders. Drug Discov Today Dis Mech. 2005;2:307–312. doi: 10.1016/j.ddmec.2005.08.003. [DOI] [Google Scholar]
- VOLCKMAR AL, SÜLTMANN H, RIEDIGER A, FIORETOS T, SCHIRMACHER P, ENDRIS V, STENZINGER A, DIETZ S. A field guide for cancer diagnostics using cell-free DNA: From principles to practice and clinical applications. Genes Chromosomes Cancer. 2018;57:123–139. doi: 10.1002/gcc.22517. [DOI] [PubMed] [Google Scholar]
- WHO, World Health Organization. [accessed 22 March 2019];Obesity and overweight. 2018 [Google Scholar]
- XU Y, SONG Y, CHANG J, ZHOU X, QI Q, TIAN X, LI M, ZENG X, XU M, ZHANG W, CRAM DS, LIU J. High levels of circulating cell-free DNA are a biomarker of active SLE. Eur J Clin Invest. 2018;48:1–10. doi: 10.1111/eci.13015. [DOI] [PubMed] [Google Scholar]