Summary
Background
Given the importance of flexible use of different COVID-19 vaccines within the same schedule to facilitate rapid deployment, we studied mixed priming schedules incorporating an adenoviral-vectored vaccine (ChAdOx1 nCoV-19 [ChAd], AstraZeneca), two mRNA vaccines (BNT162b2 [BNT], Pfizer–BioNTech, and mRNA-1273 [m1273], Moderna) and a nanoparticle vaccine containing SARS-CoV-2 spike glycoprotein and Matrix-M adjuvant (NVX-CoV2373 [NVX], Novavax).
Methods
Com-COV2 is a single-blind, randomised, non-inferiority trial in which adults aged 50 years and older, previously immunised with a single dose of ChAd or BNT in the community, were randomly assigned (in random blocks of three and six) within these cohorts in a 1:1:1 ratio to receive a second dose intramuscularly (8–12 weeks after the first dose) with the homologous vaccine, m1273, or NVX. The primary endpoint was the geometric mean ratio (GMR) of serum SARS-CoV-2 anti-spike IgG concentrations measured by ELISA in heterologous versus homologous schedules at 28 days after the second dose, with a non-inferiority criterion of the GMR above 0·63 for the one-sided 98·75% CI. The primary analysis was on the per-protocol population, who were seronegative at baseline. Safety analyses were done for all participants who received a dose of study vaccine. The trial is registered with ISRCTN, number 27841311.
Findings
Between April 19 and May 14, 2021, 1072 participants were enrolled at a median of 9·4 weeks after receipt of a single dose of ChAd (n=540, 47% female) or BNT (n=532, 40% female). In ChAd-primed participants, geometric mean concentration (GMC) 28 days after a boost of SARS-CoV-2 anti-spike IgG in recipients of ChAd/m1273 (20 114 ELISA laboratory units [ELU]/mL [95% CI 18 160 to 22 279]) and ChAd/NVX (5597 ELU/mL [4756 to 6586]) was non-inferior to that of ChAd/ChAd recipients (1971 ELU/mL [1718 to 2262]) with a GMR of 10·2 (one-sided 98·75% CI 8·4 to ∞) for ChAd/m1273 and 2·8 (2·2 to ∞) for ChAd/NVX, compared with ChAd/ChAd. In BNT-primed participants, non-inferiority was shown for BNT/m1273 (GMC 22 978 ELU/mL [95% CI 20 597 to 25 636]) but not for BNT/NVX (8874 ELU/mL [7391 to 10 654]), compared with BNT/BNT (16 929 ELU/mL [15 025 to 19 075]) with a GMR of 1·3 (one-sided 98·75% CI 1·1 to ∞) for BNT/m1273 and 0·5 (0·4 to ∞) for BNT/NVX, compared with BNT/BNT; however, NVX still induced an 18-fold rise in GMC 28 days after vaccination. There were 15 serious adverse events, none considered related to immunisation.
Interpretation
Heterologous second dosing with m1273, but not NVX, increased transient systemic reactogenicity compared with homologous schedules. Multiple vaccines are appropriate to complete primary immunisation following priming with BNT or ChAd, facilitating rapid vaccine deployment globally and supporting recognition of such schedules for vaccine certification.
Funding
UK Vaccine Task Force, Coalition for Epidemic Preparedness Innovations (CEPI), and National Institute for Health Research. NVX vaccine was supplied for use in the trial by Novavax.
Introduction
The COVID-19 pandemic that began in 2019 has resulted in more than 5 million deaths to date.1 As of Oct 20, 2021, over 3 billion people globally have received at least one dose of a SARS-COV-2 vaccine, but only 2·8% of people in low-income countries.1 Although there are now 24 different vaccines approved worldwide, manufacturing issues, raw material shortages, and surges in infection have led to supply chain disruption and delays.2, 3, 4 The emergence of new safety concerns with available vaccines led to changes in vaccine deployment policy. In early 2021, multiple countries implemented age restrictions for the ChAdOx1 n-CoV-19 vaccine (AstraZeneca, hereafter referred to as ChAd) after the emergence of vaccine-induced thrombocytopenia and thrombosis, and recent pauses in immunisation of young people with mRNA vaccines due to concerns about myocarditis.5, 6, 7, 8 Such changes have disrupted roll-out plans, and have the potential to do so in future. Additionally, concerns about waning vaccine immunity, and the potential for existing schedules to protect against new SARS-CoV-2 variants of concern have led to questions on the optimisation of vaccine schedules.9
Research in context.
Evidence before this study
Most high-income countries have now vaccinated their adult populations with a primary course of COVID-19 vaccine, but deployment remains low across lower income regions. Heterologous prime-boost schedules are a measure that could enhance deployment flexibility and improve access to vaccines. Evidence to support the use of mixed schedules is rapidly evolving. We searched PubMed for articles published between database inception and Oct 20, 2021 using the terms “(COVID) AND (heterologous) AND (vaccin*) NOT (BCG)” with no language restrictions. An additional search was done of the medRxiv preprint server. There are few randomised controlled trials examining heterologous schedules, but many observational studies. Heterologous schedules studied included: ChAdOx1 nCoV-19 (ChAd)/BNT162b2 (BNT), BNT/ChAd, ChAd/mRNA-1273, Ad26/Ad5, ChAd/BBV152, Coronavac/ChAd, Coronavac/Convidecia, and Ad26/BNT. Where a homologous comparator was included, reactogenicity appeared increased in heterologous boost, but was tolerated. There were no safety concerns identified in any study. Heterologous schedules were immunogenic, with mRNA boost after ChAd prime inducing higher concentrations of neutralising antibodies when compared with homologous ChAd. mRNA boost after ChAd prime induced a T-cell response above that of homologous comparators. Adenoviral-vectored boost after inactivated prime appears to enhance immunogenicity over that of inactivated prime-boost.
Added value of this study
These data are the first from a randomised controlled trial of COVID-19 vaccines of heterologous mRNA boost and protein-subunit boost. We have shown that reactogenicity at boost is consistently increased in heterologous versus homologous schedules of ChAd and mRNA vaccines, but not increased by NVX-CoV2373 (NVX) boost after ChAd or BNT prime. mRNA-1273 as a heterologous boost after ChAd or BNT prime induces a higher binding and neutralising antibody response than either homologous schedule. Heterologous boost with NVX after ChAd prime was superior to homologous ChAd for induction of humoral and cellular immunity. NVX after BNT prime did not meet non-inferiority criteria for binding antibodies when compared with homologous BNT; however, in a non-randomised comparison, binding antibody concentrations were still well above the geometric mean concentration observed following ChAd/ChAd. Decrements in neutralising antibody responses to beta and delta variants of concern were largely conserved across schedules, whereas T-cell responses were not affected.
Implications of all the available evidence
This study adds to the body of evidence that heterologous COVID-19 vaccination is safe, tolerated, and immunogenic. Flexibility of schedules should be considered to improve access to COVID-19 vaccination globally.
Heterologous prime-boost COVID-19 vaccination might be useful in responding to these challenges. Many national immunisation advisory groups implemented this strategy on a pragmatic basis following ChAd-related vaccine-induced thrombocytopenia and thrombosis events before evidence had accrued.10, 11, 12 Since then, a number of studies have shown an acceptable short-term safety profile of ChAd and BNT162b2 (Pfizer–BioNTech, hereafter referred to as BNT) heterologous vaccination, although with some transient increased reactogenicity.13, 14, 15, 16, 17 There is accruing evidence that the use of an mRNA boost after adenoviral-vector prime might enhance humoral and cellular responses against SARS-CoV-2, and that this response translates to efficacy against COVID-19.8, 14, 15, 16, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 The only other available data regarding heterologous schedules comes from preprints that suggest that adenoviral vaccines might be an effective boost for inactivated whole-virion prime.30, 31
To date, there are no published data from randomised controlled trials examining the immunogenicity and safety of heterologous schedules of primary course COVID-19 vaccination with mixed mRNA, and none examining regimes containing a protein-subunit vaccine. NVX-CoV2373 (Novavax, hereafter referred to as NVX), a Matrix-M adjuvanted recombinant nanoparticle spike protein vaccine, has shown safety and efficacy in phase 3 trials and has been submitted to multiple regulatory agencies, including WHO, for licensing.32 This vaccine can be transported and stored at standard refrigeration temperatures, making it particularly suitable for deployment in low-resource settings.33 An mRNA vaccine mRNA-1273 (Moderna, hereafter referred to as m1273) has also shown high efficacy in clinical trials, with an acceptable safety profile, although with appreciable reactogenicity after second dose.34
We have previously reported on the preliminary results of the Com-COV study, a randomised controlled trial of ChAd and BNT heterologous vaccination.8, 13 Here, we present findings from the related Com-COV2 study, a non-inferiority randomised controlled trial examining safety, reactogenicity, and immunogenicity of heterologous COVID-19 regimens including m1273 and NVX as boost vaccines for people who received a first dose of ChAd or BNT in the community COVID-19 vaccination programme in the UK, given after the prime at 8–12 weeks.
Methods
Study design
Com-COV2 is a UK multicentre, single-blinded, randomised, phase 2, non-inferiority study, investigating the safety, reactogenicity, and immunogenicity of heterologous boost COVID-19 vaccine schedules. Recruitment occurred at nine National Health Service and academic institutions in England (appendix p 4). The trial was reviewed and approved by the South-Central Berkshire Research Ethics Committee (21/SC/0119), the University of Oxford, and the Medicines and Healthcare products Regulatory Agency.
Most participants were enrolled into the general cohort while a subset (n=150, selected on the basis of site capacity and participant availability) were enrolled into an immunology cohort that had mucosal and salivary samples collected, along with two additional blood tests to explore the kinetics of the immune responses.
We report the primary endpoint results, and reactogenicity profile from the day 28 visit. Safety data were collected up until Oct 5, 2021. The protocol is provided in the appendix (pp 45–123), and online.
Participants
The study inclusion criteria were being aged 50 years or older and having received a single dose of either ChAd or BNT by routine immunisation 8–12 weeks earlier. Important exclusion criteria were history of confirmed SARS-CoV-2 infection, comorbidities that were considered severe or poorly controlled, anaphylaxis or allergy to a vaccine component, pregnancy, or intent to conceive, breastfeeding, and use of anticoagulants. Full details of the inclusion and exclusion criteria can be found in the protocol (appendix pp 69–71).
Randomisation and masking
Computer generated randomisation lists were prepared by the study statistician. Participants were randomised (1:1:1) to receive a single dose of either the same vaccine as their prime dose (BNT or ChAd homologous schedule), m1273, or NVX (heterologous schedules).
Randomisation was done by random block sizes of three and six. Randomisation was stratified by study site, cohort (immunology or general), and prime vaccine (ChAd/BNT). Clinical research nurses who were not involved in endpoint evaluation did the randomisation using REDCap version 10.6.13 and prepared and administered the vaccine.
Participants were masked to boost vaccine allocation at enrolment. Blinding was maintained by completing pages and by preparing vaccines out of sight of participants, and use of the same syringe type across vaccines, with the drawn volume concealed by applying a masking tape. Following the introduction of COVID-19 vaccine certification in the UK, the study was amended on June 21, 2021, to allow individual participant unblinding where necessary to prevent disadvantage in accessing facilities or travel. This took place after all participants had completed the 28 day postvaccination diary monitoring period. Staff involved in study delivery, including in the assessment of adverse events, were aware of vaccine allocation. Laboratory staff processing the immunology samples remained blinded to vaccine allocation.
Procedures
Participants meeting inclusion and exclusion criteria at the online screening (and additional subsequent telephone screening for some participants) were invited to attend a screening and enrolment visit (day 0). Participants who met final eligibility criteria and provided written informed consent were randomly assigned to a study group and vaccinated at the day 0 visit. Baseline haematological and biochemical blood tests were taken before vaccination at day 0.
Four vaccines were used in this study. ChAd was given as a 0·5 mL intramuscular injection into the upper arm. BNT was given as a 0·3 mL intramuscular injection into the upper arm. m1273 was given as a 0·5 mL intramuscular injection into the upper arm, and NVX as a 0·5 mL intramuscular dose injection into the upper arm.
Participants were observed for at least 15 min after vaccination. During the day 0 visit, participants were given an oral thermometer, tape measure, and diary card (electronic or paper) to record solicited, unsolicited, and medically attended adverse events with instructions. Study-site physicians reviewed the diary card regularly to identify, clinically action, and record adverse events, adverse events of special interest, and serious adverse events, with additional recording of events that were not entered into the diaries collected in person at study visits. The follow-up visit schedule can be found in the protocol (appendix pp 116–17).
Participants were instructed to notify trial teams if they received a positive SARS-CoV-2 test in the community. They were then invited for a study visit to allow for clinical assessment, collection of blood samples, and a nasopharyngeal swab, and were asked to complete a COVID-19 symptom diary for 7 days.
Sera were analysed at Nexelis (Laval, QC, Canada) to determine SARS-CoV-2 anti-spike IgG concentrations by ELISA (reported as ELISA laboratory units [ELU]/mL) and the 50% neutralising antibody titre (NT50) for SARS-CoV-2 pseudotype virus neutralisation assay, using a vesicular stomatitis virus backbone adapted to bear the 2019-nCOV SARS-CoV-2 spike protein.35 The conversion factors to international standard units can be found in the appendix (pp 3–4). Sera from day 0 were analysed at Porton Down, Public Health England, by ECLIA (Cobas platform, Roche Diagnostics) to determine anti-SARS-CoV-2 nucleocapsid IgG status (reported as negative if below a cutoff index of 1·0). The samples from the immunology cohort (n=25) were also tested at Porton Down, UK Health Security Agency to measure the normalised 80% neutralising antibody titre (NT80) for live SARS-CoV-2 virus (lineage Victoria/01/2020) by microneutralisation assays.35 For participants from five of nine selected sites, IFN-γ secreting T cells specific to whole spike protein epitopes, designed based on the Wuhan-Hu-1 sequence (YP_009724390.1), were detected using a modified T-SPOT-Discovery test done at Oxford Immunotec (Abingdon, UK) within 32 h of venepuncture, using the addition of T-Cell Xtend reagent to extend peripheral blood mononuclear cell (PBMC) survival. Participants at the other four sites did not have these taken as their sites were too far from the processing laboratory to enable sample integrity. T-cell frequencies were reported as spot forming cells (SFC) per 250 000 PBMCs with a lower limit of detection of one in 250 000 PBMCs. Intracellular cytokine staining was done on cryopreserved PBMCs, stimulated for 16 h with SARS-CoV-2 antigens (based on the Wuhan-Hu-1 sequence). Cells were stained with viability dye and fluorochrome-conjugated antibodies to CD3, CD4, and CD8 and further stained with fluorochrome-conjugated antibodies specific to IFN-γ, TNF-α, IL-2, IL-4, IL-5, and IL-13. Data are presented as percentage of CD4 or CD8 T cells expressing specific cytokines.36
Samples collected at day 28 after boost immunisation from a subgroup of seronegative participants (roughly 50 per arm) were selected pragmatically to test immunogenicity against Victoria/01/2020 (representative of wild-type), beta, and delta variants. Cryopreserved samples were tested against full spike protein from these variants using ELISpot at Oxford Immunotec. Microneutralisation assays to determine 50% focus reduction neutralisation titres (FRNT50) for live SARS-CoV-2 virus lineages (Victoria/01/2020, beta variant B.1.351, delta variant B.1.617.1) were done at the University of Oxford, Oxford, UK. The reduction in the number of infected foci is compared with a negative control well without an antibody.37
Outcomes
The primary outcome was non-inferiority of serum SARS-CoV-2 anti-spike IgG concentration 28 days after heterologous boost in comparison with homologous boost (the geometric mean ratio [GMR]) in participants who were seronegative for SARS CoV-2 nucleocapsid IgG at enrolment.
Secondary outcomes included safety and reactogenicity, measured through local and systemic solicited adverse events for 7 days after the boost, unsolicited adverse events for 28 days after the boost, medically attended adverse events up to 3 months after boost, and adverse events of special interest and serious adverse events throughout the study. Haematological and biochemical blood parameters were measured at day 0 and day 28 after the boost for all participants, and additionally at day 7 for those in the immunology cohort. Full definitions of safety outcomes can be found in the protocol (appendix pp 95–103). Immunological secondary outcomes include kinetics of SARS-CoV-2 anti-spike binding IgG concentration, live virus neutralisation titres, pseudotype virus neutralisation titres, cellular responses (measured by IFNγ ELISpot) in peripheral blood, and intracellular cytokines. The full list of outcome measures can be found in the protocol (appendix pp 66–67).
Statistical analysis
The primary analysis of SARS-CoV-2 anti-spike IgG was carried out on a per-protocol basis. The per-protocol population consisted of participants who were seronegative for SARS-CoV-2 at baseline (defined by anti-nucleocapsid IgG negativity), had no confirmed SARS-CoV-2 infection within 14 days after boost vaccination, received the study vaccine as randomly assigned, had primary endpoint data available, and had no protocol deviations, whereas the modified intention-to-treat (mITT) population included the per-protocol population and participants with protocol deviations. The GMR was calculated as the antilogarithm of the difference between the mean of the log10 transformed titre in the heterologous arms and the corresponding homologous arm as the reference adjusting for study site and cohort (general or immunology) in the linear regression model. Results are presented separately for participants primed with ChAd and BNT in the community. We did four primary comparisons and therefore we presented one-sided 98·75% CIs to adjust for multiple testing for the primary outcome. Non-inferiority of a heterologous arm to its corresponding homologous arm was concluded if the lower 98·75% CI of a GMR lay above the non-inferiority margin of 0·63. This margin was chosen after discussion with policy makers and regulatory agencies to allow a sample size consistent with rapid study delivery, while still being close to the WHO criterion of 0·67 for licensing of new vaccines.38 We also presented geometric means, adjusted GMRs, and corresponding two-sided 95% CIs in the mITT population. A heterologous arm was considered superior to its homologous arm if the lower limit of the two-sided 95% CI lies above one, and the homologous boost arm was superior if the upper limit of the two-sided 95% CI lies below one. The analyses for secondary immunological outcome were also carried out among the mITT population. For all immunological outcomes, censored data reported as below the lower limit of detection or lower limit of quantification were imputed with a value equal to half of the threshold before transformation. Correlations between different immunological outcomes were evaluated by Pearson correlation coefficients. Participants who received at least one dose of the study vaccines were included in the safety analysis. The proportion of participants with at least one safety event was reported by vaccine schedule.
The sample size calculation assumed a non-inferiority margin of a 0·63 fold difference between the geometric mean concentration (GMC) in the heterologous and homologous boost arms, a SD of 0·456 on log10 scale, and a true difference in GMC on log10 scale of zero. The study needed to recruit 175 participants in each arm to achieve 90% power at a one-sided 1·25% significance level, after adjusting for an attrition rate of 25% due to baseline SARS-CoV-2 seropositivity or loss to follow-up. Statistical analyses were done using R version 4.1.1 and SAS version 9.4.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
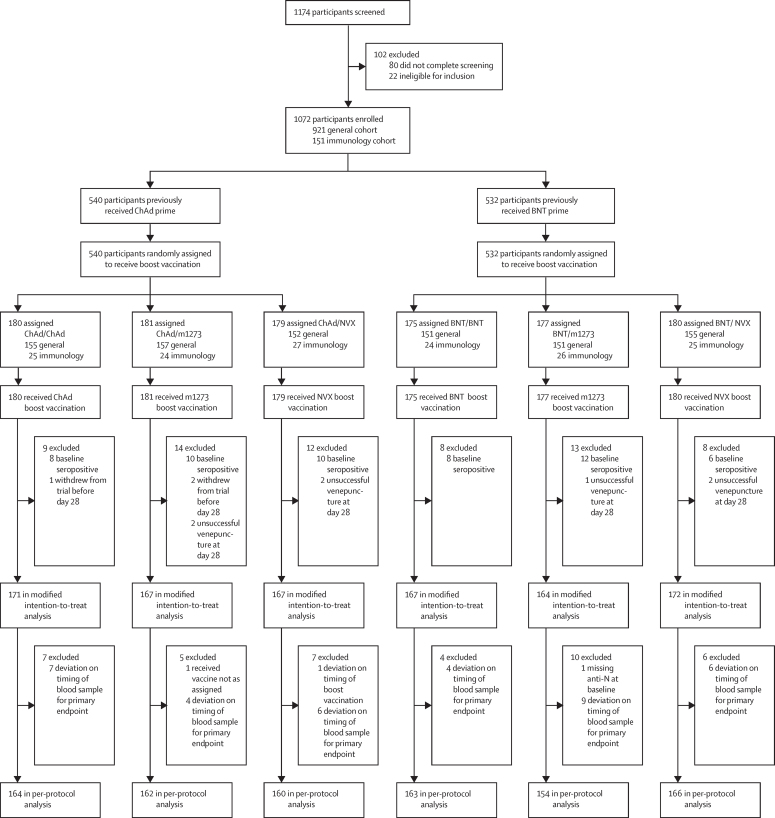
From April 19 to May 14, 2021, 1174 participants across nine study sites in England were screened, with a total of 1072 enrolled and randomly assigned to a study group: 921 to the general cohort and 151 to the immunology cohort (figure 1).
Figure 1.
Trial profile
BNT=BNT162b2 vaccine, Pfizer–BioNTech. ChAd=ChAdOx1 nCoV-19 vaccine, AstraZeneca. m1273=mRNA-1273 vaccine, Moderna. NVX=NVX-CoV2373 vaccine, Novavax.
Recruitment was stratified by community prime vaccines, with 540 participants having received ChAd and 532 BNT. Study site recruitment by community prime is presented in the appendix (p 4). All participants were immunised.
The median age of participants primed with ChAd was 63 years (range 50–75 years), 241 (45%) were female, and 50 (9%) were from minority ethnicities. In participants primed with BNT, the median age was 62 years (range 50–78 years), 210 (40%) were female, and 28 (5%) from minority ethnicities (table 1). There were higher rates of cardiovascular, respiratory, and diabetic comorbidity in the BNT primed groups; reflective of the earlier availability of BNT in the UK and vaccination prioritisation in the initial phases of the national vaccine roll-out. Median prime-boost interval was 9·4 (range 4·7–12·0) weeks for the ChAd community-prime group, and 9·6 (8·0–12·0) weeks for the BNT community-prime group (table 1).
Table 1.
Baseline characteristics by study arm
|
Prime with ChAd |
Prime with BNT |
|||||||
|---|---|---|---|---|---|---|---|---|
| ChAd (n=180) | m1273 (n=181) | NVX (n=179) | Overall (n=540) | BNT (n=175) | m1273 (n=177) | NVX (n=180) | Overall (n=532) | |
| Age | ||||||||
| Mean (SD) | 63·0 (5·51) | 63·3 (5·55) | 63·1 (5·76) | 63·2 (5·60) | 61·9 (5·37) | 62·0 (5·92) | 62·2 (5·56) | 62·0 (5·61) |
| Median (range) | 64·4 (50·1–74·2) | 64·1 (50·2–74·4) | 64·2 (50·1–74·6) | 64·2 (50·1–74·6) | 62·3 (50·4–77·1) | 62·4 (50·0–77·7) | 62·7 (50·2–78·1) | 62·4 (50·0–78·1) |
| Gender | ||||||||
| Female | 87 (48%) | 80 (44%) | 74 (41%) | 241 (45%) | 80 (46%) | 68 (38%) | 62 (34%) | 210 (40%) |
| Male | 93 (52%) | 101 (56%) | 105 (59%) | 299 (55%) | 95 (54%) | 109 (62%) | 118 (66%) | 322 (61%) |
| Ethnicity | ||||||||
| White | 169 (94%) | 159 (88%) | 162 (91%) | 490 (91%) | 166 (95%) | 166 (94%) | 172 (96%) | 504 (95%) |
| Black | 1 (1%) | 1 (1%) | 3 (2%) | 5 (1%) | 3 (2%) | 2 (1%) | 3 (2%) | 8 (2%) |
| Asian | 4 (2%) | 11 (6%) | 9 (5%) | 24 (4%) | 3 (2%) | 5 (3%) | 2 (1%) | 10 (2%) |
| Mixed | 3 (2%) | 7 (4%) | 3 (2%) | 13 (2%) | 1 (1%) | 1 (1%) | 2 (1%) | 4 (1%) |
| Other | 3 (2%) | 3 (2%) | 2 (1%) | 8 (2%) | 2 (1%) | 3 (2%) | 1 (1%) | 6 (1%) |
| Comorbidities* | ||||||||
| Cardiovascular | 49 (27%) | 55 (30%) | 40 (22%) | 144 (27%) | 63 (36%) | 46 (26%) | 57 (32%) | 166 (31%) |
| Respiratory | 15 (8%) | 18 (10%) | 19 (11%) | 52 (10%) | 30 (17%) | 34 (19%) | 31 (17%) | 95 (18%) |
| Diabetes | 9 (5%) | 10 (6%) | 14 (8%) | 33 (6%) | 22 (13%) | 21 (12%) | 24 (13%) | 67 (13%) |
| Prime-boost interval (weeks) | ||||||||
| Mean (SD) | 9·4 (0·96) | 9·5 (0·95) | 9·5 (1·01) | 9·5 (0·97) | 9·5 (0·98) | 9·5 (0·95) | 9·6 (0·96) | 9·5 (0·96) |
| Median (range) | 9·4 (8·0–12·0) | 9·4 (8·0–12·0) | 9·4 (4·7† −11·9) | 9·4 (4·7–12·0) | 9·6 (8·0–11·9) | 9·4 (8·0–12·0) | 9·6 (8·0–11·9) | 9·6 (8·0–12·0) |
Data are n (%), mean (SD), or median (range). BNT=BNT162b2 vaccine, Pfizer–BioNTech. ChAd=ChAdOx1 nCoV-19 vaccine, AstraZeneca. m1273=mRNA-1273 vaccine, Moderna. NVX=NVXCoV2373 vaccine, Novavax.
Comorbidities were self-reported by participants, with review by study team doctor for assessment of severity. General practitioner confirmation was sought where needed. Included severities were those classified as mild, moderate, or well controlled.
Single participant boosted in error at 33 days, protocol deviation.
Within each prime group, characteristics were well balanced between the three randomised arms, except for a male predominance in the BNT/NVX group (34% female; table 1). At baseline, 54 (5%) of 1072 participants were positive for anti-nucleocapsid IgG (cutoff index ≥1·0), with a range of 4–6% in the ChAd-primed groups and 3–7% in the BNT-primed groups (figure 1).
Participants who received a community prime with ChAd had a SARS-CoV-2 anti-spike IgG GMC at 28 days of 20 114 ELU/mL (95% CI 18 160 to 22 279) in the m1273 group, 5597 ELU/mL (4756 to 6586) in the NVX group, and 1971 ELU/mL (1718 to 2262) in the ChAd homologous group (per-protocol analysis; table 2). GMRs in the per-protocol population were 10·2 (one-sided 98·75% CI 8·4 to ∞) for ChAd/m1273 and 2·8 (2·2 to ∞) for ChAd/NVX when compared with ChAd/ChAd, with lower limits of both the CIs above the non-inferiority margin. Similar GMRs were observed in the mITT population, and the SARS-CoV-2 anti-spike IgG response to ChAd/m1273 and ChAd/NVX were both statistically superior to that of homologous ChAd/ChAd (table 2).
Table 2.
Summary of immunogenicity between heterologous and homologous prime-boost schedules at 28 days post boost
|
Prime with ChAd |
Prime with BNT |
||||||
|---|---|---|---|---|---|---|---|
| ChAd/ChAd | ChAd/m1273 | ChAd/NVX | BNT/BNT | BNT/m1273 | BNT/NVX | ||
| Per-protocol analysis | |||||||
| SARS-CoV-2 anti-spike IgG, ELU/mL | |||||||
| n/N | 163/164 | 162/162 | 158/160 | 159/163 | 153/154 | 163/166 | |
| GMC | 1971 (1718 to 2262) | 20 114 (18 160 to 22 279) | 5597 (4756 to 6586) | 16 929 (15 025 to 19 075) | 22 978 (20 597 to 25 636 | 8874 (7391 to 10 654) | |
| GMR* | Ref | 10·2 (8·4 to ∞) | 2·8 (2·2 to ∞) | Ref | 1·3 (1·1 to ∞) | 0·5 (0·4 to ∞) | |
| Modified intention-to-treat analysis | |||||||
| SARS-CoV-2 anti-spike IgG, ELU/mL | |||||||
| n/N | 170/171 | 167/167 | 165/167 | 163/167 | 163/164 | 169/172 | |
| GMC | 1959 (1704 to 2253) | 20 360 (18 411 to 22 517) | 5440 (4632 to 6390) | 16 838 (14 985 to 18 921) | 23 187 (20 891 to 25 735) | 8913 (7464 to 10 644) | |
| GMR* | Ref | 10·5 (8·9 to 12·3) | 2·8 (2·2 to 3·4) | Ref | 1·3 (1·2 to 1·6) | 0·53 (0·43 to 0·65) | |
| Live virus neutralising antibody (Victoria†), FRNT50 | |||||||
| n/N | 47/171 | 48/167 | 51/167 | 46/167 | 48/164 | 49/172 | |
| GMC | 109 (70 to 168) | 1684 (1313 to 2162) | 432 (301 to 618) | 1501 (1188 to 1896) | 1883 (1546 to 2294) | 1109 (805 to 1529) | |
| GMR* | Ref | 16·9 (10·1 to 28·0) | 4·2 (2·4 to 7·2) | Ref | 1·3 (1·0 to 1·8) | 0·8 (0·6 to 1·2) | |
| Live virus neutralising antibody (Victoria†), normalised NT80 | |||||||
| n/N | 19/171 | 18/167 | 20/167 | 17/167 | 19/164 | 21/172 | |
| GMC | 331 (213 to 514) | 2244 (1737 to 2901) | 630 (398 to 997) | 3216 (2336 to 4427) | 3252 (2416 to 4376) | 868 (494 to 1527) | |
| GMR* | Ref | 7·5 (4·4 to 12·7) | 2·0 (1·0 to 3·9) | Ref | 1·0 (0·6 to 1·6) | 0·3 (0·1 to 0·6) | |
| Pseudotype virus neutralising antibody, NT50 | |||||||
| n/N | 169/171 | 154/167 | 158/167 | 159/167 | 157/164 | 163/172 | |
| GMC | 132 (113 to 154) | 1358 (1182 to 1562) | 473 (399 to 561) | 883 (751 to 1039) | 1260 (1106 to 1436) | 787 (631 to 981) | |
| GMR* | Ref | 10·0 (8·1 to 12·3) | 3·4 (2·7 to 4·3) | Ref | 1·4 (1·2 to 1·7) | 0·9 (0·7 to 1·2) | |
| Cellular response (wild-type), SFC per million PBMCs‡ | |||||||
| n/N | 95/171 | 101/167 | 98/167 | 96/167 | 98/164 | 102/172 | |
| GMC | 45 (34 to 61) | 148 (118 to 187) | 190 (159 to 227) | 49 (39 to 63) | 76 (58 to 99) | 29 (22 to 38) | |
| GMR* | Ref | 3·5 (2·5 to 4·8) | 4·8 (3·6 to 6·6) | Ref | 1·5 (1·1 to 2·2) | 0·6 (0·4 to 0·9) | |
In the per-protocol analysis, data are n/N, GMC (95% CI), and GMR (98·75% CI); in the modified intention-to-treat analysis, data are n/N, GMC (95% CI), and GMR (95% CI). BNT=BNT162b2 vaccine, Pfizer–BioNTech. ChAd=ChAdOx1 nCoV-19 vaccine, AstraZeneca. ELU=ELISA laboratory units. FRNT50=50% focus reduction neutralising antibody titre. GMC=geometric mean concentration. GMR=geometric mean ratio. m1273=mRNA–1273 vaccine, Moderna. NT80=80% neutralising antibody titre. NVX=NVXCoV2373 vaccine, Novavax. NT50=50% neutralising antibody titre. SFC=spot-forming cells. PBMC=peripheral blood mononuclear cell.
GMRs were adjusted for randomisation stratification variables, including study site and cohort, with one-sided 98·75% CIs in per-protocol analyses and were further adjusted for interval between first and second dose and baseline immunogenicity, with two-sided 95% CIs in the modified intention-to-treat analyses; non-inferiority margin is 0·63.
A Wuhan-related strain isolated early in the pandemic from Australia.
Cellular response data were available in around 60% of sites, the rest of the study sites did not collect plasma samples due to logistical challenges.
In those who received BNT community prime, anti-spike IgG GMC was 22 978 ELU/mL (95% CI 20 597 to 25 636) in the m1273 group, 16 929 ELU/mL (15 025 to 19 075) in the BNT homologous group, and 8874 ELU/mL (7391 to 10 654) in the NVX group (per-protocol analysis, table 2). The BNT/m1273 group GMR was 1·3 (one-sided 98·75% CI 1·1 to ∞), with the lower limit of the CI above the non-inferiority margin, and was statistically superior to BNT/BNT. Although BNT/NVX did not meet the non-inferiority criterion at 0·5 (one-sided 98·75% CI 0·4 to ∞) there was an 18-fold rise (95% CI 15 to 21) from baseline (appendix pp 8–10). Similar results were reported for the mITT analysis (table 2).
In the mITT population, the GMRs between the live virus neutralising antibody titres (normalised FRNT50) against the Victoria strain, the live virus neutralising antibody titres (normalised NT80 by microneutralisation assays), and the pseudotype virus neutralising (NT50) antibody titres from the mITT analysis at 28 days after boost are broadly consistent with those of binding antibodies (table 2).
Cellular responses by T-cell ELISpot for ChAd community-primed participants were greatest in those boosted with NVX at 190 SFC per million PBMCs (95% CI 159–227), with a GMR of 4·8 (3·6–6·6) compared with participants receiving homologous ChAd (45 SFC per million PBMCs, 34–61). For those boosted with m1273 these respective values were 148 SFC per million PBMCs (118–187) and a GMR of 3·5 (2·5–4·8; table 2).
In BNT community-primed groups, cellular responses by T-cell ELISpot were higher in those receiving m1273 at 76 SFC per million PBMCs (95% CI 58–99) than in the homologous boost group at 49 SFC per million PBMCs (39–63); however, the GMC for NVX boost was below that of the homologous boost at 29 SFC per million PBMCs (22–38; table 2).
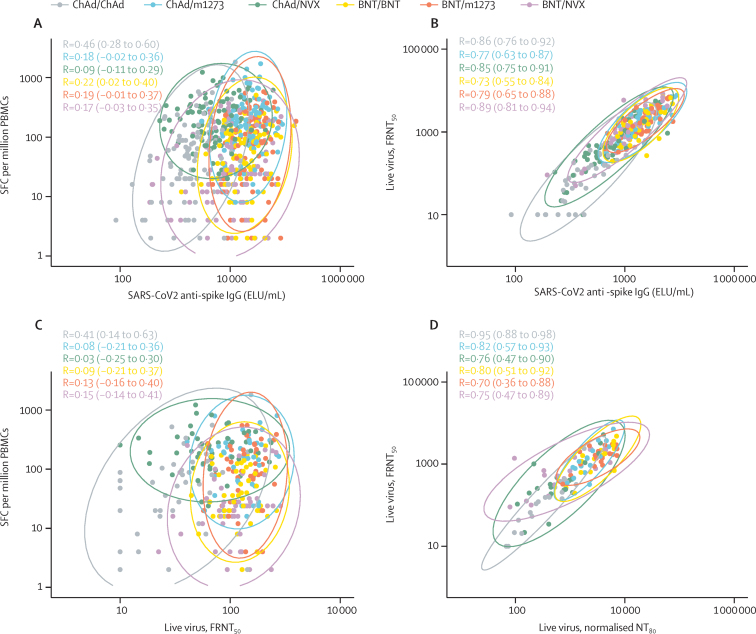
Correlations between cellular and humoral responses show a moderate correlation for the ChAd/ChAd group (correlation coefficient of 0·46), and weak correlation (Pearson correlation coefficients <0·3) for all other schedules (figure 2A). There is a strong correlation between live neutralising assays done at national reference and local laboratories (figure 2D).
Figure 2.
Correlation in the modified intention-to-treat population
(A) SARS-CoV-2 anti-spike IgG and cellular response by IFN-γ ELISpot (the subset with cellular data only, n=582), (B) SARS-CoV-2 anti-spike IgG and live virus neutralising antibodies (the subset with live virus neutralising antibodies data only, n=289), (C) live virus neutralising antibodies and cellular response by IFN-γ ELISpot at 28 days after boost (the subset with live virus neutralising antibodies data only, n=289), (D) live virus neutralising antibodies tested at University of Oxford and those tested at Porton Down, UK Health Security Agency (the immunology cohort, n=123). Ellipses show the 95% CI for different vaccine schedules assuming multivariate normal distributions. Pearson correlation coefficients (95% CI) are presented for each vaccine schedule. BNT=BNT162b2 vaccine, Pfizer–BioNTech. ChAd=ChAdOx1 nCoV-19 vaccine, AstraZeneca. ELU=ELISA laboratory units. FRNT50=50% focus reduction neutralising antibody titre. m1273=mRNA-1273 vaccine, Moderna. NT80=80% neutralising antibody titre. NVX=NVXCoV2373 vaccine, Novavax. PBMCs=peripheral blood mononuclear cells. SFC=spot forming cells.
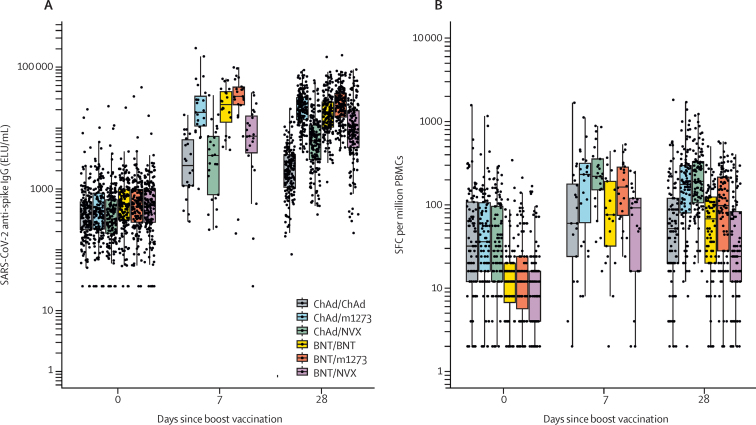
In the modified intention-to-treat population, the GMCs of anti-spike IgG at baseline were 381 (95% CI 347–418) and 526 (480–577) in the populations previously immunised with ChAd and BNT, respectively. While an increase in antibody concentrations at day 7 compared with baseline was observed in all groups, these tended to remain stable or decrease across all groups by day 28 except those receiving an NVX-CoV2373 boost, where there was a further increase from day 7 to day 28 (figure 3, appendix pp 6–9).
Figure 3.
Kinetics of immunogenicity by vaccine schedule
Cellular response data were available in around 60% of sites, the other study sites did not collect plasma samples due to logistical challenges; data presented at day 7 and day 14 were from the immunology cohort only. Data presented from day 0 and day 28 are for all participants in the modified intention-to-treat-population. Boxplots represent the median and 25th and 75th percentiles; the whiskers extend up to the largest value, not greater than 1·5 times the IQR beyond the box. (A) Anti-spike IgG and (B) T-cell response. BNT=BNT162b2 vaccine, Pfizer–BioNTech. ChAd=ChAdOx1 nCoV-19 vaccine, AstraZeneca. ELU=ELISA laboratory units. m1273=mRNA−1273 vaccine, Moderna. NVX=NVXCoV2373 vaccine, Novavax. PBMCs=peripheral blood mononuclear cells. SFC=spot-forming cells.
With regard to cellular responses, participants who had received a community prime with ChAd had numerically higher baseline frequencies than did the BNT groups; responses peaked in all groups at day 14 but were maintained above baseline at day 28 (figure 3B). Responses of intracellular cytokines to SARS-COV-2 spike peptides by flow cytometry at day 0 and 14 after boost were similar across groups, with a predominantly T-helper-1 cell response (appendix pp 11–14).
In ChAd-primed groups, live virus neutralising antibody titres (FRNT50) were reduced according to point estimates across all groups for beta and delta variants, relative to Victoria, with the greatest decrement noted for beta (table 3, appendix pp 15–17). However, both heterologous groups maintained numerically higher titres than ChAd/ChAd, with the highest titres in ChAd/m1273 recipients, with GMRs of 15·8 (95% CI 9·6–26·1) against the beta variant and 17·4 (10·2–29·5) against the delta variant. Similarly, in BNT-primed groups, live virus neutralising antibody titres (FRNT50) across groups were numerically lower against beta and delta variants than to Victoria (table 3). There was no evidence of a difference in neutralising activity against Victoria, beta, and delta variants between the BNT-primed groups. Across all groups, little difference was noted in cellular responses to variants (table 3, appendix p 15).
Table 3.
Summary of immunogenicity against variants of concern between heterologous and homologous prime-boost schedules at 28 days after boost
|
Prime with ChAd |
Prime with BNT |
|||||
|---|---|---|---|---|---|---|
| ChAd/ChAd (n=47) | ChAd/m1273 (n=48) | ChAd/NVX (n=51) | BNT/BNT (n=46) | BNT/m1273 (n=48) | BNT/NVX (n=49) | |
| Live virus neutralising antibody (Victoria*), FRNT50 | ||||||
| n | 47 | 48 | 51 | 46 | 48 | 49 |
| GMC | 109 (70–168) | 1684 (1313–2162) | 432 (301–618) | 1501 (1188–1896) | 1883 (1546–2294) | 1109 (805–1529) |
| GMR† | Ref | 16·9 (10·1–28·0) | 4·2 (2·4–7·2) | Ref | 1·3 (1·0–1·8) | 0·8 (0·6–1·2) |
| Live virus neutralising antibody (beta), FRNT50 | ||||||
| n | 47 | 48 | 51 | 46 | 48 | 49 |
| GMC | 25 (18–34) | 376 (260–545) | 109 (71–167) | 405 (290–565) | 603 (442–822) | 451 (305–666) |
| GMR† | Ref | 15·8 (9·6–26·1) | 4·2 (2·4–7·4) | Ref | 1·6 (1·0–2·5) | 1·3 (0·8–2·2) |
| Live virus neutralising antibody (delta), FRNT50 | ||||||
| n | 47 | 48 | 51 | 46 | 48 | 49 |
| GMC | 41 (27–64) | 672 (506–891) | 153 (99–237) | 697 (520–933) | 873 (688–1107) | 629 (444–891) |
| GMR† | Ref | 17·4 (10·2–29·5) | 3·7 (2·0–6·9) | Ref | 1·3 (0·9–2·0) | 1 (0·7–1·6) |
| Cellular response (wild-type, frozen samples), SFC per million PBMCs | ||||||
| n | 44 | 47 | 50 | 44 | 44 | 46 |
| GMC | 41 (27–62) | 100 (73–136) | 160 (129–198) | 35 (25–49) | 71 (52–97) | 20 (14–29) |
| GMR† | Ref | 2·9 (1·8–4·6) | 4·5 (3·0–6·8) | Ref | 1·9 (1·2–3·0) | 0·60 (0·4–1·0) |
| Cellular response (beta, frozen samples), SFC per million PBMCs | ||||||
| n | 44 | 47 | 50 | 44 | 44 | 46 |
| GMC | 41 (28–60) | 104 (77–141) | 150 (120–187) | 34 (23–48) | 69 (52–92) | 22 (17–30) |
| GMR† | Ref | 3·1 (2·0–4·7) | 4·2 (2·9–6·0) | Ref | 2·0 (1·2–3·1) | 0·72 (0·46–1·1) |
| Cellular response (delta, frozen samples), SFC per million PBMCs | ||||||
| n | 44 | 47 | 50 | 44 | 44 | 46 |
| GMC | 35 (23–54) | 102 (76–136) | 155 (123–196) | 36 (26–51) | 64 (47–86) | 19 (13–28) |
| GMR† | Ref | 3·4 (2·1–5·4) | 5·0 (3·3–7·6) | Ref | 1·6 (1·0–2·5) | 0·56 (0·3–0·9) |
Data shown are geometric mean (95% CI), unless otherwise specified. BNT=BNT162b2 vaccine, Pfizer–BioNTech. ChAd=ChAdOx1 nCoV-19 vaccine, AstraZeneca. ELU=ELISA laboratory units. FRNT50=50% focus reduction neutralising antibody titre. GMC=geometric mean concentration. GMR=geometric mean ratio. m1273=mRNA-1273 vaccine, Moderna. NVX=NVXCoV2373 vaccine, Novavax. PBMCs=peripheral blood mononuclear cells. SFC=spot-forming cells.
A Wuhan-related strain isolated early in the pandemic from Australia.
The two-sided 95% CIs of GMRs were adjusted for study site, cohort, interval between first and second dose, and baseline immunogenicity.
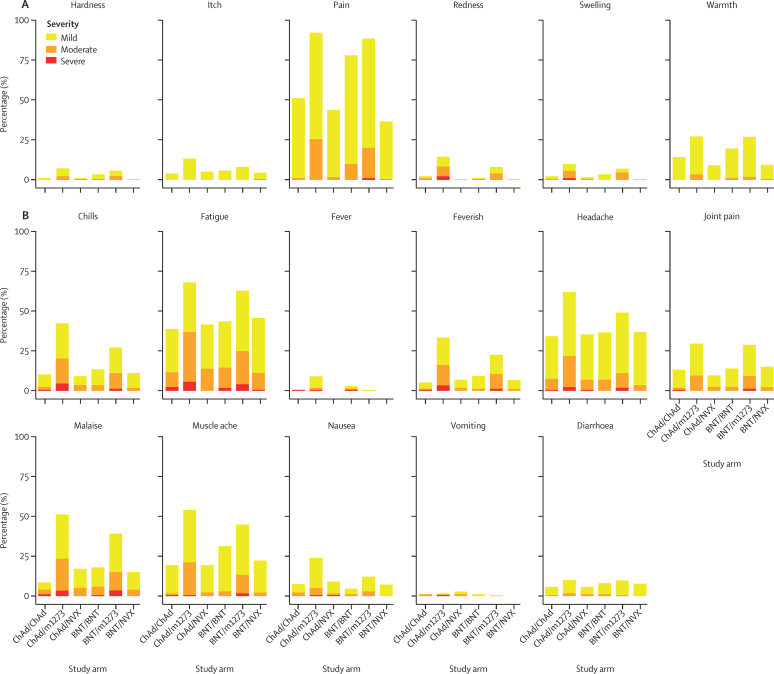
Local and systemic reactions were more frequent after m1273 boost vaccination compared with the homologous boost groups, with feverishness reported by 60 (33%) of 181 recipients of ChAd/m1273 compared with nine (5%) of 176 recipients of ChAd/ChAd (difference 28%, 95% CI 20–36), and by 39 (22%) of 176 recipients of BNT/m1273, compared with 16 (9%) of 175 recipients of BNT/BNT (13%, 5–21; figure 4). Similar increases were observed for chills, fatigue, fever, headache, joint pain, malaise, muscle ache, and nausea (figure 4). Most of the increase in reactogenicity was observed within the 48 h after immunisation (appendix pp 18–19).
Figure 4.
Severity of solicited local and systemic reactions in days 0–7 following boost vaccination by study arm as self-reported in participant electronic diaries
Data are (A) local at vaccination site and (B) systemic. The severity presented is the participant's highest severity across 7 days after vaccination for each solicited adverse event. Fever was defined as mild if 38·0°C to <38·5°C, moderate if 38·5°C to <39°C, and severe if ≥39·0°C. Feverish was self-reported feeling of feverishness. For systemic symptoms, grading was classified as mild if easily tolerated with no limitation on normal activity; moderate if some limitation of daily activity; severe if unable to perform normal daily activity. BNT=BNT162b2 vaccine, Pfizer–BioNTech. ChAd=ChAdOx1 nCoV-19 vaccine, AstraZeneca. m1273=mRNA-1273 vaccine, Moderna. NVX=NVXCoV2373 vaccine, Novavax.
By contrast, for NVX recipients, similar patterns were observed in systemic reactogenicity compared with the homologous study schedules. Local reactions were generally less frequent for NVX recipients, for example a difference in injection site pain of −49% (95% CI −50 to −30) for BNT/NVX compared with BNT/BNT. Paracetamol use mirrored the profile of systemic reactogenicity (appendix p 20).
Between enrolment and Oct 5, 2021, there were 589 adverse events in 357 participants (appendix p 21). Numbers of adverse events were similar across vaccine groups. Descriptions of all non-serious adverse events of grade 3 or worse are presented in the appendix (pp 22–25). There were five adverse events of special interest (excluding SARS-CoV-2 and COVID-19 events) and one deemed possibly related to study vaccination (eosinophilia, grade 2 severity; appendix p 26).
18 participants tested positive for SARS-CoV-2 or had a COVID-19 episode after enrolment (appendix pp 26–28). All cases occurred at least 2 months after a boost, coinciding with an epidemiological surge of infection nationally. A single participant was hospitalised but did not require invasive ventilation (appendix pp 28–30). There were 15 serious adverse events, none of which was considered related to study vaccination (appendix pp 28–30).
Biochemical and haematological blood results were taken at enrolment, day 7, and day 28 after boost and graded by modified US Food and Drug Administration toxicity scale (appendix pp 31–32, 120). There were no notable differences in biochemical adverse events between groups, with some minor variations reported in white blood cell indices. A single participant had grade 3 neutropenia at enrolment and day 7, which progressed to grade 4 at day 28 and was reported as an unrelated serious adverse event.
Discussion
Findings from this trial demonstrate that the immunogenicity of heterologous boost with m1273 following community prime with ChAd or BNT was non-inferior to the homologous-boost schedule. When heterologous boost was with NVX, only those primed with ChAd had titres of SARS-CoV-2 anti-spike IgG that were non-inferior to the homologous schedule, whereas BNT/NVX did not meet the non-inferiority threshold against homologous BNT. Nevertheless, within the limitations of comparing between non-randomised cohorts, SARS-CoV-2 anti-spike IgG titres induced by BNT/NVX were still above that of homologous ChAd, a schedule with demonstrated effectiveness of 65–70% against symptomatic SARS-CoV-2 infection and over 90% against hospitalisation and death.39 Additionally, the T-cell response across all groups was greatest with ChAd/NVX, which might prove important in terms of durability of protection and protection against new SARS-CoV-2 variants.40 These data align with real-world evidence of robust effectiveness of mixed schedules against disease.24, 25, 26 Together, these findings support use of the mixed schedules studied here in rapid and flexible global deployment of COVID-19 vaccines. Accordingly, these schedules should be widely recognised for travel and certification.
Consistent with our previous results, schedules containing at least one mRNA dose showed the greatest binding and neutralising antibody responses, with the heterologous mRNA vaccination studied (BNT/m1273) generating a greater humoral immune response than the homologous BNT/BNT schedule. Given that m1273 contains an mRNA dose (100 μg) over three times that of BNT (30 μg), and that the m1273 containing schedules (BNT/m1273 and ChAd/m1273) generated the two highest antibody concentrations observed across Com-COV and this study, this might reflect a greater humoral immunogenicity of m1273 when compared with BNT, rather than a specific benefit of mixing mRNA vaccines.8 It is interesting to note that in the groups receiving an NVX boost, there is a modest increase in antibody titres after day 7; although the samples' size are small, this finding warrants further investigation.
The strongest cellular immune response was seen with heterologous ChAd-primed schedules. ChAd/NVX showed the highest cellular response as measured by IFN-γ ELISPOT, and is similar to that seen with ChAd/BNT at a 28 day boost interval.8 The ChAd/m1273 schedule also produced a substantial cellular response, whereas both heterologous and homologous mRNA schedules (BNT/m1273 and BNT/BNT) showed lower responses. Strikingly, the BNT/NVX schedule produced a response below that of all other schedules studied, suggesting important differences in the ability of adenoviral-vectored and mRNA vaccines to prime T cells for subsequent stimulation by protein antigens.
These data do not show any convincing evidence of additional benefit of mixed schedules in maintaining neutralising activity against variants of concern, beyond that conferred by greater neutralising activity against the Victoria strain—ie, the decrease in neutralising activity elicited by mixed and homologous schedules was broadly similar. However, for the BNT/NVX recipients, it is notable that the apparent differences in neutralising activity against Victoria were less evident when testing against beta and delta strains. Whether this result is an artefact of a relatively small sample size, or suggestive of better preservation of neutralisation against variants of concern for this schedule, this finding warrants further research. Consistent with previous work, the T-cell response was maintained across all variants of concern, regardless of the schedule.40
This study supports our previous finding of mixed adenoviral-vectored and mRNA COVID-19 vaccine schedules being more reactogenic than homologous schedules, consistent with other studies examining BNT/ChAd.13, 41 Additionally, there was evidence of increased reactogenicity for the heterologous over the homologous mRNA schedule (BNT/m1273 vs BNT/BNT). This effect could be due to the mixing of different mRNA vaccines, or m1273 being more reactogenic than BNT, potentially consistent with higher mRNA dosage; however, it is not possible to differentiate these possible causes in our study. By contrast, there was no evidence of increased reactogenicity for the NVX containing schedules. Importantly, within the limits of the sample size of this study, none of the schedules raised any safety concerns.
While most high-income countries have completed two-dose primary courses of COVID-19 vaccinations in adults, these data remain extremely relevant to the 94% of people in low-income countries who are yet to receive any doses.1 Of the mixed schedules studied, perhaps the most relevant to these countries is the ChAd/NVX, given that neither of these vaccines require ultra-low temperature storage and the low cost of ChAd. NVX has recently been the subject of a WHO Emergency Use Licence and should become available under the COVAX scheme.42 Furthermore data for mixed schedules across platforms could help to inform the use of third-dose boosters, in addition with data from CoV-BOOST (ISRCTN12348322)43 and the recent work by Atmar and colleagues44 supporting use of mRNA vaccines for adenoviral-primed individuals. Such schedules could also allow avoidance of a second mRNA vaccine dose in adolescents, given concerns regarding myocarditis, and this hypothesis is being further studied in Com-COV3.45, 46
This study has a number of limitations. The age range (50–78 years) and ethnicity (90·7% of participants primed with ChAd and 94·7% of those primed with BNT self-identified as White) of the study cohort limit generalisability of the reactogenicity and immunology results to younger populations and people who are not of White ethnicity. These groups might be more likely to receive heterologous COVID-19 vaccine schedules as a primary course, given age-associated safety concerns with the use of ChAd, and logistic constraints in lower-income regions.47 Any increased reactogenicity with heterologous regimes might be amplified in younger groups, as reported in studies of homologous vaccine schedules.48, 49, 50, 51 As participants were recruited after community prime, this dose was not randomised, meaning that some caution is required when comparing ChAd versus BNT community-primed groups, especially as there were higher rates of comorbidity in the BNT-primed groups. Additionally, the proportion of participants primed with ChAd versus BNT differed according to site, creating the potential for different amounts of exposure to SARS-CoV-2 between the groups. This fact is especially important when comparing the number of breakthrough infections observed between ChAd-primed and BNT-primed groups. Due to logistical and pragmatic constraints, we were not able to incorporate a full comparative schedule for all vaccines, and thus cannot provide evidence for the use of mixed mRNA schedules in both permutations, nor the effect of prime with a protein-based vaccine followed by ChAd or mRNA.
This research confirms previous evidence of mixed adenoviral and mRNA schedules as being safe, tolerable, and immunogenic alternatives to homologous schedules when given at an 8–12 weeks interval. It also provides new evidence on the response to mixed mRNA vaccinations in a randomised trial, and novel data for the incorporation of protein-based COVID-19 vaccines into heterologous schedules. These results provide reassurance that there are multiple appropriate options to complete primary immunisation in individuals primed with BNT or ChAd, which will facilitate rapid vaccine deployment globally.
Data sharing
The study protocol is provided in the appendix. Individual participant data will be made available when the trial is complete, upon requests directed to the corresponding author; after approval of a proposal, data can be shared through a secure online platform.
For the protocol online see https://comcovstudy.org.uk
Declaration of interests
MDS acts on behalf of the University of Oxford as an investigator on studies funded or sponsored by vaccine manufacturers, including AstraZeneca, GlaxoSmithKline, Pfizer, Novavax, Janssen, Medimmune, and MCM. He receives no personal financial payment for this work. JSN-V-T is seconded to the Department of Health and Social Care, England. AMC and DMF are investigators on studies funded by Pfizer and Unilever. They receive no personal financial payment for this work. AF is a member of the Joint Committee on Vaccination and Immunisation and chair of the WHO European Technical Advisory Group of Experts on Immunisation. He is an investigator or provides consultative advice on clinical trials and studies of COVID-19 vaccines produced by AstraZeneca, Janssen, Valneva, Pfizer, and Sanofi, and of other vaccines from these and other manufacturers, including GlaxoSmithKline, VPI Pharmaceuticals, Takeda, and Bionet Asia. He receives no personal remuneration or benefits for any of this work. SNF acts on behalf of University Hospital Southampton NHS Foundation Trust as an investigator or provides consultative advice on clinical trials and studies of COVID-19 and other vaccines funded or sponsored by vaccine manufacturers, including Janssen, Pfizer, AstraZeneca, GlaxoSmithKline, Novavax, Seqirus, Sanofi, Medimmune, Merck, and Valneva. He receives no personal financial payment for this work. PTH acts on behalf of St George's University of London as an investigator on clinical trials of COVID-19 vaccines funded or sponsored by vaccine manufacturers, including Janssen, Pfizer, AstraZeneca, Novavax, and Valneva. He receives no personal financial payment for this work. CAG acts on behalf of University Hospitals Birmingham NHS Foundation Trust as an investigator on clinical trials and studies of COVID-19 and other vaccines funded or sponsored by vaccine manufacturers, including Janssen, Pfizer, AstraZeneca, Novavax, CureVac, Moderna, and Valneva. He receives no personal financial payment for this work. VL acts on behalf of University College London Hospitals NHS Foundation Trust as an investigator on clinical trials of COVID-19 vaccines funded or sponsored by vaccine manufacturers including Pfizer, AstraZeneca, and Valneva. He receives no personal financial payment for this work. TL is named as an inventor on a patent application covering the ChAd vaccine and is an occasional consultant to Vaccitech, unrelated to this work. ALG is named as an inventor on a patent covering use of a particular promoter construct that is often used in ChAdOx1-vectored vaccines and is incorporated in the ChAdOx1 nCoV-19 vaccine. ALG benefits from royalty income paid to the University of Oxford from sales of this vaccine by AstraZeneca and its sublicensees under the University's revenue sharing policy. Oxford University has entered into a partnership with AstraZeneca for further development of ChAdOx1 nCoV-19. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
The study is funded by the UK Government through the National Institute for Health Research, the Vaccine Task Force, and the Coalition for Epidemic Preparedness Innovations. This research was supported by the NIHR Oxford Biomedical Research Centre, NIHR Guy's and St Thomas' Biomedical Research Centre, NIHR King's Clinical Research Facility and NIHR Policy Research Programme (PR-R17-0916-22001), the Southampton NIHR Biomedical Research Centre and Southampton NIHR Clinical Research Facility and delivered through the NIHR-funded National Immunisation Schedule Evaluation Consortium. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. NVX vaccine was supplied for use in the trial by Novavax. MDS and SNF are NIHR senior investigators. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. The investigators express their gratitude for the contribution of all trial participants, and for the invaluable advice of the data safety monitoring board. We additionally acknowledge the broader support from the various teams within the University of Oxford, including the Department of Paediatrics, Clinical Trials Research Governance, Research Contracts, and the Public Affairs Directorate.
Contributors
MDS and JSN-V-T conceived the trial and MDS is the chief investigator. MDS, ASVS, RHS, and XL contributed to the protocol and design of the study. ASVS, ELP, RHS, and RW led the implementation of the study. XL and MG did the statistical analysis and have verified the underlying data. ASVS, RHS, MG, XL, and MDS drafted the report. All other authors contributed to the implementation and data collection. All authors reviewed and approved the final report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Our world in data. Statistics and research coronavirus pandemic (COVID-19). https://doi.org/10/2021 2021. https://ourworldindata.org/coronavirus (accessed Nov 30, 2021).
- 2.Li X, Ghadami A, Drake JM, Rohani P, Epureanu BI. Mathematical model of the feedback between global supply chain disruption and COVID-19 dynamics. Sci Rep. 2021;11 doi: 10.1038/s41598-021-94619-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Padma TV. India's COVID-vaccine woes—by the numbers. Nature. 2021;592:500–501. doi: 10.1038/d41586-021-00996-y. [DOI] [PubMed] [Google Scholar]
- 4.UNICEF COVID-19 Vaccine Market Dashboard. 2021. https://www.unicef.org/supply/covid-19-vaccine-market-dashboard
- 5.Paterlini M. Covid-19: Sweden, Norway, and Finland suspend use of Moderna vaccine in young people “as a precaution”. BMJ. 2021;375 doi: 10.1136/bmj.n2477. [DOI] [PubMed] [Google Scholar]
- 6.European Medicines Agency AstraZeneca's COVID-19 vaccine: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets. April 7, 2021. https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood
- 7.Department of Health and Social Care Use of the AstraZeneca COVID-19 (AZD1222) vaccine: updated JCVI statement. 7 May 2021. https://www.gov.uk/government/publications/use-of-the-astrazeneca-covid-19-vaccine-jcvi-statement-7-may-2021/use-of-the-astrazeneca-covid-19-azd1222-vaccine-updated-jcvi-statement-7-may-2021
- 8.Liu X, Shaw RH, Stuart ASV, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398:856–869. doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cevik M, Grubaugh ND, Iwasaki A, Openshaw P. COVID-19 vaccines: keeping pace with SARS-CoV-2 variants. Cell. 2021;184:5077–5081. doi: 10.1016/j.cell.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danish Health Authority Denmark continues its vaccine rollout without the COVID-19 vaccine from AstraZeneca. May 19, 2021. https://www.sst.dk/en/English/Corona-eng/Vaccination%20against%20COVID-19/AstraZeneca%20vaccine%20paused
- 11.Public Health Agency of Canada Recommendations on the use of COVID-19 vaccines. 2021. https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines.html
- 12.Haute Autorite de Sante Vaccins contre la Covid-19 : la HAS maintient la limite d'âge de 55 ans pour Vaxzevria. 12/05/2021. 2021. https://www.has-sante.fr/jcms/p_3266654/en/vaccins-contre-la-covid-19-la-has-maintient-la-limite-d-age-de-55-ans-pour-vaxzevria
- 13.Shaw RH, Stuart A, Greenland M, Liu X, Nguyen Van-Tam JS, Snape MD. Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. Lancet. 2021;397:2043–2046. doi: 10.1016/S0140-6736(21)01115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borobia AM, Carcas AJ, Pérez-Olmeda M, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398:121–130. doi: 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kant R, Dwivedi G, Zaman K, et al. Serendipitous COVID-19 vaccine-mix in Uttar Pradesh, India: safety and immunogenicity assessment of a heterologous regime. medRxiv. 2021 doi: 10.1101/2021.08.06.21261716. published online Aug 13. (preprint). [DOI] [Google Scholar]
- 16.Hillus D, Schwarz T, Tober-Lau P, et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir Med. 2021;9:1255–1265. doi: 10.1016/S2213-2600(21)00357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powell AA, Power L, Westrop S, et al. Real-world data shows increased reactogenicity in adults after heterologous compared to homologous prime-boost COVID-19 vaccination, March-June 2021, England. Euro Surveill. 2021 doi: 10.2807/1560-7917.ES.2021.26.28.2100634. published online July 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barros-Martins J, Hammerschmidt SI, Cossmann A, et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med. 2021;27:1525–1529. doi: 10.1038/s41591-021-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benning L, Töllner M, Hidmark A, et al. Heterologous ChAdOx1 nCoV-19/BNT162b2 prime-boost vaccination induces strong humoral responses among health care workers. Vaccines (Basel) 2021;9:857. doi: 10.3390/vaccines9080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimeglio C, Herin F, Da-Silva I, et al. Heterologous ChAdOx1-S/BNT162b2 vaccination: neutralizing antibody response to SARS-CoV-2. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab705. published online Aug 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groß R, Zanoni M, Seidel A, et al. Heterologous ChAdOx1 nCoV-19 and BNT162b2 prime-boost vaccination elicits potent neutralizing antibody responses and T cell reactivity. medRxiv. 2021 doi: 10.1101/2021.05.30.21257971. published online June 1. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt T, Klemis V, Schub D, et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat Med. 2021;27:1530–1535. doi: 10.1038/s41591-021-01464-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tenbusch M, Schumacher S, Vogel E, et al. Heterologous prime-boost vaccination with ChAdOx1 nCoV-19 and BNT162b2. Lancet Infect Dis. 2021;21:1212–1213. doi: 10.1016/S1473-3099(21)00420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pozzetto B, Legros V, Djebali S, et al. Immunogenicity and efficacy of heterologous ChadOx1/BNT162b2 vaccination. Nature. 2021 doi: 10.1038/s41586.021.04120-y. published online Oct 21. [DOI] [PubMed] [Google Scholar]
- 25.Gram MA, Nielsen J, Schelde AB, et al. Vaccine effectiveness when combining the ChAdOx1 vaccine as the first dose with an mRNA COVID-19 vaccine as the second dose. medRxiv. 2021 doi: 10.1101/2021.07.26.21261130. published online July 28. (preprint). [DOI] [Google Scholar]
- 26.Nordström P, Ballin M, Nordström A. Effectiveness of heterologous ChAdOx1 nCoV-19 and mRNA prime-boost vaccination against symptomatic Covid-19 infection in Sweden: a nationwide cohort study. Lancet Regional Health Europe. 2021 doi: 10.1016/j.lanepe.2021.100249. published online Oct 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vallée A, Vasse M, Mazaux L, et al. An immunogenicity report for the comparison between heterologous and homologous prime-boost schedules with ChAdOx1-S and BNT162b2 vaccines. J Clin Med. 2021;10 doi: 10.3390/jcm10173817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Normark J, Vikström L, Gwon YD, et al. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 vaccination. N Engl J Med. 2021;385:1049–1051. doi: 10.1056/NEJMc2110716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fabricius D, Ludwig C, Scholz J, et al. mRNA vaccines enhance neutralizing immunity against SARS-CoV-2 variants in convalescent and ChAdOx1-primed subjects. Vaccines (Basel) 2021;9:918. doi: 10.3390/vaccines9080918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Hou L, Guo X, et al. Heterologous prime-boost immunization with CoronaVac and Convidecia. medRxiv. 2021 doi: 10.1101/2021.09.03.21263062. published online Sept 6. (preprint). [DOI] [Google Scholar]
- 31.Yorsaeng R, Vichaiwattana P, Klinfueng S, et al. Immune response elicited from heterologous SARS-CoV-2 vaccination: Sinovac (CoronaVac) followed by AstraZeneca (Vaxzevria) medRxiv. 2021 doi: 10.1101/2021.09.01.21262955. published online Sept 3. (preprint). [DOI] [Google Scholar]
- 32.Novavax Novavax and Serum Institute of India announce submission to World Health Organization for emergency use listing of Novavax' COVID-19 vaccine. Sept 23, 2021. https://ir.novavax.com/2021-09-23-Novavax-and-Serum-Institute-of-India-Announce-Submission-to-World-Health-Organization-for-Emergency-Use-Listing-of-Novavax-COVID-19-Vaccine
- 33.Heath PT, Galiza EP, Baxter DN, et al. Safety and efficacy of NVX-CoV2373 COVID-19 vaccine. N Engl J Med. 2021;385:1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bewley KR, Coombes NS, Gagnon L, et al. Quantification of SARS-CoV-2 neutralizing antibody by wild-type plaque reduction neutralization, microneutralization and pseudotyped virus neutralization assays. Nat Protoc. 2021;16:3114–3140. doi: 10.1038/s41596-021-00536-y. [DOI] [PubMed] [Google Scholar]
- 36.Peng Y, Mentzer AJ, Liu G, et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. 2020;21:1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Payne RP, Longet S, Austin JA, et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell. 2021;184:5699–5714.e11. doi: 10.1016/j.cell.2021.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO Guidelines on clinical evaluation of vaccines: regulatory expectations. 2016. https://www.who.int/biologicals/BS2287_Clinical_guidelines_final_LINE_NOs_20_July_2016.pdf
- 39.UK Health Security Agency COVID-19 vaccine surveillance report: 14 October 2021 (week 41) https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1025358/Vaccine-surveillance-report-week-41.pdf
- 40.Geers D, Shamier MC, Bogers S, et al. SARS-CoV-2 variants of concern partially escape humoral but not T cell responses in COVID-19 convalescent donors and vaccine recipients. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiu NC, Chi H, Tu YK, et al. To mix or not to mix? A rapid systematic review of heterologous prime-boost covid-19 vaccination. Expert Rev Vaccines. 2021;20:1211–1220. doi: 10.1080/14760584.2021.1971522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gavi. the Vaccine Alliance Gavi signs agreement with Novavax to secure doses on behalf of COVAX Facility. May, 6, 2021. https://www.gavi.org/news/media-room/gavi-signs-agreement-novavax-secure-doses-behalf-covax-facility
- 43.Munro APS, Janani L, Cornelius V. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021 doi: 10.1016/S0140-6736(21)02717-3. published online 2 Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atmar RL, Lyke KE, Deming ME, et al. Heterologous SARS-CoV-2 booster vaccinations—preliminary report. medRxiv. 2021 https://doi:10.1101/2021.10.10.21264827 published online Oct 15. (preprint). [Google Scholar]
- 45.ISRCTN registry . ISRCTN; 2021. ISRCTN12348322. Comparing COVID-19 vaccine schedule combinations in adolescents (Com-COV3) [Google Scholar]
- 46.Joint Committee on Vaccination and Immunisation JCVI statement on COVID-19 vaccination of children aged 12 to 15 years. Sept 3, 2021. https://www.gov.uk/government/publications/jcvi-statement-september-2021-covid-19-vaccination-of-children-aged-12-to-15-years/jcvi-statement-on-covid-19-vaccination-of-children-aged-12-to-15-years-3-september-2021
- 47.The Lancet Infectious Diseases COVID-19 vaccine equity and booster doses. Lancet Infect Dis. 2021;21 doi: 10.1016/S1473-3099(21)00486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walsh EE, Frenck RW, Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.The Lancet Infectious Diseases COVID-19 vaccine equity and booster doses. Lancet Infect Dis. 2021;21 doi: 10.1016/S1473-3099(21)00486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study protocol is provided in the appendix. Individual participant data will be made available when the trial is complete, upon requests directed to the corresponding author; after approval of a proposal, data can be shared through a secure online platform.
For the protocol online see https://comcovstudy.org.uk