Newly developed vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have shown efficacy against severe illness and death caused by coronavirus infection 2019 (COVID-19). Among the rare adverse events, there are cases of immune thrombocytopenic purpura (ITP) reported in association with COVID-19 vaccination. ITP is a condition of low platelet counts characterized by immune-mediated platelet destruction. Detection of platelet autoantibodies is often challenging, and due to the absence of clear biomarkers, diagnosis of ITP is made after excluding other causes of thrombocytopenia. Here, we report a case of acute ITP that occurred after the second dose of BNT162b2 mRNA vaccine in which we demonstrate the presence of antiplatelet antibodies, particularly anti-GPIbα and related pathophysiology involving hepatic platelet clearance and transient TPO upregulation.
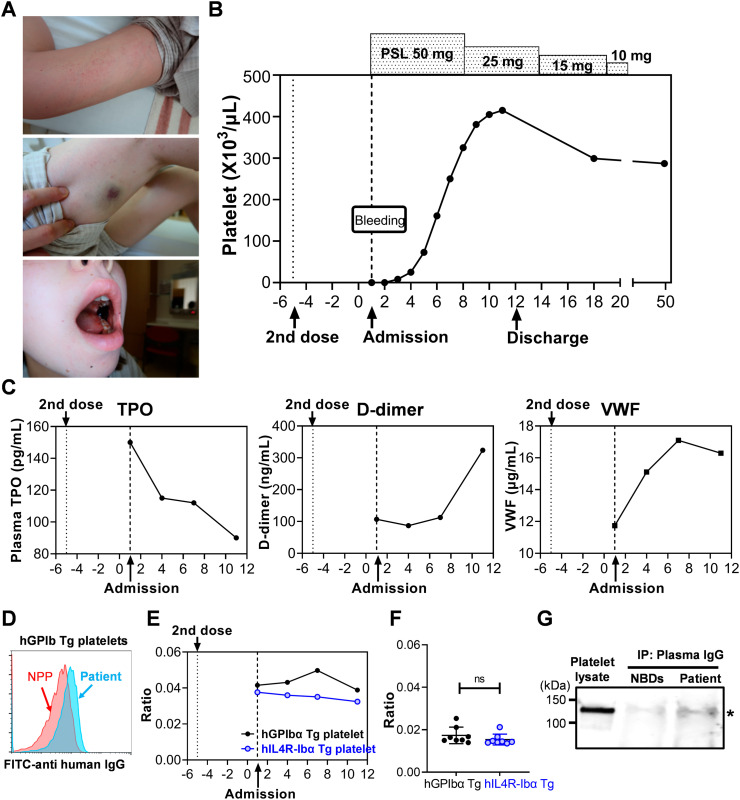
A 32-year-old female with no medical history developed bleeding symptoms 5 days after receiving the second dose of BNT16B2b2 mRNA COVID-19 vaccine. One day after receiving the second dose, she developed fever and headache, which resolved with acetaminophen. Five days after the second dose, petechiae and purpura on the extremities, as well as gingival and oral bleeding developed. The patient sought medical attention on the following day and was admitted to Kurume University Hospital because of persistent bleeding and thrombocytopenia. On examination, she had wet purpura on the left buccal mucosa and petechiae and purpura on the extremities (Fig. 1A). Laboratory tests showed severe thrombocytopenia (<1 × 103/μL) and Platelet-associated IgG (PA-IgG) (1880 ng/107 cells). Of note, this patient had normal platelet count (210 × 103/μL) when tested 3 months before the first dose COVID-19 vaccination. Other laboratory results, including coagulation tests and complement, were normal and antibodies against Platelet factor-4 (PF4), PF4-heparin complex were not detected, excluding the possibility of Vaccine-induced Thrombotic Thrombocytopenia (VITT) (Table 1 ) [1]. Bone marrow (BM) smear demonstrated a normal megakaryocyte count and lack of platelet adhesion around megakaryocytes. BM biopsy revealed an increased number of megakaryocytes with no obvious morphological abnormalities. Cytogenetic analysis showed normal chromosomes of 46XX. Based on these results, the patient was diagnosed as ITP and treatment was initiated with prednisolone (PSL; 50 mg daily). The patient did not receive platelet transfusion or intravenous immunoglobulin (IVIG). On day 3, platelet count started to increase (8 × 103 /μL), albeit still low, and the persistent bleeding in the oral cavity resolved. Platelet count recovered to the normal range on day 6 and continued to increase beyond her normal count (415 × 103/μL on day 11). PSL was tapered to 25 mg on day 8, and after confirming the maintained platelet count and resolution of bleeding symptoms, the patient was discharged on day 12 (Fig. 1B). PSL was further tapered and stopped on day 25.
Fig. 1.
Clinical course and testing results of the patient. (A, B) Initial presentation of the bleeding symptoms on admission and platelet count responses to treatment. (C) Plasma levels of TPO, D-dimer, and VWF. TPO level in the plasma was measured using Human Thrombopoietin ELISA Kit (Sigma-Aldrich, St. Louis, MO) according to manufacturer's instruction. The minimal detectable dose of human TPO using this assay kit is 90 pg/mL. D-dimer was measured using monoclonal antibodies (DD189cc and biotinylated DD255cc; HyTest, Turku, Finland). Plasma D-dimer levels in the normal donors tested by this assay ranged from 26.7 to 142.7 ng/mL (mean 78.2 ± 39.2, n = 15). VWF was measured using anti-human VWF monoclonal antibody (2.2.9, produced at The Scripps Research Institute) and rabbit anti-human VWF polyclonal antibody (produced at The Scripps Research Institute). Plasma VWF levels in the normal donors tested by this assay ranged from 7.2 to 13.2 μg/mL (mean 10.4 ± 2.3, n = 8). (D) Flow cytometry analysis of the patient's plasma or normal pooled plasma (NPP; George King Bio-Medical) binding to hGPIbα Tg mouse platelets. Blood samples collected from transgenic mouse strains hGPIbα Tg and hIL4R-Ibα Tg were fixed with 1% paraformaldehyde and incubated with plasma samples of the patient or normal controls and then, bound IgG was detected with FITC-anti-human IgG (Biolegend, San Diego CA). Platelet population was gated by staining with APC-anti-mCD41 antibody (MWReg30, Biolegend). Samples were analyzed on a Novocyte flow cytometer (ACEA Biosciences), and the results were analyzed with FlowJo software. (E, F) Patient's plasma was incubated with hGPIbα Tg or hIL4R-Ibα Tg platelets, and the bound IgG was detected by FITC-anti-human IgG. Platelet population was gated by staining with APC-anti-mCD41 antibody. Median Fluorescence Intensity (MFI) of FITC-anti-human IgG was divided by MFI of APC-anti-mCD41 signal to correct platelet size difference, and the relative binding ratio are plotted. Results of the plasma samples obtained from normal healthy donors (n = 8) are shown in (F). (G) Immunoprecipitation and Western blot analysis of platelet lysate using Protein G agarose bearing IgG of the patient or normal healthy donors (NBDs). Plasma samples collected from the patient at four time points were pooled (total volume of 360 μL) and incubated with Protein G agarose beads. After washing, IgG bearing Protein G beads were washed and incubated with normal donor platelet lysates solubilized by 1% NP-40, then coprecipitated proteins were analyzed by sodium dodecyl sulfate gel electrophoresis (SDS-PAGE) followed by Western blotting. PVDF membrane was probed with anti- glycocalicin polyclonal antibody, and the signal was detected with IRDye 800 goat anti-rabbit IgG (LI-COR Biosciences, Lincoln, NE). The image was obtained using LI-COR Odyssey imaging system.
Table 1.
Laboratory test results.
| Variable | Measurements | Reference range |
|---|---|---|
| Red-cell count (×10^6/μL) | 4.32 | 3.86–4.92 |
| Hemoglobin (g/dL) | 13.1 | 11.6–14.8 |
| Hematocrit (%) | 37.2 | 35.1–44.4 |
| Platelet count (×10^3/μL) | <1 | 158–148 |
| White-cell count (/μL) | 3.6 | 3.3–8.6 |
| Stab cell (%) | 1.5 | 2.0–13.0 |
| Segmented cell (%) | 56.0 | 38.0–58.9 |
| Lymphocytes (%) | 37.0 | 26.0–46.6 |
| Monocytes (%) | 3.5 | 2.3–7.7 |
| Eosinophils (%) | 0.5 | 0.0–5.0 |
| Atypical lymphocytes (%) | 1.5 | 0.0 |
| Aspartate aminotransferase (U/L) | 20 | 13–30 |
| Alanine aminotransferase (U/L) | 15 | 7–30 |
| Lactate dehydrogenase (U/L) | 160 | 124–222 |
| Alkaline phosphatase (U/L) | 52 | 38–113 |
| γ-glutamyl transpeptidase (U/L) | 12 | 9–32 |
| Total bilirubin (mg/dL) | 1.1 | 0.4–1.2 |
| Direct bilirubin (mg/dL) | 0.1 | ≤0.2 |
| Total protein (g/dL) | 7.7 | 6.6–8.1 |
| Albumin (g/dL) | 4.6 | 4.1–5.1 |
| Cholinesterase (U/L) | 298 | 201–421 |
| Urea nitrogen (mg/dL) | 9 | 8–20 |
| Creatinine (mg/dL) | 0.44 | 0.46–0.79 |
| Sodium (mmol/L) | 140 | 138–145 |
| Potassium (mmol/L) | 3.4 | 3.6–4.8 |
| Chloride (mmol/L) | 104 | 101–108 |
| Calcium (mg/dL) | 9.0 | 8.8–10.1 |
| Uric acid (mg/dL) | 2.1 | 2.6–7.0 |
| C-reactive protein (mg/dL) | 0.04 | ≤0.14 |
| Iron (μg/dL) | 77 | 40–188 |
| Unsaturated iron-binding capacity (μg/dL) | 193 | 191–269 |
| Ferritin (ng/mL) | 92.4 | 10.0–60.0 |
| Immunoglobulin G (mg/dL) | 1705 | 861–1747 |
| Immunoglobulin A (mg/dL) | 121 | 93–393 |
| Immunoglobulin M (mg/dL) | 83 | 50–269 |
| Prothrombin time (seconds) | 12.5 | |
| International normalized ratio | 0.96 | 0.85–1.15 |
| Activated partial-thromboplastin time (seconds) | 29.9 | 24.0–39.0 |
| Fibrinogen (mg/dL) | 316 | 200–400 |
| Fibrin degradation products (μg/mL) | <2.5 | <5.0 |
| Antithrombin activity (%) | 110 | 80–130 |
| Complement component 3 (mg/dL) | 92 | 73–138 |
| Complement component 4 (mg/dL) | 29 | ≤15 |
| Antinuclear antibody (times) | 40 | <40 |
| Rheumatoid factor (IU/mL) | <1 | ≤15 |
| Anticardiolipin antibody IgG (U/mL) | ≤8 | <10.0 |
| Anticardiolipin β2-glycoprotein complex antibody (U/mL) | ≤1.2 | <3.5 |
| Platelet-associated IgG (ng/10^7 cells) | 1880 | ≤46 |
| Platelet factor 4-heparin complex antibody (U/mL) | <0.6 | <1.0 |
| Antiplatelet factor 4 (ng/mL) | 2 | ≤20 |
| Hepatitis B surface antigen | (−) | (−) |
| Hepatitis B core antibody | (−) | (−) |
| Hepatitis C antibody | (−) | (−) |
| Helicobacter pylori antibody IgG (U/mL) | 3 | <10.0 |
To study in more detail, plasma samples collected at different time points were studied. Thrombopoietin (TPO) levels were within normal range, but the highest on the day of admission and rapidly decreased (Fig. 1C, left). This result, together with the platelet overshoot observed later during the treatment (day 9–11), which was about 2-fold higher than her normal count, indicates that TPO level was elevated at an earlier time point before admission when circulating platelets was rapidly cleared. TPO upregulation is not generally observed in patients with ITP [2]. However, it has been known that clearance of desialylated platelets from circulation upregulates hepatic TPO production via Ashwell-Morell receptor (AMR) signaling [3]. It is also reported in mouse that platelets opsonized with anti-GPIbα antibodies are desialylated, resulting in Fc-independent hepatic clearance via the AMR [4]. In agreement with previous studies, we previously reported in a mouse model that intravenous administration of anti-GPIbα antibody induced platelet microaggregates and clearance in the liver at early time points (30 min) with transient increase of hepatic TPO mRNA (peaking at 8 h) and TPO protein (peaking at 24 h) [5]. Thus, we hypothesized that anti-GPIbα antibodies developed in this patient, which induced platelet desialylation, acute hepatic clearance, leading to transient TPO upregulation. To test this hypothesis, plasma samples were incubated with fixed platelets of mice expressing human GPIbα (hGPIbα Tg) in the background of GPIbα knockout [6], and bound antibodies were detected with FITC-anti human IgG. Unlike human platelets, mouse platelets do not express Fc receptors. Therefore, the binding of plasma IgG to platelets via Fc receptors can be excluded in this setting. As shown in Fig. 1D, IgG in the patient's plasma showed higher binding to hGPIbα Tg mouse platelets compared to normal pooled plasma (NPP). To further investigate the presence of IgG binding specifically to hGPIbα, another transgenic strain in which extracellular domain of hGPIbα is replaced with IL4Rα (hIL4R-Ibα Tg) was used as controls [7]. Of note, due to the difference in platelet size of these two mouse strains, median fluorescent intensity (MFI) of FITC-anti human IgG was corrected by MFI of APC-anti-mCD41 antibody and shown as relative binding ratio [5]. As expected, relative binding ratio of FITC-anti-human IgG to hGPIbα Tg platelets was higher than that to hIL4R-Ibα Tg, and the difference peaked on day 7 (Fig. 1E). There was no difference between these signals when plasma samples of healthy donors (n = 8) were tested (Fig. 1F). The relative binding ratio of the patient's plasma to hIL4R-Ibα Tg platelets was stable with the average of 0.035 ± 0.002, which was about 2-fold higher than that of healthy donors (0.015 ± 0.003, n = 8). We do not have a clear explanation for this, but there might be a possibility that the patient's plasma contains autoantibodies not only against GPIbα but also against other platelet epitopes conserved between human and mouse. To further confirm the presence of autoantibodies against GPIbα, normal human platelet lysate was immunoprecipitated with the patient's plasma IgG. The same volume of NPP was used as control. Immunoprecipitated proteins were analyzed by SDS-PAGE and Western blotting detected by anti-glycocalicin antibody. The result showed the higher signal of GPIbα precipitated with the patient's plasma IgG compared to that of normal blood donors (Fig. 1G).
Results obtained in this study demonstrate that antiplatelet antibodies, specifically anti-hGPIbα antibodies, were present in the patient's plasma after the second dose of COVID-19 vaccination. Anti-hGPIbα antibodies binding to platelets may have led to hepatic sequestration with associated transient TPO upregulation and platelet overshooting in response to steroid treatment. Detection of the autoantibodies is often challenging in the diagnosis of ITP. In this patient, difference in the binding of the plasma antibodies to hGPIbα Tg platelets versus hIL4R-Ibα Tg platelets analyzed by flow cytometry was not obvious on admission and was the highest on day 7. This might be explained by the rapid clearance of opsonized platelets and/or the antibody internalization in platelets and megakaryocytes at early phase of the disease [5]. Although D-dimer levels were within the normal range in our observation period and kept low during the thrombocytopenic phase, it increased later with rapid platelet recovery on day 12 (Fig. 1C, middle). This result may suggest the presence of circulating activated platelets or microaggregates/microthrombi during the recovery phase. Plasma VWF levels also increased concurrently with recovery of platelets (Fig. 1C, right). VWF might be released either from platelets or the vascular endothelium activated by the spike protein and/or the immune complex containing it.
To our knowledge, there are over 40 cases of ITP reported in association with COVID-19 vaccination. More than half of these patients had chronic medical conditions, including autoimmune diseases, hypothyroidism, or preexisting ITP. Our case is one of the few cases with no underlying medical conditions and the first report in which the target of the anti-platelet antibody was determined as GPIbα. It is not yet known how the antiplatelet antibodies are induced following COVID-19 vaccination, antigen presentation, and/or immune-complex formation, and what is the major epitope for the anti-platelet antibodies. Autoimmune responses following other vaccines, including those against Measles, Mumps and Rubella (MMR), Influenza, Polio, and Human Papillomavirus have been previously reported, and molecular mimicry has been implicated as a mechanism of undesired immune responses. Interestingly, the recent COVID-19 vaccination follow-up study of the patients with pre-existing ITP showed higher exacerbation risk in those post-splenectomy and those with more refractory disease [8]. Previous studies reported that resistance to IVIG treatment was more frequent when ITP was caused by anti-GPIb/IX compared to anti-GPIIb-IIIa [9] [10]. Thus, we speculate that patients who present recurrence or exacerbation of thrombocytopenia after COVID-19 vaccination may include those having anti-GPIbα autoantibodies. In addition, our previous study using mouse models demonstrated that platelet hepatic sequestration and TPO upregulation occurs when platelets are acutely depleted by high-dose anti-GPIbα [5]. Higher exacerbation risk of thrombocytopenia in patients with prior splenectomy [8] may indicate hepatic platelet clearance mediated by anti-GPIbα. Further investigation is warranted to determine the mechanism by which the administration of mRNA vaccine and the expression of spike proteins not only trigger T cell responses against spike proteins but also potentiate the production of anti-GPIbα antibodies. Understanding the pathophysiology for the new onset or recurrence of ITP following COVID-19 vaccination, especially identification of the autoantibodies will help clarify the mechanisms of vaccination-associated ITP.
Credit authorship contribution statement.
T.N. was responsible for the treatment of the patient, collected the clinical data, and wrote the first draft of the manuscript. Y.M. and S.K. performed plasma analyses, interpreted the data and helped manuscript preparation. T.O. has overseen the study and provided helpful discussions. K.N. and T.K. designed experiments, directed the study, and revised the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by National Institutes of Health grants HL129011 (TK), by fellowships and additional financial support from MERU Foundation (Italy) to YM, SK, and TK; and by the National Foundation for Cancer Research (SK and TK). We thank Dr. Zaverio M. Ruggeri for helpful discussions and suggestions.
References
- 1.Nazy I., Sachs U.J., Arnold D.M., McKenzie S.E., Choi P., Althaus K., Ahlen M.T., Sharma R., Grace R.F., Bakchoul T. Recommendations for the clinical and laboratory diagnosis of VITT against COVID-19: communication from the ISTH SSC subcommittee on platelet immunology. J. Thromb.Haemost. 2021 doi: 10.1111/jth.15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuter D.J., Gernsheimer T.B. Thrombopoietin and platelet production in chronic immune thrombocytopenia. Hematol. Oncol. Clin. North Am. 2009;23:1193–1211. doi: 10.1016/j.hoc.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grozovsky R., Begonja A.J., Liu K., Visner G., Hartwig J.H., Falet H., Hoffmeister K.M. The Ashwell-Morell receptor regulates hepatic thrombopoietin production via JAK2-STAT3 signaling. Nat. Med. 2015;21:47–54. doi: 10.1038/nm.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J., van der Wal D.E., Zhu G., Xu M., Yougbare I., Ma L., Vadasz B., Carrim N., Grozovsky R., Ruan M., Zhu L., Zeng Q., Tao L., Zhai Z.M., Peng J., Hou M., Leytin V., Freedman J., Hoffmeister K.M., Ni H. Desialylation is a mechanism of Fc-independent platelet clearance and a therapeutic target in immune thrombocytopenia. Nat. Commun. 2015;6:7737. doi: 10.1038/ncomms8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morodomi Y., Kanaji S., Won E., Ruggeri Z.M., Kanaji T. Mechanisms of anti-GPIbα antibody-induced thrombocytopenia in mice. Blood. 2020;135:2292–2301. doi: 10.1182/blood.2019003770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ware J., Russell S., Ruggeri Z.M. Generation and rescue of a murine model of platelet dysfunction: the Bernard-Soulier syndrome. Proc. Natl. Acad. Sci. U. S. A. 2000;97:2803–2808. doi: 10.1073/pnas.050582097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanaji T., Russell S., Ware J. Amelioration of the macrothrombocytopenia associated with the murine Bernard-Soulier syndrome. Blood. 2002;100:2102–2107. doi: 10.1182/blood-2002-03-0997. [DOI] [PubMed] [Google Scholar]
- 8.Lee E.J., Beltrami Moreira M., Al-Samkari H., Cuker A., DiRaimo J., Gernsheimer T., Kruse A., Kessler C.M., Kruse C., Leavitt A.D., Lee A.I., Liebman H.A., Newland A.C., Ray A.E., Tarantino M.D., Thachil J., Kuter D.J., Cines D.B., Bussel J.B. SARS-CoV-2 vaccination and immune thrombocytopenia in de novo and pre-existing ITP patients. Blood. 2021 doi: 10.1182/blood.2021013411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Go R.S., Johnston K.L., Bruden K.C. The association between platelet autoantibody specificity and response to intravenous immunoglobulin G in the treatment of patients with immune thrombocytopenia. Haematologica. 2007;92:283–284. doi: 10.3324/haematol.10667. [DOI] [PubMed] [Google Scholar]
- 10.Peng J., Ma S.-H., Liu J., Hou Y., Liu X.-M., Niu T., Xu R.-R., Guo C.-S., Wang X.-M., Cheng Y.-F., Ni H., Hou M. Association of autoantibody specificity and response to intravenous immunoglobulin G therapy in immune thrombocytopenia: a multicenter cohort study. J. Thromb. Haemost. 2014;12:497–504. doi: 10.1111/jth.12524. [DOI] [PubMed] [Google Scholar]