The tumor suppressor p53 plays an important role in the inhibition of cancer progression, particularly in response to chemotherapy or target-specific therapy. Inactivation or mutation of p53 often becomes a cancer’s tactic for drug resistance. One of the clinically applied therapeutic strategies is to inhibit poly(ADP-ribose) polymerase (PARP) activity, as PARP inhibitors are widely used for subsets of tumors with homologous recombination deficiency due to mutation of BRCA1/2 or other DNA repair-associated genes. It has been shown that p53 deficiency or mutation enhances the cytotoxicity of PARP inhibition in various tumors (Williamson et al., 2012). A possible mechanism underlying this is that loss of p53 impairs DNA repair pathways, creating additional tumor vulnerability to PARP inhibition, as wild-type (wt) p53 transcriptionally induces the expression of genes involved in DNA repair (Vousden and Prives, 2009). Alternatively, missense mutant p53 proteins, which comprise the majority of p53 mutations, may interact with replicating DNA in association with PARP to promote aberrant DNA repair, establishing a strong tumor dependency on the PARP-associated repair pathway (Polotskaia et al., 2015; Xiao et al., 2020). However, recent studies also suggested that wt p53 activity may contribute to tumor response to PARP inhibition, including a subset of colorectal cancer (CRC) (Smeby et al., 2020), as PARP inhibitors were found to activate the p53 pathway (Hong et al., 2021). These seemingly contradictory findings warrant the need of further understanding the role of p53 in response to PARP inhibition.
In our recent study (Chen et al., 2021), we unveiled a long noncoding RNA, the RNA component of mitochondrial RNA-processing endoribonuclease (RMRP), as a novel inhibitor of p53 upon PARP inhibition. RMRP is responsible for mitochondrial DNA replication and biosynthesis of ribosomal RNA. It possesses tumor-promoting activity in different human cancers (Yeganeh and Hernandez, 2020). Consistent with this, we found that RMRP is highly expressed in CRCs, which is associated with unfavorable prognosis. Our RNA-sequencing analysis revealed that knockout of RMRP in CRC cells dramatically activates the p53 pathway. Overexpression of RMRP promoted, whereas depletion of RMRP suppressed, CRC cell growth and proliferation in vitro and in vivo. These cellular functions of RMRP were executed by suppressing wt p53 activity, as RMRP had a minimal effect on p53-null CRC cells. To elucidate how exactly RMRP suppresses p53 activity, we performed co-immunoprecipitation coupled with mass spectrometry analysis. As a result, we identified SNRPA1 as an RMRP-binding partner to bridge the interplay between RMRP and p53. SNRPA1 is a component of the spliceosome, but its role in CRC remained elusive. Interestingly, SNRPA1 underwent lysosomal degradation in the cytoplasm via the chaperone-mediated autophagy pathway upon RMRP depletion. In RMRP-highly expressing CRC cells, RMRP sequestered SNRPA1 in the nucleus to block the lysosomal degradation of the latter. The nuclear SNRPA1 then interacted with p53 and promoted MDM2-mediated degradation of p53, consequently accelerating CRC cell growth. Further supporting this, knockdown of SNRPA1 undermined RMRP’s function to regulate p53 activity and CRC cell growth. More interestingly, we also found that the transcription factor C/EBPβ can activate the transcription of RMRP by directly binding to the RMRP promoter. This finding is coincident with a previous report showing that PARP-1 represses C/EBPβ activity by inducing its ADP-ribosylation (or PARylation), while inhibition of PARP-1 increases C/EBPβ activity (Luo et al., 2017). In line with this, we showed that inhibition of PARP-1 by siRNAs or PARP inhibitors leads to the increase of RMRP expression. Also, we demonstrated that targeting RMRP significantly enhances cytotoxicity of PARP inhibitors in wt p53-harboring CRC cells.
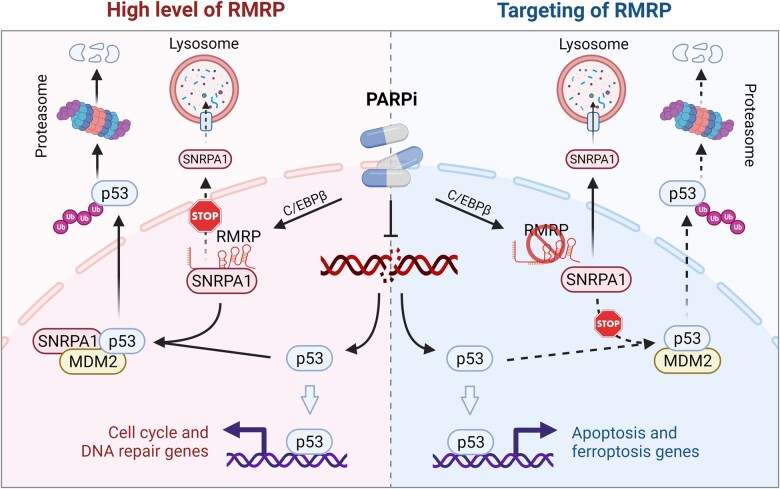
Our study suggests a promising strategy to sensitize cancer cells to PARP inhibition by reactivating p53. Post-treatment of relapsed or drug-resistant cancer cells with a PARP inhibitor could turn on the C/EBPβ‒RMRP‒SNRPA1‒p53 signaling pathway, leading to wt p53 inactivation and consequent resistance to PARP inhibition as depicted here (Figure 1). Thus, we propose that, by specifically targeting this pathway, such as targeting RMRP or SNRPA1 via exosome-mediated delivery of antisense oligonucleotides or proteolysis targeting chimeras against one of them, one could develop the strategy of synthetic lethality (dual inhibition of PARP and RMRP or SNRPA1, leading to cell death through re-activation of inactive wt p53 as a result of the PARP inhibition) as a potential therapy against those PARP inhibitor-resistant CRC cancer cells that harbor wt p53 (Figure 1).
Figure 1.
The role of the C/EBPβ‒RMRP‒SNRPA1‒p53 signaling pathway in tumor response to PARP inhibition. PARP inhibitors (PARPi) lead to the increase of RMRP expression by the transcription factor C/EBPβ. RMRP then interacts with and retains SNRPA1 in the nucleus to prevent the lysosomal degradation of the latter via the chaperone-mediated autophagy pathway. The nuclear SNRPA1 binds to p53 and promotes MDM2-mediated p53 ubiquitination and degradation, which compromises PARPi-triggered p53 activation, resulting in the protective upregulation of cell cycle and DNA repair genes (left panel). Targeting RMRP prompts the nuclear export and lysosomal degradation of SNRPA1. In the absence of SNRPA1, p53 can be fully activated by PARPi, thus eliminating cancer cells by inducing apoptosis or ferroptosis (right panel) (created with BioRender.com).
[The work described was supported in part by grants to X.Z. and Q.H. from the National Natural Science Foundation of China (81874053, 82072879, and 81702352) and in part by the Reynolds and Ryan Families Chair Fund of Translational Cancer to H.L.]
References
- Chen Y., Hao Q., Wang S., et al. (2021). Inactivation of the tumor suppressor p53 by long noncoding RNA RMRP. Proc. Natl Acad. Sci. USA 118, e2026813118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong T., Lei G., Chen X., et al. (2021). PARP inhibition promotes ferroptosis via repressing SLC7A11 and synergizes with ferroptosis inducers in BRCA-proficient ovarian cancer. Redox Biol. 42, 101928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X., Ryu K.W., Kim D.S., et al. (2017). PARP-1 controls the adipogenic transcriptional program by PARylating C/EBPβ and modulating its transcriptional activity. Mol. Cell 65, 260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polotskaia A., Xiao G., Reynoso K., et al. (2015). Proteome-wide analysis of mutant p53 targets in breast cancer identifies new levels of gain-of-function that influence PARP, PCNA, and MCM4. Proc. Natl Acad. Sci. USA 112, E1220–E1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeby J., Kryeziu K., Berg K.C.G., et al. (2020). Molecular correlates of sensitivity to PARP inhibition beyond homologous recombination deficiency in pre-clinical models of colorectal cancer point to wild-type TP53 activity. EBioMedicine 59, 102923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden K.H., Prives C. (2009). Blinded by the light: the growing complexity of p53. Cell 137, 413–431. [DOI] [PubMed] [Google Scholar]
- Williamson C.T., Kubota E., Hamill J.D., et al. (2012). Enhanced cytotoxicity of PARP inhibition in mantle cell lymphoma harbouring mutations in both ATM and p53. EMBO Mol. Med. 4, 515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G., Lundine D., Annor G.K., et al. (2020). Gain-of-function mutant p53 R273H interacts with replicating DNA and PARP1 in breast cancer. Cancer Res. 80, 394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeganeh M., Hernandez N. (2020). RNA polymerase III transcription as a disease factor. Genes Dev. 34, 865–882. [DOI] [PMC free article] [PubMed] [Google Scholar]