Abstract
Aims
To assess differences in long-term outcome and functional status of patients with cardiogenic shock (CS) treated by percutaneous mechanical circulatory support (pMCS) and intra-aortic balloon pump (IABP).
Methods and results
Long-term follow-up of the multicentre, randomized IMPRESS in Severe Shock trial (NTR3450) was performed 5-year after initial randomization. Between 2012 and 2015, a total of 48 patients with severe CS from acute myocardial infarction (AMI) with ST-segment elevation undergoing immediate revascularization were randomized to pMCS by Impella CP (n = 24) or IABP (n = 24). For the 5-year assessment, all-cause mortality, functional status, and occurrence of major adverse cardiac and cerebrovascular event (MACCE) were assessed. MACCE consisted of death, myocardial re-infarction, repeat percutaneous coronary intervention, coronary artery bypass grafting, and stroke. Five-year mortality was 50% (n = 12/24) in pMCS patients and 63% (n = 15/24) in IABP patients (relative risk 0.87, 95% confidence interval 0.47–1.59, P = 0.65). MACCE occurred in 12/24 (50%) of the pMCS patients vs. 19/24 (79%) of the IABP patients (P = 0.07). All survivors except for one were in New York Heart Association Class I/II [pMCS n = 10 (91%) and IABP n = 7 (100%), P = 1.00] and none of the patients had residual angina. There were no differences in left ventricular ejection fraction between the groups (pMCS 52 ± 11% vs. IABP 48 ± 10%, P = 0.53).
Conclusions
In this explorative randomized trial of patients with severe CS after AMI, there was no difference in long-term 5-year mortality between pMCS and IABP-treated patients, supporting previously published short-term data and in accordance with other long-term CS trials.
Keywords: Cardiogenic shock, Mechanical circulatory support, Acute myocardial infarction, Randomized controlled trial, Intra-aortic balloon pump
Graphical Abstract
Key message
What is known?
Although percutaneous mechanical circulatory support (pMCS) is frequently used in cardiogenic shock (CS), there is limited information on long-term outcome. The IMPRESS in Severe Shock is a pivotal trial that evaluated the effect of pMCS in CS.
What is new?
We performed a 5-year follow-up of the IMPRESS in Severe shock trial. Cardiogenic shock patients treated with IABP or Impella CP had similar survival rates, but IABP supported patients had a numerical higher occurrence of MACCE (major adverse cardiac and cerebrovascular events).
What are the implications?
Future studies regarding pMCS in CS should evaluate the effect on long-term outcome, such as MACCE and functional status.
Introduction
The role of percutaneous mechanical circulatory support (pMCS) in cardiogenic shock (CS) is still a matter of debate.1 So far, limited randomized data on the effect of pMCS in patients with CS from acute myocardial infarction (AMI) are available.2 The IMPRESS in Severe Shock trial is a pivotal randomized trial reporting outcome of CS patients treated with pMCS.3 In this trial, treatment with pMCS by Impella CP (Abiomed, Danvers, MA, USA) was compared with the intra-aortic balloon pump (IABP). The short-term outcome of CS patients treated with these two devices did not differ. However, the effect of pMCS on long-term outcome of patients treated in the IMPRESS in Severe Shock trial has not yet been reported.
We therefore performed 5-year follow-up of the IMPRESS in Severe Shock trial to assess differences in clinical outcomes and functional status between pMCS and IABP supported patients.
Methods
The IMPRESS in Severe Shock trial (NTR3450) is an investigator-initiated, multicentre, randomized, open-label trial. The trial design was approved by the ethics committee at each participating centre. The study design, informed consent procedure, and primary endpoint results (30-day all-cause mortality) have been previously published.3
Between June 2012 and September 2015, a total of 48 patients with severe CS complicating acute ST-segment elevation myocardial infarction undergoing immediate revascularization were randomized to either pMCS by Impella CP (n = 24) or IABP (n = 24). Severe CS was defined as systolic blood pressure <90 mmHg or the need for inotropes or vasopressors, and the requirement for mechanical ventilation. Exclusion criteria were: severe aorto-iliac arterial disease impeding placement of either IABP or pMCS, known severe cardiac aortic valvular disease, serious known concomitant disease with a life expectancy of <1 year, known participation in this study or any other trial within the previous 30 days, or coronary artery bypass grafting (CABG) within the preceding week.
For the prespecified 5-year assessment, all-cause mortality was retrieved from the Dutch population (BRP; in Dutch ‘Basisregistratie Personen’) register. Major adverse cardiac and cerebrovascular event (MACCE) assessment consisted of death, myocardial re-infarction, repeat percutaneous coronary intervention (PCI), CABG, and stroke. In survivors, a structured phone interview was conducted to assess residual angina and functional status according to the New York Heart Association (NYHA) classification. Also, follow-up echocardiography was obtained to assess the left ventricular ejection fraction (LVEF).
Statistical analysis
At the interim analysis of the initial study, mortality in the control group was much lower than anticipated, and there was no difference in mortality between the two groups. Therefore, the Executive Committee decided to complete the study with 48 patients as an exploratory safety study. There was no formal power analysis for the long-term follow-up. Data were analysed according to the intention-to-treat principle. Normally distributed data were described as mean ± standard deviation and compared with the t-test. Non-normally distributed data were described as median with interquartile range and compared using the Mann–Whitney U test. Categorical data were described as frequencies with percentages and compared using the Fisher’s exact or Chi-squared test, whichever appropriate. Kaplan–Meier curves were used to show event rates over time and relative risk (RR) was calculated with 95% confidence interval (CI). A P-value <0.05 was considered statistically significant for all analyses. Statistical analyses were performed using IBM SPSS Statistics version 26.0.
Results
Long-term follow-up of the IMPRESS in Severe Shock trial was performed at a median of 5.5 years (5.3–6.5) after initial randomization. The clinical and procedural characteristics of the treatment groups at baseline are presented in Table 1. At randomization, mean age was 58 ± 9 years in pMCS patients vs. 59 ± 11 years in IABP patients. Respectively, male sex in 18/24 (75%) vs. 20/24 (83%) patients and occurrence of cardiac arrest before randomization in 24/24 (100%) vs. 20/24 (83%) patients. Device placement before revascularization occurred in 5/24 (21%) of the pMCS patients vs. 3/24 (13%) of the IABP patients. The baseline characteristics were well balanced between the two groups.
Table 1.
Clinical and procedural characteristics and clinical course of patients treated with percutaneous mechanical support (Impella CP) or intra-aortic balloon pump
| pMCS (n = 24) | IABP (n = 24) | |
|---|---|---|
| Clinical characteristics at baseline | ||
| Age, years | 58 ± 9 | 59 ± 11 |
| Male sex | 18/24 (75) | 20/24 (83) |
| Diabetes mellitus | 2/22 (9) | 3/23 (13) |
| Prior myocardial infarction | 1/22 (5) | 1/23 (4) |
| Prior stroke | 0/22 (0) | 1/23 (4) |
| Prior PCI or CABG | 1/22 (5) | 0/23 (0) |
| Mean arterial pressure, mmHg | 66 ± 15 | 66 ± 15 |
| Heart rate, beats/min | 81 ± 21 | 83 ± 28 |
| Catecholamine or inotrope use | 24/24 (100) | 22/24 (92) |
| Cardiac arrest | 24/24 (100) | 20/24 (83) |
| Time till return of spontaneous circulation, min | 21 (15–46) | 27 (15–52) |
| Lactate, mmol/L | 7.5 ± 3.2 | 8.9 ± 6.6 |
| Haemoglobin, mmol/L | 8.6 ± 1.2 | 8.6 ± 1.2 |
| Creatinine, mg/dL | 96 ± 29 | 102 ± 22 |
| Procedural characteristics | ||
| Device placement before revascularization | 5/24 (21) | 3/24 (13) |
| Device placement after revascularization | 19/24 (80) | 21/24 (88) |
| TIMI flow post-PCI | ||
| 0 or 1 | 1/24 (4) | 0/24 (0) |
| 2 or 3 | 23/24 (96) | 24/24 (100) |
| SYNTAX score pre-PCI | 23.2 ± 8.7 | 28.2 ± 10.6 |
| Clinical course during admission | ||
| Duration of support (h) | 49 (28–76) | 48 (24–77) |
| Crossover or upgrading to device with more support | 1/24 (4.2) | 3/24 (12.5) |
| Renal replacement therapy | 8/24 (33) | 7/24 (29) |
| Blood products during admission | 11/24 (46) | 8/24 (33) |
| Placement of implantable cardioverter-defibrillator | 2/24 (8) | 1/24 (4) |
| Intensive care unit stay duration | 7 (3–16) | 7 (4–10) |
Values are given as mean ± standard deviation, median (IQT) or n/N (%).
CABG: coronary artery bypass grafting; IABP, intra-aortic balloon pump; PCI, percutaneous coronary intervention; pMCS, percutaneous mechanical circulatory support.
The clinical course during admission is presented in Table 1. Median duration of support was 49 h (28–76) for pMCS patients vs. 48 h (24–77) for IABP patients. Renal replacement therapy was used in 8/24 (33%) of the pMCS patients vs. 7/24 (29%) of the IABP patients. Median length of intensive care unit stay was 7 days (3–16) for pMCS patients vs. 7 (4–10) for IABP patients.
Long-term mortality and major adverse cardiac and cerebrovascular event
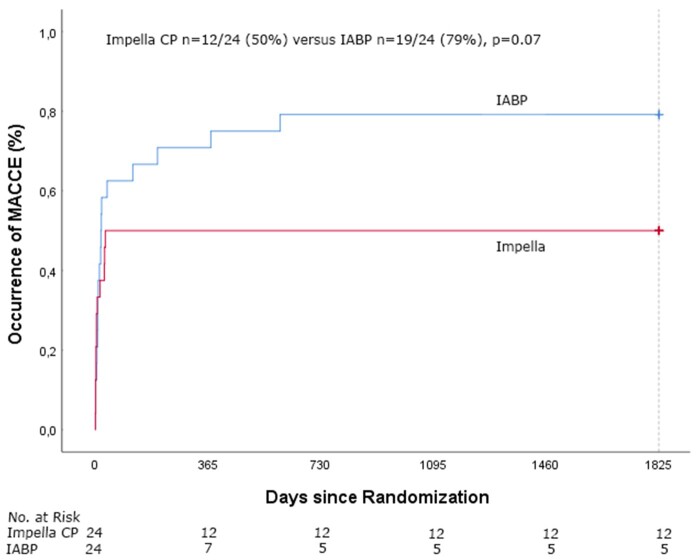
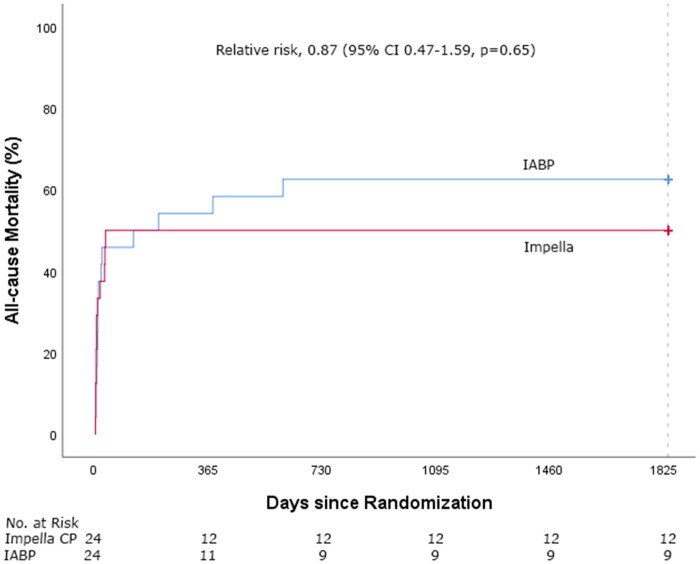
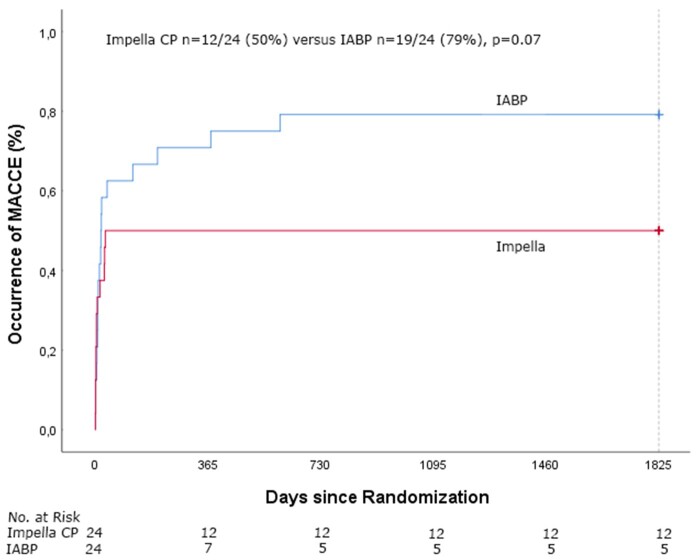
Clinical outcome of patients treated with pMCS and IABP is presented in Table 2. Follow-up was completed for all patients regarding the mortality status. Five-year mortality was 50% (12/24) in the pMCS group and 63% (15/24) in the IABP group (RR 0.87, 95% CI 0.47–1.59, P = 0.65, Figure 1). Cause of death was post-anoxic neurological damage in 13 patients (48%), refractory CS in 7 patients (26%) and other reason in 7 patients (26%). After 6 months, only 3 additional deaths had occurred, all in the IABP group. One patient underwent surgical durable left ventricular assist device placement but died 19 months later from a stroke. Causes of death in the other two patients were sudden cardiac arrest and multiple sclerosis.
Table 2.
Five-year clinical outcome of patients treated with percutaneous mechanical support (Impella CP) or intra-aortic balloon pump
| pMCS (n = 24) | IABP (n = 24) | P-value | |
|---|---|---|---|
| Clinical outcome | |||
| 5-year mortality | 12/24 | 15/24 | 0.65 |
| Cause of death | |||
| Post-anoxic neurological damage | 6 | 7 | |
| Refractory cardiogenic shock | 3 | 4 | |
| Other cause | 3 | 4 | |
| MACCE | 12/24 | 19/24 | 0.07 |
| Death | 12 | 15 | |
| Myocardial re-infarction | 1 | 2 | |
| Repeat PCI | 3 | 0 | |
| CABG | 0 | 1 | |
| Stroke | 2 | 2 | |
| Heart transplantation | 0/24 | 0/24 | 1.00 |
| Durable LVAD placement | 0/24 | 1/24 | 1.00 |
| Functional status | |||
| Severe acquired disability | 0/11 | 0/7 | 1.00 |
| NYHA class I/II | 10/11 | 7/7 | 1.00 |
| Residual angina | 0/11 | 0/7 | 1.00 |
| LVEF (%) | 52 ± 11 | 48 ± 10 | 0.53 |
Values are given as mean ± standard deviation or n/N (%).
CABG, coronary artery bypass grafting; IABP, intra-aortic balloon pump; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; MACCE, major adverse cardiac and cerebrovascular event; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; pMCS, percutaneous mechanical circulatory support.
Figure 1.
Time-to-event Kaplan–Meier curves of all-cause mortality in Impella CP and intra-aortic balloon pump-treated patients.
Figure 2.
Time-to-event Kaplan–Meier curves for the occurrence of major adverse cardiac and cerebrovascular events in Impella CP and intra-aortic balloon pump-treated patients.
MACCE occurred in 12/24 (50%) of the pMCS patients vs. 19/24 (79%) of the IABP patients (P = 0.07, Figure 2). Myocardial re-infarction occurred in one pMCS patient and in two IABP patients, repeat PCI in three pMCS patients, CABG in one IABP patient and stroke in two pMCS patients and in two IABP patients. All pMCS patients with a MACCE had died at 5-year follow-up. For the four IABP patients who survived at 5 years and had a MACCE, the MACCE occurred on day 13 (CABG), day 17 (myocardial infarction, MI), day 19 (stroke), and day 38 (MI) after randomization. Three of the survivors in the pMCS group had received an implantable cardioverter-defibrillator at 5-year follow-up.
Functional status
Among 5-year survivors, a follow-up interview was successfully conducted in 11/12 (92%) pMCS and 7/9 (78%) IABP patients. None of the pMCS or IABP supported patients had a severe acquired disability. All patients except for one were in NYHA class I or II [pMCS n = 10 (91%) and IABP n = 7 (100%), P = 1.00] and none of the patients had residual angina complaints. Follow-up echocardiography was conducted at a median of 5.0 years (5.0–6.0) and successfully obtained in 10/12 (83%) pMCS and 6/9 (67%) IABP patients. There were no differences in LVEF between the two groups (pMCS 52 ± 11% vs. IABP 48 ± 10%, P = 0.53).
Discussion
In this randomized trial of patients with CS after AMI, there was no difference in long-term 5-year all-cause mortality between pMCS and IABP treated patients, supporting previously published 30-day and 6-month data.
The long-term results of the SHOCK, IABP-SHOCK II, and CULPRIT-SHOCK trials show similar overall mortality rates.4–6 These trials show that the mortality of CS patients is mostly determined in the acute phase. The most frequent causes of death, irreversible post-anoxic neurological damage and refractory CS, are usually evident during hospital admission and contribute to the high mortality of patients in the acute phase. The long-term follow-up of the IMPRESS in Severe Shock confirms this finding, with only an additional absolute mortality increase of ∼6% at 5 years compared with 30-day outcomes.
The functional status of patients at 5 years was relatively good, with more than 90% of patients in NYHA class I or II. These findings are similar to the results of previous trials reporting long-term outcomes of CS patients.4–6
Interestingly, we did observe a numerically higher occurrence of MACCE in the IABP group, compared with patients treated with pMCS by Impella CP (P = 0.07). We speculate that the use of a mechanical support device which offers a higher output than IABP, such as the Impella CP, may yield better overall patient outcome. Nevertheless, the IMPRESS in Severe Shock trial was not powered to show a potential difference in mortality and there was no formal power analysis conducted regarding MACCE.
Late effects after mechanical support have been reported. For instance, the BCIS study showed better survival in IABP supported patients during elective PCI.7 Therefore, future trials should not only focus on the important endpoint of short-term mortality but also take other clinically relevant measures into account at long-term follow-up, such as long-term mortality, the occurrence of stroke, re-MI, repeat PCI or CABG. Furthermore, the quality of life and functional status of patients at long-term is of great relevance for patients and should also be evaluated.
A minority of the patients included in the IMPRESS in Severe Shock trial underwent device placement before revascularization. Current evidence suggests that early initiation of mechanical support may be beneficial.8–10 Restoring and maintaining adequate systemic circulation might be of higher priority than coronary revascularization in the setting of severe CS to overcome the high mortality rate. In addition, experimental research suggests that unloading prior to revascularization may result in an additional reduction in infarct size. Experimental models with Impella CP specifically showed improved coronary flow and infarct zone perfusion, which might provide a rationale for the beneficial effect on a cellular level.11 The concept of early initiation of mechanical support is now being studied in the ‘Door-to-Unloading’ trial in anterior ST-elevation myocardial infarction patients without CS (DTU-STEMI trial: NCT 03947619).12
The IMPRESS in Severe Shock trial showed that conducting a RCT in CS regarding mechanical circulatory support can be challenging. However, to have a definitive answer on whether these devices are effective in improving patient outcome, we need to conduct trials that are adequately powered. Several promising randomized trials are anticipated or currently being conducted designed to evaluate the efficacy of pMCS in CS patients. The DanGer Shock trial (NCT01633502) was initiated in 2012 and randomizes CS patients to either Impella CP or standard care with 6-month all-cause mortality as their primary outcome. Due to slow enrolment, this trial was expanded with more sites in 2018 and currently more than 100 patients are included.13 The anticipated patient enrolment is 360 patients. Also, the RECOVER IV trial is anticipated to start enrolment in 2021 and will assess whether Impella initiation before PCI is superior to PCI without Impella in patients with CS from AMI.
Currently, there is no randomized data that shows the superiority of any support device over the other. However, it may be speculated that devices offering more hemodynamic support may be beneficial in this population. The role of high-output devices such as the venoarterial extracorporeal membrane oxygenation (VA-ECMO) system need to be clarified. Several trials regarding the use of VA-ECMO in CS patients are currently being conducted or are anticipated [e.g. ECLS-SHOCK trial (NCT02301819), EURO-SHOCK trial (NCT03813134), ECMO-CS trial (NCT03637205)]. Future studies should also assess the role of (concomitant) right ventricular failure as this particular patient population may have worse outcome and require a different treatment strategy.
The strengths of the present long-term follow-up study are recruitment of severe CS patients representing a real-world population, complete follow-up regarding long-term mortality status and information on functional status. Several limitations of the initial study remain, such as the lack of blinding due to the nature of the study and the small sample size. The initial study was completed as an exploratory safety study and there was no formal power analysis for the long-term follow-up. Therefore, the additional (post hoc) analyses should also be considered hypotheses generating. Also, these results may not be generalizable to other CS aetiologies, as more than 90% of the patients included in this trial presented with a cardiac arrest. Although limited by sample size, the IMPRESS in Severe Shock trial remains a pivotal randomized trial investigating the effectiveness of pMCS in CS patients.
Conclusion
At long-term 5-year follow-up of the explorative randomized IMPRESS in Severe Shock trial, there were no differences in all-cause mortality and functional status between pMCS and IABP treated patients, supporting previously published short-term data and in accordance with other long-term CS trials.
Conflict of interest: none declared.
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Schramm R, Morshuis M, Schoenbrodt M, Boergermann J, Hakim-Meibodi K, Hata M, Gummert JF.. Current perspectives on mechanical circulatory support. Eur J Cardiothorac Surg 2019;55:i31–i37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Combes A, Price S, Slutsky AS, Brodie D.. Temporary circulatory support for cardiogenic shock. Lancet 2020;396:199–212. [DOI] [PubMed] [Google Scholar]
- 3. Ouweneel DM, Eriksen E, Seyfarth M, Henriques JPS.. Percutaneous mechanical circulatory support versus intra-aortic balloon pump for treating cardiogenic shock: meta-analysis. J Am Coll Cardiol 2017;69:358–360. [DOI] [PubMed] [Google Scholar]
- 4. Hochman JS, Sleeper LA, Webb JG, Dzavik V, Buller CE, Aylward P, Col J, White HD; SHOCK Investigators. Early revascularization and long-term survival in cardiogenic shock complicating acute myocardial infarction. JAMA 2006;295:2511–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thiele H, Akin I, Sandri M, de Waha-Thiele S, Meyer-Saraei R, Fuernau G, Eitel I, Nordbeck P, Geisler T, Landmesser U, Skurk C, Fach A, Jobs A, Lapp H, Piek JJ, Noc M, Goslar T, Felix SB, Maier LS, Stepinska J, Oldroyd K, Serpytis P, Montalescot G, Barthelemy O, Huber K, Windecker S, Hunziker L, Savonitto S, Torremante P, Vrints C, Schneider S, Zeymer U, Desch S; CULPRIT-SHOCK Investigators. One-year outcomes after PCI strategies in cardiogenic shock. N Engl J Med 2018;379:1699–1710. [DOI] [PubMed] [Google Scholar]
- 6. Thiele H, Zeymer U, Thelemann N, Neumann FJ, Hausleiter J, Abdel-Wahab M, Meyer-Saraei R, Fuernau G, Eitel I, Hambrecht R, Böhm M, Werdan K, Felix SB, Hennersdorf M, Schneider S, Ouarrak T, Desch S, de Waha-Thiele S; IABPSHOCK II Trial (Intraaortic Balloon Pump in Cardiogenic Shock II) Investigators. Intraaortic balloon pump in cardiogenic shock complicating acute myocardial infarction: long-term 6-year outcome of the randomized IABP-SHOCK II trial.Circulation. 2019;139:395–403 [DOI] [PubMed] [Google Scholar]
- 7. Perera D, Stables R, Clayton T, De Silva K, Lumley M, Clack L, Thomas M, Redwood S.. Long-term mortality data from the balloon pump-assisted coronary intervention study (BCIS-1): a randomized, controlled trial of elective balloon counterpulsation during high-risk percutaneous coronary intervention. Circulation 2013;127:207–212. [DOI] [PubMed] [Google Scholar]
- 8. Schrage B, Ibrahim K, Loehn T, Werner N, Sinning J-M, Pappalardo F, Pieri M, Skurk C, Lauten A, Landmesser U, Westenfeld R, Horn P, Pauschinger M, Eckner D, Twerenbold R, Nordbeck P, Salinger T, Abel P, Empen K, Busch MC, Felix SB, Sieweke J-T, Møller JE, Pareek N, Hill J, MacCarthy P, Bergmann MW, Henriques JPS, Möbius-Winkler S, Schulze PC, Ouarrak T, Zeymer U, Schneider S, Blankenberg S, Thiele H, Schäfer A, Westermann D.. Impella support for acute myocardial infarction complicated by cardiogenic shock. Circulation 2019;139:1249–1258. [DOI] [PubMed] [Google Scholar]
- 9. Hemradj VV, Karami M, Sjauw KD, Engström AE, Ouweneel DM, de Brabander J, Vis MM, Wykrzykowska JJ, Beijk MA, Koch KT, Baan J, de Winter RJ, Piek JJ, Driessen AHG, Lagrand WK, Vlaar APJ, Ottervanger JP, Henriques JPS.. Pre-PCI versus immediate post-PCI Impella initiation in acute myocardial infarction complicated by cardiogenic shock. PLoS One 2020;15:e0235762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iannaccone M, Albani S, Giannini F, Colangelo S, Boccuzzi GG, Garbo R, Brilakis ES, D'ascenzo F, de Ferrari GM, Colombo A.. Short term outcomes of Impella in cardiogenic shock: a review and meta-analysis of observational studies. Int J Cardiol 2021;324:44–51. [DOI] [PubMed] [Google Scholar]
- 11. Watanabe S, Fish K, Kovacic JC, Bikou O, Leonardson L, Nomoto K, Aguero J, Kapur NK, Hajjar RJ, Ishikawa K.. Left ventricular unloading using an Impella CP improves coronary flow and infarct zone perfusion in ischemic heart failure. J Am Heart Assoc 2018;7:e006462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kapur NK, Esposito ML.. Door to unload: a new paradigm for the management of cardiogenic shock. Curr Cardiovasc Risk Rep 2016;10:41. [Google Scholar]
- 13. Udesen NJ, Møller JE, Lindholm MG, Eiskjær H, Schäfer A, Werner N, Holmvang L, Terkelsen CJ, Jensen LO, Junker A, Schmidt H, Wachtell K, Thiele H, Engstrøm T, Hassager C; DanGer Shock investigators. Rationale and design of DanGer shock: Danish-German cardiogenic shock trial. Am Heart J 2019;214:60–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.