ABSTRACT
Tregs infiltrate tumors and inhibit antitumor immunity. KW-0761 (Mogamulizumab) is a humanized anti-CCR4 monoclonal antibody that could eliminate activated Tregs with high immunosuppressive activity that express CCR4. In this phase Ib trial, KW-0761 was used as a cancer immunotherapeutic reagent to deplete Tregs in patients with advanced or recurrent solid CCR4-negative tumors. Thirty-nine patients with solid cancer were treated with KW-0761 at a dose of 0.1 or 1.0 mg/kg. The safety, clinical responses, and effects of Treg depletion were analyzed. Any grade and grade 3–4 treatment-related adverse events (AEs) were observed in 36 (92%) and 14 (36%) out of 39 patients, respectively. All treatment-related AEs were manageable. One and 5 patients achieved a partial response and stable disease, respectively, during treatment and were long survivors. The efficient depletion of Treg in peripheral blood was confirmed in both cohorts. Therefore, the administration of KW-0761 was safe, resulting in the depletion of Tregs in peripheral blood and potential immune responses in patients with solid cancer. The combined use of KW-0761 to deplete Tregs and other immunotherapies is a promising approach to augment immune responses.
Key Words: solid cancer patients, mogamulizumab, regulatory T cells, clinical trial, CCR4
INTRODUCTION
Immunotherapy, primarily with immune checkpoint inhibitors (ICIs) such as anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and anti-programmed cell death protein 1 (PD-1) monoclonal antibodies (mAbs), has markedly improved the survival rate of patients with cancer, even in the advanced stage1,2,3; however, the efficacy of ICIs is unsatisfactory in the majority of patients and, thus, other therapies, including immunotherapy, are urgently needed. In the escape phase of the concept of cancer immunoediting, tumor cells have developed strategies to establish an immunosuppressive state within the tumor microenvironment (TME) by producing immunosuppressive cytokines, such as vascular endothelial growth factor (VEGF), transforming growth factor-β (TGF-β), and indoleamine 2,3-dioxygenase (IDO), and recruiting regulatory immune cells, including regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs).4 Therefore, the manipulation of immunosuppressive cells or TME is an anticancer therapeutic strategy.
Tregs constitute 5–10% of CD4+ T cells in the periphery and play an important role in maintaining immune tolerance.4,5 Tregs inhibit the development of antitumor immunity, thereby hindering the immune surveillance of cancer and preventing effective antitumor immune responses in tumor-bearing hosts. The presence of a large number of Tregs and a low ratio of CD8+ T cells to Tregs in the TME correlate with a poor prognosis in various cancer types.6,7 Tregs promote tumor progression by suppressing antitumor immunity,8,9 manipulating Tregs, or targeting the immunosuppressive factors produced by these cells, and, thus, have potential as an anticancer treatment strategy. Despite promising pre-clinical studies, Treg cell-targeted therapy has not yet been successfully applied to clinical settings.
KW-0761 (mogamulizumab) is a humanized anti-CCR4 immunoglobulin G1 (IgG1) mAb with enhanced antibody-dependent cellular cytotoxicity.10,11 It was developed as an orphan drug for adult T-cell leukemia-lymphoma (ATLL), which expresses CCR4 on cell surfaces, and has been approved for T-cell lymphomas, including ATLL and other peripheral T-cell lymphomas, by the Pharmaceuticals and Medical Devices Agency, Food and Drug Administration, and European Medicines Agency.12,13,14 Evidence is emerging to show that KW-0761 suppresses the function of effector Tregs (eTregs), which is a subpopulation of Tregs with high expression levels of FoxP3 and CCR4, and exhibits strong immunosuppressive activity against tumors, leading to robust CD8+ T-cell proliferation in ATLL.15,16 Therefore, we conducted a clinical trial to examine the treatment effects of KW-0761 on solid cancers based on a novel concept, namely, Treg depletion. In a previous phase Ia clinical trial, KW-0761 was administered to 7 patients with lung cancer and 3 with esophageal cancer with a dose escalation design of 0.1, 0.5, and 1.0 mg/kg.17 All doses were confirmed to be safe and well tolerated. Four out of the 10 patients achieved stable disease (SD) during the treatment and were long survivors. The monitoring of FoxP3+ Tregs in peripheral blood mononuclear cells (PBMCs) during the treatment indicated efficient depletion even at the lowest dose of 0.1 mg/kg.
To investigate the safety and efficacy of eTreg depletion and clinical responses in patients with solid cancer in more detail, we conducted a phase Ib clinical trial on KW-0761 in 39 patients with CCR4-negative cancers.
PATIENTS AND METHODS
Patients
Eligible patients had CCR4-negative treatment-refractory or advanced cancer with target lesions. Archival or newly obtained tumor samples from patients were screened for the expression of CCR4 by immunohistochemistry (IHC) as previously described.18 CCR4 expression was confirmed by the review committee with a central evaluation. Inclusion criteria were described in the phase Ia study.17
Study Design
This study was designed as a multi-institutional, open-label, two-arm, investigator-initiated phase Ib clinical trial on KW-0761. The investigational drug KW-0761was provided by Kyowa Hakko Kirin. The study was registered with ClinicalTrials.gov as NCT01929486 and was approved by local Institutional Review Boards. All participating patients provided written informed consent before enrolment, in accordance with the Declaration of Helsinki. The primary objectives were to characterize the safety and efficacy of eTreg depletion in PBMCs by KW-0761 for patients with advanced or recurrent solid cancer. The secondary objectives were to assess clinical responses, including the overall response rate, progression-free survival (PFS), and overall survival (OS), and select the recommended dose for a phase II trial. Twenty and nineteen patients were randomly enrolled into cohorts with doses of 0.1 and 1.0 mg/kg, respectively, and received 8 intravenous infusions of KW-0761 weekly followed by monthly infusions until disease progression. These two doses of KW-0761 were established in the phase Ia study as the maximum-tolerated and minimal doses, respectively. Oral antihistamines and acetaminophen were administered prior to each KW-0761 treatment, and hydrocortisone was simultaneously infused intravenously with the first KW-0761 treatment to prevent infusion reactions.
Toxicity Evaluation
Toxicity was evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Patients were assessed for toxicity weekly during the first 8 treatments and every 4 weeks thereafter until 24 weeks after the last treatment. The independent data monitoring committee evaluated safety data for each dose level.
Clinical Response Evaluation
Responses were evaluated 12 weeks after the first KW-0761 treatment or at the study discontinuation based on computed tomography (CT) scans according to RECIST (ver. 1.1),19 and/or immune-related (ir) RECIST. OS was defined as the duration from the first day of KW-0761 treatment until the day of death from any cause. PFS was defined as the duration from the first day of KW-0761 treatment until either the day of progressive disease (PD) detection or death from any cause. Tumor responses, OS, and PFS were confirmed by a central evaluation for each patient.
Evaluation of eTreg Depletion on PBMCs
Blood samples were obtained at baseline, 4 and 8 weeks after the first KW-0761 treatment, and every 4 weeks during the continuous treatment until study-off. PBMCs were isolated from heparinized blood by density gradient centrifugation using Ficoll-Paque Plus (GE Healthcare, Fairfield, CT). Cells were stored in liquid N2 until used. Treg depletion was evaluated by flow cytometry. After thawing, PBMCs were incubated with mAbs at 4°C for 20 minutes. Cells were stained with anti-CD4-PerCP (clone SK3; BD Biosciences, San Jose, CA), anti-CD25-APC (clone 2A3; BD Biosciences), and anti-CD45RA-FITC (clone ALB11; Beckman Coulter, Brea, CA) mAbs. The intracellular staining of FOXP3 was performed with anti-FoxP3-PE (clone PCH101; eBioscience) mAb and a FoxP3/Transcription Factor Staining Buffer Set (eBioscience, San Diego, CA) according to the manufacturer’s instructions. After the incubation, cells were washed and analyzed by FACSCalibur (BD Biosciences). CD45RA+ FoxP3 lo resting/naïve Tregs, CD45RA– FoxP3 hi activated/effector Tregs (eTregs), and CD45RA– FoxP3 lo, non-Tregs were analyzed as previously described.15
RESULTS
Patient Characteristics
In this phase Ib study, 39 patients with advanced CCR4-negative solid cancer were randomly assigned to receive a treatment with KW-0761 at 0.1 mg/kg (n=20) or 1.0 mg/kg (n=19) between October 2013 and April 2016 (Table 1). The median age of patients was 65 years. The cohort included 11 patients with esophageal cancer, 9 with lung cancer, 6 with malignant melanoma, 5 with gastric cancer, 5 with ovarian cancer, and 3 with mesothelioma with 2 to 23 infusions of KW-0761. Eleven patients dropped out before the completion of the first cycle with 8 infusions of KW-0761 due to the withdrawal of consent in 1 patient, disease progression in 4, and a decision by the principal investigators in 6. The median number of infusions was 8 (2–14) in the 0.1 mg/kg cohort and 8 (2–23) in the 1.0 mg/kg cohort. The median follow-up was 94 (21–498) days in the 0.1 mg/kg cohort and 99 (25–511) in the 1.0 mg/kg cohort. Seven (35%) patients in the 0.1 mg/kg cohort and 7 (36%) in the 1.0 mg/kg cohort died, and the cause of all deaths was disease progression.
Table 1.
Patient characteristics
| Dose
(mg/kg) |
ID | Age | Sex | Tumor Type | No. of
Infusions |
Best
overall response |
Time to
progression (days) |
OS
(days) |
Treatment-
related AEs (All) |
Treatment-
related AEs (Grade 3–4) |
Treg
depletion |
| 0.1 | B-01 | 66 | M | Esophageal cancer (SCC) | 6 | PD | 42 | 96 | + | + | + |
| 0.1 | B-03 | 70 | F | NSCLC | 8 | PD | 63 | 422 | + | – | + |
| 0.1 | B-05 | 67 | M | Melanoma | 5 | PD | 28 | 73 | + | – | + |
| 0.1 | B-07 | 68 | F | Melanoma | 8 | PD | 77 | 77 | + | – | + |
| 0.1 | B-10 | 64 | F | Esophageal cancer (SCC) | 8 | PD | 63 | 249 | + | + | + |
| 0.1 | B-11 | 70 | M | Esophageal cancer (Other) | 4 | PD | 25 | 391 | + | + | NA |
| 0.1 | B-12 | 66 | M | Melanoma | 8 | PD | 76 | 150 | + | – | + |
| 0.1 | B-15 | 57 | M | NSCLC | 8 | PD | 67 | 77 | + | – | + |
| 0.1 | B-19 | 76 | M | NSCLC | 8 | SD | 63 | 92 | + | + | + |
| 0.1 | B-21 | 67 | M | Melanoma | 8 | PD | 77 | 91 | + | + | + |
| 0.1 | B-22 | 53 | F | Ovarian cancer | 8 | PD | 67 | 224 | + | + | + |
| 0.1 | B-23 | 61 | M | NSCLC | 8 | PD | 63 | 498 | + | + | + |
| 0.1 | B-24 | 70 | M | Gastric cancer | 8 | PD | 70 | 271 | + | – | + |
| 0.1 | B-26 | 85 | M | Gastric cancer | 2 | PD | 21 | 21 | + | – | + |
| 0.1 | B-28 | 71 | M | Mesothelioma | 4 | SD | 40 | 40 | – | – | + |
| 0.1 | B-32 | 65 | F | NSCLC | 8 | PD | 63 | 395 | + | – | + |
| 0.1 | B-33 | 63 | F | Ovarian cancer | 8 | PD | 69 | 77 | + | – | + |
| 0.1 | B-35 | 47 | F | Esophageal cancer (Other) | 4 | PD | 29 | 31 | + | – | + |
| 0.1 | B-37 | 65 | M | Esophageal cancer (SCC) | 9 | SD | 96 | 284 | + | – | + |
| 0.1 | B-39 | 59 | F | Esophageal cancer (SCC) | 14 | PD | 70 | 251 | + | + | + |
| 1.0 | B-02 | 65 | F | Ovarian cancer | 8 | PD | 66 | 413 | + | – | + |
| 1.0 | B-04 | 68 | F | Melanoma | 8 | PD | 79 | 142 | – | – | + |
| 1.0 | B-06 | 49 | M | Melanoma | 8 | PD | 56 | 233 | + | – | + |
| 1.0 | B-08 | 51 | F | Esophageal cancer (SCC) | 2 | PD | 27 | 93 | + | – | + |
| 1.0 | B-09 | 64 | M | Esophageal cancer (SCC) | 23 | PR | 491 | 511 | + | + | + |
| 1.0 | B-13 | 54 | F | Ovarian cancer | 8 | PD | 65 | 318 | + | – | + |
| 1.0 | B-14 | 68 | F | Gastric cancer | 4 | PD | 35 | 35 | – | – | + |
| 1.0 | B-16 | 57 | M | NSCLC | 3 | PD | 25 | 25 | + | + | NA |
| 1.0 | B-17 | 67 | M | Gastric cancer | 5 | PD | 47 | 47 | + | – | + |
| 1.0 | B-18 | 68 | M | NSCLC | 8 | PD | 62 | 65 | + | + | + |
| 1.0 | B-20 | 54 | M | SCLC | 8 | PD | 56 | 58 | + | – | + |
| 1.0 | B-25 | 61 | M | NSCLC | 8 | PD | 63 | 497 | + | – | + |
| 1.0 | B-27 | 64 | M | Esophageal cancer (SCC) | 5 | PD | 36 | 99 | + | + | + |
| 1.0 | B-29 | 45 | M | Gastric cancer | 8 | PD | 70 | 272 | + | – | + |
| 1.0 | B-30 | 77 | M | Esophageal cancer (SCC) | 8 | PD | 63 | 68 | + | – | + |
| 1.0 | B-31 | 68 | M | Mesothelioma | 10 | SD | 124 | 124 | + | + | + |
| 1.0 | B-34 | 65 | M | Esophageal cancer (Other) | 8 | PD | 81 | 88 | + | + | + |
| 1.0 | B-36 | 80 | M | Mesothelioma | 10 | SD | 160 | 168 | + | – | + |
| 1.0 | B-38 | 73 | F | Ovarian cancer | 8 | PD | 76 | 84 | + | – | + |
NSCLC: non-small cell lung cancer
SCLC: small cell lung cancer
SCC: squamous cell carcinoma
PD: progressive disease
SD: stable disease
PR: partial response
AEs: adverse events
NA: not assessed
Adverse events (AEs)
Any grade treatment-related AEs occurred in 19 (95%) out of 20 patients in the 0.1 mg/kg cohort and in 17 (89%) out of 19 in the 1.0 mg/kg cohort; grade 3–4 treatment-related AEs occurred in 7 (35%) patients in the 0.1 mg/kg cohort and in 6 (32%) in the 1.0 mg/kg cohort (Table 1). Table 2 lists all treatment-related AEs, with 65 and 49 AEs being observed in the 0.1 and 1.0 mg/kg cohorts, respectively. The most frequently observed categories of treatment-related AEs were skin disorders and lymphopenia. Grade 3–4 treatment-related AEs were lymphopenia (4 [20%] in the 0.1 mg/kg cohort and 4 [21%] in the 1.0 mg/kg cohort), rash, increased alanine aminotransferase, increased aspartate aminotransferase, hypophosphatemia, increased gamma-glutamyltransferase, and appetite loss. All treatment-related AEs were manageable or recovered without any treatment, and no drug-related deaths were observed.
Table 2.
Treatment-related adverse events
| Grade | 0.1 mg/kg (N=20) | 1.0 mg/kg (N=19) | |||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | ||
| Cases | 16 | 12 | 6 | 1 | 10 | 14 | 4 | 2 | |
| Total events | 34 | 23 | 7 | 1 | 23 | 20 | 4 | 2 | |
| Non-hematological | |||||||||
| General | |||||||||
| Fever | 1 | 1 | 2 | ||||||
| Fatigue | 1 | 2 | |||||||
| Appetite loss | 1 | 1 | |||||||
| Edema | 1 | ||||||||
| Weight loss | 1 | ||||||||
| Skin and subcutaneous tissue | |||||||||
| Rash | 8 | 5 | 1 | 5 | 5 | ||||
| Pruritus | 1 | ||||||||
| Drug eruption | 1 | ||||||||
| Gastrointestinal | |||||||||
| Diarrhea | 1 | 1 | |||||||
| Nausea | 1 | ||||||||
| Vomiting | 1 | ||||||||
| Ear and labyrinth | |||||||||
| Vertigo positional | 1 | ||||||||
| Ear discomfort | 1 | ||||||||
| Cardiac disorders | |||||||||
| Arrhythmia | 1 | 2 | |||||||
| Electrocardiogram T wave inversion | 1 | ||||||||
| Hypertension | 1 | ||||||||
| Thoracic disorders | |||||||||
| Pleural effusion | 1 | ||||||||
| Nervous system disorders | |||||||||
| Dizziness | 1 | ||||||||
| Dysgeusia | 1 | ||||||||
| Headache | 3 | ||||||||
| Neuropathy peripheral | 1 | ||||||||
| Infections | |||||||||
| Oral candidiasis | 1 | ||||||||
| Upper respiratory tract Inflammation | 1 | ||||||||
| Cheilitis | 1 | ||||||||
| Endocrine | |||||||||
| Hypothyroidism | 1 | 1 | |||||||
| Gynecomastia | 1 | ||||||||
| Hematological | |||||||||
| Leukopenia | 2 | 1 | |||||||
| Lymphopenia | 1 | 10 | 3 | 1 | 7 | 2 | 2 | ||
| Thrombocytopenia | 1 | ||||||||
| Eosinophilia | 1 | ||||||||
| Hypokalemia | 1 | 2 | |||||||
| Hyponatremia | 1 | ||||||||
| Hypophosphatemia | 1 | ||||||||
| Hypoalbuminemia | 1 | 1 | |||||||
| GGT increased | 1 | ||||||||
| ALT increased | 3 | 1 | |||||||
| AST increased | 2 | 1 | |||||||
| Amylase increased | 1 | ||||||||
| LDH increased | 1 | 1 | |||||||
| ALP increased | 1 | 1 | |||||||
| TSH increased | 1 | ||||||||
| Glucose urine present | 1 | ||||||||
ALT: alanine aminotransferase
AST: aspartate aminotransferase
LDH: lactate dehydrogenase
TSH: thyroid-stimulating hormone
GGT: gamma-glutamyltransferase
ALP: alkaline phosphatase
Clinical Responses
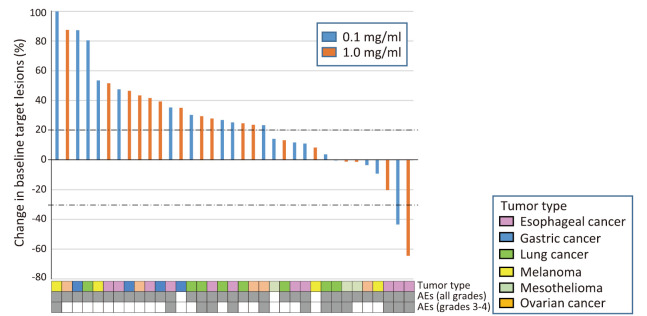
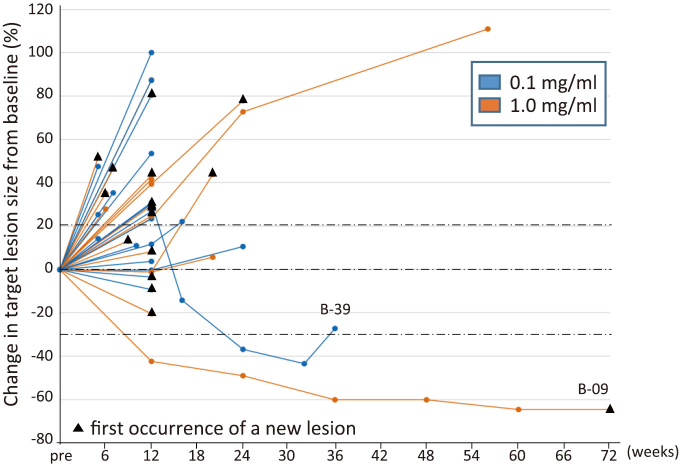
Confirmed objective responses with RECIST were SD in 3 patients with NSCLC, esophageal cancer, or mesothelioma in the 0.1 mg/kg cohort, and a partial response in 1 patient with esophageal cancer and SD in 2 patients with mesothelioma in the 1.0 mg/kg cohort (Table 1, Fig. 1). Median PFS was 67 and 65 days and OS was 271 and 272 days in the 0.1 and 1.0 mg/dl cohorts, respectively (Table 1). A durable response was observed in 2 patients (B-09, B-39) (Fig. 2).
Fig. 1.
Best percentage change in the target lesion tumor burden from baseline in patients with a CT assessment
Waterfall plot for the maximum percentage reduction in the target lesion tumor burden until disease progression (study-off) according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 progression. A positive change in the tumor burden indicates tumor growth; a negative change in the tumor burden indicates a tumor reduction. The horizontal dotted lines denote a 30% decrease and 20% increase, indicating an objective response and progressive disease, respectively, as per RECIST version 1.1. The tumor type and presence or absence of adverse events (AEs) are annotated for each patient. 0.1 mg/ml (n=18), blue bar; 1.0 mg/ml (n=17), orange bar.
Fig. 2.
Percentage change in the target lesion tumor burden from baseline over time
A spaghetti plot for changes from the baseline in the tumor burden, measured as the sum of the longest diameters of the target lesions by patients over time. The black triangle indicates the presence of a new lesion. Horizontal dotted lines denote a 30% decrease and 20% increase. 0.1 mg/ml (n=20), blue line; 1.0 mg/ml (n=19), orange line.
Efficacy of eTreg Depletion on PBMCs
eTreg depletion by KW-0761 was examined with PBMCs at baseline and during the KW-0761 treatment using flow cytometry. The percentage of eTregs in CD4+ T cells markedly decreased in all patients, except for 2 for whom blood samples were not available after the start of the KW-0761 treatment (Table 1). The median percentages of eTregs in CD4+ T cells in the 0.1 and 1.0 mg/kg cohorts were 2.1 and 2.1% at baseline and 0.22 and 0.21% after 4 infusions, respectively. The percentage of eTregs in blood remained low during the treatment with KW-0761.
DISCUSSION
The results of this phase Ib study confirmed the safety of KW-0761. Some treatment-related AEs were observed, including lymphopenia, which was the most frequent grade 3–4 AE; however, these AEs were all manageable and no drug-related deaths occurred. All AEs related to skin reactions in the present study were grades 1–2, except for one patient with a rash, which were less severe than those previously reported in ATLL patients.11,13 No significant difference was noted in the incidence of AEs between the 0.1 and 1.0 mg/kg cohorts.
The manipulation of Tregs has attracted increasing attention as a novel therapeutic strategy for cancer. In this phase Ib study, eTregs were efficiently depleted on PBMCs after the KW-0761 treatment. Several potential strategies to target Tregs are currently being clinically and preclinically investigated alone or in combination, mostly with ICIs. One approach is the depletion of Tregs by targeting molecules specifically expressed on Tregs, such as CD25, CTLA-4, CCR4, OX40 (CD134), inducible T cell co-stimulator (ICOS), and glucocorticoid-induced TNFR-related protein (GITR), or signals that are crucial for Treg cell survival and function, including T cell receptor (TCR) and IL-2 receptor signaling. Another approach for targeting Tregs is to control or modulate Treg cell function and infiltration, such as TGF-β, tyrosine kinase, phosphoinositide 3-kinase (PI3K), IDO, the CD39-CD73 axis, and vascular endothelial growth factor receptor (VEGFR) signaling. In these strategies, anti-CTLA-4 mAbs has attracted special attention because it yielded durable responses in a subset of cancer patients.3,20 Recent findings from several preclinical studies indicated that Treg depletion is one of the mechanisms underlying the antitumor effects of anti-CTLA-4 mAbs used as a checkpoint inhibitor.21,22 Anti-CTLA-4 mAb reportedly deplete Tregs from the TME, which may contribute to the clinical benefits of this agent.20 However, direct Treg cell-targeted therapy has not yet been applied to clinical settings. Treg recruitment at local sites is driven by combinations of chemokines and their receptors, such as CCL22 to CCR4 and CCL1 to CCR8.23,24 CCR4 is differentially expressed on the surface of various types of lymphocyte subpopulations. In a previous phase Ia study, we confirmed the high expression of CCR4 on eTregs and CCR4+ eTreg enrichment in tumor specimens.17,25 Moreover, an in vitro experiment on an anti-CCR4 mAb treatment with PBMCs showed the efficient induction of NY-ESO-specific CD4+ and CD8+ T cells due to the depletion of CCR4+ eTregs. ATLL patients treated with KW-0761 showed the induction of NY-ESO-1-specific CD8+ T cells, potentially contributing to the prolongation of survival. These findings suggest the contribution of KW-0761 to antitumor immune enhancements through Treg depletion.
In this clinical trial, while KW-0761 effectively depleted eTregs in PBMCs, most of the treated patients did not exhibit tumor regression, which is consistent with the findings of our previous phase Ia study; however, a durable clinical response was observed in 2 patients with esophageal cancer. A possible explanation for the low clinical efficacy of KW-0761 is that KW-0761 may deplete another type of CCR4+ T-cell subset. The comprehensive assessment conducted in the previous phase Ia study showed the high expression of CCR4 on eTregs, Th2 CD4+ T cells, and Th17 CD4+ T cells and the low expression of CCR4 on Th1 CD4+ T cells and CD8+ T cells.17 The efficacy of KW-0761 depends on the balance between the depletion of eTregs, which may improve antitumor immunity, and the depletion of other types of cells, including CD8+ T cells or Th1 CD4+ T cells, which may attenuate antitumor immunity. Another plausible explanation for impaired clinical responses by KW-0761 is that eTregs were not sufficiently depleted in the TME. It currently remains unclear whether these drugs selectively deplete Tregs in the TME. The density of Tregs in the TME is not always reflected in peripheral blood. In this clinical trial, we observed Treg reductions in the tumor of a patient with biopsy specimens that were collected at baseline and post-treatment (data not shown). Therefore, further basic and translational research is warranted to obtain a more detailed understanding of Treg cell functions, particularly in the TME.
In summary, KW-0761 was safely administered and efficiently depleted eTreg in PBMCs, with potential immune responses in solid CCR4-negative cancers. We are currently conducting a phase I clinical trial on the preoperative administration of KW-0761 combined with anti-PD-1 mAb to patients with advanced or recurrent solid cancer, with the expectation of the synergistic effects of these immunologically different functional treatments. Although further refinement of the regimen is required, the combined use of KW-0761 to deplete Tregs and other immunotherapies is a promising strategy to augment immune responses.
IN MEMORIAM
This study is dedicated to the memory of the late Dr. E. Nakayama.
ACKNOWLEDGMENTS
We thank all the patients and their families who participated in this clinical trial. We also thank all the nurses, clinical research coordinators in Kawasaki Medical School Hospital, Nagoya City University Hospital, Osaka University Hospital, and National Cancer Center Hospital East, Tokyo University Hospital, Keio University Hospital, review committees, and medical experts who were involved in this study. We are grateful to Drs. Y. Ueda, H. Nagase, Y. Ohue, T. Oguri, A. Arakawa, M. Nakamura, Y. Mori, T. Ishida, H. Matsushita, M. Anraku, and M. Sugaya for their clinical support, Dr. H. Ito for statistical support, and Drs. K. Iwata, S. Kihara, and T. Hida for data and safety monitoring support. We are also grateful to Kyowa Hakko Kirin for providing us with the investigational drug KW-0761.
FUNDING/SUPPORT
This study was supported by Grants-in-Aid for Scientific Research (S) grant no. 17H06162 (H.N.), Challenging Exploratory Research grant no. 16K15551 (H.N.), Research Activity Start-up grant no. 15H06878 (Y.M.), Young Scientists (B) grant no. 17K15738 (Y.M.), and Scientific Research (B) grant no. 19H03729 (H.W.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; by the Projects for Cancer Research by Therapeutic Evolution [P-CREATE, no. 16cm0106301h0001 (H.N.) and no. 17cm0106322h0002 (Y.M.)], by the Development of Technology for Patient Stratification Biomarker Discovery grant [no.19ae0101074s0401 (R.U. and H.N.)] from the Japan Agency for Medical Research and Development (AMED), and by the National Cancer Center Research and Development Fund [no. 28-A-7 and 31-A-7 (H.N.)].
CONFLICTS OF INTEREST
T.K. received honoraria and research funding from Ono Pharmaceutical, MSD, Shionogi, Bristol-Myers Squibb, Chugai Pharmaceutical, Amgen, Astellas Pharmaceutical, Oncolys BioPharma, Parexel, and Merck Serono outside of this study. T.F. received research funding from Ono Pharmaceutical outside of this study. H.N. received honoraria and research funding from Ono Pharmaceutical, Chugai Pharmaceutical, MSD, and Bristol-Myers Squibb, and research funding from Taiho Pharmaceutical, Daiichi-Sankyo, Kyowa Kirin, Zenyaku Kogyo, Oncolys BioPharma, Debiopharma, Asahi-Kasei, Sysmex, Fujifilm, SRL, Astellas Pharmaceutical, Sumitomo Dainippon Pharma, and BD Japan outside of this study. K.K. received research funding from TAKARA BIO and MSD outside of this study. The Department of Immunotherapeutics, The University of Tokyo Hospital is endowed by TAKARA BIO. S.I. received honoraria and research funding from Ono Pharmaceutical, Takeda, Sanofi, Bristol-Myers Squibb, Janssen, Celgene and Daichi-Sankyo, and research funding from Kyowa Kirin, Abbvie, Chugai Pharmaceutical, MSD, and Gilead outside of this study. Y.D. received honoraria and research funding from Ono Pharmaceutical, Taiho Pharmaceutical, and research funding from Chugai Pharmaceutical, Covidien Japan, Johnson & Johnson, and honoraria from Otsuka Pharmaceutical outside of this study. M.O. received research funding from Thyas, Sysmex, and Pole Star outside of this study. R.U. received research funding from Ono Pharmaceutical, Chugai Pharmaceutical, and Kyowa Kirin outside of this study. H.W. received research funding from Ono Pharmaceutical and Kyowa Kirin, and honoraria from Ono Pharmaceutical, Chugai Pharmaceutical, MSD, and Bristol-Myers Squibb outside of this study. The Department of Clinical Research in Tumor Immunology, Osaka University Graduate School of Medicine is a joint research laboratory with Shionogi & Co., Ltd.
Abbreviations
- Tregs
regulatory T cells
- AEs
adverse events
- TME
tumor microenvironment
- mAbs
antibodies
- PBMCs
blood mononuclear cells
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4
- PD-1
programmed cell death protein 1
- IDO
indoleamine 2,3-dioxygenase
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
- ICOS
inducible T cell co-stimulator
- GITR
glucocorticoid-induced TNFR-related protein
- PI3K
phosphoinositide 3-kinase
- TCR
T cell receptor
REFERENCES
- 1.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed]
- 2.Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed]
- 3.Hodi FS, O’Day SJ, McDermott DF, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1402685. [DOI] [PMC free article] [PubMed]
- 4.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed]
- 5.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11(1):7–13. doi: 10.1038/ni.1818. [DOI] [PubMed]
- 6.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102(51):18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed]
- 7.Fridman WH, Pagès F, Saut`s-Fridman C, Galon J. The immune contexture in human tumours: Impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed]
- 8.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor α) monoclonal antibody. Cancer Res. 1999;59(13):3128–3133. [PubMed]
- 9.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163(10):5211–5218. [PubMed]
- 10.Ishii T, Ishida T, Utsunomiya A, et al. Defucosylated humanized aanti-CCR4 monoclonal antibody KW-0761 as a novel immunotherapeutic agent for adult T-cell leukemia/lymphoma. Clin Cancer Res. 2010;16(5):1520–1531. doi: 10.1158/1078-0432.CCR-09-2697. [DOI] [PubMed]
- 11.Yamamoto K, Utsunomiya A, Tobinai K, et al. Phase I study of KW-0761, a defucosylated humanized anti-CCR4 antibody, in relapsed patients with adult T-cell leukemia-lymphoma and peripheral T-cell lymphoma. J Clin Oncol. 2010;28(9):1591–1598. doi: 10.1200/JCO.2009.25.3575. [DOI] [PubMed]
- 12.Yoshie O, Fujisawa R, Nakayama T, et al. Frequent expression of CCR4 in adult T-cell leukemia and human T-cell leukemia virus type 1-transformed T cells. Blood. 2002;99(5):1505–1511. doi: 10.1182/blood.V99.5.1505. [DOI] [PubMed]
- 13.Ishida T, Joh T, Uike N, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: A multicenter phase II study. J Clin Oncol. 2012;30(8):837–842. doi: 10.1200/JCO.2011.37.3472. [DOI] [PubMed]
- 14.Yonekura K, Kusumoto S, Choi I, et al. Mogamulizumab for adult T-cell leukemia-lymphoma: a multicenter prospective observational study. Blood Adv. 2020;4(20):5133–5145. doi: 10.1182/bloodadvances.2020003053 [DOI] [PMC free article] [PubMed]
- 15.Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed]
- 16.Saito M, Ishii T, Urakawa I, et al. Robust CD81 T-cell proliferation and diversification after mogamulizumab in patients with adult T-cell leukemia-lymphoma. Blood Adv. 2020;4(10):2180–2191. doi: 10.1182/bloodadvances.2020001641. [DOI] [PMC free article] [PubMed]
- 17.Kurose K, Ohue Y, Wada H, et al. Phase Ia Study of FoxP3+ CD4 Treg Depletion by Infusion of a Humanized Anti-CCR4 Antibody, KW-0761, in Cancer Patients. Clin Cancer Res. 2015;21(19):4327–4336. doi: 10.1158/1078-0432.CCR-15-0357. [DOI] [PubMed]
- 18.Ohue Y, Kurose K, Karasaki T, et al. Serum Antibody Against NY-ESO-1 and XAGE1 Antigens Potentially Predicts Clinical Responses to Anti–Programmed Cell Death-1 Therapy in NSCLC. J Thorac Oncol. 2019;14(12):2071–2083. doi: 10.1016/j.jtho.2019.08.008. [DOI] [PubMed]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed]
- 20.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed]
- 21.Simpson TR, Li F, Montalvo-Ortiz W, et al. Fc-dependent depletion of tumor-infiltrating regulatory t cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210(9):1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed]
- 22.Vargas FA, Furness AJS, Litchfield K, et al. Fc Effector Function Contributes to the Activity of Human Anti-CTLA-4 Antibodies. Cancer Cell. 2018;33(4):649–663.e4. doi: 10.1016/j.ccell.2018.02.010. [DOI] [PMC free article] [PubMed]
- 23.Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: Chemokines in control of T cell traffic. Nat Immunol. 2008;9(9):970–980. doi: 10.1038/ni.f.213. [DOI] [PubMed]
- 24.Spranger S, Spaapen RM, Zha Y, et al. Up-regulation of PD-L1, IDO, and Tregs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci Transl Med. 2013;5(200) 200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed]
- 25.Sugiyama D, Nishikawa H, Maeda Y, et al. Anti-CCR4 mAb selectively depletes effector-Type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci U S A. 2013;110(44):17945–17950. doi: 10.1073/pnas.1316796110. [DOI] [PMC free article] [PubMed]