ABSTRACT
The efficacy and safety of cyclooxygenase 2 (COX2) inhibitors for the treatment of desmoid-type fibromatosis (DF) are unclear. Therefore, we systematically reviewed related literature to assess the efficacy and safety of COX2 inhibitors for DF treatment. We searched pertinent literature between January 1999 and August 2017 to identify relevant studies using the keywords “Fibromatosis, aggressive” and “Cyclooxygenase inhibitors.” Thereafter, we screened and determined the quality of the studies using the Grading of Recommendations Assessment, Development, and Evaluation system and extracted the article data. The critical outcomes selected were the efficacy and adverse effects of COX2 inhibitors. Efficacy was evaluated in terms of clinical benefit when patients showed complete response, partial response, and stable disease. Thirty-one articles were identified from the database search, and one was identified through the reviewers’ manual search. Finally, we retrieved six studies, including three case reports, comprising 89 patients after the first and second screenings. Fifty-three patients were excluded because three studies were reported from the same institution; hence, in total, 36 patients were included. Clinical benefit was noted in 64% of the patients. Three adverse effects were identified from the records of the six extracted studies. The strategy of watchful waiting using COX2 inhibitors with few side effects is weakly recommended for DF, especially DF patients with pain.
Key Words: cyclooxygenase 2 inhibitors, desmoid, response rate, adverse effect, systematic review
INTRODUCTION
Desmoid-type fibromatosis (DF) is a rare monoclonal, fibroblastic proliferation marked by infiltrative growth and an inability to metastasize. Historically, the management of DF involved surgical resection with negative margins as the standard treatment. However, occasionally, complete surgical resection can be difficult to achieve. Furthermore, the rate of local recurrence is high even after wide resection and is accompanied by the risk of chronic pain, loss of function, and impaired quality of life because DF infiltrates the surrounding structures and spreads along planes and muscle.1
Given the unpredictable nature of the disease, including the possibility of spontaneous regression, increasing attention has been directed toward initial nonoperative management.2,3 Nonoperative treatment includes watchful waiting using nonsteroidal anti-inflammatory drugs (NSAIDs) with or without hormonal manipulation, chemotherapy, or radiation therapy. Aggressive chemotherapy should be avoided because it is associated with significant morbidities. NSAIDs are a relatively safe treatment for DF. Pharmacological blockade of cyclooxygenase 2 (COX2), which was reported to decrease cell proliferation in animal models, may contribute to therapeutic effects in DF.4 However, the antitumor activity of COX2 inhibitors for clinical use in humans is not well understood. Hence, the objective of this study was to systematically review the pertinent available literature to assess the efficacy and safety of COX2 inhibitors for treating patients with DF.
METHODS
We established a guideline committee for extra-abdominal DF to develop optimal clinical guidelines for DF management under the direction of the Ministry of Health, Labour and Welfare of Japan with the cooperation of the Japan Orthopedic Association (JOA) and the Bone and Soft Tissue Oncology Committee of the JOA.5 The committee outlined the initial project scope; key principles; and relevant patient/population, intervention, comparison, and outcome (PICO) questions. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) system6 was employed to combine the results of the systematic review with expert opinion to address the following clinical question for extra-abdominal DF: “Is nonoperative treatment using COX2 inhibitors effective for patients with extra-abdominal DF?”
Conflicts of interest and selection of systematic review members
No specific industry played any roles in this consensus meeting, including the systematic review committee. Members of the systematic review committee were invited to join by the chair (Y.N.) and the clinical practice guidelines development committee.
Search strategy
An expert librarian (N.Y. mentioned in the acknowledgments) searched PubMed/MEDLINE, the Japan Medical Abstract Society (JMAS; Ichushi in Japanese), the Cochrane library, the Guideline in National Quality Measures Clearinghouse, and the National Institute for Health and Care Excellence databases for potentially relevant literature between January 1999 and August 2017. The following search keywords were used: “Fibromatosis, aggressive” and “Cyclooxygenase inhibitors“. We screened and determined the quality of the studies using the GRADE system and extracted the data from the articles.7
Study selection
The eligibility of the selected articles was determined in two phases. In the first phase of study selection, we evaluated the studies for eligibility by screening the titles and abstracts. In the second phase, we obtained and screened the full text of all papers according to our inclusion and exclusion criteria. This systematic review for the PICO question, “Is nonoperative treatment using COX2 inhibitors effective for patients with extra-abdominal DF?,” was composed of (P) participants: patients with extra-abdominal DF; (I) intervention: nonoperative treatment using COX2 inhibitors; (C) comparison: watchful waiting, and (O) outcomes: the critical outcomes selected were efficacy and adverse events. The articles selected included studies with all types of study designs: cases reports, case series, case-control studies, and clinical trials. The exclusion criteria were as follows: (a) non-English and non-Japanese language; (b) animal subjects; (c) review articles or comments; and (d) experimental studies.
Data extraction and quality appraisal
Data were extracted by an experienced investigator belonging to the Japan Medical Library Association (N.Y.). The data recorded were the first author, publication year, study design, type of COX2 inhibitor, and efficacy of and adverse effects of COX2 inhibitors. The primary outcome to be evaluated was the efficacy of COX2 inhibitors in DF patients, which was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST). Efficacy was evaluated in terms of clinical benefit when patients showed complete response (CR), partial response (PR), and stable disease (SD). The second outcome to be evaluated was adverse effects. The methodological quality of the included studies was assessed by two investigators (M.E. and Y.M.) independently according to the methods proposed by the Minds Tokyo GRADE center.
Rating the quality of evidence
The two reviewers independently rated the quality of the evidence according to the GRADE approach.8 We used the Cochrane risk of bias tool for the assessment of bias risk.9 The two reviewers evaluated the extracted individual studies and created ‘an evaluation sheet’ for each critical and important outcome, taking into account limitations of the study design, integrated bias risk, increasing factor and indirectness to PICO, and event number of patients/risk number of patients. Based on the evaluation sheets of all outcomes, the reviewers created the ‘Body of Evidence’ for the clinical question in consideration of limitations of the study design, risk of bias, inconsistency, indirectness, imprecision and other factors according to the methods proposed by Minds Tokyo GRADE center as A (strong), B (moderate), C (weak) and D (very weak). The reviewers created a ‘Qualitative Systematic Review’ and a ‘Summary of Systematic Review Report’, which they provided to the clinical guideline committee. A recommendation was decided in consideration of the balance of relative benefits and harm of the COX2 treatment, the quality of the evidence and patients’ preferences, under GRADE approach. The clinical guideline committee voted on the recommendation and its strength related to this clinical question. To decide them, 75% consent was required. If 75% agreement was not obtained in the first vote, the voting members discussed again before re-voting. For the developed recommendation, a written explanation was given that led to the decision by the guideline member in charge of this clinical question.
RESULTS
Study selection and study characteristics
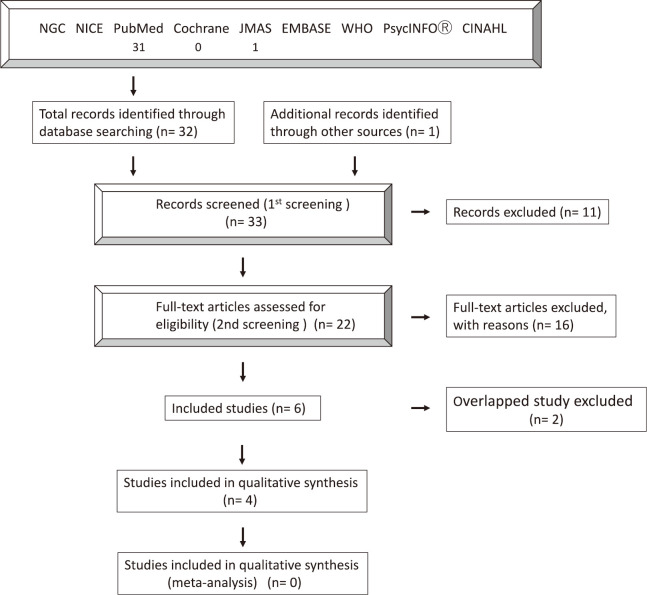
In total, 32 articles were identified from the database search and one article was identified through other sources by the reviewers’ manual search. Of the 33 articles, 11 were excluded as their title or abstract was not relevant to the outcome of interest for the review. The full texts of the remaining 22 articles were examined as the second screening. In total, 16 articles were excluded for not meeting the eligibility criteria for evaluation. The most common reason for exclusion was the combined use of COX2 inhibitors with other drugs (n = 10), comment or editorial (n = 3), and experimental model (n = 3). Finally, six articles met the eligibility criteria for evaluation in this systematic review. A detailed flowchart of the selection process is shown in Figure 1. All studies were single-center, retrospective, and case series. The number of participants in each study ranged from one to 33; the studies enrolled a total of 89 patients. However, two12,14 studies out of three12-14 were excluded to prevent the inclusion of duplicate cases, because three research papers were published from the same institution. Of the three papers, the study with the largest number of cases was selected. Finally, 4 studies and 36 patients were included in this review. The characteristics of the selected studies are shown in Table 1. The medication period ranged from 2 to 113 months. In this systematic review, a comparison with watchful waiting alone was not possible because no comparative study was available.
Fig. 1.
Detailed flowchart of the study selection process
Table 1.
The characteristics of the 4 studies after 1st and 2nd screening
| Reference | Study | Patient
number |
COX2
inhibitors |
Rate of
Outcome (%) |
Response (patient number) | Adverse
effect patient number |
|||
|---|---|---|---|---|---|---|---|---|---|
| Complete
response |
Partial
response |
Stable
disease |
Progressive
disease |
||||||
|
Hamada
et al13 |
case series,
retrospective |
33 | meloxicam
10mg/day |
60 | 1 | 7 | 12 | 13 | 0 |
|
Yang
et al11 |
case report,
retrospective |
1 | celecoxib
200mg/day |
100 | 1 | 0 | 0 | 0 | 0 |
|
Tanaka
et al15 |
case report,
retrospective |
1 | etodolac
200mg/day |
100 | 1 | 0 | 0 | 0 | 1
(hot flushes) |
|
Wang
et al10 |
case report,
retrospective |
1 | celecoxib
200mg/day |
100 | 1 | 0 | 0 | 0 | 0 |
Efficacy of NSAIDs
Table 1 shows the results of each study and case report with regard to the outcomes of clinical benefit and adverse events. Three kinds of medication were used: celecoxib (200 mg/day),10,11 meloxicam (10 mg/day),12-14 and etodolac (200 mg/day).15 Of the 36 patients evaluated, 4 showed CR, 7 showed PR, 12 showed SD, and 13 showed progressive disease. Therefore, the clinical benefit was 64%.
Adverse effects of NSAIDs
Among the included studies and case reports (the initial six studies), only one study and a case report provided information regarding adverse effects.8,13 There were only three adverse effects, gastritis, diarrhea, and hot flushes. Only one adverse effect was described in the four studies selected (Table 1).
Body of evidence, Qualitative Systematic Review, and Summary of Systematic Review Report
The reviewers created the “Body of Evidence” of important outcomes (efficacy and adverse events) for the clinical question and integrated it (Table 2).7 Since all articles were case series or case reports, the strength of evidence began with C. There were several “Serious” biases in the selected studies; therefore, we considered that the evidence level should be lowered from C to D for efficacy. Regarding adverse events, the existence of several biases could lead to a very low rate of incidence. Hence, the strength level was also reduced from C to D for adverse events. “Qualitative Systematic Review” for clinical question was summarized (Table 3). The reviewers also created the “Summary of Systematic Review Report” as follows, “there were no articles comparing the results of clinical benefit between watchful waiting with COX2 inhibitor administration and without. All articles on clinical benefit in the COX2 inhibitors administration are retrospective case-accumulation studies, and the target patients are also inconsistent with the first and recurrence cases, and the evidence is extremely low.” Clinical benefit was favorable and adverse effect was very rare with COX-2 inhibitor administration. Therefore, the guideline committee considered the conservative treatment using COX2 inhibitors was more valuable and preferable than conservative treatment without COX2 inhibitors. Furthermore, the conservative treatment using COX2 inhibitors gave little burden to patients with extra-abdominal desmoid fibromatosis because treatment using COX2 inhibitors is within health care services provided by health insurance in Japan.
Table 2.
Study quality of evidence according to GRADE guidelines
| Outcomes | No.
of studies |
Study
design |
Risk
of bias |
Inconsistency | Imprecision | Indirectness | Others | Rate of
outcome (%) |
Level
of evidencea |
Significance
of outcomeb |
|---|---|---|---|---|---|---|---|---|---|---|
|
Efficacy of
COX2 inhibitors* (CR+ PR+ SD) |
4 | Case
series and case report |
Serious | Not serious | Not serious | Serious | Serious | 64 | D | 8 |
| Adverse events | 1 | Case
report |
Serious | Serious | Serious | Serious | Serious | 3 | D | 6 |
*Complete remission + partial response + stable disease
aA, strong; B, moderate; C, low; D, very low
bSignificance of outcome was determined by guideline committee when setting up PICO by a 1–10 point evaluation. (10: most important)
Table 3.
Qualitative systematic review
| Clinical question | Is nonoperative treatment using COX2 inhibitors effective for patients with extra-abdominal desmoid fibromatosis? |
|---|---|
| P | Patients with extra-abdominal desmoid fibromatosis |
| I | Nonoperative treatment using COX2 inhibitors |
| C | Watchful waiting |
| Clinical context | Since desmoid fibromatosis shrink spontaneously in some cases, careful follow-up by “wait and see” may be performed. Verify whether COX2 inhibitors are more useful for extra-abdominal desmoid fibromatosis than “wait and see” as a drug treatment. |
| Outcome | Response rate |
| Summary of indirectness | All 4 studies do not have a watchful waiting group |
| Summary of bias risk | All are retrospective studies and have a high risk of bias |
| Summary of inconsistencies | Subjects include first-onset and recurrent cases, and are inconsistent |
| Comment | All are retrospective and do not include the watchful waiting group, so the level of evidence is low. |
The “Body of Evidence,” “Qualitative Systematic Review,” and “Summary of Systematic Review Report” created by the reviewers were submitted to the guidelines committee. Based on these reports, the guideline committee discussed the following points. Clinical benefit was favorable and adverse effect was very rare with COX-2 inhibitor administration. The conservative treatment using COX2 inhibitors was more valuable and preferable than conservative treatment without COX2 inhibitors in patients with pain. Furthermore, the conservative treatment using COX2 inhibitors gave little burden to patients with extra-abdominal desmoid fibromatosis because treatment using COX2 inhibitors is within health care services provided by health insurance in Japan. All together, the guidelines committee in charge of the clinical question proposed the following recommendation: “We weakly recommend nonoperative treatment using COX2 inhibitors in patients with DF.” This recommendation gained a consensus rate of 94% in the entire guidelines group.
DISCUSSION
In this systematic review, four studies including case reports were studied to evaluate the efficacy and safety of COX2 inhibitors for DF treatment. The clinical benefit rate of COX2 inhibitors for DF treatment was 64% and the rate of adverse effects was very low (3 cases in six studies, 1 case in 4 studies). Patients with DF could be treated using “watchful waiting” with COX2 inhibitors because they afford favorable local control and are associated with a low rate of adverse effects. Watchful waiting with COX2 inhibitors allows patients to avoid the significant morbidity risk associated with surgery or radiation and invasive intervention.
Therapy for DF is aimed at local control and/or reduction of symptoms that bother patients because DF does not have metastatic potential. Historically, complete surgical resection with wide margins has been the standard care; however, resection of this type often results in significant functional impairment. Moreover, the rates of local recurrence after resection, even with wide surgical margins, are high, ranging from 20 to 60% depending on the series.16-18 While DF has an unpredictable natural history, some cases show rapid growth followed by periods of stabilization; occasionally, spontaneous regression is noted. Spontaneous regression is reported in up to 20% of DF patients.19 Therefore, watchful waiting is increasingly advocated for DF patients. With regard to real-world clinical experience, many DF patients have complained of pain, leading to poor quality of life. Although COX2 inhibitors could control DF based on the results of an animal study,4 the efficacy of COX2 inhibitors in human DF is controversial because of a lack of randomized controlled studies. In this review, the clinical benefit achieved with COX2 inhibitors was favorable; however, comparison with watchful waiting alone was not possible. Watchful waiting with COX2 inhibitors could be considered one of the treatment choices because of the favorable local control and low rate of adverse effects associated with these drugs; moreover, DF patients experience severe pain. Our findings support the current consensus released by the European Organization for Research and Treatment of Cancer (EORTC). The EORTC advocates that observation should be the first-line treatment, irrespective of pain or clinical symptoms.2,20 However, pain management is a major issue for patients with DF. The pathogenetic mechanisms of pain in DF are certainly complex and multifactorial. DF-related pain is associated with the overexpression of COX2.21 Therefore, watchful waiting with COX2 inhibitors may be suitable for DF patients with pain.
The course of DF is difficult to predict; therefore, it is important to identify the predictors of DF progression during watchful waiting. Cassidy et al reported that the percentage of baseline tumor volume showing a hyperintense signal on T2 magnetic resonance imaging is a useful tool for clinical decision-making for patients with DF considering watchful waiting.22 DF patients with a hyperintense T2 score of ≥90% have a high likelihood of progression. Therefore, for these DF patients, watchful waiting with or without COX2 inhibitor administration might not be sufficient as a treatment modality.
There are limitations to the current systematic review that are common to most papers evaluating rare diseases. The evidence from the included studies was of low quality, and there were many case reports. Moreover, three studies of case series were from the same institution, two of which should be excluded from this systematic review. In addition, there are some variations in the medication. Clinical outcomes of watchful waiting with or without COX2 inhibitor should be evaluated in the future.
CONCLUSION
The efficacy of COX2 inhibitor treatment was favorable among patients with DF, although there is no comparative study with no treatment. Further, the incidence rate of adverse events was very low. Considering that many DF patients experience pain that cannot be ignored, we weakly recommend watchful waiting using COX2 inhibitors for DF patients. However, clinical outcomes of watchful waiting with or without COX2 inhibitor should be evaluated in the future.
ACKNOWLEDGEMENTS
We thank Japan Medical Library Association and Mr Naohiko Yamaguchi for their support to literature search.
DISCLOSURE STATEMENT
The authors declare that they have no conflict of interest.
Abbreviations
- COX
cyclooxygenase
- CR
complete response
- DF
desmoid-type fibromatosis
- NSAIDs
nonsteroidal anti-inflammatory drugs
- PR
partial response
- SD
stable disease
REFERENCES
- 1.Peng PD, Hyder O, Mavros MN, et al. Management and recurrence patterns of desmoids tumors: a multi-institutional analysis of 211 patients. Ann Surg Oncol. 2012;19(13):4036–4042. doi: 10.1245/s10434-012-2634-6. [DOI] [PMC free article] [PubMed]
- 2.Kasper B, Baumgarten C, Bonvalot S, et al. Management of sporadic desmoid-type fibromatosis: a European consensus approach based on patients’ and professionals’ expertise - a sarcoma patients EuroNet and European Organisation for Research and Treatment of Cancer/Soft Tissue and Bone Sarcoma Group initiative. Eur J Cancer. 2015;51(2):127–136. doi: 10.1016/j.ejca.2014.11.005. [DOI] [PubMed]
- 3.Ghert M, Yao X, Corbett T, et al. Treatment and follow-up strategies in desmoid tumours: a practice guideline. Curr Oncol. 2014;21(4):e642–e649. doi: 10.3747/co.21.2112. [DOI] [PMC free article] [PubMed]
- 4.Poon R, Smits R, Li C, et al. Cyclooxygenase-two (COX-2) modulates proliferation in aggressive fibromatosis (desmoid tumor). Oncogene. 2001;20(4):451–460. doi: 10.1038/sj.onc.1204107. [DOI] [PubMed]
- 5.Shimizu K, Hamada S, Sakai T, Koike H, Yoshida M, Nishida Y. Efficacy of low-dose chemotherapy with methotrexate and vinblastine for patients with extra-abdominal desmoid-type fibromatosis: a systematic review. Jpn J Clin Oncol. 2020;50(4):419–424. doi: 10.1093/jjco/hyz204. [DOI] [PubMed]
- 6.Andrews JC, Schunemann HJ, Oxman AD, et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation’s direction and strength. J Clin Epidemiol. 2013;66(7):726–735. doi: 10.1016/j.jclinepi.2013.02.003. [DOI] [PubMed]
- 7.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed]
- 8.Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed]
- 9.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed]
- 10.Wang YC, Wong JU. Complete remission of pancreatic head desmoid tumor treated by COX-2 inhibitor-a case report. World J Surg Oncol. 2016;14(1):190. doi: 10.1186/s12957-016-0944-z. [DOI] [PMC free article] [PubMed]
- 11.Yang S, Wang X, Jiang H, Wang Y, Li Z, Lu H. Effective treatment of aggressive fibromatosis with celecoxib guided by genetic testing. Cancer Biol Ther. 2017;18(10):757–760. doi: 10.1080/15384047.2017.1373215. [DOI] [PMC free article] [PubMed]
- 12.Nishida Y, Tsukushi S, Shido Y, Wasa J, Ishiguro N, Yamada Y. Successful treatment with meloxicam, a cyclooxygenase-2 inhibitor, of patients with extra-abdominal desmoid tumors: a pilot study. J Clin Oncol. 2010;28(6):e107–9. doi: 10.1200/jco.2009.25.5950. [DOI] [PubMed]
- 13.Hamada S, Futamura N, Ikuta K, et al. CTNNB1 S45F mutation predicts poor efficacy of meloxicam treatment for desmoid tumors: a pilot study. PloS One. 2014;9(5):e96391. doi: 10.1371/journal.pone.0096391. [DOI] [PMC free article] [PubMed]
- 14.Hamada S, Urakawa H, Kozawa E, et al. Nuclear expression of beta-catenin predicts the efficacy of meloxicam treatment for patients with sporadic desmoid tumors. Tumour Biol. 2014;35(5):4561–4566. doi: 10.1007/s13277-013-1600-7. [DOI] [PubMed]
- 15.Tanaka K, Yoshikawa R, Yanagi H, et al. Regression of sporadic intra-abdominal desmoid tumour following administration of non-steroidal anti-inflammatory drug. World J Surg Oncol. 2008;6:17. doi: 10.1186/1477-7819-6-17. [DOI] [PMC free article] [PubMed]
- 16.Gronchi A, Casali PG, Mariani L, et al. Quality of surgery and outcome in extra-abdominal aggressive fibromatosis: a series of patients surgically treated at a single institution. J Clin Oncol. 2003;21(7):1390–7. doi: 10.1200/jco.2003.05.150. [DOI] [PubMed]
- 17.Merchant NB, Lewis JJ, Woodruff JM, Leung DH, Brennan MF. Extremity and trunk desmoid tumors: a multifactorial analysis of outcome. Cancer. 1999;86(10):2045–2052. [PubMed]
- 18.Lev D, Kotilingam D, Wei C, et al. Optimizing treatment of desmoid tumors. J Clin Oncol. 2007;25(13):1785–1791. doi: 10.1200/jco.2006.10.5015. [DOI] [PubMed]
- 19.Bonvalot S, Ternes N, Fiore M, et al. Spontaneous regression of primary abdominal wall desmoid tumors: more common than previously thought. Ann Surg Oncol. 2013;20(13):4096–4102. doi: 10.1245/s10434-013-3197-x. [DOI] [PubMed]
- 20.Kasper B, Baumgarten C, Garcia J, et al. An update on the management of sporadic desmoid-type fibromatosis: a European Consensus Initiative between Sarcoma PAtients EuroNet (SPAEN) and European Organization for Research and Treatment of Cancer (EORTC)/Soft Tissue and Bone Sarcoma Group (STBSG). Ann Oncol. 2017;28(10):2399–2408. doi: 10.1093/annonc/mdx323. [DOI] [PMC free article] [PubMed]
- 21.Emori M, Kaya M, Mitsuhashi T, Asanuma H, Yamashita T. Desmoid tumor-associated pain is dependent on mast cell expression of cyclooxygenase-2. Diagn Pathol. 2014;9:14. doi: 10.1186/1746-1596-9-14. [DOI] [PMC free article] [PubMed]
- 22.Cassidy MR, Lefkowitz RA, Long N, et al. Association of MRI T2 Signal Intensity With Desmoid Tumor Progression During Active Observation: A Retrospective Cohort Study. Ann Surg. 2018;doi: 10.1097/sla.0000000000003073. [DOI] [PMC free article] [PubMed]