Abstract
OBJECTIVES
To investigate complications within 30-days following first-time ablation for atrial fibrillation (AF), including a composite of cardiac tamponade, hematoma requiring intervention, stroke or death, in patients ≥ 75 years of age, compared to patients aged 65−74 years. In addition, one-year all-cause mortality and AF relapse were compared.
METHODS & RESULTS
All patients receiving their first catheter ablation for AF between 2012 and 2016 were identified using Danish nationwide registries. Patients aged 65−74 years served as the reference group for patients ≥ 75 years. Relapse of AF within one year was defined as cardioversion following a three-month blanking period, re-ablation or confirmed relapse within follow-up. The composite complication outcome did not differ between the two age groups, with 39/1554 (2.8%) in patients 65−74 years of age, versus 5/199 (2.5%) in older patients (adjusted HR = 0.94), 95% CI: 0.37−2.39, P = 0.896). Patients ≥ 75 years or older had no increased hazard of death within 30 days after the procedure, with an incidence of 3/1554 (0.2%) in younger patients and 2/199 (1.0%) in patients ≥ 75 years of age (adjusted HR = 4.71, 95% CI: 0.78−28.40, P = 0.091). There was no difference in relapse of AF after one year between age groups (≥ 75 years adjusted HR = 1.00, 95% CI: 0.78-1.26, P = 0.969).
CONCLUSION
In patients ≥ 75 years of age selected for catheter ablation for AF, the incidence of periprocedural complications, as well as one-year freedom from AF showed no statistical difference, when compared to patients 65−74 years of age.
Pulmonary vein isolation by means of catheter ablation has become a commonly used therapy for drug-resistant atrial fibrillation (AF) and as a result of a steadily increase in life expectancy along with improved methods to diagnose AF, the incidence of AF is on the rise.[1] Pulmonary vein isolation has a success rate of 50%−70% after one procedure, and short-term procedural complications including death, stroke, cardiac tamponade and atrio-esophageal fistula vary from 2%−3%.[1,2] Other complications include pericardial effusion, phrenic nerve injury, pulmonary vein stenosis and vascular complications, including hematomas and pseudo-aneurisms, with complication rates ranging from < 1%−4%. [1] Regarding the treatment of elderly patients with catheter ablation, the 2016 ESC guidelines for the management of atrial fibrillation states that “In general, better rhythm outcome and lower procedure-related complications can be expected in younger patients with a short history of AF and frequent, short AF episodes in the absence of significant structural heart disease”, and “although the evidence base is smaller for other treatment options in AF, the available data support the use of available rate and rhythm control interventions, including pacemakers and catheter ablation, without justification to discriminate by age group”.[3] However, many studies referred to in this guideline have a relatively low volume of elderly patients, as the mean age of patients included were between 52.6−66 years. The 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation stated that outcome data was needed in “high-risk populations”, including the very elderly.[4]
In the latest 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation, AF catheter ablation is stated as it “may be an effective and safe option in selected older individuals with success rates comparable to younger patients and acceptable complication rates”.[1] This change in recommendation compared to 2016 guidelines is based on several observational studies.[5-8] Using a nationwide register-based cohort, consisting of all patients receiving their first catheter ablation procedure for AF in Denmark, the present study aimed to investigate the incidence of periprocedural complications within 30 days post-ablation. These include cardiac tamponade, hematoma requiring intervention, stroke or death and a composite endpoint of these complications in patients ≥ 75 years of age, compared to patients aged 65-74 years. In addition, one-year outcomes including AF relapse and all-cause mortality after catheter ablation were compared between the two age groups.
METHODS
Registries
Data was collected from the Danish National Patient Register, the Danish National Prescription Register, the Danish Register of Causes of Death and the National Danish Ablation Register. The Danish National Patient Register has data on all patient hospital admissions and discharges with diagnosis codes since 1977, as well as outpatient and emergency room contacts since 1994. The database also includes date of admission and performed procedures coded according to the Danish version of the Nordic Classification of Surgical Procedures (NCSP), and discharge diagnoses coded according to the ICD.[9] The Danish National Prescription Register contains data on all prescription drugs dispensed at Danish pharmacies since 1995, including date of dispensation and identification of the drug through the anatomic therapeutic chemical (ATC) classification code.[10] The Danish Register of Causes of Death contains information regarding cause and time of in-country deaths since 1970.[11] The National Danish Ablation Register contains information of catheter ablations performed in Denmark since 2010, including procedural data and complications. Since 2012, all ablation procedures performed by national ablation centers are registered in the National Danish Ablation Register. All citizens in Denmark are given a unique Civil Personal Registration (CPR) number, which are included in the databases and registries. This enables identification and exact linkage across registries.[12]
Study Design and Patient Population
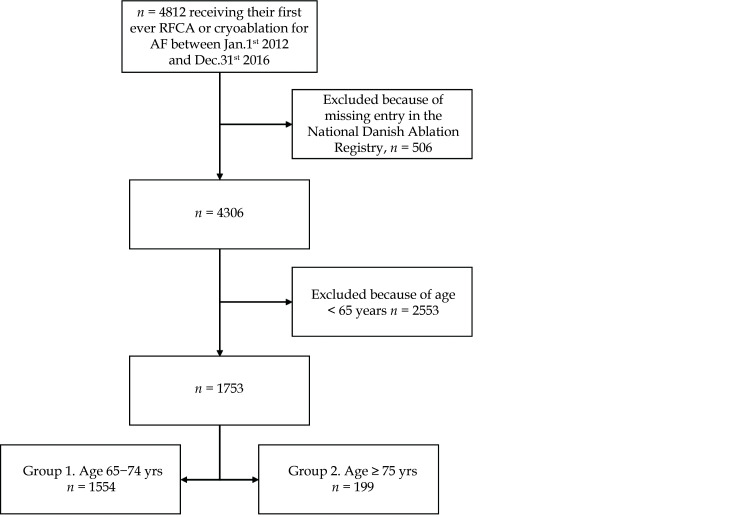
In this retrospective nationwide cohort study, we used the Danish National Patient Register to identify all patients undergoing their first RFCA (n = 4747) or cryoablation (n = 65) for paroxysmal, persistent and long standing AF between January 1st, 2012 and December 31st, 2016 (n = 4812), when coded as either BFFB (NCSP: RFCA for AF) or KFPB10 (NCSP: cryoablation for heart focus). The lower cut-off date was chosen since data collection from all ablation centers was not complete until 2012. The upper cut-off date was chosen in order to ensure a one-year follow-up for all included patients, since the last data available in the Danish National Patient Register and the Danish Register of Causes of Death are from December 31st, 2017. To make sure that the population only consisted of patients strictly receiving RFCA or cryoablation for AF, we only included patients, if the same procedure was registered in the National Danish Ablation Register (nexcluded = 506), as the same NCSP codes are used in patients treated with ablation during heart surgery. Finally, patients younger than 65 at the time of the procedure were excluded (n = 2553). The final study population consisted of 1753 patients receiving either RFCA (n = 1728) or cryoablation (n = 25). The population was divided into two groups according to age. Group 1 aged 60−74 years (n = 1554, 88.6%), and group 2 aged ≥ 75 years (n = 199, 11.4%). Figure 1 illustrates the patient inclusion and exclusion process.
Figure 1.
Flowchart summarizing the selection process of the study population.
RFCA: radiofrequency catheter ablation.
Index was set as the date of ablation procedure. Comorbidities were identified from the Danish National Patient Register using ICD-10 codes for the following diseases: diabetes mellitus (DM), chronic kidney disease (CKD), hypertension, ischemic heart disease (IHD), previous myocardial infarction (MI), previous stroke, previous percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) surgery, valvular heart disease and heart-failure. For all comorbidities assessed using diagnosis codes, only conditions registered 10 years prior to the index date were assessed. As patients with DM and hypertension are most often diagnosed and treated in the primary care setting outside of hospital, we assessed relevant drug redemptions for these two conditions from the Danish National Prescription Register. For DM, we assessed any antidiabetic drug redemptions under the A10 ATC-code up to six months prior to the index date. For hypertension, we required at least two antihypertensive drug redemptions in two consecutive quarters prior to the index date. The following antihypertensive drugs were included: antiadrenergic agents, diuretics excluding loop-diuretics, vasodilators, beta-blockers, calcium channel blockers, or agents acting on the renin-angiotensin system. Specific diagnosis, procedure and ATC-codes used in this study can be found in Supplementary Table 1.
Table 1. Baseline characteristics.
| Age 65−74 yrs (n = 1554) | Age ≥ 75 yrs (n = 199) | Total (n = 1753) | P-value | |
| Data are expressed as n (%) or median [interquartile range]. AF: atrial fibrillation; CABG: coronary artery bypass graft; CKD: chronic kidney disease; DM: diabetes mellitus; IHD: ischemic heart disease; MI: myocardial infarction; PCI: percutaneous coronary intervention. *In 80 (5%) patients AF type was not classified. | ||||
| Age, yrs | 69 [67−71] | 76 [75−78] | 69 [67−72] | − |
| Males | 953 (61.3%) | 104 (52.3%) | 1057 (60.3%) | 0.017 |
| Duration of AF, yrs | 3 [1−7] | 4 [1−8] | 3 [1−7] | 0.035 |
| Paroxysmal AF | 917 (61.9%) | 119 (62.3%) | 1036 (59%) | − |
| Persistent AF | 413 (27.9%) | 53 (27.7%) | 466 (27%) | − |
| Longstanding AF* | 152 (10.3%) | 19 (9.9%) | 171 (10%) | 0.989 |
| Ejection fraction | 60% [55%−60%] | 60% [55%−60%] | 60% [55%−60%] | 0.665 |
| Diabetes | 52 (3.3%) | 8 (4.0%) | 60 (3.4%) | 0.776 |
| CKD | 23 (1.5%) | 4 (2.0%) | 27 (1.5%) | 0.790 |
| Hypertension | 1191 (76.6%) | 168 (84.4%) | 1359 (77.5%) | 0.017 |
| IHD | 304 (19.6%) | 46 (23.1%) | 350 (20.0%) | 0.277 |
| MI | 134 (8.6%) | 20 (10.1%) | 154 (8.8%) | 0.592 |
| Stroke | 96 (5.2%) | 8 (4.0%) | 104 (5.9%) | 0.591 |
| PCI or CABG | 120 (7.7%) | 11 (5.5%) | 131 (7.5%) | 0.334 |
| Valvular disease | 71 (4.6%) | 13 (6.5%) | 84 (4.8%) | 0.296 |
| Heart-failure | 161 (10.4%) | 23 (11.6%) | 184 (10.5%) | 0.692 |
| Pacemaker | 85 (5.5%) | 10 (5.0%) | 95 (5.4%) | 0.925 |
Outcomes
We sought to investigate both periprocedural complications and death within 30 days following ablation as well as AF relapse following a three-month blanking period and all-cause mortality within one-year post-ablation. Periprocedural complications were defined as one of the following: pericardial effusion requiring drainage or cardiac tamponade, stroke, hematoma requiring surgical treatment or death. Individual components and a composite endpoint of at least one of these complications were evaluated. All complications were investigated using discharge diagnosis codes or procedural codes from the Danish National Patient Register, and through complications registered in the National Danish Ablation Register. Death was examined through the Danish Register of Causes of Death. Using the aforementioned registries, we followed patients for one year after index procedure, investigating the primary and secondary endpoint. The primary outcome being relapse of AF, and the secondary endpoint all-cause mortality. Relapse was defined as a composite of RFCA or cryoablation ablation for AF (NCSP codes BFFP or KFPB10) after index procedure, synchronized cardioversion (NCSP code BFFA0) > 90 days after procedure or confirmed relapse at a follow-up registered in the National Danish Ablation Register, all within one year after index procedure. Re-ablation and synchronized cardioversion were investigated through the Danish National Patient Register. End of study was 365 days after index procedure. However, to accurately assess one-year follow-up visits, we expanded the definition for follow-up visits up to 15 months. It was found that 9.5% of the population did not have a follow-up performed within 365 days, and in order to minimize selection bias, a sensitivity-analysis was performed, investigating relapse as described above, but substituting confirmed relapse at a follow-up with an admission to hospital with diagnosis code I48 (AF and/or atrial flutter) being the main diagnosis. Furthermore, we investigated the use of antiarrhythmic drugs redeemed from pharmacies 90 days after index procedure and until end of study. Included were beta-blockers, verapamil, digoxin, amiodarone, dronedarone, class 1c antiarrhythmics and sotalol.
The use of data for this study was approved by the data responsible unit in Region North Denmark (ID-number 2019-60).
Validation of Complications in the National Danish Ablation Register
In order to examine if procedural complications were registered correctly in the National Danish Ablation Register, 610 randomly selected patient entries were crosschecked with information from patient’s medical records and positive predictive values (PPV) and negative predictive values (NPV) were calculated. The complications studied were: cardiac tamponade, embolism, death, deep-vein thrombosis, hematoma, heart block, infection, left ventricle stenosis, oesophageal fistula, phrenicus paresis, pneumothorax, TIA and stroke.
Statistical Analysis
Continuous variables are presented with median and 25%−75% percentiles (1st−3rd quartile). Categorical data are reported with percentages and frequencies. Univariate comparisons were performed using unpaired Student’s t test for continuous variables, and chi-square tests for categorical and binary variables. Hazard of periprocedural complications were examined using cause-specific Cox regression analysis, with death being a competing risk. Due to a low number of procedural complications, the regression analysis of complications was performed unadjusted, except for death and a composite of all complications.
One-year outcome was from index procedure until either AF relapse as defined above, death or 365 days post ablation, whichever came first. We did not record cardioversion in the first three months. In order to visualize the development of relapse after catheter ablation, a cumulative incidence plot was made. A cause-specific Cox regression was used in order to examine the association between the two age groups and relapse, with death being a competing risk. The analysis was performed both unadjusted and adjusted. We also performed unadjusted and adjusted Cox regression analyses investigating one-year death.
The following covariates were included in all adjusted analyses: sex, previous DM, CKD, hypertension, IHD, MI, stroke, PCI or CABG, valvular heart disease, heart-failure and pacemaker implantation. Proportional hazards were examined and found non-violated using Schoenfeld residuals. In a subgroup analysis, we matched patients ≥ 75 years of age from the study population 1: 2 to AF patients, who were not treated with catheter ablation, using CHA2DS2-VASc and time suffering from AF as matching criteria. In total, 25 (12.6%) cases were matched to 50 controls. Using the controls as reference group, we examined all-cause mortality 30 days post-ablation and within one year. A two-sided P-value < 0.05 was considered statistically significant. In total, we were able to match 25 (12.6%) patients with 50 controls. Using the controls as reference group, we examined all-cause mortality 30 days post-ablation and within one year. A two-sided P-value < 0.05 was considered statistically significant. These specific matching criteria were chosen in order to counter selection bias, as one must assume that elderly patients undergoing catheter ablation for AF are carefully selected by physicians, since guidelines suggested an upper age limit of 75.
All data management and analyses were done in SAS 9.4 (SAS Institute Inc., Cary, NC, USA) and Rstudio version 3.6.1 (Rstudio inc., Boston, MA, USA).
RESULTS
Baseline Characteristics
We identified 1753 patients, ≥ 65 years, receiving their first radiofrequency catheter ablation or cryoablation for AF. Patients ≥75 years accounted for 11.4% (n = 199) of the entire study population (Figure 1). Patient characteristics are shown in Table 1. Relative to patients aged 65-74 (n = 1554), older patients were less frequently of male sex (52.3% vs. 61.3%, P = 0.017), and suffered more often from hypertension (84.4% vs. 76.6%, P = 0.017). Remaining factors were more evenly distributed between the two age groups (all P > 0.05, Table 1). The total number of patients ≥ 18 years of age, receiving their first catheter ablation for AF, increased with 102% between 2012 and 2016: 582 procedures in 2012, 728 in 2013, 847 in 2014, 969 in 2015 and 1180 in 2016. Patients 65−74 years of age increased with 143%: 184 procedures in 2012, 245 in 2013, 306 in 2014, 371 in 2015 and 448 in 2016. Patients ≥ 75 years of age increased with 268%, with 22 procedures in 2012, 16 in 2013, 31 in 2014, 49 in 2015 and 81 in 2016. Of the 1753 patients included in the study, 81.5% had a follow-up performed and registered in the National Danish Ablation Register within 15 months.
In addition, patients who had an event and/or were treated with amiodarone were excluded from the calculation of follow-up, resulting in a total of 9.5% of patients who did not have a registered follow-up or an event. Regarding the two age groups, 9.9% of the younger population and 6.5% of the older population did not have a registered follow-up or an event.
As for ablation strategy, there was no significant difference between the two age groups. Pulmonary vein isolation was performed in all patients. Complex Fractionated Atrial Electrograms ablation was only performed in 38 patients in the 65−74 age group with permanent AF. Mitral line ablation was also performed in only four patients with permanent AF in the 65−74 age group. Cavo-tricuspid line lesion was performed in 202 (14.6%) of in the 65−74 age group compared to 29 (15.8%) of the patients ≥ 75 years of age (P = 0.73).
There was no significant difference in median procedure time between the 65−74 age group and the patients ≥ 75 years of age (130 min [25%−75%; 100−170] vs. 128 min [25%−75%; 100, 155]; P = 0.48). The X-ray time was also comparable and without significant difference in the two groups (13 min [25%−75%; 8, 19] vs. 12 [25%−75%; 7, 18] min; P = 0.12).
Left atrial size values are not available in the database. However, objective grading of left atrial size (normal, moderately enlarged and severely enlarged) are inserted. Nine hundred seventy seven (64.8%) of the 65−74 age group and 129 (65.5%) of the patients ≥ 75 years of age had a normal left atria size, respectively 402 (26.7%) and 44 (22.3%) had moderately enlarged, and 129 (8.6%) of the 65−74 age group and 24 (12%) had severely enlarged left atrium (P = 0.15).
Procedural Complications
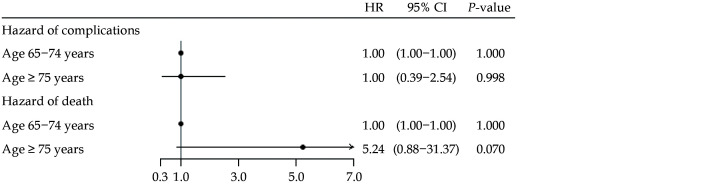
The frequency of procedural complications within 30 days after initial procedure is depicted in Table 2. In both age groups, a total of 2.5% experienced a complication. Using patients aged 65−74 years as reference, an unadjusted and adjusted Cox regression analysis revealed a non-significant HR of 1.00 and 0.94 for the older population (95% CI: 0.39−2.54,P = 0.837 and 95% CI: 0.37−2.39,P = 0.896). There were no significant differences among the groups regarding post-procedural cardiac tamponade, hematoma or stroke individually, whose complication rates ranged from 0−1.5% (allP > 0.05). The same was true regarding death within 30 days, with three (0.2%) deaths among patients aged 65−74 and two (1.0%) deaths in patients ≥75 years. The unadjusted and adjusted Cox regression analysis revealed a non-significant HR of 5.24 and 4.71 for the older population (95% CI: 0.88−31.37, P = 0.070 and 95% CI: 0.78−28.40,P = 0.091). Results of the unadjusted analysis are depicted as a forest plot in Figure 2. The cause of death for the five patients who died within 30 days after ablation was retrieved from patient medical records. Two died of unknown causes, one died during the procedure due to acute and fulminant heart failure following pericardiocentesis, one died of cerebral hemorrhage and one died due to septic embolism to cerebrum which we may assume was due to esophagus fistula.
Table 2. Periprocedural complications within 30 days after index procedure.
| Age 65−74 yrs (n = 1554) | Age ≥ 75 yrs (n = 199) | P-value | |
| P-value of crude cox regression analysis. Stroke includes hemorrhagic stroke, stroke due to embolism and transient ischemic attack. | |||
| Cardiac tamponade or pericardial effusion | 17 (1.1%) | 3 (1.5%) | 0.912 |
| Significant hematoma | 9 (0.6%) | 1 (0.5%) | 0.901 |
| Stroke | 10 (0.6%) | 0 | 0.998 |
| Death | 3 (0.2%) | 2 (1.0%) | 0.070 |
| At least one of the above | 40 (2.5%) | 5 (2.5%) | 0.998 |
Figure 2.
Periprocedural complications.
Forest plot showing the unadjusted HR of overall periprocedural complications including pericardial effusion or cardiac tamponade, stroke, hematoma requiring treatment or death, as well as 30-day all-cause mortality in patients ≥ 75 years of age, relative to patients aged 65−74 years old.
Validation of Complications in the National Danish Ablation Register
We consistently found a high PPV (95%−99%) among the majority of the registered complications in the DARD.DK. There were three procedural or late-term complications that had PPV’s below 50%: unspecified procedural complications (PPV: 40%, 95% CI: 5%−95%), cardiac tamponade (PPV: 50%, 95% CI: 1%−99%) and occurrence of atrial flutter (PPV: 44%, 95% CI: 14%−79%).
NPV values were correspondingly high (99%) among all registered complications apart from the “No registered complication” (procedural, post procedural, long-term)-variables, which had NPV as low as 17% (95% CI: 11%−24%).
One-year Outcome
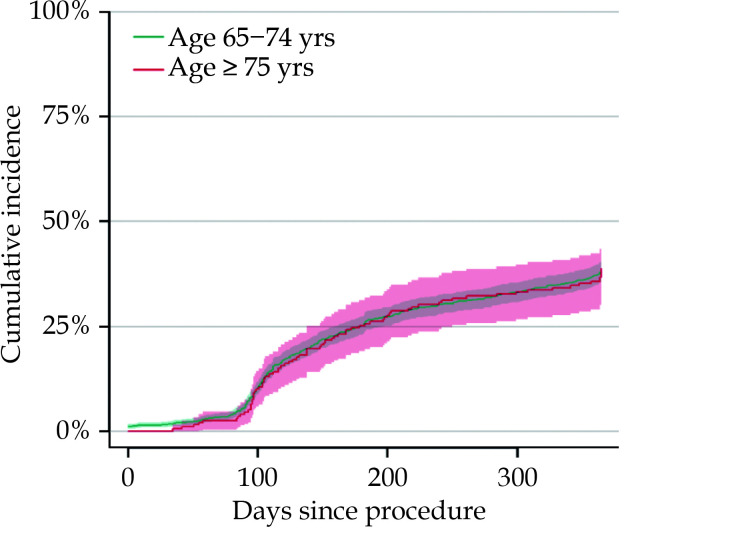
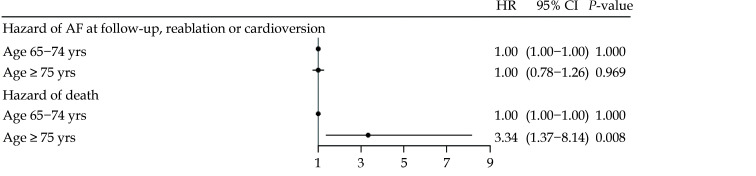
The cumulative incidence of relapse within one year, in patients aged 65−74 years and ≥ 75 years respectively is shown inFigure 3, whereas the cumulative incidence of individual outcomes of synchronized cardioversion and re-ablation can be found in Supplementary Figures 1 and 2. When investigating the composite endpoint of AF relapse, the unadjusted and adjusted Cox regression analysis revealed no difference between the two age groups (HR = 1.01, 95% CI: 0.80−1.28,P = 0.949 and HR = 1.00, 95% CI: 0.78−1.26,P = 0.969). The same held true when investigating relapse at follow-up (unadjusted HR = 0.88, 95% CI: 0.65−1.20,P = 0.428), synchronized cardioversion (unadjusted HR = 1.07, 95% CI: 0.74−1.56,P = 0.725) and re-ablation (unadjusted HR = 0.88, 95% CI: 0.61−1.27,P = 0.489) individually. The older age group had a higher hazard of one-year mortality in both the unadjusted and the adjusted analysis (HR = 3.27, 95% CI: 1.35−7.87,P = 0.008 and HR = 3.34, 95% CI: 1.37−8.14,P = 0.008). Results of the adjusted analysis is depicted as a forest plots in Figure 4. A forest plot of individual outcomes can be seen in Supplementary Figure 3.
Figure 3.
Cumulative incidence of atrial fibrillation relapse.
Figure 4.
The adjusted HR of one-year AF relapse and death, using patients aged 65-74 as the reference group.
AF: atrial fibrillation; HR: hazard ratio.
Cumulative relapse defined as confirmed relapse at follow-up within one year, cardioversion following a 90 days blanking period or re-ablation within one year after index procedure.
AF relapse defined as confirmed relapse at follow-up within one year, cardioversion following a 90 days blanking period or re-ablation within one year after index procedure (Table 3).
Table 3. Distribution of age groups, according to one-year outcome.
| Age group | No relapse | AF relapse* | Competing risk of death | Total |
| *AF relapse defined as confirmed relapse at follow-up within one year, cardioversion following a 90 days blanking period or re-ablation within one year after index procedure. AF: atrial fibrillation. | ||||
| 65−75 yrs | 938 (60.4%) | 603 (38.8%) | 13 (0.8%) | 1554 |
| ≥ 75 yrs | 117 (58.8%) | 77 (38.7%) | 5 (2.5%) | 199 |
Among patients ≥ 75 years, a total of 3.5% died within one year, compared to 1.1% of patients 65−74 years of age. In total, there were 16 deaths in the younger age group, and 81.3% were due to non-cardiovascular causes. In the older age group, we observed five deaths, and 80% were due to non-cardiovascular causes. When 25 patients ≥ 75 years of age were matched 1: 2 to 50 other AF patients who were not treated with catheter ablation, using CHA2DS2-VASc and time suffering from AF as matching criteria, a Cox regression analysis investigating one-year mortality, revealed no increased hazard of death in the older age group when compared to the matched controls (HR = 0.24, 95% CI: 0.03−1.90, P = 0.176). The number of patients redeeming a prescription for antiarrhythmic drugs between 90 days and 365 days after index procedure, compared to the redeemed prescriptions before the procedure, can be seen in Table 4. After the procedure, only the use of class 1c antiarrhythmic drugs differed between groups, with 88 (5.7%) patients 65−74 years of age having redeemed a prescription, compared to four (2%) of patients ≥ 75 years (P = 0.045).
Table 4. The use of antiarrhythmics before and between 90 days and 365 days after index procedure.
| Age 65-74 yrs (n = 1554) | Age ≥ 75 yrs (n = 199) | P-value | ||||
| Before procedure | After procedure | Before procedure | After procedure | |||
| Beta-blockers | 1194 (76.8%) | 1008 (64.9%) | 156 (78.4%) | 127 (63.8%) | 0.832 | |
| Verapamil | 135 (8.7%) | 86 (5.5%) | 25 (12.6%) | 11 (5.5%) | 1.0 | |
| Digoxin | 264 (17.0%) | 109 (7.0%) | 39 (19.6%) | 19 (9.5%) | 0.251 | |
| Amiodarone | 336 (21.6%) | 184 (11.8%) | 59 (29.6%) | 31 (15.6%) | 0.162 | |
| Dronedarone | 39 (2.5%) | 14 (0.9%) | 4 (2.0%) | < 3 | 0.868 | |
| Class 1c antiarrhythmics | 253 (16.3%) | 88 (5.7%) | 18 (9.0%) | 4 (2.0%) | 0.045 | |
| Sotalol | 10 (0.6%) | 6 (0.4%) | 3 (1.5%) | < 3 | 0.509 | |
DISCUSSION
In this nationwide study of AF patients ≥ 65 years of age undergoing first-time RFCA or cryo-balloon ablation, the incidence of periprocedural complications, as well as one-year freedom from AF showed no statistical difference, when comparing patients of ≥ 75 years of age to patients 65−74 years of age. Pulmonary vein isolation using RFCA or cryo-balloon ablation appears to be safe and as efficient for selected patients ≥ 75 years of age as for patients of 65−74 years of age.
Pulmonary vein isolation (PVI) using RFCA or cryo-balloon ablation technique has been extensively examined and proved to be an effective therapy for patients with paroxysmal AF.[1] Even though the prevalence of AF increases with age, to the authors’ knowledge, all randomized studies examining the effect of ablation compared to medical therapy, have been conducted in patients below the age of 70.[2,13,14] Only few trials have examined the safety and efficacy of catheter ablation of paroxysmal AF in elderly, in which many of the studies suffered from a limited population size.[7,8,15,16]
A retrospective study by Dagres, et al.[17] from 2009 investigated 30-day procedural outcomes after RFCA for AF. They included patients treated between 2005 and 2008 and found age ≥ 75 (n = 11) to be associated with a higher risk of procedural complications deemed life-threatening, causing permanent harm, or requiring interventions or prolonged hospitalization (HR = 3.98, 95% CI: 1.21−13.01,P = 0.022). In contrast, we found no differences between age groups in the total number of procedural complications, which may be due the difference in procedure years, i.e., a decline in complications could be due to better operator experience over time and/or technical changes. Another reason could be that we used patients aged 65−74 year as the reference group in contrast to the population by Dagres, et al.,[17] who had a younger study population (mean age 58 ± 10 years). The small number of patients included in the study by Dagres et al. may also explain the difference in our findings.[17]
A more recent study by Heeger, et al.[7] from 2019 investigated the efficacy and safety of PVI using the 2nd generation Cryoballoon in the elderly. They included 104 patients ≥ 75 years, who were matched to 104 patients < 75 years of age. Both groups received PVI using the 2 nd generation cryo-balloon, and Heeger, et al.[7] analyzed periprocedural complications within 30 days after procedure resulting in permanent injury or death, requiring intervention or prolonging or requiring hospitalization > 48 h. In each group, 6.7% had major complications ( P = 0.999). One death in the entire population was observed, belonging to the elderly patient group (P = 0.124). The study concluded that cryoablation for AF in patients ≥ 75 years of age is safe, associated with short procedure and fluoroscopy times, and the long-term clinical success rate is comparable to younger patients.[7] Like our study, there were no differences in the incidence of periprocedural complications between age groups. They observed one death (1%) within the older age group, analogous to the 1.0% observed in our study. A study by Metzner, et al.[8] from 2016 investigated RFCA for AF in patients ≥ 75 years of age. They included 94 patients, and the rate of observed serious complications was 5.8% without any fatal events.
The absolute and relative numbers of deaths observed among the elderly patients are generally low in both this study and the studies by Heeger, et al.[7] and Metzner, et al.[8] (two (1.0%), one (1.0%) and zero, respectively).
In our study, 38.8% of patients aged 65-74 and 38.7% of patients ≥ 75 years experienced relapse of AF within one year, showing no statistical difference between age groups. This percentage of relapse was to be expected, as several studies have shown similar re-occurrence of AF after one ablation procedure.[7,8,16] In the study by Metzner, et al.[8] 35/93 (38%) of the patients, aged > 75 years, were free of relapse after a single RFCA procedure after a mean follow-up periode of 37 ± 20 months. Furthermore, Santangeli, et al.[18] have published a study of 103 patients ≥ 80 years treated with RFCA for AF and compared the results with 2651 patients < 80 years of age. There were no differences in safety or effectiveness between the two groups. Another single-center study has shown comparable complication rates in octogenarians ( n = 49) compared to patients who were 70−79 years (n = 151) and 60−69 years (n = 177).[19]
To best of our knowledge, our study is the first nationwide study of efficacy and safety outcomes of PVI in elderly with a follow-up period of one year including registration of the post-ablation use of anti-arrhythmic drugs.
Our study indicates that catheter ablation of selected patients ≥ 75 years of age is as effective and safe as when treating younger patients of 65−74 years of age.
LIMITATIONS
Being a register-based observational study, statistical associations may not be causal. Patient characteristics are limited to the data available in registries. Furthermore, register-based studies are highly dependent on the information in the registries being correct and of high quality. Due to the low number of complications in the study, one could suspect complications to be under-reported. However, as the Danish National Patient Register since the year 2000 has been used to determine funding for hospitals, it is considered to be of good quality.[7] As described above, we also found high PPV of registrations of procedural complications in the National Danish Ablation Register. The population in the older age group consisted of 199 patients. Therefore, one could assume that the study is underpowered. However, since this is a nationwide study, there simply were not any more patients to include, and the results should be viewed as being observational. Lastly, one must assume that elderly patients referred for catheter ablation must be some of the healthier elderly, and as such, our results cannot directly be transferred to the general population of elderly patients suffering from AF.
CONCLUSIONS
In patients ≥ 75 years of age selected for catheter ablation for AF, the incidence of periprocedural complications as well as one-year freedom from AF showed no difference, when compared to patients 65−74 years of age, suggesting that catheter ablation for AF in selected patients of 75 years or older appear to be safe and as efficient as patients below 75 years of age.
FUNDING
This study was funded by Obel Family Foundation.
CONFLICT OF INTEREST
Jesper Nielsen, Kristian Kragholm, Sam Riahi, Peter Søgaard, Steen B. Kristensen, Christoffer Polcwiartek, Peter Karl Jacobsen, Anna Margrethe Thøgersen and Peter Steen Hansen have no involvements that might raise the question of bias in this study. Christian Torp Petersen has received grants for studies from Byer and Novo Nordisk, not related to the current study.
DATA AVAILABILITY
The data underlying this article cannot be shared publicly due to Danish legislation and Statistics Denmark regulations.
References
- 1.Hindricks G, Potpara T, Dagres N, et al 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2021;42:546–547. doi: 10.1093/eurheartj/ehaa945. [DOI] [PubMed] [Google Scholar]
- 2.Pappone C, Vicedomini G, Augello G, et al Radiofrequency catheter ablation and antiarrhythmic drug therapy: a prospective, randomized, 4-year follow-up trial: the APAF study. Circ Arrhythm Electrophysiol. 2011;4:808–814. doi: 10.1161/CIRCEP.111.966408. [DOI] [PubMed] [Google Scholar]
- 3.Kirchhof P, Benussi S, Kotecha D, et al 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 4.Calkins H, Hindricks G, Cappato R, et al 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. Europace. 2018;20:157–208. doi: 10.1093/europace/eux275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bulava A, Hanis J, Dusek L Clinical outcomes of radiofrequency catheter ablation of atrial fibrillation in octogenarians-10-year experience of a one high-volume center. J Geriatr Cardiol. 2017;14:575–581. doi: 10.11909/j.issn.1671-5411.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhargava M, Marrouche NF, Martin DO, et al Impact of age on the outcome of pulmonary vein isolation for atrial fibrillation using circular mapping technique and cooled-tip ablation catheter. J Cardiovasc Electrophysiol. 2004;15:8–13. doi: 10.1046/j.1540-8167.2004.03266.x. [DOI] [PubMed] [Google Scholar]
- 7.Heeger CH, Bellmann B, Fink T, et al Efficacy and safety of cryoballoon ablation in the elderly: a multicenter study. Int J Cardiol. 2019;278:108–113. doi: 10.1016/j.ijcard.2018.09.090. [DOI] [PubMed] [Google Scholar]
- 8.Metzner I, Wissner E, Tilz RR, et al Ablation of atrial fibrillation in patients ≥ 75 years: long-term clinical outcome and safety. Europace. 2016;18:543–549. doi: 10.1093/europace/euv229. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt M, Schmidt SA, Sandegaard JL, et al The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kildemoes HW, Sørensen HT, Hallas J The Danish National Prescription Registry. Scand J Public Health. 2011;39(7 Suppl):S38–S41. doi: 10.1177/1403494810394717. [DOI] [PubMed] [Google Scholar]
- 11.Helweg-Larsen K. The Danish Register of Causes of Death. Scand J Public Health. 2011; 39(7 Suppl): 26-29.
- 12.Pedersen CB. The Danish Civil Registration System. Scand J Public Health 2011; 39(7 Suppl): 22-25.
- 13.Jais P, Cauchemez B, Macle L, et al Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008;118:2498–2505. doi: 10.1161/CIRCULATIONAHA.108.772582. [DOI] [PubMed] [Google Scholar]
- 14.Cosedis Nielsen J, Johannessen A, Raatikainen P, et al Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012;367:1587–1595. doi: 10.1056/NEJMoa1113566. [DOI] [PubMed] [Google Scholar]
- 15.Dagres N, Piorkowski C, Kottkamp H, et al Contemporary catheter ablation of arrhythmias in geriatric patients: patient characteristics, distribution of arrhythmias, and outcome. Europace. 2007;9:477–480. doi: 10.1093/europace/eum048. [DOI] [PubMed] [Google Scholar]
- 16.Pedrinazzi C, Durin O, Agricola P, et al Efficacy and safety of radiofrequency catheter ablation in the elderly. J Interv Card Electrophysiol. 2007;19:179–185. doi: 10.1007/s10840-007-9153-6. [DOI] [PubMed] [Google Scholar]
- 17.Dagres N, Hindricks G, Kottkamp H, et al Complications of atrial fibrillation ablation in a high-volume center in 1, 000 procedures: still cause for concern? J Cardiovasc Electrophysiol. 2009;20:1014–1019. doi: 10.1111/j.1540-8167.2009.01493.x. [DOI] [PubMed] [Google Scholar]
- 18.Santangeli P, Biase LD, Mohanty P, et al Catheter ablation of atrial fibrillation in octogenarians: safety and outcomes. J Cardiovasc Electrophysiol. 2012;23:687–693. doi: 10.1111/j.1540-8167.2012.02293.x. [DOI] [PubMed] [Google Scholar]
- 19.Tan HW, Wang XH, Shi HF, et al Efficacy, safety and outcome of catheter ablation for atrial fibrillation in octogenarians. Int J Cardiol. 2010;145:147–148. doi: 10.1016/j.ijcard.2009.06.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article cannot be shared publicly due to Danish legislation and Statistics Denmark regulations.