Abstract
BACKGROUND
Tanscatheter left atrial appendage (LAA) closure and minimally invasive thoracoscopic LAA occlusion are local interventions of LAA for stroke prevention in patients with nonvalvular atrial fibrillation (NVAF). However, the safety and efficacy of these methods have not been compared. This prospective cohort study aimed to assess the safety and efficacy of those two treatment approaches for stroke prevention in NVAF patients.
METHODS
Two hundred and nine recurrent NVAF patients who received radiofrequency ablation were enrolled. These patients were treated with transcatheter LAA closure or thoracoscopic LAA occlusion. The patients were followed up from the first postoperative day and evaluated for efficacy endpoints (stroke/transient ischemic attack (TIA), systemic embolism (SE), and death) and a safety endpoint (bleeding events). Perioperative complications were recorded.
RESULTS
After a median follow-up of 1.8 years (383 patient-years), the overall rate of the composite efficacy endpoints was similar between the two groups (3.8 vs. 2.7 events per 100 patient-years; HR = 0.71; 95% CI: 0.225−2.237; P = 0.559). However, regarding primary safety endpoint, there were 1.5 bleeding events per 100 patient-years in the thoracoscopic LAA occlusion group, compared with 6.4 in transcatheter LAA closure group (HR = 0.246; 95% CI: 0.074−0.819; P = 0.022). The incidence of operative complications was 3/138 (2.17%) in thoracoscopic LAA occlusion group and 1/71 (1.41%) in transcatheter LAA closure group.
CONCLUSIONS
Thoracoscopic LAA occlusion and transcatheter LAA closure have similar efficacy in preventing stroke in NVAF patients. However, the thoracoscopic group had fewer bleeding events than the transcatheter group, but the former group required a longer hospital stay.
The left atrial appendage (LAA) is the most likely site of thrombosis in patients with nonvalvular atrial fibrillation (NVAF),[1-3] and left atrial thrombus is the main cause of stroke which can reduce survival and increase risk of serious disability.[4] Therefore, thrombus formation within the LAA is a crucial component of stroke prophylaxis. Both thoracoscopic LAA occlusion and transcatheter LAA closure are local interventions to the LAA for stroke prevention in NVAF patients.
Currently, several clinical trials have demonstrated that transcatheter LAA closure is not inferior to warfarin in stroke prevention.[5,6] However, the transcatheter LAA closure has several limitations such as additional risk of thrombosis (device-related thrombi and residual peri-device flow), higher incidence of bleeding events due to combined antithrombotic therapy after the device implantation.[7,8] Thoracoscopic LAA occlusion does not have these drawbacks (device implantation and long-term antithrombotic therapy), but there is a lack of strong prospective evidence internally in the comparison between thoracoscopic LAA occlusion and transcatheter LAA closure.
Methods
Study Patients
From 2014/3/1 to 2018/4/30, patients with recurrent NVAF were enrolled continuously from Beijing Anzhen Hospital. The inclusion criteria was as follows: age ≥ 18 years; recurrent atrial fibrillation after radiofrequency ablation and treatment with LAA intervention; and CHADS2 score (congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke) ≥ 2. The exclusion criteria was as follows: need for anticoagulation therapy; concomitant disease affecting the valves and coronary arteries; condition leading to a need for long-term antiplatelet therapy, such as coronary heart disease and peripheral vascular disease; condition leading to a high risk of thrombosis, including congenital heart disease, mechanical cardiac valve repair, thrombosis in the aorta and intracardiac or neurological symptoms of carotid artery disease; contraindication to aspirin, clopidogrel or warfarin and so on. According to the inclusion and exclusion criteria, 228 patients treated with local LAA interventions for stroke prevention were enrolled. In total, 19 were lost to follow-up, and 209 patients were finally eventually enrolled. Of these, 71 and 138 patients were treated with transcatheter LAA closure and thoracoscopic LAA occlusion respectively. Neurological examinations were performed during follow-up. Repeated head CT or MRI was performed for patients previously diagnosed with stroke and considered as baseline before follow-up.
Study Procedures
In the thoracoscopic LAA occlusion group, the atrial appendages were sutured with a modern stapler (Johnson & Johnson EZ-45G) using two lines of staples. The sutured border was close to the border area of the left atrial appendage entrance to prevent the occurrence of a stump (prone to thrombosis). Warfarin was administered according to the patients’ INR (international normalized ratio) for three months postoperatively. Transesophageal echocardiography (TEE) was performed at the third/12 th month, if the LAA was completely occluded or the residual width was less than 1 cm, warfarin was discontinued, otherwise warfarin treatment was continued. Antiplatelet and anticoagulant drugs were not used after warfarin was discontinued.
The WATCHMAN device is a self-expanding nickel titanium (nitinol) frame structure with fixation barbs and a permeable polyester fabric cover. It is implanted via a transseptal approach using of a catheter-based delivery system to seal the ostium of the LAA and implanted under fluoroscopy or TEE guidance.[9] Warfarin and aspirin (100 mg) were administered during the first 45 days. TEE examination was performed 45 days/6 months/12 months, and the eligibility criteria of TEE examination include: the width of residual efflux around the device less than 5 mm and there was no device-related thrombus. Clopidogrel (75 mg) replaced warfarin until the 6th month after the transcatheter therapy, if the TEE results met the standard 45 days after the surgery. Otherwise, the medication regimen was not changed, after which only aspirin was continued. If the TEE didn’t get eligibility criteria, the patients need to continue taking warfarin until TEE is satisfactory.
Follow-up and Data Collection
Patients were followed up by telephone or at the outpatient clinic at 1 week/45 days/3 months/6 months/12 months/twice annually after one year, and the relevant events were recorded. Neurologic examinations were performed 12 months/once annually after one year. Detailed neurological examination were required when patients developed neurological symptoms.
This study had four endpoints. The first efficacy endpoint was a composite endpoint for stroke/SE and death. The second late efficacy endpoint was the composite endpoint for events from the 3rd month after surgery to the end of follow-up. The BARC (Bleeding Academic Research Consortium) standard (record at least grade 2 or above) was mainly used to assess the safety endpoints. Bleeding events mainly included operation-related (pericardial effusion/hematoma of the chest wall/bleeding from the suture incision of the left auricle) and drug-related (gastrointestinal bleeding/nasal bleeding/intracranial storage bleeding/hematuria/other types of bleeding requiring transfusion) events. Operation-related stroke was an efficacy end point, not a safety endpoint. The fourth endpoint was the difference in complications between the two groups. The follow-up of each patient lasted until the endpoint/death or the end of follow-up, whichever came first. Patients lost follow-up were not included in the statistical analysis.
Statistical Analysis
Continuous variables of distribution and categorical variables were expressed as mean ± SD, median/quartile and counts (percentages), respectively. The differences were quantified through independent t-test, rank sum test and chi-square test, respectively. The incidence of events was expressed in terms of the number of incidents per 100 patient-years. Kaplan-Meier method was used for graphical evaluation of time-related events and evaluated by Log-rank tests. All analyses were performed with reference to the intervention group. Statistical significance was set as P ≤ 0.05. SPSS software for Windows (version 22.0, SPSS Inc., Chicago, Illinois) was used for statistical analyses.
RESULTS
Baseline Characteristics
A total of 209 NVAF patients were enrolled in this study (Table 1). Thoracoscopic LAA occlusion group and transcatheter LAA closure group have 138 and 71 patients, respectively. The length of hospital stay in thoracoscopic LAA occlusion group was significantly longer than that in transcatheter LAA closure group (4.0 (3.0, 4.0) vs. 7.0 (4.0, 7.0), P < 0.001). There were no significant differences between the two groups (including age, BMI, sex, type of nonvalvular atrial fibrillation, CHADS2 score, CHA2DS2-VASc score and HAS-BLED score).
The First Primary Efficacy Endpoint
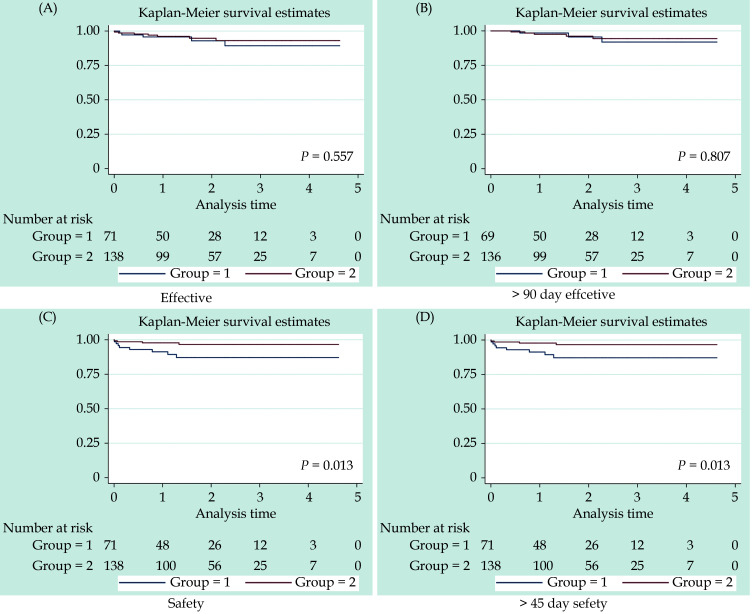
The two groups had similar results regarding the efficacy endpoints (3.8 vs. 2.7 events per 100 patient-years, HR = 0.71, 95% CI: 0.225−0.237, P = 0.559) (Table 2). The incidence of TIA/stroke was 1.9/3.1 in the resection and intervention groups (HR = 0.633, 95% CI: 0.170−0.59, P = 0.496). The Kaplan-Meier survival curves were not significantly between the two groups (Figure 1A). The late efficacy end points remained unchanged between the two groups from three months after treatment to the end of follow-up (Table 3). Kaplan-Meier curve survival analysis also show no significance difference between groups (Figure 1B).
Table 2. Primary efficacy results.
| Transcatheter LAA closure (n = 71) | Thoracoscopic LAA occlusion (n = 138) | HR (thoracoscopic
group/transcatheter group) |
95% CI | P | ||||
| Events/
patient-years |
Observed rate:
events per 100 patient-years (95% CI) |
Events/
patient-years |
Observed rate:
events per 100 patient-years (95% CI) |
|||||
| HR: hazard ratio; LAA: left atria appendage; TIA: transient ischemic attack. | ||||||||
| Primary efficacy | 5/130.0 | 3.8 (1.6−9.2) | 7/259.5 | 2.7 (1.3−5.7) | 0.71 | 0.225−2.237 | 0.559 | |
| Stroke/TIA | 4/130.0 | 3.1 (1.2−8.2) | 5/261.2 | 1.9 (0.8−4.6) | 0.633 | 0.170−2.359 | 0.496 | |
| Systemic embolism | 0/135.3 | 0 | 1/264.6 | 0.4 (0.1−2.7) | − | − | − | |
| All-cause mortality | 1/ 135.3 | 0.7 (0.1−5.2) | 1/ 266.4 | 0.4 (0.1−2.7) | 0.505 | 0.031−8.066 | 0.629 | |
Figure 1.
Kaplan-Meier curve survival.
(A): The first primary efficacy endpoint; (B): the late efficacy endpoint; (C): the first primary safety endpoint; and (D): late primary safety endpoint.
Table 3. The late primary efficacy from three months after procedure to the end of follow-up.
| Transcatheter LAA closure (n = 69) | Thoracoscopic LAA occlusion (n = 136) | HR (thoracoscopic
group/transcatheter group) |
95% CI | P | ||||
| Events/
patient-years |
Observed rate:
events per 100 patient-years (95% CI) |
Events/
patient-years |
Observed rate:
events per 100 patient-years (95% CI) |
|||||
| HR: hazard ratio; LAA: left atria appendage; TIA: transient ischemic attack. | ||||||||
| Late primary efficacy | 3/129.7 | 2.3 (0.7−7.2) | 5/259.4 | 1.9 (0.8−4.6) | 0.837 | 0.200−3.502 | 0.807 | |
| Stroke/TIA | 2/129.7 | 1.5 (0.4−6.2) | 3/261.2 | 1.1 (0.4−3.6) | 0.748 | 0.125−4.475 | 0.75 | |
| Systemic embolism | 0/131.8 | 0 | 1/262.6 | 0.4 (0.1−2.7) | − | − | − | |
| All-cause mortality | 1/131.8 | 0.8 (0.1−5.4) | 1/264.3 | 0.4 (0.1−2.7) | 0.489 | 0.031−7.810 | 0.612 | |
Safety Endpoint
The first primary safety endpoint, risk scores and bleeding events are shown in Table 1 and 4. The incidence of bleeding in the resection group was lower than that in the intervention group. The difference was statistically significant. The incidence of gastrointestinal bleeding was similar. The Kaplan-Meier curve survival analysis is shown in Figure 1C. For the late primary safety endpoint, bleeding events beyond 45 days post-operation were differed in the resection group and the intervention group (listed in Table 5). Kaplan-Meier curve survival analysis showed no significant difference between groups, beyond 45 days post-operation (Figure 1D).
Table 1. Baseline characteristics of the study population.
| Transcatheter LAA closure (n = 71) | Thoracoscopic LAA occlusion (n = 138) | P | |
| Data are presented as mean ± SD or n (%). BMI: body mass index; CHA2DS2-VASc: congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke/transient ischemic attack, vascular disease, age 65–74 years, sex category; HAS-BLED: hypertension, abnormal liver and kidney function, stroke, bleeding, international normalized ratio instability, age ≥ 65 years, medication and alcohol; LAA: left atria appendage; TIA: transient ischemic attack. | |||
| Age, yrs | 69.1 ± 10.9 | 69.4 ± 9.7 | 0.843 |
| BMI, kg/m2 | 26.0 ± 5.4 | 26.5 ± 5.2 | 0.516 |
| Sex | 0.655 | ||
| Male | 21 (29.6%) | 45 (32.6%) | |
| Female | 50 (70.4%) | 93 (67.5%) | |
| Type of nonvalvular atrial fibrillation | 0.926 | ||
| Paroxysmal | 10 (14.1%) | 17 (12.3%) | |
| Persistent | 27 (38.0%) | 55 (39.9%) | |
| Permanent | 34 (47.9%) | 66 (47.8%) | |
| Risk factors for stroke | |||
| Age > 65 yrs | 24 (33.8%) | 53 (38.4%) | 0.514 |
| Chronic heart failure | 5 (7.0%) | 10 (7.2%) | 0.505 |
| Hypertension | 53 (74.6%) | 98 (71.0%) | 0.578 |
| Diabetes mellitus | 22 (31.0%) | 49 (35.5%) | 0.957 |
| Previous stroke/TIA | 52 (73.2%) | 100 (72.5%) | 0.905 |
| CHADS2 score (continuous) | 3.1 ± 1.0 | 3.0 ± 0.9 | 0.387 |
| CHADS2 score (categorical) | 0.607 | ||
| 2 | 26 (36.6%) | 56 (40.6%) | |
| 3 | 21 (29.6%) | 40 (29.0%) | |
| 4 | 17 (23.9%) | 35 (25.4%) | |
| 5 | 7 (9.9%) | 7 (5.1%) | |
| CHA2DS2-VASc score (continuous) | 4.2 ± 1.2 | 4.1 ± 1.4 | 0.647 |
| CHA2DS2-VASc score (categorical) | 0.402 | ||
| 1 | 0 | 0 | |
| 2 | 6 (8.5%) | 23 (16.7%) | |
| 3 | 13 (18.3%) | 18 (13.0%) | |
| 4 | 25 (35.2%) | 44 (31.9%) | |
| 5 | 15 (21.1%) | 31 (22.5%) | |
| 6 | 11 (15.5%) | 16 (11.6%) | |
| 7 | 1 (1.4%) | 6 (4.3%) | |
| HAS-BLED score (continuous) | 2.7 ± 1.0 | 2.7 ± 1.0 | 0.969 |
| HAS-BLED score (categorical) | 0.83 | ||
| 1 | 9 (12.7%) | 16 (11.6%) | |
| 2 | 20 (28.2%) | 40 (29.0%) | |
| 3 | 26 (36.6%) | 54 (39.1%) | |
| 4 | 16 (22.5%) | 26 (18.8%) | |
| 5 | 0 | 2 (1.4%) | |
| Hospital stay, day | 4.0 (3.0, 4.0) | 7.0 (4.0, 7.0) | < 0.001 |
Table 4. Primary safety results.
| Transcatheter LAA closure (n = 71) | Thoracoscopic LAA occlusion (n = 138) | HR (thoracoscopic
group/transcatheter group) |
95% CI | P | ||||
| Events/
patient-years |
Observed rate:
events per 100 patient-years (95% CI) |
Events/
patient-years |
Observed rate:
events per 100 patient-years (95% CI) |
|||||
| HR: hazard ratio; LAA: left atria appendage. | ||||||||
| Total hemorrhage events | 8/124.2 | 6.4 (3.2−12.9) | 4/258.9 | 1.5 (0.6−4.1) | 0.246 | 0.074−0.819 | 0.022 | |
| Digestive tract hemorrhage | 4/129.6 | 3.1 (1.2−8.2) | 2/263.6 | 0.8 (0.2−3.0) | 0.252 | 0.046− 1.374 | 0.111 | |
| Hematuria | 1/134.3 | 0.7 (0.1−5.3) | 0/266.4 | 0 | − | − | − | |
| Intracranial hemorrhage | 1/134.5 | 0.7 (0.1−5.3) | 0/266.4 | 0 | − | − | − | |
| Nasal bleeding | 1/132.5 | 0.8 (0.1−5.4) | 1/ 265.3 | 0.4 (0.1−2.7) | 0.5 | 0.031−8.003 | 0.625 | |
| Surgery-related bleeding | 1/134.7 | 0.7 (0.1−5.3) | 1/262.7 | 0.4 (0.1−2.7) | 0.516 | 0.032− 8.255 | 0.64 | |
Table 5. Bleeding events beyond 45 days post-operation to the end of follow-up.
| Transcatheter LAA closure (n = 67) | Thoracoscopic LAA occlusion(n = 136) | HR (thoracoscopic
group/transcatheter group) |
95% CI | P | ||||
| Events/
patient-years |
Observed rate:
events per 100 patient-years (95% CI) |
Events/
patient-years |
Observed rate:
events per 100 patient-years (95% CI) |
|||||
| HR: hazard ratio; LAA: left atria appendage. | ||||||||
| Total hemorrhage events | 4/124.0 | 3.2 (1.2−8.6) | 2/258.8 | 0.8 (0.2−3.1) | 0.246 | 0.074−0.819 | 0.022 | |
| Digestive tract hemorrhage | 2/127.9 | 1.6 (0.4−6.3) | 1/259.9 | 0.4 (0.1−2.7) | 0.252 | 0.046−1.374 | 0.111 | |
| Hematuria | 0/130.0 | 0 | 0/262.1 | 0 | − | − | − | |
| Intracranial hemorrhage | 1/127.2 | 0.8 (0.1−5.5) | 0/262.1 | 0 | − | − | − | |
| Nasal bleeding | 1/132.5 | 0.8 (0.1−5.6) | 1/261.0 | 0.4 (0.1−2.7) | 0.5 | 0.031−78.003 | 0.625 | |
Complications
Three complications (3/138, 2.17%) occurred in the thoracoscopic group, including perioperative cerebral embolism, pericardial effusion, and bleeding from the chest wall incision, which was 1/71 (1.41%) in the transcatheter group, including pericardial effusion developing in one case. Surgery-related cerebral embolism was included as part of the first primary efficacy endpoint. Pericardial effusion was included as part of the first primary safety endpoint for both groups, while the chest wall wound bleeding of the surgery group was excluded, because the amount of bleeding is very small.
DISCUSSION
At present, transcatheter LAA closure is considered as an alternative to oral anticoagulation for prevention of stroke or bleeding in patients with AF. Although this approach avoids the bleeding risk associated with long-term oral anticoagulation,[10,11] the PROTECT AF/PROVAIL studies only proved that it is not inferior to warfarin in preventing stroke and bleeding events.[12,13] The high rate of residual peri-implant flow and implant-related thrombi greatly affect prognosis. In the PROTECT AF[12] and ASAP[14] studies, the risk of device-related thrombi were 4.2% and 4.0%, respectively. Viles-Gonzalez, et al.[15] observed that approximately 1/3 of the patients with WATCHMAN implantation had peri-device flow, and stroke or TIA events increased in patients with residual shunts > 5 mm. Implantation of foreign substances in the heart increases the inherent risk of thrombosis. Therefore, antithrombotic therapy is necessary until endocardialization of the device is complete. The process of endocardialization is associated with increased bleeding risk. In the ROCKET AF study, [16-18] more than half of bleeding events occurred the first 45 days combined antithrombotic period. Thoracoscopic LAA occlusion does not present these troubles.
Thoracoscopic LAA occlusion has the following advantages: first, additional risk of thrombosis is avoidable with no foreign materials implantation. Second, it does not have an endothelization of the implant that does not require antithrombotic therapy. There are many thoracoscopic procedures to remove the LAA in the world. The two lines of staples used in this study were used to close the atrial appendages, which had an advantage over single-layer sutures in resilience to pneumatic pressure. The sutured border is close to the border area of the LAA entrance to prevent the occurrence of the stump (prone to thrombosis). In thoracoscopic resection, the accurate location of suture is the most critical and even the prognostic factor. The effect of stapler in this experiment is similar to that of Ohtsuka’s study.[19] The complete closure of the LAA is an important prognostic factor, so we ensured that LAA resection was performed by an experienced cardiovascular surgeon. It is reported that our unit leads the Asia-Pacific region in the number of operations in this field.
Efficacy of Transcatheter LAA Closure and Thoracoscopic LAA Occlusion
The first primary efficacy endpoint and the late primary efficacy endpoint have no significant difference between the two groups. This may contradict our hypothesis (additional risk of thrombosis after the device implantation). The subgroup analysis of the PROTECT-AF showed that the occurrence of thrombus events in the LAA closure group was independent of residual peri-implant flow.[15] Similarly, residual peri-implant flow (11.6%) and implant-related thrombi (4.6%) of patients with ACP treated were observed, but these did not cause stroke or TIA.[20] In our study, the incidence of residual peri-device flow (up to 7.04% at the first day, 5.63% at 45 days, 4.23% at 12 months) and device-related thrombi (up to 4.23%) was significantly reduced. Ischemic stroke occurred in only one patient with the peri-device flow or device-related thrombi, which higher than the patients without peri-device flow (20% vs. 6.06%). Interestingly, three patients were found to have left atrial thrombosis during follow-up and one of whom developed a stroke.
The success rate of thoracoscopic LAA occlusion was 80.43% (111/138, LAA stump < 1 cm). Twelve patients chose to continue warfarin anticoagulation and 15 refused to use warfarin in exchange for aspirin among 27 (19.57%) patients who had residual stumps. During the follow-up, stroke or SE events and death occurred in 3 of the 27 patients with residual stumps significantly higher than 4 of 111 (11.11% vs. 3.60%) patients with complete resection, which was also consistent with the increased incidence of previous unsuccessful thoracoscopic resection of embolism events.[21] It is worth noting that the efficacy of thoracoscopic resection was significantly better than that of transcatheter closure in all patients with successful LAA closure (significant residual leaks in 3.7% vs. 6.1%).
Safety of Transcatheter LAA Closure and Thoracoscopic LAA Occlusion
The higher incidence of bleeding events due to combined antithrombotic therapy after the device implantation were remarkable. During the follow-up, one patient in the transcatheter closure group had to stop warfarin because of perioperative bleeding. Three patients had to discontinue due to bleeding during the first 45 days warfarin combined with aspirin therapy. These bleeding events account for 50.0% of all bleeding. In thoracoscopic resection group, one patient stopped warfarin because of perioperative bleeding, two patients had to discontinue due to bleeding during the first three months warfarin therapy, accounting for 75.0% of all bleeding. The above data confirmed that the control of hemorrhage was crucial, especially during shorten-warfarin application, both in internal medicine and surgery. Fortunately, the short-term or even no anticoagulation after transcatheter LAA closure were used by many institutions, and real-world studies suggest that the safety and efficacy are comparable to that of long-term oral anticoagulation.[22-24] With the improvement of postoperative anticoagulation, the incidence of bleeding events in patients with transcatheter LAA closure should be further reduced.
Complications, Hospital Stays, the Success Rate of LAA Intervention
Thoracoscopic and transcatheter LAA local treatments have their own unique complications. The complications of thoracoscopic group during the first week was significantly higher than transcatheter group. The length of hospital stay in surgery was significantly longer than that in internal medicine. The success rate of transcatheter LAA closure is significantly higher than thoracoscopic LAA occlusion.
Finally, with the further exploration of the local treatment of left atrial appendage, cooperation between internal medicine and surgery is expected.
LIMITATIONS
Some limitations and strengths of the present study have to be acknowledged. First, this study was only a single-center study, which might weaken the statistical power of the conclusions. Second, the type of local treatment to the LAA was chosen at the discretion of the patient with a full explanation from the physician. Therefore, the results may need to be confirmed.
CONCLUSIONS
On the premise of similar effects in preventing stroke, Thoracoscopic LAA occlusion has the advantage of low risk of bleeding, but it is accompanied by long hospital stay. There is still plenty of room to explore for local treatment of left atrial appendage.
AUTHORS’ CONTRIBUTIONS
JLW and KZ as the the co-first authors analysis the data and write the original draft. YJZ as the corresponding author design this study. ZQ, WJC, LZZ and YJZ made substantial contributions in data acquisition. All authors have read and approved the manuscript.
FUNDING
This work was supported by the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (code: ZYLX201303, XMLX201601), and the grant from National Key Research and Development Program of China (2017YFC0908800).
DATA AVAILABILITY
The raw data used and/or analyzed during the current study are available from the corresponding author on reasonable request.
ETHICS
The study was reviewed by the Clinical Research Ethics Committee of Beijing Anzhen Hospital, Capital Medical University and obtained the written informed consent of each patient.
CONFLICT OF INTERESTS
None.
References
- 1.Prosper A, Shinbane J, Maliglig A, et al Left atrial appendage mechanical exclusion: procedural planning using cardiovascular computed tomographic angiography. J Thorac Imaging. 2020;35:W107–W118. doi: 10.1097/RTI.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 2.Marin N, Shiv S, Ryan R, et al Percutaneous left atrial appendage occlusion in atrial fibrillation patients with a contraindication to oral anticoagulation: a focused review. Europace. 2018;20:1412–1419. doi: 10.1093/europace/eux313. [DOI] [PubMed] [Google Scholar]
- 3.Tomlin AM, Lloyd HS, Tilyard MW, et al Atrial fibrillation in New Zealand primary care: prevalence, risk factors for stroke and the management of thromboembolic risk. Eur J Prev Cardiol. 2017;24:311–319. doi: 10.1177/2047487316674830. [DOI] [PubMed] [Google Scholar]
- 4.Stoddard MF, Dawkins PR, Price CR, et al Left atrial appendage thrombus is not uncommon in patients with acute atrial fibrillation and a recent embolic event: a transesophageal echocardiographic study. J Am Coll Cardiol. 1995;25:452–459. doi: 10.1016/0735-1097(94)00396-8. [DOI] [PubMed] [Google Scholar]
- 5.Phillips KP, Pokushalov E, Romanov A, et al Combining Watchman left atrial appendage closure and catheter ablation for atrial fibrillation: multi-centre registry results of feasibility and safety during implant and 30 days follow-up. Europace. 2018;20:949–955. doi: 10.1093/europace/eux183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddy VY, Doshi SK, Kar S, et al 5-year out comes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol. 2017;70:2964–2975. doi: 10.1016/j.jacc.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Fauchier L, Cinaud A, Brigadeau F, et al Device-related thrombosis after percutaneous left atrial appendage occlusion for atrial fibrillation. J Am Coll Cardiol. 2018;71:1528–1536. doi: 10.1016/j.jacc.2018.01.076. [DOI] [PubMed] [Google Scholar]
- 8.Price MJ Safety and efficacy of transcatheter left atrial appendage closure for stroke prevention in patients with atrial fibrillation. Prog Cardiovasc Dis. 2018;60:542–549. doi: 10.1016/j.pcad.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Fountain RB, Holmes DR, Chandrasekaran K, et al The PROTECT AF (WATCHMAN left atrial appendage system for embolic protection in patients with atrial fibrillation) trial. Am Heart J. 2006;151:956–961. doi: 10.1016/j.ahj.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 10.WANG N, QIU S, YANG Y, et al Physician pharmacist collaborative clinic model to improve anticoagulation quality in atrial fibrillation patients receiving warfarin: an analysis of time in therapeutic range and a nomogram development. Front Pharmacol. 2021;12:673302. doi: 10.3389/fphar.2021.673302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jurin I, Lucijanić M, Šakić Z, et al Patterns of anticoagulation therapy in atrial fibrillation: results from a large real-life single-center registry. Croat Med J. 2020;61:440–449. doi: 10.3325/cmj.2020.61.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy VY, Doshi SK, Sievert H, et al Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2. 3-year follow-up of the PROTECT AF (Watchman left atrial appendage system for embolic protection in patients with atrial fibrillation) Trial. Circulation. 2013;127:720–729. doi: 10.1161/CIRCULATIONAHA.112.114389. [DOI] [PubMed] [Google Scholar]
- 13.Holmes DR, Kar S, Price MJ, et al Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 14.Reddy VY, Möbius-Winkler S, Miller MA, et al Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology) J Am Coll Cardiol. 2013;61:2551–2556. doi: 10.1016/j.jacc.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 15.Viles-Gonzalez JF, Kar S, Douglas P, et al The clinical impact of incomplete left atrial appendage closure with the Watchman Device in patients with atrial fibrillation: a PROTECT AF (percutaneous closure of the left atrial appendage versus warfarin therapy foe prevention of stroke in patients witn atrial fibrillation) substudy. J Am Coll Cardiol. 2012;59:923–929. doi: 10.1016/j.jacc.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 16.Asmarats L, O'Hara G, Champagne J, et al Short-term oral anticoagulation versus antiplatelet therapy following transcatheter left atrial appendage closure. Circ Cardiovasc Interv. 2020;13:e009039. doi: 10.1161/CIRCINTERVENTIONS.120.009039. [DOI] [PubMed] [Google Scholar]
- 17.Washam JB, Stevens SR, Lokhnygina Y, et al Digoxin use in patients with atrial fibrillation and adverse cardiovascular outcomes: a retrospective analysis of the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF) Lancet. 2015;385:2363–2370. doi: 10.1016/S0140-6736(14)61836-5. [DOI] [PubMed] [Google Scholar]
- 18.Hollon M, Faloye A Utilization of the transgastric view of the left atrial appendange for procedural guidance during left atrial appendage clip via video-assisted thoracoscopic surgery. J Cardiovasc Echogr. 2020;30:211–213. doi: 10.4103/jcecho.jcecho_45_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohtsua T, Ninomiya M, Nonaka T, et al Thoracoscoic standalone left atrial aendectomy for thromboembolism revention in nonvalvular atrial fibrillation. J Am Col Cardiol. 2013;62:103–107. doi: 10.1016/j.jacc.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Tzikas A, Shakir S, Gafoor S, et al Left atrial appendage occlusion for stroke prevention in atrial fibrillation: multicenter experience with the AMPLATZER cardiac plug. EuroIntervention. 2016;11:1170–1179. doi: 10.4244/EIJY15M01_06. [DOI] [PubMed] [Google Scholar]
- 21.García-Fernández MA, Pérez-David E, Quiles J, et al Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study. J Am College Cardiol. 2003;42:1253–1258. doi: 10.1016/S0735-1097(03)00954-9. [DOI] [PubMed] [Google Scholar]
- 22.Glikson M, Wolff R, Hindricks G, et al EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion--an update. EuroIntervention. 2020;15:1133–1180. doi: 10.4244/EIJY19M08_01. [DOI] [PubMed] [Google Scholar]
- 23.Widimsky P, Osmancik P. Left atrial appendage closure-ready for widespread clinical use? EuroIntervention 2020; 16: e701−e702.
- 24.Osmancik P, Herman D, Neuzil P, et al Left atrial appendage closure versus direct oral anticoagulants in high-risk patients with atrial fibrillation. J Am Coll Cardiol. 2020;75:3122–3135. doi: 10.1016/j.jacc.2020.04.067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data used and/or analyzed during the current study are available from the corresponding author on reasonable request.