Abstract
Background
Despite remarkable progress in the reduction of under-five mortality, preterm birth associated mortality and morbidity remains a major public health problem in Sub-saharan Africa. In Ethiopia, study findings on the association of preterm birth with intimate partner violence and maternal malnutrition have been inconsistent. Therefore, this systematic review and meta-analysis estimates the pooled effect of intimate partner violence and maternal malnutrition on preterm birth.
Methods
International databases including PubMed, Web of Science, SCOPUS, CINAHL, PsycINFO, Google Scholar, Science Direct, and the Cochrane Library, were systematically searched. All identified observational studies and/or predictors were included. I2 statistics and Egger's test were used to assess the heterogeneity and publication biases of the studies. A random-effects model was computed to estimate the prevalence and its determinants of preterm birth.
Results
The random effects meta-analysis showed that a pooled national prevalence of preterm birth was 13% (95% CI: 10.0%, 16.0%). The highest prevalence of preterm birth was 25% (95% CI: 21.0%, 30.0%) in Harar, and the lowest prevalence was 8% in Southern Nations Nationalities People of Representatives. The meta-analysis suggested a decrease in preterm birth of up to 61% among women receiving antenatal care [POR = 0.39 (95% CI: 0.21, 0.72)]. Women who experienced intimate partner violence [POR = 2.52 (95% CI: 1.68, 3.78)], malnutrition during pregnancy [POR = 2.00 (95% CI: 1.16, 3.46)], and previous preterm birth [POR = 3.73 (95% CI: 2.37, 5.88)] had significantly higher odds of preterm birth.
Conclusion
One in every eight live births in Ethiopia were preterm. Women who experienced intimate partner violence, malnutrition, and had previous preterm exposure were significantly associated with preterm birth. Thus, improving antenatal care visits and screening women who experience previous preterm birth are key interventions. The Federal Ministry of Health could be instrumental in preventing intimate partner violence and improving the nutritional status of pregnant women through proper and widespread implementation of programs to reduce preterm birth.
Keywords: Preterm birth, Antenatal care visit, Meta-analysis, Ethiopia
Preterm birth; Antenatal care visit; Meta-analysis; Ethiopia.
1. Introduction
Despite advancements in antenatal care and a greater reduction in mortality among children under-five, neonatal death remains a public health challenge across a range of countries; most specifically in Sub-saharan Africa (SSA) [1] where nearly three-quarters of neonatal deaths are estimated to occur during the first week of life [2]. In 2013, 2.8 million (44%) of the 6.3 million deaths in under-five children occurred during the neonatal period [3]. Globally, preterm birth is the leading cause of neonatal morbidity and mortality and is also categorized as a global burden of disease condition due to its high mortality and the risk of lifelong impairment among survivors [4, 5]. Preterm births are the leading cause of neonatal deaths accounting for 35% of the world's annual deaths [5, 6].
The healthcare requirements of preterm infants impose a huge economic burden on health systems globally. However, the burden is significantly higher in low-income countries due to higher rates of preterm birth and limited availability of the required resources in conjunction with understaffed and ill-equipped hospitals [7], underutilization of available antenatal steroids [8], commonly occurring readmissions [9, 10, 11], increased length of hospital stays [12], and hospital bill cost increases related to the care of premature newborns [13]. Premature birth leads to severe morbidity with 45.7% premature infants requiring assisted ventilation and 3.2% still requiring supplemental oxygen at 36 weeks of age [12, 14]. Neonatal morbidity was 34%, 24%, and 17% at 34, 35, and 36 weeks of gestation, respectively, compared to 39 weeks of gestation [13]. Premature birth is associated with increased mortality among infants [12, 15, 16, 17], throughout adulthood [16, 18], and offspring morbidity across the lifespan [12, 19], including psychiatric disorders [20] and social difficulties [21, 22], with the risk of epilepsy during childhood increasing in premature newborns [23].
Preterm birth also predicts the likelihood of parenthood as those born premature face a decreased likelihood of parenthood [24, 25] and a decreased chance of romantic partnership and sexual intercourse [25, 26]. Global strategic plans published by the United Nations Sustainable Development Goals are in place in an effort to reduce preterm births associated with neonatal mortality and morbidity as well as to lower newborn mortality to children under 12 per 1,000 live births in every country by 2030. Globally, the burden of preterm births is high, and the morbidity or mortality associated with preterm births is higher in Eastern Africa [27], including Ethiopia [28, 29, 30, 31]. Currently, there is growing national attention focused on reducing neonatal mortality in Ethiopia; however, information regarding the relative importance of preventative strategies and predictors for newborn morbidity and mortality remains limited in Ethiopia. A meta-analysis reported on preterm birth and the effect of hypertension, multiple pregnancies, antepartum hemorrhage, and chronic illness on preterm birth [32], yet the pooled prevalence was limited to only four of the nine regions in the country and did not address modifiable risk factors for preterm birth such as intimate partner violence (IPV) and maternal malnutrition. Hence, there was inconclusive and inconsistent findings across the country [29, 30, 33, 34, 35, 36, 37, 38]. Therefore, this systematic review and meta-analysis aims to estimate the pooled prevalence of preterm birth and its association with IPV and maternal malnutrion among women in Ethiopia.
2. Methods
2.1. Reporting of systematic review, data sources, and search strategies
The findings of this systematic review and meta-analysis have been reported based on the recommendations of the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) 2009 statement checklist (S1 Table) [39]. All published articles were searched from major international databases including: PubMed, Cochrane Library, Web of Science, Science Direct, Google Scholar, African Journals Online databases, and Google hand searches. Additionally, a search was made for the reference list of studies already identified in order to retrieve additional articles. The PECO (Population, Exposure, Comparison, and Outcomes) search formula was used for this review.
The population of interest was all live births delivered between 28 weeks of gestation and 37 weeks of gestation in Ethiopia. The exposure determinants of preterm birth included maternal area of residence, presence of antenatal care visits, frequency of antenatal care visits, IPV, malnutrition during pregnancy, and previous exposure of preterm birth. Comparisons were defined for each predictor with the respective reported reference group for each predictor per variable.
2.2. Study outcomes
The outcome of interest was preterm birth. The secondary outcomes included the predictors and adverse perinatal or neonatal outcomes of preterm birth. For each of the selected components of PECO, electronic databases were searched using the keyword search and the medical subject heading [MeSH] words. The keywords include preterm birth, adverse perinatal outcomes, determinants, predictors, associated factors, and Ethiopia. The search terms are combined by the Boolean operators “OR” and “AND”. Preterm birth is defined as a newborn being born between 28 and 37 weeks of gestation. IPV is defined as “any behaviour within an intimate relationship that causes physical, psychological or sexual harm to those in the relationship. Such behaviour includes acts of physical aggression, such as slapping, hitting, kicking and beating, as well as psychological abuse, such as intimidation, constant belittling and humiliation, and forced intercourse and other forms of sexual coercion based on the WHO definition. It can involve various controlling behaviours, such as isolating a person from their family and friends, monitoring their movements, and restricting their access to information [40]. Malnutrition during pregnancy was defined based on the mid-upper arm circumference (MUAC). MUAC <23 cm (cm) was considered as being malnourished [41].
2.3. Eligibility criteria and study selection
This review included studies that reported preterm birth or predictors, antenatal care visits, IPV, and previous preterm birth. All published studies published in the English language until the end of our search on July 4, 2020 have been retrieved to assess eligibility for inclusion in this review and critical assessment. The review excluded studies that were case reports of populations, surveillance data (Demographic Health Surveys), abstracts for conferences, and articles without full access. First, through title, abstract, and full review, the two reviewers (MD and FA) evaluated the articles for inclusion. Any disagreement between the two reviewers was resolved by consensus. There was then a full-text analysis of those potentially qualifying studies, whether or not the specified set of criteria had been met, and for duplicated records. During the encounter of duplication, only the full-text article was retained.
2.4. Quality assessment and data collection
The Newcastle-Ottawa Scale (NOS) quality assessment tool was used to assess the quality of the included studies based on three components: the selection of the study groups, comparability of the study groups, and ascertainment of exposure or outcome [42]. The main component of the tool was graded from five stars and mainly emphasized the methodological quality of each primary study. The other component of the tool graded from two stars and mainly scored the comparability of each study, and the last component of the tool graded from three stars and was used to evaluate the results and statistical analysis of each original study. The NOS included three categorical criteria with a maximum score of 9 points. The quality of each study was assessed using the following score algorithms: ≥7 points was considered “good”, 4 to 6 points was considered “moderate”, and ≤3 points was considered “poor” quality studies. In order to improve the validity of this systematic review result, only primary studies of fair to good quality were included. The two reviewers (MD and TYA) independently assessed or extracted articles for overall study quality and/or included in the review articles using a standardized data extraction format. The data extraction format included primary author, year of publication, geographic region of the study, sample size, the reported outcome (preterm birth), and the number of cases of live births developing the respective outcome. Selected predictors of preterm birth including association with antenatal care visits, IPV, previous preterm birth, and adverse neonatal outcomes were also extracted.
2.5. Publication bias and statistical analysis
Publication bias was assessed using the Egger's [43] and Begg's [44] tests with a p-value of less than 0.05. The I [2] statistic was used to assess heterogeneity between studies, and a p-value of less than 0.05 was used. As a result of the presence of heterogeneity, a random-effects model was used as a method of analysis [45] resulting in the use of a random-effects meta-analysis model to estimate the pooled effect based on the metaprop software of the double arcsine transformations [46]. The proportions contain inadmissible values near the boundary resulting in computation of confidence intervals not being possible. Hence, the estimated standard error is set to zero and one. Data were extracted in Microsoft Excel and exported to Stata version 11 for analysis. Subgroup analysis was conducted by geographic region and study design. A meta-regression model based on sample size, geographic region, study design, and year of publication was used to identify the sources of random variations in the included studies. The effect of selected determinant variables was analyzed using separate categories of meta-analysis [47]. The findings of the meta-analysis were presented using a forest plot and Odds Ratio (OR) with 95% confidence intervals (CI). In addition, we conducted a sensitivity analysis to assess whether the pooled prevalence estimates were influenced by individual studies.
3. Results
3.1. Study identification and characteristics of included studies
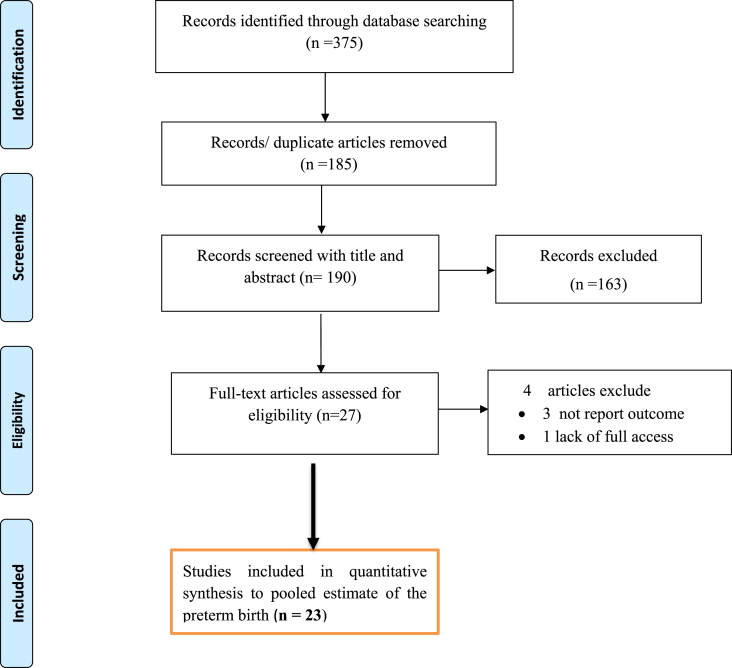
This systematic review and meta-analysis included published studies on the pooled prevalence of preterm birth and its common determinants in Ethiopia using international electronic databases. A total of 375 data sources were found in the review. Of these, 185 duplicated records were deleted and 163 articles were excluded by the screening of titles and abstracts. Subsequently, a total of 27 full-text papers were assessed for eligibility on the basis of the inclusion and exclusion criteria. Four studies were excluded due to lack of an outcome of interest or no reported outcome of interest [48], and the remaining one article was excluded due to a lack of access to the full text [49]. 23 studies [29, 30, 31, 34, 35, 37, 38, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62] were included in the final quantitative meta-analysis to estimate pooled preterm birth (Figure 1).
Figure 1.
PRISMA flow diagram of preterm birth in Ethiopia.
The review was conducted among live births delivered after 28 weeks of gestation to estimate the pooled prevalence of preterm births. Regarding the design of the included studies to estimate preterm birth, 17 (81%) were cross-sectional studies and five (19%) studies were cohort studies [57, 60, 63]. Of those, 20 (95.2%) studies were facility-based studies, and only one study was a community-based study. The articles were published between 2012 and 2020. The largest sample size was 50,787 of live births in a national level study, and the smallest sample was 220 live births from one geographic region conducted in the Oromia Region [50]. All studies were conducted in the six geographic regions of Ethiopia; 8 studies (38.1%) were from the Amhara region [29, 38, 51, 52, 58, 60, 61, 62], 7 (33.3%) were from the Tigray Region [30, 35, 55, 63], four (18.2%) were from Southern Nations, Nationalities, and Peoples’ Representative (SNNPR) [53, 54, 56], three (9.5%) were from the Oromia Region [50, 57, 59], and the remaining two (9.5%) were from the Addiss Ababa [34] and Harar Regions (Table 1) [31]. The included studies were deemed fair to good quality based on the NOS quality assessment tool (Supplement 2).
Table 1.
Characteristics of included studies to estimate preterm birth in Ethiopia.
| Author | Year | Region | Sample | Case | Prevalence | Design |
|---|---|---|---|---|---|---|
| Bekele I et al [50] | 2017 | Oromia | 220 | 57 | 25.9 | FBCS |
| Aregawi G et al [35] | 2019 | Tigray | 472 | 63 | 13.3 | FBCS |
| Brhane M et al [38] | 2019 | Amhara | 480 | 50 | 10.4 | FBCoS |
| W/Yohannes D et al [56] | 2019 | SNNPR | 322 | 42 | 13 | FBCS |
| Mekonen DG et al [29] | 2019 | Amhara | 548 | 70 | 12.8 | FBCS |
| Adane AW et al [52] | 2014 | Amhara | 481 | 69 | 14.3 | FBCS |
| Gebreslasie K [51] | 2016 | Amhara | 540 | 24 | 4.4 | FBCS |
| Abdo RA et al [53] | 2017 | SNNPR | 327 | 28 | 8.6 | FBCS |
| Deressa TA et al [34] | 2018 | AA | 23115 | 3732 | 16.15 | FBCS |
| Cherie & Mebratu [62] | 2017 | Amhara | 462 | 70 | 15.2 | FBCS |
| Eshete A et al [61] | 2013 | Amhara | 295 | 22 | 7.45 | FBCS |
| Mengesha YG et al [63] | 2016 | Tigray | 1152 | 93 | 8.1 | FBCoS |
| Kelkay B et al [30] | 2017 | Tigray | 325 | 55 | 16.9 | FBCS |
| Kebede B et al [60] | 2013 | Amhara | 416 | 69 | 16.6 | FBCoS |
| Kassahun EB et al [58] | 2019 | Amhara | 462 | 61 | 13.2 | FBCS |
| Lolaso T et al | 2019 | SNNPR | 718 | 13 | 1.8 | FBCS |
| Berhe T et al [55] | 2019 | Tigray | 413 | 51 | 12.4 | FBCS |
| Deressa AT et al | 2018 | AA | 384 | 45 | 11.7 | FBCS |
| Mehari M et al | 2020 | Tigray | 752 | 105 | 14 | FBCS |
| Zewde GT [31] | 2020 | Harar | 325 | 81 | 24.9 | FBCS |
| Zerfu TA et al [59] | 2016 | Oromia | 432 | 59 | 13.6 | FBCos |
| Abdo RA et al [54] | 2020 | SNNPR | 304 | 47 | 15.5 | FBCS |
| Brhane M et al [38] | 2019 | Oromia | 1486 | 143 | 10.2 | FBCos |
3.2. Meta-analysis of preterm in Ethiopia
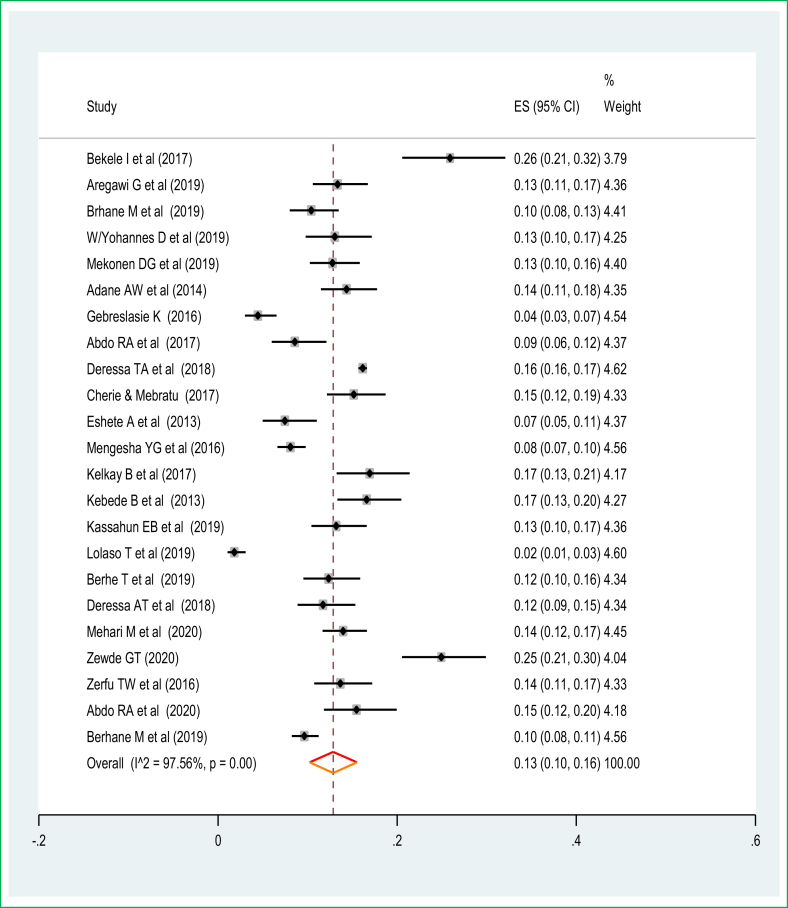
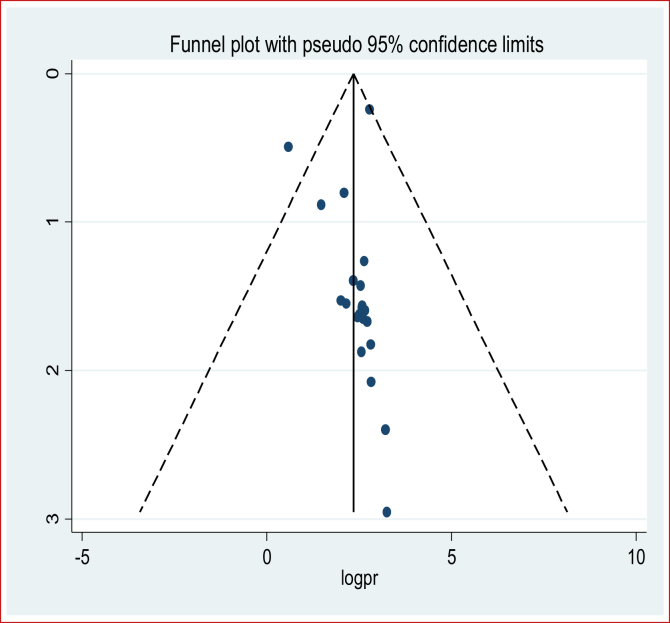
The greatest rate of preterm births was 57 preterm births per 220 total live births making the prevalence 25.9%, which was reported from Jimma University Specialized Teaching and Referral Hospital in the Oromia Region [50] followed by 81 cases per 325 live births with a prevalence of 24.9% of preterm births in the Harar Region [31]. The lowest was 13 cases of preterm births per 718 live births and a prevalence of 1.8% of preterm births from a facility-based cross-sectional study in SNNPR. The meta-analysis of 21 studies showed that the pooled national prevalence of preterm births was 13% (95% CI: 10.0%, 16.0%). A random-effects model was used due to significant heterogeneity (I2 = 97.7%, p-value < 0.05) (Figure 2). The funnel plot observation also showed that there is a symmetrical distribution, and the objective assessment of the Egger's and Begg's tests revealed that there was no publication bias with a p-value of 0.54 and 0.75. The univariate meta-regression model was used to identify possible sources of heterogeneity based on year of publication, sample size, and study design, but none of these variables were found to be statistically significant (p-value ≤ 0.05). Sensitivity analyses using a random-effects model showed that no single study had unduly influenced the pooled prevalence of preterm birth among live births in Ethiopia. The funnel plot showed that there was a symmetrical distribution (Figure 3).
Figure 2.
The pooled prevalence of preterm birth among live births in Ethiopia.
Figure 3.
Funnel plot of symmetry on the prevalence of preterm birth in Ethiopia.
3.3. Subgroup analysis
The subgroup analysis was conducted based on the geographic region and the study design. Therefore, this random-effects meta-analysis based on the geographic region revealed that the highest pooled prevalence of preterm birth was observed in the Harar Region at 25% (95% CI: 21%, 30%) followed by the Oromia Region at 17% (95% CI: 14%, 19%), and the SNNPR Region at 8% (95% CI: 1%, 10%) (Table 2). In addition, the pooled subgroup analysis showed that the prevalence of preterm births was highest in the cross-sectional studies at 13% (95% CI: 10%, 17%).
Table 2.
Subgroup analysis of preterm birth by geographic region and study design.
| Outcome | Category | No of studies | Prevalence [95%CI] | I2 | p-value |
|---|---|---|---|---|---|
| Geographic region | Amhara | 8 | 12.0 (8.00, 15.0) | 91.3 | <0.0001 |
| Tigray | 5 | 13.0 (9.0, 7.01, 16.0) | 85.6% | <0.0001 | |
| Oromia | 3 | 17.0 (14.0, 19.0) | 0% | <0.0001 | |
| SNNPR | 4 | 8.00 (1.00, 15.0) | 0% | <0.0001 | |
| Addiss Abeba | 2 | 16.0 (15.00, 17.00) | - | - | |
| Harar | 1 | 25.0 (21.0, 30.0) | - | - | |
| Study design | Cohort | 5 | 12.3 (8.00, 16.0) | 87.3% | <0.0001 |
| Cross-sectional | 18 | 13.0 (10.0, 17.0) | 98.1% | <0.0001 |
3.4. Association of maternal area of residence and preterm birth
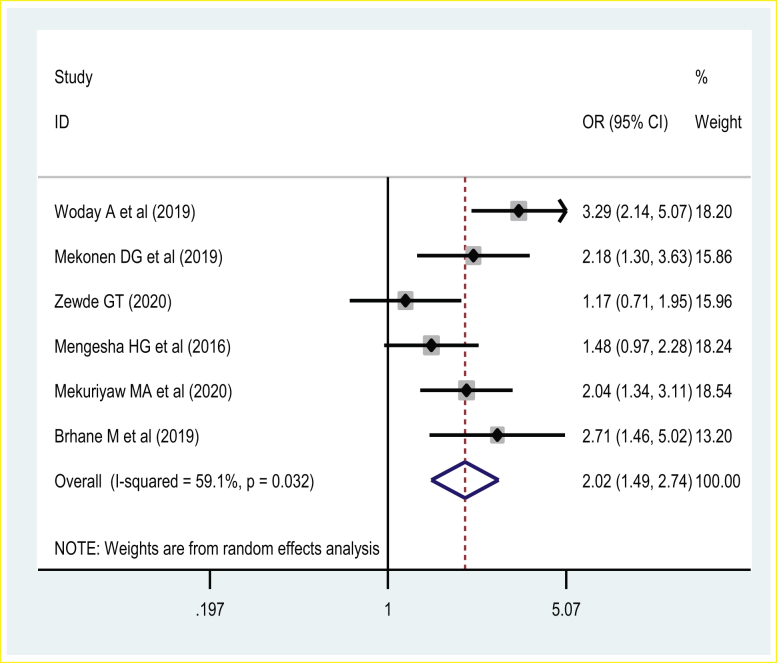
Six studies were included in investigating the association between the maternal area of residency and preterm birth. The pooled effect showed that women who reside in a rural area were at increased risk of preterm birth. Thus, women who reside in rural areas were two times [POR = 2.02 (95% CI: 1.49, 2.74)] more likely to have a preterm birth than those women who reside in urban areas. A random-effects model was used due to the presence of a significant source of moderate heterogeneity (I2 = 59%, p-value < 0.03) (Figure 4).
Figure 4.
The association of maternal rural residency and preterm birth.
3.5. Impact of antenatal care visit to reduce preterm birth
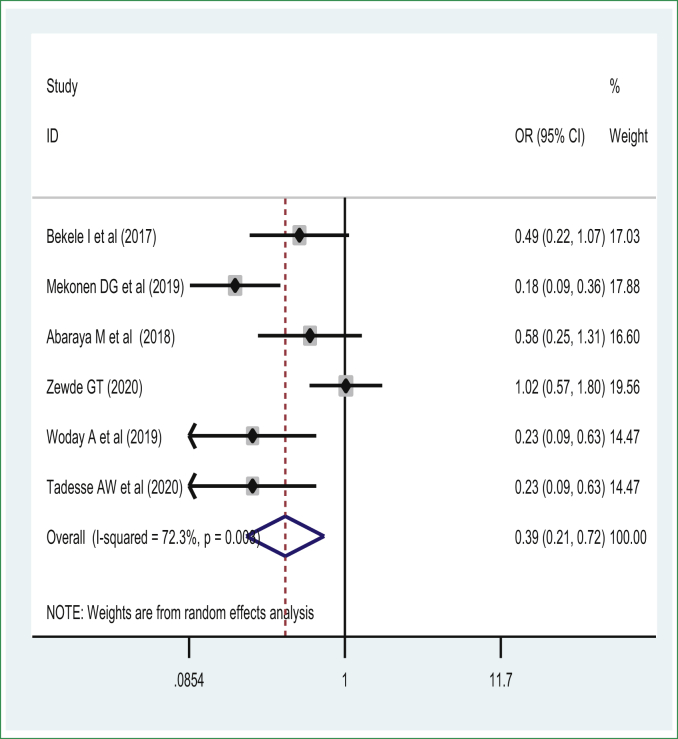
The presence of antenatal care (ANC) visits and frequency of ANC visits impacting preterm birth was investigated to assess the pooled effect on the rate of preterm birth due to presence of inconsistent findings. Of those, three studies showed a significant association between the presence of at least one ANC visit with preterm birth [33, 38, 56, 64] versus zero visits, and four studies showed absence of association between the presence of at least one ANC visit and preterm birth [29, 37, 50, 55]. Seven primary studies showed there was a significant association between the frequency of four or more ANC visits versus less than or equal to four visits and preterm birth [30, 33, 51] and no association based on four studies [54, 64, 65]. Thus, the meta-analysis revealed that the presence of at least 1 ANC visit as well as having four or more frequent antenatal care visit interventions are modifiable predictors that reduce the odds of preterm birth.
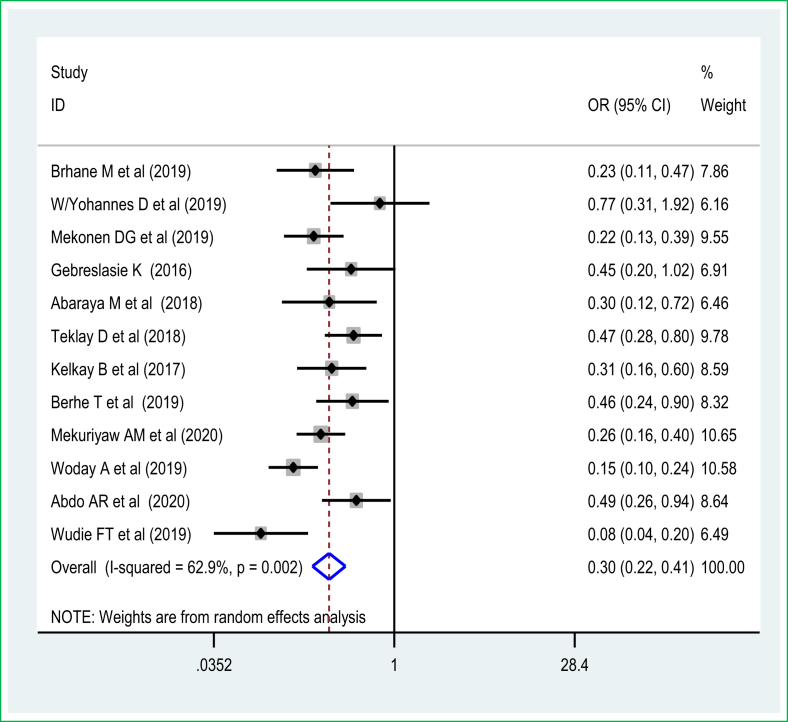
The pooled effect of 6 studies [29, 31, 33, 50, 54, 64] showed that women who completed ANC visits had a reduced risk of preterm birth among live births in Ethiopia. Thus, women who had ANC were 61% [POR = 0.39 (95% CI: 0.21, 0.72)] less likely to have preterm birth than those women who didn't have any ANC visits during pregnancy. A random-effects model was used due to the presence of significant sources of moderate heterogeneity (I2 = 72.4%, p-value < 0.05) (Figure 5). There was no publication bias based on Egger's test with a p-value of 0.25. The pooled effect meta-analysis of eleven studies [29, 30, 33, 51, 54, 56, 65] also revealed that women who have four or more ANC visits experienced reduced odds of preterm birth and were 70% less likely to have a preterm birth [POR = 0.30 (95% CI: 0.22, 0.41)] than women who had less than four ANC visits during pregnancy. A random-effects model of the meta-analysis was used due to the presence of a significant source of heterogeneity (I2 = 88.2%, p-value < 0.05) (Figure 6). There was no significant publication bias based on the Egger's test.
Figure 5.
The association of presence of antenatal care visit and preterm birth.
Figure 6.
The association of frequency of antenatal care visit and preterm birth.
3.6. Association of IPV, maternal malnutrition, and preterm birth
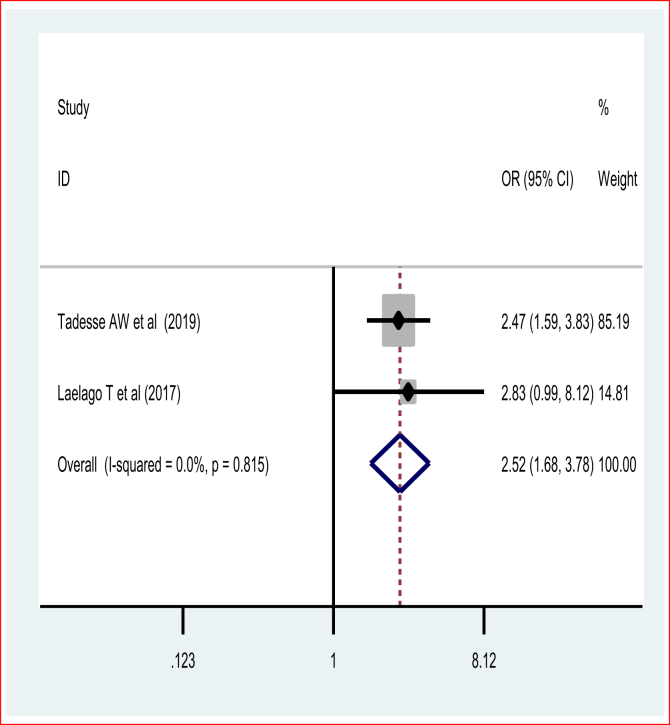
This systematic review and meta-analysis assessed the effect of IPV and previous preterm deliveries on recent preterm birth. The pooled odds ratio of the two studies revealed [66] that women who have encountered IPV were two and half times [POR = 2.52 (95%CI: 1.68, 3.78)] more likely to experience preterm birth than those who have not encountered IPV during pregnancy. The fixed-effect model of the meta-analysis was used due to the absence of a statistically significant source of heterogeneity (I2 = 0% and p = 0.82) (Figure 7). Egger's test showed a non-significant publication bias.
Figure 7.
Pooled odds ratio of effect of intimate partner violence on preterm birth.
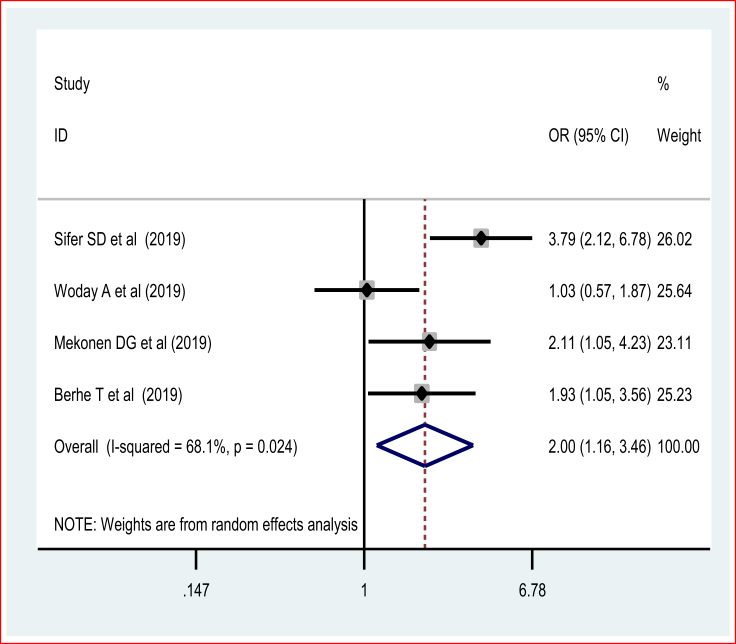
This systematic review and meta-analysis also investigated the association between maternal nutritional status during pregnancy and preterm birth. The pooled odds ratio of four studies using the random-effects model of the meta-analysis showed that the odds of preterm birth were two folds higher [POR = 2.00 (95% CI: 1.16, 3.46, I2 = 68%)] among women who experienced malnutrition (Figure 8). There was no publication bias based on the Egger's test.
Figure 8.
Forest plot of association of malnutrition in pregnancy and preterm birth.
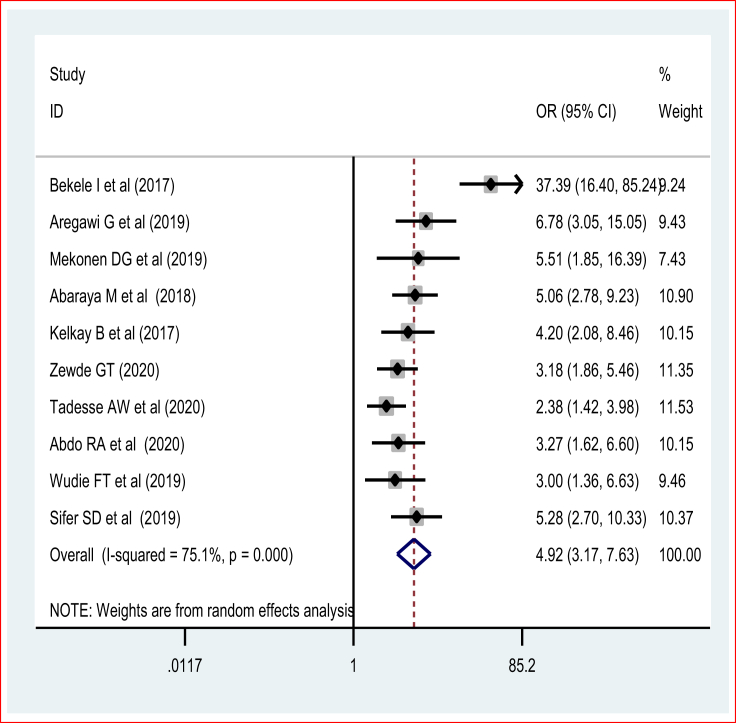
3.7. Impact of previous preterm delivery on recent preterm birth
The pooled effect of the meta-analysis [29, 30, 31, 35, 50, 54, 67] showed that women who had a history of preterm delivery were four times [POR = 4.92 (95% CI: 3.17, 7.13)] more likely to experience preterm birth compared to their counterparts. The random-effects model of the meta-analysis was used due to the presence of evidence of significant heterogeneity (I2 = 83.5% and p = 0.0001) (Figure 9). There was no publication bias based on the Egger's and Begg's tests.
Figure 9.
The association of previous preterm birth and preterm birth.
4. Discussion
This systematic review and meta-analysis revealed that the national level pooled estimate of preterm birth in Ethiopia was 13% (95% CI: 10.0%, 16.0%) based on the random-effects model. The pooled prevalence in the current meta-analysis was comparable with the estimated global preterm birth rate of 10.6% [68]: National study reports in North America were at 11.2%, reports in SSA countries were at 12%, reports from Indonesia were at 10.4% [69], and 10.4% was reported in Asian countries [68]. The fact that the results are comparable to estimates from North America reflects the high prevalence of chronic disease in the latter– namely obesity, heart disease, and multiple gestations possibly related to assisted reproduction and unintended pregnancies. Women are exposed to stress loads that are known to disrupt physiological functions and are a known risk factor in the increase of preterm labor despite having an improved healthcare system than that of Ethiopia [70]. This pooled estimate was higher compared with the national prevalence of South Korea in which 7.1% [71] of live births were preterm. This variation might be due to the differences in socio-demographic characteristics and also an indicator that Ethiopia has limited quality of maternal and perinatal healthcare systems that are not yet as committed and competitive as higher income countries in regard to implementing different strategies and preventive modalities to reduce preterm birth and also reduce a higher burden of malnutrition among pregnant women in Ethiopia [72, 73]. Thus, the government, in collaboration other stakeholders and non-governmental organizations, need to increase strategies to reduce preterm births to meet the global WHO targets. The high prevalence of preterm births was reported in the Oromia Region based on the pooled results from the subgroup analysis due to the fact that the prevalence of IPV was higher among pregnant women in Oromia than other regions based on a recent meta-analysis done in Ethiopia by Alebel A and his colleagues [74].
This systematic review and meta-analysis also revealed that compared to women with fewer than four antenatal visits, women who completed four or more as recommended by the Ethiopian Ministry of Health, had reduced odds of preterm birth. The provision of information on essential micronutrient supplementation during pregnancy prevents anemia [75] and may increase the adherence to iron and folic-acid (IFA) supplementation as may reduce the rate of preterm births [76, 77, 78]. A high number of ANC visits may use for the identification of abnormalities occurring in pregnancy and may be associated with the management of a selected subgroup with very high-risk groups (like pre-eclampsia, antepartum haemorrhage, and previous exposure).
This systematic review and meta-analysis found that those who experienced IPV were more likely to have preterm birth from their pregnancy. This finding has been consistent with other systematic reviews and meta-analyses undertaken across a range of settings [79, 80, 81]. A variety of direct and indirect factors may be involved in this association. Physical violence/assault to the abdomen or sexual trauma experienced during pregnancy may increase the risk of spontaneous intiation of labour and preterm delivery [82]. Direct physical assault or sexual abuse has been associated with placentalabruption, uterine contractions, premature rupture of membranes, and genitourinary infections all which can result in preterm birth. Violence may influence behavioural risk factors such as drug use, smoking, inadequate nutrition, or prenatal care. Women in a violent relationship tend to have less support from their partners, increased stress levels, and reduced autonomy in terms of access to material resources, which contributes preterm delivery [83]. Chronic maternal stress in particular has been associated with preterm birth [84, 85] through the maternal-fetal endocrinological and immunological pathways. Psychosocial stress leads to increased production of proinflammatory cytokines [86] which may lead to overexpression of receptors in the chorioamniotic membranes [87], an increase in the production of prostaglandins [88], a weakening of the fetal membranes, and ripening of the cervix [89] which all increase the odds of preterm birth [90]. Women who experience IPV are less likely to access maternal healthcare services and have reduced antenatal care uptake and which therefore results in less opportunity to receive preventative healthcare for medical disorders and complications in pregnancy [91]. A meta-analysis provided evidence that the experience of IPV reduces ANC visits [92]. Thus, a strategy to reduce IPV and its impact on mental health should be an area of priority to decrease preterm birth.
As in previous studies [93, 94, 95] our anlaysis revealed that malnourished pregnant women had a twofold higher odds of preterm birth than their healthy counterparts. . It is well documented that maternal diet affects placental and fetal growth directly by contributing to anemia and impacting the number of fetal nutrients available to be transported through the placenta. Maternal diet also indirectly impacts the fetal endocrine system by modulating gene activity [96]. Indeed, maternal malnutrition plays a significant role in increased risk for acute and chronic diseases both in mother, neonate and child [97]. Maternal malnutrition leading to insufficient gestational weight gain is considered independent risk factors for the occurrence of spontaneous preterm birth [98]. Malnutrion raises the risk of developing hypertensive disorders - mainly preeclampsia [99, 100] which in turn increases the odds of preterm birth. Moreover, malnourished pregnant women might be more likely to give small for gestational child further increases the risk of preterm birth [101]. Malnourished women might also acquire infections more easily which releases inflammatory cytokines leading to initiation of preterm labor and birth. Therefore, the Ministry of Health, in collaboration with other concerned bodies, should emphasize the national nutrition targets to reduce the adverse pregnancy outcomes associated with malnutrition.
This systematic review and meta-analysis show that completion of at least one ANC visit was a significant protective predictor of experiencing preterm birth and aligns with a study done in Eastern Africa [102] that revealed the absence of ANC visits increased the risk of preterm birth. This is because women who adhere to ANC visits during pregnancy are more likely to receive obstetric care earlier within the golden time when to seek care at the point of childbirth [103, 104]. They are also more likely to have increased birth preparedness and have a complication readiness plan to prevent any form of delay in women with comorbidities such as preeclampsia or antepartum hemorrhage, which commonly results in preterm Caesarean delivery to save the life of the mother or the neonate.
Moreover, this meta-analysis revealed that women who had previous history of preterm delivery have increased the risk of preterm birth which supported a study done in Eastern Africa [102] that revealed the presence of previous preterm birth increased the risk of preterm birth. This can be explained due to the thought that women who have had an experience of pre-term birth may have increased maternal anxiety and distress, which have the increased the odds of preterm birth. The possible reason for the significant increase of preterm birth among those women might be more likely to develop maternal stress associated with the previous exposure. The maternal stress increased the risk of preterm birth [84, 85] through increased production of proinflammatory cytokines [86], cause the production of prostaglandins [88], weaken fetal membranes and ripen the cervix [89] increased the risk of preterm birth. Thus, improving the care provision, integrating with women with previous bad obstetric history is an area of improvements.
Despite our extensive literature search and a meta-analysis of articles from all regions across the country, the results may not be generalizable as the studies were conducted only in 6 of the X regions. Further, there was high heterogeneity among studies which may be explained by the heterogeneity in the characteristics of the studies and may have led to insufficient statistical power to detect statistically significant association. A meta-regression analysis revealed that there was no variation due to sample size and publication year. The effect of IPV and malnutrition have included small studies with have small sample sizes which might affect the estimation. The meta-analysis cannot assess the impact of the type of IPV on preterm birth nor on the type of preterm birth - either spontaneous or medically indicated. Future prospective studies need to assess the contribution of the spectrum of IPV on spontaneous and iatrogenic preterm birth.
5. Conclusions
A significant number of live births in Ethiopia are preterm with a wide range of short- and long-term complications as a result. A range of issues increase the odds of this occurrence, including improving women's empowerment, increasing ANC visits, and screening women for previous preterm birth. There is also a critical need to address and mitigate IPV and maternal malnutrition during pregnancy in order to reduce preterm birth. The Federal Ministry of Health and concerned bodies should work toward prevention of preterm birth through focusing on the identified modifiable behavioural factors.
Declarations
Author contribution statement
Melaku Desta and Temesgen Getaneh: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Peter Memiah, Kirsten I Black, Tadesse Yirga Akalu, Wondimeneh Shibabaw Shiferaw, Nigus Bililign Yimer and Biachew Asmare: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank the authors who conducted the primary studies.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Oza S., Cousens S.N., Lawn J.E. Estimation of daily risk of neonatal death, including the day of birth, in 186 countries in 2013: a vital-registration and modelling-based study Lancet. Glob. Health. 2014;2:e635–e644. doi: 10.1016/S2214-109X(14)70309-2. [DOI] [PubMed] [Google Scholar]
- 2.Lawn J.E., Cousens S., Bhutta Z.A., et al. Why are 4 million newborn babies dying each year? Lancet. 2004;364(9432):399–401. doi: 10.1016/S0140-6736(04)16783-4. [DOI] [PubMed] [Google Scholar]
- 3.UNICEF, WHO, World Bank, UN-DESA Population Division . United Nations Children’s Fund; New York, NY: 2014. Levels and Trends in Child Mortality—Report 2014. Estimates Developed by the UN Inter-agency Group for Child Mortality Estimation. [Google Scholar]
- 4.Murray C.J.V.T., Lozano R., Naghavi M., Flaxman A.D., Michaud C., Ezzati M., Shibuya K., Salomon J.A., Abdalla S., et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 5.Liu L.J.H., Cousens S., Perin J., Scott S., Lawn J., Ruden I., Campbell H., Cibulskis R., Mengying L., et al. Global, regional and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 6.Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet (London, England) 2017;390(10100):1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruiz-Peláez J.G., Charpak N., Cuervo L.G. Kangaroo Mother Care, an example to follow from developing countries. Br. Med. J. 2004;329(7475):1179–1181. doi: 10.1136/bmj.329.7475.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawn J., Kerber K., Enweronu-Laryea C., Massee Bateman O. Newborn survival in low resource settings—are we delivering? BJOG An Int. J. Obstet. Gynaecol. 2009;116:49–59. doi: 10.1111/j.1471-0528.2009.02328.x. [DOI] [PubMed] [Google Scholar]
- 9.Paul I.M., Lehman E.B., Hollenbeak C.S., Maisels M.J. Preventable newborn readmissions since passage of the newborns’ and mothers’ health protection act. Pediatrics. 2006;118(6):2349–2358. doi: 10.1542/peds.2006-2043. [DOI] [PubMed] [Google Scholar]
- 10.Tseng Y.H., Chen C.W., Huang H.L., et al. Incidence of and predictors for short-term readmission among preterm low-birthweight infants. Pediatr. Int. 2010;52(5):711–717. doi: 10.1111/j.1442-200X.2010.03129.x. [DOI] [PubMed] [Google Scholar]
- 11.Kunle-Olowu O.E., Peterside O., Adeyemi O.O. Prevalence and outcome of preterm admissions at the neonatal unit of a tertiary health centre in Southern Nigeria. Open J. Pediatr. 2014;4(1):67. [Google Scholar]
- 12.Khashu M., Narayanan M., Bhargava S., Osiovich H. Perinatal outcomes associated with preterm birth at 33 to 36 Weeks’ gestation: a population-based cohort study. Pediatrics. 2009;123(1):109. doi: 10.1542/peds.2007-3743. [DOI] [PubMed] [Google Scholar]
- 13.McIntire D.D., Leveno K.J. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet. Gynecol. 2008;111(1):35–41. doi: 10.1097/01.AOG.0000297311.33046.73. [DOI] [PubMed] [Google Scholar]
- 14.Escobar G.J., McCormick M.C., Zupancic J.A., et al. Unstudied infants: outcomes of moderately premature infants in the neonatal intensive care unit. Arch. Dis. Child. Fetal Neonatal Ed. 2006;91(4):F238–F244. doi: 10.1136/adc.2005.087031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fellman V., Hellström-Westas L., Norman M., et al. One-year survival of extremely preterm infants after active perinatal care in Sweden. Obstet. Anesth. Digest. 2010;30(1):22–23. doi: 10.1001/jama.2009.771. [DOI] [PubMed] [Google Scholar]
- 16.Swamy G.K., Østbye T., Skjærven R. Association of preterm birth with long-term survival, reproduction, and next-generation preterm birth. J. Am. Med. Assoc. 2008;299(12):1429–1436. doi: 10.1001/jama.299.12.1429. [DOI] [PubMed] [Google Scholar]
- 17.Kramer M.S., Demissie K., Yang H., et al. The contribution of mild and moderate preterm birth to infant mortality. JAMA. 2000;284(7):843–849. doi: 10.1001/jama.284.7.843. [DOI] [PubMed] [Google Scholar]
- 18.Crump C., Sundquist K., Sundquist J., Winkleby M.A. Gestational age at birth and mortality in young adulthood. JAMA. 2011;306(11):1233–1240. doi: 10.1001/jama.2011.1331. [DOI] [PubMed] [Google Scholar]
- 19.McCormick M.C., Litt J.S., Smith V.C., Zupancic J.A. Prematurity: an overview and public health implications. Annu. Rev. Publ. Health. 2011;32:367–379. doi: 10.1146/annurev-publhealth-090810-182459. [DOI] [PubMed] [Google Scholar]
- 20.Gardener H., Spiegelman D., Buka S.L. Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics. 2011;128(2):344–355. doi: 10.1542/peds.2010-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGowan J.E., Alderdice F.A., Holmes V.A., Johnston L. Early childhood development of late-preterm infants: a systematic review. Pediatrics. 2011;127(6):1111–1124. doi: 10.1542/peds.2010-2257. [DOI] [PubMed] [Google Scholar]
- 22.Bilgin A., Mendonca M., Wolke D. Preterm birth/low birth weight and markers reflective of wealth in adulthood: a meta-analysis. Pediatrics. 2018;142(1) doi: 10.1542/peds.2017-3625. [DOI] [PubMed] [Google Scholar]
- 23.Li W., Peng A., Deng S., et al. Do premature and postterm birth increase the risk of epilepsy? An updated meta-analysis. Epilepsy Behav. 2019;97:83–91. doi: 10.1016/j.yebeh.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 24.D’Onofrio B.M., Class Q.A., Rickert M.E., Larsson H., Långström N., Lichtenstein P. Preterm birth and mortality and morbidity: a population-based quasi-experimental StudyPreterm birth and mortality and Morbidity Preterm birth and mortality and morbidity. JAMA Psychiat. 2013;70(11):1231–1240. doi: 10.1001/jamapsychiatry.2013.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendonça M., Bilgin A., Wolke D. Association of preterm birth and low birth weight with romantic partnership, sexual intercourse, and parenthood in adulthood: a systematic review and meta-analysis. JAMA Netw. Open. 2019;2(7) doi: 10.1001/jamanetworkopen.2019.6961. e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mannisto T., Vaarasmaki M., Sipola-Leppanen M., et al. Independent living and romantic relations among young adults born preterm. Pediatrics. 2015;135(2):290–297. doi: 10.1542/peds.2014-1345. [DOI] [PubMed] [Google Scholar]
- 27.Marchant T.W.B., Katz J., Clarke S., Kariuki S., Kuile Ft, et al. Neonatal mortality risk associated with preterm birth in East Africa, adjusted by weight for gestational age: individual participant level meta-analysis. PLoS Med. 2012;9(8) doi: 10.1371/journal.pmed.1001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aynalem Y.A. Survival status and predictor of mortality among premature neonate that was admitted to neonatal intensive care unit from 2013-2017 at Tikur Anbessa Hospital, Addis Ababa Ethiopia: a retrospective cohort study with survival analysis. Prim. Health Care. 2018;8 [Google Scholar]
- 29.Mekonen D.G., Yismaw A.E., Nigussie T.S., Ambaw W.M. Proportion of Preterm birth and associated factors among mothers who gave birth in Debretabor town health institutions, northwest, Ethiopia. BMC Res. Notes. 2019;12(1):2. doi: 10.1186/s13104-018-4037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelkay B., Omer A., Teferi Y., Moges Y. Factors associated with singleton preterm birth in Shire Suhul general hospital, northern Ethiopia. J. Pregnancy. 2018:2019. doi: 10.1155/2019/4629101. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zewde G.T. Preterm birth and associated factors among mother who gave birth in public health hospitals in harar town eastern Ethiopia 2019. OSP J. Health Care Med. 2020;1(1):1–3. [Google Scholar]
- 32.Muchie K.F., Lakew A.M., Teshome D.F., et al. Epidemiology of preterm birth in Ethiopia: systematic review and meta-analysis. BMC Pregnancy Childbirth. 2020;20(1):574. doi: 10.1186/s12884-020-03271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abaraya M., Seid S.S., Ibro S.A. Determinants of preterm birth at Jimma university medical center, Southwest Ethiopia. Pediatr. Health Med. Therapeut. 2018;9:101. doi: 10.2147/PHMT.S174789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deressa A.T., Cherie A., Belihu T.M., Tasisa G.G. Factors associated with spontaneous preterm birth in Addis Ababa public hospitals, Ethiopia: cross sectional study. BMC Pregnancy Childbirth. 2018;18(1):332. doi: 10.1186/s12884-018-1957-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aregawi G., Assefa N., Mesfin F., et al. Preterm births and associated factors among mothers who gave birth in Axum and Adwa Town public hospitals, Northern Ethiopia, 2018. BMC Res. Notes. 2019;12(1):640. doi: 10.1186/s13104-019-4650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdo R.A., Halil H.M., Muhammed M.A., Karebo M.S. Magnitude of preterm birth and its associated factors: a cross-sectional study at butajira hospital, southern Nations, Nationalities, and people’s region, Ethiopia. Int. J. Pediatr. 2020;2020:6303062. doi: 10.1155/2020/6303062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berhe T., Gebreyesus H., Desta H. Determinants of preterm birth among mothers delivered in central zone hospitals, Tigray, northern Ethiopia. BMC Res. Notes. 2019;12(1):266. doi: 10.1186/s13104-019-4307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brhane M., Hagos B., Abrha M.W., Weldearegay H.G. Does short inter-pregnancy interval predicts the risk of preterm birth in Northern Ethiopia? BMC Res. Notes. 2019;12(1):405. doi: 10.1186/s13104-019-4439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moher D., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4(1) doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krug E.G., et al. Chapter 4. Violence by Intimate Partners. World Health Organization; Geneva: 2002. World report on violence and health; p. 89. [Google Scholar]
- 41.Ververs M-t, Antierens A., Sackl A., Staderini N., Captier V. Which anthropometric indicators identify a pregnant woman as acutely malnourished and predict adverse birth outcomes in the humanitarian context? PLoS Curr. 2013;5 doi: 10.1371/currents.dis.54a8b618c1bc031ea140e3f2934599c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singer M., Deutschman C.S., Seymour C.W., et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Egger M.S.G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Begg C.B.M.M. Operating characteristics of a rank correlation testfor publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 45.Higgins J.P.T.S., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nyaga V.N., Arbyn M., Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch. Publ. Health. 2014;72(1):39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deeks J.J., Higgins J.P., Altman D.G. Analysing data and undertaking meta-analyses. Cochrane Handbook Syst. Rev. Intervent. 2008:241–284. [Google Scholar]
- 48.Tsegaye B., Kassa A. Prevalence of adverse birth outcome and associated factors among women who delivered in Hawassa town governmental health institutions, south Ethiopia. Reprod. Health. 2017;15(1):193. doi: 10.1186/s12978-018-0631-3. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gebremeskel G., Fm M., Welay F., Hailu T., Gebremedhin M., Gg A. 2018. Preterm Births and Associated Factors Among Mothers Who Gave Birth in Axum and Adwa Town Public Hospitals, Tigrai, Northern Ethiopia. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bekele I., Demeke T., Dugna K. Prevalence of preterm birth and its associated factors among mothers delivered in Jimma university specialized teaching and referral hospital, Jimma Zone, Oromia Regional State, South West Ethiopia. J. Women's Health Care. 2017;6:356. [Google Scholar]
- 51.Gebreslasie K. Preterm birth and associated factors among mothers who gave birth in Gondar Town Health Institutions. Adv. Nurs. 2016;2016 [Google Scholar]
- 52.Adane A.A., Ayele T.A., Ararsa L.G., Bitew B.D., Zeleke B.M. Adverse birth outcomes among deliveries at Gondar University hospital, Northwest Ethiopia. BMC Pregnancy Childbirth. 2014;14(1):90. doi: 10.1186/1471-2393-14-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abdo R., Endalemaw T., Tesso F. Prevalence and associated factors of adverse birth outcomes among women attended maternity ward at Negest Elene Mohammed Memorial General Hospital in Hosanna Town, SNNPR, Ethiopia. J. Women's Health Care. 2016;5(4) [Google Scholar]
- 54.Abdo R.A., Halil H.M., Muhammed M.A., Karebo M.S. Magnitude of preterm birth and its associated factors: a cross-sectional study at butajira hospital, southern Nations, Nationalities, and people’s region, Ethiopia. Int. J. Pediatr. 2020:2020. doi: 10.1155/2020/6303062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mekuriyaw A.M., Mihret M.S., Yismaw A.E. Determinants of preterm birth among women who gave birth in Amhara region referral hospitals, northern Ethiopia, 2018: institutional based case control study. Int. J. Pediatr. 2020;2020:1854073. doi: 10.1155/2020/1854073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woldeyohannes D., Kene C., Gomora D., Seyoum K., Assefa T. Factors associated with preterm birth among mothers who gave birth in dodola town hospitals, southeast Ethiopia: institutional based cross sectional study. Clin. Mother Child Health. 2019;16(317):2. [Google Scholar]
- 57.Berhane M., Workineh N., Girma T., et al. Prevalence of low birth weight and prematurity and associated factors in neonates in Ethiopia: results from a hospital-based observational study. Ethiop. J. Health Sci. 2019;29(6) doi: 10.4314/ejhs.v29i6.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kassahun E.A., Mitku H.D., Getu M.A. Adverse birth outcomes and its associated factors among women who delivered in North Wollo zone, northeast Ethiopia: a facility based cross-sectional study. BMC Res. Notes. 2019;12(1):357. doi: 10.1186/s13104-019-4387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zerfu T.A., Umeta M., Baye K. Dietary diversity during pregnancy is associated with reduced risk of maternal anemia, preterm delivery, and low birth weight in a prospective cohort study in rural Ethiopia. Am. J. Clin. Nutr. 2016;103(6):1482–1488. doi: 10.3945/ajcn.115.116798. [DOI] [PubMed] [Google Scholar]
- 60.Kebede B., Andargie G., Gebeyehu A. 2013. Birth Outcome and Correlates of Low Birth Weight and Preterm Delivery Among Infants Born to HIV-Infected Women in Public Hospitals of Northwest Ethiopia. [Google Scholar]
- 61.Eshete A., Birhanu D., Wassie B. 2013. Birth Outcomes Among Laboring Mothers in Selected Health Facilities of north Wollo Zone, Northeast Ethiopia: a Facility Based Cross-Sectional Study. [Google Scholar]
- 62.Cherie N., Mebratu A. Adverse birth out comes and associated factors among delivered mothers in dessie referral hospital. North East Ethiopia. 2018:1–6. [Google Scholar]
- 63.Mengesha H.G., Lerebo W.T., Kidanemariam A., Gebrezgiabher G., Berhane Y. Pre-term and post-term births: predictors and implications on neonatal mortality in Northern Ethiopia. BMC Nurs. 2016;15(1):48. doi: 10.1186/s12912-016-0170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Woday A., Muluneh M.D., Sherif S. Determinants of preterm birth among mothers who gave birth at public hospitals in the Amhara region, Ethiopia: a case-control study. PLoS One. 2019;14(11) doi: 10.1371/journal.pone.0225060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wudie F., Tesfamicheal F., Fisseha H., et al. Determinants of preterm delivery in the central zone of Tigray, northern Ethiopia: a case-control study. South. Afr. J. Child Health. 2019;13(3):108–114. [Google Scholar]
- 66.Tadesse A.W., Deyessa N., Wondimagegnehu A., Biset G., Mihret S. Intimate partner violence during pregnancy and preterm birth among mothers who gave birth in public hospitals, Amhara Region, Ethiopia: a case-control study. Ethiop. J. Health Dev. 2020;34(1) [Google Scholar]
- 67.Sifer S., Kedir B., Demisse G. Determinants of preterm birth in neonatal intensive care units at public hospitals in Sidama zone, South East Ethiopia; case control study. J. Pediatr. Neonatal. Care. 2019;9(6):180–186. [Google Scholar]
- 68.Chawanpaiboon S.V.J., Moller A.B., Lumbiganon P., Petzold M., Hogan D., Landoulsi S.J.N., Kongwattanakul K., Laopaiboon M., Lewis C. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob. Health. 2019;7(1):e37–46. doi: 10.1016/S2214-109X(18)30451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Su D., Samson K., Garg A., et al. Birth history as a predictor of adverse birth outcomes: evidence from state vital statistics data. Prevent. Med. Rep. 2018;11:63–68. doi: 10.1016/j.pmedr.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bronstein J.M., Wingate M.S., Brisendine A.E. Why is the U.S. Preterm birth rate so much higher than the rates in Canada, great Britain, and western Europe? Int. J. Health Serv. 2018;48(4):622–640. doi: 10.1177/0020731418786360. [DOI] [PubMed] [Google Scholar]
- 71.Choi Y., Lee G., Song I.G., Kim O.J., Park S.M. Prevalence of adverse birth outcomes and disparity of unmarried women in South Korea: a systematic review. J. Global Health Rep. 2018;2 [Google Scholar]
- 72.Assefa N., Berhane Y., Worku A. Wealth status, mid upper arm circumference (MUAC) and antenatal care (ANC) are determinants for low birth weight in Kersa, Ethiopia. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0039957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Desyibelew H.D., Dadi A.F. Burden and determinants of malnutrition among pregnant women in Africa: a systematic review and meta-analysis. PLoS One. 2019;14(9) doi: 10.1371/journal.pone.0221712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alebel A., Kibret G.D., Wagnew F., et al. Intimate partner violence and associated factors among pregnant women in Ethiopia: a systematic review and meta-analysis. Reprod. Health. 2018;15(1):1–12. doi: 10.1186/s12978-018-0637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Molla T., Guadu T., Muhammad E.A., Hunegnaw M.T. Factors associated with adherence to iron folate supplementation among pregnant women in West Dembia district, northwest Ethiopia: a cross sectional study. BMC Res. Notes. 2019;12(1):6. doi: 10.1186/s13104-019-4045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Desta M., Kassie B., Chanie H., et al. Adherence of iron and folic acid supplementation and determinants among pregnant women in Ethiopia: a systematic review and meta-analysis. Reprod. Health. 2019;16(1):1–14. doi: 10.1186/s12978-019-0848-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Imdad A., Bhutta Z.A. Routine iron/folate supplementation during pregnancy: effect on maternal anaemia and birth outcomes. Paediatr. Perinat. Epidemiol. 2012;26:168–177. doi: 10.1111/j.1365-3016.2012.01312.x. [DOI] [PubMed] [Google Scholar]
- 78.Liu J-m, Mei Z., Ye R., Serdula M.K., Ren A., Cogswell M.E. Micronutrient supplementation and pregnancy outcomes: double-blind randomized controlled trial in China. JAMA Intern. Med. 2013;173(4):276–282. doi: 10.1001/jamainternmed.2013.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hill A., Pallitto C., McCleary-Sills J., Garcia-Moreno C. A systematic review and meta-analysis of intimate partner violence during pregnancy and selected birth outcomes. Int. J. Gynecol. Obstet. 2016;133(3):269–276. doi: 10.1016/j.ijgo.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 80.Donovan B., Spracklen C., Schweizer M., Ryckman K., Saftlas A. Intimate partner violence during pregnancy and the risk for adverse infant outcomes: a systematic review and meta-analysis. BJOG An Int. J. Obstet. Gynaecol. 2016;123(8):1289–1299. doi: 10.1111/1471-0528.13928. [DOI] [PubMed] [Google Scholar]
- 81.De Mola C.L., De França G.V.A., de Avila Quevedo L., Horta B.L. Low birth weight, preterm birth and small for gestational age association with adult depression: systematic review and meta-analysis. Br. J. Psychiatr. 2014;205(5):340–347. doi: 10.1192/bjp.bp.113.139014. [DOI] [PubMed] [Google Scholar]
- 82.Heaman M.I. Relationships between physical abuse during pregnancy and risk factors for preterm birth among women in Manitoba. J. Obstet. Gynecol. Neonatal Nurs. 2005;34(6):721–731. doi: 10.1177/0884217505281906. [DOI] [PubMed] [Google Scholar]
- 83.O'Doherty L., Hegarty K., Ramsay J., Davidson L.L., Feder G., Taft A. Screening women for intimate partner violence in healthcare settings. Cochrane Database Syst. Rev. 2015;(7) doi: 10.1002/14651858.CD007007.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lilliecreutz C., Larén J., Sydsjö G., Josefsson A. Effect of maternal stress during pregnancy on the risk for preterm birth. BMC Pregnancy Childbirth. 2016;16(1):5. doi: 10.1186/s12884-015-0775-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Staneva A., Bogossian F., Pritchard M., Wittkowski A. The effects of maternal depression, anxiety, and perceived stress during pregnancy on preterm birth: a systematic review. Women Birth: J. Aust. Coll. Midwives. 2015;28(3):179–193. doi: 10.1016/j.wombi.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 86.Christian L.M. Psychoneuroimmunology in pregnancy: immune pathways linking stress with maternal health, adverse birth outcomes, and fetal development. Neurosci. Biobehav. Rev. 2012;36(1):350–361. doi: 10.1016/j.neubiorev.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blank V., Hirsch E., Challis J.R., Romero R., Lye S.J. Cytokine signaling, inflammation, innate immunity and preterm labour - a workshop report. Placenta. 2008;29(Suppl A):S102–S104. doi: 10.1016/j.placenta.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 88.Challis J.R., Lockwood C.J., Myatt L., Norman J.E., Strauss J.F., Petraglia F. Inflammation and pregnancy. Reprod. Sci. 2009;16(2):206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 89.Snegovskikh V., Park J.S., Norwitz E.R. Endocrinology of parturition. Endocrinol. Metabol. Clin. 2006;35(1):173–191. doi: 10.1016/j.ecl.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 90.Wei S.-Q., Fraser W., Luo Z.-C. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet. Gynecol. 2010;116(2):393–401. doi: 10.1097/AOG.0b013e3181e6dbc0. [DOI] [PubMed] [Google Scholar]
- 91.Mulualem G., Wondim A., Woretaw A. The effect of pregnancy induced hypertension and multiple pregnancies on preterm birth in Ethiopia: a systematic review and meta-analysis. BMC Res. Notes. 2019;12(1):91. doi: 10.1186/s13104-019-4128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Musa A., Chojenta C., Geleto A., Loxton D. The associations between intimate partner violence and maternal health care service utilization: a systematic review and meta-analysis. BMC Wom. Health. 2019;19(1):36. doi: 10.1186/s12905-019-0735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Urooj Asna, Rao Kamini, Sesikeran B. Maternal malnutrition in low-income and middle-income countries: a closer look at the Indian scenario. EC Paed. 2018;7:295–311. 4 (2018) [Google Scholar]
- 94.Cates J.E., Unger H.W., Briand V., et al. Malaria, malnutrition, and birthweight: a meta-analysis using individual participant data. PLoS Med. 2017;14(8) doi: 10.1371/journal.pmed.1002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Organization W.H. World Health Organization; 2016. The Double burden of Malnutrition: Policy Brief. [Google Scholar]
- 96.Black R.E., Allen L.H., Bhutta Z.A., et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371(9608):243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 97.Triunfo S., Lanzone A. Impact of maternal under nutrition on obstetric outcomes. J. Endocrinol. Invest. 2015;38(1):31–38. doi: 10.1007/s40618-014-0168-4. [DOI] [PubMed] [Google Scholar]
- 98.Triunfo S., Lanzone A. Impact of maternal under nutrition on obstetric outcomes. J. Endocrinol. Invest. 2015;38(1):31–38. doi: 10.1007/s40618-014-0168-4. [DOI] [PubMed] [Google Scholar]
- 99.Sibley C., Glazier J., D'Souza S. Placental transporter activity and expression in relation to fetal growth. Exp. Physiol.: Trans. Integr. 1997;82(2):389–402. doi: 10.1113/expphysiol.1997.sp004034. [DOI] [PubMed] [Google Scholar]
- 100.Charnock-Jones D., Kaufmann P., Mayhew T. Aspects of human fetoplacental vasculogenesis and angiogenesis. I. Molecular regulation. Placenta. 2004;25(2-3):103–113. doi: 10.1016/j.placenta.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 101.Christian P., Lee S.E., Donahue Angel M., et al. Risk of childhood undernutrition related to small-for-gestational age and preterm birth in low-and middle-income countries. Int. J. Epidemiol. 2013;42(5):1340–1355. doi: 10.1093/ije/dyt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Laelago T., Yohannes T., Tsige G. Determinants of preterm birth among mothers who gave birth in East Africa: systematic review and meta-analysis. Ital. J. Pediatr. 2020;46(1):10. doi: 10.1186/s13052-020-0772-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gabrysch S., Campbell O.M. Still too far to walk: literature review of the determinants of delivery service use. BMC Pregnancy Childbirth. 2009;9(1):34. doi: 10.1186/1471-2393-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Berhe Y., Wall L.L. Uterine rupture in resource-poor countries. Obstet. Gynecol. Surv. 2014;69(11):695–707. doi: 10.1097/OGX.0000000000000123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.



 , Email:
, Email: