Abstract
Background
Older adults are at higher risk for cardiovascular disease and functional decline, often leading to deterioration and dependency. Cardiac rehabilitation (CR) provides opportunity to improve clinical and functional recovery, yet participation in CR decreases with age. Modified Application of CR in Older Adults (MACRO) is a National Institute on Aging (NIA)-funded pragmatic trial that responds to this gap by aiming to increase enrollment of older adults into CR and improving functional outcomes. This article describes the methodology and novel features of the MACRO trial.
Methods
Randomized, controlled trial of a coaching intervention (MACRO-I) vs. usual care for older adults (age ≥ 70 years) eligible for CR after an incident cardiac hospitalization. MACRO-I incorporates innovations including holistic risk assessments, flexible CR format (i.e., helping patients to select a CR design that aligns with their personal risks and preferences), motivational prompts, nutritional emphasis, facilitated deprescription, enhanced education, and home visits. Key modifications were necessitated by the COVID-19 pandemic, including switching from a performance-based primary endpoint (Short Physical Performance Battery) to a patient-reported measure (Activity Measure for Post-Acute Care Computerized Adaptive Testing). Changes prompted by COVID-19 maintain the original intent of the trial and provide key methodologic advantages.
Conclusions
MACRO is exploring a novel individualized coaching intervention to better enable older patients to participate in CR. Due to COVID-19 many aspects of the MACRO protocol required modification, but the primary objective of the trial is maintained and the updated protocol will more effectively achieve the original goals of the study.
Keywords: Cardiac rehabilitation, Geriatric cardiology, Frailty, Physical activity
1. Introduction
The US population aged 65 years and over will almost double between 2020 and 2050 [1]. As adults survive into old age, the biology and physiology associated with aging predisposes them to cardiovascular disease (CVD) in a context of high clinical complexity [2]. CVD prevalence increases from ~40% in men and women 40–59 years, to 70–75% in those 60–79, and to 79–86% in those aged ≥80 years [3]. Age-related CVD complexity stems from pervasiveness of comorbid diagnoses, frailty, cognitive decline, sensory deficits, incontinence, and associated sequelae of polypharmacy, falls, diminished adherence, poor quality of life, and suboptimal procedural outcomes [4]. While cardiac rehabilitation (CR) has been proven to benefit older adults who participate [5], only 24.4% of CR-eligible Medicare fee-for-services beneficiaries attend even a single session, and just 26.9% of those complete the full program [6]. Barriers to participation include the encumbering effects of geriatric conditions as well as logistical obstacles and lack of motivation amidst mounting health conditions and lifestyle limitations.
Functional capacity also declines with age and disease, with additional risks of rehospitalizations, disability, and mortality among CVD patients. Exercise training and structured physical activity through CR are especially beneficial for older adults, as many tend to become sedentary long before the incident CVD event occurs, with deconditioning that often then accelerates during incident hospitalizations. Overcoming exercise intolerance and fears associated with restarting activity are critical elements of successful recovery [7]. Contemporary CR also includes components of risk factor reduction, nutrition, lifestyle modifications, medication adherence, education, and stress relief to further advance clinical stability and well-being, which in turn can provide disproportionate benefit amidst the high mortality and morbidity risks of old age [8].
Given the compelling evidence for the value of CR as part of current-day CVD therapeutics, it has been elevated by the Centers for Disease Control and Prevention (CDC) as a healthcare priority. The CDC's Million Hearts initiative to improve cardiovascular health targets enrollment of 70% of all eligible patients into CR by 2022 [9]. Nonetheless, most older adults do not attend, and it remains unclear if CR implementation can be improved to enhance the incentive for and process of CR for the untreated majority.
Modified Application of Cardiac Rehabilitation in Older Adults (MACRO; NCT03922529) is a pragmatic [10], randomized controlled trial (RCT) funded by the National Institute on Aging (NIA) that investigates the effectiveness of an intervention, MACRO-I, designed to increase participation of older adults in CR as a key means to reduce disability by improving their physical function. The overarching intent of the MACRO-I is to enhance the health and wellbeing of older adults by overcoming barriers to CR participation and by broadening the scope of CR to address distinctive (and relevant) geriatric challenges. In this paper, we describe the original MACRO clinical trial designed pre-COVID, and modifications for the trial and MACRO-I delivery necessitated by the COVID-19 pandemic.
2. Original design and methods
2.1. Design
Adults aged ≥70 years are randomized to a MACRO intervention (MACRO-I) versus usual care. MACRO-I is a person-centered coaching intervention to facilitate CR. Coaching incorporates innovative techniques to better align CR with the priorities and capacities of eligible older adults. These innovations (explained below) include holistic risk assessments, flexible conceptualization of the CR format (i.e., choosing a CR design that responds to each patient's risks and preferences), motivational prompts, nutritional emphasis, facilitated deprescription, enhanced education, and integrated home visits.
2.2. Specific aims
Aim 1 of MACRO is to establish effectiveness, safety and acceptability of MACRO-I versus usual care, with the hypothesis that patients randomized to MACRO-I would achieve greater improvements in function. The primary outcome is the change in the Short Physical Performance Battery (SPPB) [11] from baseline to 3 months, with lower scores indicating more severe functional impairment. Complementary performance and self-reported functional measures are also assessed to more fully characterize physical and cognitive function.
Aim 2 of MACRO is to demonstrate the sustainability of the functional benefits of the MACRO-I at 6 and 12 months. Aim 3 is exploratory and aims to delineate characteristics of patients who benefit the most from MACRO-I. It is hypothesized that patients who are relatively more burdened by frailty, multimorbidity and other vulnerabilities of age may benefit more than patients who are relatively robust and/or less clinically complex.
2.3. Recruitment procedures
Eligibility for MACRO includes hospitalization for coronary heart disease (CHD), acute myocardial infarction (AMI), coronary artery revascularization (PCI or CABG), heart failure with reduced or preserved ejection fraction (HFrEF or HFpEF), valve repair or replacement (surgical or transcatheter), or heart transplant. The enrollment window to MACRO is up to 10 days after the incident event, with the intent to initiate the MACRO-I promptly (for those in the intervention arm) to mitigate hospital-related functional decline and disability [12].
Potential participants are pre-screened daily through inpatient records to assess for eligible diagnoses and absence of contraindications. Candidate participants are approached while inpatient, at a follow-up visit, by phone, and/or by letter after discharge. Study personnel describe the study to the patient. The Short Blessed test [13] is used to screen for dementia. Patients with severe cognitive impairment (i.e., diagnosis of dementia in the medical record or Short Blessed ≥13), unstable medical conditions, life expectancy less than 12 months, residing in a long-term care living situation prior to the time of hospitalization (with no plans to return to independent living after the hospitalization), or inability or unwillingness to consent are excluded. The consent process varies according to each hospital's governance. At the VA Pittsburgh Healthcare System, consent is obtained either in person on hard copy forms or over the phone. At Barnes-Jewish, Missouri Baptist, and Shadyside Hospitals, consent is obtained either in person or by mail on hard copy forms, or by electronic consent.
MACRO is approved by the Institutional Review Boards at the University of Pittsburgh, Veterans Affairs Pittsburgh Medical Center, and the Washington University School of Medicine, and all participants provide written informed consent.
2.4. Participants, recruitment, randomization
Based on the SPPB, the original MACRO study enrollment goal is 480 participants, which yields 80% statistical power to detect a between treatment arm difference of 0.77 point in SPPB with a two-tailed α = 0.05, assuming a retention rate of 80% and a standard deviation of 2.7 for SPPB change [[14], [15], [16], [17], [18]]. This corresponds to a moderate effect size. Randomization is stratified by site and baseline SPPB score (i.e., 0–6, 7–9 or 10–12) to ensure a balance between the treatment groups with respect to the baseline value of the primary outcome.
Enrollees are randomized to MACRO-I or usual care in a 1:1 ratio using a blocked scheme with random block size. All patients are eligible for CR as determined by their physicians, but CR is facilitated by coaching only in the MACRO-I arm. MACRO-I coaches supplement care to increase the accessibility, sustainability and patient-centeredness of CR such that it may become more available and pragmatic for eligible older patients.
2.5. MACRO intervention
MACRO-I coaches first meet study patients during the incident hospitalization when deemed stable by their medical teams. MACRO-I coaches follow these participants daily while they are still hospitalized. Coaches ensure that inpatient CR (phase I) is ordered and also provide additional supportive coaching.
MACRO-I innovations include novel holistic risk assessments that link functional and psychosocial risks to risks from CVD. This perspective is used to guide recommendations for different formats of outpatient CR, i.e., site-based, remote-based (aka home-based), or hybrid CR (site-based that transitions to remote-based). For holistic risk assessment, details of the patient's current illness, past medical history, physical functional assessment at baseline (e.g., gait speed [19], SPPB score, grip strength) [20], and psychosocial factors assessed at baseline (e.g., Readiness to Change [21], Patient Health Questionnaire [PHQ]-9 [22] score) are integrated with one another. Table 1 shows the elements incorporated into the holistic risk assessment. This risk assessment is used to enrich the rationale for CR, and to inform the coach's patient-specific recommendations for site-based, remote-based or hybrid-based approaches to CR. High risk in any category increases consideration for site-based or hybrid CR, as it may provide greater supervision and support. However, if high-risk patients still prefer remote-based CR, risk assessment provides opportunity to develop pragmatic approaches that specifically mitigate CVD and non-CVD dangers.
Table 1.
Pre-COVID MACRO risk stratification.
| Medical Criteria |
Functional Criteria |
Psychosocial Criteria |
|---|---|---|
| CVD based on medical record | Risk based on baseline assessments | Risk based on baseline assessments |
High Risk
|
High Risk
|
High Risk
|
Moderate Risk
|
Moderate Risk
|
Moderate Risk
|
Low Risk
|
Low Risk
|
Low Risk
|
LVEF-Left ventricular ejection fraction; MI-myocardial infaction; PCI-percutaneous coronary intervention; CABG-coronary artery bypass grafting surgery; PTCA-percutaneous transluminal coronary angioplasty; ECG-electrocardiogram; FRIDs-Fall risk-increasing drugs; SPPB-Short Physical Performance Battery; METs-metabolic equivalents; PHQ-Patient Health Questionnaire; REALM-Rapid Estimate of Adult Literacy in Medicine.
FRIDs: Fall risk-increasing drugs (includes: opioids, antipsychotics [lithium excluded], anxiolytics, hypnotics and sedatives, antidepressants, vasodilators used in cardiac disease, antihypertensives, and dopaminergic agents).
Estimate of METs is based on the Duke Activity Status Index.
MACRO-I innovations also include flexible conceptualization of CR. Whereas most hospital systems promote their own CR programs, there are significant differences between programs (e.g., site-based versus remote-based, but also intensive CR and other variations) and some programs are better suited to some patients than others. MACRO-I coaches conceptualize CR more broadly, and suggest formats that best match each patient's risk profile and preferences. Site-, remote-, and hybrid-based CR formats are all described and potentially facilitated. The MACRO-I coach explains how different formats of CR may better align with each patient's health risks and preferences, and includes issues of logistics, costs, and the home environment. A patient prone to fall risks may, for example, benefit most by starting with site-based CR, with the plan to prioritize learning chair-based exercises such that (s)he can then transition safely and effectively to remote-based CR. Regardless of which type of CR program the patient selects, the MACRO-I coach serves as a common denominator to supplement each patient's experience with feedback and reinforcement to best ensure that (s)he derives a personally-centered experience from CR that helps him/her recover.
MACRO-I enhances motivation for CR by applying each patient's goals of care as an important motivational stimulus. To clarify these goals of care, the MACRO-I coach uses a standardized goals assessment process wherein (s)he displays a series of images that convey a broad range of life-goal choices. Once each patient's goals are identified, the MACRO-I coach applies them with the premise that CR is the principal means for goal attainment. This goals assessment technique was adapted from Enhanced Medical Rehabilitation (E-MR) developed by Lenze et al. [23]
Coaches also employ novel approaches to nutrition, education, and deprescription of sedating medications. MACRO-I coaches ensure that referral to dietary assessment and education is achieved irrespective of the CR format and whether or not a nutritionist is part of the patient's CR program. In contrast to standard precepts of dietary restrictions in most cardiac programs, MACRO-I coaches encourage sufficient caloric intake for patients prone to sarcopenia, frailty and malnutrition.
To enhance education, MACRO-I transition resources were developed and are utilized by the coaches both during the inpatient and the immediate post-hospitalization phases of care. MACRO-I transition educational booklets are disease-specific (e.g., MI, PCI, CABG, HF) and concise to provide basic information about the event that occurred, essentials of therapeutics, and the utility of CR as part of recovery. The transition documents highlight the centrality of CR in recovery, with language, font, and pictures designed for an older population.
MACRO-I includes deprescription as a means to augment functional recovery [24,25]. MACRO-I coaches identify benzodiazepines and anticholinergic/antihistamine medications, as these drug classes predispose to fatigue, falls, poor functional recovery, and other risks in an older population and are considered potentially inappropriate [26]. Medical regimens in these patients are then reviewed by a MACRO-I geriatric psychiatrist and/or a pharmacist with deprescribing expertise. If the participant and the PCP both agree, deprescription guidance is provided by the MACRO experts (i.e., benzodiazepines and anticholinergics are usually tapered slowly, decreased ~25% every two weeks). MACRO-I coaches also provide follow-up with the patient to identify potential adverse effects of deprescribing. Whereas co-I Dr. Lenze brings distinctive expertise to refine deprescription in this research protocol [27] based on his prior research efforts, ultimately MACRO aims to refine the role of a pharmacist as a more practical and generalizable deprescription staffing model.
In MACRO-I, the safety and organization of the home environment are regarded as important aspects of recovery. The MACRO-I coach provides two home visits, at one and four weeks after enrollment, with a primary objective to ensure that exercise training can be completed safely and effectively. At home visit 1, the MACRO-I coach emphasizes safety of the home environment for function and exercise and goals of care. The criteria of assessment and recommendations were adapted for the MACRO-I from Chiu et al.'s Safer-Home [28] checklist. At home visit 2, the MACRO-I coach confirms the success of changes made to improve the environment or takes steps to mitigate residual barriers. Home assessments occur irrespective of enrollment into or type of CR.
MACRO-I coaches follow patients by telephone after discharge with weekly calls for 3 months and monthly calls for the subsequent 9 months. Phone calls last 30–60 min and, in addition to continuing with the innovative elements (physical activity, motivation, nutrition, education, and deprescription), the coaches also specifically review the patient's overall health course and symptoms since the prior call and their adherence to medications, activity, dietary recommendations, and CR participation, and address lapses, with the goal to achieve solutions. Coaches follow the MACRO-I patients whether or not they enroll in CR.
2.6. Usual care
Patients randomized to usual care receive standard care for the hospital in which they are treated. Patients are eligible to receive all treatments, including CR, as prescribed by their medical team, but they do not receive the MACRO-I. While CR is an indicated therapy for CVD, implementation is inconsistent.
2.7. Assessments
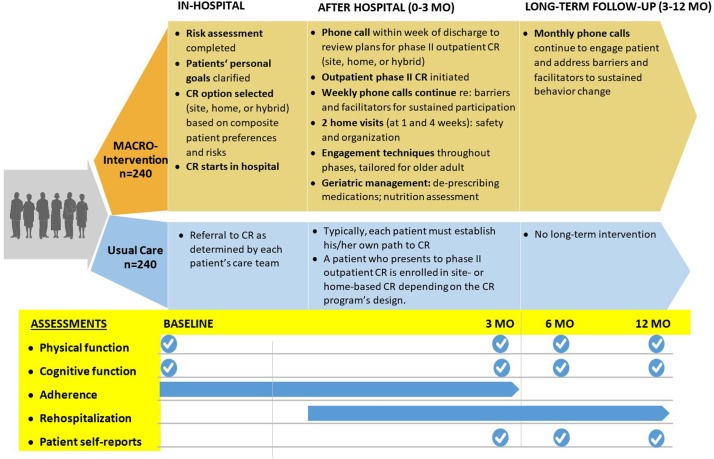
Assessments for MACRO-I and usual care study patients are completed at baseline, 3, 6 and 12 months (Fig. 1 ). The SPPB is a composite of balance, gait speed, and strength that correlates directly with capacity for independent living in older adults. Poor function measured with the SPPB is a harbinger of eroding independence and institutionalization, as well as poor quality of life, increased hospitalizations, and increased mortality in older populations [11,29]. Using the SPPB as the primary outcome of interest contrasts with most prior CR trials that tend to focus principally on cardiorespiratory fitness measured as peak oxygen uptake (VO2) and/or 6-min walk distance [30]. Nobably, SPPB was used in the recently published REHAB-HF Trial [31].
Fig. 1.
In the Modified Application of Cardiac Rehabilitation in Older Adults (MACRO) Trial, participants are randomized between a MACRO-intervention versus Usual Care. Whereas Usual Care may include cardiac rehabilitation, relatively few eligible adults participate. The MACRO-I both facilitates CR enrollment and enriches the CR process of care, and endpoints comparing MACRO-I and Usual Care are oriented primarily to improved physical and cognitive function.
Assessments complementary to SPPB include performance metrics (grip strength [32] and accelerometry [33]) and patient-reported evaluations (Duke Activity Status Index [DASI] [34] and Activity Measure for Post-Acute Care with Computerized Adaptive Testing [AM-PAC-CAT]) [35]. The DASI questionnaire is used to quantify each participant's self-report of function immediately prior to their incident cardiac event or hospitalization and to also provide an estimate of cardiorespiratory fitness. The AM-PAC-CAT is used to quantify basic patient-reported daily activities using a scoring system. The initial assessment of the AM-PAC-CAT is collected one week after discharge to quantify post-event daily activity.
Other MACRO assessments include frailty (Survey of Health, Ageing and Retirement in Europe-Frailty Index [SHARE-FI]) [36]; cognition (Trails A&B [37] and Brief Test of Adult Cognition by Telephone [BTACT]) [38]; mood (Patient Health Questionnaire [PHQ-9]) [22]; quality of life (Veterans RAND 12 Item Health Survey [VR-12]) [39]; cardiac self-efficacy [40]; literacy (Rapid Estimate of Adult Literacy in Medicine [REALM]) [41]; and Readiness for change [21]. Other evaluations include diet (Rate Your Plate [42] and 3-day food diary), as well as comprehensive assessments of medications, comorbidities, hospitalizations and CR participation. Stringent methods for fidelity of assessments and quality control are maintained. We plan a multivariable analysis that takes into account pre-randomization covariates, multiple post-randomization time points, potential for intervention effect to vary at different follow-up time points, multiple follow-up assessments over time from each participant and the resulting stochastic non-independence of observations. In addition, we plan to employ multiple imputation to mitigate any biases potentially arising from missing data.
Data from all enrolling sites are stored in a common REDCap database provided through University of Pittsburgh. An independent Data and Safety Monitoring Board approved the MACRO study protocol and meets regularly with the study investigators to ensure safety and appropriateness of the study procedures, and to monitor the progress of the study.
3. MACRO during COVID
COVID profoundly disrupted the original MACRO protocol because face-to-face engagements were no longer feasible. Thus, methods had to change to be practical and safe in a pandemic environment. Per Data and Safety and Monitoring Board (DSMB) decision, all 43 study patients who enrolled in the trial before March 2020 were released. The MACRO investigators were challenged to modify process without undercutting the essence of the original aims and the embedded innovation. A related challenge was the need to develop remote or virtual alternative approaches that were safe, feasible and effective for an older adult population that is inherently prone to geriatric syndromes (e.g., mild cognitive impairments, sensory limitations, movement disorders and multimorbidity) and related technology limitations that may preclude computer- and app-based strategies. Therefore, the decision was made to revise the MACRO protocol and re-start the trial using assessments that could be achieved entirely by telephone, since all MACRO candidates had access and capacity to use a telephone. Furthermore, a related decision was made that all telephone-based evaluations be limited to one hour in total, as brevity was deemed essential for participants who were also prone to fatigue and inattention. Given these major constraints in format and time, considerable revision and paring of the pre-COVID protocol was essential. All modifications to the MACRO protocol were reviewed and approved by the DSMB.
Overall, the updated MACRO protocol responds to the COVID pandemic, but still remains an RCT of older adults with CVD, aiming to increase physical function by facilitating the use of CR. While the inclusion and exclusion criteria are the same, the window of eligibility expanded to 24 days initially, and then to 90 days to increase flexibility and time, given the more limited access to patients on the hospital wards during COVID. Nonetheless, it is a priority to enroll patients into MACRO as close as possible to their incident events to activate MACRO-I expeditiously for those randomized to the intervention arm.
While the aims of the updated MACRO protocol did not change, the use of the SPPB performance measure as a primary endpoint was no longer feasible, as it was neither safe nor reliable to administer remotely. In contrast, the AM-PAC-CAT Basic Mobility Scale and Daily Activity domains in the original assessment battery could still be assessed at baseline, 3, 6, and 12 months. Change in AM-PAC-CAT Basic Mobility Scale from baseline to 3 months was selected to replace SPPB as the primary outcome.
AM-PAC-CAT has been used mostly by physical and occupational therapists when assessing patients with medical, orthopedic, and neurologic impairments, and it has not previously been applied to CR interventions. The Basic Mobility and the Daily Activity Domains use specific subsets of AM-PAC-CAT criteria to characterize categories of function. The Basic Mobility Scale quantifies basic movement and physical functioning activities, such as bending, walking, carrying, and climbing stairs. The Daily Activity domain quantifies difficulty of daily activities (reaching, dressing, turning locks, opening jars). The computer adaptive technology (CAT) selects items that correspond to the participant's previous responses, thereby reducing the number of total questions while increasing the test's sensitivity and validity [43]. Whereas the AM-PAC Basic Mobility Domain draws on 101 potential criteria to assess capacity, with CAT, the selection narrows to about a dozen, and the assessment can usually be completed in less than 2 min. Although the CAT relies on a computer interface, the computer is used only by the investigator administering the test, with no technological demands on the participant.
AM-PAC-CAT is reliable for a wide range of patient capacities and provides high capacity to discriminate change [35]. Assuming a standard deviation of 7.97 for the baseline to follow-up change in AM-PAC-CAT Basic Mobility Scale [35] and a minimally clinically important difference based on minimum detectable change of 2.60 points [35], a sample size of 374 was calculated as adequate to retain the same level of statistical power as the original protocol. Moreover, to improve flexibility when the would-be trajectory of the pandemic was unknown, randomization in the updated protocol is now stratified only by the enrolling site.
Despite the novel attributes of AM-PAC-CAT, a patient-reported index still provides less reliability than a performance measure [44]. Therefore, in the updated protocol, accelerometry is also prioritized as a complementary performance measure (secondary endpoint). Accelerometers are watch-like devices that record the frequency and intensity of movements throughout the day as an objective measure of physical activity patterns. Prior studies have shown that participants with cognitive and physical challenges can use them reliably. Accelerometers are mailed to participants and returned via mail after 7 full days of wear time. A novel accelerometry index of gait acceleration has been shown to correlate with the SPPB [45] and is being computed from the raw accelerometer data collected at 80 hz using an ActiGraph Link device (GT9X, ActiGraph, LLC, Pensacola, FL) on the non-dominant wrist. Cadence (steps-per-second) will also be extracted from the free-living raw accelerometry signals and evaluated as another objective indicator of physical performance [46].
In addition to AM-PAC-CAT, the updated protocol includes as many components of the original protocol as possible within a one-hour constraint for composite evaluations at baseline, 3, 6 and 12 months (Table 2 ). The DASI, VR-12, PHQ-9, Readiness for change, and cardiac self-efficacy assessment questionnaires are all amenable to remote administration and are also part of the updated protocol. Similarly, the Short Blessed cognition assessment tool ≥13 is still being used remotely to screen patients for severe cognitive impairment, but the additional assessments of cognitive function that had been used in the pre-COVID MACRO protocol are not feasible in respect to remote administration (Trails A&B) and length (BTACT), and are no longer being used. Likewise, the pre-COVID assessment of frailty utilizing the SHARE-FI tool depended on grip strength assessments that are now only optional (i.e., deferred until deemed practical in respect to COVID safety concerns); therefore, the Morley Frail scale has replaced the SHARE-FI. REALM literacy assessments also require face-to-face interaction and are now optional.
Table 2.
Assessments pre- and during COVID-19.
| Assessment | Original | COVID-19 |
|---|---|---|
| Short Physical Performance Battery (SPPB)⁎ | ✓ | ✓‡ |
| Activity Measure for Post-Acute Care with Computerized Adaptive Testing (AM-PAC-CAT); Basic Mobility Domain† | ✓ | ✓ |
| AM-PAC-CAT; Daily Living Domain | ✓ | ✓ |
| Grip Strength | ✓ | ✓‡ |
| Accelerometry | ✓ | ✓ |
| Short Blessed | ✓ | ✓ |
| Brief Test of Adult Cognition by Telephone (BTACT) | ✓ | |
| Trails A&B | ✓ | |
| Patient Health Questionnaire (PHQ9) | ✓ | ✓ |
| Rapid Estimate of Adult Literacy in Medicine (REALM) | ✓ | ✓‡ |
| Frailty: Survey of Healthy, Aging and Retirement in Europe-Frailty Index (SHARE-FI) | ✓ | |
| Morley Frail Scale | ✓ | |
| Duke Activity Status Index (DASI) | ✓ | ✓ |
| Readiness for Change | ✓ | ✓ |
| Home Assessment (based on Safer-Home) | ✓ | |
| Self-Efficacy | ✓ | ✓ |
| Veterans RAND 12 Item Health Survey (VR-12) | ✓ | ✓ |
| Rate Your Plate | ✓ | |
| 3-day food diary | ✓ | |
| Rapid Eating Assessment for Participants (REAP-S) | ✓ | |
| Medications | ✓ | ✓ |
| Comorbidities | ✓ | ✓ |
Original primary outcome measure.
New primary outcome measure in revised protocol.
Optional assessment contingent on COVID-19 risk.
Nutrition assessments using the Rate your Plate questionnaire and 3-day food diaries proved to be too cumbersome and impractical to administer remotely. The Rapid Eating Assessment for Participants – shortened version (REAP-S) [47] has been added as a shorter and more practical tool in the updated protocol.
4. Preserving the MACRO-I coaching innovations during COVID
While face-to-face interactions with inpatients became less certain amidst fluctuations in prevailing COVID prevalence and risk, the MACRO-I coaches now meet with patients in the hospital when feasible and by telephone when in-person contact is not feasible. The key principles of innovation that enrich MACRO-I coaching were adapted for virtual administration.
-
1.
Holistic risk assessment. Risk assessments are still completed by MACRO-I coaches, but functional risk stratification based on the SPPB, gait speed, and grip strength is no longer feasible. Therefore, the revised MACRO protocol integrates AM-PAC-CAT as the primary index for functional risk determination (i.e., AM-PAC-CAT <34 for high risk, 34–52 for moderate risk, and > 52 for low risk). Most other elements used to categorize medical and psychosocial risks remain accessible from the medical record and phone-based assessments (i.e., DASI, Readiness for Change, PHQ, Short Blessed). Only literacy had to be removed as a risk criterion, as the REALM assessment requires face-to-face engagement.
-
2.
Flexible conceptualization of CR. MACRO-I coaches still align CR formats with each patient's circumstances and preferences. Yet amidst COVID, concerns regarding infectivity often dominate preference for CR care format. As a pragmatic trial, MACRO incorporates the shift in site-based CR availability dictated by the pandemic, which has decreased, while remote-based options have escalated. Whichever CR program the patients utilize, the MACRO-I coaches continue to follow them regularly.
-
3.
Goals of Care as a motivational prompt: While CR is still applied as a means for goal attainment, MACRO-I coaches now use specific phrases instead of images to identify life-goal choices. Using methodology that the coaching team standardized and rehearsed, this virtual approach to goal clarification was practiced and refined.
MACRO-I coaching also includes most other innovation elements as previously described. Emphasis on transitions, nutrition, and education are all especially topical during the prevailing circumstances of a pandemic. Implementation of deprescription of benzodiazepine and anti-cholinergic medications continue as previously described.
A key change in the updated protocol pertains to the home visits in the MACRO-I. Home visits remain curtailed until COVID risks have been sufficiently mitigated. In lieu of home visits, a Centers for Disease Control and Prevention checklist [48] is offered to MACRO-I participants, and they are encouraged to review it for home safety. MACRO-I patients are also asked if they have ever had a home visit from clinical services and made any changes to best attain successful aging in place. These options are encouraged if they can be achieved safely amidst COVID risks.
5. Net effect of COVID on MACRO
The COVID pandemic placed enormous pressure on the MACRO study team to pivot midway through a trial to preserve the essence of the protocol while avoiding the hazards of the virus.
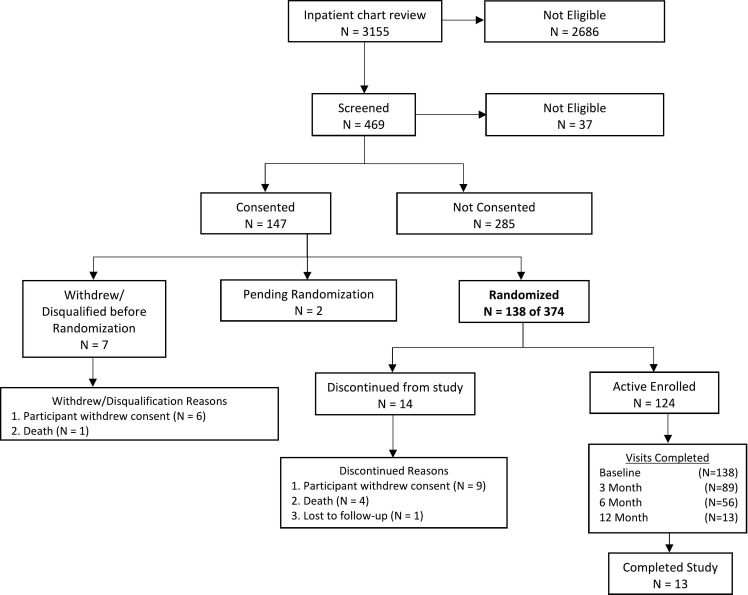
MACRO started as a 2-site study. Original recruitment extended over 30 months (11/2019 to 4/2022) and aimed to recruit 16 subjects per month and achieve 12 months of follow-up for 480 participants. Post-COVID MACRO expanded to include 2 additional sites: i.e., Shadyside Hospital in Pittsburgh, and Missouri Baptist Hospital in St Louis. The post-COVID MACRO has a 27-month recruitment window (Oct 2020-Dec 2022) and aims to recruit 14 patients per month and achieve 12 months of follow-up for 374 participants. Assessments for the MACRO intervention and Usual Care study participants in the original and post-COVID protocols are completed at baseline, 3, 6 and 12 months. A current study status diagram (Fig. 2 ) hightlights the success of post-COVID MACRO progress.
Fig. 2.
Study status as of November 2021 showing successful MACRO enrollment and progression.
In some respects, the restrictions imposed by COVID served to exacerbate logistical challenges already inherent to many older adults with CVD. Limited access to providers and hospitals, limited communication, and the propensity to social isolation are common barriers of older CVD patients struggling with frailty, cognitive decline, and other debilitating factors, and are only compounded by the pandemic. Therefore, the challenge to overcome barriers attributable to COVID also helped to overcome barriers related to geriatric conditions. Furthermore, COVID has catalyzed increased reliance on virtual approaches to care that are likely to continue even after current infectious risks have diminished [49]. It is anticipated that MACRO-I will serve as a model for assessment and manangement for future therapeutics and trials.
Thus, not only does the updated protocol enable safe resumption of the trial despite COVID, but it enables the study team to better recruit candidates who were previously unable to carry out the travel and logistic demands required for the endpoint assessments. MACRO recruitment now extends to a more diverse pool of candidates who are more willing and able to participate using the abbreviated telephone-based format, including many who are relatively more frail as well as many who live in more remote locations. Likewise, recruitment has been expanded to multiple new sites.
6. Summary
In summary, MACRO is an innovative, multi-center NIA-funded trial that seeks to transform the concept of CR by integrating an enriched coaching model into the current paradigm of care using greater flexibility in CR models to better enable each patient to participate in a CR program that is responsive to their personal needs and preferences. After the successful launch of MACRO, COVID had an overwhelming impact that necessitated modifying the protocol and starting anew. Whereas many aspects of the trial methods had to be modified, the investigative team believes that the updated protocol is in many respects superior and will more effectively achieve the original goals of the trial.
Disclosures
Daniel Forman: Dr. Forman receives funds from the National Institute On Aging through grants R01AG060499, R01AG058883, U19AG065188, and P30AG024827.
Michael Rich: Home-based cardiac rehabilitation in the time of COVID-19. Funded by the Foundation at Barnes-Jewish Hospital. July 2020 to June 2021. Role: PI.
The views and conclusions expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the U.S. Government.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.https://www.nih.gov/news-events/news-releases/worlds-older-population-grows-dramatically
- 2.Bell S.P., Orr N.M., Dodson J.A., et al. What to expect from the evolving field of geriatric cardiology. J. Am. Coll. Cardiol. 2015;66(11):1286–1299. doi: 10.1016/j.jacc.2015.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yazdanyar A., Newman A.B. The burden of cardiovascular disease in the elderly: morbidity, mortality, and costs. Clin. Geriatr. Med. 2009;25(4):563–577. doi: 10.1016/j.cger.2009.07.007. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forman D.E., Maurer M.S., Boyd C., et al. Multimorbidity in older adults with cardiovascular disease. J. Am. Coll. Cardiol. 2018;71(19):2149–2161. doi: 10.1016/j.jacc.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Neill D., Forman D.E. Never too old for cardiac rehabilitation. Clin. Geriatr. Med. 2019;35(4):407–421. doi: 10.1016/j.cger.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ritchey M.D., Maresh S., McNeely J., et al. Tracking cardiac rehabilitation participation and completion among medicare beneficiaries to inform the efforts of a National Initiative. Circ. Cardiovasc. Qual. Outcome. 2020;13(1) doi: 10.1161/CIRCOUTCOMES.119.005902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forman D.E., Arena R., Boxer R., et al. Prioritizing functional capacity as a principal end point for therapies oriented to older adults with cardiovascular disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2017;135(16):e894–e918. doi: 10.1161/CIR.0000000000000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldasseroni S., Pratesi A., Francini S., et al. Cardiac rehabilitation in very old adults: effect of baseline functional capacity on treatment effectiveness. J. Am. Geriatr. Soc. 2016;64(8):1640–1645. doi: 10.1111/jgs.14239. [DOI] [PubMed] [Google Scholar]
- 9.https://millionhearts.hhs.gov/index.html
- 10.Loudon K., Treweek S., Sullivan F., Donnan P., Thorpe K.E., Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. Bmj. 2015;350 doi: 10.1136/bmj.h2147. [DOI] [PubMed] [Google Scholar]
- 11.Guralnik J.M., Simonsick E.M., Ferrucci L., et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 12.Krumholz H.M. Post-hospital syndrome--an acquired, transient condition of generalized risk. N. Engl. J. Med. 2013;368(2):100–102. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heun R., Papassotiropoulos A., Jennssen F. The validity of psychometric instruments for detection of dementia in the elderly general population. Int. J. Geriatr. Psychiatr. 1998;13(6):368–380. doi: 10.1002/(sici)1099-1166(199806)13:6<368::aid-gps775>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Perera S., Mody S.H., Woodman R.C., Studenski S.A. Meaningful change and responsiveness in common physical performance measures in older adults. J. Am. Geriatr. Soc. 2006;54(5):743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 15.Julious S.A. Chapman & Hall/CRC; Boca Raton, FL: 2010. Sample Sizes for Clinical Trials. [Google Scholar]
- 16.Chow S.-C. 2nd ed. 2008. Sample Size Calculations in Clinical Research. [Google Scholar]
- 17.Machin D.C.M., Fayers P., Pinol A. Blackwell Science; Malden, MA: 1997. Sample Size Tables for Clinical Studies 2ed. [Google Scholar]
- 18.Zar J.H. 2 ed. Prentice Hall; Englewood Cliffs, NJ: 1984. Biostatistical Analysis. [Google Scholar]
- 19.Studenski S., Perera S., Patel K., et al. Gait speed and survival in older adults. Jama. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leong D.P., Teo K.K., Rangarajan S., et al. Prognostic value of grip strength: findings from the prospective urban rural epidemiology (PURE) study. Lancet. 2015;386(9990):266–273. doi: 10.1016/S0140-6736(14)62000-6. [DOI] [PubMed] [Google Scholar]
- 21.Prochaska. The transtheoretical model and stages of change. Health Behav. Health Educ. 2008:97–148. [Google Scholar]
- 22.Levis B., Benedetti A., Thombs B.D. Accuracy of patient health questionnaire-9 (PHQ-9) for screening to detect major depression: individual participant data meta-analysis. Bmj. 2019;365 doi: 10.1136/bmj.l1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenze E.J., Lenard E., Bland M., et al. Effect of enhanced medical rehabilitation on functional recovery in older adults receiving skilled nursing care after acute rehabilitation: a randomized clinical trial. JAMA Netw. Open. 2019;2(7) doi: 10.1001/jamanetworkopen.2019.8199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnaswami A., Steinman M.A., Goyal P., et al. Deprescribing in older adults with cardiovascular disease. J. Am. Coll. Cardiol. 2019;73(20):2584–2595. doi: 10.1016/j.jacc.2019.03.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goyal P., Gorodeski E.Z., Marcum Z.A., Forman D.E. Cardiac rehabilitation to optimize medication regimens in heart failure. Clin. Geriatr. Med. 2019;35(4):549–560. doi: 10.1016/j.cger.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Geriatrics Society 2019 updated AGS beers criteria® for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 2019;67(4):674–694. doi: 10.1111/jgs.15767. [DOI] [PubMed] [Google Scholar]
- 27.Iaboni A., Rawson K., Burkett C., Lenze E.J., Flint A.J. Potentially inappropriate medications and the time to full functional recovery after hip fracture. Drugs Aging. 2017;34(9):723–728. doi: 10.1007/s40266-017-0482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiu T., et al. Safety Assessment of Function and the Environment for Rehabilitation Health Outcome Measurement and Evaluation (SAFER–HOME) Version 3. Toronto, ON: COTA Health. 2006 [Google Scholar]
- 29.Guralnik J.M., Ferrucci L., Simonsick E.M., Salive M.E., Wallace R.B. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N. Engl. J. Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reeves G.R., Gupta S., Forman D.E. Evolving role of exercise testing in contemporary cardiac rehabilitation. J. Cardiopulm. Rehab. Prev. 2016;36(5):309–319. doi: 10.1097/HCR.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 31.Kitzman D.W., Whellan D.J., Duncan P., et al. Physical rehabilitation for older patients hospitalized for heart failure. N. Engl. J. Med. 2021;385(3):203–216. doi: 10.1056/NEJMoa2026141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sallinen J., Stenholm S., Rantanen T., Heliövaara M., Sainio P., Koskinen S. Hand-grip strength cut points to screen older persons at risk for mobility limitation. J. Am. Geriatr. Soc. 2010;58(9):1721–1726. doi: 10.1111/j.1532-5415.2010.03035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karas M., Bai J., Strączkiewicz M., et al. Accelerometry data in health research: challenges and opportunities. Stat. Biosci. 2019;11(2):210–237. doi: 10.1007/s12561-018-9227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hlatky M.A., Boineau R.E., Higginbotham M.B., et al. A brief self-administered questionnaire to determine functional capacity (the Duke activity status index) Am. J. Cardiol. 1989;64(10):651–654. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 35.Jette A.M., Haley S.M., Tao W., et al. Prospective evaluation of the AM-PAC-CAT in outpatient rehabilitation settings. Phys. Ther. 2007;87(4):385–398. doi: 10.2522/ptj.20060121. [DOI] [PubMed] [Google Scholar]
- 36.Romero-Ortuno R., Walsh C.D., Lawlor B.A., Kenny R.A. A frailty instrument for primary care: findings from the survey of health, ageing and retirement in Europe (SHARE) BMC Geriatr. 2010;10:57. doi: 10.1186/1471-2318-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenlief C.L., Margolis R.B., Erker G.J. Application of the trail making test in differentiating neuropsychological impairment of elderly persons. Percept. Mot. Skills. 1985;61(3 Pt 2):1283–1289. doi: 10.2466/pms.1985.61.3f.1283. [DOI] [PubMed] [Google Scholar]
- 38.Lachman M.E., Agrigoroaei S., Tun P.A., Weaver S.L. Monitoring cognitive functioning: psychometric properties of the brief test of adult cognition by telephone. Assessment. 2014;21(4):404–417. doi: 10.1177/1073191113508807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kazis L.E., Miller D.R., Clark J.A., et al. Improving the response choices on the veterans SF-36 health survey role functioning scales: results from the veterans health study. J. Ambul. Care Manage. 2004;27(3):263–280. doi: 10.1097/00004479-200407000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan M.D., LaCroix A.Z., Russo J., Katon W.J. Self-efficacy and self-reported functional status in coronary heart disease: a six-month prospective study. Psychosom. Med. 1998;60(4):473–478. doi: 10.1097/00006842-199807000-00014. [DOI] [PubMed] [Google Scholar]
- 41.Arozullah A.M., Yarnold P.R., Bennett C.L., et al. Development and validation of a short-form, rapid estimate of adult literacy in medicine. Med. Care. 2007;45(11):1026–1033. doi: 10.1097/MLR.0b013e3180616c1b. [DOI] [PubMed] [Google Scholar]
- 42.Gans K.M. Rate your plate: an eating pattern assessment and educational tool used at cholesterol screening and education programs. J. Nutr. Educ. 1993;25:29–36. [Google Scholar]
- 43.Haley S.M., Siebens H., Coster W.J., et al. Computerized adaptive testing for follow-up after discharge from inpatient rehabilitation: I. Activity outcomes. Arch. Phys. Med. Rehabil. 2006;87(8):1033–1042. doi: 10.1016/j.apmr.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y., Li H., Ding N., Wang N., Wen D. Functional status assessment of patients with COPD: a systematic review of performance-based measures and patient-reported measures. Medicine (Baltimore) 2016;95(20) doi: 10.1097/MD.0000000000003672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urbanek J.K., Zipunnikov V., Harris T., Crainiceanu C., Harezlak J., Glynn N.W. Validation of gait characteristics extracted from raw accelerometry during walking against measures of physical function, mobility, fatigability, and fitness. J. Gerontol. A Biol. Sci. Med. Sci. 2018;73(5):676–681. doi: 10.1093/gerona/glx174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karas M., Stra Czkiewicz M., Fadel W., Harezlak J., Crainiceanu C.M., Urbanek J.K. Adaptive empirical pattern transformation (ADEPT) with application to walking stride segmentation. Biostatistics. 2021;22(2):331–347. doi: 10.1093/biostatistics/kxz033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Segal-Isaacson C.J., Wylie-Rosett J., Gans K.M. Validation of a short dietary assessment questionnaire: the rapid eating and activity assessment for participants short version (REAP-S) Diabetes Educ. 2004;30(5) doi: 10.1177/014572170403000512. 774, 776, 778 passim. [DOI] [PubMed] [Google Scholar]
- 48.https://www.cdc.gov/steadi/pdf/patient/customizable/CheckforSafety-Brochure-Final-Customizable-508.pdf
- 49.Wosik J., Fudim M., Cameron B., et al. Telehealth transformation: COVID-19 and the rise of virtual care. J. Am. Med. Inform. Assoc. 2020;27(6):957–962. doi: 10.1093/jamia/ocaa067. [DOI] [PMC free article] [PubMed] [Google Scholar]