Abstract
Lectins or clusters of carbohydrate-binding proteins of non-immune origin are distributed chiefly in the Plantae. Lectins have potent anti-infectivity properties for several RNA viruses including SARS-CoV-2. The primary purpose of this review is to review the ability of lectins mediated potential biotherapeutic and bioprophylactic strategy against coronavirus causing COVID-19. Lectins have binding affinity to the glycans of SARS-COV-2 Spike glycoprotein that has N-glycosylation sites. Apart from this, the complement lectin pathway is a “first line host defense” against the viral infection that is activated by mannose-binding lectins. Mannose-binding lectins deficiency in serum influences innate immunity of the host and facilitates infectious diseases including COVID-19. Our accumulated evidence obtained from scientific databases particularly PubMed and Google Scholar databases indicate that mannose-specific/mannose-binding lectins (MBL) have potent efficacies like anti-infectivity, complement cascade induction, immunoadjuvants, DC-SIGN antagonists, or glycomimetic approach, which can prove useful in the strategy of COVID-19 combat along with the glycobiological aspects of SARS-CoV-2 infections and antiviral immunity. For example, plant-derived mannose-specific lectins BanLac, FRIL, Lentil, and GRFT from red algae can inhibit and neutralize SARS-CoV-2 infectivity, as confirmed with in-vitro, in-vivo, and in-silico assessments. Furthermore, Bangladesh has a noteworthy resource of antiviral medicinal plants as well as plant lectins. Intensifying research on the antiviral plant lectins, adopting a glyco-biotechnological approach, and with deeper insights into the “glycovirological” aspects may result in the designing of alternative and potent blueprints against the 21st century's biological pandemic of SARS-CoV-2 causing COVID-19
Keywords: Mannose-specific/mannose-binding lectins, Glycoprotein, Glycosylation, SARS-CoV-2 glycobiology, Antiviral plant lectins, Glycobiotechnology
Graphical Abstract
1. Introduction
Lectins are a diverse group of carbohydrate-binding natural proteins that bind reversibly to mono and oligosaccharides with high specificity [1]. The first study of lectin began more than 130 years ago in 1888 by Peter Hermann Stillmark with the finding that the seed extracts of Ricinus communis (Castor bean) can agglutinate red blood cells and the isolated lectin was named Ricin [2]. During World War I and II ricin was utilized as a potential weapon by the United States and British military, respectively [3]. The modern age of lectinology began in 1972 with the purification of lectins from different plant sources [4]. Seeds, tubers, leaves, stems, roots, and fruits of medicinal plants are rich sources to isolate and purify lectins [5], specially from the Leguminosae family [6], and large numbers of lectins are present in seed cotyledons [7].
Lectins can target the sugar complex of glycoproteins and all the lectins possess two or more carbohydrate-binding sites with the essential property of agglutinating ability to the erythrocytes without altering the carbohydrates properties [8]. Lectins can be classified based on binding specificity as glucose/mannose, galactose and N-acetyl-D-galactosamine, N-acetylglucosamine, L-fucose, and sialic acids [9]. Lectins may also be categorized into merolectins, holoectins, chimerolectins, and superlectins based on the number of binding sites [9]. Plant lectins can be classified into 12 families based on species and lectin domain [10]. Man-specific or mannose-specific lectins have specificity for mannose and mannose containing glycoproteins and they are widely present in all living organisms, and especially have been isolated and identified from plants, algae, fungi, and cyanobacteria [11].
The application of lectins is dependent on their properties, and some of the lectins derived from the natural resources have in-vivo and in-vitro antiviral activities [12]; therefore, some of these novel lectins have been considered for the potential development of therapeutic agents against viral infections [12]. Lectins are highly potent in virus neutralization activities and their modes of action can target enveloped viruses that share the feature of glycosylated proteins on their surfaces [13]. Thus, lectins can inhibit the replication of viruses by interacting with viral envelope proteins. Such antiviral lectins have extensively been evaluated in-vitro for their neutralization effects on different enveloped viruses including coronaviruses and HIV [13], because lectins can interfere with the virus entry and inhibit the viral proteins production [14]. Lectins have also been used as glyco-analytical tools in the development of biosensors for the diagnosis of infectious diseases and detection of viral pathogens [15]. Example includes, Concanavalin A (ConA) lectin can recognize the structural glycoproteins of the arboviruses [16]. In another study, five lectins such as Dolichos biflorus lectin (DBA), Helix pomatia lectin (HPA), peanut lectin (PNA), soybean lectin (SBA), and Ulex europaeus lectin (UEA-1) were evaluated for the detection of hepatitis A virus (HAV) and amongst the five lectins, SBA showed significant activity in the detection of HAV [17].
In addition, some lectins may have mitogenic activity that leads to systemic inflammation [18], and increase viral transmission because of their ability to activate T cells [19]. Lectin-induced mitogenicity can be overcome by attempting glycoengineering techniques such as BanLac, which was engineered to eliminate its mitogenicity by amino acid mutation at position 83–84 from histidine to threonine [19], without compromising antiviral activity against Ebola and influenza viruses [20], [21]. In another recent study published in April 2021, a site-specific engineered lectin based on structural insights, Pseudomonas taiwanensis lectin (PTL), reportedly enhanced antiviral activity against the influenza virus and such site-specific engineering of lectins can be a potential strategy to boost the antiviral activity of lectins [22]. The data from 2015 to 2020 showed that numerous potential antiviral lectins were discovered along with studies of structural modifications when needed [23].
1.1. Antiviral plant lectins and their modes of action
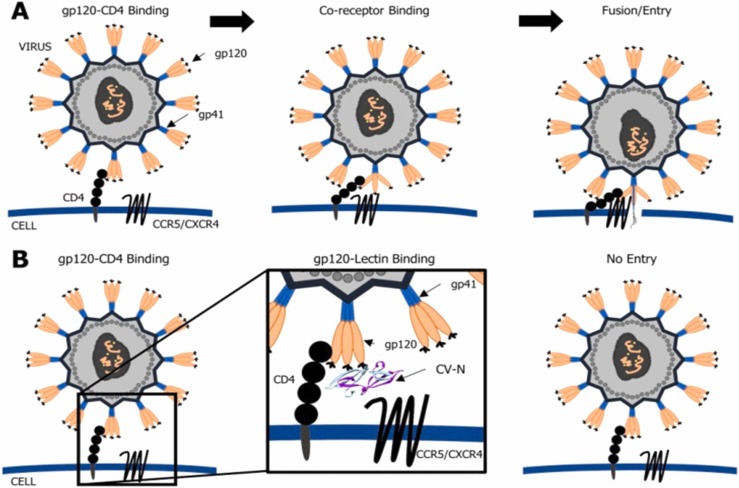
The contribution of plant lectins as antiviral activity was first reported in 1988 wherein D-mannose-specific plant lectins blocked the binding of HIV in-vitro [24]. Glycosylated envelope proteins (GEP) are a particular protein for the regulation of virus recognition and virus entry. They exert affinity for cell-surface proteins of host cells, and therefore the antiviral lectins react with the high-mannose glycan to trigger glycosylation of viral GEP [12]. A glycosylated envelope protein complex of HIV has transmembrane trimer, gp31 and extracellular trimer, gp120 that contain N-linked oligosaccharide attachment sites. These structures assist viral evasion of the host immune system and entry into the host cells, mediated by recognition of CD4+ triggering. The antiviral lectins inhibit the conformational reorganization of the glycosylated envelope protein complex and thus result in suppressing virus entry into host cells [12], [25]. Specific carbohydrate-binding lectins are considered as potential anti-HIV agents which can block the host-virus interactions at their earliest stage because of the glycosylated action of the viral envelope proteins [26]. The following figure, Fig. 1, represents the role of cyanobacterial lectin against viral infection.
Fig. 1.
“Schematic representation of viral infection (A) and the role of cyanobacterial lectin, cyanovirin (CV-N) on inhibition of viral entry and fusion (B). CV-N blocks the interaction between the viral gp120 and the CD4 receptor on the host cell. It prevents the interaction with the associated co-receptors CXCR4/CCR5. As a consequence, the virus cannot enter into the cell.”
Adapted from [27] distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Since antiviral medicinal plants and their bioactive compounds are potential sources that may play a significant role in the development of COVID-19 biotherapeutics [28], [29], [30], [31], we documented some plant lectins from reported antiviral medicinal plants available in Bangladesh in Table 1.
Table 1.
Source of plant lectins from reported in-vivo, in-vitro and in-silico antiviral medicinal plants available in Bangladesh.
| Botanical name | Family | Used plant part | Lectin identification, isolation and purification (reference) | Specificity | Antiviral activity (reference) |
|---|---|---|---|---|---|
| Abelmoschus esculentus (L.) Moench | Malvaceae | Seed | [32] | Non-specified | Not reported |
| Abrus precatorius L. | Fabaceae | Seed | [33] | Galactose | Coronavirus[34] |
| Aegle marmelos (L.) Correa | Rutaceae | Fruit pulp | [35] | N-acetylgalactosamine, Mannose and sialic acid | Coxsackie viruses B1–B6, BmNPV[36], [37] |
| Alisma plantago-aquatica var. orientale Sam. | Alismataceae | Rhizome | [38] | Non-specified | HBV, HSV-1[39], [40] |
| Amaranthus viridis L. | Amaranthaceae | Seed | [41] | T-antigen and N-acetyl-D-lactosamine | Measles virus[42] |
| Areca catechu L. | Arecaceae | Nut | [43] | Non-specified | HIV-1, NDV[44], [45] |
| Artocarpus heterophyllus Lam. | Moraceae | Seed | [46] | Galactose and N-acetylgalactosamine | HIV, HSV-2, CMV, HCV[47], [48], [49] |
| Bauhinia variegata L. | Fabaceae | Seed | [50] | Glucose/Galactose | Coxsackievirus B3, Rotavirus[51], [52] |
| Benincasa hispida (Thunb.) Cogn. | Cucurbitaceae | Fruit | [53] | N-acetylglucosamine | Not reported |
| Butea monosperma (Lam.) Taub | Leguminosae | Seed | [54], [55] | N-acetylgalactosamine, N-acetyl d-galactosamine, galactose and lactose | Unknown[56] |
| Cajanus cajan (L.) Millsp. | Fabaceae | Root | [57] | Mannose/glucose | Measles virus[58] |
| Cassia fistula L. | Fabaceae | Seed | [59] | Galactose | IBR[60] |
| Clitoria ternatea L. | Fabaceae | Seed | [61] | Galactose and N-acetylgalactosamine | MCV, HSV[62] |
| Coccinia indica Wight & Arn. | Cucurbitaceae | Fruit | [63] | Chito | HBV[64] |
| Corchorus olitorius L. | Tiliaceae | Leaf | [65] | Glucose/mannose, galactose | Measles virus[66] |
| Cucurbita maxima Duchesne | Cucurbitaceae | Seed kernels | [67] | Galactose | Not reported |
| Datura stramonium L. | Solanaceae | Seed | [68] | N-Acetylglucosamine | Potato virus X[69] |
| Erythrina variegata L. | Fabaceae | seed | [70], [71] | Galactose, N-acetylgalactosamine | Not reported |
| Glycyrrhiza glabra L. | Fabaceae | Root | [72] | Non-specified | HAV, HBV, HCV, HIV, SARS-CoV[73], [74] |
| Kaempferia rotunda L. | Zingiberaceae | Rhizome | [75] | Mannose | H5N1[76] |
| Kaempferia parviflora Wall. ex Baker | Zingiberaceae | Rhizome | [77] | Non-specified | HIV-1, HCV, HCMV[78] |
| Lathyrus sativus L. | Fabaceae | Seed | [79] | Mannose | Not reported |
| Litchi chinensis Sonn. | Sapindaceae | Seed | [80] | Glucose/mannose | HSV-1[81] |
| Mangifera indica L. | Anacardiaceae | Fruit seed | [82] | Non-specified | HSV, influenza virus[83], [84] |
| Momordica charantia L | Cucurbitaceae | Seed | [85] | Galactose/ N-Acetylgalactosamine | HIV, HSV-1, H1N1, H3N2, H5N1[86], [87], [88] |
| Mucuna pruriens (L.) DC. | Fabaceae | Seed | [89] | Mannose | HCV[90] |
| Musa paradisiaca L. | Musaceae | Ripe fruit pulp | [91] | Mannose | HSV-1, HSV-2[91] |
| Oryza sativa L. | Poaceae | – | [82] | N-Acetylglucosamine | CMV, HSV[92], [93] |
| Phaseolus vulgaris L. | Fabaceae | Seed | [94], [95] | Galactose | HIV-1 RT[94], [95] |
| Psidium guajava L. | Myrtaceae | Fruit | [96] | Galactose | H1N1[97] |
| Pisum sativum L. | Fabaceae | Seed | [82], [98] | Mannose/galatose | HCV, ADV[98], [99] |
| Pterocarpus indicus Willd | Fabaceae | Seed | [100] | Mannose/glucose | Dengue virus[101] |
| Senna tora (L.) Roxb. | Fabaceae | Seed | [102] | Mannose/Galactose | SARS-CoV 3CL protease[103] |
| Senna occidentalis (L.) Link | Fabaceae | Seed | [104] | Non-specified | BHV-1, SHV-1[105] |
| Sesbania bispinosa (Jacq.) W.Wight | Fabaceae | Stem | [106] | Glucose | Not reported |
| Solanum lycopersicum L. | Solanaceae | Fruit | [107] | N-acetylglucosamine | Not reported |
| Solanum melongena L. | Solanaceae | Fruit | [108] | Non-specified | HSV-1[109] |
| Tamarindus indica L. | Fabaceae | Seed | [110] | Mannose/maltose | NDV, mosaic viruses[111], [112] |
| Trichosanthes cucumerina L. | Cucurbitaceae | Seed | [113] | Galactose | Not reported |
| Trichosanthes dioica Roxb. | Cucurbitaceae | Seed | [114] | Galactose and N-acetylgalactosamine | Not reported |
| Urtica dioica L. | Urticaceae | Root | [115] | N-acetylglucosamine | SARS-CoV; HIV, CMV, RSV, H1N1[12], [116], [117] |
| Vigna mungo (L.) Hepper | Fabaceae | Seed | [118] | Galactose | Urdbean Leaf Crinkle Virus[119] |
| Vigna radiata (L.) R.Wilczek | Fabaceae | Seed | [120] | Galactose | Influenza A virus, HSV-1, RSV[121], [122] |
| Vigna unguiculata (L.) Walp | Fabaceae | Seed | [123] | Non-specified | HIV[124] |
| Withania somnifera (L.) Dunal | Solanaceae | Leaf | [125] | Mannose | SARS-CoV-2, HIV, HSV, H1N1[126], [127] |
HSV= Herpes simplex virus; HAV= Hepatitis A virus; HBV= Hepatitis B virus; HCV= Hepatitis C virus; CMV= Cytomegalovirus; RSV= Respiratory Syncytial Virus; ADV= Adenoviruses; MCV= Molluscum contagiosum virus; BmNPV= Bombyx mori nucleopolyhedrovirus; IBR= Infectious Bovine Rhinotracheitis; HIV= Human immunodeficiency viruses; H1N1, H3N2, H5N1= Subtypes of Influenza A virus; NDV= Newcastle disease virus; BHV= Bovine herpesvirus-1; SHV= swine herpesvirus 1.
The in-vitro antiviral potency of bioactive compounds or drugs can be determined by the two most commonly reported parameters such as EC50 (EC for effective concentration), the concentration of bioactive compounds (drugs) in cell culture media that provides 50% maximal protection against virus induced cytopathicity and IC50 (IC for inhibitory concentration), the concentration of bioactive compounds (drugs) that yields 50% of the maximum inhibitory effect.
In a study of antiviral activity, Gondim and coauthors have evaluated 4 leguminous lectins, namely Canavalia /brasiliensis (ConBr), C. maritima (ConM), Dioclea lasiocarpa (DLasiL) and D. sclerocarpa (DSclerL), and 5 algal lectins: Amansia multifida (AML), Bryothamniom seaforthii (BSL), Hypnea musciformis (HML), Meristiella echinocarpa (MEL) and Solieria filiformis (SfL) from the Brazilian biodiversity, which are active against 18 different viruses, including influenza viruses and HIV. On the basis of EC50 values, the most potential lectins were DLasiL and DSclerL, which showed EC50 values ranging from 9 nM to 46 nM for HIV-1 and respiratory syncytial virus, respectively; DSclerL, ConBr and ConM showed EC50 ranging from 0.4 to 6 nM against influenza A virus strain H3N2 and influenza B virus, and DLasiL showed EC50 of 5 nM against feline coronavirus [128]. In a review study published in August 2021, Carneiro and co-authors have demonstrated antimicrobial applications of patented lectins and listed some patented lectins that exhibited in-vitro antiviral activity [129] (see Table 2).
Table 2.
List of some patented lectins that exhibited antiviral activity.
| Name of lectin | Virus |
|---|---|
| Momordica balsamina lectin (MOMO30) | HIV-1 |
| Mistletoe lectin I (ml-I) | HSV-1, Ad5 |
| Singapore mistletoe lectin (SML) | DENV |
| Sambucus nigra agglutinin IV (SNA IV) | Influenza A |
| Sambucus nigra agglutinin V (SNA V) | Influenza A |
| Sambucus nigra protein derived from lectin (SNL RP | Influenza A |
| Vicia villosa agglutinin (VVA-G) | Influenza A |
| Wisteria floribunda lectin (WFL) | Influenza A |
| Aleuria aurantia lectin (AAL) | Influenza A |
| Aspergillus oryzae lectin (AOL) | Influenza A |
HIV-1= human immunodeficiency virus 1; HSV-1= herpes simplex virus 1; Ad5= adenovirus 5; DENV= dengue virus.
Modified from Carneiro et al. [129].
2. Potentials of lectins against SARS-CoV-2 infectivity
Lectins can be used to identify and characterize the structure of glycans [130]. Glycans are the carbohydrate portion of glycoproteins which have crucial roles in the immune system of humans [131] and pathobiology of viral infections [132]. Antiviral lectins can block the entry of virus by binding to glycans from either the virus or host cell [129]. Antiviral lectins can bind to the viral Spike (S) protein of SARS-CoV [116]; specifically, lectins like mannose/glucose and N-acetylglucosamine (GlcNAc)- specific lectins have been found to inhibit entry of several coronaviruses such as SARS-CoV [133], [134], MERS-CoV [135], and other coronaviruses [136], [137].
The glucose/mannose-specific plant lectin FRIL, derived from Lablab purpureus, is effective both in-vivo and in-vitro for neutralizing SARS-CoV-2 binding to complex-type-N-glycans on viral glycoproteins [138]. Another plant lectin which is mannose-specific, lentil, isolated from Lens culinaris, showed strong inhibitory SARS-CoV-2 activity at the early steps of infections by blocking the ACE2-S trimer binding to oligomannose-type glycans and N-acetylglucosamine at glycosylation sites N165, N234, and N343, which are located around the receptor binding domain [139].
The agal lectin, GRFT (Griffithsin), derived from red algae Griffithsia sp., is a high mannose-specific lectin, which significantly inhibits SARS-CoV-2 pseudovirus infection in-vitro with an IC50 of 63 nmol/L compared to remdesivir with effective concentration of 0.77 μmol/L; GRFT also inhibits SARS-CoV-2 S-mediated cell to cell fusion with an IC50 of 323 nmol/L [140]. GRFT has also been reported to inhibit SARS-CoV infectivity in previous in-vivo and in-vitro studies [133]. The following table, Table 3 lists some algae-derived mannose-specific antiviral lectins that have been reported to be potent inhibitors of different RNA viruses summarized by Alam et al. [141], and can be potent inhibitors of coronaviruses as well.
Table 3.
List of mannose specific antiviral lectins derived from marine algae.
| Name of antiviral lectin | Algae source |
|---|---|
| Microvirin | Microcystis aeruginosa (Kützing) Lemmermann |
| Cyanovirin | Nostoc ellipsosporum Rabenhorst ex Bornet & Flahault |
| AML, BSL, HML, MEL, Sfl | Amansia multifida J.V.Lamouroux, Bryothamnion seaforthii (Turner) Kützing, Hypnea musciformis (Wulfen) Lamouroux, Meristiella echinocarpa (J.E. Areschoug) D.P.Cheney & P.W.Gabrielson and Solieria filiformis (Kützing) Gabrielson |
| ESA-2 | Eucheuma serra (J.Agardh) J.Agardh |
| KAA-2 | Kappaphycus alvarezii (Doty) Doty ex P.C.Silva |
| BCA | Boodlea coacta (Dicke) Murray and De Toni |
| HRL40 | Halimeda renschii Hauck |
| MVL | Microcystis viridis (A.Braun) Lemmermann |
| Scytovirin | Scytonema varium Kützing ex Bornet & Flahault |
From molecular docking and MD simulation studies, Banana derived mannose-specific lectin, BanLec, can target N-glycans of the Spike glycoproteins to neutralize SARS-CoV-2 infectivity [142]. The following table, Table 4 is listed with some antiviral plant lectins, which are reportedly potent inhibitor of coronaviruses by targeting both the early virus replication cycle and the end of the infectious virus cycle (modified from Bah et al. [143]).
Table 4.
List of antiviral lectins derived from plant sources.
| Name of lectin | Plant source | Specificity | Anti-viral activity |
|---|---|---|---|
| EAPL | Phaseolus vulgaris L. | Galactose | HIV-1 |
| DBL | Musa acuminata L. (Del Monte banana) | Fructose | HIV-1 |
| APA | Allium ampeloprasum L. | Mannose | SARS-CoV |
| SGBSL | Glycine max (L.) Merr. | Melibiose | HIV-1 |
| PAL | Pholiota adipose (Batsch) P.Kumm. mushroom | Inulin | HIV-1 |
| TDL | Typhonium divaricatum (L.) Decne | Mannose | HSV-2 |
| CLL | Crinum latifolium L. | Mannose | Pox virus |
The antiviral medicinal plant Withania somnifera (L.) Dunal, which is called Indian ginseng has potential for COVID-19 management [127]. An in-silico docking and molecular dynamics results have showed its (phytochemicals of W. somnifera) ability to inhibit SARS-CoV-2 host entry and replication [144], and the plant has effective roles on the host ACE2 receptor complex and receptor binding domain (RBD) of virus [145]. It is worth noting that a mannose-specific lectin was isolated from leaves of W. somnifera [125].
2.1. Lectin mediated DC-SIGN antagonists and glycomimetic approach
C-type lectins, which according to their property of Ca2+ dependent carbohydrate-binding lectins, were identified as the key susceptibility factors that interact with multiple viruses, and then induce infection [146]. DC-SIGN (Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin) is such a mannose-specific C-type lectin and pathogen recognition receptor of human innate immune system expressed in dendritic cells [147]. DC-SIGN can recognize N-linked high-mannose oligosaccharides and branched fucosylated structures [148]. It has been reported that DC-SIGN can act as an alternative receptor for SARS-COV-2 entry, therefore enhancing infectivity [149], [150]. The glycomimetic antagonists of DC-SIGN and L-SIGN which is another C-type lectin, highly expressed in human type II alveolar cells and the endothelial cells of the lung, liver, and lymph nodes can be promising candidates for the inhibition of SARS-CoV-2 entry to host cell receptors [150]. Therefore, DC-SIGN antagonist that act as a glycomimetic approach, are potential anti-infectives [151], and several glycomimetics are already in clinical trials [152]. In-vitro assays have confirmed ability of the plasma-derived human mannose-binding lectin to block binding of SARS-CoV to DC-SIGN [153]. Furthermore, the algal lectin GRFT and cyanobacterial lectins Cyanovirin-N, and Scytovirin can block HIV-1 binding to the DC-SIGN receptor and the DC-SIGN-mediated HIV-1 infection of CD4(+) cells [154].
2.2. Mannose-binding lectins and SARS-CoV-2 possible interactions
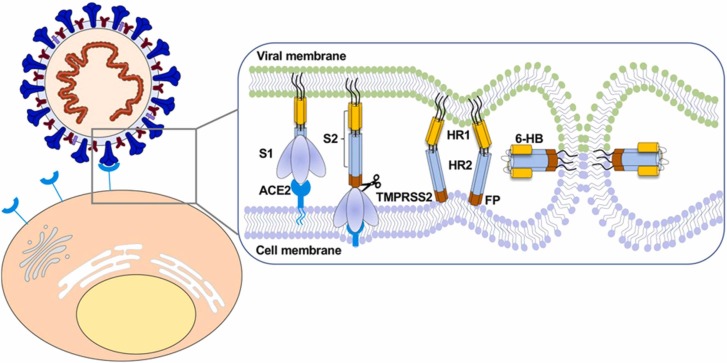
The SARS-CoV-2 Spike (S) glycoprotein has S1 and S2 subunits linked through transmembrane protease serine 2 (TMPRSS2) and furin cleavage sites [155]. The S1 subunit is involved in the attachment to host cell receptors facilitated by a receptor binding domain (RBD), and S2 is involved in the fusion of cellular membranes in between virus and human [155]. The entry of SARS-CoV-2 into human host cells is shown schematically in Fig. 2.
Fig. 2.
“Diagram of SARS-CoV-2 entry into host cells. S protein binding to ACE2 receptor and virus attachment to the cell; S protein cleaved by TMPRSS2 produces S1 and S2 subunits. HR1 and HR2 of the S2 subunit gradually approach each other and form a six-helix bundle (6-HB), which causes the virus envelope and host cell membrane to complete fusion.”
Adapted from Zhang et al. [156] with permission.
Watanabe et al. [157] and Zhou et al. [158] has identified the location of 22 N-linked highly glycosylated sites where glycans are attached to the SARS-CoV-2 Spike glycoproteins and the types of sugar at each site. High mannose-type glycans were identified on the site N234 of the S glycoprotein of SARS-CoV-2, and Complex-type N-glycans and high mannose-type glycans were identified at the sites N165, N331, and N343 [155]. The SARS-CoV-2 Spike proteins are coated with sugars (glycans) [158]; neutralization of the sugar-coated Spike protein by using lectins, which behaves as sugar-binding proteins, can be a promising strategy for the COVID-19 therapeutics. For instance, the two mannose-specific mammalian lectins (Clec4g and CD209c) strongly bind to the N-glycan site N343 of the SARS-CoV-2 Spike protein, which can be visualized and quantified using atomic force microscopy [159].
High mannose-specific seaweed lectins have the ability to interfere both with the virus entry in the host cell and virus release from the host cell by targeting the Spike glycoprotein and heavily glycosylated ACE2 receptor [160]. ACE2 stands for Angiotensin converting enzyme 2, is expressed in human organs and play a chief role in the entry of SARS-CoV-2 [161] by binding of S1 subunit to ACE2 receptors [162] (see Fig. 2 as well) and the molecular mechanisms of SARS-CoV-2 binding to the ACE2 receptor has been discussed by Ramírez Hernández et al. [163].
An in-vitro study [153] showed that plasma-derived human mannose-binding lectin (pdMBL) selectively binds to SARS Spike (SARS-S) glycoprotein and can inhibit SARS-CoV infection in susceptible cell lines. This experiment has identified a single N- glycosylation site, N330, on S glycoprotein as the target for the specific interactions between pdMBL and SARS-CoV. Comparably, Mannose-binding plant lectins (MBPLs) can interfere during virus entry by binding to the high-mannose type N-glycans of SARS-CoV Spike (S) protein, and by blocking viral attachment to the host cell [116], [164] wherein N-linked glycosylation plays a critical role in the specific interaction between the MBPLs and SARS-CoV Spike glycoprotein [165].
It is worth noting that the lower levels of serum mannose-binding lectin (sMBL) in human blood is associated with the occurrence of many infectious diseases including SARS, as MBL has a pivotal role in innate immune response [166], [167]. Mannose-binding lectin deficiency (< 70 ng/ml) in serum has been reported in 25 patients with viral upper respiratory infections and in 13 patients with immunodeficiencies [168]. A case report study of a 2-times COVID-19 affected patient showed MBL deficiency (< 50 ng/ml), which indicated that patients with decreased levels of MBL may have greater risk of COVID-19 re-infection than the general people [169]. Serum MBL levels test in COVID-19 patients should be carried out (by using a sample of the patient’s blood and Enzyme-linked immunosorbent assay) to avoid critical conditions and targeting the mannose-binding pathway (see Fig. 3) can be a potential treatment for COVID-19 as well for thrombosis in COVID-19 [170]. Changes in the MBL2 gene can lead to MBL (produced in the liver) deficiency that is very common in the general population and reduced MBL levels in blood serum (< 500 ng/ml) may be considered as susceptibility for recurrent infection including respiratory tract infections by pathogens as well as to inflammatory and autoimmune diseases [171].
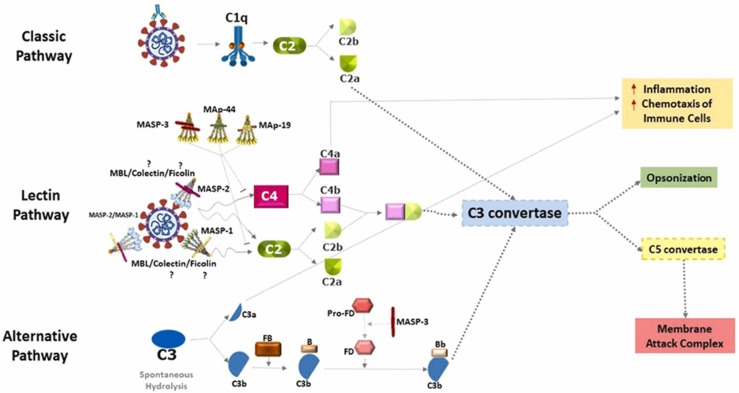
Fig. 3.
“Complement pathways in SARS-CoV-2 infection. The activation of the classical pathway occurs through the C1 complex, after recognition of antibodies complexed to SARS-CoV-2. This leads to the cleavage of the C2 component into C2a and C2b. C2a joins the common pathway of the three complement pathways to form the C3 convertase. After binding of MBL/MASP complexes to the surface of pathogens, MASP-1 autoactivates, transactivates MASP-2, and C2 and C4 components are cleaved (C2 and C4 by MASP-2 and C2 by MASP-1), generating the C3 convertase. The alternative pathway is initiated by the spontaneous hydrolysis of component C3, generating C3a and C3b. C3b binds to factor B and is cleaved by factor D, forming the C3 convertase of the alternative pathway. After this step, the three pathways converge into a single pathway. The C3 convertase enzyme cleaves component C3 into C3a and C3b. C3a and C4a are anaphylatoxins that contribute to an increase in inflammatory processes and to the chemotaxis of neutrophils and macrophages (red arrows), while C3b performs viral opsonization. The formation of C5 convertase occurs in different ways through the three pathways, but all generate C5a and C5b. C5a is an anaphylatoxin (as also C3a) that contributes to inflammatory processes, and regulates innate and adaptive immune responses [172], while C5b joins the last C6-C9 components of the cascade and forms the membrane attack complex.” (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Adapted from Bumiller-Bini et al. [173] with permission, and some modifications in the text body.
A clinical investigation of 284 PCR-confirmed COVID-19 patients and 100 healthy controls revealed that mannose-binding lectin 2 (MBL2) gene B variant is common in patients with COVID-19 cases compared to the control group because MBL2 gene is related to lower levels of MBL [174]. Changes in mannose-binding lectin (MBL)- associated serine proteases, MASP-1 and/or MASP-2 levels have been related with COVID-19 risk factors such as sex, diabetes, kidney, cardiovascular, cerebrovascular, and chronic obstructive pulmonary disease (COPD), and association of MASPs and COVID-19 comorbidities has been demonstrated by Bumiller-Bini et al. [173]. Human coronaviruses SARS, MERS, and SARS-CoV-2-induced hyperactivation of MASP-2 aggravates lung injury, and this hyperactivation of MASP-2 is caused through a direct interaction between MASP-2 and coronavirus nucleocapsid protein [175].
Moreover, mannose-binding lectins induce complement cascade, which is a defense against invading pathogens in mucosal immunity [176] and trigger the production of pro-inflammatory cytokines [177]. The complement pathway in SARS-CoV-2 infection has been mentioned as having a “double-edged sword”; it can control mild or asymptomatic cases in COVID-19, but exacerbate local and systemic damage in severe COVID-19 [178]. Complement pathways in SARS-CoV-2 infection has been demonstrated by Bumiller-Bini et al. [173] and shown in Fig. 3.
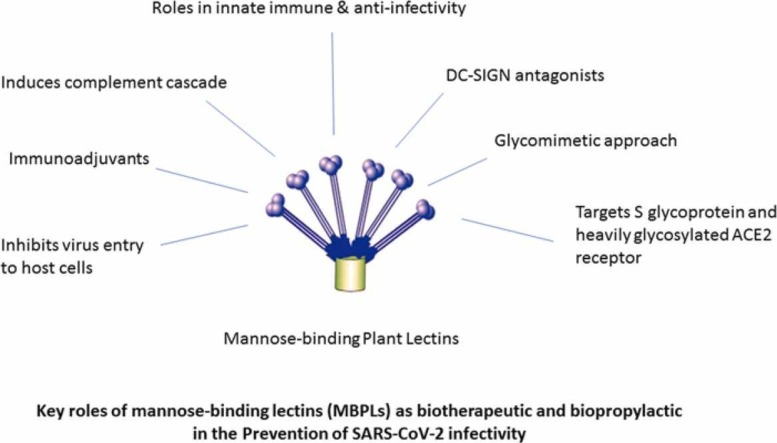
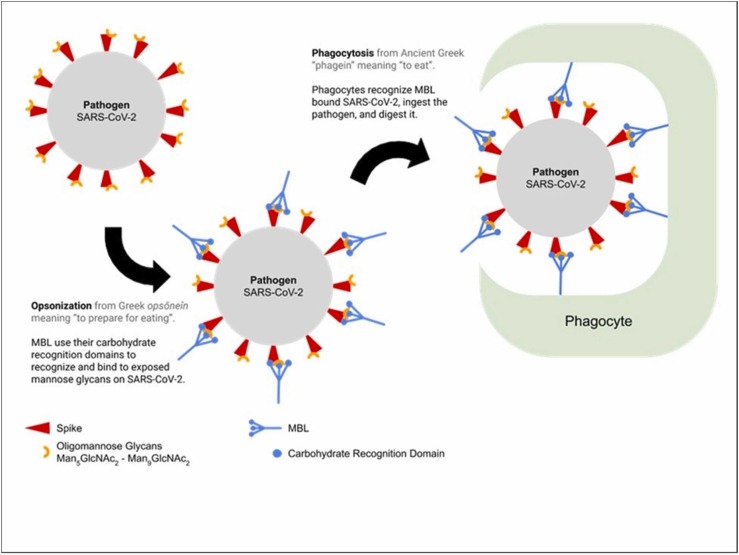
However, in COVID-19 therapeutic strategy, the complement cascade pathway, particularly lectin pathway has received negligible attention [179] dues to the complex pattern of immune dysregulation in COVID-19 patients with acute respiratory failure [180]. Since serum MBL has a key role in innate immunity [181], and can inhibit SARS-CoV in-vitro [166] by preventing ACE2 binding with Spike glycoprotein and also enhances phagocytosis function as an opsonin [182], thus MBPLs may offer potent and alternative biotherapeutic and bioprophylactic strategy against SARS-CoV-2 infections. The following Fig. 4 demonstrates potential role of mannose-binding lectins in prevention of SARS-CoV-2.
Fig. 4.
“MBL binding and complement activation enhances phagocytosis by acting as an opsonin.”
Adapted from Lau [206] with permission.
2.3. Glycosylation for the SARS-CoV-2 antiviral therapeutics and vaccines design
Glycosylation is a ubiquitous post-translational modification of proteins that plays significant roles both in the virus life cycle for stability, antigenicity and infectivity, and in glycans of the host cell receptors for the attachment and entry of the virus [183]. N-glycosylation or N-acetylglucosamine (GlcNAc) and O-glycosylation or N-acetylgalactosamine (GalNAc) are mainly two types of glycosylation sites [183]. Lectins can be used as microarray for high-throughput glycosylation analysis that can aid to screen glycan patterns of therapeutic glycoproteins [184], [185]. Viral pathogens use glycans and lectins for the replication and spread, but recent advances in glycobiological research showed that specific glycan-lectin interactions on the basis of viral infectivity and antiviral immunity, can be useful in antiviral strategy [131].
Numerous studies have been performed on Lectins as antimicrobial agents and virucidal agents against several enveloped viruses [186] considering glycan-lectin interactions in antiviral strategies [131]. Besides the ones mentioned before, anti-viral lectins have been isolated from various plants like Scilla campanulata, Narcissus pseudonarcissus, Galanthus nivalis, Polygonatum cyrtonema, as reviewed by Mitchell et al. [12].
Lectins have been used in immunotherapeutic studies for potential prophylactic and therapeutic strategies against microbial infectious diseases, especially plant lectins [187], [188]. Lectins from mushroom [189], [190], and sea mussel [191] have also been demonstrated to exhibit immunomodulatory activities. Since lectins have exhibited immunomodulatory properties, they can also be used as vaccine bio-adjuvants to improve the efficacy of immunization [192], which has been demonstrated in the laboratory against enveloped viruses such as influenza, hepatitis, and herpes virus, for example see Table 5.
Table 5.
Reported lectins used as adjuvants in the antiviral vaccine strategy.
| Name of Lectin [source] | Specificity | Administration route in mice | Vaccine category against viruses |
|---|---|---|---|
| AAL [Agrocybe aegerita (V.Brig.) Singer] | N-acetylglucosamine | subcutaneous injection | Inactivated vaccine for H9N2 virus |
| POL [Pleurotus ostreatus (Jacq.) P. Kumm.] | N-acetylgalactosamine | Intramuscular injection | DNA vaccine for Hepatitis B virus |
| KML-C [Viscum album coloratum (Kom.) Nakai] | Galactose/N-acetylgalactosamine | Intranasal route | Inactivated vaccine for H1N1 virus |
| MLI, MLII, MLIII [Viscum album L.] | Galactose/N-acetylgalactosamine | Intranasal route | Subunit vaccine for Herpes simplex virus |
Modified from Nascimento da Silva et al. [193].
Lectins are potential biomolecules that can induce IL-12, IFN-γ, and T helper type 1 (Th1) protective immunity against viral infections and can also modulate the expression of toll-like receptors (TLRs) which initiate the early immune recognition of the pathogens and the release of proinflammatory cytokines [193]. Current reports strongly suggest that glycobiological and/or “glycovirological” contribution, particularly in glycan-lectin interactions can help for highly effective COVID-19 vaccines and drugs development [131], [132] and focusing on glycosylation of Spike glycoprotein can be a novel strategy in the development of both anti-viral vaccine and anti-viral drugs designs against SARS-CoV-2 [157], [183], [194].
3. Concluding remarks
The idea of Lectin-based specific drug delivery was first reported in 1988 via the use of tomato lectin (TL) to target the luminal surface of the small intestine [4]. The lectin-based drug targeting system can be attained via two mechanisms: (i) direct lectin targeting system, which includes carbohydrate molecules that are recognized by endogenous cell surface lectins, and (ii) reverse lectin targeting system, which include exogenous lectins that recognize synthesized carbohydrate molecules on glycolipids and glycoproteins [4], [195], [196], [197]. Ribosome inactivating lectins (Ulex europaeus I and Wheat Germ Agglutinin) containing HIV peptides, hepatitis B surface antigen, TLR receptor were used as bioactive molecules for drug targeting as excipients in vaccine application [198]. Antiviral lectins are potential microbicide molecules for their exhibition of lower toxicity than any other currently used antiviral therapeutics, best for topical applications, odorless, resistant to low pH and high temperatures [143]. Furthermore, rigorous characterization and identification of glycosylation motifs in viral glycoproteins are essential to the design of vaccines and anti-viral drugs [199].
COVID-19 is an infectious disease caused by the zoonotic virus SARS-CoV-2, which has created catastrophe among Homo sapiens worldwide. Scientists and health experts are still working to find out effective therapeutics and vaccines that can eradicate COVID-19. To date, there are six WHO-recognized experimental vaccines available but their efficacy is still under consideration due to continuous SARS-CoV-2 mutations and wane of immunity over time. Bangladesh has also joined the global COVID-19 vaccine race with developing an mRNA-based vaccine candidate [200] with hopes for human trials in November 2021 after completion of successful clinical trials that is happening on non-human primates and monkeys [201].
Numerous attempts have been made with the hope of glycan-based effective antiviral molecules for preventing 2019-nCoV infections. According to our information obtained from established databases, mannose-binding lectins (MBL) have been found to be highly effective against coronaviruses, and mannose-binding plant lectins should have significance for its potent antiviral properties particularly against SARS-CoV-2 as MBL has properties of anti-infectivity, immunoadjuvant, DC-SIGN antagonist, or glycomimetic approach, and specially MBL induces complement cascade pathway, which is a first-line host defense, but MBL has been given limited attention in the COVID-19 biotherapeutic and bioprophylactic strategy. Moreover, decreased levels of serum MBL is a susceptible factor for severe COVID-19 infections and MBL levels in COVID-19 patients should be diagnosed to avoid greater risk of re-infection and disease severity.
Nonetheless, researchers are working hard on plant-based COVID-19 vaccine development via glycoengineering technology and plant-based vaccines have low cost production, rapidity, scalability, and safety [202]. The pharmacological properties and stability, solubility, bioavailability, pharmacokinetics, and immunogenicity of glycosylated biotherapeutics can be determined by the glyco-biotechnological approach, which can propel scientific research to the development of the next generation of biotherapeutics and glycoengineered vaccines [203]. In addition, antiviral lectins for COVID-19 may suffer from low sale production, high cost purification and manufacturing process that can be resolved by the applications of plant biotechnology, as for example molecular farming or transient expression of Griffithsin and Cyanovirin-N in plants [204] as well as Griffithsin (GRFT) in engineered Escherichia coli [205].
To be a consideration, “glycovirology” is an emerging discipline covering both glycobiology and virology, and Bangladesh is a resource of anti-viral medicinal plants with lectins. Altogether, deeper insights into “glycovirological” aspects, antiviral lectins, and glyco-biotechnology may lead to developing highly effective next-generation antiviral biotherapeutic and bioprophylactic against the 21st century's biological pandemic of SARS-CoV-2 causing COVID-19 and quite possibly other new emerging viruses.
4. Methodological approach in literature search
The authors have performed translating mind derived research questions to keywords in pursuit of specific evidence-based literature to accumulate further information on antiviral lectins against COVID-19. The literature search was performed in PubMed, Google Scholar, Google Search databases by randomly using below keywords: (lectins and coronavirus; lectins and SARS-CoV; medicinal plants and antiviral activity; antiviral plan lectins; molecular mechanisms of actions of antiviral lectins; bioactive compounds and SARS-coronavirus; glycans-lectin interactions for antiviral therapy; glycan and lectins interplay; classifications of lectins; etc.).
The articles were screened and included for this review are proof of concept studies that paid attention to the involvement of inhibitory activity of lectins against SARS-CoV, the antiviral mechanisms of actions of lectins, and studies related to antiviral plant lectins. Furthermore, we also focused on all the relevant articles that were investigated lectins for the antiviral activity. Finally, this review deals with the literature discussion on the potential role of glycosylation for the designing of biotherapeutics and vaccines against SARS-CoV-2.
Funding
This study has received no external or internal funding.
CRediT authorship contribution statement
Md. Nasir Ahmed: Conceptualization, Visualization, Writing – original draft. Rownak Jahan: Validation, Writing – review & editing. Veeranoot Nissapatorn: Writing – review & editing. Polrat Wilairatana: Funding acquisition. Mohammed Rahmatullah: Supervision, Validation, Writing – review & editing.
Conflict of interest statement
The authors declare have no conflict of interest.
Acknowledgments
This study received no funding. Authors are grateful to scientists and experts worldwide who have contributed to the lectin and glycobiology research.
Data availability
No data was used for the research described in the article.
References
- 1.Lagarda-Diaz I., Guzman-Partida A.M., Vazquez-Moreno L. Legume lectins: proteins with diverse applications. J. Mol. Sci. 2017;18(6):1242. doi: 10.3390/ijms18061242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Damme E.J.M. In: Lectins: Methods and Protocols. Hirabayashi J., editor. Vol. 1200. Humana Press; New York, NY: 2014. History of plant lectin research; pp. 3–13. (Methods in Molecular Biology). [DOI] [Google Scholar]
- 3.Sharon N., Lis H. History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology. 2004;14(11):53R–62R. doi: 10.1093/glycob/cwh122. [DOI] [PubMed] [Google Scholar]
- 4.Bies C., Lehr C.M., Woodley J.F. Lectin-mediated drug targeting: history and applications. Adv. Drug Deliv. Rev. 2004;56(4):425–435. doi: 10.1016/j.addr.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 5.Kabir S.R., Hasan I., Zubair A. In: Recent Progress in Medicinal Plants-Nutraceuticals and Functional Foods. Govil J.N., editor. Vol. 42. Studium Press LLC; Houston, Texas, USA: 2014. Lectins from medicinal plants: characterizations and biological properties; pp. 339–356. [Google Scholar]
- 6.Ingale A.G., Hivrale A.U. Plant as a plenteous reserve of lectin. Plant Signal Behav. 2013;8(12) doi: 10.4161/psb.26595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moreira R., Ainouz I.L., De Oliveira J.T., Cavada B.S. Plant lectins, chemical and biological aspects. Mem. Inst. Oswaldo Cruz. 1991;86(2):211–218. doi: 10.1590/s0074-02761991000600048. [DOI] [PubMed] [Google Scholar]
- 8.Lam S.K., Ng T.B. Lectins: production and practical applications. Appl. Microbiol Biotechnol. 2011;89(1):45–55. doi: 10.1007/s00253-010-2892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chettri D., Boro M., Sarkar L., Verma A.K. Lectins: biological significance to biotechnological application. Carbohydr. Res. 2021;506 doi: 10.1016/j.carres.2021.108367. [DOI] [PubMed] [Google Scholar]
- 10.François Bonnardel, Bioinformatics study of lectins: new classification and prediction in genomes. Structural Biology [q-bio.BM]. Université Grenoble Alpes [2020-..]; Université de Genève, 2021. English. 〈https://www.theses.fr/2021GRALV010〉.
- 11.Barre A., Van Damme E.J.M., Simplicien M., et al. Man-specific lectins from plants, fungi, algae and cyanobacteria, as potential blockers for SARS-CoV, MERS-CoV and SARS-CoV-2 (COVID-19) coronaviruses: biomedical perspectives. Cells. 2021;10(7):1619. doi: 10.3390/cells10071619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell C.A., Ramessar K., O’Keefe B.R. Antiviral lectins: selective inhibitors of viral entry. Antivir. Res. 2017;142:37–54. doi: 10.1016/j.antiviral.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazalovska M., Kouokam J.C. Lectins as promising therapeutics for the prevention and treatment of HIV and other potential coinfections. BioMed. Res. Int. 2018:3750646. doi: 10.1155/2018/3750646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barton C., Kouokam J.C., Lasnik A.B., Foreman O., Cambon A., Brock G., Montefiori D.C., Vojdani F., McCormick A.A., O’Keefe B.R., Palmer K.E. Activity of and effect of subcutaneous treatment with the broad-spectrum antiviral lectin griffithsin in two laboratory rodent models. Antimicrob. Agents Chemother. 2014;58(1):120–127. doi: 10.1128/aac.01407-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Souza Barbosa P.P., Araújo F.N. de, Almeida J.M. de, Gadelha T.S. Leguminosae lectins as biological tools in medical research: a review. Braz. Arch. Biol. Technol. 2021;64 doi: 10.1590/1678-4324-2021200170. [DOI] [Google Scholar]
- 16.Simão E.P., Silva D., Cordeiro M.T., Gil L., Andrade C., Oliveira M. Nanostructured impedimetric lectin-based biosensor for arboviruses detection. Talanta. 2020;208 doi: 10.1016/j.talanta.2019.120338. [DOI] [PubMed] [Google Scholar]
- 17.Ko S.M., Kwon J., Vaidya B., Choi J.S., Lee H.M., Oh M.J., Bae H.J., Cho S.Y., Oh K.S., Kim D. Development of lectin-linked immunomagnetic separation for the detection of hepatitis a virus. Viruses. 2014;6(3):1037–1048. doi: 10.3390/v6031037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huskens D., Vermeire K., Vandemeulebroucke E., Balzarini J., Schols D. Safety concerns for the potential use of cyanovirin-N as a microbicidal anti-HIV agent. Int J. Biochem Cell Biol. 2008;40(12):2802–2814. doi: 10.1016/j.biocel.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Swanson M.D., Boudreaux D.M., Salmon L., Chugh J., Winter H.C., Meagher J.L., André S., Murphy P.V., Oscarson S., Roy R., King S., Kaplan M.H., Goldstein I.J., Tarbet E.B., Hurst B.L., Smee D.F., de la Fuente C., Hoffmann H.H., Xue Y., Rice C.M., Markovitz D.M. Engineering a therapeutic lectin by uncoupling mitogenicity from antiviral activity. Cell. 2015;163(3):746–758. doi: 10.1016/j.cell.2015.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Covés-Datson E.M., Dyall J., DeWald L.E., King S.R., Dube D., Legendre M., Nelson E., Drews K.C., Gross R., Gerhardt D.M., Torzewski L., Postnikova E., Liang J.Y., Ban B., Shetty J., Hensley L.E., Jahrling P.B., Olinger G.G., Jr., White J.M., Markovitz D.M. Inhibition of Ebola virus by a molecularly engineered banana lectin. PLoS Negl. Trop. Dis. 2019;13(7) doi: 10.1371/journal.pntd.0007595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Covés-Datson E.M., King S.R., Legendre M., Gupta A., Chan S.M., Gitlin E., Kulkarni V.V., Pantaleón García J., Smee D.F., Lipka E., Evans S.E., Tarbet E.B., Ono A., Markovitz D.M. A molecularly engineered antiviral banana lectin inhibits fusion and is efficacious against influenza virus infection in vivo. Proc. Nat. Acad. Sci. USA. 2020;117(4):2122–2132. doi: 10.1073/pnas.1915152117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matoba Y., Sato Y., Oda K., Hatori Y., Morimoto K. Lectins engineered to favor a glycan-binding conformation have enhanced antiviral activity. J. Biol. Chem. 2021;296 doi: 10.1016/j.jbc.2021.100698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moura RBde Pereira J.únior F.N., Santos G.F.A., de Souza Rodrigues A.R. Importance of Lectins in Virology – an integrative review. Res. Soc. Dev. 2020;9(11) doi: 10.33448/rsd-v9i11.10083. [DOI] [Google Scholar]
- 24.Müller W.E., Renneisen K., Kreuter M.H., Schröder H.C., Winkler I. The D-mannose-specific lectin from Gerardia savaglia blocks binding of human immunodeficiency virus type I to H9 cells and human lymphocytes in vitro. J. Acquir Immune Defic. Syndr. 1988;1(5):453–458. [PubMed] [Google Scholar]
- 25.Hwang H.J., Han J.W., Jeon H., Cho K., Kim J.H., Lee D.S., Han J.W. Characterization of a novel mannose-binding lectin with antiviral activities from Red Alga, Grateloupia chiangii. Biomolecules. 2020;10(2):333. doi: 10.3390/biom10020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang L., Zhang L., Chen C.H. Potential drug targets on the HIV-1 envelope glycoproteins, gp120 and gp41. Curr. Pharm. Des. 2003;9(18):1453–1462. doi: 10.2174/1381612033454720. [DOI] [PubMed] [Google Scholar]
- 27.Mazur-Marzec H., Cegłowska M., Konkel R., Pyrć K. Antiviral cyanometabolites – a review. Biomolecules. 2021;11(3):474. doi: 10.3390/biom11030474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benarba B., Pandiella A. Medicinal plants as sources of active molecules against COVID-19. Front. Pharmacol. 2020;11:1189. doi: 10.3389/fphar.2020.01189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khadka D., Dhamala M.K., Li F., Aryal P.C., Magar P.R., Bhatta S., Thakur M.S., Basnet A., Cui D., Shi S. The use of medicinal plants to prevent COVID-19 in Nepal. J. Ethnobiol. Ethnomed. 2021;17(1):26. doi: 10.1186/s13002-021-00449-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel B., Sharma S., Nair N., Majeed J., Goyal R.K., Dhobi M. Therapeutic opportunities of edible antiviral plants for COVID-19. Mol. Cell Biochem. 2021;476(6):2345–2364. doi: 10.1007/s11010-021-04084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tegen D., Dessie K., Damtie D. Candidate anti-COVID-19 medicinal plants from Ethiopia: a review of plants traditionally used to treat viral diseases. Evid. Based Complement Altern. Med. 2021;2021:6622410. doi: 10.1155/2021/6622410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Sousa Ferreira Soares G., Assreuy A.M., de Almeida Gadelha C.A., de Morais Gomes V., Delatorre P., da Conceição Simões R., Cavada B.S., Leite J.F., Nagano C.S., Pinto N.V., de Luna Freire Pessoa H., Santi-Gadelha T. Purification and biological activities of Abelmoschus esculentus seed lectin. Protein J. 2012;31(8):674–680. doi: 10.1007/s10930-012-9447-0. [DOI] [PubMed] [Google Scholar]
- 33.Herrmann M.S., Behnke W.D. Physical studies on three lectins from the seeds of Abrus precatorius. Biochim. Biophys. Acta. 1980;621(1):43–52. doi: 10.1016/0005-2795(80)90060-4. [DOI] [PubMed] [Google Scholar]
- 34.Adeleye O.A., Femi-Oyewo M.N., Bamiro O.A., Bakre L.G., Alabi A., Ashidi J.S., Balogun-Agbaje O.A., Hassan O.M., Fakoya G. Ethnomedicinal herbs in African traditional medicine with potential activity for the prevention, treatment, and management of coronavirus disease 2019. Future J. Pharm. Sci. 2021;1:72. doi: 10.1186/s43094-021-00223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raja S.B., Murali M.R., Kumar N.K., Devaraj S.N. Isolation and partial characterisation of a novel lectin from Aegle marmelos fruit and its effect on adherence and invasion of Shigellae to HT29 Cells. PLoS One. 2011;6(1) doi: 10.1371/journal.pone.0016231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Badam L., Bedekar S.S., Sonawane K.B., Joshi S.P. In vitro antiviral activity of Bael (Aegle marmelos Corr) upon human coxsackieviruses B1–B6. J. Commun. Dis. 2002;34(3):88–99. [PubMed] [Google Scholar]
- 37.Somu C., Karuppiah H., Sundaram J. Antiviral activity of seselin from Aegle marmelos against nuclear polyhedrosis virus infection in the larvae of silkworm, Bombyx mori. J. Ethnopharmacol. 2019;245 doi: 10.1016/j.jep.2019.112155. [DOI] [PubMed] [Google Scholar]
- 38.Shao B., Wang S., Zhou J., Ke L., Rao P. A novel lectin from fresh rhizome of Alisma orientale (Sam.) Juzep. Process Biochem. 2011;46:1554–1559. doi: 10.1016/j.procbio.2011.04.007. [DOI] [Google Scholar]
- 39.Jiang Z.Y., Zhang X.M., Zhang F.X., Liu N., Zhao F., Zhou J., Chen J.J. A new triterpene and anti-hepatitis B virus active compounds from Alisma orientalis. Planta Med. 2006;72(10):951–954. doi: 10.1055/s-2006-947178. [DOI] [PubMed] [Google Scholar]
- 40.Kang B.J., Lee H.H., Hong W.S., Park K.J. Activities of Korean medicinal herbs and traditional prescriptions against herpes simplex Virus type-1. Pharm. Biol. 1998;36(4):287–294. doi: 10.1076/phbi.36.4.287.4582. [DOI] [Google Scholar]
- 41.Kaur N., Dhuna V., Kamboj S.S., Agrewala J.N., Singh J. A novel antiproliferative and antifungal lectin from Amaranthus viridis Linn seeds. Protein Pept. Lett. 2006;13(9):897–905. doi: 10.2174/092986606778256153. [DOI] [PubMed] [Google Scholar]
- 42.Obi R.K. iroagba II and Ojiako OA. Virucidal potential of some edible Nigerian vegetables. Afr. J. Biotechnol. 2006;5(19):1785–1788. [Google Scholar]
- 43.BIRD GW ANTI-T’ AGGLUTININS FROM Areca catechu LINN. Experientia. 1965;21:5–6. doi: 10.1007/BF02136349. [DOI] [PubMed] [Google Scholar]
- 44.Kusumoto I.T., Nakabayashi T., Kida H., Miyashiro H., Hattori M., Namba T., Shimotohno K. Screening of various plant extracts used in ayurvedic medicine for inhibitory effects on human immunodeficiency virus type 1 (HIV-1) protease. Phytother. Res. 1995;9:180–184. doi: 10.1002/ptr.2650090305. [DOI] [Google Scholar]
- 45.Lee D., Boo Kyung-hwan, Kim Y.C., Lee Jin-Man, Kang S., Lee W.S., Riu K.Z., Lee D.Sun. The antiviral effects of Areca catechu L. Extract. J Korean Soc. Food Sci. Nutr. 2014;46(2):245–248. doi: 10.9721/KJFST.2014.46.2.245. [DOI] [Google Scholar]
- 46.Kabir S. The isolation and characterization of jacalin [Artocarpus heterophyllus (jackfruit) lectin] based on its charge properties. Int. J. Biochem Cell Biol. 1995;27(2):147–156. doi: 10.1016/1357-2725(94)00071-i. [DOI] [PubMed] [Google Scholar]
- 47.Favero J., Corbeau P., Nicolas M., Benkirane M., Travé G., Dixon J.F., Aucouturier P., Rasheed S., Parker J.W., Liautard J.P., Devaux C., Dornand J. Inhibition of human immunodeficiency virus infection by the lectin jacalin and by a derived peptide showing a sequence similarity with gp120. Eur. J. Immunol. 1993;23(1):179–185. doi: 10.1002/eji.1830230128. [DOI] [PubMed] [Google Scholar]
- 48.Wetprasit N., Threesangsri W., Klamklai N., Chulavatnatol M. Jackfruit lectin: properties of mitogenicity and the inhibition of herpesvirus infection. Jpn. J. Infect. Dis. 2000;53(4):156–161. [PubMed] [Google Scholar]
- 49.Hafid A.F., Aoki-Utsubo C., Permanasari A.A., Adianti M., Tumew L., Widyawaruyanti A., Wahyuningsih S.P.A.A., Wahyuni T.S., Lusida M.I., Soetjipto Hotta H. Antiviral activity of the dichloromethane extracts from Artocarpus heterophyllus leaves against hepatitis C virus. Asian Pac. J. Trop. Biomed. 2017;7:633–639. doi: 10.1016/j.apjtb.2017.06.003. [DOI] [Google Scholar]
- 50.Chan Y.S., Ng T.B. Bauhinia variegata var. variegata lectin: isolation, characterization, and comparison. Appl. Biochem Biotechnol. 2015;175(1):75–84. doi: 10.1007/s12010-014-1261-z. [DOI] [PubMed] [Google Scholar]
- 51.Shaheen M., El-Gamal M., Mousa A., Mostafa S., El-Esnawy N. Antiviral activity of Bauhinia variegata extracts against rotavirus in vitro. Curr. Sci. Int. 2014;3(3):172–178. [Google Scholar]
- 52.Shaheen M., Borsanyiova M., Mostafa S., Bopegamage S., El-Esnawy N. In vitro and in vivo evaluation of Bauhinia variegata extracts to prevent coxsackievirus B3 infection. J. Proteom. Bioinform. 2017;10:73–78. doi: 10.4172/jpb.1000426. [DOI] [Google Scholar]
- 53.Singh R., Gaikwad S.M., Suresh C.G. A chito-specific, adenine binding agglutinin from Benincasa hispida shows high structural and functional stability. Int. J. Biochem. Res. Rev. 2016;9(4):1–14. doi: 10.9734/IJBCRR/2016/23270. [DOI] [Google Scholar]
- 54.Abhilash J., Geethanandan K., Bharath S.R., Sabu A., Sadasivan C., Haridas M. The crystal structure of a lectin from Butea monosperma: insight into its glycosylation and binding of ligands. Int. J. Biol. Macromol. 2015;72:1376–1383. doi: 10.1016/j.ijbiomac.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 55.Hiremath K.Y., Hegde P., Sharma M., Inamdar S.R. A modified method for purification of N-acetylgalactosamine specific lectin from Butea monosperma seeds and its effect on human hepatocellular carcinoma cell growth. J. Plant Biochem. Biotechnol. 2019;28:397–404. doi: 10.1007/s13562-019-00488-1. [DOI] [Google Scholar]
- 56.Yadava R.N., Tiwari L. A potential antiviral flavone glycoside from the seeds of Butea monosperma O. Kuntze. J. Asian Nat. Prod. Res. 2005;7(2):185–188. doi: 10.1080/1028602042000204054. [DOI] [PubMed] [Google Scholar]
- 57.Naeem A., Khan R.H., Vikram H., Akif M. Purification of Cajanus cajan root lectin and its interaction with rhizobial lipopolysaccharide as studied by different spectroscopic techniques. Arch. Biochem. Biophys. 2001;396(1):99–105. doi: 10.1006/abbi.2001.2595. [DOI] [PubMed] [Google Scholar]
- 58.Nwodo U.U., Ngene A.A., Iroegbu C.U., Onyedikachi O.A., Chigor V.N., Okoh A.I. In vivo evaluation of the antiviral activity of Cajanus cajan on measles virus. Arch. Virol. 2011;156(9):1551–1557. doi: 10.1007/s00705-011-1032-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ali M.A., Sayeed M.A., Absar N. Purification and characterization of three lectins extracted from Cassia fistula seeds and effect of various physical and chemical agents on their stability. J. Chin. Chem. Soc. 2004;51:647–654. doi: 10.1002/jccs.200400097. [DOI] [Google Scholar]
- 60.Anubhuti S., Vijay L., Anjana G., Viney S., AK B. Anti-viral activity of Cassia fistula against IBR virus. J. Immunol. Immunopathol. 2010;12(2):114–119. [Google Scholar]
- 61.Naeem A., Haque S., Khan R.H. Purification and characterization of a novel beta-D-galactosides-specific lectin from Clitoria ternatea. Protein J. 2007;26(6):403–413. doi: 10.1007/s10930-007-9080-5. [DOI] [PubMed] [Google Scholar]
- 62.Vimalanathan S., Ignacimuthu S., Hudson J.B. Medicinal plants of Tamil Nadu (Southern India) are a rich source of antiviral activities. Pharm. Biol. 2009;47(5):422–429. doi: 10.1080/13880200902800196. [DOI] [Google Scholar]
- 63.Bobbili K.B., Datta D., Mondal S., Polepalli S., Pohlentz G., Mormann M., Swamy M.J. Purification, chitooligosaccharide binding properties and thermal stability of CIA24, a new PP2-like phloem exudate lectin from ivy gourd (Coccinia indica) Int. J. Biol. Macromol. 2018;110:588–597. doi: 10.1016/j.ijbiomac.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 64.Arbab A.H., Parvez M.K., Al-Dosari M.S., Al-Rehaily A.J. In vitro evaluation of novel antiviral activities of 60 medicinal plants extracts against hepatitis B virus. Exp. Ther. Med. 2017;14(1):626–634. doi: 10.3892/etm.2017.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khan M.M.H., Rahman A.T.M.M., Uddin M.S., Khatun S., Pervin F., Absar N. Purification and characterization of lectins from jute (Chorchorus olitorius) leaves. J. Chin. Chem. Soc. 2008;55:1171–1177. doi: 10.1002/jccs.200800173. [DOI] [Google Scholar]
- 66.Hasan H., Kadhim E.J. Phytochemical investigation of Corchorus olitorius L. leaves cultivated in Iraq and its in Vitro antiviral activity. Iraqi J. Pharm. Sci. 2018;27(2):115–122. doi: 10.31351/vol27iss2pp115-122. [DOI] [Google Scholar]
- 67.Sarkar S.K., Hossain M.T., Uddin M.B., Absar N. Purification, characterization and physico-chemical properties of three galactose-specific lectins from pumpkin (Cucurbita maxima) seed kernels. J. Chin. Chem. Soc. 2007;54:1433–1442. doi: 10.1002/jccs.200700203. [DOI] [Google Scholar]
- 68.Nishimoto K., Tanaka K., Murakami T., Nakashita H., Sakamoto H., Oguri S. Datura stramonium agglutinin: cloning, molecular characterization and recombinant production in Arabidopsis thaliana. Glycobiology. 2015;25(2):157–169. doi: 10.1093/glycob/cwu098. [DOI] [PubMed] [Google Scholar]
- 69.Miraj S. Datura stramonium: an updated review. Der Pharma Chem. 2016;8(17):253–257. [Google Scholar]
- 70.Datta T.K., Basu P.S. Identification, isolation and some properties of lectin from the seeds of Indian coral tree [Erythrina variegata (Linn.) var. orientalis (Linn.) Merrill] Biochem. J. 1981;197(3):751–753. doi: 10.1042/bj1970751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamasaki N., Kimura M., Yamaguchi O., Araki M. Isolation and characterization of isolectins from Erythrina variegata seeds. J. Chromatogr. A. 1992;597(1–2):207–211. doi: 10.1016/0021-9673(92)80112-8. [DOI] [PubMed] [Google Scholar]
- 72.Makhlouf A., Al-Sohaimy S.A., Moustafa Y., Saadani M., Makhlouf H., ISOLATION A.N.D. IDENTIFICATION OF LECTIN GENE IN LICO- RICE, Glycorrhiza glabra L., PLANT IN EGYPT. Egypt J. Genet. Cytol. 2013;42:183–193. [Google Scholar]
- 73.Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361(9374):2045–2046. doi: 10.1016/s0140-6736(03)13615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fiore C., Eisenhut M., Krausse R., Ragazzi E., Pellati D., Armanini D., Bielenberg J. Antiviral effects of Glycyrrhiza species. Phytother. Res. 2008;22(2):141–148. doi: 10.1002/ptr.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kabir S.R., Hossen A., Zubair A., Alom J., Islam F., Hossain A., Kimura Y. A new lectin from the tuberous rhizome of Kaempferia rotunda: isolation, characterization, antibacterial and antiproliferative activities. Protein Pept. Lett. 2011;18(11):1140–1149. doi: 10.2174/092986611797200896. [DOI] [PubMed] [Google Scholar]
- 76.Aznam N., Atun S., Arianingrum R., Nurestri S. Isolation, identification and antiviral activity of bioactive compounds of Kaempferia rotunda, in: 3rd International Conference on Chemistry and Chemical Engineering. IPCBEE, 2012, 38, pp. 27–30. 12, IACSIT Press, Singapore.
- 77.Konkumnerd W., Karnchanatat A., Sangvanich P. A thermostable lectin from the rhizomes of Kaempferia parviflora. J. Sci. Food Agric. 2010;90(11):1920–1925. doi: 10.1002/jsfa.4033. [DOI] [PubMed] [Google Scholar]
- 78.Sookkongwaree K., Geitmann M., Roengsumran S., Petsom A., Danielson U.H. Inhibition of viral proteases by Zingiberaceae extracts and flavones isolated from Kaempferia parviflora. Die Pharm. 2006;61(8):717–721. [PubMed] [Google Scholar]
- 79.Sletten K., Kolberg J. The primary structure of the alpha chain of a mitogenic lectin from the seeds of Lathyrus sativus. Hoppe Seylers Z. Physiol. Chem. 1983;364(8):1047–1051. doi: 10.1515/bchm2.1983.364.2.1047. [DOI] [PubMed] [Google Scholar]
- 80.Bose P.P., Bhattacharjee S., Singha S., Mandal S., Mondal G., Gupta P., Chatterjee B.P. A glucose/mannose binding lectin from litchi (Litchi chinensis) seeds: biochemical and biophysical characterizations. Biochem. Biophys. Rep. 2016;6:242–252. doi: 10.1016/j.bbrep.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hsu C.M., Chiang S.T., Chang Y.Y., Chen Y.C., Yang D.J., Chen Y.Y., Lin H.W., Tseng J.K. Lychee flower extract inhibits proliferation and viral replication of HSV-1-infected corneal epithelial cells. Mol. Vis. 2016;22:129–137. [PMC free article] [PubMed] [Google Scholar]
- 82.Slifkin M., Doyle R.J. Lectins and their application to clinical microbiology. Clin. Microbiol Rev. 1990;3(3):197–218. doi: 10.1128/cmr.3.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parvez G.M. Pharmacological activities of mango (Mangifera indica): a review. J. Pharmacogn. Phytochem. 2016;5:01–07. [Google Scholar]
- 84.Rawi AAS A.L., AL Dulaimi H.S.H., AL Rawi M.A.A. Antiviral activity of Mangifera extract on influenza virus cultivated in different cell cultures. J. Pure Appl. Micro. 2019;13(1):455–458. doi: 10.22207/JPAM.13.1.50. [DOI] [Google Scholar]
- 85.Huang L., Adachi T., Shimizu Y., Goto Y., Toyama J., Tanaka H., Akashi R., Sawaguchi A., Iwata H., Haga T. Characterization of lectin isolated from Momordica charantia seed as a B cell activator. Immunol. Lett. 2008;121(2):148–156. doi: 10.1016/j.imlet.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 86.Lee-Huang S., Huang P.L., Chen H.C., Huang P.L., Bourinbaiar A., Huang H.I., Kung H.F. Anti-HIV and anti-tumor activities of recombinant MAP30 from bitter melon. Gene. 1995;161(2):151–156. doi: 10.1016/0378-1119(95)00186-a. [DOI] [PubMed] [Google Scholar]
- 87.Praseno Saleh S., Ning R. Antiviral activity of Momordica charantia: a preliminary study on in vitro anti-herpes simplex virus. Berk. llmu Kedokt. 1997;29(3):121–123. [Google Scholar]
- 88.Pongthanapisith V., Ikuta K., Puthavathana P., Leelamanit W. Antiviral protein of Momordica charantia L. inhibits different subtypes of Influenza A. Evid. Based Complement. Altern. Med. 2013;20013 doi: 10.1155/2013/729081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lacerda R.R., Moreira I.C., do Nascimento J.S.J., de Lacerda A.C.S., Cabral N.L., Lucetti D.L., de Barros Viana G.S., Felipe C.F.B., de Luna Freire Pessoa H., de Almeida Gadelha C.A., Santi-Gadelha T. Lectin isolated from Brazilian seeds of velvet bean (Mucuna pruriens (L) DC.) presents analgesic, anti-inflammatory and antihemolytic action. J. Med. Plant Res. 2015;9(8):231–242. doi: 10.5897/JMPR2014.5693. (e) [DOI] [Google Scholar]
- 90.Taghizadeh S.F., Azizi M., Asili J., Madarshahi F.S., Rakhshandeh H., Fujii Y. Therapeutic peptides of Mucuna pruriens L.: anti-genotoxic molecules against human hepatocellular carcinoma and hepatitis C virus. Food Sci. Nutr. 2021;9(6):2908–2914. doi: 10.1002/fsn3.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mahaboob Batcha A.T., Wadhwani A., Subramaniam G. In vitro antiviral activity of BanLec against herpes simplex viruses type 1 and 2. Bangladesh J. Pharmacol. 2020;15(1):11–18. doi: 10.3329/bjp.v15i1.42320. [DOI] [Google Scholar]
- 92.Aoki H., Akaike T., Abe K., Kuroda M., Arai S., Okamura R., Negi A., Maeda H. Antiviral effect of oryzacystatin, a proteinase inhibitor in rice, against herpes simplex virus type 1 in vitro and in vivo. Antimicrob. Agents Chemother. 1995;39(4):846–849. doi: 10.1128/aac.39.4.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ray B., Hutterer C., Bandyopadhyay S.S., Ghosh K., Chatterjee U.R., Ray S., Zeitträger I., Wagner S., Marschall M. Chemically engineered sulfated glucans from rice bran exert strong antiviral activity at the stage of viral entry. J. Nat. Prod. 2013;76(12):2180–2188. doi: 10.1021/np4003977. [DOI] [PubMed] [Google Scholar]
- 94.Ye X.Y., Ng T.B., Tsang P.W., Wang J. Isolation of a homodimeric lectin with antifungal and antiviral activities from red kidney bean (Phaseolus vulgaris) seeds. J. Protein Chem. 2001;20(5):367–375. doi: 10.1023/a:1012276619686. [DOI] [PubMed] [Google Scholar]
- 95.Fang E.F., Lin P., Wong J.H., Tsao S.W., Ng T.B. A lectin with anti-HIV-1 reverse transcriptase, antitumor, and nitric oxide inducing activities from seeds of Phaseolus vulgaris cv. extralong autumn purple bean. J. Agric. Food Chem. 2010;58(4):2221–2229. doi: 10.1021/jf903964u. [DOI] [PubMed] [Google Scholar]
- 96.Coutiño-Rodríguez R., Hernández-Cruz P., Giles-Ríos H. Lectins in fruits having gastrointestinal activity: their participation in the hemagglutinating property of Escherichia coli O157:H7. Arch. Med. Res. 2001;32(4):251–257. doi: 10.1016/s0188-4409(01)00287-9. [DOI] [PubMed] [Google Scholar]
- 97.Sriwilaijaroen N., Fukumoto S., Kumagai K., Hiramatsu H., Odagiri T., Tashiro M., Suzuki Y. Antiviral effects of Psidium guajava Linn. (guava) tea on the growth of clinical isolated H1N1 viruses: its role in viral hemagglutination and neuraminidase inhibition. Antivir. Res. 2012;94(2):139–146. doi: 10.1016/j.antiviral.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 98.Al-Sohaimy S.A., Hafez E.E., Abdelwahab A.E., El-Saadani M.A. Anti -HCV Lectin from Egyptian Pisum sativum. Aust. J. Basic Appl. Sci. 2007;1(3):213–219. [Google Scholar]
- 99.Chiang L.C., Cheng H.Y., Liu M.C., Chiang W., Lin C.C. Antiviral activity of eight commonly used medicinal plants in Taiwan. Am. J. Chin. Med. 2003;31(6):897–905. doi: 10.1142/s0192415x03001582. [DOI] [PubMed] [Google Scholar]
- 100.Echemendia Blanco D., Van Driessche E., De Greve H., Beeckmans S. 2005. Characterization and expression of the seed lectin from Pterocarpus indicus. In Abstracts of the 191st Meeting of the Belgian Society of Biochemistry and Molecular Biology (electronic).
- 101.Dewi B., Angelina M., meilawati l, Hartati S., Dewijanti I., Santi M., Desti H., Sudiro M. Antiviral Effect of pterocarpus indicus willd leaves extract against replication of Dengue Virus (DENV) in vitro. Trop. Life Sci. Res. 2018;8(1):55–61. [Google Scholar]
- 102.Pawar H.A., Lalitha K.G. Isolation, purification and characterization of galactomannans as an excipient from Senna tora seeds. Int. J. Biol. Macromol. 2014;65:167–175. doi: 10.1016/j.ijbiomac.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 103.Wen C.C., Shyur L.F., Jan J.T., Liang P.H., Kuo C.J., Arulselvan P., Wu J.B., Kuo S.C., Yang N.S. Traditional Chinese medicine herbal extracts of Cibotium barometz, Gentiana scabra, Dioscorea batatas, Cassia tora, and Taxillus chinensis inhibit SARS-CoV replication. J. Tradit. Complement. Med. 2011;1(1):41–50. doi: 10.1016/s2225-4110(16)30055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sultana M., Shakil Ahmed F., Alam M. Identification of lectins from the seeds of Bangladeshi plants Sesbania bispinosa and Senna occidentalis by hemagglutination assay. Asian J. Green Chem. 2019;3(4):518–524. doi: 10.33945/SAMI/AJGC/2019.4.8. [DOI] [Google Scholar]
- 105.Lombardo M., Ikuno A.A., Baldassi L., Ferreira V.C.A., Kiyota S. Evaluation of protein fractions from Senna occidentalis seeds extracts for cytotoxic, antiviral and antibacterial activities. Virus Rev. Res. 2004;9(2):61–68. [Google Scholar]
- 106.Biswas S., Agrawal P., Saroha A., Das H.R. Purification and mass spectrometric characterization of Sesbania aculeata (Dhaincha) stem lectin. Protein J. 2009;28(9–10):391–399. doi: 10.1007/s10930-009-9206-z. [DOI] [PubMed] [Google Scholar]
- 107.Nachbar M.S., Oppenheim J.D., Thomas J.O. Lectins in the U.S. Diet. Isolation and characterization of a lectin from the tomato (Lycopersicon esculentum) J. Biol. Chem. 1980;255(5):2056–2061. [PubMed] [Google Scholar]
- 108.Zubcević N., Fočak M., Suljević D. Highly specifi c hemagglutination activity of plant lectins in specific species: case of Fabaceae and Solanaceae. Bulg. J. Agric. Sci. 2018;24(3):391–397. [Google Scholar]
- 109.Di Sotto A., Di Giacomo S., Amatore D., Locatelli M., Vitalone A., Toniolo C., Rotino G.L., Lo Scalzo R., Palamara A.T., Marcocci M.E., Nencioni L. A polyphenol rich extract from Solanum melongena L. DR2 peel exhibits antioxidant properties and anti-herpes simplex virus type 1 activity in vitro. Molecules. 2018;23(8):2066. doi: 10.3390/molecules23082066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Osman M.E.M., Awadallah A.K.E., Konozy E.H.E. Isolation, purification and partial characterization of three lectins from Tamarindus indica seeds with a novel sugar specificity. Int. J. Plant Res. 2016;6(1):13–19. doi: 10.5923/j.plant.20160601.03. [DOI] [Google Scholar]
- 111.Okoh O.O., Obiiyeke G.E., Nwodo U.U., Okoh A.I. Ethanol extract and chromatographic fractions of Tamarindus indica stem bark inhibits Newcastle disease virus replication. Pharm. Biol. 2017;55(1):1806–1808. doi: 10.1080/13880209.2017.1331364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kuru P. Tamarindus indica and its health-related effects. Asian Pac. J. Trop. Biomed. 2014;4:676–681. doi: 10.12980/APJTB.4.2014APJTB-2014-0173. [DOI] [Google Scholar]
- 113.Padma P., Komath S.S., Nadimpalli S., Swamy M.J. Purification in high yield and characterisation of a new galactose-specific lectin from the seeds of Trichosanthes cucumerina. Phytochemistry. 1999;50:363–371. doi: 10.1016/S0031-9422(98)00544-5. [DOI] [Google Scholar]
- 114.Sultan N.A., Kenoth R., Swamy M.J. Purification, physicochemical characterization, saccharide specificity, and chemical modification of a Gal/GalNAc specific lectin from the seeds of Trichosanthes dioica. Arch. Biochem. Biophys. 2004;432(2):212–221. doi: 10.1016/j.abb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 115.Shibuya N., Goldstein I.J., Shafer J.A., Peumans W.J., Broekaert W.F. Carbohydrate binding properties of the stinging nettle (Urtica dioica) rhizome lectin. Arch. Biochem. Biophys. 1986;249(1):215–224. doi: 10.1016/0003-9861(86)90577-1. [DOI] [PubMed] [Google Scholar]
- 116.Keyaerts E., Vijgen L., Pannecouque C., Van Damme E., Peumans W., Egberink H., Balzarini J., Van Ranst M. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antivir. Res. 2007;75(3):179–187. doi: 10.1016/j.antiviral.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.van der Meer F.J., de Haan C.A., Schuurman N.M., Haijema B.J., Verheije M.H., Bosch B.J., Balzarini J., Egberink H.F. The carbohydrate-binding plant lectins and the non-peptidic antibiotic pradimicin A target the glycans of the coronavirus envelope glycoproteins. J. Antimicrob. Chemother. 2007;60(4):741–749. doi: 10.1093/jac/dkm301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Suseelan K., Bhatia A., Mitra R. Purification and characterization of two major lectins from Vigna mungo (blackgram) J. Biosci. 1997;22:439–455. doi: 10.1007/BF02703190. [DOI] [Google Scholar]
- 119.Karthikeyan G., Doraisamy S., Rabindran R., Ganapathy T. Evaluation of antiviral principles for the induction of systemic resistance in blackgram (Vigna mungo) against Urdbean Leaf Crinkle Virus. Arch. Phytopathol. Pflanzenschutz. 2009;42:1172–1186. doi: 10.1080/03235400701652334. [DOI] [Google Scholar]
- 120.Suseelan K.N., Bhatia C.R., Mitra R. Characteristics of two major lectins from mungbean (Vigna radiata) seeds. Plant Foods Hum. Nutr. 1997;50(3):211–222. doi: 10.1007/bf02436058. [DOI] [PubMed] [Google Scholar]
- 121.Hafidh R.R., Abdulamir A.S., Abu Bakar F., Sekawi Z., Jahansheri F., Jalilian F.A. Novel antiviral activity of mung bean sprouts against respiratory syncytial virus and herpes simplex virus -1: an in vitro study on virally infected Vero and MRC-5 cell lines. BMC Complement. Alter. Med. 2015;15:179. doi: 10.1186/s12906-015-0688-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lo C.W., Pi C.C., Chen Y.T., Chen H.W. Vigna radiata (L.) R. Wilczek extract inhibits Influenza A virus by targeting viral attachment, penetration, assembly, and release. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.584973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Roberson B.J., Strength D.R. Characterization of a lectin from cowpeas. Prep. Biochem. 1983;13(1):45–56. doi: 10.1080/00327488308068734. [DOI] [PubMed] [Google Scholar]
- 124.Ye X.Y., Wang H.X., Ng T.B. Structurally dissimilar proteins with antiviral and antifungal potency from cowpea (Vigna unguiculata) seeds. Life Sci. 2000;67(26):3199–3207. doi: 10.1016/s0024-3205(00)00905-x. [DOI] [PubMed] [Google Scholar]
- 125.George B.S., Silambarasan S., Senthil K., Jacob J.P., Ghosh Dasgupta M. Characterization of an insecticidal protein from Withania somnifera against lepidopteran and hemipteran pest. Mol. Biotechnol. 2018;60(4):290–301. doi: 10.1007/s12033-018-0070-y. [DOI] [PubMed] [Google Scholar]
- 126.Chikhale R.V., Gurav S.S., Patil R.B., Sinha S.K., Prasad S.K., Shakya A., Shrivastava S.K., Gurav N.S., Prasad R.S. Sars-cov-2 host entry and replication inhibitors from Indian ginseng: an in-silico approach. J. Biomol. Struct. Dyn. 2021;39(12):4510–4521. doi: 10.1080/07391102.2020.1778539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Saggam A., Limgaokar K., Borse S., Chavan-Gautam P., Dixit S., Tillu G., Patwardhan B. Withania somnifera (L.) Dunal: opportunity for clinical repurposing in COVID-19 management. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.623795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gondim A., Roberta da Silva S., Mathys L., Noppen S., Liekens S., Holanda Sampaio A., Nagano C.S., Renata Costa Rocha C., Nascimento K.S., Cavada B.S., Sadler P.J., Balzarini J. Potent antiviral activity of carbohydrate-specific algal and leguminous lectins from the Brazilian biodiversity. MedChemComm. 2019;10(3):390–398. doi: 10.1039/c8md00508g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Carneiro D.C., Fernandez L.G., Monteiro-Cunha J.P., Benevides R.G., Cunha Lima S.T. A patent review of the antimicrobial applications of lectins: perspectives on therapy of infectious diseases. J. Appl. Microbiol. 2021 doi: 10.1111/jam.15263. 10.1111/jam.15263. [DOI] [PubMed] [Google Scholar]
- 130.Wu A.M., Lisowska E., Duk M., et al. Lectins as tools in glycoconjugate research. Glycoconj. J. 2009;26:899. doi: 10.1007/s10719-008-9119-7. [DOI] [PubMed] [Google Scholar]
- 131.Van Breedam W., Pöhlmann S., Favoreel H.W., de Groot R.J., Nauwynck H.J. Bitter-sweet symphony: glycan-lectin interactions in virus biology. FEMS Microbiol. Rev. 2014;38(4):598–632. doi: 10.1111/1574-6976.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lardone R.D., Garay Y.C., Parodi P., de la Fuente S., Angeloni G., Bravo E.O., Schmider A.K., Irazoqui F.J. How glycobiology can help us treat and beat the COVID-19 pandemic. J. Biol. Chem. 2021;296 doi: 10.1016/j.jbc.2021.100375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.O’Keefe B.R., Giomarelli B., Barnard D.L., Shenoy S.R., Chan P.K., McMahon J.B., Palmer K.E., Barnett B.W., Meyerholz D.K., Wohlford-Lenane C.L., McCray P.B., Jr. Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family Coronaviridae. J. Virol. 2010;84(5):2511–2521. doi: 10.1128/jvi.02322-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kumaki Y., Wandersee M.K., Smith A.J., Zhou Y., Simmons G., Nelson N.M., Bailey K.W., Vest Z.G., Li J.K., Chan P.K., Smee D.F., Barnard D.L. Inhibition of severe acute respiratory syndrome coronavirus replication in a lethal SARS-CoV BALB/c mouse model by stinging nettle lectin, Urtica dioica agglutinin. Antivir. Res. 2011;90(1):22–32. doi: 10.1016/j.antiviral.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Millet J.K., Séron K., Labitt R.N., Danneels A., Palmer K.E., Whittaker G.R., Dubuisson J., Belouzard S. Middle East respiratory syndrome coronavirus infection is inhibited by griffithsin. Antivir. Res. 2016;133:1–8. doi: 10.1016/j.antiviral.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Greig A.S., Bouillant A.M. Binding effects of concanavalin A on a coronavirus. Can. J. Comp. Med. 1977;41(1):122–126. [PMC free article] [PubMed] [Google Scholar]
- 137.Hsieh L.E., Lin C.N., Su B.L., Jan T.R., Chen C.M., Wang C.H., Lin D.S., Lin C.T., Chueh L.L. Synergistic antiviral effect of Galanthus nivalis agglutinin and nelfinavir against feline coronavirus. Antivir. Res. 2010;88(1):25–30. doi: 10.1016/j.antiviral.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu Y.M., Shahed-Al-Mahmud M., Chen X., Chen T.H., Liao K.S., Lo J.M., Wu Y.M., Ho M.C., Wu C.Y., Wong C.H., Jan J.T., Ma C. A carbohydrate-binding protein from the edible lablab beans effectively blocks the infections of influenza viruses and SARS-CoV-2. Cell Rep. 2020;32(6) doi: 10.1016/j.celrep.2020.108016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang W., Li Q., Wu J., Hu Y., Wu G., Yu C., Xu K., Liu X., Wang Q., Huang W., Wang L., Wang Y. Lentil lectin derived from Lens culinaris exhibit broad antiviral activities against SARS-CoV-2 variants. Emerg. Microbes Infect. 2021;10(1):1519–1529. doi: 10.1080/22221751.2021.1957720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cai Y., Xu W., Gu C., Cai X., Qu D., Lu L., Xie Y., Jiang S. Griffithsin with a broad-spectrum antiviral activity by binding glycans in viral glycoprotein exhibits strong synergistic effect in combination with a pan-coronavirus fusion inhibitor targeting SARS-CoV-2 spike S2 subunit. Virol. Sin. 2020;35(6):857–860. doi: 10.1007/s12250-020-00305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Alam M.A., Parra-Saldivar R., Bilal M., Afroze C.A., Ahmed M.N., Iqbal H., Xu J. Algae-derived bioactive molecules for the potential treatment of SARS-CoV-2. Molecules. 2021;26(8):2134. doi: 10.3390/molecules26082134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lokhande K.B., Apte G.R., Shrivastava A., Singh A., Pal J.K., Venkateswara Swamy K., Gupta R.K. Sensing the interactions between carbohydrate-binding agents and N-linked glycans of SARS-CoV-2 spike glycoprotein using molecular docking and simulation studies. J. Biomol. Struct. Dyn. 2020:1–19. doi: 10.1080/07391102.2020.1851303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bah C.S.F., Fang E.F., Ng T.B. Antitumor Potential and Other Emerging Medicinal Properties of Natural Compounds. Springer Netherlands; 2012. Medicinal applications of plant lectins; pp. 55–74. [DOI] [Google Scholar]
- 144.Chikhale R.V., Gurav S.S., Patil R.B., Sinha S.K., Prasad S.K., Shakya A., Shrivastava S.K., Gurav N.S., Prasad R.S. Sars-CoV-2 host entry and replication inhibitors from Indian ginseng: an in-silico approach. J. Biomol. Struct. Dyn. 2021;39(12):4510–4521. doi: 10.1080/07391102.2020.1778539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kumar N., Shala A.Y., Paul Khurana S.M. Antiviral and immuno-boosting potential of ashwagandha (Withania somnifera l.) Med. Plants – Int J. Phytomed. Relat. Ind. 2021;13(2):237–244. doi: 10.5958/0975-6892.2021.00026.5. [DOI] [Google Scholar]
- 146.Liu Y., Liu J., Pang X., Liu T., Ning Z., Cheng G. The roles of direct recognition by animal lectins in antiviral immunity and viral pathogenesis. Molecules. 2015;20(2):2272–2295. doi: 10.3390/molecules20022272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gordts S.C., Renders M., Férir G., Huskens D., Van Damme E.J., Peumans W., Balzarini J., Schols D. NICTABA and UDA, two GlcNAc-binding lectins with unique antiviral activity profiles. J. Antimicrob. Chemother. 2015;70(6):1674–1685. doi: 10.1093/jac/dkv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Geurtsen J., Driessen N.N., Appelmelk B.J. Mannose–fucose recognition by DC-SIGN. Microb. Glycobiol. 2010:673–695. doi: 10.1016/B978-0-12-374546-0.00034-1. [DOI] [Google Scholar]
- 149.Amraei R., Yin W., Napoleon M.A., Suder E.L., Berrigan J., Zhao Q., Olejnik J., Chandler K.B., Xia C., Feldman J., Hauser B.M., Caradonna T.M., Schmidt A.G., Gummuluru S., Mühlberger E., Chitalia V., Costello C.E., Rahimi N. CD209L/L-SIGN and CD209/DC-SIGN Act as Receptors for SARS-CoV-2. ACS Cent. Sci. 2021;7(7):1156–1165. doi: 10.1021/acscentsci.0c01537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Thépaut M., Luczkowiak J., Vivès C., Labiod N., Bally I., Lasala F., Grimoire Y., Fenel D., Sattin S., Thielens N., Schoehn G., Bernardi A., Delgado R., Fieschi F. DC/L-SIGN recognition of spike glycoprotein promotes SARS-CoV-2 trans-infection and can be inhibited by a glycomimetic antagonist. PLoS Pathog. 2021;17(5) doi: 10.1371/journal.ppat.1009576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Anderluh M., Jug G., Svajger U., Obermajer N. DC-SIGN antagonists, a potential new class of anti-infectives. Curr. Med. Chem. 2012;19(7):992–1007. doi: 10.2174/092986712799320664. [DOI] [PubMed] [Google Scholar]
- 152.Valverde P., Ardá A., Reichardt N.C., Jiménez-Barbero J., Gimeno A. Glycans in drug discovery. MedChemComm. 2019;10(10):1678–1691. doi: 10.1039/C9MD00292H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhou Y., Lu K., Pfefferle S., Bertram S., Glowacka I., Drosten C., Pöhlmann S., Simmons G. A single asparagine-linked glycosylation site of the severe acute respiratory syndrome coronavirus spike glycoprotein facilitates inhibition by mannose-binding lectin through multiple mechanisms. J. Virol. 2010;84(17):8753–8764. doi: 10.1128/jvi.00554-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Alexandre K.B., Gray E.S., Mufhandu H., McMahon J.B., Chakauya E., O’Keefe B.R., Chikwamba R., Morris L. The lectins griffithsin, cyanovirin-N and scytovirin inhibit HIV-1 binding to the DC-SIGN receptor and transfer to CD4(+) cells. Virology. 2012;423(2):175–186. doi: 10.1016/j.virol.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shajahan A., Pepi L.E., Rouhani D.S., Heiss C., Azadi P. Glycosylation of SARS-CoV-2: structural and functional insights. Anal. Bioanal. Chem. 2021:1–15. doi: 10.1007/s00216-021-03499-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang Q., Xiang R., Huo S., Zhou Y., Jiang S., Wang Q., Yu F. Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy. Signal Transduct. Target Ther. 2021;6(1):233. doi: 10.1038/s41392-021-00653-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Watanabe Y., Bowden T.A., Wilson I.A., Crispin M. Exploitation of glycosylation in enveloped virus pathobiology. Biochim Biophys. Acta Gen. Subj. 2019;1863(10):1480–1497. doi: 10.1016/j.bbagen.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhou D., Tian X., Qi R., Peng C., Zhang W. Identification of 22 N-glycosites on spike glycoprotein of SARS-CoV-2 and accessible surface glycopeptide motifs: implications for vaccination and antibody therapeutics. Glycobiology. 2021;31(1):69–80. doi: 10.1093/glycob/cwaa052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hoffmann D., Mereiter S., Jin Oh Y., Monteil V., Elder E., Zhu R., Canena D., Hain L., Laurent E., Grunwaldd-Gruber C., Klausberger M., Jonsson G., Kellner M.J., Novatchkova M., Ticevic M., Chabloz A., Wirnsberger G., Hgelkruys A., Altmann F., Mach L., Stadlmann J., Oostenbrink C., Mirazimi A., Hinterdorfer P., Penningeer J.M. Identification of lectin receptors for conserved SARS-CoV-2 glycosylation sites. EMBO J. 2021 doi: 10.15252/embj.2021108375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Barre A., Damme E., Simplicien M., Benoist H., Rougé P. Man-specific, GalNAc/T/Tn-specific and Neu5Ac-specific seaweed lectins as glycan probes for the SARS-CoV-2 (COVID-19) coronavirus. Mar. Drugs. 2020;18(11):543. doi: 10.3390/md18110543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yang J., Petitjean S., Koehler M., Zhang Q., Dumitru A.C., Chen W., Derclaye S., Vincent S.P., Soumillion P., Alsteens D. Molecular interaction and inhibition of SARS-CoV-2 binding to the ACE2 receptor. Nat. Commun. 2020;11(1):4541. doi: 10.1038/s41467-020-18319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ramírez Hernández E., Hernández-Zimbrón L.F., Martínez Zúñiga N., Leal-García J.J., Ignacio Hernández V., Ucharima-Corona L.E., Pérez Campos E., Zenteno E. The role of the SARS-CoV-2 S-protein glycosylation in the interaction of SARS-CoV-2/ACE2 and immunological responses. Viral Immunol. 2021;34(3):165–173. doi: 10.1089/vim.2020.0174. [DOI] [PubMed] [Google Scholar]
- 164.Ritchie G., Harvey D.J., Feldmann F., Stroeher U., Feldmann H., Royle L., Dwek R.A., Rudd P.M. Identification of N-linked carbohydrates from severe acute respiratory syndrome (SARS) spike glycoprotein. Virology. 2010;399(2):257–269. doi: 10.1016/j.virol.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fung T.S., Liu D.X. Post-translational modifications of coronavirus proteins: roles and function. Future Virol. 2018;13(6):405–430. doi: 10.2217/fvl-2018-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ip W.K., Chan K.H., Law H.K., Tso G.H., Kong E.K., Wong W.H., To Y.F., Yung R.W., Chow E.Y., Au K.L., Chan E.Y., Lim W., Jensenius J.C., Turner M.W., Peiris J.S., Lau Y.L. Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. J. Infect. Dis. 2005;191(10):1697–1704. doi: 10.1086/429631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gupta A., Gupta G.S. Status of mannose-binding lectin (MBL) and complement system in COVID-19 patients and therapeutic applications of antiviral plant MBLs. Mol. Cell Biochem. 2021;476(8):2917–2942. doi: 10.1007/s11010-021-04107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kaplan B., McInerney A. Infections and associated diseases in patients with mannose-binding lectin deficiency, presenting to a tertiary care immunology clinic. J. Allergy Clin. Immunol. 2021;147(2):AB74. doi: 10.1016/j.jaci.2020.12.289. [DOI] [Google Scholar]
- 169.Hayes B., Stanley J., Peppers B.P. COVID-19 Recurrence Without Seroconversion in a Patient with Mannose-Binding Lectin Deficiency. Allergy Rhinol. (Provid.) 2021;12 doi: 10.1177/21526567211024140. 21526567211024140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Eriksson O., Hultström M., Persson B., Lipcsey M., Ekdahl K.N., Nilsson B., Frithiof R. Mannose-binding lectin is associated with thrombosis and coagulopathy in critically Ill COVID-19 patients. Thromb. Haemost. 2020;120(12):1720–1724. doi: 10.1055/s-0040-1715835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gupta A. Animal Lectins: Form, Function and Clinical Application. Springer; Vienna: 2012. MBL deficiency as risk of infection and autoimmunity. [DOI] [Google Scholar]
- 172.Peng Q., Li K., Sacks S.H., Zhou W. The role of anaphylatoxins C3a and C5a in regulating innate and adaptive immune responses. Inflamm. Allergy Drug Targets. 2009;8(3):236–246. doi: 10.2174/187152809788681038. [DOI] [PubMed] [Google Scholar]
- 173.Bumiller-Bini V., de Freitas Oliveira-Toré C., Carvalho T.M., Kretzschmar G.C., Gonçalves L.B., Alencar N.M., Gasparetto Filho M.A., Beltrame M.H., Winter Boldt A.B. MASPs at the crossroad between the complement and the coagulation cascades – the case for COVID-19. Genet. Mol. Biol. 2021;44(1 Suppl 1) doi: 10.1590/1678-4685-gmb-2020-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Medetalibeyoglu A., Bahat G., Senkal N., Kose M., Avci K., Sayin G.Y., Isoglu-Alkac U., Tukek T., Pehlivan S. Mannose binding lectin gene 2 (rs1800450) missense variant may contribute to development and severity of COVID-19 infection. Infect. Genet. Evol. 2021;89 doi: 10.1016/j.meegid.2021.104717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Flude B.M., Nannetti G., Mitchell P., Compton N., Richards C., Heurich M., Brancale A., Ferla S., Bassetto M. Targeting the complement serine protease MASP-2 as a therapeutic strategy for coronavirus infections. Viruses. 2021;13:312. doi: 10.3390/v13020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Matricardi P.M., Dal Negro R.W., Nisini R. The first, holistic immunological model of COVID-19: Implications for prevention, diagnosis, and public health measures. Pediatr. Allergy Immunol. 2020;31(5):454–470. doi: 10.1111/pai.13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kalia N., Singh J., Kaur M. The ambiguous role of mannose-binding lectin (MBL) in human immunity. Open Med. 2021;16(1):299–310. doi: 10.1515/med-2021-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Santiesteban-Lores L.E., Amamura T.A., da Silva T.F., Midon L.M., Carneiro M.C., Isaac L., Bavia L. A double-edged sword – the complement system during SARS-CoV-2 infection. Life Sci. 2021;272 doi: 10.1016/j.lfs.2021.119245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Stahel P.F., Barnum S.R. Complement inhibition in coronavirus disease (COVID)-19: a neglected therapeutic option. Front. Immunol. 2020;11:1661. doi: 10.3389/fimmu.2020.01661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992–1000. doi: 10.1016/j.chom.2020.04.009. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dommett R.M., Klein N., Turner M.W. Mannose-binding lectin in innate immunity: past, present and future. Tissue Antigens. 2006;68(3):193–209. doi: 10.1111/j.1399-0039.2006.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chatterjee S.K., Saha S., Munoz M. Molecular pathogenesis, immunopathogenesis and novel therapeutic strategy against COVID-19. Front. Mol. Biosci. 2020;7:196. doi: 10.3389/fmolb.2020.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li Y., Liu D., Wang Y., Su W., Liu G., Dong W. The importance of glycans of viral and host proteins in enveloped virus infection. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.638573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Van Damme E.J. Lectins as tools to select for glycosylated proteins. Methods Mol. Biol. 2011;753:289–297. doi: 10.1007/978-1-61779-148-2_19. [DOI] [PubMed] [Google Scholar]
- 185.Zhang L., Luo S., Zhang B. The use of lectin microarray for assessing glycosylation of therapeutic proteins. MAbs. 2016;8(3):524–535. doi: 10.1080/19420862.2016.1149662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Breitenbach Barroso Coelho L.C., Marcelino Dos Santos Silva P., Felix de Oliveira W., de Moura M.C., Viana Pontual E., Soares Gomes F., Guedes Paiva P.M., Napoleão T.H., Dos Santos Correia M.T. Lectins as antimicrobial agents. J. Appl. Microbiol. 2018;125(5):1238–1252. doi: 10.1111/jam.14055. [DOI] [PubMed] [Google Scholar]
- 187.Jandú J., Moraes Neto R.N., Zagmignan A., de Sousa E.M., Brelaz-de-Castro M., Dos Santos Correia M.T., da Silva L. Targeting the immune system with plant lectins to combat microbial infections. Front. Pharmacol. 2017;8:671. doi: 10.3389/fphar.2017.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Souza M.A., Carvalho F.C., Ruas L.P., Ricci-Azevedo R., Roque-Barreira M.C. The immunomodulatory effect of plant lectins: a review with emphasis on ArtinM properties. Glycoconj. J. 2013;30(7):641–657. doi: 10.1007/s10719-012-9464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ditamo Y., Rupil L.L., Sendra V.G., Nores G.A., Roth G.A., Irazoqui F.J. In vivo immunomodulatory effect of the lectin from edible mushroom Agaricus bisporus. Food Funct. 2016;7(1):262–269. doi: 10.1039/C5FO00360A. [DOI] [PubMed] [Google Scholar]
- 190.Wang Y., Zhang Y., Shao J., Wu B., Li B. Potential immunomodulatory activities of a lectin from the mushroom Latiporus sulphureus. Int. J. Biol. Macromol. 2019;130:399–406. doi: 10.1016/j.ijbiomac.2019.02.150. [DOI] [PubMed] [Google Scholar]
- 191.Chernikov O.V., Wong W.T., Li L.H., Chikalovets I.V., Molchanova V.I., Wu S.H., Liao J.H., Hua K.F. A GalNAc/Gal-specific lectin from the sea mussel Crenomytilus grayanus modulates immune response in macrophages and in mice. Sci. Rep. 2017;7(1):6315. doi: 10.1038/s41598-017-06647-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Reyna-Margarita H.R., Irais C.M., Mario-Alberto R.G., Agustina R.M., Luis-Benjamín S.G., David P.E. Plant phenolics and lectins as vaccine adjuvants. Curr. Pharm. Biotechnol. 2019:20. doi: 10.2174/1389201020666190716110705. [DOI] [PubMed] [Google Scholar]
- 193.Nascimento da Silva L.C., Mendonça J., de Oliveira W.F., Batista K., Zagmignan A., Viana I., Dos Santos Correia M.T. Exploring lectin-glycan interactions to combat COVID-19: lessons acquired from other enveloped viruses. Glycobiology. 2021;31(4):358–371. doi: 10.1093/glycob/cwaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Reis C.A., Tauber R., Blanchard V. Glycosylation is a key in SARS-CoV-2 infection. J. Mol. Med. 2021;99(8):1023–1031. doi: 10.1007/s00109-021-02092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Minko T. Drug targeting to the colon with lectins and neoglycoconjugates. Adv. Drug Deliv. Rev. 2004;56(4):491–509. doi: 10.1016/j.addr.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 196.Rek A., Krenn E., Kungl A.J. Therapeutically targeting protein-glycan interactions. Br. J. Pharmacol. 2009;157(5):686–694. doi: 10.1111/j.1476-5381.2009.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Plattner V.E., Ratzinger G., Engleder E.T., Gallauner S., Gabor F., Wirth M. Alteration of the glycosylation pattern of monocytic THP-1 cells upon differentiation and its impact on lectin-mediated drug delivery. Eur. J. Pharm. Biopharm. 2009;73(3):324–330. doi: 10.1016/j.ejpb.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 198.de Juan L.L., Recio V.G., López P.J., Juan T.G., Cordoba-Diaz M., Cordoba-Diaz D. Pharmaceutical applications of lectins. J. Drug Deliv. Sci. Technol. 2017;42:126–133. doi: 10.1016/j.jddst.2017.05.018. [DOI] [Google Scholar]
- 199.Bagdonaite I., Vakhrushev S.Y., Joshi H.J., Wandall H.H. Viral glycoproteomes: technologies for characterization and outlook for vaccine design. FEBS Lett. 2018;592(23):3898–3920. doi: 10.1002/1873-3468.13177. [DOI] [PubMed] [Google Scholar]
- 200.Nag K., Chandra Baray J., Rahman Khan M., Mahmud A., Islam J., Myti S., Ali R., Sarker E.H., Kumar S., Chowdhury M.H., Roy R., Islam F., Barman U., Khan H., Chakraborty S., Badsha A., Hossain M., ahammad S., Chowdhury M.R., Ghosh P., Shimul R.I., Ahmmed R., Bhuiya E.H., Biswas B.K., Mohiuddin M.M., Sultana N. An mRNA-based vaccine candidate against SARS-CoV-2 elicits stable immuno-response with single dose. Vaccine. 2021;39(28):3745–3755. doi: 10.1016/j.vaccine.2021.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Antara, AF. Globe Biotech hopes for Bangavax human trials in September, 2021. 〈https://www.dhakatribune.com/bangladesh/2021/08/13/globe-biotech-hopes-for-bangavax-human-trials-september〉 (Accessed 8 September 2021).
- 202.Maharjan P.M., Choe S. Plant-based COVID-19 vaccines: current status, design, and development strategies of candidate vaccines. Vaccines. 2021;9(9):992. doi: 10.3390/vaccines9090992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Alagesan K., Hoffmann M., Rapp E., Kolarich D. Glycoproteomics technologies in glycobiotechnology. Adv. Biochem. Eng. Biotechnol. 2021;175:413–434. doi: 10.1007/10_2020_144. [DOI] [PubMed] [Google Scholar]
- 204.Capell T., Twyman R.M., Armario-Najera V., Ma J.K., Schillberg S., Christou P. Potential applications of plant biotechnology against SARS-CoV-2. Trends Plant Sci. 2020;25(7):635–643. doi: 10.1016/j.tplants.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Decker J.S., Menacho-Melgar R., Lynch M.D. Low-cost, large-scale production of the anti-viral lectin griffithsin. Front. Bioeng. Biotechnol. 2020;8:1020. doi: 10.3389/fbioe.2020.01020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lau K.K. Reducing COVID-19 risk through dietary supplementation of plant mannose binding lectins. Int. J. Corona. 2020;1(4):4–11. doi: 10.14302/issn.2692-1537.ijcv-20-3492. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.