Abstract
Background
Sarcopenia was reported to be associated with poor clinical outcome, higher incidence of community-acquired pneumonia, increased risk of infections and reduced survival in different clinical settings. The aim of our work is to evaluate the prognostic role of sarcopenia in patients with the 2019 novel coronavirus disease (COVID-19).
Materials and methods
272 COVID-19 patients admitted to the University Hospital of Modena (Italy) from February 2020 to January 2021 were retrospectively studied. All included patients underwent a chest computed tomography (CT) scan to assess pneumonia during their hospitalization and showed a positive SARS-CoV-2 molecular test. Sarcopenia was defined by skeletal muscle area (SMA) evaluation at the 12th thoracic vertebra (T12). Clinical, laboratory data and adverse clinical outcome (admission to Intensive Care Unit and death) were collected for all patients.
Results
Prevalence of sarcopenia was high (41.5%) but significantly different in each pandemic wave (57.9% vs 21.6% p < 0.0000). At the multivariate analysis, sarcopenia during the first wave (Hazard Ratio 2.29, 95% confidence intervals 1.17 to 4.49 p = 0.0162) was the only independent prognostic factor for adverse clinical outcome. There were no significant differences in comorbidities and COVID19 severity in terms of pulmonary involvement at lung CT comparing during the first and second wave. Mixed pattern with peripheral and central involvement was found to be dominant in both groups.
Conclusion
We highlight the prognostic impact of sarcopenia in COVID-19 patients hospitalized during the first wave. T12 SMA could represent a potential tool to identify sarcopenic patients in particular settings. Further studies are needed to better understand the association between sarcopenia and COVID-19.
Keywords: COVID-19, SARS-CoV-2, Sarcopenia, Nutritional status, Steatosis
1. Background
The 2019 novel coronavirus disease (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), manifests as systemic disorders, particularly severe pneumonia and acute respiratory distress syndrome [1]. COVID-19 is a pandemic that swept around the world. COVID-19 and obesity share metabolic, cardiovascular or pulmonary comorbidities. Obesity has been recognized as a major risk factor for COVID-19-related prognosis, contributing to worse outcomes in those with established COVID-19 [2]. However, other nutritional disorders could affect clinical outcomes in COVID-19.
Sarcopenia, defined as a syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength, is observed in some physiological conditions (aging, inactivity) and in several pathologic processes, such as acute and chronic diseases and nutritional deficiencies [3].
Sarcopenia not only affects respiratory function, motor skill and swallowing profile, but also impairs the immune response [[4], [5], [6], [7]]. In different clinical setting sarcopenia was reported to be associated with poor clinical outcome, higher incidence of community-acquired pneumonia, increased risk of infections and reduced survival in various solid tumors and other diseases [[8], [9], [10], [11], [12], [13]]. Conventionally, measurements of skeletal muscle cross-sectional area and index, at the level of the third lumbar (L3) vertebra, utilizing clinical computed tomography (CT) scans, are considered the gold standard for the assessments of muscle mass [4]. However, when L3 is not available, skeletal muscle area (SMA) evaluation at the 12th thoracic vertebra (T12) level permits the diagnosis of sarcopenia and could be used to correlate sarcopenia with outcome parameters in patients undergoing CT limited to the chest [14,15]. Indeed, a direct relationship between SMA in L3 and T12 level has been recognized [14,15]. Old age and chronic diseases, which were involved in the etiologies of sarcopenia, were identified as risk factors for COVID-19 infection and mortality [[16], [17], [18]]; in addition, sarcopenic patients had compromised respiratory muscle strength and respiratory function, which were detrimental in the treatment of severe pneumonia and acute respiratory distress syndrome [5]. This evidence partially supports the hypothesis of a negative impact of sarcopenia on clinical outcome of patients with COVID-19 [19]. Conversely, COVID-19 could be considered as a risk factor for the onset and progression of sarcopenia because of the reduced physical activity and inadequate protein intake caused by social isolation [20,21].
Researchers have mostly tried to solve the pandemic by studying drugs and developing vaccines; although underappreciated, recognition of and intervention for adverse physical states, particularly sarcopenia, represent novel methods to promote COVID-19 treatment [19].
Recently, the association between sarcopenia and adverse clinical outcomes of COVID-19 has been investigated in some preliminary studies [22,23].
The aim of our retrospective observational study is to investigate the prevalence of sarcopenia and the potential relationship between sarcopenia and clinical outcome in a cohort of hospitalized patients with coronavirus disease.
2. Materials and methods
This retrospective study was approved by the local Ethics Committee (n◦423/2020/OSS/AOUMO) and all alive patients provided written informed consent. Patients with a positive SARS-CoV-2 molecular test, that have been hospitalized in the University Hospital of Modena and have undergone chest Computed Tomography (CT) during hospitalization were included in the study.
Two study groups were identified according to different pandemic periods: the first from February 2020 to August 2020 (first wave) and the second from September 2020 to March 2021 (second wave). Adverse clinical outcomes (admission to intensive care unit, ICU, and death) were recorded in each study group. The following clinical and laboratory data were collected for all patients: age, gender, comorbidities, C-reactive protein (CRP) and albumin level and length of hospital stay.
2.1. Body composition parameter measurements
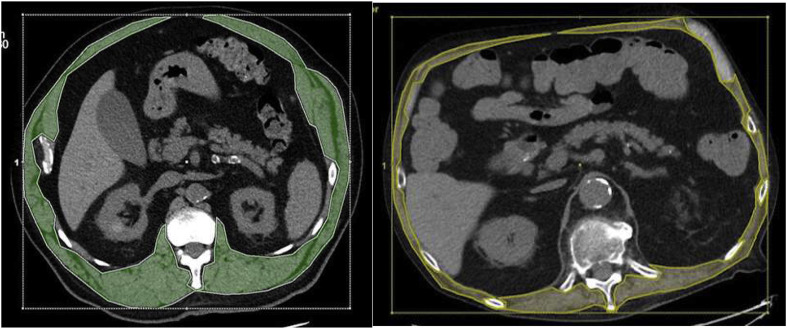
CT exams were performed at our hospital using a 64-slice CT scanner (Lightspeed VCT, GE Healthcare, Milwaukee, WI, USA). CT examinations were loaded on an Advantage Workstation (VolumeShare 7, GE Healthcare, Milwaukee, WI, USA) and non-contrast images at the level of the 12th dorsal vertebra (D12) were used for reconstructions and measurements of quantitative and qualitative body composition parameters. According to literature [15], 12th vertebra skeletal muscle cross-sectional areas include erector spinae, latissimus dorsi, external and internal oblique, rectus abdominis and external and internal intercostal muscles. The muscle components were identified using the pre-established Hounsfield Unit (HU) thresholds for muscle (HU −30 to 150) (Fig. 1 ). The skeletal muscle cross-sectional areas were made manually and the skeletal muscle area (SMA) value was automatically reported. We used specific cut-off values for SMA to define sarcopenic state in accordance with their prognostic role highlighted in two large cohorts [16]: sarcopenia was defined as SMA <92.3 cm2 in male patients and <56.1 cm2 in female patients (Fig. 1).
Fig. 1.
Muscle mass at the 12th thoracic vertebra. In both images, the area defined in green and yellow represents the muscle component at the level of the 12th thoracic vertebra, manually traced after setting the typical HU interval of the muscle. In A, the SMA values are compatible with a non-sarcopenic patient; in B, the SMA values are characteristic for a sarcopenic patient.
We also measured liver and splenic density using manual ROI (region of interest) on CT scan: according to literature, a difference between liver and splenic density <10 HU is considered statistically significant for steatosis [24].
2.2. Assessment of pneumonia and COVID-19 severity
We analyzed the chest CT scans of COVID-19 patients in relation to type and extent of lung involvement [25]; particularly, we considered:
-
•
Extention: number of pulmonary lobes, a value of “0” is awarded when less than three lobes were pathological, otherwise a value of “1”.
-
•
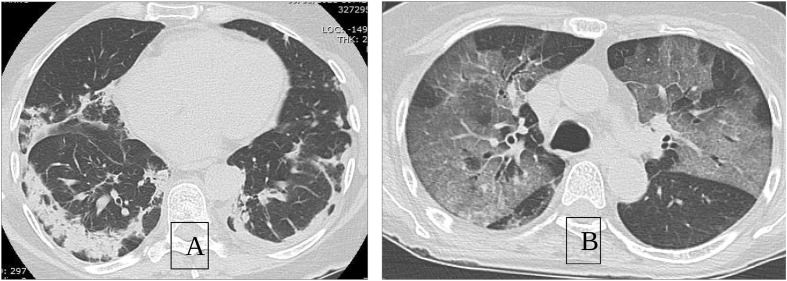
Pattern: ground glass opacities, defined as a circumscribed area of increased pulmonary attenuation with preservation of the bronchial and vascular margins, or consolidations or both (Fig. 2 ).
-
•
Distribution: central, peripheral (subpleural) or both
Fig. 2.
Radiological pattern of SARS-COV pneumonia. The images show two different pulmonary implications during SARS-CoV pneumonia. A: predominantly consolidative pattern with involvement of the subpleural peripheral parenchyma. B: ground glass pattern with prevalent central distribution.
2.3. Statistical analysis
Numerical variables were described as the mean and the standard deviation (SD) or as the median and the interquartile range (IQR), whereas categorical variables as the absolute and percentage frequencies. Between groups comparison of numerical variables was assessed with Wilcoxon–Mann–Whitney test, whereas comparison of categorical variables was assessed with the Fisher's exact test. The effect of patient's characteristics on the risk of ICU admission or death (composite outcome) was assessed by using a multivariable Cox regression model. The independent variables that were considered in the model were: wave (2-nd vs 1-st) sarcopenia (yes vs no), steatosis (yes vs no), age (years), gender (male vs female), all relevant comorbidities such as (heart diseases, hypertension, diabetes, dyslipidemia, cerebrovascular diseases, asthma, chronic obstructive pulmonary diseases, chronic kidney disease, cancer, endocrinopathies -yes vs no), sarcopenia × wave interaction and steatosis × wave interaction. The results were expressed as the Hazard Ratio (HR) with 95% confidence intervals. HRs for sarcopenia and steatosis were calculated separately for the 1-st wave and 2-nd wave periods, by using linear combinations of model parameters. Analyses were carried out with R 3.4.3 statistical software (The R Foundation for Statistical Computing, Wien), considering a significance level equal to p-value < 0.05.
3. Results
3.1. Patients characteristics
272 consecutive patients with a confirmed diagnosis of COVID-19 and treated in medical wards of University Hospital of Modena from February 2020 to January 2021 were retrospectively identified and included in the study. The main characteristics of patients enrolled in the study are summarized in Table 1 . The median age was 71 (IQR 61–78) and 62.9% were male. Concerning comorbidities, hypertension was the most common (59%). Mean SMA was 89.1 cm2 (±34.4) and prevalence of sarcopenia and steatosis was 41.5% and 66.9%, respectively. The mean length of hospital stay was 26.7 (±21.1) days.
Table 1.
General characteristics – Missing values were excluded from calculations.
| Gender | M | n % | 171 | 62.9% |
| Age | years | median IQR | 71.0 | 61–78 |
| Albumin | g/dl | mean sd | 3.3 | 0.5 |
| PCR | mg/dl | mean sd | 9.6 | 9.3 |
| Heart diseases | yes | n % | 72 | 27.1% |
| Hypertension | yes | n % | 157 | 59.0% |
| Cerebrovascular diseases | yes | n % | 21 | 7.9% |
| Diabetes | yes | n % | 61 | 22.9% |
| Dyslipidemia | yes | n % | 70 | 26.3% |
| COPD | yes | n % | 31 | 11.7% |
| Asthma | yes | n % | 7 | 2.6% |
| CKD | yes | n % | 37 | 13.9% |
| Cancer | yes | n % | 59 | 21.7% |
| Endocrinopathies | yes | n % | 24 | 8.8% |
| SMA | cm2 | mean sd | 89.1 | 34.4 |
| Sarcopenia | yes | n % | 113 | 41.5% |
| Steatosis | yes | n % | 182 | 66.9% |
| LOS | days | mean sd | 26.7 | 21.1 |
| ICU admission | yes | n % | 77 | 28.3 |
| Death | yes | n % | 54 | 19.9 |
Abbreviations: M: male, n: number, SD: standard deviation, IQR: interquartile range, PCR: c-reactive protein, COPD: chronic obstructive pulmonary disease, CKD: Chronic Kidney Disease, SMA: skeletal muscle area, LOS: length of stay, ICU: intensive care unit.
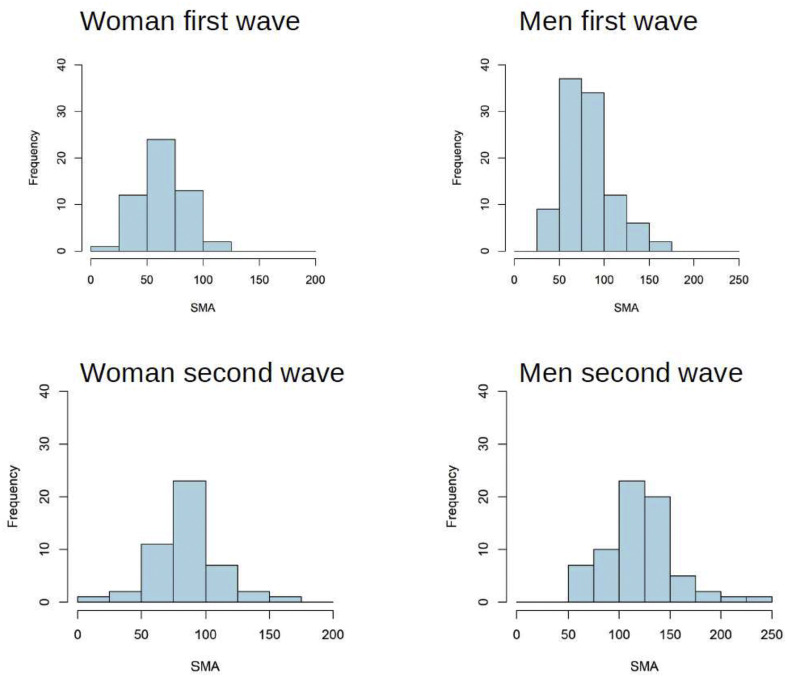
During two different epidemic waves, in particular from February 2020 to May 2020 (first wave) and from September 2020 to January 2021 (second wave), we observed a different prevalence rate of sarcopenia among patients: 57.9% vs 21.6%, respectively (p < 0.0000). Conversely, the prevalence of steatosis was similar (73.5% vs 73.3%) in both groups (Table 2 ). In both genders, SMA values during the second wave showed a different pattern of distribution characterized by a greater dispersion (Fig. 3 ).
Table 2.
Comparison of general characteristics in first wave and second wave. Missing values were excluded from calculations.
| 1-st wave |
2-nd wave |
p-value | |||||
|---|---|---|---|---|---|---|---|
| N = 155 | N = 117 | ||||||
| Gender | M | n % | 101 | 65.2% | 70 | 59.8% | 0.3779 |
| Age | years | median IQR | 70.0 | 61–78 | 71.0 | 63–78 | 0.3436 |
| Albumin | g/dl | mean sd | 3.2 | 0.5 | 3.4 | 0.5 | 0.0024 |
| PCR | mg/dl | mean sd | 10.2 | 9.4 | 8.9 | 9.2 | 0.1951 |
| Heart diseases | yes | n % | 35 | 22.7% | 37 | 33.0% | 0.0699 |
| Hypertension | yes | n % | 87 | 56.5% | 70 | 62.5% | 0.3770 |
| Cerebrovascular diseases | yes | n % | 14 | 9.1% | 7 | 6.3% | 0.4925 |
| Diabetes | yes | n % | 31 | 20.1% | 30 | 26.8% | 0.2377 |
| Dyslipidemia | yes | n % | 42 | 27.3% | 28 | 25.0% | 0.7781 |
| COPD | yes | n % | 19 | 12.3% | 12 | 10.7% | 0.8469 |
| Asthma | yes | n % | 4 | 2.6% | 3 | 2.7% | 1.0000 |
| CKD | yes | n % | 21 | 13.6% | 16 | 14.3% | 1.0000 |
| Cancer | yes | n % | 24 | 15.5% | 35 | 29.9% | 0.0049 |
| Endocrinopathies | yes | n % | 13 | 8.4% | 11 | 9.4% | 0.8306 |
| SMA | cm2 | mean sd | 75.0 | 26.4 | 107.6 | 35.1 | 0.0000 |
| Sarcopenia | yes | n % | 88 | 57.9% | 25 | 21.6% | 0.0000 |
| Steatosis | yes | n % | 97 | 73.5% | 85 | 73.3% | 1.0000 |
| LOS | days | mean sd | 25.5 | 19.5 | 28.4 | 23.1 | 0.3033 |
| ICU admission | yes | n % | 39 | 25.2% | 38 | 32.5% | 0.2212 |
| Death | yes | n % | 23 | 14.8% | 31 | 26.5% | 0.0211 |
| ICU admission + Death | yes | n % | 54 | 34.8% | 54 | 46.2% | 0.6193 |
Abbreviations: M: male, n: number, SD: standard deviation, IQR: interquartile range, PCR: c-reactive protein, COPD: chronic obstructive pulmonary disease, CKD: Chronic Kidney Disease, SMA: skeletal muscle area, LOS: length of stay, ICU: intensive care unit.
Bold characters are used to stressed significant data.
Fig. 3.
Different distribution of SMA values for men and women in the first and second wave.
Mean albumin concentration differed between the two groups (3.2 g/dl ± 0.5 vs 3.4 g/dl ± 0.5 p = 0.0024). We registered a different mortality between first wave group and second wave group: 14.8% vs 26.5%, respectively (p < 0.0211). Other clinical characteristics were well balanced between the two groups of patients as shown in Table 2.
In sarcopenic patients subgroup, mean albumin concentration was 3.1 ± 0.5 g/dl during the first wave and 3.4 ± 0.4 g/dl during the second wave (p < 0.0033); mean PCR was 12.2 ± 9.9 mg/dl during the first wave and 6.9 ± 7.4 mg/dl during the second wave (p < 0.0071) (Table 3 ). Additionally, we observed a different prevalence of steatosis between the two waves (69.7% vs 48%) (Table 3).
Table 3.
Comparison of general characteristics of sarcopenic patients during first and second wave: missing values were excluded from calculations.
| Sarcopenic patients |
|||||||
|---|---|---|---|---|---|---|---|
| 1-st wave n = 88 | 2-nd wave n = 25 | p-value | |||||
| Gender | M | n % | 68 | 77.3% | 12 | 48.0% | 0.0066 |
| Age | years | median IQR | 71.0 | 62–78 | 73.0 | 63–84 | 0.3597 |
| Albumin | g/dl | mean sd | 3.1 | 0.5 | 3.4 | 0.4 | 0.0033 |
| PCR | mg/dl | mean sd | 12.2 | 9.9 | 6.9 | 7.4 | 0.0071 |
| Heart diseases | yes | n % | 19 | 21.6% | 9 | 36.0% | 0.1885 |
| Hypertension | yes | n % | 50 | 56.8% | 15 | 60.0% | 0.8223 |
| Cerebrovascular diseases | yes | n % | 11 | 12.5% | 3 | 12.0% | 1.0000 |
| Diabetes | yes | n % | 18 | 20.5% | 5 | 20.0% | 1.0000 |
| Dyslipidemia | yes | n % | 29 | 33.0% | 3 | 12.0% | 0.0459 |
| COPD | yes | n % | 11 | 12.5% | 2 | 8.0% | 0.7296 |
| Asthma | yes | n % | 2 | 2.3% | 1 | 4.0% | 0.5313 |
| CKD | yes | n % | 12 | 13.6% | 2 | 8.0% | 0.7316 |
| Cancer | yes | n % | 13 | 14.8% | 7 | 28.0% | 0.1433 |
| Endocrinopathies | yes | n % | 5 | 5.7% | 3 | 12.0% | 0.3721 |
| LOS | days | mean sd | 26.8 | 18.5 | 26.6 | 20.5 | 0.8409 |
| SMA | cm2 | mean sd | 62.3 | 17.5 | 67.8 | 16.7 | 0.1899 |
| Steatosis | yes | n % | 53 | 69.7% | 12 | 48.0% | 0.0577 |
Abbreviations: M: male, n: number, SD: standard deviation, IQR: interquartile range, PCR: c-reactive protein, COPD: chronic obstructive pulmonary disease, CKD: Chronic Kidney Disease, SMA: skeletal muscle area, LOS: length of stay.
Bold characters are used to stressed significant data.
3.2. Role of sarcopenia
Our analysis was aimed at searching for clinical and anthropometric prognostic parameters. We evaluated the prognostic impact of body composition and steatosis, finding a significant association between sarcopenia and poor clinical outcome during first wave (HR 2.29; 95% CI 1.22 to 4.30, p < 0.0101). Following adjustment for age, gender and comorbidities covariates, the multivariate analysis confirmed sarcopenia as the only independent prognostic factor in terms of adverse clinical outcome during the first wave (HR 2.29, 95% CI 1.17 to 4.49 p = 0.0162) (Table 4 ). Conversely, no association was found between steatosis or sarcopenia and clinical outcome during the second wave.
Table 4.
Univariate and Multivariate analysis for the risk of adverse clinical outcomes.
| Univariate |
Multivariate |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | ||||
| Sarcopenia at 1-st wave | yes vs no | 2.29 | 1.22 | 4.30 | 0.0101 | 2.29 | 1.17 | 4.49 | 0.0162 |
| Sarcopenia at 2-nd wave | yes vs no | 0.74 | 0.35 | 1.57 | 0.4312 | 0.97 | 0.44 | 2.12 | 0.9345 |
| Steatosis at 1-st wave | yes vs no | 1.60 | 0.81 | 3.16 | 0.1741 | 1.64 | 0.82 | 3.29 | 0.1654 |
| Steatosis at 2-nd wave | yes vs no | 0.80 | 0.42 | 1.56 | 0.5188 | 0.74 | 0.37 | 1.49 | 0.4020 |
| Age | +1 year | – | – | – | – | 0.99 | 0.97 | 1.00 | 0.0916 |
| Sex | M vs F | – | – | – | – | 1.75 | 1.05 | 2.90 | 0.0320 |
| Heart diseases | yes vs no | – | – | – | – | 0.96 | 0.56 | 1.63 | 0.8727 |
| Hypertension | yes vs no | – | – | – | – | 1.38 | 0.86 | 2.20 | 0.1845 |
| Cerebrovascular diseases | yes vs no | – | – | – | – | 1.30 | 0.77 | 2.19 | 0.3243 |
| Diabetes | yes vs no | – | – | – | – | 1.62 | 1.00 | 2.63 | 0.0491 |
| Dyslipidemia | yes vs no | – | – | – | – | 0.97 | 0.44 | 2.16 | 0.9454 |
| COPD | yes vs no | – | – | – | – | 0.52 | 0.23 | 1.19 | 0.1205 |
| Asthma | yes vs no | – | – | – | – | 1.58 | 0.35 | 7.25 | 0.5539 |
| CKD | yes vs no | – | – | – | – | 1.15 | 0.63 | 2.13 | 0.6449 |
| Cancer | yes vs no | – | – | – | – | 1.43 | 0.86 | 2.37 | 0.1711 |
| Endocrinopathies | yes vs no | – | – | – | – | 0.55 | 0.23 | 1.33 | 0.1873 |
Abbreviations: HR: hazard ratio, CI: confidence interval, COPD: chronic obstructive pulmonary disease, CKD: Chronic Kidney Disease.
Bold characters are used to stressed significant data.
3.3. Pneumonia radiological characteristics
There were no significant differences in COVID19 severity in terms of pulmonary involvement at lung CT comparing sarcopenic vs not sarcopenic patients, either during the first and second wave. In 76% of patients in the first wave and 71% in the second wave, lung involvement was >3 lobes, indicating that the extension of lung impairment is a characteristic of COVID-19 pneumonia in both group. Carefully analyzing the lung involvement, we found common characteristics in both waves, such as a very low percentage of cases with an exclusive involvement of the central lung. On the other hand, an exclusive and typical peripheral lung involvement was present in first and second wave (32% and 29%, respectively). In light of these reasons, COVID-19 severity was not included in the multivariate analysis for risk of adverse clinical outcome. Mixed pattern with peripheral and central involvement was found to be dominant in both groups.
In relation to the type of pulmonary alteration at CT scan, the predominant aspect was that of ground glass (53%) opacities in patients of the first wave, whereas ground glass opacities associated to consolidating components (39%) or exclusive pulmonary alteration (33%) in patients of the second wave (Table 5 ).
Table 5.
Comparison of radiological aspects of pneumonia in first and second wave: missing values were excluded from calculations.
| 1-st wave |
2-nd wave |
p-value | |||||
|---|---|---|---|---|---|---|---|
| N = 155 | N = 117 | ||||||
| Lobes | 1 | n % | 113.0 | 76.4% | 83.0 | 70.9% | 0.3276 |
| Lung consolidation | C | n % | 16.0 | 12.3% | 14.0 | 14.3% | 0.000 |
| C-G | n % | 45.0 | 34.6% | 7.0 | 7.1% | ||
| G | n % | 69.0 | 53.1% | 39.0 | 39.8% | ||
| G-C | n % | 0.0 | 0.0% | 38.0 | 38.8% | ||
| Distribution | C | n % | 4.0 | 3.1% | 1.0 | 1.0% | 0.000 |
| C-P | n % | 80.0 | 61.5% | 37.0 | 37.8% | ||
| P | n % | 45.0 | 34.6% | 34.0 | 34.7% | ||
| P-C | n % | 1.0 | 0.8% | 26.0 | 26.5% | ||
Abbreviations: C: central, G: ground glass, P: peripheral; 1 = ≥3 lobes.
Bold characters are used to stressed significant data.
Sarcopenic patients with an extended pulmonary involvement (≥3 lobes) were 80% in the first wave and 56% in the second wave (Table 6 ).
Table 6.
Comparison of radiological characteristics of pneumonia in sarcopenic patients in first and second wave: missing values were excluded from calculations.
| Sarcopenic patients |
p-value | ||||||
|---|---|---|---|---|---|---|---|
| 1-st wave | 2-nd wave | ||||||
| Lobes | 1 | n % | 68 | 80.0% | 14 | 56.0% | 0.0205 |
| Lung consolidation | C | n % | 10 | 12.8% | 4 | 20.0% | 0.0001 |
| C-G | n % | 29 | 37.2% | 2 | 10.0% | ||
| G | n % | 39 | 50.0% | 9 | 45.5% | ||
| G-C | n % | 0 | 0.0% | 5 | 25.0% | ||
| Distribution | C | n % | 1 | 1.3% | 0 | 0.0% | 0.0019 |
| C-P | n % | 49 | 62.8% | 8 | 40.0% | ||
| P | n % | 28 | 35.9% | 8 | 40.0% | ||
| P-C | n % | 0 | 0.0% | 4 | 20.0% | ||
Abbreviations: C: central, G: ground glass, P: peripheral; 1 = ≥3 lobes.
Bold characters are used to stressed significant data.
4. Discussion
Based on empirical data, some authors suggested that patients with sarcopenia have increased infection rates and poor prognosis during the current 2019 novel coronavirus disease epidemic [19]. Recently, the association between sarcopenia and adverse clinical outcomes in COVID-19 has been investigated in small observational studies that examined CT-defined sarcopenia in patients with SARS-CoV-2 infection: sarcopenia was found to be associated with prolonged hospital stay [22] and higher mortality [23,26]. The aim of our retrospective observational study was to investigate the prevalence of sarcopenia and the potential relationship between sarcopenic state and clinical outcome in hospitalized patients with coronavirus disease.
In our study, the prevalence of sarcopenia was high (41.5%) but significantly different in each epidemic wave (57.9% vs 21.6%); different health policies could partially explain this data. During the first wave of the pandemic, health authorities suggested to not test but only home quarantine for 14 days individuals with mild to moderate symptoms possibly related to SARS-CoV-2 infection. In this initial phase of the emergency, COVID-19 patients showed compromised general conditions (fever, pneumonia, anorexia, catabolism) at the moment of hospital admission, probably due to several days of disease and home isolation, which are considered risk factors for sarcopenia [20,21]. After the first wave of the pandemic, the structure of the health system underwent significant changes to try to stem a second wave: total number of ICU beds increased, primary care doctors were directly involved in the initial management of COVID-19 patients and an earlier hospitalization was promoted.
In our study, sarcopenia was identified as an independent negative prognostic factor (HR 2.29, 95% CI 1.17 to 4.49 p = 0.0162) only during the first epidemic wave. Conversely, no relationship was found between sarcopenia and poor clinical outcome during the second wave. These findings suggest that body composition might have an important role in predicting clinical outcome of COVID-19 patients.
Overall, 73.4% of patients presented steatosis at CT analysis, with similar incidence rates in the two waves (73.5% vs 73.3%). However, when taking into account only the sarcopenic populations in the first and second waves, the percentage of patients with steatosis was 69.7% and 48% respectively, indicating a higher incidence of hepatic steatosis in sarcopenic patients during the first wave compared to the second one.
The results of our study highlight a different risk profile between patients of the first and second wave and a strong association between sarcopenia and steatosis in the first wave. Notably, sarcopenia and steatosis are metabolic factors associated with chronic inflammatory state and malnutrition that can condition the immune response to systemic therapies and pathologies, as some authors suggested [19].
In our study, sarcopenia emerged as a significant risk factor for predisposition to COVID-19 pneumonia during the first wave, but not in the second one, indicating a different setting of patients. This relationship is not highlighted in the literature and needs confirmation in larger populations with different characteristics.
Regarding radiological characteristics and severity of COVID-19 pneumonia in the two groups, we found patterns in line with the literature: extensive lung involvement, prevalence of ground glass opacities and predominantly peripheral parenchymal involvement [25,27,28].
Interestingly, no significant difference in the pattern of lung involvement could be supported by different timings in the execution of CT in relation to the clinical course of the disease. The only relevant element is that 80% of sarcopenic patients in the first wave had a more extensive pulmonary involvement versus 56% of patients in the second wave. Sarcopenia, steatosis and extensive pulmonary involvement appeared to be mainly associated during the first wave.
In our study, COVID-19 patients showed different metabolic and pulmonary features; sarcopenia was more frequently associated with steatosis, inflammation, lower albumin and extensive lung involvement during the first wave than the second one.
Our analysis has several limitations. Firstly, the retrospective design of the study. Secondly, patients without available CT scans were excluded from our analysis, leading to a possible selection bias. Moreover, a comprehensive report of the relation between body composition parameters and adverse outcome was not available due to limited medical records about muscle strength, prealbumin level, body mass index, body weight and height, likely due to the emergency situation and the critical clinical condition of patients. These limitations prevent the functional diagnosis of sarcopenia [3]. In our retrospective study, the diagnosis of sarcopenia relied only on CT findings of 12th vertebra skeletal muscle cross-sectional areas and no muscle function evaluation (handgrip strength) was performed. Some data suggest that T12 SMA permits the diagnosis of low muscle mass and could be used to correlate sarcopenia with outcome of patients undergoing CT scans limited to the chest [14,15]. These findings definitely need to be confirmed in larger prospective studies, since the validation of T12 SMA as parameter to assess low muscle quantity or quality and to confirm the diagnosis of sarcopenia could be a useful tool in the clinical practice when abdominal CT is not available.
5. Conclusions
As reported in other different clinical settings, we highlight the prognostic impact of sarcopenia in COVID-19 patients hospitalized during the first wave of the pandemic. However, the role of sarcopenia in COVID-19 patients deserve further confirmation in larger prospective studies. T12 SMA might represent a potential tool to identify sarcopenic patients in particular settings.
Author contributions
Conception and design: Menozzi R, Valoriani F, Pecchi AR, D'amico R and Banchelli F. Analysis and interpretation of data: all authors. Statistical analysis: D'amico R and Banchelli F. Final approval of manuscript: all authors. Supervision: Menozzi R, Pecchi AR, Pantaleoni M.
Additional contributions
We are grateful to all the employees of the University Hospital of Modena, for their courageous efforts in struggling against the clinical and social COVID-19 emergency.
Funding statement
This research received no external funding.
Institutional review board statement
The Ethical Review Board of each Institutional Hospital approved the present study. This study was performed in line with the principles of the Declaration of Helsinki.
Data availability statement
Data available on request from the authors.
Declaration of competing interest
None of the authors have conflicts of interest to disclose.
List of abbreviations
- COVID-19
2019 novel coronavirus disease
- SARS-CoV-2
acute respiratory syndrome coronavirus 2
- L3
third lumbar vertebra
- CT
computed tomography
- SMA
skeletal muscle area
- T12
12th thoracic vertebra
- ICU
intensive care unit
- CRP
C-reactive protein
- D12
12th dorsal vertebra
- HU
Hounsfield Unit
- SD
standard deviation
- IQR
interquartile range
- HR
hazard ratio
- M
male
- n
number
- COPD
chronic obstructive pulmonary disease
- CKD
Chronic Kidney Disease
- LOS
length of stay
- C
central
- G
ground glass
- P
peripheral
References
- 1.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cava Edda, Neri Barbara, Carbonelli Maria Grazia, Riso Sergio, Carbone Salvatore. Obesity pandemic during COVID-19 outbreak: narrative review and future considerations. Clin Nutr. 2021;40:1637–1643. doi: 10.1016/j.clnu.2021.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okazaki T., Ebihara S., Mori T., Izumi S., Ebihara T. Association between sarcopenia and pneumonia in older people. Geriatr Gerontol Int. 2020;20:7–13. doi: 10.1111/ggi.13839. [DOI] [PubMed] [Google Scholar]
- 5.Ohara D.G., Pegorari M.S., Oliveira Dos Santos N.L., de Fatima Ribeiro Silva C., Oliveira M.S.R., Matos A.P., et al. Cross-sectional study on the association between pulmonary function and sarcopenia in Brazilian community-dwelling elderly from the Amazon region. J Nutr Health Aging. 2020;24:181–187. doi: 10.1007/s12603-019-1290-y. [DOI] [PubMed] [Google Scholar]
- 6.Nelke C., Dziewas R., Minnerup J., Meuth S.G., Ruck T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EbioMedicine. 2019;49:381–388. doi: 10.1016/j.ebiom.2019.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lutz C.T., Quinn L.S. Sarcopenia, obesity, and natural killer cell immune senescence in aging: altered cytokine levels as a common mechanism. Aging (Albany NY) 2012;4:535–546. doi: 10.18632/aging.100482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altuna-Venegas S., Aliaga-Vega R., Maguina J.L., Parodi J.F., Runzer-Colmenares F.M. Risk of community-acquired pneumonia in older adults with sarcopenia of a hospital from Callao, Peru 2010-2015. Arch Gerontol Geriatr. 2019;82:100–105. doi: 10.1016/j.archger.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sepúlveda-Loyola Walter, Osadnik Christian, Phu Steven, Morita Andrea A., Duque Gustavo, Probst Vanessa S. Diagnosis, prevalence, and clinical impact of sarcopenia in COPD: a systematic review and metaanalysis. J Cachexia Sarcopenia Muscle. 2020;11:1164–1176. doi: 10.1002/jcsm.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vugt J., et al. Systematic review and meta-analysis of the impact of computed tomography–assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. Am J Transplant. 2016;16:2277–2292. doi: 10.1111/ajt.13732. [DOI] [PubMed] [Google Scholar]
- 11.Wang P.Y., Xu L.D., Chen X.K., Xu L., Yu Y.K., Zhang R.X., et al. Sarcopenia and short-term outcomes after esophagectomy: a meta-analysis. Ann Surg Oncol. 2020;27:3041–3051. doi: 10.1245/s10434-020-08236-9. [DOI] [PubMed] [Google Scholar]
- 12.Feliciano E.M.C., Kroenke C.H., Meyerhardt J.A., Prado C.M., Bradshaw P.T., Kwan M.L., et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS study. JAMA Oncol. 2017;3 doi: 10.1001/jamaoncol.2017.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pamoukdjian Frédéric, Bouillet Thierry, Vincent Lévy, Soussan Michael, Zelek Laurent, Elena Paillaud. Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: a systematic review. Clin Nutr. 2018 Aug;37(4):1101–1113. doi: 10.1016/j.clnu.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Gariballa S., Alessa A. Sarcopenia: prevalence and prognostic significance in hospitalized patients. Clin Nutr. 2013;32:772–776. doi: 10.1016/j.clnu.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Nemec Ursula, Heidinger Benedikt, Sokas Claire, Chu Louis, Eisenberg Ronald L. Diagnosing sarcopenia on thoracic computed tomography: quantitative assessment of skeletal muscle mass in patients undergoing transcatheter aortic valve replacement. Acad Radiol. 2017 Sep;24(9):1154–1161. doi: 10.1016/j.acra.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Derstine Brian A., Holcombe Sven A., Ross Brian E., Wang Nicholas C., Su Grace L., Wang Stewart C. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci Rep. 2018;8:11369. doi: 10.1038/s41598-018-29825-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Lusignan S., Dorward J., Correa A., Jones N., Akinyemi O., Amirthalingam G., et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020;20:1034–1042. doi: 10.1016/S1473-3099(20)30371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Pei-yu, Yin Li, Wang Qin. Sarcopenia: an underlying treatment target during the COVID-19 pandemic. Nutrition. 2021;84:111104. doi: 10.1016/j.nut.2020.111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morley J.E., Kalantar-Zadeh K., Anker S.D. COVID-19: a major cause of cachexia and sarcopenia? J Cachexia Sarcopenia Muscle. 2020;11:863–865. doi: 10.1002/jcsm.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akseer N., Kandru G., Keats E.C., Bhutta Z.A. COVID-19 pandemic and mitigation strategies: implications for maternal and child health and nutrition. Am J Clin Nutr. 2020;112:251–256. doi: 10.1093/ajcn/nqaa171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J.W., Yoon J.S., Kim E.J., Hong H.L., Kwon H.H., Jung C.Y., et al. Prognostic implication of baseline sarcopenia for length of hospital stay and survival in patients with coronavirus disease 2019. J Gerontol A Biol Sci Med Sci. 2021 Jul 13;76(8):e110–e116. doi: 10.1093/gerona/glab085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGovern J., Dolan R., Richards C., Laird B.J., McMillan D.C., Maguire D. Relation between body composition, systemic inflammatory response, and clinical outcomes in patients admitted to an urban teaching hospital with COVID-19. J Nutr. 2021 Jun 3:nxab142. doi: 10.1093/jn/nxab142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwasaki M., Takada Y., Hayashi M., Minamiguchi S., Haga H., Maetani Y., et al. Noninvasive evaluation of graft steatosis in living donor liver transplantation. Transplantation. 2004 Nov 27;78(10):1501–1505. doi: 10.1097/01.tp.0000140499.23683.0d. [DOI] [PubMed] [Google Scholar]
- 25.Kwee T.C., Kwee R.M. Chest CT in COVID-19: what the radiologist needs to know. Radiographics. 2020 Nov-Dec;40(7):1848–1865. doi: 10.1148/rg.2020200159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ufuk F., Demirci M., Sagtas E., Akbudak I.H., Ugurlu E., Sari T. The prognostic value of pneumonia severity score and pectoralis muscle Area on chest CT in adult COVID-19 patients. Eur J Radiol. 2020 Oct;131:109271. doi: 10.1016/j.ejrad.2020.109271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufman A.E., Naidu S., Ramachandran S., Kaufman D.S., Fayad Z.A., Mani V. Review of radiographic findings in COVID-19. World J Radiol. 2020 Aug 28;12(8):142–155. doi: 10.4329/wjr.v12.i8.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salehi S., Abedi A., Balakrishnan S., Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19) imaging reporting and data system (COVID-RADS) and common lexicon: a proposal based on the imaging data of 37 studies. Eur Radiol. 2020 Sep;30(9):4930–4942. doi: 10.1007/s00330-020-06863-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.