Abstract
Background
SARS-CoV-2 vaccine has been recommended to pregnant women, but survey studies showed contrasting findings worldwide in relation to the willingness to accept vaccination during pregnancy.
Objective
To evaluate the evidence from the literature regarding the acceptance rate of the SARS-CoV-2 vaccine in pregnant and breastfeeding women.
Study design
We performed a systematic review on the main databases (MEDLINE (PubMed), Scopus, ISI Web of Science) searching for all the peer-reviewed survey studies analyzing the eventual acceptance rate of the SARS-CoV-2 vaccine among pregnant and breastfeeding women. To combine data meta-analyses of proportions and pooled proportions with their 95% confidence intervals (CI) were calculated.
Results
15 studies including 25,839 women were included in the analysis. The proportion of women actually willing to be vaccinated during pregnancy is 49.1% (95% CI, 42.3–56.0), and the proportion of breastfeeding women is 61.6% (95% CI, 50.0–75.0).
Conclusion
The cumulative SARS-CoV-2 vaccine acceptance rate among pregnant women appears still low. Vaccinal campaign are urgently needed to drive more confidence into the vaccine to help reducing the spread of the infection and the possible consequences during pregnancy.
Keywords: SARS-CoV-2, COVID-19 in pregnancy, Vaccine, Prevention, Infection during pregnancy
Introduction
It has been more than a year since SARS-CoV-2 pandemic was declared. At the time of writing >4 million and 400 thousand deaths have been registered worldwide. [1] Italy was the one of the first European countries to be severely hit by the spread of the pandemic. There, evidence and guidance on how to manage obstetrics and gynecology patients during this period have been soon released and taken as example by other nations [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]. Notably, pregnant women were soon considered a population at increased risk for complications and more severe COVID-19 course [16], [17], [18], [19], [20], [21], [22]. Very soon, it appeared as the vaccination could have been one of the most useful solutions to counteract the pandemic, but historically pregnant women have not been included into vaccine trials [23], and therefore uncertainty about its safety in this specific population opened a debate on the need to administer SARS-CoV-2 vaccine during pregnancy [24], [25], [26], [27], [28]; indeed, counseling becomes of striking value in such a context [29], [30], [31], [32], [33]. National and international societies endorsed this suggestion, initially considering the high-risk pregnancies and working as health practitioners as the main indications to the vaccine, as well as the need to include pregnant women into future vaccine trials, and then stating that pregnancy should not be considered a contraindication to the vaccine and that pregnant women are “de facto” a population at risk [34], [35], [36], [37], [38], [39], [40], [41], [42]. Preliminary data seem to reassure regarding safety issues and immunization properties of SARS-CoV-2 vaccines in pregnant women, demonstrating also that neonates born from vaccinated mothers possess antibodies against SARS-CoV-2 [43], [44], [45].
In this scenario, it is still unclear whether women are really likely to request vaccination during pregnancy. For this reason, we previously conducted two surveys in two Italian teaching hospitals to evaluate the willingness of women to undergo the SARS-CoV-2 vaccine, with contrasting findings [46], [47]. In light of the increasing number of surveys on the matter, the aim of this systematic review was to elucidate what pregnant women worldwide really think about the chance to receive the vaccine against COVID-19.
Materials and methods
Search strategy
We conducted a systematic search using the MEDLINE (PubMed), Scopus, ISI Web of Science databases to identify all relevant studies published before 22 August 2021. Combinations of the following keywords and MESH search terms were used: (“vaccine” OR “vaccination”) AND (“SARS-CoV-2” OR “COVID-19” OR “coronavirus”) AND (“pregnancy” OR “pregnant” OR “pregnant women” OR “during pregnancy” AND (“acceptance” OR “hesitancy” OR “belief” OR “perspectives” OR “willingness”) AND (“survey”). Search strategy was limited to only English studies. The reference lists of relevant reviews and articles were also hand-searched to complement database search. We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [48], and provide its checklist (Supplementary material 1).
Selection of studies
Only studies reporting the willingness of pregnant women to receive anti-SARS-CoV-2 vaccine on a survey basis were included. We excluded studies on vaccines other than SARS-COV-2. We also excluded studies reporting the rate of currently SARS-CoV-2-vaccinated women in pregnancy. Two reviewers (LC, RDG) independently evaluated titles and abstracts. Duplications were removed using Endnote online software and also manually. Disagreements were resolved by discussion among authors, and if required, with the involvement of the most experienced authors (GR, GMM). Only studies published in peer-reviewed journals were evaluated.
Data extraction
Data were extracted independently by two reviewers (RDG, VDV) using predefined data fields, and study quality indicators. In detail, we developed a data extraction sheet based on the Cochrane data extraction template for non RCTs (https://dplp.cochrane.org/data-extraction-forms).
Quality assessment
The risk of bias and quality assessment of the included studies were performed using the Quality Assessment Checklist for Survey Studies in Psychology (Q-SSP) [49]. This checklist is divided into 4 domains (Introduction: Rationale/Variables – 4 items; Participants: Sampling/Recruitment – 3 items; Data: Collection/Analyses/Measures/Results/Discussion – 10 items; Ethics – 3 items). Two authors (IM, GS) independently assessed the risk of bias for each study. If for >70% of items a YES response has been found, the study is considered of acceptable quality, otherwise questionable.
Outcomes
The primary outcome was the evaluation of SARS-CoV-2 vaccine acceptance rate in pregnant or breastfeeding women. The main reasons declared as determinants for vaccine acceptance/refusal were also described.
Statistical analysis
Prevalence of SARS-CoV-2 vaccine acceptance was calculated by the total number of women accepting to be vaccinated in that whole population group, then considering the number of women accepting to be vaccinated in pregnant and breastfeeding groups alone, respectively. Prevalence was calculated for each included study and as pooled estimate, and graphically reported on forest plots with 95% confidence interval (CI). Statistical heterogeneity among studies was assessed by the inconsistency index I2. Heterogeneity was categorized as: null for I2 = 0%, minimal for I2 < 25%, low for I2 < 50%, moderate for I2 < 75% and high for I2 ≥ 75%. The association between the prevalence of socio-demographic and medical features and SARS-CoV-2 vaccine acceptance was assessed using odds ratio (OR) with 95% confidence interval (CI). In details, advanced maternal age, Caucasian ethnicity, medium–high education, occupation, health status features and trimesters of pregnancy were analyzed comparing with non-variables as reference groups. P value < 0.05 was considered significant. The random effect model of DerSimonian and Laird was adopted for all analyses. Egger's test was used to assess potential publication bias and funnel plots were created for visual inspection. Tests for funnel plot asymmetry were not used when the total number of publications included for each outcome was <10, as the tests lack power to detect real asymmetry in this case. The analysis was performed using Stats direct 3.0.171 (Stats Direct Ltd) and Revman 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) statistical software.
Results
Study characteristics and quality assessment
Initially, 232 articles were identified; of these, 84 articles were duplications and thus removed. The titles and abstracts of 148 articles were scrutinized and ultimately 32 were selected for full text retrieval and eligibility assessment. Finally, 15 articles [46], [47], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62] were included in the quantitative and qualitative analyses (Fig. 1 ). Two of them were multicenter international surveys [50], [59], two came from Italy [46], [47], two from Ethiopia [53], [56] and three from USA [54], [57], [61]. In relation to the period of analysis, Ceulemans et al. [50] were the first ones to ask for vaccine acceptance among pregnant women, followed by Skjefte et al. [59], Mohan et al. [55], Tao et al. [62] and Mappa et al. [47], during a period in which there was still no vaccine or it was not yet widely proposed to pregnant women (Table 1 ). The study by Stukelberger et al. [60] is a part of Ceulemans et al. [50], but it was included in the analysis since it was not possible to extract data for secondary analysis from Ceulemans et al. [50]; however, in order not to duplicate the study population, we subtracted from the total cases of Ceulemans et al. [50] those of Stukelberger et al. [60].
Fig. 1.
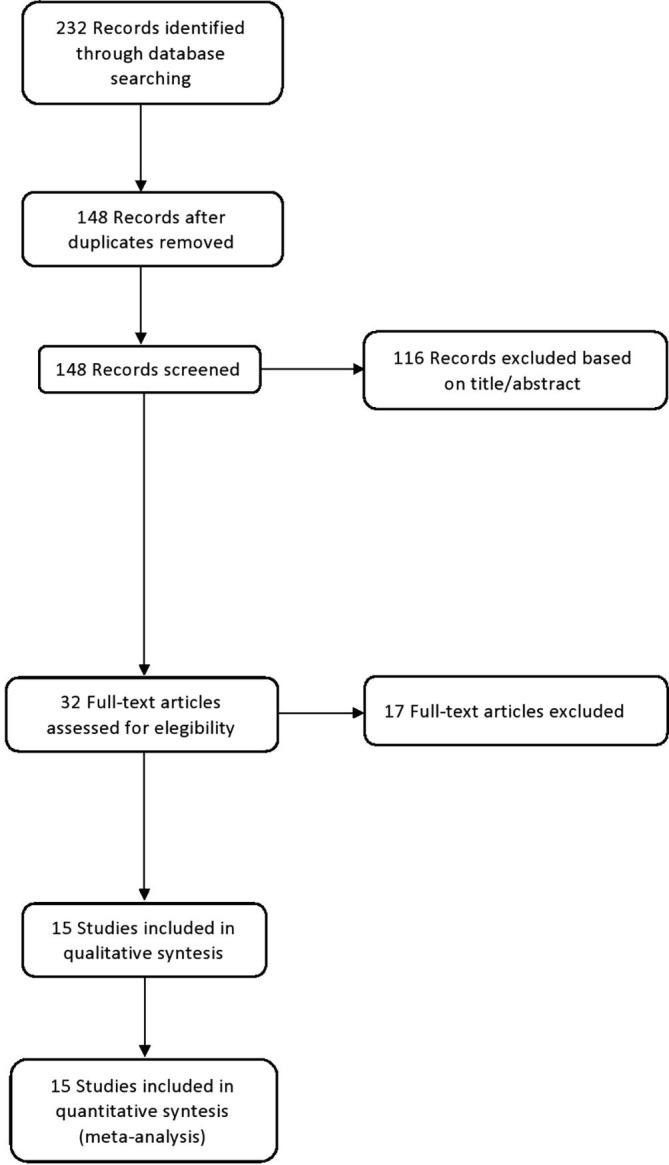
Flow-chart diagram of studies’ inclusion in the systematic review.
Table 1.
Features of included studies.
| Authors, Year | Study location | Study design | Sample size | Period considered | Inclusion criteria | Exclusion criteria | Intervention | Outcomes | Findings |
|---|---|---|---|---|---|---|---|---|---|
| Carbone et al., 202146 | Italy | Multicenter cross-sectional survey. | 142 (119 pregnant and 23 early post-partum period). | January 2021 | Pregnant women attending the two centers for outpatient visits, and early post-partum inpatient women who were asked to participate to a survey on the possible uptake of the SARS-CoV-2 vaccine during pregnancy and puerperium. | Inability to comprehend the text and to sign the informed consent. | After signing an informed consent and reading the position paper ad interim on “Pregnancy and COVID-19 vaccine”, an anonymous online semi-structured questionnaire developed using google forms was administered; Part A (socio-cultural and demographic variables, past and current obstetrical history, maternal age and gestational age at the receipt of the questionnaire); Part B (women’s knowledge and concerns about vaccines). | Women were specifically asked if they were in favor or against the SARS-CoV-2 vaccine during pregnancy. Furthermore, questions were asked regarding general acceptance of vaccines, whether the acceptance of SARS-CoV-2 vaccine was dependent on the pregnant or breastfeeding status and if they would receive other vaccine recommended during pregnancy (referring to the trivalent DTPa and the influenza vaccines). Patients were grouped according to their response to the survey (acceptance or decline of the SARS- CoV-2 vaccine during pregnancy or breastfeeding). |
Most of the included women did not express their agreement to eventually receive SARS-CoV-2 vaccine during pregnancy (40, 28.2% vs 102, 71.8%). No statistically significant differences were found in relation to nationality, marital status, education, employment, smoke, pre-existing diseases, type of conception, pregnancy trimester at survey and pregnancy complications during current pregnancy, between women who would undergo SARS-CoV-2 vaccine in pregnancy and women who would not. Women who had a previous pregnancy (irrespectively from the outcome) and women still pregnant at survey time would preferably decline the SARS-CoV-2 vaccine in a statistically significant manner. |
| Ceulemans et al., 202150 | Ireland, Norway, Switzerland, the Netherlands, United Kingdom, Belgium. | Multinational, cross-sectional, web-based survey. | 16,063 (6661 pregnant and 9402 breast- feeding women). | Between June 16 and July 14, 2020, and between April 10 and May 31, 2020. | Pregnant and breastfeeding women up to 3 months postpartum (or up to 4 weeks after delivery in Belgium) and who were older than 18 years. | NS | Data collection occurred through a uniform, anonymous web survey. Women’s perceptions about the coronavirus and COVID-19 vaccine willingness were assessed by seven statements (five in the pregnancy and two in the breastfeeding survey), rated on a 4-point Likert scale ranging from “strongly agree” to “strongly disagree”. | Pregnant and breastfeeding women’s beliefs about the coronavirus and COVID-19 vaccine willingness in pregnancy and breastfeeding (for all countries) and on the self- reported impact of the pandemic on the four thematic areas of maternity care (for all countries except Belgium). | Primigravida pregnant women and breastfeeding women who delivered in the last 6 months were more likely to be willing to get a vaccine. In contrast, pregnant and breastfeeding women with low and medium levels of education and without employment were less in favor of COVID-19 vaccination. Finally, pregnant women working in healthcare were also less likely to be willing to get a vaccine compared to women employed outside healthcare. In the univariable analyses, pregnant women who had a positive test result for SARS- CoV-2 were more in favor of getting a COVID-19 vaccine in pregnancy compared to women without a positive test result, but the finding was not significant. A similar observation was noted among breastfeeding women. |
| Desai et al., 202157 | USA | Cross-sectional survey | 124 | Between February and March 2021. | Pregnant women of 18 years old or older. | NS | Anonymous survey | Patients were queried about their willingness to receive the COVID-19 vaccine before they were given any information. Data on demographic factors, including race, religion, education level, marital status, and prior vaccine history, were also collected. Patients were then asked to read a fact sheet about the safety of the COVID-19 vaccines in pregnant women and discussed the information with a provider. A comparison between the pre- and post- willingness to receive the COVID-19 vaccine was conducted. | Those who received the annual influenza vaccine were significantly more likely to get the COVID-19 vaccine (50% vs. 9.7%). Additionally, those who had previously discussed the COVID-19 vaccine with a physician were significantly more likely to receive the vaccine (45.8% vs. 26.0%). There was a statistically significant effect of discussing the vaccine with a healthcare provider and providing patients with a fact sheet regarding patients’ willingness to receive the COVID-19 vaccine. |
| Gheoghean et al., 202151 | Ireland | Single center cross sectional survey. | 300 | Between December 4, 2020 and January 14, 2021. | Participants were recruited both in person and via online platforms. Obstetricians and midwives distributed study information leaflets with a link to the survey to women attending for clinic appointments. A short video that explained the purpose of the research and provided a link to the survey appeared on the hospital’s Instagram and Twitter accounts. A member of the research team also recruited women in-person during OGTT. | NS | The survey instrument was developed and hosted online using Sawtooth software. Likelihood was measured on a 10-point scale where 1 represented “very unlikely” and 10 “very likely.” An open-ended question, “What do you think would most affect your choice about receiving or not receiving a COVID- 19 vaccine during pregnancy?” accompanied the scale question. Survey items included demographic information, obstetric factors, and prior vaccination experience. | Women rated their likelihood of receipt of a COVID-19 vaccine, when pregnant and when not pregnant, and their likelihood of receipt of routinely recommended vaccines in pregnancy. | 113 (38%) women rated likelihood of receipt of a COVID-19 vaccine during pregnancy as 8 or higher, while 108 (36%) respond with a score of ≤ 2. On the other hand, 63% of women rated their likelihood of receipt of a COVID-19 vaccine if not pregnant as ≥ 8 and 75% of women rated their likelihood of receipt of routine vaccines during pregnancy as ≥ 8. On bivariate analysis having a college degree, attending private or semi-private clinic, being aged 30–35 y, and gestational age > 31 weeks were associated with a score of ≥ 8. However, only later gestational age and being aged 30–35 y remained associated with increased likelihood of a COVID-19 vaccine receipt on multivariable logistic regression. |
| Goncu Ayan et al., 202152 | Turkey | single center cross-sectional survey. | 300 | Between January 1, 2021 and February 1, 2021. | Pregnant women who were seen for prenatal care. | NS | The face-to-face questionnaire contained 40 questions about socio-demographic features, vaccination history, perception of risk related to the COVID-19 pandemic, the impact of the COVID-19 pandemic, and acceptance of and attitude toward future COVID-19 vaccination. | NS | 111 (37%) stated their intent to receive the vaccine if it were recommended for pregnant women. 92 (30.7%) of the participants were regarded as experiencing high-risk pregnancies. We did not find a significant difference between high-risk and low-risk groups. Pregnant women who said they would refuse the vaccine stated their most important concerns as: (1) a lack of data about COVID-19 vaccine safety in the pregnant population, and (2) the possibility of harm to the fetus. When we compared first-trimester with second- and third-trimester pregnant women, women in their first trimester expressed greater interest in receiving the COVID-19 vaccination than others. |
| Levy et al., 202154 | USA | Single center cross sectional survey. | 662 | From December 14, 2020 to January 14, 2021. | ≥18 years old and English-speaking during their nuchal translucency or anatomic survey sonogram appointment. | NS | 31 questions regarding socio-demographics, vaccination history, previous COVID-19 symptoms and diagnoses, attitudes toward vaccines in pregnancy, and beliefs about the COVID-19 vaccination specifically. | Primary outcome was COVID-19 vaccine acceptance rate. Univariate analyses were performed to estimate the effect of different variables on acceptance of COVID-19 vaccination and are reported as OR with 95% CI. To better understand certain subpopulations’ vaccine acceptance rate, we performed crosswise analyses of a priori variables of interest (race, educational attainment, and influenza vaccine status). | Overall, 381/653 (58.3%) women would accept the COVID-19 vaccine while pregnant. Among the women who declined vaccination, the most common primary concern was risk to the fetus or neonate (45.8%), followed by vaccine side effects (17.7%). On univariate analyses, younger age, Black or African American race, Hispanic ethnicity, having less than a bachelor’s degree, and declining the seasonal influenza vaccine were associated with non-acceptance of COVID-19 vaccination in pregnancy. Trust in the information received about vaccinations was the strongest predictor of COVID-19 vaccination acceptance. On crosswise comparisons, educational status did not affect COVID-19 vaccine acceptance rate among Black or African American women; however, among White women, lower education was associated with lower odds of vaccine acceptance. Additionally, among those who decline influenza vaccination in pregnancy, no Black or African American women would accept COVID-19 vaccination, while 25.0% of White women would accept the vaccine. |
| Mappa et al., 202147 | Italy | Single center cross-sectional survey. | 161 | On December 27, 2020. | Women that have attended the antenatal clinic of Ospedale Cristo Re, Università di Roma Tor Vergata, Rome, Italy, in the last 2 weeks. | NS | Anonymous online semi-structured questionnaire was sent through emails the first day of starting SARS-CoV-2 vaccinations in Italy; the questionnaire is structured in two sections: part A, finalized to acquire in 16 items maternal characteristics and to test women’s knowledge and concerns about vaccines; part B, containing the STAI. To evaluate the maternal concern about perinatal complication induced by SARS-CoV-2 vaccine the following fears were also considered: fetal structural anomalies, growth anomalies and preterm birth. |
NS | 136 (84.5%) felt vaccination as a possible breakthrough in resolving the pandemic (vaccine positive) while the remaining 25 (25.5%) considered the vaccine not useful (vaccine negative). Among the former group, 72 women (52.9%) were favorable to obtain the vaccine during pregnancy, a percentage significantly higher when compared to the vaccine negative group (7; 28%). Further, women negative to SARS-CoV-2 vaccine showed a lower educational level and a higher prevalence of unemployment when compared to the vaccine positive group. No differences were found among the other parameters tested. No differences were found between groups in basal anxiety as expressed by the presence of STAI T scale values > 40, while there was significant higher prevalence of abnormal STAI S values in the group of women negative to vaccine. |
| Mohan et al., 202155 | Qatar | Single center cross-sectional survey. | 341 | From October 15, 2020 to November 15, 2020. | Pregnant or lactating women. | NS | Voluntary participation in the survey via an online link was made available to all the residents of Qatar via the HMC social media platforms. A composite questionnaire incorporating a validated vaccine hesitancy measurement tool called VAX was used to explore attitudes to vaccination, participants’ concerns around the vaccine and relevant background information. | Intentions to vaccinate: whether participant would accept vaccination or has degree of hesitancy. Influences determining vaccine hesitancy: a. Contextual factors influencing vaccine attitude: education level, ethnicity/cultural factors. b. Group or individual influences: previous vaccination choices, impact of available information and endorsements, general beliefs around immunization, trust in health systems and pharmaceutical companies. c. Vaccine specific issues: knowledge around COVID-19 pandemic and vaccine, concerns around the COVID-19 vaccine and perceived risks to health of children and adults. |
As many as a quarter of the respondents demonstrated vaccine hesitancy saying that they probably or definitely would not take it (25%) and another quarter (25.9%) remained unsure. Notably, Qatari respondents demonstrated much higher hesitancy rates at 75%. As many as 18.3% would probably or definitely not recommend the vaccine to family and 28.3% would not get their children vaccinated if a vaccine became available for them. When considering vaccination before travel, while 65.9% would consider taking the vaccine, over a third (34.1%) still remained unsure. 75% of Qatari women, 50% of Non-Qatari Arabic women, 9% of women from other Asian countries and 39% of women from Non-Asian countries would refuse a vaccine. A similar analysis found there was no significant effect of completing childhood vaccinations, chronic illness, past mental health problems, educational status or age on vaccine hesitancy. Of 154 respondents who usually accept influenza vaccination, 18 (11.7%) showed hesitancy towards COVID-19 vaccination. |
| Mose et al., 202156 | Ethiopia | Institutional-based cross-sectional survey. | 396 | From January 1 up to 30, 2021. | All pregnant mothers who were attending regular antenatal care follow-up at Gurage Zone public hospitals during data collection period were included. | Pregnant women who were critically ill during the study period, who had history of mental illness and hearing impairment, which were unable to provide the required information by themself. | The questionnaire contains: socio-demographic characteristic of the study respondents, knowledge, attitude, practice of the study respondents on COVID-19 preventive measures, and intention of COVID-19 vaccine acceptance among pregnant women. | COVID-19 vaccine acceptance: “Will you get vaccinated if you get COVID-19 vaccine?” those who respond “Yes” for this question were considered as vaccine acceptance and those pregnant women who respond “No” were considered as vaccine hesitancy. The respondents’ level of knowledge about COVID-19 was reported as good knowledge if the study participant correctly responded to more than or equal to 80% of knowledge assessment tools, and poor for < 80%. The attitude of the participants was categorized as positive or favorable if responded above or equal to 80% of the attitude related items and negative if below 80%. The respondents’ level of practice of COVID-19 preventive measures was reported as good practice if the study participant correctly responded to more than or equal to 80% of practice assessment tools, and poor for < 80%. |
The COVID-19 vaccine acceptance if it is available was found to be 70.7%. The reasons for refusal of accepting COVID-19 vaccine were due to fear of side effect, 54 (13.6%), the vaccine might be ineffective, 24 (6.1%), I used other methods of COVID-19 prevention, 20 (5.1%), the vaccine might turn into COVID-19, 15 (3.8%) and the vaccine might affect my fetuses, 3 (0.8%). The multivariate analysis showed that maternal age (34–41 years), maternal primary educational status, good knowledge, and good practice of pregnant women towards COVID-19 and its preventive measures were significantly associated with acceptance of the COVID-19 vaccine. The odds of acceptability of COVID-19 vaccine among pregnant mothers found between 34 and 41 years age group were nearly 1.5 times more likely than those pregnant mothers found in the age group between 18 and 24 years. Those pregnant mothers who had completed primary education were 3.5 times more likely to accept COVID-19 vaccine compared to pregnant mothers who had no formal education. Pregnant mothers who had good knowledge of COVID-19 and its preventive measures were approximately 6 times more likely to accept COVID-19 vaccine compared to those mothers who had poor knowledge. The odds of acceptability of COVID-19 vaccine among pregnant mothers who had good practice of COVID-19 preventive measures were 9 times more likely to accept COVID-19 vaccine compared to those mothers who had poor practice towards COVID-19 preventive measures. |
| Skjefte et al., 202159 | 16 countries (USA, India, Brazil, Russia, Spain, Argentina, Colombia, UK, Peru, Mexico, South Africa, Italy, Chile, the Philippines, Australia and New Zealand). | Multicenter cross-sectional survey. | 5294 | Between October 28 and November 18, 2020. | Women aged 18 years or older, currently pregnant or with at least one child under 18 years of age. | NS | Anonymous online survey, containing 63 questions divided into five sections, assessing COVID-19 vaccine acceptance and confidence, negative experiences with COVID-19, perception on the risk of COVID-19, public trust, general vaccine attitude, as well as demographics and socioeconomic status. Responses to multiple-choice questions measured agreement with 5- or 7-point Likert scales. Participants were allowed to skip any questions or withdraw from the survey at any time without penalty. No follow-up was conducted. | Covid-19 vaccine acceptance; attitudes towards vaccines; top reasons for COVID‐19 vaccine reluctance; predictors of COVID‐19 vaccine acceptance. | Among pregnant women, 52.0% (n = 2747) intended to receive COVID-19 vaccination during their pregnancy if an efficacy of 90% were achieved. Responses among pregnant women varied substantially by country (range: 28.8–84.4%). COVID-19 vaccine acceptance level was above 80% for pregnant women in Mexico and India; and below 45% for USA, Australia and Russia. Among non-pregnant women, 73.4% (n = 9214/12,562) intended to receive vaccination. The top three reasons for pregnant women to decline COVID-19 vaccination during pregnancy even if the vaccine were safe and free were that they did not want to expose their developing baby to any possible harmful side effects (65.9%), were concerned that approval of the vaccine would be rushed for political reasons (44.9%) and would like to see more safety and effectiveness data among pregnant women (48.8%). Health care providers had a limited impact: only 45.9% of pregnant women and 54.6% of non-pregnant women would be more likely to have themselves/children vaccinated if recommended by healthcare providers. A sensitivity analysis was conducted to see if there was any difference in vaccine acceptance within-country before and after November 9th, 2020, the day in which Pfizer-BioNTech announced news of the first COVID-19 vaccine efficacy results. No significant difference was found in vaccine acceptance outcomes from this test. Demographic factors, such as younger age, lower income, lower education level, non-married and no health insurance were slightly linked to COVID-19 vaccine non-acceptance. Strongest predictors of COVID-19 vaccine acceptance were confidence in COVID-19 vaccine safety and efficacy, belief in the importance of vaccines/mass vaccination to their own country, confidence in routine childhood vaccines, worried about COVID-19, trust of public health agencies/health science, as well as compliance to mask guidelines. These predictors were similar for pregnant and non-pregnant women, for self-vaccination and for child vaccination acceptance. The AUC was 0.84, 0.94 and 0.92 for the models of pregnant women, non-pregnant women self-vaccination and child vaccination acceptance, respectively. |
| Stuckelberger et al., 202169 | Switzerland | Part of the multicenter cross-sectional survey by Ceulemans et al. 2021.50 | 1551 (515 pregnant and 1036 breastfeeding women). | From June 18 to July 12, 2020. | Women needed to be at least 18 years old and be pregnant at the time of the survey or have breastfed within the past three months. | NS | Data collection occurred through a uniform, anonymous web survey. Women’s perceptions about the coronavirus and COVID-19 vaccine willingness were assessed by seven statements (five in the pregnancy and two in the breastfeeding survey), rated on a 4-point Likert scale ranging from “strongly agree” to “strongly disagree”. Information on vaccination practices was obtained through a dichotomic question on vaccination against influenza within the past year (yes or no) and multi-choice questions assessing their opinion on influenza vaccine usefulness during pregnancy and breastfeeding, the fear of maternal and fetal/neonatal side effects, and overall vaccination acceptance. | COVID-19 vaccine willingness of pregnant and breastfeeding women if a vaccine had been available. Information on sociodemographic characteristics (i.e., age, primary language, marital status, working status, education level), medical history (i.e., gravidity, parity, co-morbidities, smoking during pregnancy, main practitioner for the pregnancy follow-up, clinical course of the neonate for breastfeeding mothers), exposure to SARS- CoV-2 or presence in an at-risk setting (i.e., symptoms potentially related to COVID-19, hospitalization related to COVID-19, testing by RT-PCR, serology or computed tomography, living with someone who tested positive, co-habiting with an elderly person (>65 years old)). | Only 29.7% (153/515) of pregnant and 38.6% (400/1036) of breastfeeding women were willing to get vaccinated against SARS-CoV-2 if a vaccine had been available during the first wave. More specifically, 8.1% (127/1551) fully agreed, 27.5% (426/1551) somewhat agreed, 40.4% (626/1551) somewhat disagreed, and 24% (372/1551) fully disagreed. Potential predictors of SARS-CoV-2 vaccine acceptance. Sociodemographic factors such as a maternal age above 40 years old, an educational level higher than high school, and Italian as a primary language were associated with a higher rate of vaccine acceptance. On the other hand, German-speaking participants were less likely to get vaccinated. Having had the influenza vaccination in the past year was a positive predictor for SARS-CoV-2 vaccine acceptance. Women who usually declined influenza vaccination were less likely to be willing to get the SARS-CoV-2 vaccine. When assessing the impact of the SARS-CoV-2 pandemic, none of the variables showed statistically significant influence on the willingness to get vaccinated. However, a trend toward COVID-19 vaccine willingness can be observed among women having a positive diagnosis of SARS-CoV-2 and living with someone older than 65 years old. Among the pregnant participants, those who had an obstetrician following their pregnancy and who were in their third trimester of pregnancy were more likely to be willing to receive the SARS-CoV-2 vaccine. |
| Sutton et al., 202161 | USA | Single center cross-sectional survey. | 338 (216 pregnant and 122 breastfeeding women). | From January 7, 2021 to January 29, 2021. | Respondents were conveniently recruited through three primary sources with no restrictions to participation except that respondents were to be of the female sex. | NS | An anonymous web-based survey was created in RedCap©, a secure web-based application designed to support data capture for research studies, and a URL link created for respondents to complete the survey. | Respondents were asked their age, pregnancy status, breastfeeding status, race, ethnicity, chronic medical conditions, employment and their health care provider. On our 9th multiple choice question respondents were asked if they planned on taking the vaccine once it was available to them. Respondents who responded “yes” or “I have already been vaccinated” were classified under vaccine acceptance. The remainder of their survey inquired about factors associated with vaccine acceptance. Those who responded “no” were classified under vaccine declination and the remainder of their survey focused on factors associated with declination. Those who responded “unsure” were classified as undecided and answered all questions associated with vaccine acceptance and declination. All respondents were then queried on factors that would influence their decision to accept and decline the COVID-19 vaccine. Factors against vaccination included concerns of its effect on pregnancy, suffering side effects, permanent injury, infertility, and risk of infection with COVID-19 from the vaccine. Factors in favor or vaccination included fear of severe COVID-19 infection, fear of infecting others with COVID-19, current available data from vaccine trials, healthcare workers acceptance of vaccination, current employment in healthcare and fear of suffering severe illness due to their race and or ethnicity. | Breastfeeding respondents were the second most likely to accept vaccination with an overall acceptance rate of 55.2%, with 60 (49.2%) reporting plan to take the vaccine and 4 (3.3%) reporting already have received the vaccine. Pregnant respondents had the lowest percentage of responses indicating vaccine acceptance with an overall rate of 44.3%, with only 82 (38.0%) respondents planning to be vaccinated and 4 (1.9%) respondents who were already vaccinated. Additionally, pregnant respondents had the highest percentage responses indicating vaccine declination with 59 (27.3%) stating they did not plan on getting the vaccine. Breastfeeding respondents were the most likely to report indecision towards vaccination with 32 (26.2%) stating that they were “not sure” if they would accept or decline the vaccine compared to 49 (22.7%) of pregnant respondents and 91 (13.9%) of non-pregnant respondents. |
| Tao et al., 202162 | China | Multi-center hospital-based cross-sectional survey. | 1392 | From November 13 to 27, 2020 | 1) women aged 18 years or above; 2) pregnant women who attended antenatal clinics in the participating obstetric hospitals during 13 November 2020 to 27 November 2020; 3) voluntary agreement to participant in the present study. | NS | In the present study, knowledge toward COVID-19 infection consisted of 17 items, including source of infection, route of transmission, susceptible population, common symptoms, high-risk population for severe illness and death, individual preventive measures for COVID-19 infection. There were three possible responses (yes, no, or not sure). For each item, if correct answer was chosen, the respondent received 1 score. Wrong answer or responses “unknow” received zero score. The sum of the scores for all the 17 items was calculated as the total knowledge score on COVID-19, which ranged from 0 to 17. The higher the score, the more knowledge participants got. The total knowledge score was divided into three groups (low, moderate, high) by tertiles. | The primary outcome is the acceptance of a potential COVID-19 vaccine. The acceptance of a potential COVID-19 vaccine was collected by the question “If a vaccine for the COVID-19 infection becomes available, will you get vaccinated during pregnancy? (yes, no or not sure)”. Pregnant women who responded “no or not sure” were then asked the reasons for vaccine hesitation by the question “What makes you unwilling (or unsure) to get the vaccine?”. Acceptable price for the COVID-19 vaccine was also collected among all participants by the question “How much do you think the price of the COVID-19 vaccine is acceptable? (cost of whole stage of vaccination)” followed by the response options “only acceptable for free”, “<200 RMB”, “201–400 RMB”, ”401–600 RMB”, and “>600 RMB”. To further assess the factors related to the attitude toward COVID-19 vaccine, we developed several questions based on the health belief model. The health belief model included five dimensions that might influence individuals’ health behaviors, namely perceptions of susceptibility, severity, barriers, benefits and cues to action. In the present study, there were totally 12 items focused on factors related to the attitude toward COVID-19 vaccine, including perceived susceptibility to COVID-19 infection for mother and infant (2 items), perceived severity of COVID-19 infection for mother and infant (2 items), perceived barriers of COVID-19 vaccination (3 items), benefits of COVID-19 vaccination (3 items) and cues to action (2 items). The response answers of “very concerned or agree”, “moderate concerned or not sure”, “not concerned or disagree” was recorded as 3, 2, and 1 score, respectively. The summed scores for each dimension of the health belief model framework were calculated accordingly. The participants were divided into three groups (low, moderate, high) by tertiles according to the summed score for each HBM dimension. | The proportion of acceptance of a COVID-19 vaccine were 77.4% among all participants. The acceptance rates decreased significantly along with the increasing age, from 81.7% in women aged 25 years or below to 66.7% in women aged above 40 years. Pregnant women with younger age, lower education, living in western region, second and third gestational trimester, with gestational complications, and higher knowledge score on COVID-19 infection were more likely to accept COVID-19 vaccination. The acceptance rates of a COVID-19 vaccine were significantly increased with the increasing total knowledge score on COVID-19 infection by locally weighted scatterplot smoothing regression analysis. Nearly one quarter (24.4%) of all participants only accept the COVID-19 vaccination for free. There were totally 80.4% of pregnant women who responded that the acceptable price of the COVID-19 vaccine (cost for the whole stage of vaccination) was < 200 RMB. Pregnant women who were concerned about getting COVID-19 were more likely to accept COVID-19 vaccination (79.0%) than those not concerned. Pregnant women who agreed with the benefit of vaccination to her fetus and baby had higher level of acceptance (78.7%) than those not agreed. Pregnant women perceived cues to action (receiving vaccine recommendation from doctors) were more likely to accept COVID-19 vaccine (80.6%) than those not perceived (33.3%). The acceptance rates of a COVID-19 vaccine were significantly higher in pregnant women with high level of perceived susceptibility to COVID-19 infection, severity of COVID-19 infection, benefits of COVID-19 vaccination, and cues to action than those with low level, while it was significantly lower in pregnant women with higher level of perceived barriers of vaccination (50.8% vs 91.3%). In the multivariable regression model, the acceptance of a COVID-19 vaccine was associated with young age, western region, low level of education, late pregnancy, high knowledge score on COVID-19, high level of perceived susceptibility, low level of perceived barriers, high level of perceived benefit, and high level of perceived cues to action. Among the 315 (22.6%) pregnant women with vaccine hesitancy, 54% of them refuse any vaccination during pregnancy due to their worry on any side effect. 47.0% of them concerned about the safety and 44.1% concerned about the efficacy of the COVID-19 vaccine on pregnant women and unborn baby. |
| Hailemariam et al., 202153 | Ethiopia | A facility-based cross-sectional survey. | 412 | From February 1 to March 1, 2021. | Pregnant women within the age group > 18 and residing for > 6 months in the area. | Pregnant women who were unable to respond due to illness or other physical impairment. | Interviewer-administered structured tool was used to collect the data. The tool has six sections: sociodemographic characteristics, clinical and reproductive features, knowledge of the COVID-19 vaccine, perception toward COVID-19 vaccine, compliance with COVID-19 guidelines, intention to vaccinate against COVID-19. | Knowledge of the COVID-19 vaccine was measured by five items, and it was analyzed as a binary variable. Participants who had correctly answered three and more questions were designated as having “Good knowledge,” otherwise “Poor knowledge.” The items were as follows: “have you heard vaccine for COVID-19?” “The vaccine doesn’t interfere with the pregnancy,” “the vaccine can decrease the risk of COVID-19 transmission,” “the vaccine can severely affect my health condition,” and “the vaccine can cure already affected people.” Perception toward COVID-19 vaccine was measured by four items, and the responses were rated on a 6-point scale. The items were as follows: “I have a mistrust of vaccine benefits,” “I worry about unforeseen future effects,” “I have a concern that it may cause infertility,” and “I prefer natural immunity.” It was dichotomized into “Negative perception” and “Positive perception”. The response to the question on compliance with government COVID-19 guidelines was on a scale from 1 = “none at all” to 7 = “very much so.” This was analyzed as a binary variable reflecting higher (6–7) and lower (1– 5) compliance. Intention to vaccinate against COVID-19 when available was measured based on one item (“How likely do you think you are to get a COVID-19 vaccine when one is available?”). Response options ranged from 1 to 6. It was dichotomized into “Intended to vaccinate” (if greater than or equal to mean score) and “Unwilling to vaccinate” (if less than mean score). |
About half (211; 51.2%) of the participants prefer for natural immunity rather than COVID-19 vaccine, and more than half (229; 55.6%) of the participants had a mistrust of vaccine benefit. Overall, 217 (52.6%) of the participants had a positive perception to the COVID-19 vaccine. Only 31.3% of the participants had an intention to take the COVID-19 vaccine when available. From the total seven candidate variables entered into multivariable analysis, four variables—namely, residence, education level, compliance to COVID-19 guidelines, and perception toward COVID-19 vaccine—were found to be independently associated with intention to take the COVID-19 vaccine. The OR of intention to take COVID-19 vaccine were nearly 2.6 times higher among women who live in urban residences. Women with secondary and higher education were four times more likely intended to take the COVID-19 vaccine than women with no formal education. Compared with those women who had not made compliance with the COVID-19 guidelines, women who had made compliance with the COVID-19 guidelines were nearly six times more likely to have an intention for COVID-19 vaccination. Moreover, women who had good knowledge were three times more likely to have an intention for COVID-19 vaccination than those who had poor knowledge. |
| Nguyen et al., 202158 | Vietnam | Cross-sectional survey | 651 | From January to February 2021 | (1) aged over 18 years; (2) being pregnant or had just given birth; (3) providing written informed consent to participate. | Women who suffered from serious illnesses or could not answer the questionnaire (for instance, inability to read/write or having a cognitive impairment which might influence the ability for responding to questions). | An online questionnaire on SurveyMonkey’s platform was designed. The survey link was sent to participants by the research team, and the participants answered the questionnaire via their smartphones or tablets. The researchers stayed in the same room with participants to answer their questions or clarify unclear terminology. The survey had four components: 1) socio- demographic information; 2) maternal characteristics; 3) affected by COVID-19 pandemic; 4) willingness to receive and pay for COVID-19 vaccine. Demographic information, maternal features and effect of COVID-19 epidemic during antenatal care were also collected. | The primary outcomes were the acceptance to receive the COVID-19 vaccine, willingness to pay for the vaccine and the amount of money participants were willing to pay (“amount of WTP”). To measure the outcomes, we asked them the following questions: “Do you want to get a COVID-19 vaccine?” and “Are you willing to pay for a COVID-19 vaccine for yourself and your household members?”. We asked this question because pregnant women might perceive that they were not eligible for the vaccination but they were willing to pay for other family members to be vaccinated. Those who answered “No” were asked to provide their reasons. Meanwhile, pregnant women answering “Yes” were then asked the amount of money they would be willing to pay for the COVID vaccination by asking “How much do you want to pay for a COVID vaccination?”. | Significant differences in willingness to receive COVID-19 vaccine were observed between respondents in Hanoi and Ca Mau, whereby 67.8% of women in Hanoi were willing to get the vaccine, whereas in Ca Mau only 39.9%. The majority of respondents were willing to pay for the COVID-19 vaccine with a mean amount of WTP of USD 15.2; however, there were notable differences in WTP for the vaccine between Hanoi and Ca Mau. <35% and 50% of participants were willing to pay 15.2 USD and 4.5 USD for the vaccine, respectively. Regarding acceptance, pregnant women living in rural/mountainous areas (48.4%), those with high school education or below (51.6%), and those with private health insurance (31.5%) were more likely to refuse vaccination. White-collar workers were more likely to accept to be immunized (67.1%) and pay for the vaccine (87.3%) than other groups. Pregnant women living with husband and children were less likely to be willing to pay (92.2%) for the vaccine. Those willing to be vaccinated and pay for it had significantly higher monthly household income than those not willing be vaccinated and to pay. |
GDM, gestational diabetes mellitus; NS, Not specified; OGTT, oral glucose tolerance testing; PIH, pregnancy induced hypertension; PTL, preterm labor; USA, United States of America;
DTPa, Diphtheria, Tetanus, acellular Pertussis; OR, Odds Ratio; STAI, State–Trait Anxiety inventory; HMC, Hamad Medical Corporation; AUC, area under the curve; RT-PCR, real time polymerase chain reaction; URL, Uniform Resource Locator; RMB, Renminbi; HBM, health belief model; USD, USA dollar; WTP, willingness to pay.
When performing the quality assessment according to the Q-SSP checklist, it was decided to not take into consideration two items (n. 3 and n. 12). Item n. 3 is related to the definition of a proper hypothesis to guide the survey, while item n. 12 requests the use of validated instruments to perform the survey. In our opinion, given that the main aim of these surveys was to understand an epidemiological issue and not to prove some theory or hypothesis, and also just to know pregnant women’s willingness to receive the SARS-CoV-2 vaccine (a simple and direct question, without the use of specific tools), this would have led to lower total scores since no studies but one clearly declared a hypothesis [52], and only a study declared the use of a validated tool [55]. Finally, only 2 studies were considered of questionable quality (Table 2 ). However, since the purpose of our study is to understand the worldwide attitude of pregnant women to receive the SARS-CoV-2 vaccine, we decided to include all of them into the systematic review.
Table 2.
Quality assessment of included studies.
|
Quality Assessment Checklist for Survey Studies in Psychology (Q-SSP) | ||||||
|---|---|---|---|---|---|---|
| Studies | Introduction | Participants | Data | Ethics | TOTAL | Result |
| Carbone et al., 2021 | 3/3 | 2/3 | 7/9 | 2/3 | 14/18 | A |
| Ceulemans et al., 2021 | 3/3 | 2/3 | 6/9 | 2/3 | 13/18 | A |
| Geoghegan et al., 2021 | 3/3 | 1/3 | 8/9 | 2/3 | 14/18 | A |
| Goncu Ayan et al., 2021 | 3/3 | 1/3 | 6/9 | 2/3 | 13/18 | A |
| Hailemariam et al., 2021 | 3/3 | 3/3 | 7/9 | 2/3 | 15/18 | A |
| Levy et al., 2021 | 3/3 | 3/3 | 7/9 | 2/3 | 15/18 | A |
| Mappa et al., 2021 | 3/3 | 2/3 | 7/9 | 2/3 | 14/18 | A |
| Mohan et al., 2021 | 3/3 | 2/3 | 7/9 | 2/3 | 14/18 | A |
| Mose et al., 2021 | 3/3 | 3/3 | 8/9 | 2/3 | 16/18 | A |
| Desai et al., 2021 | 3/3 | 1/3 | 5/9 | 1/3 | 10/18 | Q |
| Nguyen et al., 2021 | 3/3 | 2/3 | 8/9 | 2/3 | 15/18 | A |
| Skjefte et al., 2021 | 3/3 | 2/3 | 6/9 | 2/3 | 13/18 | A |
| Stuckelberger et al., 2021 | 3/3 | 2/3 | 7/9 | 2/3 | 14/18 | A |
| Sutton et al., 2021 | 3/3 | 2/3 | 6/9 | 2/3 | 13/18 | A |
| Tao et al., 2021 | 3/3 | 2/3 | 5/9 | 2/3 | 12/18 | Q |
A, acceptable quality; Q, questionable quality.
Synthesis of results
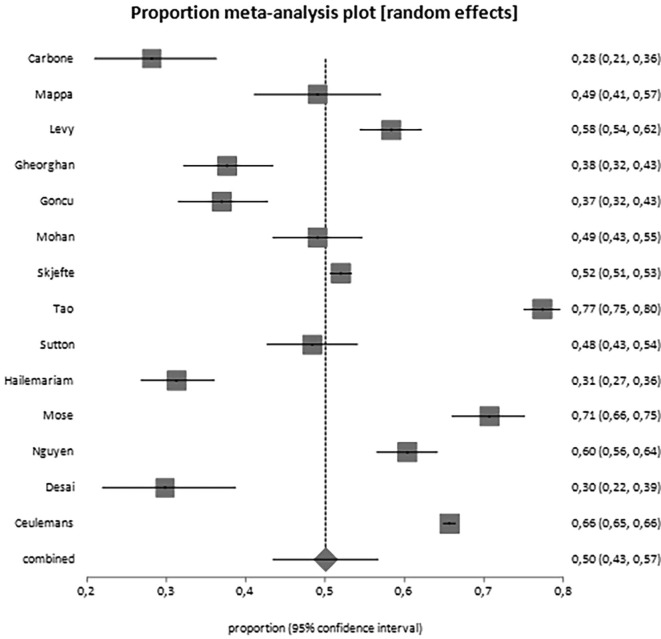
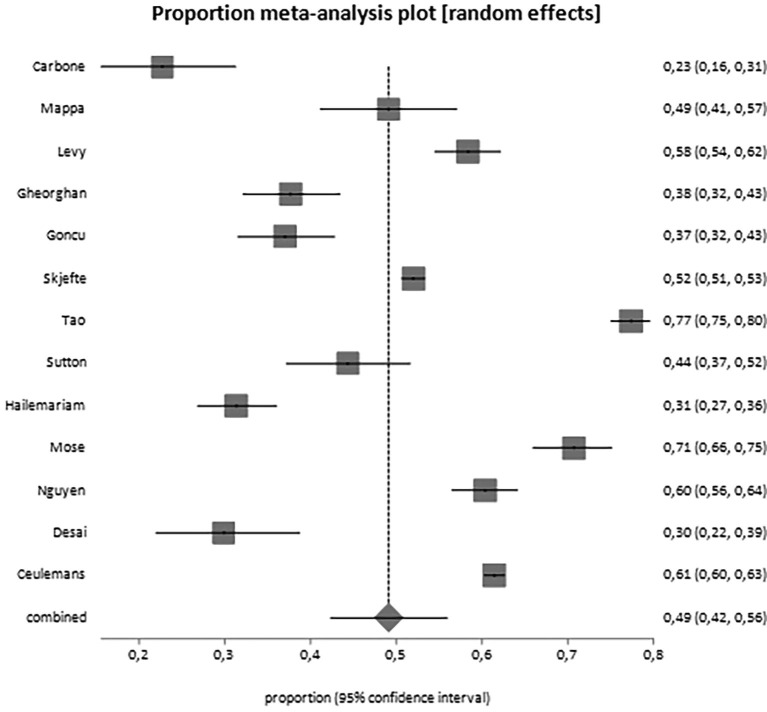
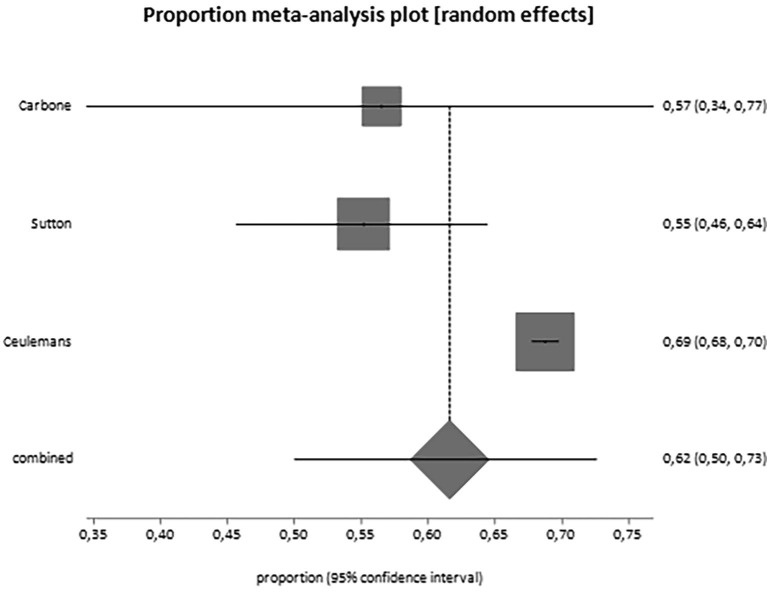
Overall, 25,839 women have been included in the analysis. From the data reported by included studies, it appears that 50% of them (95% CI, 43.4–56.7) would accept the vaccine during pregnancy or breastfeeding (Table 3 ; Fig. 2 ). When we analyzed pregnant women separately from breastfeeding women, it came out that the proportion of women actually willing to be vaccinated during pregnancy is 49.1% (95% CI, 42.3–56.0; Fig. 3 ), but the proportion of breastfeeding women is higher, reaching the 61.6% (95% CI, 50.0–75.0; Fig. 4 ) (Table 3). Moreover, we observed increased acceptance rate in Caucasian women (OR 1.93, 95% CI 1.0–3.5) and women usually accepting influenza vaccine (also eventually when pregnant; OR 5.18, 2.6–10.1). No differences were noted in relation to advanced maternal age, civil status, education, occupation, trimester of pregnancy and other features (Table 4 ). A potential publication bias could be found for the primary outcome (Egger's test: −6,762783, 95% CI = -13,057623 to −0,467942; P = 0.0373) (Supplementary material 2), while this was not found for the pregnant women alone subgroup (Egger's test: −5,363621, 95% CI = -13,159764 to 2,432523; P = 0.1582) (Supplementary material 3).
Table 3.
Pooled proportions (95% CI) for the main outcomes observed in the present systematic review.
| Studies | Pregnancies | Pooled proportions (95% CI) | I2 (%) | |
|---|---|---|---|---|
| General population | ||||
| Vaccine acceptance | 14 | 15809/25839 | 50.0 (43.4–56.7) | 98.7 |
| Sub-analysis considering only pregnant women | ||||
| Vaccine acceptance | 13 | 9403/16404 | 49.1 (42.3–56.0) | 98.4 |
| Sub-analysis considering only breastfeeding women | ||||
| Vaccine acceptance | 3 | 6251/9119 | 61.6 (50.0–70.5) | 81.2 |
Fig. 2.
Forest plot for the worldwide acceptance rate of SARS-CoV-2 vaccine among pregnant and breastfeeding women.
Fig. 3.
Forest plot for the acceptance rate of SARS-CoV-2 vaccine among pregnant women only.
Fig. 4.
Forest plot for the acceptance rate of SARS-CoV-2 vaccine among breastfeeding women only.
Table 4.
Pooled Odds Ratios (OR) for the different categorical outcomes explored in the present systematic review about women accepting SARS-CoV-2 vaccine compared to those who refused or were unsecure.
| Outcome | Studies | Pregnancies Acceptance vs refusal |
Pooled OR (95% CI) | I2 (%) | p-value (P < 0.05) |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Advanced maternal age (>35 years) | 7 | 967/5040 vs 874/4692 | 1.02 (0.8–1.3) | 69.4 | 0.84 |
| Caucasian | 3 | 412/534 vs 408/561 | 1.93 (1.0–3.5) | 32 | 0.03 |
| Civil status | |||||
| Unmarried | 3 | 1057/3180 vs 814/2895 | 1.33 (0.6–2.8) | 92.1 | 0.46 |
| Education | |||||
| Medium-high | 9 | 4703/5713 vs 3727/5066 | 1.60 (0.9–2.6) | 93.2 | 0.07 |
| Occupation | |||||
| Employed | 6 | 1869/2724 vs 1396/2061 | 1.15 (0.8–1.5) | 72.7 | 0.31 |
| Health status | |||||
| Pre-existing disease | 5 | 256/2343 vs 202/1789 | 1.22 (0.9–1.5) | 3.0 | 0.08 |
| Vaccinated for influenza | 6 | 838/2312 vs 580/2150 | 5.18 (2.6–10.1) | 85.6 | < 0.001 |
| Nulliparous | 7 | 2454/5031 vs 1698/3785 | 1.01 (0.8–1.3) | 77.1 | 0.90 |
| Complication in pregnancy | 3 | 374/1510 vs 133/675 | 1.24 (0.8–1.8) | 55.8 | 0.29 |
| Trimester of pregnancy | |||||
| 1st trimester | 3 | 295/1670 vs 198/1415 | 0.75 (0.3–1.4) | 80.5 | 0.40 |
| 2nd trimester | 3 | 363/1670 vs 247/1415 | 0.85 (0.4–1.6) | 85.2 | 0.62 |
| 3rd trimester | 3 | 599/1670 vs 323/1415 | 0.85 (0.4–1.8) | 91.5 | 0.68 |
Discussion
Main findings
Worldwide, the acceptance rate of the vaccination against SARS-CoV-2 is about 50% in pregnant women and 60% in breastfeeding women. This data show that vaccinal campaign should still be largely implemented to increase the proportion of women receiving the vaccine during pregnancy. Having received influenza vaccine is associated with an increased acceptance of anti-SARS-CoV-2 vaccine, demonstrating that people usually confident in vaccines do not avoid this one. Although not statistically significant, it appears that well-educated women would accept the vaccine compared with less educated women, as well as employed compared to unemployed, women with pre-existing disease or complications during pregnancy, and unmarried women compared to married ones.
Results in the context of what is known
Since the launch of the vaccinal campaign against SARS-CoV-2, the issue of pregnant women has been raised by the scientific community and the most important Obstetrics and Gynecology societies released recommendations in favor of it, but on the basis of a free choice of the pregnant woman, given the lack of data. However, reports showed quite soon that the vaccines were able to induce a response in pregnant women [43], [44], and that neither obvious safety issues were noticed in pregnant women and their fetuses or newborns [45], nor a true change in pregnancy complications’ rates [63]. Moreover, the vaccine during pregnancy has been shown to reduce the risk of SARS-CoV-2 infection [64]. Indeed, a retrospective analysis conducted in the United Kingdom showed that less than one third of women delivering between March and July 2021 received the vaccine against SARS-CoV-2, with a lower acceptance rate among younger women, non-white ethnicity, and lower socioeconomic background [65]. These data are consistent with the findings from our analysis and need a thorough attention, as pregnant women suffering from COVID-19 are at increased risk of severe course of the disease [66].
Strength and limitations
This is the first systematic review to evaluate the overall worldwide acceptance rate of SARS-CoV-2 vaccine among pregnant women. The review protocol was not registered a priori. Adherence to PRISMA guidelines and reporting of all peer-reviewed survey studies are among the strength of our analysis. Indeed, given that no specific tools or shared and uniform questionnaires have been distributed among the different populations, there is a wide heterogeneity of data both as evaluated and as reported, making it difficult to compare and unify the results. Furthermore, we did not plan any sensitivity analysis. In addition, having asked the willingness towards SARS-CoV-2 vaccination in different periods, before and during vaccines’ distribution, could have provoked different reactions among respondents. A potential publication bias was found for the primary outcome, but not when only pregnant women were considered, excluding breastfeeding ones. Also, a selection bias may have skewed the results of many included surveys, due to the voluntary participation and the large inclusion and few exclusion criteria adopted by each study. Last but not least, we did not plan to perform an individual-patient-data meta-analysis asking raw data to authors of the included surveys, and therefore, an evaluation of potential confounders could not be performed.
Clinical and research implications
During pandemic and lockdown, it has been observed that the access to emergency units for obstetrical and gynecological issues was reduced compared to the previous year [67], [68], mostly as a consequence of the anxiety to get the infection in public and crowded places [69], [70], [71], [72]. Currently, it seems that the anxiety of pregnant women is greater about receiving the vaccine than about contracting the infection, mainly in relation to unknown effects on the health of the fetuses and newborn as reported by various studies [46], [47], [52], [54], [56]. A recent study explored if the possibility of onsite vaccination against SARS-CoV-2 offered to a high-risk obstetric population would increase the uptake in pregnant women, with scarce results [73], showing that vaccine hesitancy is the most important reason for refusal and not a limited vaccine access as claimed by Centers for Disease Control and Prevention (CDC) [74]. In this scenario, it is of paramount importance that governments adopt all the needed strategies to inform this subgroup of the population that the consequences of the disease may be significantly more severe than the potential and unproven consequences of the vaccine, which are causing pregnant women’s avoidance. Furthermore, reporting of the acceptance/refusal of the vaccine against SARS-CoV-2 in all obstetrical and delivery settings will help to acquire more data on the safety of it, to be shared as soon as possible.
Conclusions
Our systematic review showed that 49.1% of pregnant women and 61.6% of breastfeeding women would accept SARS-CoV-2 vaccination. These rates appear still too low given the high rate of complications associated with COVID-19 course during pregnancy. Therefore, different strategies with stronger and more informative messages regarding the pros and cons of getting vaccinated should be carried out, in order to reduce the spread of the infection.
Funding
None.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejogrb.2021.12.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
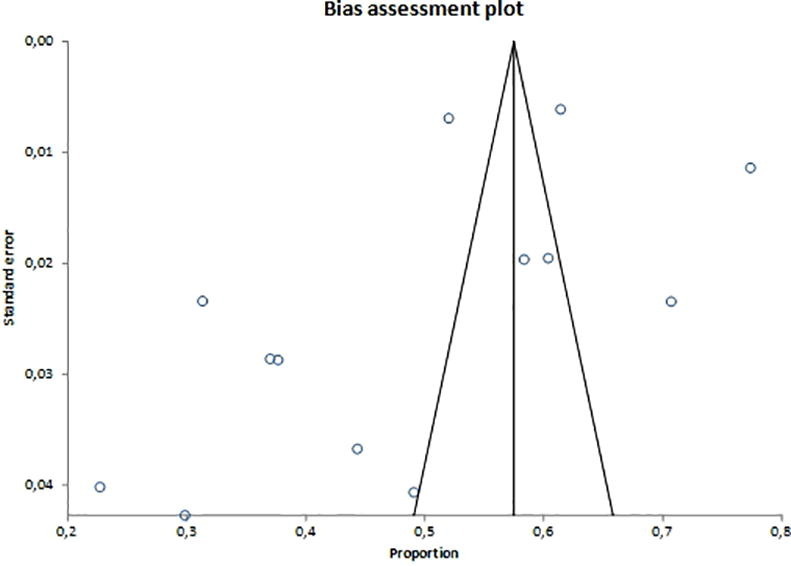
Supplementary figure 1.
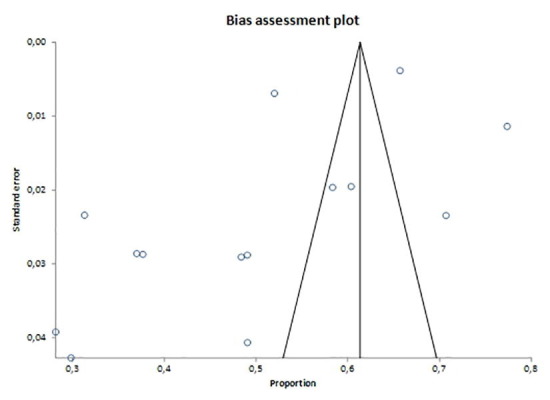
Supplementary figure 2.
References
- 1.<https://covid19.who.int> [last accessed 22 August 2021].
- 2.Saccone G. Get your obstetric inpatient and outpatient units ready for COVID-19. Minerva Ginecol. 2020;72(4):185–186. doi: 10.23736/S0026-4784.20.04560-8. Epub 2020 May 13. [DOI] [PubMed] [Google Scholar]
- 3.Franchi M., Bosco M., Garzon S., Lagana A.S., Cromi A., Barbieri B., et al. Management of obstetrics and gynaecological patients with COVID-19. Italian. J Obstet Gynecol. 2020;32(01):06. doi: 10.36129/jog.32.01.01. [DOI] [Google Scholar]
- 4.Dell’Utri C., Manzoni E., Cipriani S., Spizzico C., Dell’Acqua A., Barbara G., et al. Effects of SARS Cov-2 epidemic on the obstetrical and gynecological emergency service accesses. What happened and what shall we expect now? Eur J Obst Gynecol Reprod Biol. 2020;254:64–68. doi: 10.1016/j.ejogrb.2020.09.006. Epub 2020 Sep 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gagliardi L., Danieli R., Suriano G., Vaccaro A., Tripodi G., Rusconi F., et al. Universal severe acute respiratory syndrome coronavirus 2 testing of pregnant women admitted for delivery in 2 Italian regions. Am J Obstet Gynecol. 2020;223(2):291–292. doi: 10.1016/j.ajog.2020.05.017. Epub 2020 May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salsi G., Seidenari A., Diglio J., Bellussi F., Pilu G., Bellussi F. Obstetrics and gynecology emergency services during the coronavirus disease 2019 pandemic. Am J Obst Gynecol MFM. 2020;2(4):100214. doi: 10.1016/j.ajogmf.2020.100214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Findeklee S., Morinello E. Clinical implications and economic effects of the Coronavirus pandemic on gynecology, obstetrics and reproductive medicine in Germany: learning from Italy. Minerva Ginecol. 2020;72(3):171–177. doi: 10.23736/S0026-4784.20.04558-X. Epub 2020 May 13. [DOI] [PubMed] [Google Scholar]
- 8.Giannubilo S.R., Giannella L., Delli Carpini G., Carnielli V.P., Ciavattini A. Obstetric network reorganization during the COVID-19 pandemic: suggestions from an Italian regional model. Eur J Obst Gynecol Reprod Biol. 2020;249:103–105. doi: 10.1016/j.ejogrb.2020.04.062. Epub 2020 Apr 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrazzi E.M., Frigerio L., Cetin I., Vergani P., Spinillo A., Prefumo F., et al. COVID-19 Obstetrics Task Force, Lombardy, Italy: executive management summary and short report of outcome. Int J Gynaecol Obstet. 2020;149(3):377–378. doi: 10.1002/ijgo.13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carbone I.F., Conforti A., Farina A., Alviggi C. A practical approach for the management of obstetric and infertile women during the phase two of the novel coronavirus disease 2019 (COVID -19) pandemic. Eur J Obstet Gynecol Reprod Biol. 2020 Aug;251:266–267. doi: 10.1016/j.ejogrb.2020.06.006. Epub 2020 Jun 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alviggi C., Esteves S.C., Orvieto R., Conforti A., La Marca A., Fischer R., et al. POSEIDON (Patient-Oriented Strategies Encompassing IndividualizeD Oocyte Number) group. COVID-19 and assisted reproductive technology services: repercussions for patients and proposal for individualized clinical management. Reprod Biol Endocrinol. 2020;18(1) doi: 10.1186/s12958-020-00605-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaiarelli A., Bulletti C., Cimadomo D., Borini A., Alviggi C., Ajossa S., et al. COVID-19 and ART: the view of the Italian Society of Fertility and Sterility and Reproductive Medicine. Reprod Biomed Online. 2020;40(6):755–759. doi: 10.1016/j.rbmo.2020.04.003. Epub 2020 Apr 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carbone L., Conforti A., La Marca A., Cariati F., Vallone R., Raffone A., et al. The negative impact of most relevant infections on fertility and Assisted Reproduction Technology. Minerva Obstet Gynecol. 2021 doi: 10.23736/S2724-606X.21.04870-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Picarelli S., Conforti A., Buonfantino C., Vallone R., De Rosa P., Carbone L., et al. IVF during coronavirus pandemic: who comes first? The POSEIDON viewpoint. Italian. J Obstet Gynecol. 2020;32(04):223. doi: 10.36129/jog.32.04.01. [DOI] [Google Scholar]
- 15.Alviggi C., Borini A., Costa M., D’Amato G., Gianaroli L., Colacurci N. Sterility Special Interest Group position paper on ART treatments and COVID 19 pandemic. Italian. J Obstet Gynecol. 2020;32(3):154–162. doi: 10.36129/jog.32.03.01. [DOI] [Google Scholar]
- 16.Carbone L., Esposito R., Raffone A., Verrazzo P., Carbone I.F., Saccone G. Proposal for radiologic diagnosis and follow-up of COVID-19 in pregnant women. J Matern Fetal Neonatal Med. 2020:1–2. doi: 10.1080/14767058.2020.1793325. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 17.Rizzo G., Mappa I., Maqina P., Bitsadze V., Khizroeva J., Makatsarya A., et al. Effect of SARS‐CoV‐2 infection during the second half of pregnancy on fetal growth and hemodynamics: a prospective study. Acta Obstet Gynecol Scand. 2021;100(6):1034–1039. doi: 10.1111/aogs.14130. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurol-Urganci I., Jardine J.E., Carroll F., Draycott T., Dunn G., Fremeaux A., Harris T., Hawdon J., Morris E., Muller P., Waite L., Webster K., Der V.A.N., Meulen J., Khalil A. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: national cohort study. Am J Obstet Gynecol. 2021 doi: 10.1016/j.ajog.2021.05.016. S0002-9378(21)00565-2, Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WAPM (World Association of Perinatal Medicine) Working Group on COVID-19. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection. Ultrasound Obstet Gynecol; 2021 Feb;57(2):232-241. Epub 2021 Jan 21. 10.1002/uog.23107. [DOI] [PubMed]
- 20.D'Antonio F., Sen C., Mascio D.D., Galindo A., Villalain C., Herraiz I., et al. Maternal and perinatal outcomes in high compared to low risk pregnancies complicated by severe acute respiratory syndrome coronavirus 2 infection (phase 2): the World Association of Perinatal Medicine working group on coronavirus disease 2019. Am J Obst Gynecol MFM. 2021;3(4):100329. doi: 10.1016/j.ajogmf.2021.100329. Epub 2021 Feb 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Mascio D., D'Antonio F. Perinatal mortality and morbidity of SARS-COV-2 infection during pregnancy in European countries: Findings from an international study. Eur J Obstet Gynecol Reprod Biol. 2021;256:505–507. doi: 10.1016/j.ejogrb.2020.10.009. Epub 2020 Oct 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Girolamo R., Khalil A., Alameddine S., D’Angelo E., Galliani C., Matarrelli B., et al. Placental histopathology after SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis. Am J Obst Gynecol MFM. 2021 doi: 10.1016/j.ajogmf.2021.100468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackin D.W., Walker S.P. The historical aspects of vaccination in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2020 doi: 10.1016/j.bpobgyn.2020.09.005. S1521-6934(20)30158-9, Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitehead C.L., Walker S.P. Consider pregnancy in COVID-19 therapeutic drug and vaccine trials. The Lancet. 2020;395(10237):e92. doi: 10.1016/S0140-6736(20)31029-1. Epub 2020 May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rasmussen S.A., Kelley C.F., Horton J.P., Jamieson D.J. Coronavirus Disease 2019 (COVID-19) vaccines and pregnancy: what obstetricians need to know. Obstet Gynecol. 2021;137(3):408–414. doi: 10.1097/AOG.0000000000004290. Erratum. In: Obstet Gynecol. 2021 May 1;137(5):962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heath P.T., Le Doare K., Khalil A. Inclusion of pregnant women in COVID-19 vaccine development. Lancet Infect Dis. 2020 Sep;20(9):1007–1008. doi: 10.1016/S1473-3099(20)30638-1. Epub 2020 Aug 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maykin M.M., Heuser C., Feltovich H. Pregnant people deserve the protection offered by SARS-CoV-2 vaccines. Vaccine. 2021;39(2):171–172. doi: 10.1016/j.vaccine.2020.12.007. Epub 2020 Dec 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beigi R.H., Krubiner C., Jamieson D.J., Lyerly A.D., Hughes B., Riley L., et al. The need for inclusion of pregnant women in COVID-19 vaccine trials. Vaccine. 2021;39(6):868–870. doi: 10.1016/j.vaccine.2020.12.074. Epub 2021 Jan 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Mascio D., Buca D., Berghella V., Khalil A., Rizzo G., Odibo A., et al. Counseling in maternal-fetal medicine: SARS-CoV-2 infection in pregnancy. Ultrasound Obstet Gynecol. 2021;57(5):687–697. doi: 10.1002/uog.23628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saccone G., Zullo F., Di Mascio D. Coronavirus disease 2019 vaccine in pregnant women: not so far! The importance of counseling and the need for evidence-based data. Am J Obstet Gynecol MFM. 2021;3(3) doi: 10.1016/j.ajogmf.2021.100324. Epub 2021 Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Craig A.M., Hughes B.L., Swamy G.K. Coronavirus disease 2019 vaccines in pregnancy. Am J Obstet Gynecol MFM. 2021;3(2) doi: 10.1016/j.ajogmf.2020.100295. Epub 2020 Dec 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chervenak F.A., McCullough L.B., Bornstein E., Johnson L., Katz A., McLeod-Sordjan R., et al. Professionally responsible coronavirus disease 2019 vaccination counseling of obstetrical and gynecologic patients. Am J Obstet Gynecol. 2021;224(5):470–478. doi: 10.1016/j.ajog.2021.01.027. Epub 2021 Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stafford I.A., Parchem J.G., Sibai B.M. The coronavirus disease 2019 vaccine in pregnancy: risks, benefits, and recommendations. Am J Obstet Gynecol. 2021 May;224(5):484–495. doi: 10.1016/j.ajog.2021.01.022. Epub 2021 Jan 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The International Federation of Gynecology and Obstetrics (FIGO). <https://www.figo.org/sites/default/files/2021-03/FIGO%20Statement_COVID-19%20vaccination%20pregnant%20breastfeeding%20women_EN.pdf> [last accessed 22 August 2021].
- 35.The Italian Society of Gynaecology and Obstetrics (SIGO). <https://www.sigo.it/comunicati-stampa/la-ginecologia-italiana-con-un-documento-unitario-scende-in-campo-circa-la-campagna-vaccinale-e-le-reali-esigenze-della-donna-e-bambino-il-vaccino-deve-essere-offerto-a-tutte-le-donne-durante/> [last accessed 22 August 2021]
- 36.The Royal College of Obstetricians and Gynecologists (RCOG). <https://www.rcog.org.uk/en/guidelines-research-services/coronavirus-covid-19-pregnancy-and-womens-health/covid-19-vaccines-and-pregnancy/covid-19-vaccines-pregnancy-and-breastfeeding/> [last accessed 22 August 2021].
- 37.Centers for disease Control and Prevention (CDC). <https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html> [last accessed 22 August 2021].
- 38.The American College of Obstetricians and Gynecologists (ACOG). <https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care> [last accessed 22 August 2021].
- 39.The Society of Obstetricians and Gynaecologists of Canada (SOGC). <https://sogc.org/common/Uploaded%20files/Latest%20News/SOGC_Statement_COVID-19_Vaccination_in_Pregnancy.pdf> [last accessed 22 August 2021].
- 40.The Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG). <https://ranzcog.edu.au/news/midwives-and-obstetricians-encourage-covid-19-vacc> [last accessed 22 August 2021].
- 41.Donders G.G.G., Grinceviciene S., Haldre K., Lonnee-Hoffmann R., Donders F., Tsiakalos A., Adriaanse A., Martinez de Oliveira J., Ault K., Mendling W., On The Behalf Of The Covid-Isidog Guideline Group ISIDOG consensus guidelines on COVID-19 vaccination for women before, during and after pregnancy. J Clin Med. 2021;10(13):2902. doi: 10.3390/jcm10132902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martins I., Louwen F., Ayres-de-Campos D., Mahmood T. EBCOG position statement on COVID-19 vaccination for pregnant and breastfeeding women. Eur J Obstet Gynecol Reprod Biol. 2021;262:256–258. doi: 10.1016/j.ejogrb.2021.05.021. Epub 2021 May 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gray K.J., Bordt E.A., Atyeo C., Deriso E., Akinwunmi B., Young N., et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;225(3) doi: 10.1016/j.ajog.2021.03.023. S0002-9378(21)00187-3.Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collier A.-R., McMahan K., Yu J., Tostanoski L.H., Aguayo R., Ansel J., et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021;325(23):2370. doi: 10.1001/jama.2021.7563. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shimabukuro T.T., Kim S.Y., Myers T.R., Moro P.L., Oduyebo T., Panagiotakopoulos L., Marquez P.L., Olson C.K., Liu R., Chang K.T., Ellington S.R., Burkel V.K., Smoots A.N., Green C.J., Licata C., Zhang B.C., Alimchandani M., Mba-Jonas A., Martin S.W., Gee J.M., Meaney-Delman D.M. CDC v-safe COVID-19 pregnancy registry team. preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021 doi: 10.1056/NEJMoa2104983. NEJMoa2104983. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carbone L., Mappa I., Sirico A., Di Girolamo R., Saccone G., Di Mascio D., et al. Pregnant women perspectives on SARS-COV-2 vaccine: condensation: Most of Italian pregnant women would not agree to get the SARS-COV-2 vaccine, irrespective of having features of high risk themselves, or being high-risk pregnancies. Am J Obstet Gynecol MFM. 2021;3(4):100352. doi: 10.1016/j.ajogmf.2021.100352. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mappa I., Luviso M., Distefano F.A., Carbone L., Maruotti G.M., Rizzo G. Women perception of SARS-CoV-2 vaccination during pregnancy and subsequent maternal anxiety: a prospective observational study. J Matern Fetal Neonatal Med. 2021:1–4. doi: 10.1080/14767058.2021.1910672. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 48.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Bmj. 2009;339 doi: 10.1136/bmj.b2700. b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Protogerou C., Hagger M.S. A checklist to assess the quality of survey studies in psychology. Methods Psychol. 2020;3:100031. doi: 10.1016/j.metip.2020.100031. [DOI] [Google Scholar]
- 50.Ceulemans M., Foulon V., Panchaud A., Winterfeld U., Pomar L., Lambelet V., et al. Vaccine willingness and impact of the COVID-19 pandemic on women's perinatal experiences and practices-a multinational, cross-sectional study covering the first wave of the pandemic. Int J Environ Res Public Health. 2021;18(7):3367. doi: 10.3390/ijerph18073367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Geoghegan S., Stephens L.C., Feemster K.A., Drew R.J., Eogan M., Butler K.M. “This choice does not just affect me.” Attitudes of pregnant women toward COVID-19 vaccines: a mixed-methods study. Hum Vaccines Immunotherapeutics. 2021;17(10):3371–3376. doi: 10.1080/21645515.2021.1924018. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goncu Ayhan S., Oluklu D., Atalay A., Menekse Beser D., Tanacan A., Moraloglu Tekin O., et al. COVID-19 vaccine acceptance in pregnant women. Int J Gynaecol Obstet. 2021;154(2):291–296. doi: 10.1002/ijgo.13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hailemariam S., Mekonnen B., Shifera N., Endalkachew B., Asnake M., Assefa A., Qanche Q. Predictor of pregnant women's intention to vaccinate against coronavirus disease in 2019. SAGE Open Med. 2021;9:1–8. doi: 10.1177/20503121211038454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levy A.T., Singh S., Riley L.E., Prabhu M. Acceptance of COVID-19 vaccination in pregnancy: a survey study. Am J Obst Gynecol MFM. 2021;3(5):100399. doi: 10.1016/j.ajogmf.2021.100399. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mohan S., Reagu S., Lindow S., Alabdulla M. COVID-19 vaccine hesitancy in perinatal women: a cross sectional survey. J Perinat Med. 2021;49(6):678–685. doi: 10.1515/jpm-2021-0069. [DOI] [PubMed] [Google Scholar]
- 56.Mose A., Yeshaneh A. COVID-19 Vaccine acceptance and its associated factors among pregnant women attending antenatal care clinic in Southwest Ethiopia: institutional-based cross-sectional study. Int J Gen Med. 2021;8(14):2385–2395. doi: 10.2147/IJGM.S314346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Desai P., Kaur G., Dong F., Rodriguez M. COVID-19 vaccine acceptance in pregnancy. Neonatol Today. 2021;16(7):11–15. doi: 10.51362/neonatology.today/202171671115. [DOI] [Google Scholar]
- 58.Nguyen L.H., Hoang M.T., Nguyen L.D., Ninh L.T., Nguyen H.T.T., Nguyen A.D., et al. Acceptance and willingness to pay for COVID‐19 vaccines among pregnant women in Vietnam. Trop Med Int Health. 2021;26(10):1303–1313. doi: 10.1111/tmi.13666. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skjefte M., Ngirbabul M., Akeju O., Escudero D., Hernandez-Diaz S., Wyszynski D.F., et al. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol. 2021;36(2):197–211. doi: 10.1007/s10654-021-00728-6. Epub 2021 Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stuckelberger S., Favre G., Ceulemans M., Nordeng H., Gerbier E., Lambelet V., et al. SARS-CoV-2 vaccine willingness among pregnant and breastfeeding women during the first pandemic wave: a cross-sectional study in Switzerland. Viruses. 2021;13(7):1199. doi: 10.3390/v13071199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sutton D., D'Alton M., Zhang Y., Kahe K.a., Cepin A., Goffman D., et al. COVID-19 vaccine acceptance among pregnant, breastfeeding, and nonpregnant reproductive-aged women. Am J Obst Gynecol MFM. 2021;3(5):100403. doi: 10.1016/j.ajogmf.2021.100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tao L., Wang R., Han N.a., Liu J., Yuan C., Deng L., et al. Acceptance of a COVID-19 vaccine and associated factors among pregnant women in China: a multi-center cross-sectional study based on health belief model. Hum Vaccines Immunother. 2021;17(8):2378–2388. doi: 10.1080/21645515.2021.1892432. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trostle M.E., Limaye M.A., Avtushka V., Lighter J.L., Penfield C.A., Roman A.S. COVID-19 vaccination in pregnancy: early experience from a single institution. Am J Obst Gynecol MFM. 2021;3(6):100464. doi: 10.1016/j.ajogmf.2021.100464. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Theiler R.N., Wick M., Mehta R., Weaver A.L., Virk A., Swift M. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am J Obst Gynecol MFM. 2021 doi: 10.1016/j.ajogmf.2021.100467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blakeway H., Prasad S., Kalafat E., Heath P.T., Ladhani S.N., Le Doare K., Magee L.A., O’brien P., Rezvani A., Dadelszen Pv., Khalil A. COVID-19 vaccination during pregnancy: coverage and safety. Am J Obst Gynecol MFM. 2021 doi: 10.1016/j.ajog.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rose C.H., Wyatt M.A., Narang K., Lorenz K.E., Szymanski L.M., Vaught A.J. Timing of delivery with coronavirus disease 2019 pneumonia requiring intensive care unit admission. Am J Obst Gynecol MFM. 2021;3(4):100373. doi: 10.1016/j.ajogmf.2021.100373. Epub 2021 Apr 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carbone L., Raffone A., Travaglino A., Sarno L., Conforti A., Gabrielli O., De Vivo V., De Rosa M., Migliorini S., Saccone G., Locci M., Alviggi C., Mollo A., Guida M., Zullo F., Maruotti G.M. Obstetric A&E unit admission and hospitalization for obstetrical management during COVID-19 pandemic in a third-level hospital of southern Italy. Arch Gynecol Obstet. 2021:1–9. doi: 10.1007/s00404-021-06212-6. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carbone L., Raffone A., Sarno L., Travaglino A., Saccone G., Gabrielli O., Migliorini S., Sirico A., Genesio R., Castaldo G., Capponi A., Zullo F., Rizzo G., Maruotti G.M. Invasive prenatal diagnosis during COVID-19 pandemic. Arch Gynecol Obstet. 2021:1–5. doi: 10.1007/s00404-021-06276-4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mappa I., Distefano F.A., Rizzo G. Effects of coronavirus 19 pandemic on maternal anxiety during pregnancy: a prospectic observational study. J Perinat Med. 2020;48(6):545–550. doi: 10.1515/jpm-2020-0182. [DOI] [PubMed] [Google Scholar]
- 70.Esposito V., Rania E., Lico D., Pedri S., Fiorenza A., Strati M.F., et al. Influence of COVID-19 pandemic on the psychological status of infertile couples. Eur J Obstet Gynecol Reprod Biol. 2020;253:148–153. doi: 10.1016/j.ejogrb.2020.08.025. Epub 2020 Aug 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saccone G., Florio A., Aiello F., Venturella R., De Angelis M.C., Locci M., et al. Psychological impact of coronavirus disease 2019 in pregnant women. Am J Obstet Gynecol. 2020;223(2):293–295. doi: 10.1016/j.ajog.2020.05.003. Epub 2020 May 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Di Mascio D., Saccone G., D’Antonio F., Berghella V. Psychopathology associated with coronavirus disease 2019 among pregnant women. Am J Obst Gynecol MFM. 2021;3(1):100290. doi: 10.1016/j.ajogmf.2020.100290. Epub 2020 Dec 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hirshberg J.S., Huysman B.C., Oakes M.C., Cater E.B., Odibo A.O., Raghuraman N., Kelly J.C. Offering onsite COVID-19 vaccination to high-risk obstetric patients: initial Findings. Am J Obst Gynecol MFM. 2021 doi: 10.1016/j.ajogmf.2021.100478. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Centers for Disease Control and Prevention (CDC). Expanding COVID-19 vaccine distribution to primary care providers to address disparities in immunization: guide for jurisdictions; 2021. <https://www.cdc.gov/vaccines/covid-19/downloads/Guide-for-Jurisdictions-on-PCP-COVID-19-Vaccination.pdf>.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.