Abstract
Botulinum toxin type A (BoNTA) products are widely used for therapeutic and aesthetic indications, but there is a need for longer-lasting treatments that maintain symptom relief between injections and reduce the frequency of re-treatment. DaxibotulinumtoxinA for Injection (DAXI) is a novel BoNTA product containing highly purified 150-kDa core neurotoxin and is the first to be formulated with a proprietary stabilizing excipient peptide (RTP004) instead of human serum albumin. The positively charged RTP004 has been shown to enhance binding of the neurotoxin to neuronal surfaces, which may enhance the likelihood of neurotoxin internalization. DAXI produces robust, extended efficacy across both aesthetic and therapeutic indications. In an extensive glabellar lines clinical program, DAXI showed a high degree of efficacy, a consistent median time to loss of none or mild glabellar line severity of 24 weeks, and median time until return to baseline of up to 28 weeks. In adults with cervical dystonia, DAXI at 125 U and 250 U significantly improved Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) total scores, with a median duration of efficacy of 24 and 20 weeks, respectively, which compares favorably with the 12–14 weeks’ duration reported for approved BoNTA products. Overall, DAXI was well tolerated, and the consistent extended duration of effect suggests that DAXI has the potential to improve the management of both aesthetic and therapeutic conditions.
Plain Language Summary
Botulinum toxin is used to block the nerve signals that cause muscles to contract. Products containing botulinum toxin are commonly given by injection to treat muscle spasms (such as cervical dystonia, a painful condition where the neck muscles contract involuntarily) and for cosmetic treatment of frown lines. However, the effects of the currently approved botulinum toxin products typically wear off about 3–4 months after injection and so the injections must be repeated regularly. A new product called DAXI (DaxibotulinumtoxinA for Injection) has been developed. In this product, the botulinum toxin is formulated with a unique protein (called RTP004) that has been designed to help deliver the botulinum toxin to the nerve cells. Research suggests that the RTP004 protein in DAXI adheres the botulinum toxin to the nerves close to the injection site, potentially making its effect last longer. To date, DAXI has been studied in over 3800 patients. The studies have shown that DAXI is effective for treating neck spasms (cervical dystonia) and for reducing the appearance of frown lines. Importantly, the effects of DAXI lasted up to 6 months, which is longer than seen with other botulinum toxin products. The side effects seen with DAXI are consistent in nature and frequency with those seen with other botulinum toxin products. These findings suggest that DAXI can improve both medical and cosmetic treatments due to its longer-lasting effect.
Key Points
| Longer-lasting botulinum toxin type A (BoNTA) products are needed for therapeutic and aesthetic indications to maintain stable clinical effects between injections and potentially reduce the frequency of re-treatment. |
| DaxibotulinumtoxinA for Injection (DAXI) is a novel BoNTA utilizing a proprietary stabilizing excipient peptide (RTP004) in place of human serum albumin. |
| DAXI has consistently demonstrated a median effect duration of 24 weeks in the treatment of cervical dystonia and glabellar lines, suggesting that DAXI has the potential to improve the management of both aesthetic and therapeutic conditions. |
Introduction
Over the past several decades, a large volume of clinical data has demonstrated the efficacy and safety of botulinum toxin type A (BoNTA) products for treating medical conditions, such as cervical dystonia, upper limb spasticity, chronic migraine headaches, overactive bladder, and blepharospasm, as well as aesthetic concerns, such as facial lines [1–3]. Despite the widespread use of BoNTAs for therapeutic and aesthetic indications, a limitation of all currently available BoNTA products is their duration of effect. Most BoNTAs currently approved in the USA have a recommended re-treatment interval of 12–16 weeks (3–4 months) [4–7]. In cervical dystonia, however, patients have reported declines in symptom relief after a mean of 9.5 weeks [8], re-emergence of cervical dystonia symptoms between injections [9, 10], and low satisfaction with BoNTA treatment prior to reinjection [8], with many saying they would like a longer-lasting BoNTA treatment and more stable symptom relief [9, 10]. Product labelling prohibits re-treatment intervals shorter than 12 weeks [4, 5, 7] and health insurance will typically not reimburse for more frequent treatments. As a result, patients may face a period of inadequate symptom relief.
Similar evidence suggests that patients treated for glabellar lines desire longer-lasting effects. The reported duration of effect on glabellar lines for currently approved BoNTA products is approximately 3–4 months [5–7], such that patients require 3 or 4 treatments annually to maintain the clinical benefit. In 2018, an online survey found that 88% of respondents (among 1004 women aged 25–70 years who had used a neuromodulator in the last 5 years) indicated a BoNTA product offering long-lasting aesthetic results was absolutely essential or very important when considering a neuromodulator [11]. Further, while reimbursement decisions do not drive re-treatment timing in aesthetic indications, patients often limit themselves to twice yearly treatments [11], resulting in periods of suboptimal correction.
To date, none of the currently available BoNTA products has consistently demonstrated a clinically distinct peak efficacy (response rate), duration of therapeutic benefit, or safety profile. DaxibotulinumtoxinA for Injection (DAXI; Revance Therapeutics, Inc., Newark, CA) is a novel BoNTA product, containing purified 150-kDa core neurotoxin and a proprietary excipient peptide, and is in clinical development for multiple therapeutic (cervical dystonia and upper limb spasticity) [12–14] and aesthetic indications (upper facial lines including glabellar lines, forehead lines, and lateral canthal lines) [15–17]. The formulation of DAXI and the clinical data from the extensive development program establish its role as a novel BoNTA with potential to improve upon the efficacy and safety of currently approved BoNTAs. This article describes the unique product characteristics and manufacturing of DAXI and summarizes the currently available DAXI clinical data.
Background on DAXI
Product Characteristics
The neurotoxin in DAXI and all other BoNTA products is derived from the Hall strain of Clostridium botulinum [18]; however, DAXI is the only BoNTA product that is wholly manufactured and formulated in the USA. The formulation consists of highly purified daxibotulinumtoxinA (RTT150, a 150-kDa BoNTA), a stabilizing peptide that binds the neurotoxin with high avidity, and other excipients, including polysorbate-20 (a surfactant), buffers, and a sugar [15]. Whereas all other BoNT products rely on human serum albumin (HSA) as an excipient to limit the aggregation of BoNT molecules and their adsorption to glass surfaces [19], the unique formulation of DAXI, combining the novel, proprietary stabilizing excipient peptide (RTP004) along with polysorbate-20, allows for an HSA-free product [20] (summarized in Table 1).
Table 1.
Botulinum toxin type A products for aesthetic indications in the USA: summary of product characteristics
| DaxibotulinumtoxinA | OnabotulinumtoxinA [4, 6] | AbobotulinumtoxinA [5] | IncobotulinumtoxinA [7] | PrabotulinumtoxinA [36] | |
|---|---|---|---|---|---|
| Molecular weight (kDa) | 150 | 900 | ~400 | 150 | 900 |
| Contains accessory proteins | No | Yes | Yes | No | Yes |
| Contains HSA | No | Yes; 500 µg | Yes; 125 µg | Yes; 1 mg | Yes |
| Excipients | PS20, sugar, buffer, excipient peptide (RTP004) | Sodium chloride, HSA | Lactose, HSA | Sucrose, HSA | Sodium chloride, HSA |
| Stabilization | Lyophilization | Vacuum drying | Lyophilization | Lyophilization | Vacuum drying |
| Solubilization | Normal saline | Normal saline | Normal saline | Normal saline | Normal saline |
| Shelf-life once reconstituted (h) | 72 | 36 | 24 | 36 | 24 |
| Can be stored at room temperature unreconstituted | Yes | No | No | Yes | No |
| Purification method | Chromatography | Crystallization | Chromatography | NA | NA |
| 100% sourced and manufactured in USA | Yes | No | No | No | No |
| Glabellar line dose (U) | 40 | 20 | 50 | 20 | 20 |
| Mass of core neurotoxin in glabellar line dose (ng) [36, 53] | 0.18 | 0.18 | 0.27 | 0.08 | 0.12 |
| Glabellar line response rate (≥ 2-point improvement from baseline based on investigator and subject assessment) (%) | 74 | NA | 52–60 | 48–60 | 67–71 |
| Median duration of effect | 24 weeks/6 months | 3–4 months | Up to 4 months | Up to 3 months | Only 1-month data reported |
HSA human serum albumin, NA not applicable, PS20 polysorbate-20
RTP004 is a 35-amino acid peptide that is highly positively charged at physiologic pH and forms a strong electrostatic bond with daxibotulinumtoxinA. RTP004 is wholly synthetic and is constructed with two distinct domains: a core domain of 15 consecutive lysines that is flanked at each terminus by a 10-amino acid protein transduction domain (PTD). The cationic, arginine-rich PTD in RTP004 was originally identified as a region between residues 48–60 of the 86-amino acid transcriptional activator (TAT) coded for by HIV-1, a sequence that permitted transduction of proteins across cell membranes [21]. TAT-PTD complexes are thought to enter cells by endocytosis [22] and are in development for the intracellular delivery of therapeutic proteins for conditions such as central nervous system disorders, eye diseases, diabetes, and several types of cancer [23–26]. The RTP004 structure, with a PTD at each terminus, makes DAXI unlike other PTD-containing products in development that typically comprise a single PTD conjugated to the protein of interest.
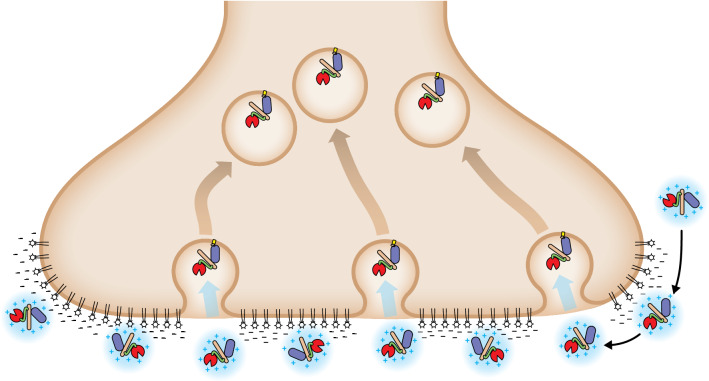
The strong net positive charge of RTP004 drives electrostatic binding to negatively charged extracellular structures, such as neuronal surfaces and extracellular matrix proteins. Specifically, in vitro surface plasmon resonance, a sensor of biomolecular interactions used to measure the kinetics of binding/dissociation, has demonstrated that RTP004 increases binding of DAXI to a lipid membrane preparation [11]. Similarly, results from an in vitro binding study with synaptosomes indicate that RTP004 increased maximal percentage binding of BoNTA heavy chain to rat brain nerve terminals [27]. Enhanced binding to nerve terminals and extracellular matrix elements may facilitate the localization of BoNTA and reduce diffusion from the injection site [28], a finding that was observed in a study of DAXI and onabotulinumtoxinA in mice [18]. In that study, a DAXI dose of 2.5 times that of the onabotulinumtoxinA dose was required to see comparable diffusion. When comparing diffusion-matched doses of the two BoNTA products, it was observed that DAXI had an extended duration of drug effect (100–126% increase) versus onabotulinumtoxinA [18]. The strong positive charge on the peptide has been demonstrated to drive an association between the peptide and the neurotoxin, and between the peptide-toxin complex and the negatively charged presynaptic nerve terminal. The stronger binding to the nerve terminal will increase the duration of the anchoring of BoNTA at the presynaptic terminal in comparison with a neurotoxin formulation without peptide. We hypothesize that this prolonged binding will increase the likelihood of DAXI encountering its protein receptor, SV2 (synaptic vesicle glycoprotein 2), over time and thereby increase the number of BoNT molecules internalized in comparison with “non-sticky” BoNTs that may be washed away shortly after injection (Fig. 1). Pharmacologically, a greater amount of available botulinum toxin light chain inside the neuron will correlate with the longer duration of effect observed clinically with DAXI and discussed below.
Fig. 1.
Proposed mechanism of enhanced DAXI binding and internalization. In vitro data suggest that RTP004, the positively-charged stabilizing excipient peptide in the DAXI formulation, increases the affinity of the daxibotulinumtoxinA for neuronal membranes, which enhances localization of DAXI at the presynaptic terminal and may, therefore, facilitate increased internalization of the botulinum toxin molecule.  = DAXI/peptide complex
= DAXI/peptide complex
Cationic amino acids (arginine and lysine), such as those in the PTD of RTP004, protect against thermally induced aggregation of protein therapeutics. At physiological pH, the highly positively charged RTP004 peptide forms a noncovalent electrostatic interaction with anionic surfaces of the core 150-kDa neurotoxin [29]. These electrostatic interactions with RTP004 likely shield the core neurotoxin from self-aggregating, allowing DAXI to be formulated without the excipient HSA found in all other BoNT products [19]. In contrast, there is evidence to suggest that the presence of HSA slightly destabilizes DAXI with respect to thermally induced aggregation [20]. The DAXI formulation permits the product to be stable as a lyophilized product at room temperature for up to 3 years before reconstitution [11]. Once reconstituted with normal saline, DAXI is stable for at least 72 h at 2–8 °C, which is considerably longer than the 24–36 h reported for other BoNTA products (Table 1).
RTP004 prevents adsorption of DAXI to container surfaces during manufacturing and also prevents adsorption of the core neurotoxin to the vial (likely by blocking the neurotoxin from interacting with hydrophobic areas on container surfaces) [30]. Specifically, the addition of RTP004 or polysorbate-20 alone reduces DAXI adsorption, but the combination of RTP004 and polysorbate-20 at levels present in DAXI achieve complete protection against surface adsorption [30].
Manufacturing
DAXI is manufactured using a complex process of fermentation, purification, and formulation. After fermentation, DAXI is purified via a tightly controlled process that includes three column chromatography steps: hydrophobic interaction chromatography, anion exchange chromatography, and cation exchange chromatography. This process removes bacterial impurities and non-toxin accessory proteins and concentrates the final product, yielding the highly purified 150-kDa core neurotoxin. Following formulation with the peptide and other excipients, the final drug product is dried by lyophilization, forming an even cake at the bottom of the vial.
DAXI Clinical Evidence
DAXI has been administered to 3824 subjects receiving treatment within 17 clinical studies for seven clinical indications (glabellar lines, forehead lines, lateral canthal lines, upper facial lines, cervical dystonia, upper limb spasticity, and plantar fasciitis). This section summarizes results from the two Phase 3 clinical programs: glabellar lines and cervical dystonia. Of note, these two indications represent the earliest and largest applications of conventional BoNTAs.
Overview of DAXI Glabellar Lines Clinical Program
The glabellar lines clinical program included a Phase 1/2 dose-escalation study [31], a Phase 2 dose-ranging study (which also compared DAXI to onabotulinumtoxinA 20 U [32]), and three Phase 3 studies (two pivotal trials [15, 33] and an open-label safety study [16, 34]; Table 2). A total of 3139 adults participated in this development program, with 2994 subjects receiving 4444 DAXI treatments (at any dose). The DAXI Phase 3 clinical program in glabellar lines was the largest to date in aesthetics. Overall, the results demonstrate that DAXI is a well-tolerated treatment, offering a high degree of efficacy and up to 28 weeks’ duration to return to baseline following a single treatment across the Phase 2 and 3 studies [15, 16, 33, 34], as well as durable efficacy following repeated DAXI treatment [16]. A remarkable degree of consistency in the response rates and duration profile was observed between studies and between treatment cycles in the repeat-dose, open label study.
Table 2.
Summary of the DAXI clinical program for the treatment of glabellar lines
| Study design | Sites | Treatment | Follow-up period (weeks) | N | |
|---|---|---|---|---|---|
| Phase 1/2 [31] | Randomized, open-label, uncontrolled, dose-escalation, single-center | 1 in Mexico |
DAXI ~ 25 U DAXI ~ 50 U DAXI ~ 75 U DAXI ~ 100U |
36 | 48 |
|
Phase 2 (Belmont) (NCT02303002) [32] |
Randomized, double-blind, placebo-controlled, parallel-group, multicenter | 9 in Canada |
DAXI 20 U DAXI 40 U DAXI 60 U OnabotulinumtoxinA 20 U Placebo |
24 | 286 |
|
Phase 3 (SAKURA 1) (NCT03014622) [15, 33] |
Randomized, double-blind, placebo-controlled, parallel-group, multicenter | 15 in USA |
DAXI 40 U Placebo |
36 | 303 |
|
Phase 3 (SAKURA 2) (NCT03014635) [15, 33] |
Randomized, double-blind, placebo-controlled, parallel-group, multicenter |
6 in Canada 9 in USA |
DAXI 40 U Placebo |
36 | 306 |
|
Phase 3 (SAKURA 3/OLS) (NCT03004248) [16, 34] |
Open-label, multicenter | 65 in USA and Canada | DAXI 40 U | 84 | 2691 |
DAXI DaxibotulinumtoxinA for Injection, OLS open-label study
Phase 1/2 Dose-Escalation Study
The Phase 1/2 clinical study was designed to evaluate the safety of escalating doses of DAXI for the treatment of moderate-to-severe glabellar lines [31]. In total, 48 subjects were randomized to four cohorts for escalating doses of DAXI. DAXI was well tolerated and highly effective based on ratings taken at maximum frown at Week 4 and, overall, 60% of subjects had wrinkle severity of none or mild at 6 months [32]. This study indicated that DAXI may provide a benefit of extended duration and lead to the continued clinical development of DAXI.
Phase 2 Dose-Ranging Study
The randomized, double-blind, placebo-controlled, Phase 2 ‘Belmont’ study was designed to evaluate DAXI for the treatment of glabellar lines compared to the approved dose of onabotulinumtoxinA (20 U) and placebo [32, 35]. A total of 268 adults with moderate or severe glabellar lines at maximum frown were enrolled and randomly assigned (with equal allocation) to DAXI 20 U, DAXI 40 U, DAXI 60 U, onabotulinumtoxinA 20 U, or placebo. All subjects received a single treatment and were followed for up to 24 weeks.
At Weeks 4 and 24, compared with placebo, all doses of DAXI resulted in a significantly greater proportion of subjects achieving a ≥ 1-point improvement from baseline in glabellar line severity at maximum frown based on the four-point Investigator Global Assessment-Frown Wrinkle Severity (IGA-FWS) scale (Week 4: 100% for all DAXI doses vs 3% for placebo; Week 24: 18%, 36%, and 29% for DAXI 20 U, 40 U, and 60 U, respectively, vs 3% for placebo; p < 0.05 for all) [32, 35]. DAXI 40 U also demonstrated significantly greater efficacy compared with onabotulinumtoxinA 20 U; at Weeks 16 and 24, significantly greater proportions of subjects treated with DAXI 40 U achieved a rating of none or mild on the IGA-FWS score (Week 16: 66.7%; Week 24: 30.8%) versus subjects treated with onabotulinumtoxinA 20 U (Week 16: 31.7%; Week 24: 11.9%; p < 0.05 for both) [32]. Furthermore, the median duration of effect (time to loss of a ≥ 1-point improvement from baseline on IGA-FWS score) was significantly longer with DAXI 40 U than with onabotulinumtoxinA 20 U (p = 0.03) [32].
Safety and tolerability were similar across the DAXI doses and when compared with onabotulinumtoxinA 20 U [32, 35]. No serious adverse events (AEs) occurred. Most AEs were predominantly localized to the injection site, transient, and mild in severity. Eyelid ptosis occurred in four subjects treated with DAXI 60 U and one subject treated with onabotulinumtoxinA 20 U. Overall, the DAXI 40 U dose had the most favorable risk:benefit profile [32, 35] and was subsequently evaluated in the Phase 3 pivotal trials.
Phase 3 Pivotal Trials
Two identical randomized, double-blind, placebo-controlled, parallel-group, multicenter Phase 3 pivotal trials (SAKURA 1 and SAKURA 2) were conducted to evaluate the efficacy and safety of DAXI 40 U [15, 33]. In total, 609 adults with moderate or severe glabellar lines at maximum frown were enrolled. All subjects received a single treatment and were followed for up to 36 weeks [15]. In both trials, the proportion of subjects who achieved a ≥ 2-point improvement from baseline in glabellar line severity at maximum frown based on both the IGA-FWS and Patient Frown Wrinkle Severity (PFWS) score at Week 4 was significantly higher with DAXI than with placebo at 73.6% vs 0.0%, respectively, in SAKURA 1 and 74.0% vs 1.0%, respectively, in SAKURA 2 (p < 0.0001 for both) [15]. The proportion of subjects achieving glabellar line severity of none or mild at Week 4 was 97.5% with DAXI in both trials compared with ≤ 4.9% with placebo (p < 0.0001 for both). Median duration of effect (defined as time over which none or mild glabellar line severity score was maintained) was 24.0 weeks, and median time to return to baseline levels was 27.1 weeks [33].
The safety and tolerability profile of DAXI in the SAKURA trials was similar to that reported in the Phase 2 study [15, 33]. Treatment-related AEs were primarily mild, with headache and injection site AEs (pain, erythema, and edema) being the most common treatment-related AEs. Eyelid ptosis occurred in 2.2% of DAXI-treated subjects; all cases resolved without sequelae. No subjects developed neutralizing antibodies to DAXI, assessed using the validated mouse protection assay [15, 33].
Open-Label Safety Study
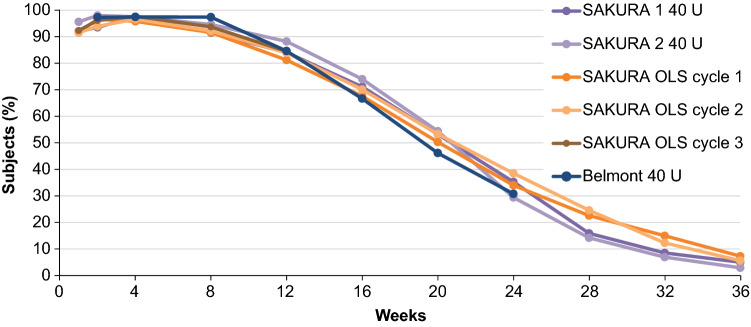
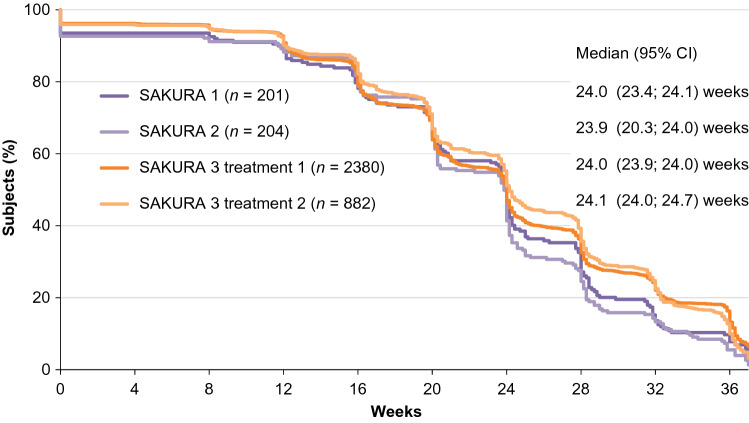
SAKURA 3 was an open-label, multicenter Phase 3 trial that evaluated single and repeat treatments of DAXI 40 U for up to 84 weeks [16, 34]. This study was an extension of the pivotal trials, including substantially more subjects (n = 2691) at 65 clinical sites in the USA and Canada. Efficacy outcomes and duration of effect were consistent with those reported in the Phase 2 and Phase 3 pivotal trials. The proportion of subjects who achieved none or mild glabellar line severity at maximum frown over time based on IGA-FWS score was consistent across the Phase 3 studies and after repeated doses, and also showed consistency with the Phase 2 dose-ranging study (Fig. 2). Median duration of effect (time to loss of none or mild glabellar line severity at maximum frown based on both the IGA-FWS and PFWS scores) was 24 weeks, which was remarkably consistent across the Phase 3 studies (Fig. 3), and median time to return to baseline severity on both scales was 28 weeks.
Fig. 2.
Consistency in response to DaxibotulinumtoxinA for Injection (DAXI) treatment across Phase 2 and 3 clinical trials. Response was defined as achievement of none or mild glabellar line (GL) severity at maximum frown based on investigator assessment via the validated 4-point Investigator Global Assessment-Frown Wrinkle Severity score (ranging from none [0] to severe [3]). OLS open-label study
Fig. 3.
Consistency in duration of effect (time to loss of none or mild glabellar line severity at maximum frown based on both IGA-FWS and PFWS) following DaxibotulinumtoxinA for Injection (DAXI) treatment in the Phase 3 SAKURA clinical trials. CI confidence interval, IGA-FWS Investigator Global Assessment-Frown Wrinkle Severity, PFWS Patient Frown Wrinkle Severity
The safety and tolerability profile of DAXI in SAKURA 3 was similar to that reported in the SAKURA 1 and SAKURA 2 trials with no new safety signals [34]. Headache and injection site AEs (pain, erythema, and edema) were the most common treatment-related AEs. These generally resolved within 1–2 days and were mild in severity. The frequency of AEs tended to decrease over subsequent DAXI treatments. No serious AEs were considered related to DAXI treatment. Treatment-related eyelid ptosis occurred with 0.9% of DAXI treatments; most ptosis events (82.4%) were mild in severity, and the median time to resolution of eyelid ptosis was 43 days. These rates are comparable to rates reported in prescribing information for onabotulinumtoxinA (3%), abobotulinumtoxinA (2%), prabotulinumtoxinA (2%), and incobotulinumtoxinA (0.2%) [5–7, 36]. No subjects developed neutralizing antibodies to DAXI after exposure to three cycles of treatment [34].
Overview of DAXI Cervical Dystonia Clinical Program
DAXI is being investigated for several therapeutic indications, including the treatment of cervical dystonia. Cervical dystonia, also known as spasmodic torticollis, is a chronic neurological condition resulting in painful and debilitating twisting of the neck and shoulders [37]. BoNTA treatment is currently recommended as first-line therapy for adults with cervical dystonia [2, 38], but patients report declining symptom relief at approximately 10–16 weeks after injection with currently approved BoNTAs [8–10, 39–41], necessitating 3–5 treatments per year to manage symptoms, which is a burden on patients and the healthcare system. Furthermore, as many payors do not permit re-treatment before the recommended reinjection interval of at least 12 weeks for cervical dystonia [5, 7, 10, 40], patients must endure periods when their recurring symptoms are not managed. Thus, there is a need for a safe and effective treatment that provides a longer duration of cervical dystonia symptom relief.
Phase 2 Dose-Ranging Study
An open-label, dose-escalation, Phase 2 study evaluated the efficacy and safety of three doses of DAXI (200 U, 200–300 U, and 300–450 U) in adults with moderate-to-severe isolated (primary) cervical dystonia [12]. Overall, 37 subjects were enrolled, received a single DAXI treatment, and were followed for up to 24 weeks. Using the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) total score, a measure of cervical dystonia severity, disability, and pain on a scale of 0–85 with higher scores indicating greater impairment [42], mean change from baseline was −16.86 (38%) at Week 4 (primary endpoint), −21.3 (50%) at Week 6, and −12.8 (30%) at Week 24 across all subjects. The response rate (the proportion of subjects with ≥ 20% reduction in TWSTRS total score from baseline) was 83% at Week 4, 94% at Week 6, and 68% at Week 24. Median duration of response was 25.3 weeks [12].
Treatment-related AEs were transient and mild or moderate in severity and no apparent dose response or dose-related increase was observed. The most common treatment-related AEs were dysphagia (14% of subjects) and injection-site erythema (8% of subjects), and these were unrelated to the dose administered. No serious AEs occurred [12]. No subjects developed neutralizing antibodies in this study [43].
Phase 3 Program
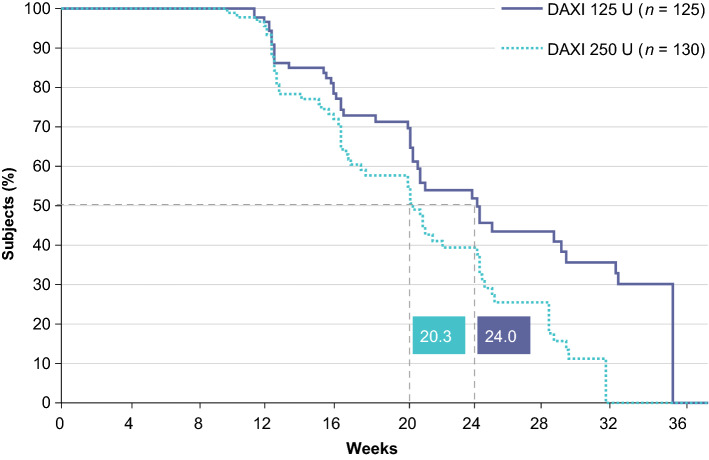
The Phase 3 program in adults with cervical dystonia includes a pivotal, randomized, double-blind, placebo-controlled Phase 3 trial (ASPEN-1) and a long-term, open-label efficacy and safety study (ASPEN-OLS) conducted in Canada, Europe, and the USA [44, 45]. ASPEN-1 (n = 301 subjects), which was conducted to evaluate the efficacy and safety of two doses of DAXI (125 U or 250 U), completed in June 2020 [44]. At peak efficacy, averaged over Weeks 4 and 6, least squares mean change from baseline in TWSTRS total score was −12.7 (31%) with DAXI 125 U and −10.9 (27%) with DAXI 250 U, which was a significantly greater improvement compared with placebo (least squares mean [standard error] difference vs placebo −8.5 [1.93] and −6.6 [1.92], respectively; p < 0.001 for each dose) [13]. These improvements in TWSTRS total score with DAXI are at least as large as reported with other BoNTAs at Week 4 (incobotulinumtoxinA difference vs placebo −7.5 with 120 U and −9.0 with 240 U; abobotulinumtoxinA difference vs placebo −8.9 and −5.9 with 500 U) [5, 46]. Median duration of effect, as determined by time to loss of 80% peak treatment benefit, was 24.0 weeks for DAXI 125 U and 20.3 weeks for DAXI 250 U (Fig. 4), which is longer than the median effect duration of 12–14 weeks reported with onabotulinumtoxinA, abobotulinumtoxinA, and incobotulinumtoxinA [4, 5, 47]. DAXI was generally safe and well tolerated, with dysphagia reported in 1.6% of subjects treated with DAXI 125 U and 3.8% of subjects treated with DAXI 250 U [13]. This is considerably lower than the incidence of dysphagia reported in registration trials of adults with cervical dystonia treated with onabotulinumtoxinA (19%), abobotulinumtoxinA (15%), and incobotulinumtoxinA (13–18%) [4, 5, 7]. The median duration of dysphagia events was 14 days, which is also substantially less than has been reported for other BoNTAs in cervical dystonia [48].
Fig. 4.
Median time to loss of ≥ 80% of peak treatment effect following DaxibotulinumtoxinA for Injection (DAXI) treatment in adults with moderate-to-severe cervical dystonia in the Phase 3 ASPEN-1 trial
Subjects enrolled in the ASPEN-OLS study (n = 358) were eligible for up to four doses of DAXI over 48 weeks [45]. The study completed in May 2021 and data were not available at the time of writing.
Clinical Differentiation of DAXI Versus Currently Available BoNTA Products
Any comparison between BoNTA products is complicated by the fact that there is no standardized dosing unit for BoNTA products because biological activity is defined by mouse LD50 assay (median lethal dose in mice), and each manufacturer has their own methodology for conducting the LD50 assay as well as their own proprietary reference standard [49]. Therefore, for BoNTAs, units are a relevant measure for comparing dosing for a given toxin product, such as in dose-escalation studies or when comparing the effective doses for aesthetic and therapeutic indications, but regulatory agencies worldwide warn against using units for comparing dosing between different toxin products.
The pharmacological effect of BoNTA is mediated by the core 150-kDa neurotoxin (the active component of all BoNTA products); however, the clinical efficacy will be a function of the amount of active ingredient and the efficiency of delivery of that active ingredient into nerve terminals. The latter is significantly influenced by the formulation.
All currently approved conventional BoNTA products are formulated with HSA as a stabilizer, which prevents loss of activity through protein aggregation and protein adsorption to vial and, potentially, syringe surfaces. These products yield very similar clinical performance at approved doses for cervical dystonia and glabellar lines, with duration in the 3- to 4-month range and similar magnitude of peak efficacy [4–7, 36].
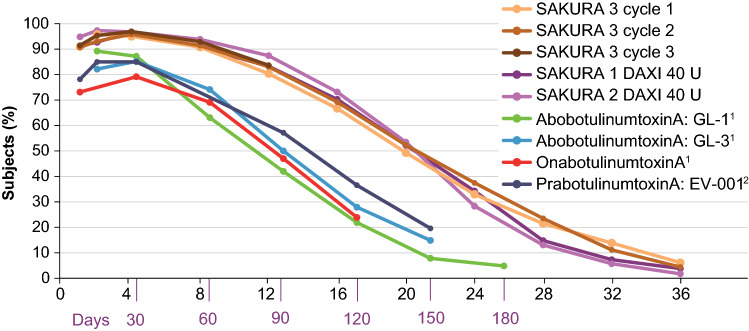
DAXI’s unique formulation, comprising the RTP004 peptide and other excipients, allows the product to be formulated without HSA and permits a greater functional efficiency to be obtained with the given amount of active core neurotoxin. For example, in the glabellar lines indication, despite a similar mass of core neurotoxin with DAXI 40 U and onabotulinumtoxinA 20 U (each 0.18 ng of core neurotoxin), results from a controlled Phase 2 study demonstrated that DAXI 40 U yielded greater efficacy and duration of effect than onabotulinumtoxinA 20 U with a similar safety profile [32]. Conversely, the mass of core neurotoxin in the 50 U dose of abobotulinumtoxinA (0.27 ng of core neurotoxin) yields a clinical efficacy profile in glabellar lines that is generally similar to that of 20 U onabotulinumtoxinA (0.18 ng of core neurotoxin) (Fig. 5), and a duration of effect of 3–4 months, despite its higher mass of neurotoxin [50]. The higher amount of neurotoxin required for efficacy of abobotulinumtoxinA is a function of the lower amount of HSA in that formulation, demonstrating insufficient stabilization [51, 52]. Lastly, in cervical dystonia, a dose of DAXI 125 U (0.56 ng of core neurotoxin) demonstrated 24 weeks of clinical benefit [13], whereas, in a separate controlled study with a similar patient population, onabotulinumtoxinA, at an average dose of 205 U (1.70 ng of core neurotoxin), produced a clinical benefit of 14 weeks [47]. These differences conclusively demonstrate that the unique formulation of DAXI is the reason for greater efficacy, and further illustrates the impact of product formulation on the clinical profile of a BoNT product.
Fig. 5.
None or mild response rates for glabellar lines on 4-point investigator assessment over time. This figure presents results from multiple randomized registration studies. Such cross-study comparisons should be interpreted with caution due to potential differences in study design and subject characteristics. 1BOTOX® and Dysport® data derived from US Prescribing Information Phase 3 studies in glabellar lines for each neuromodulator with data available through at least Day 120 conducted separately and presented for reference only 2Beer et al [54]. DAXI DaxibotulinumtoxinA for Injection
In summary, dosing of BoNTA products is complicated by the lack of standardized dosing units and, therefore, non-interchangeability of dosing guidance for each indication. Considering the complete formulation permits a more natural comparison of BoNTA products and illustrates the significant differences imparted by formulation on the clinical efficiency of the products.
Conclusion
DAXI has the potential to be the first BoNTA product approved in the USA with a demonstrated duration of effect up to 24 weeks. Importantly, a consistent duration of efficacy has been observed following DAXI treatment of cervical dystonia and glabellar lines, suggesting that DAXI has the potential to improve the management of both therapeutic and aesthetic conditions as well as requiring fewer treatments per year. Its unique formulation, utilizing a novel proprietary stabilizing excipient peptide (RTP004) rather than HSA, as well as the favorable clinical profile, high response rate, and extended duration of effect achieved with a similar or lower amount of core neurotoxin, distinguish DAXI from currently approved BoNTA products.
Declarations
Funding
Neither honoraria nor payments were made for authorship. The open access fee for the manuscript was funded by Revance Therapeutics, Inc. Writing and editorial assistance was provided to the authors by Evidence Scientific Solutions, Philadelphia, PA, and funded by Revance Therapeutics, Inc., Newark, CA.
Conflicts of interest
NS, JC, and JK are investigators for Revance Therapeutics, Inc. RGR, TMG, and CJG are employees and stockholders at Revance Therapeutics, Inc.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
All authors were involved in the drafting, critical revision, and approval of the final version of the manuscript.
References
- 1.Fonfria E, Maignel J, Lezmi S, Martin V, Splevins A, Shubber S, et al. The expanding therapeutic utility of botulinum neurotoxins. Toxins (Basel). 2018;10(5):208. doi: 10.3390/toxins10050208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simpson DM, Hallett M, Ashman EJ, Comella CL, Green MW, Gronseth GS, et al. Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2016;86(19):1818–1826. doi: 10.1212/WNL.0000000000002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sundaram H, Signorini M, Liew S, Trindade de Almeida AR, Wu Y, Vieira Braz A, et al. Global aesthetics consensus: botulinum toxin type A–evidence-based review, emerging concepts, and consensus recommendations for aesthetic use, including updates on complications. Plast Reconstr Surg. 2016;137(3):518e–e529. doi: 10.1097/01.prs.0000475758.63709.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allergan. BOTOX (onabotulinumtoxinA) for injection, for intramuscular, intradetrusor, or intradermal use prescribing information. 2020. https://media.allergan.com/actavis/actavis/media/allergan-pdf-documents/product-prescribing/20190620-BOTOX-100-and-200-Units-v3-0USPI1145-v2-0MG1145.pdf. Accessed 4 Aug 2021.
- 5.Ipsen. DYSPORT (abobotulinumtoxinA) for injection, for intramuscular use prescribing information. 2018. https://www.ipsen.com/websites/Ipsen_Online/wp-content/uploads/sites/9/2019/01/21084019/Dysport_Full_Prescribing_Information.pdf. Accessed 1 Mar 2021.
- 6.Allergan. BOTOX Cosmetic (onabotulinumtoxinA) for injection, for intramuscular use prescribing information. 2019. https://media.allergan.com/actavis/actavis/media/allergan-pdf-documents/product-prescribing/20190626-BOTOX-Cosmetic-Insert-72715US10-Med-Guide-v2-0MG1145.pdf. Accessed 12 Mar 2021.
- 7.Merz. XEOMIN (incobotulinumtoxinA) for injection, for intramuscular or intraglandular use prescribing information. 2019. https://www.xeominaesthetic.com/wp-content/uploads/2019/05/XEOMIN-Full-Prescribing-Information-including-MedGuide.pdf. Accessed 12 Mar 2021.
- 8.Sethi KD, Rodriguez R, Olayinka B. Satisfaction with botulinum toxin treatment: a cross-sectional survey of patients with cervical dystonia. J Med Econ. 2012;15(3):419–423. doi: 10.3111/13696998.2011.653726. [DOI] [PubMed] [Google Scholar]
- 9.Comella C, Ferreira JJ, Pain E, Azoulai M, Om S. Patient perspectives on the therapeutic profile of botulinum neurotoxin type A in cervical dystonia. J Neurol. 2020;286(3):903–912. doi: 10.1007/s00415-020-10217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poliziani M, Koch M, Liu X. Striving for more good days: patient perspectives on botulinum toxin for the treatment of cervical dystonia. Patient Prefer Adherence. 2016;10:1601–1608. doi: 10.2147/PPA.S106560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Revance. Data on file.
- 12.Jankovic J, Truong D, Patel AT, Brashear A, Evatt M, Rubio RG, et al. Injectable daxibotulinumtoxinA in cervical dystonia: a phase 2 dose-escalation multicenter study. Mov Disord Clin Pract. 2018;5(3):273–282. doi: 10.1002/mdc3.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jankovic J, Comella C, Hauser RA, Patel AT, Gross TM, Rubio RG, et al. ASPEN-1. A phase 3 trial evaluating the efficacy, duration of effect, and safety of DaxibotulinumtoxinA for Injection in the treatment of cervical dystonia. Poster presented at: TOXINS 2021; January 16–17, 2021; Virtual.
- 14.Overman D. DaxibotulinumtoxinA Injection for upper-limb spasticity study receives positive results [press release]. February 24, 2021. https://rehabpub.com/conditions/neurological/stroke-neurological/daxibotulinumtoxina-injection-for-upper-limb-spasticity-study-receives-positive-results/. Accessed 4 Aug 2021.
- 15.Carruthers JD, Fagien S, Joseph JH, Humphrey SD, Biesman BS, Gallagher CJ, et al. DaxibotulinumtoxinA for Injection for the treatment of glabellar lines: results from each of two multicenter, randomized, double-blind, placebo-controlled, phase 3 studies (SAKURA 1 and SAKURA 2) Plast Reconstr Surg. 2020;145(1):45–58. doi: 10.1097/PRS.0000000000006327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabi SG, Cohen JL, Green LJ, Dhawan S, Kontis TC, Baumann L, et al. DaxibotulinumtoxinA for Injection for the treatment of glabellar lines: efficacy results from SAKURA 3, a large, open-label, phase 3 safety study. Dermatol Surg. 2021;47(1):48–54. doi: 10.1097/dss.0000000000002531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solish N, Green JB, Fagien S, Bertucci V, Gallagher CJ, Liu Y, et al. A phase 2a dose-escalation study to evaluate the efficacy and safety of DaxibotulinumtoxinA for Injection for the treatment of dynamic forehead lines following glabellar line injections: an interim analysis. Poster presented at: MauiDerm 2020; January 25–29, 2020; Maui, HI.
- 18.Stone HF, Zhu Z, Thach TQ, Ruegg CL. Characterization of diffusion and duration of action of a new botulinum toxin type A formulation. Toxicon. 2011;58(2):159–167. doi: 10.1016/j.toxicon.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Walker TJ, Dayan SH. Comparison and overview of currently available neurotoxins. J Clin Aesthet Dermatol. 2014;7(2):31–39. [PMC free article] [PubMed] [Google Scholar]
- 20.Malmirchegini R, Too P, Oliyai C, Joshi A. Revance's novel peptide excipient, RTP004, and its role in stabilizing DaxibotulinumtoxinA (DAXI) against aggregation. Poster presented at: TOXINS 2019; January 16–19, 2019; Copenhagen, Denmark.
- 21.Sebbage V. Cell-penetrating peptides and their therapeutic applications. Biosci Horiz. 2009;2(1):64–72. doi: 10.1093/biohorizons/hzp001. [DOI] [Google Scholar]
- 22.Ruseska I, Zimmer A. Internalization mechanisms of cell-penetrating peptides. Beilstein J Nanotechnol. 2020;11:101–123. doi: 10.3762/bjnano.11.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vale N, Duarte D, Silva S, Correia AS, Costa B, Gouveia MJ, et al. Cell-penetrating peptides in oncologic pharmacotherapy: a review. Pharmacol Res. 2020;162:105231. doi: 10.1016/j.phrs.2020.105231. [DOI] [PubMed] [Google Scholar]
- 24.Kurrikoff K, Vunk B, Langel Ü. Status update in the use of cell-penetrating peptides for the delivery of macromolecular therapeutics. Expert Opin Biol Ther. 2021;21(3):361–370. doi: 10.1080/14712598.2021.1823368. [DOI] [PubMed] [Google Scholar]
- 25.Reissmann S, Filatova MP. New generation of cell-penetrating peptides: functionality and potential clinical application. J Pept Sci. 2021;27(5):e3300. doi: 10.1002/psc.3300. [DOI] [PubMed] [Google Scholar]
- 26.Korivi M, Huang YW, Liu BR. Cell-penetrating peptides as a potential drug delivery system for effective treatment of diabetes. Curr Pharm Des. 2020;27(6):816–825. doi: 10.2174/1381612826666201019102640. [DOI] [PubMed] [Google Scholar]
- 27.Weisemann J, Rummel A, Oliyai C, Too P, Joshi A. novel peptide excipient RTP004 enhances the binding of botulinum neurotoxin A cell binding domain Hc to rat brain synaptosomes. Poster presented at: TOXINS 2019; January 16–19, 2019; Copenhagen, Denmark.
- 28.Yin L, Masuyer G, Zhang S, Zhang J, Miyashita SI, Burgin D, et al. Characterization of a membrane binding loop leads to engineering botulinum neurotoxin B with improved therapeutic efficacy. PLoS Biol. 2020;18(3):e3000618. doi: 10.1371/journal.pbio.3000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glogau RG, Waugh JM. Preclinical transcutaneous flux experiments using a macromolecule transport system (MTS) peptide for delivery of botulinum toxin type A. Poster presented at: Annual Meeting of the American Academy of Dermatology; February 1–8, 2008; San Antonio, TX.
- 30.Smyth T, Oliyai C, Joshi A. Stabilizing effect of RTP004 on non-specific surface adsorption in drug product manufacturing of daxibotulinumtoxinA (DAXI). Poster presented at: TOXINS 2019; January 16–19, 2019; Copenhagen, Denmark.
- 31.Garcia-Murray E, Velasco Villasenor ML, Acevedo B, Luna S, Lee J, Waugh JM, et al. Safety and efficacy of RT002, an injectable botulinum toxin type A, for treating glabellar lines: results of a phase 1/2, open-label, sequential dose-escalation study. Dermatol Surg. 2015;41(Suppl 1):S47–55. doi: 10.1097/DSS.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 32.Carruthers J, Solish N, Humphrey S, Rosen N, Muhn C, Bertucci V, et al. Injectable DaxibotulinumtoxinA for the treatment of glabellar lines: a phase 2, randomized, dose-ranging, double-blind, multicenter comparison with onabotulinumtoxinA and placebo. Dermatol Surg. 2017;43(11):1321–1331. doi: 10.1097/DSS.0000000000001206. [DOI] [PubMed] [Google Scholar]
- 33.Bertucci V, Solish N, Kaufman-Janette J, Yoelin S, Shamban A, Schlessinger J, et al. DaxibotulinumtoxinA for Injection has a prolonged duration of response in the treatment of glabellar lines: Pooled data from two multicenter, randomized, double-blind, placebo-controlled, phase 3 studies (SAKURA 1 and SAKURA 2) J Am Acad Dermatol. 2020;82(4):838–845. doi: 10.1016/j.jaad.2019.06.1313. [DOI] [PubMed] [Google Scholar]
- 34.Green JB, Mariwalla K, Coleman K, Ablon G, Weinkle SH, Gallagher CJ, et al. A large, open-label, phase 3 safety study of DaxibotulinumtoxinA for Injection in glabellar lines: a focus on safety from the SAKURA 3 study. Dermatol Surg. 2021;47(1):42–46. doi: 10.1097/dss.0000000000002463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertucci V, Humphrey S, Carruthers J, Solish N, Muhn C, Swift A, et al. Comparing injectable daxibotulinumtoxinA and onabotulinumtoxinA in moderate and severe glabellar lines: additional analyses from a phase 2, randomized, dose-ranging, double-blind, multicenter study. Dermatol Surg. 2017;43:S262–S273. doi: 10.1097/DSS.0000000000001364. [DOI] [PubMed] [Google Scholar]
- 36.Evolus. JEUVEAU (prabotulinumtoxinA-xvfs) for injection, for intramuscular use prescribing information. 2020. https://info.evolus.com/hubfs/Prescribing%20Info_20200130.pdf. Accessed 12 Mar 2021.
- 37.Dystonia Medical Research Foundation. Cervical dystonia. https://dystonia-foundation.org/what-is-dystonia/types-dystonia/cervical-dystonia/. Accessed 14 Mar 2021.
- 38.Contarino MF, Van Den Dool J, Balash Y, Bhatia K, Giladi N, Koelman JH, et al. Clinical practice: evidence-based recommendations for the treatment of cervical dystonia with botulinum toxin. Front Neurol. 2017;8:35. doi: 10.3389/fneur.2017.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marsh WA, Monroe DM, Brin MF, Gallagher CJ. Systematic review and meta-analysis of the duration of clinical effect of onabotulinumtoxinA in cervical dystonia. BMC Neurol. 2014;14:91. doi: 10.1186/1471-2377-14-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evidente VG, Pappert EJ. Botulinum toxin therapy for cervical dystonia: the science of dosing. Tremor Other Hyperkinet Mov (N Y). 2014;4:273. doi: 10.7916/D84X56BF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dressler D, Tacik P, Saberi FA. Botulinum toxin therapy of cervical dystonia: duration of therapeutic effects. J Neural Transm (Vienna) 2015;122(2):297–300. doi: 10.1007/s00702-014-1253-8. [DOI] [PubMed] [Google Scholar]
- 42.Consky E, Lang A. Clinical assessments of patients with cervical dystonia. In: Jankovic J, Hallett M, editors. Therapy with botulinum toxin. New York: Marcel Dekker; 1994. pp. 211–237. [Google Scholar]
- 43.Brashear A, Truong D, Comella C, Jankovic J, Patel A, Prawdzik G, et al. DaxibotulinumtoxinA for Injection in adults with cervical dystonia from a phase 2 dose-escalation multicenter study. Poster presented at: American Academy of Neurology 2019, May 4-10, 2019; Philadelphia, PA.
- 44.ClinicalTrials.gov. Single treatment of DaxibotulinumtoxinA for Injection in adults with isolated cervical dystonia (ASPEN-1). https://clinicaltrials.gov/ct2/show/NCT03608397?term=cervical+dystonia&cond=daxibotulinumtoxinA&draw=2&rank=1. Accessed 30 July 2020.
- 45.ClinicalTrials.gov. Long-term safety and efficacy of repeat treatments of DaxibotulinumtoxinA for Injection in adults with isolated cervical dystonia (ASPEN-OLS). https://clinicaltrials.gov/ct2/show/NCT03617367?term=DaxibotulinumtoxinA&cond=Cervical+Dystonia&phase=2&fund=2&draw=2&rank=1. Accessed 19 Nov 2020.
- 46.Comella CL, Jankovic J, Truong DD, Hanschmann A, Grafe S, US XEOMIN Cervical Dystonia Study Group Efficacy and safety of incobotulinumtoxinA (NT 201, XEOMIN®, botulinum neurotoxin type A, without accessory proteins) in patients with cervical dystonia. J Neurol Sci. 2011;308(1–2):103–109. doi: 10.1016/j.jns.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 47.Comella CL, Jankovic J, Shannon KM, Tsui J, Swenson M, Leurgans S, et al. Comparison of botulinum toxin serotypes A and B for the treatment of cervical dystonia. Neurology. 2005;65(9):1423–1429. doi: 10.1212/01.wnl.0000183055.81056.5c. [DOI] [PubMed] [Google Scholar]
- 48.Naumann M, Yakovleff A, Durif F, BOTOX Cervical Dystonia Prospective Study Group A randomized, double-masked, crossover comparison of the efficacy and safety of botulinum toxin type A produced from the original bulk toxin source and current bulk toxin source for the treatment of cervical dystonia. J Neurol. 2002;249(1):57–63. doi: 10.1007/pl00007848. [DOI] [PubMed] [Google Scholar]
- 49.Frevert J. Content of botulinum neurotoxin in Botox®/Vistabel®, Dysport®/Azzalure®, and Xeomin®/Bocouture®. Drugs R D. 2010;10(2):67–73. doi: 10.2165/11584780-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.U. S. Food and Drug Administration. Center for Drug Evaluation and Research. Medical review: Dysport - abobotulinumtoxin A. 2009. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/125274Orig1s001MedR.pdf. Accessed 25 Feb 2021.
- 51.Bigalke H, Wohlfarth K, Irmer A, Dengler R. Botulinum A toxin: dysport improvement of biological availability. Exp Neurol. 2001;168(1):162–170. doi: 10.1006/exnr.2000.7583. [DOI] [PubMed] [Google Scholar]
- 52.Mohammadi B, Kollewe K, Wegener M, Bigalke H, Dengler R. Experience with long-term treatment with albumin-supplemented botulinum toxin type A. J Neural Transm (Vienna) 2009;116(4):437–441. doi: 10.1007/s00702-009-0200-6. [DOI] [PubMed] [Google Scholar]
- 53.Field M, Splevins A, Picaut P, van der Schans M, Langenberg J, Noort D, et al. AbobotulinumtoxinA (Dysport®), OnabotulinumtoxinA (Botox®), and IncobotulinumtoxinA (Xeomin®) neurotoxin content and potential implications for duration of response in patients. Toxins (Basel). 2018;10(12):535. doi: 10.3390/toxins10120535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beer KR, Shamban AT, Avelar RL, Gross JE, Jonker A. Efficacy and safety of prabotulinumtoxinA for the treatment of glabellar lines in adult subjects: results from 2 identical phase III studies. Dermatol Surg. 2019;45(11):1381–1393. doi: 10.1097/dss.0000000000001903. [DOI] [PMC free article] [PubMed] [Google Scholar]