Abstract
Introduction
Some studies report that assessing regional 123I-cardiac MIBG uptake can aid in the diagnosis of Lewy body disease, but others report heterogeneity in healthy controls. We aimed to evaluate regional cardiac MIBG uptake patterns in healthy older adults and patients with dementia.
Methods
31 older adults with normal cognition, 15 Alzheimer’s disease (AD), and 17 Dementia with Lewy bodies (DLB) patients were recruited. 5 individuals had previous myocardial infarction. Participants with sufficient cardiac uptake for regional SPECT analysis (29/31 controls, 15/15 AD, 5/17 DLB) had relative uptake pattern recorded. Controls were assessed for risk of future cardiovascular events using QRISK2, a validated online tool.
Results
In controls uptake was reduced in the inferior wall (85%), apex (23%), septum (15%), and lateral wall (8%). AD and DLB showed similar patterns to controls. Lung or liver interference was present in 61% of cases. Myocardial infarction cases showed regional reductions in uptake, but normal/borderline planar uptake. In controls, there was no relationship between cardiovascular risk score and uptake pattern.
Conclusions
Significant variability of regional cardiac 123I-MIBG uptake is common in cases with normal planar cardiac uptake. Heterogeneity of regional uptake appears non-specific and unlikely to aid in the diagnosis of Lewy body disease.
Electronic supplementary material
The online version of this article (10.1007/s12350-019-01977-5) contains supplementary material, which is available to authorized users.
Keywords: Cardiac MIBG, SPECT, sympathetic innervation, cardiac MIBG uptake homogeneity, SPECT artifacts
Background
Dementia with Lewy bodies (DLB) is the second most common form of neurodegenerative dementia after Alzheimer’s disease, accounting for 5-10% of cases.1,2 Accurate diagnosis is important for clinical management, prognosis, and carer wellbeing,3–5 but initial misdiagnosis outside the specialist setting is common.2,6,7 Cardiac 123I-metaiodobenzylguanidine sympathetic innervation imaging (“cardiac MIBG”) is an established technique for differentiating DLB from other dementias. Sympathetic denervation occurs in DLB, resulting in low cardiac MIBG uptake on the scan, whereas healthy controls and patients with other dementias such as Alzheimer’s disease have normal uptake.8–14 The technique has been used in Japan in Lewy body disease for over a decade and was recently included as an indicative biomarker in the fourth DLB consensus criteria, alongside 123I-FP-CIT SPECT.6
Cardiac MIBG studies are typically quantified on planar images using the heart-to-mediastinum ratio (HMR) as a diagnostic indicator, where HMR is the ratio between the count density in a cardiac left ventricular region (mean pixel value) and the count density in a mediastinal region of non-specific uptake. Abnormal scans have reduced cardiac uptake as indicated by a low HMR. The HMR is an assessment of the overall cardiac uptake and does not take regional differences in uptake throughout the left ventricle into account. Regional variation of uptake can be assessed using SPECT imaging.
Published studies exploring the use of SPECT cardiac MIBG imaging for diagnosing Lewy body disease have suggested that regional assessment of cardiac uptake could improve sensitivity as regional defects may occur before global loss is apparent.13,15–19 However, we have noted that regional count reductions are sometimes seen in probable AD subjects who have normal planar scan appearances, suggesting that discrete areas of reduced regional counts may be non-specific, e.g., due to unreported silent myocardial infarction, or artifactual, e.g., due to attenuation or the influence of extra-cardiac activity. It has been reported in the literature that heterogeneous cardiac MIBG count distribution can occur in healthy controls, with relative reduction most commonly seen in the inferior wall.19–26 This may be due to true physiological variation in sympathetic nerve distribution or artifacts caused by the imaging process—either from the signal being attenuated by the body or by interference from non-cardiac activity, predominantly the liver.27–29 Although several groups investigating the diagnostic accuracy of cardiac 123I-MIBG for the diagnosis of Lewy body disease have acquired SPECT data alongside planar, often it is only the planar scans that have been used for analysis with the SPECT results not reported,12,30–33 suggesting that the added value of SPECT is unclear. In their 2015 multicentre study, Yoshita et al used SPECT for visual analysis but it was not clear on what basis the SPECT images were deemed normal or abnormal.10
We recruited volunteers over 60 years of age, healthy controls, DLB patients, and Alzheimer’s disease patients, and performed cardiac MIBG SPECT imaging to gain a better understanding of cardiac MIBG distribution in these groups and therefore how to interpret patient scans. Our objective was to compare the distribution pattern of MIBG in the heart seen in these clinical groups. The underlying hope was that we may be able to distinguish confounding regional reductions in cardiac uptake due to cardiac disease from pathological ones due to Lewy body disease by looking at heterogeneity, thereby improving specificity. Sensitivity may also be improved if we were to find any characteristic patterns of reduction associated with Lewy body disease.
Methods
Participants
Older people without cognitive impairment (controls)
Thirty-one healthy adults aged over 60 years were recruited as part of an ongoing study into cardiac MIBG in a representative UK population of older adults. They underwent a detailed neurological and cognitive examination by a research physician. All had had normal cognition, no parkinsonism, and had a normal MRI brain scans. No subjects had Class II or worse heart failure according to the New York Heart Association classification,34 or had had a myocardial infarction in the previous year or were taking tramadol, labetalol or tricyclic antidepressants. Previous cardiac history, such as myocardial infarction (MI) more than 12 months prior to recruitment or atrial fibrillation was recorded as part of medical history taking. Subjects were not excluded if they had risk factors for cardiac disease, such as a history of smoking, raised blood pressure, or Type 2 diabetes because we aimed to recruit typical older adults with normal cognition. In our clinical studies in DLB we aim to assess cardiac MIBG in a “real-world” setting, where risk factors for cardiac disease are extremely common. However, a detailed medical history was taken, which later enabled an estimate of cardiac risk to be made using the QRISK2 tool, recommended by NICE in Clinical Guideline 181 Cardiovascular disease: risk assessment and reduction.35 QRISK2 uses the ratio of total cholesterol to high density lipoproteins as a risk factor, with a ratio of 4 used as default. These parameters were not part of our original study design so were not available for most of the volunteers. As the true ratios were not known, a ratio of 6, which is regarded as the upper limit by Heart UK, was input for those with high cholesterol disclosed in their medical history and the default of 4 used for everyone else. Two subjects had documented heart disease, making the QRISK2 score inappropriate—their risk scores were still calculated with this recorded as a limitation. QRISK2 scores in controls were assessed for any correlation with cardiac uptake using linear regression.
Older people with dementia
Seventeen patients meeting the clinical criteria for probable dementia with Lewy bodies and 15 meeting criteria for dementia due to Alzheimer’s disease were recruited from memory clinics, as described in our previous publications.36,37 Briefly, patients had their dementia diagnoses confirmed by an expert panel of old-age psychiatrists. They were categorized as having probable DLB if two or more of the core consensus criteria for DLB 6 (fluctuations, visual hallucinations, REM sleep behavior disorder and parkinsonism) were present, and AD if none of these were present. The same broad general inclusion criteria as discussed above for the controls were applied to ensure the patients were typical of the local population. Cardiac risk was not calculated for the dementia patients since their cardiac uptake was expected to be affected by Lewy body disease in many cases. However cardiac history was recorded to enable comparison with SPECT imaging results. We have previously showed no convincing evidence of lower cardiac MIBG uptake in patients with a previous MI, although this was a small subset.36
Image Acquisition
All participants were administered 111 ± 10% MBq I-123-MIBG via slow intravenous injection. Potassium iodate tablets (170 mg) were given before and after injection to minimize uptake of free iodine by the thyroid. Images were acquired on a dual headed Siemens Symbia Intevo or Siemens Symbia T series gamma camera (Siemens Healthcare, Munich, Germany) with medium energy low penetration (MELP) collimators. Ten minute anterior planar images were acquired at 4 h (± 30 min) after injection. SPECT imaging was carried out immediately after the planar image with the subject in the supine position with arms raised if possible; however the majority of controls (19/31) and patients (7/15 AD; 13/17 DLB) were scanned with their arms by their sides. Due to patient comfort it was not possible to complete SPECT imaging, even with arms down, for two of the 17 DLB patients, both of whom had low uptake on planar imaging. For planar imaging the energy window was 159 keV ± 10%, matrix size was 128 × 128 and no zoom was applied. SPECT images were acquired in H mode over 360 degrees with 120 projections of 20 s using a non-circular autocontoured orbit. The energy window was 159 keV ± 7.5%, matrix size 64 × 64, and zoom 1.64.
Image Processing
All images were processed on a Hermes workstation (Hermes Medical Ltd, Stockholm). Delayed anterior planar images were analyzed to obtain the heart-to-mediastinum ratio (HMR) using a 6 cm circular ROI placed over the left ventricle and 4 × 3 cm rectangular ROI between the lungs in the mediastinum as described in our previous publication.37 SPECT images were processed in Hermes Hybrid recon using filtered back projection with a Butterworth filter of 0.38 cycles per cm cut-off and order 8 applied. The images were reoriented during reconstruction to align with the horizontal and vertical long axes of the myocardium, as is standard for myocardial perfusion SPECT, with as much extra-cardiac activity (e.g., liver and lung) masked out as possible.
Image Assessment
Quantitative perfusion SPECT (QPS) software (Hermes Medical Ltd, Stockholm) was used to display the short axis, vertical long axis, and horizontal long axis slice images in rows for visual assessment of relative uptake. Polar plots of relative uptake pattern were also reviewed. These plots display the average relative uptake in the apex, anterior, lateral, inferior, and septal walls as a percentage of the maximum pixel value. The images and polar plots were reviewed blinded to the clinical groupings by a Consultant Clinical Scientist experienced in using the software for myocardial perfusion imaging (EJ). The overall degree of uniformity and the relative uptake for each of the left ventricle walls (anterior, inferior, septal, lateral, and the apex) was recorded using the categories given in Table 1. The ratings categories were developed by GR, EJ and JJL by reviewing cardiac MIBG images not included in the current study.
Table 1.
Rating categories for overall assessment of uptake pattern in the left ventricle and of uptake in each of the ventricular wall regions
| Overall rating categories | Uptake categories for each wall | Interference categories |
|---|---|---|
|
Not possible to score due to interference Definitely uniform Very slight heterogeneity Patchy Clear defect Multiple clear defects Other - see comment |
Normal Mildly reduced/patchy Moderately reduced Severely reduced No uptake |
No significant interference Liver interference Lung interference Both liver and lung interference |
Results
Controls with Normal Cognition
Of the 31 controls recruited, 2 had to be excluded due to their image appearances (see below). The remaining 29 people included were aged between 62 and 94 years (mean 75.2). Most were male (22/29) and white (28/29). Body mass indices (BMI) ranged from 22 to 38 kg/m2, with the mean value of 27.2 in the overweight category. These demographics are summarized in the controls and dementia patients are taken from concurrent clinical studies, so not all the same information was collected for dementia patients.
Table 2 alongside incidence of cardiovascular risk factors such as smoking. The controls and dementia patients are taken from concurrent clinical studies, so not all the same information was collected for dementia patients.
Table 2.
Demographics summary for the controls and patients with DLB and AD included in the study
| Controls | DLB | AD | |
|---|---|---|---|
| Age | |||
| Mean ± SD | 75.2 ± 8.3 | 77.5 ± 8.0 | 76.2 ± 6.8 |
| Range | 62 to 94 years | 60 to 89 years | 62 to 85 years |
| Sex | 7 female (24%) | 2 female (12%) | 4 female (27%) |
| 22 male (76%) | 15 male (88%) | 11 male (73%) | |
| BMI (kg/m2) | |||
| Mean ± SD | 27.8 ± 4.2 | Not measured | Not measured |
| Range | 21.6 to 37.8 | ||
| Ethnicity | 28 white (97%) | Not recorded | Not recorded |
| 1 Asian (3%) | |||
| Smoking | 13 never smoked (45%) | Not measured | Not measured |
| 13 ex-smokers (45%) | |||
| 2 current smokers (7%) | |||
| 1 not recorded (3%) | |||
| Diabetes | 0 Type I (0%) | Not recorded | Not recorded |
| 3 Type II (10%) | |||
| Systolic blood pressure | |||
| Mean ± SD | 140.5 ± 17.8 | Not recorded | Not recorded |
| Range | 112 to 173 | ||
| On blood pressure medication | 8 (28%) | 4 (24%) | 5 (33%) |
| Hypercholesterinaemia documented | 11 (34%) | Not recorded | Not recorded |
| Documented cardiac history | Previous MI and AF: 1 (3%) | Previous MI: 2 (12%) | Previous MI: 3 (20%) |
| Angina: 1 (3%) | |||
| ECG changes: 1 (3%) | |||
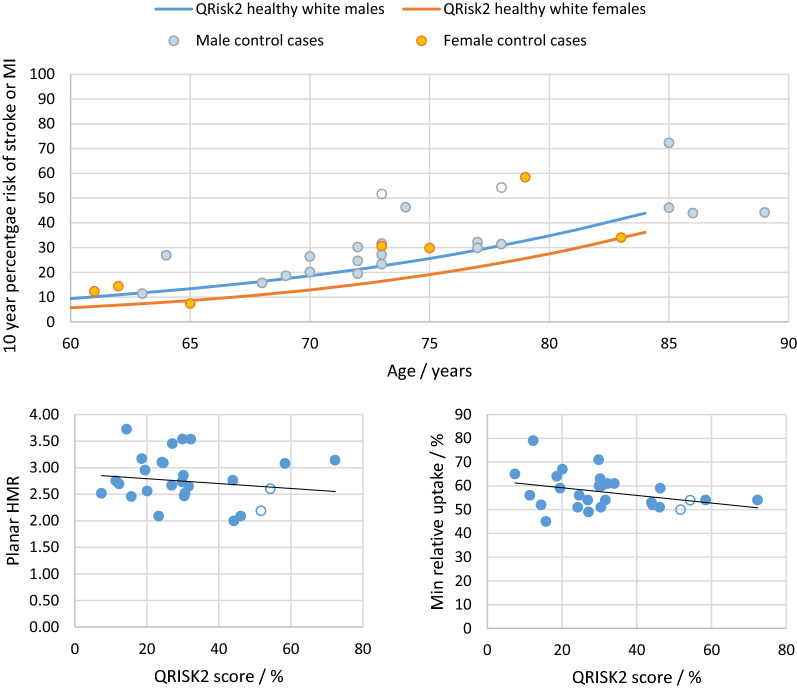
Ten-year cardiac risk scores assessed with QRISK2 showed the group to be at high risk, as expected for older adults (Figure 1). Most subjects were at higher risk than expected for age due to the inclusion of one or more cardiac risk factors in the calculation. However, despite slight downward trends, there was no significant correlation between cardiac risk and either overall cardiac MIBG uptake as given by planar HMR (P = .64) or minimum percentage relative uptake on SPECT (P = .08) (Figure 1).
Figure 1.
Top: Plot of cardiac risk for our control subjects against subject age for males (blue data points) and females (orange data points. The two males with previous cardiac history are shown in open circles. The solid lines correspond to the mean QRISK2 database scores for white males and females with no cardiac risk factors. The database is only available up to 84 years. Bottom: Plots of HMR and minimum relative uptake against cardiac risk. Controls with previous cardiac history are shown in open circles
Two controls had abnormally low uptake on planar imaging (below the normal threshold of 2.2 suggested for medium energy collimators by the Japanese normal database of controls20) and no discernible uptake on SPECT imaging. These individuals had no significant medical history and were not taking any medications suspected of interfering with cardiac MIBG uptake, including those without strong clinical evidence of an effect.38 It is well recognized that occult Lewy body disease (usually termed incidental Lewy body disease) is present in many older people,39 which may explain the findings in controls.
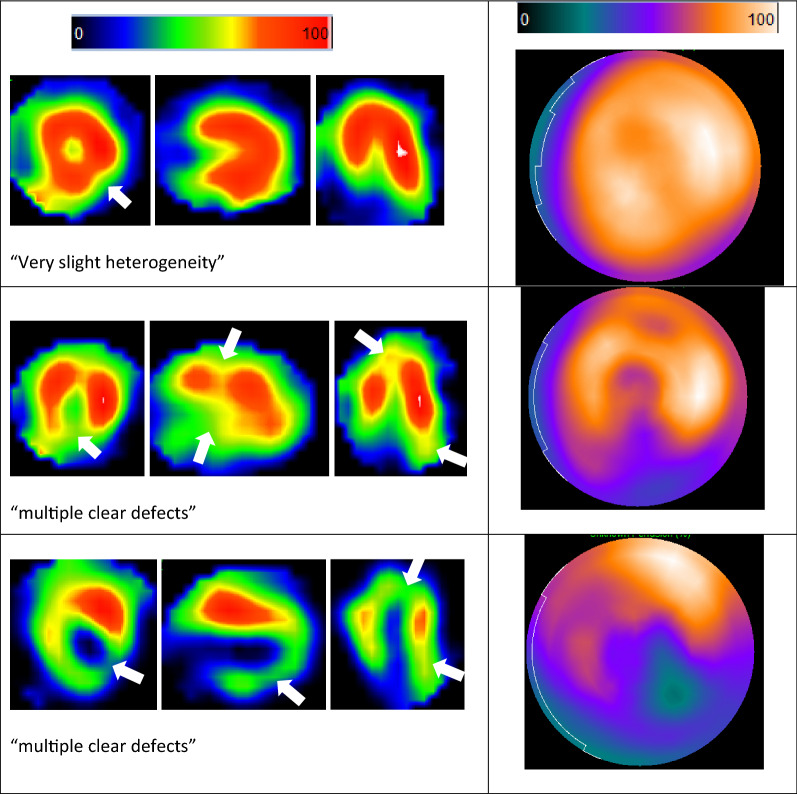
Of the remaining 29 controls, the mean planar HMR was 2.78 and standard deviation 0.46. The SPECT images for three of the controls were not possible to assess due to a high degree of lung interference. A further two with significant lung interference and two with liver interference were assessed but may be unreliable. Overall, 62% of the controls had some degree of interference; lung interference in 11/29 cases (38%) and liver interference in 7/29 (24%). Almost all controls assessed (25/26) displayed at least one area of clearly reduced relative uptake, most often in the inferior wall, which was rated as normal or mildly reduced in only 4/26 cases (15%). Some example images and ratings categories are shown in Figure 2. The results are summarized in Table 3 alongside those of the dementia patients.
Figure 2.
Top: QPS slice data and polar plot for the single control rated as within normal limits, showing almost perfectly uniform MIBG distribution, similar to that seen in normal Tc-99 m myocardial perfusion images. Middle: Slice data and polar plot for a more typical control, with reduced relative MIBG uptake inferiorly and at the apex. Bottom: A very abnormal control but without prior known cardiac events. The sites of reduced relative uptake are shown with white arrows
Table 3.
Frequency of regions showing reduced relative uptake for control, AD, and DLB groups
| Apex | Anterior wall | Lateral wall | Inferior wall | Septal wall | |
|---|---|---|---|---|---|
| Controls | 6/26 (23%) | 0 | 2/26 (8%) | 22/26 (85%) | 4/26 (15%) |
| AD | 5/15 (33%) | 0 | 2/15 (13%) | 13/14 (93%) | 4/15 (27%) |
| DLB | 1/5 (20%) | 0 | 0 | 4/5 (80%) | 1/5 (20%) |
People with Dementia
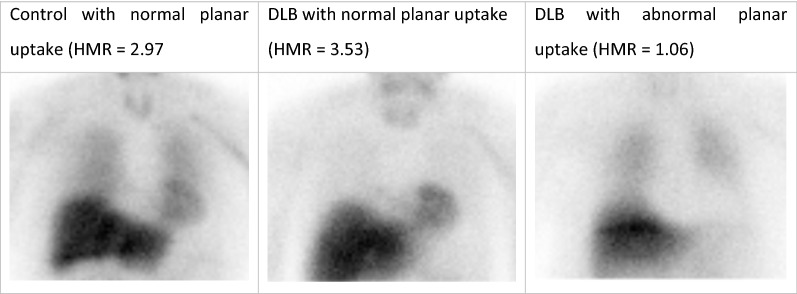
Of the 15 people with DLB who completed both planar and SPECT imaging, the mean planar HMR was 1.74 ± 0.83, with 10 scans resulting in HMR values below 2.2. One DLB participant with low planar HMR had visible cardiac uptake on SPECT images, but it was too unclear for a meaningful SPECT reconstruction to be obtained. Figure 3 shows example planar images for DLB patients with normal and abnormal cardiac uptake on planar imaging, alongside an example control image. All five of the DLB subjects with normal planar cardiac uptake had uptake visible on SPECT, i.e., the normal HMRs were not caused by overlapping liver or lung. There were therefore only five DLB SPECT scans included for SPECT regional analysis, those with normal planar imaging. Of the 15 people with AD, three had HMR values below 2.2 (mean HMR 2.51 ± 0.36); however, all 15 AD patients had sufficient cardiac uptake to reconstruct clear SPECT images.
Figure 3.
Example planar images of a control, a DLB patient with normal uptake on planar imaging, and a DLB patient with typical lack of cardiac uptake on planar imaging
The pattern of SPECT uptake was variable but overall similar to that seen in the controls for both AD and DLB (Tables 3, 4).
Table 4.
Frequency of each overall rating category for control, AD, and probable DLB groups
| Control (n = 29) | AD (n = 15) | DLB (n = 5) | |
|---|---|---|---|
| Interference present? | 18 (62%) | 10 (67%) | 2 (40%) |
| Not possible to score due to interference | 7 (24%) | 3 (20%) | 1 (20%) |
| Definitely uniform | 0 | 0 | 0 |
| Very mild heterogeneity | 1 (3%) | 0 | 0 |
| Patchy | 0 | 1 (7%) | 0 |
| Clear defect | 8 (28%) | 3 (20%) | 2 (40%) |
| Multiple clear defects | 13 (45%) | 8 (53%) | 2 (40%) |
Effect of Myocardial Infarction on Planar and SPECT Images
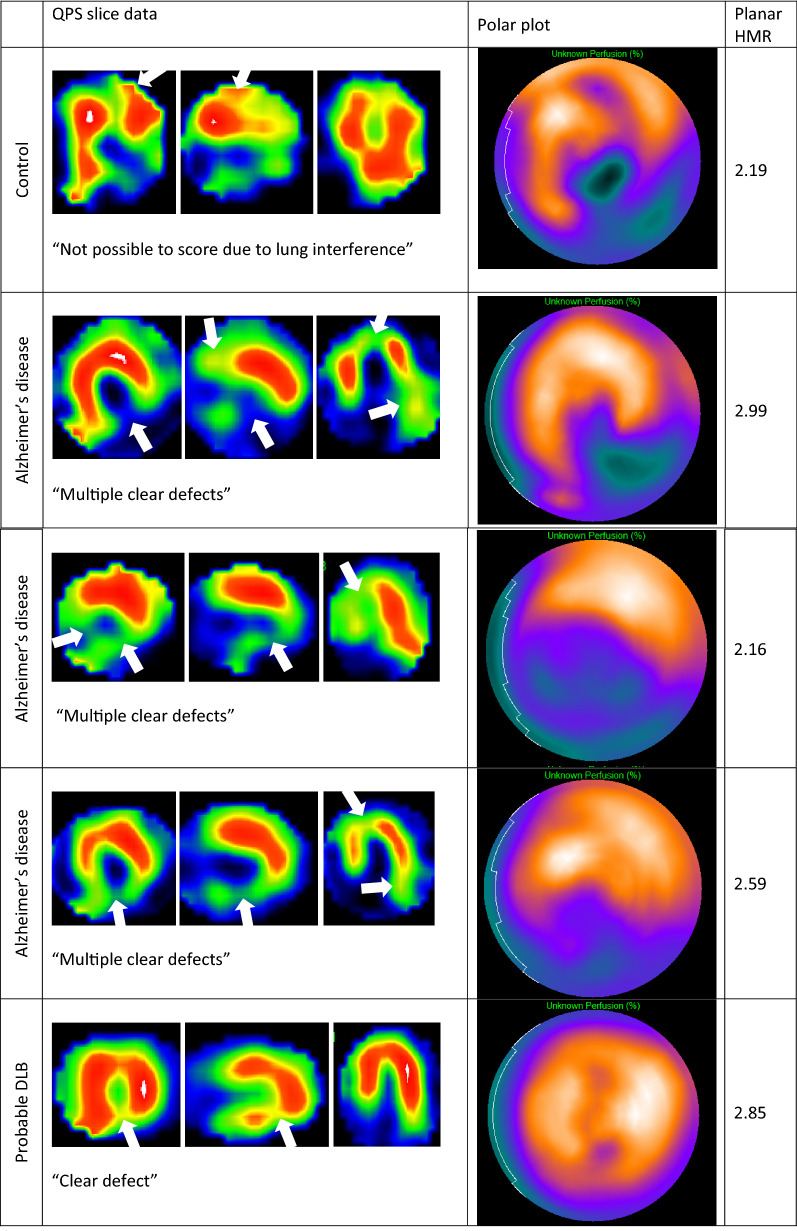
One control, three of the AD patients and two of the DLB patients had had a previous MI. Their images are displayed in Figure 4. The control had a borderline HMR of 2.19 (assuming a normal range > 2.2) despite a large area of apparently reduced uptake at the apex and inferiorly (lung interference anteriorly means this should be interpreted with caution). Of the AD MI cases, one had a borderline HMR of 2.16 and two had normal uptake. Of the DLB MI cases, one had normal HMR (2.85) and one had no visible cardiac uptake and so was one of the cases excluded from SPECT regional analysis.
Figure 4.
Slice data and polar plots for cases with previous MI, with corresponding planar HMRs. The sites of interference (top image) and relative reduced uptake are shown with arrows
The cases with previous MI showed defects of varying magnitude and extent on SPECT and variable planar HMRs, none of which were abnormally low. The number of cases is too low to establish a correlation between the extent of regional denervation and effect on global HMR but it seems likely that regional denervation would have to be widespread for planar HMR to be abnormally low.
Discussion
The majority of the controls (25/26) and all the AD patients showed at least one region of reduced relative cardiac MIBG uptake, with 45% of controls and 53% of AD patients showing relative reduction in multiple regions. This suggests that the presence of areas of reduced uptake on SPECT should not be used to diagnose Lewy body disease, as some authors have suggested, as this is likely to be highly non-specific. Our controls were almost all at high risk of coronary events over the next 10 years, so cardiac disease as a cause for the relative reduction cannot be entirely excluded. However, we note the lack of correlation between QRISK2 score and non-uniformity index suggesting the most at risk individuals are not at significantly greater risk of cardiac sympathetic denervation.
None of the control or dementia cases demonstrated reduced relative uptake in the anterior wall, which at first glance suggests that this region could be a useful target area in which to assess uptake in cases where the planar HMR is suspected of being affected by regional defects. However, 11 of the 29 control SPECT images were difficult to assess due to lung interference, three of whom were impossible to rate, making the anterior wall uptake unreliable.
The strengths of this study include the inclusion of older people thoroughly assessed to have normal cognition but who also had other morbidities associated with aging, including cardiac risk factors, making them a representative sample of older adults. The patients with dementia had an expert panel diagnostic review by three old-age psychiatrists, a method which has been validated against autopsy diagnosis and supported by regulatory authorities as a ‘gold standard’.40,41 However, a limitation is the use of only one rater for the SPECT uniformity assessments. This does however reflect clinical practice in radionuclide image reporting and the results are convincing even with a single rater. A further limitation is the lack of detailed cardiac assessment and no myocardial perfusion imaging for correlation with cardiac MIBG. However, Simula et al have shown that myocardial perfusion imaging does not correlate with cardiac MIBG SPECT in asymptomatic high coronary risk cases with mild coronary artery stenosis.42 They found a link between reduced uptake on cardiac MIBG SPECT and degree of stenosis for the left anterior descending artery (but not the left circumflex or right coronary artery) and suggest that CAD could be the cause of regional inhomogeneities on cardiac MIBG SPECT previously reported as normal variants.42 However, on our SPECT scans the areas showing reduced uptake are the inferior wall and/or apex for the majority of controls (21/26), which is against cardiac disease being the main cause as this would be expected to affect any of the cardiac territories. The pattern that we see suggests that either attenuation and scatter are causing this artifact, artifacts have been introduced in acquisition or reconstruction (e.g., due to high liver uptake), or there is a physiological reason for the apex and inferior wall to have reduced uptake.
The literature on regional sympathetic innervation in healthy controls is quite mixed. Most studies report a heterogeneous distribution within the left ventricle, although they do not agree on the sites of reduced uptake, perhaps related to small sample sizes and different age groups. Early work by Sisson and colleagues in the 1980s on 20 men aged 20-62 years showed a relative reduction at the apex only.43 The European guidelines on 123I-MIBG cardiac sympathetic imaging note that “normal cardiac MIBG distribution includes a relatively low uptake in the inferior wall, which is more pronounced in the elderly” referencing the 1993 study of Gill et al27 in seven young adults (29.4 ± 7.5 years) and eight middle aged, not elderly, adults (53 ± 5.1 years). As well as the inferior reduction Gill et al also reported septal reduction, with no difference between apex and base.27 Tsuchimochi et al studied 18 men and 11 women without cardiac diseases aged between 21 and 79 years (mean 42 ± 17 years) and reported that the inferior uptake of MIBG decreased with age in healthy controls, especially in men.19 This is important in the context of our study, as all participants were over 60 years of age and most of our controls were men. Image quality was however quite limited compared to modern gamma camera images for both this study published in 1995 and those of Sisson43 and Gill.27
Our results are similar to those of a more recent study published by Asghar et al in 201725 who reported reduced inferior wall uptake in all 14 healthy controls studied, and suggested that these effects are not predominantly caused by imaging artifacts. However, all our control cases had normal anterior wall uptake, which was not the case in the Asghar study. Interestingly, in their 2012 11C Hydroxyephedrine PET study, Wong et al showed that the lateral wall not the inferior wall shows reduced regional innervation in healthy controls, with relative sparing of anterior and septal walls.44
Post-mortem tissue studies e.g.,45,46 report non-uniform distribution of cardiac innervation, for example in the sinus node; but do not describe the relative distribution in the left ventricle so cannot be correlated with MIBG tracer uptake. However, in their post-mortem study on the distribution of autonomic nerves in the human heart, Kawano et al showed a small but statistically significant reduction in the sympathetic innervation of the inferior wall of the left ventricle compared to the anterior wall.47 The reduction is only around 20%, making this unlikely to explain the substantial differences seen in 123I-MIBG uptake between the anterior and inferior wall in many controls.
It has been hypothesized that due to its tracer kinetics, 123I-MIBG imaging is insensitive to substantial nerve losses in cardiac regions with mild to moderate levels of cardiac denervation and that regional nerve losses need to be relatively severe before a reduction in uptake becomes apparent.48 If this is the case it makes the modest physiological differences seen by Kawano et al.47 even less likely to explain the cardiac MIBG findings. We note that 11C Hydroxyephedrine (HED) has similar kinetics to 123I-MIBG, so this doesn’t explain the difference in relative uptake patterns.
This leaves artifacts as a possible contributor to the apparent regional reductions in cardiac MIBG uptake. In a study using an anthropomorphic chest phantom with uniform myocardium activity, the count density within the myocardium was inhomogeneous with both medium and low energy collimators.28 There was an overestimation of the measured count density in both the anterior and the septal wall and an underestimation of count density in the inferior wall,28 which is in line with typical control studies and which the authors assumed was due to attenuation. However, Gill considered attenuation artifacts an unlikely cause of the reduced inferior uptake in their control series, as myocardial perfusion imaging with thallium-201 did not show corresponding reductions.27 We note that although we commonly see mildly reduced relative uptake in the inferior wall on clinical myocardial perfusion images obtained with 99mTc-tetrafosmin, these reductions are typically less obvious than those seen on the majority of our control 123I-MIBG images. However, both 99mTc and 201Tl have different physical properties 123I, particularly in terms of high energy emissions and may not give the same apparent cardiac uptake patterns. This discrepancy could be potentially be resolved with the use of SPECT-CT imaging to correct for attenuation and scatter of the gamma rays from the MIBG tracer within the body. A recent phantom study by Shibutani et al demonstrated an inferior artifact in a myocardium insert (scanned with a low-medium energy general purpose collimator), which resolved with Siemens Flash3D SPECT-CT reconstruction with ACSC.49
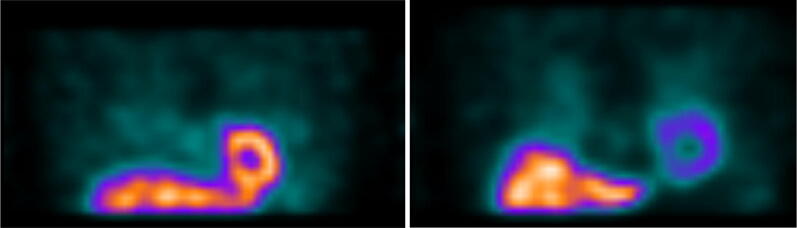
Regarding our single control regarded as having uniform relative uptake, the following observations are of note. The planar HMR was 2.69, which is a typical value in line with the other controls (mean HMR 2.78 ± 0.46), rather than an outlier. Similarly, the BMI of this individual was unremarkable at 28.6 (mean 27.8 ± 4.2). However, the relative uptake of the heart is similar to that of the liver, whereas in most of the controls the uptake is much higher in the liver than the heart (Figure 5). In addition, the positioning of the dome of the liver is just inferior to the heart, which is not the case in many of the other controls. This suggests that high liver uptake may be contributing to the apparent reduced inferior wall uptake seen in most of the controls. One of the few studies to directly compare 11C Hydroxyephedrine PET with 123I-MIBG SPECT, in patients with heart failure and coronary heart disease, reported lower liver uptake on 11C Hydroxyephedrine PET than 123I-MIBG SPECT, with increased uptake in the inferior and septal regions,50 so this may explain the difference in relative distribution reported with the two tracers. A study comparing both tracers in ten healthy young men with normal BMI showed lower relative uptake in the inferior wall and septum with 123I-MIBG SPECT compared with 11C-HED.51 This further suggests that liver uptake may be responsible for the inferior reduction, but we note that attenuation and scatter correction was used for the PET imaging but not the SPECT. To our knowledge, no other studies of 123I-MIBG and 11C-HED have been carried out in the same cohort of healthy controls and no studies at all with CT attenuation and scatter correction for 123I-MIBG. Further research in this area would help to clarify the reasons for the different pattern seen between SPECT and PET.
Figure 5.
Left: coronal MIBG slice for the control subject with uniform relative cardiac uptake. Right: a more typical control with high liver uptake relative to the heart. Images are displayed scaled to the maximum voxel
Limitations
As discussed above, a limitation of this study is the lack of definitive information on cardiac disease status of the participants. The QRISK2 score gives an idea of the likelihood of future events, but it is impossible to exclude undiagnosed asymptomatic CAD. The patients and controls were recruited to a dementia research study for which detailed cardiac assessment was not part of the study protocol; however an ECG and autonomic dysfunction tests were carried out. A further limitation of this study is the lack of data from younger subjects as only those over 60 years of age were recruited. SPECT-CT data for attenuation and scatter correction were not available for the patients so could not be assessed as part of this analysis.
The administered activity of 111 MBq is lower than reported in some cardiac MIBG studies, with 370 MBq typically used in the US. However, 111 MBq is used in Japan, where the majority of cardiac MIBG imaging studies for dementia diagnosis have been performed. Although the lower activity could give rise to slightly noisier images, it would not explain the clear defects in uptake seen in this study.
Due to the majority of DLB patients having severely reduced cardiac MIBG uptake, it was only possible to study relative uptake in a small group of five subjects with normal planar uptake. However, this group showed similar non-uniform SPECT uptake to the larger control and AD groups. Our results in the AD and control groups suggest that the presence of regional defects in uptake cannot be a specific marker of Lewy body disease. This is the case regardless of the number of DLB cases included.
New Knowledge Gained
Areas of relatively reduced cardiac 123I-MIBG uptake on SPECT images are common, but not ubiquitous, in cognitively normal older adults and patients with dementia who have normal planar cardiac uptake. Uptake is often reduced in the inferior wall and sometimes in the septal wall, lateral wall, and apex. Anterior wall uptake is often influenced by lung interference so appears unsuitable as a target region for uptake assessment, at least on images uncorrected for scatter and attenuation.
Conclusion
Reduced regional cardiac MIBG uptake in the inferior wall of the left ventricle is common in older adults and most likely related to attenuation or liver uptake. Reductions in other areas may also be artifactual or may be caused by mild underlying coronary artery disease. The pattern of reduction is in any case non-specific and therefore unlikely to aid in the diagnosis of Lewy body disease in typical clinical settings.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Electronic supplementary material 1 (PPTX 735 kb)
Acknowledgments
Disclosures
George Petrides and Gemma Roberts have received honoraria from GE Healthcare for educational presentations. Alan Thomas has received support from GE Healthcare for investigator led research. John O’Brien has acted as a consultant for GE Healthcare and received research support for investigator led research. All other authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Newcastle University and Newcastle upon Tyne NHS Foundation Trust research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Abbreviations
- DLB
Dementia with Lewy bodies
- AD
Alzheimer’s disease
- FP-CIT
I-123N-ω-fluoropropyl- 2β-carbomethoxy- 3β-(4-iodophenyl) nortropane
- MIBG
I-123 metaiodobenzylguanidine
- SPECT
Single photon emission computed tomography
- HMR
Heart-to-mediastinum ratio
- MI
Myocardial infarction
- AF
Atrial fibrillation
- CAD
Coronary artery disease
- ECG
Electrocardiogram
Footnotes
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Funding
Gemma Roberts is supported by an Alzheimer’s Society healthcare professional Fellowship. Infrastructure and support was provided to authors based at Newcastle by the National Institute for Health Research (NIHR) Newcastle Biomedical Research Centre, a partnership between Newcastle upon Tyne Hospitals NHS Foundation Trust and Newcastle University. John O’Brien was supported by the NIHR Cambridge Biomedical Research Centre.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vann Jones SA, O’Brien JT. The prevalence and incidence of dementia with Lewy bodies: a systematic review of population and clinical studies. Psychol Med. 2014;44:673–683. doi: 10.1017/S0033291713000494. [DOI] [PubMed] [Google Scholar]
- 2.Kane JPM, et al. Clinical prevalence of Lewy body dementia. Alzheimers Res Ther. 2018;10:19. doi: 10.1186/s13195-018-0350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanyu H, et al. Differences in clinical course between dementia with Lewy bodies and Alzheimer’s disease. Eur J Neurol. 2009;16:212–217. doi: 10.1111/j.1468-1331.2008.02388.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee DR, et al. Examining carer stress in dementia: the role of subtype diagnosis and neuropsychiatric symptoms. Int J Geriatr Psychiatry. 2013;28:135–141. doi: 10.1002/gps.3799. [DOI] [PubMed] [Google Scholar]
- 5.Galvin JE, et al. Lewy body dementia: the caregiver experience of clinical care. Parkinsonism Relat Disord. 2010;16:388–392. doi: 10.1016/j.parkreldis.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKeith IG, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017;89:88–100. doi: 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donaghy PC, McKeith IG. The clinical characteristics of dementia with Lewy bodies and a consideration of prodromal diagnosis. Alzheimers Res Ther. 2014;6:46. doi: 10.1186/alzrt274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung EJ, Kim SJ. (123)I-metaiodobenzylguanidine myocardial scintigraphy in lewy body-related disorders: A literature review. J Mov Disord. 2015;8:55–66. doi: 10.14802/jmd.15015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Treglia G et al. (1)(2)(3)I-MIBG cardiac scintigraphy in Lewy body-related disorders. Mov Disord 2011;26:1949-45 author reply 1950. [DOI] [PubMed]
- 10.Yoshita M, et al. Diagnostic accuracy of 123I-meta-iodobenzylguanidine myocardial scintigraphy in dementia with Lewy bodies: a multicenter study. PLoS ONE. 2015;10:e0120540. doi: 10.1371/journal.pone.0120540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estorch M, et al. Cardiac (123)I-metaiodobenzylguanidine imaging allows early identification of dementia with Lewy bodies during life. Eur J Nucl Med Mol Imaging. 2008;35:1636–1641. doi: 10.1007/s00259-008-0828-8. [DOI] [PubMed] [Google Scholar]
- 12.Hanyu H, et al. The role of 123I-metaiodobenzylguanidine myocardial scintigraphy in the diagnosis of Lewy body disease in patients with dementia in a memory clinic. Dement Geriatr Cogn Disord. 2006;22:379–384. doi: 10.1159/000095641. [DOI] [PubMed] [Google Scholar]
- 13.Lebasnier A, et al. Potential diagnostic value of regional myocardial adrenergic imaging using (123)I-MIBG SPECT to identify patients with Lewy body diseases. Eur J Nucl Med Mol Imaging. 2015;42:1043–1051. doi: 10.1007/s00259-015-2989-6. [DOI] [PubMed] [Google Scholar]
- 14.Nakajima K, et al. Iodine-123-MIBG sympathetic imaging in Lewy-body diseases and related movement disorders. Q J Nucl Med Mol Imaging. 2008;52:378–387. [PubMed] [Google Scholar]
- 15.Chen J, et al. Quantitative I-123 mIBG SPECT in differentiating abnormal and normal mIBG myocardial uptake. J Nucl Cardiol. 2012;19:8. doi: 10.1007/s12350-011-9438-0. [DOI] [PubMed] [Google Scholar]
- 16.Odagiri H, et al. On the Utility of MIBG SPECT/CT in evaluating cardiac sympathetic dysfunction in lewy body diseases. PLoS ONE. 2016;11:e0152746. doi: 10.1371/journal.pone.0152746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh JK, et al., Comparison of I-123 MIBG planar imaging and SPECT for the detection of decreased heart uptake in Parkinson disease. J Neural Transm (Vienna), 2015. [DOI] [PubMed]
- 18.Courbon Frederic, et al. Cardiac MIBG scintigraphy is a sensitive tool for detecting cardiac sympathetic denervation in Parkinson’s disease. Mov Disord. 2003;18:890–897. doi: 10.1002/mds.10461. [DOI] [PubMed] [Google Scholar]
- 19.Tsuchimochi Shinsaku, et al. Age and gender differences in normal myocardial adrenergic neuronal function evaluated by Iodine-123-MIBG imaging. J Nucl Med. 1995;36:969–974. [PubMed] [Google Scholar]
- 20.Nakajima K, et al. Normal values and standardization of parameters in nuclear cardiology: Japanese Society of Nuclear Medicine working group database. Ann Nucl Med. 2016;30:188–199. doi: 10.1007/s12149-016-1065-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morozumi Takakazu, et al. Myocardial iodine-123 -metaiodobenzyiguanidine images and autonomic nerve activity in normal subjects. J Nucl Med. 1997;38:49–52. [PubMed] [Google Scholar]
- 22.Momose M, et al. How heterogeneous is the cardiac autonomic innervation? Basic Res Cardiol. 2001;96:539–546. doi: 10.1007/s003950170004. [DOI] [PubMed] [Google Scholar]
- 23.Sakata Kazuyuki, et al. Physiological fluctuation of the human left ventricle sympathetic nervous system assessed by iodine- 123-MIBG. J Nucl Med. 1996;39:1667–1671. [PubMed] [Google Scholar]
- 24.Somsen GA, et al. Normal values and within-subject variability of cardiac I-123 MIBG scintigraphy in healthy individuals: Implications for clinical studies. J Nucl Cardiol. 2004;11:126–133. doi: 10.1016/j.nuclcard.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Asghar O, et al. Iodine-123 metaiodobenzylguanidine scintigraphy for the assessment of cardiac sympathetic innervation and the relationship with cardiac autonomic function in healthy adults using standardized methods. Nucl Med Commun. 2017;38:44–50. doi: 10.1097/MNM.0000000000000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimizu M, et al. Heterogeneity of cardiac sympathetic nerve activity and systolic dysfunction in patients with hypertrophic cardiomyopathy. J Nucl Med. 2002;43:15–20. [PubMed] [Google Scholar]
- 27.Gill JS, et al. Heterogeneity of the human myocardial sympathetic innervation: In vivo demonstration by iodine 123-labeled meta-iodobenzylguanidine scintigraphy. Am Heart J. 1993;126(2):390–398. doi: 10.1016/0002-8703(93)91056-k. [DOI] [PubMed] [Google Scholar]
- 28.Verberne HJ, et al. Influence of collimator choice and simulated clinical conditions on 123I-MIBG heart/mediastinum ratios: A phantom study. Eur J Nucl Med Mol Imaging. 2005;32:1100–1107. doi: 10.1007/s00259-005-1810-3. [DOI] [PubMed] [Google Scholar]
- 29.Verschure DO, et al. (1)(2)(3)I-MIBG heart-to-mediastinum ratio is influenced by high-energy photon penetration of collimator septa from liver and lung activity. Nucl Med Commun. 2015;36:279–285. doi: 10.1097/MNM.0000000000000238. [DOI] [PubMed] [Google Scholar]
- 30.Inui Y, et al. Comparison of (123)I-MIBG myocardial scintigraphy, brain perfusion SPECT, and voxel-based MRI morphometry for distinguishing between dementia with Lewy bodies and Alzheimer’s disease. Ann Nucl Med. 2014;28:796–804. doi: 10.1007/s12149-014-0873-2. [DOI] [PubMed] [Google Scholar]
- 31.Oide T, et al. Usefulness of [123I]metaiodobenzylguanidine ([123I]MIBG) myocardial scintigraphy in differentiating between Alzheimer’s disease and dementia with Lewy bodies. Intern Med. 2003;42:686–690. doi: 10.2169/internalmedicine.42.686. [DOI] [PubMed] [Google Scholar]
- 32.Wada-Isoe K, et al. Diagnostic markers for diagnosing dementia with Lewy bodies: CSF and MIBG cardiac scintigraphy study. J Neurol Sci. 2007;260:33–37. doi: 10.1016/j.jns.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe H, et al. Cardiac (123)I-meta-iodobenzylguanidine (MIBG) uptake in dementia with Lewy bodies: comparison with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2001;70:781–783. doi: 10.1136/jnnp.70.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marvin HM. Diseases of the heart and blood vessels: Nomenclature and criteria for diagnosis. Arch Intern Med. 1964;11(6):2. [Google Scholar]
- 35.NICE, Clinical Guideline 181: Cardiovascular disease: risk assessment and reduction, including lipid modification, N.I.f.H.a.C. Excellence, Editor. 2014.
- 36.Kane, J.P.M., et al., (123)I-MIBG scintigraphy utility and cut-off value in a clinically representative dementia cohort. Parkinsonism Relat Disord 2019. [DOI] [PubMed]
- 37.Roberts, G., et al., A comparison of visual and semiquantitative analysis methods for planar cardiac 123I-MIBG scintigraphy in dementia with Lewy bodies. Nucl Med Commun 2019. [DOI] [PubMed]
- 38.Jacobson AF, Travin MI. Impact of medications on mIBG uptake, with specific attention to the heart: Comprehensive review of the literature. J Nucl Cardiol. 2015;22:980–993. doi: 10.1007/s12350-015-0170-z. [DOI] [PubMed] [Google Scholar]
- 39.Beach TG, et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119:689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKeith I, et al. Predictive Accuracy of Clinical diagnostic criteria for dementia with lewy bodies: a prospective neuropathological validation study. Neurology 2000;54:1050-1058. [DOI] [PubMed]
- 41.McKeith I, et al. Sensitivity and specificity of dopamine transporter imaging with (123)I-FP-CIT SPECT in dementia with Lewy bodies: a phase III. Multicentre study 2007. [DOI] [PubMed]
- 42.Simula S, et al. Cardiac adrenergic innervation is affected in asymptomatic subjects with very early stage of coronary artery disease. J Nucl Med. 2002;43:1–7. [PubMed] [Google Scholar]
- 43.Sisson JC, et al. Metaiodobenzylguanidine to map scintigraphically the adrenergic nervous system in man. J Nucl Med. 1987;28:1625–1636. [PubMed] [Google Scholar]
- 44.Wong KK, et al. Pattern of cardiac sympathetic denervation in idiopathic Parkinson disease studied with 11C hydroxyephedrine PET. Radiology. 2012;265:240–247. doi: 10.1148/radiol.12112723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crick SJ, et al. Innervation of the human cardiac conduction system. A quantitative immunohistochemical and histochemical study. Circulation. 1994;89:1697–1708. doi: 10.1161/01.cir.89.4.1697. [DOI] [PubMed] [Google Scholar]
- 46.Kimura K, Ieda M, Fukuda K. Development, maturation, and transdifferentiation of cardiac sympathetic nerves. Circ Res. 2012;110:325–336. doi: 10.1161/CIRCRESAHA.111.257253. [DOI] [PubMed] [Google Scholar]
- 47.Kawano H, Okada R, Yano K. Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels. 2003;18:32–39. doi: 10.1007/s003800300005. [DOI] [PubMed] [Google Scholar]
- 48.Raffel DM, et al. Radiolabeled phenethylguanidines: Novel imaging agents for cardiac sympathetic neurons and adrenergic tumors. J Med Chem. 2007;50:2078–2088. doi: 10.1021/jm061398y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shibutani T, et al. Characteristics of iodine-123 IQ-SPECT/CT imaging compared with conventional SPECT/CT. Ann Nucl Med. 2019;33:103–111. doi: 10.1007/s12149-018-1310-8. [DOI] [PubMed] [Google Scholar]
- 50.Matsunari I, et al. Iodine-123 metaiodobenzylguanidine imaging and carbon-11 hydroxyephedrine positron emission tomography compared in patients with left ventricular dysfunction. Circ Cardiovasc Imaging. 2010;3:595–603. doi: 10.1161/CIRCIMAGING.109.920538. [DOI] [PubMed] [Google Scholar]
- 51.Yoshinaga K, et al. Prone-position acquisition of myocardial (123)I-metaiodobenzylguanidine (MIBG) SPECT reveals regional uptake similar to that found using (11)C-hydroxyephedrine PET/CT. Ann Nucl Med. 2014;28:761–769. doi: 10.1007/s12149-014-0868-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic supplementary material 1 (PPTX 735 kb)