Abstract
Objective: To investigate the persistence of symptoms compatible with COVID-19 in a real-file prospective cohort of patients at 12 months from hospital discharge.
Methods: Longitudinal, prospective, single-center, cohort telephone follow-up (FU) study in a Tertiary Care Hospital.
All consecutive patients >18 years admitted for COVID-19 were prospectively enrolled in a telephone FU program aimed at monitoring symptoms after 1,3,6,9 and 12 months from hospital discharge. The survey screened for somatic (fatigue, dyspnea, dyspnea, palpitations, cough, chest pain, abdominal pain, ageusia, anosmia, bowel symptoms) and emotional symptoms (insomnia, confusion, altered sense of reality, loss of appetite, fear, and depression) and frailty. Only patients with 12 months FU data were analyzed (N=254). Prevalence and factors associated with symptoms were the main outcomes.
Frailty was defined by the presence of ≥3 indicators: weakness, slowness/impaired mobility, weight-loss, low physical activity, and exhaustion.
Results: At 12 months, 40.5% of patients reported at least one symptom. The most common somatic ones were fatigue, exertional dyspnea, cough, bowel complaints while the most common psycho-emotional were insomnia, confusion, fear, and depression. Age, gender, gender, frailty, multiple symptoms at baseline and chronic obstructive pulmonary disease (COPD) were associated with symptoms persistence. Furthermore, frailty, COPD and multiple symptoms at baseline were associated with increased risk of somatic symptoms at 12 months, while age and gender were associated with emotional ones.
Conclusions: Burden of the long COVID-19 symptoms decreased over time but remained as high as 40% at 12 months with important gender and functional differences, highlighting potential patient categories who may benefit from specific follow up strategies.
Keywords: COVID-19, Long-COVID, Symptoms, Prognosis, Frailty, Gender medicine
1. Introduction
Since the beginning of the SARS-CoV-2 pandemic and its disease (COVID-19), over 200 million cases of infection and 4.37 million deaths have been reported world-wide [1,2]. While the most common acute phase symptoms include cough, fatigue, fever, dyspnea, musculoskeletal disorders, gastrointestinal symptoms, anosmia and dysgeusia [3,4], data on Long-COVID, defined as the persistence of symptoms after 12 weeks from hospital discharge [5,6], is limited and its relevance is still unclear. Early reports suggest that as many as 60–80% of patients with critical COVID-19 show symptoms at 1 month from hospital discharge, with fatigue and dyspnea being the most frequent ones [7]. Furthermore, evidence from non-critical cases suggests that at 60 days, up to two-thirds of adults may still complain symptoms compatible with SARS-CoV-2 infection [8]. However, little is known regarding the persistence of symptoms at 12 months from hospital discharge.
We prospectively assessed the persistence of symptoms compatible with COVID-19 in patients discharged from a tertiary care center, which served as a COVID-19 healthcare facility, via telephone interviews. We investigated potential baseline characteristics associated with the long-COVID syndrome, including frailty, a syndrome characterized by reduced homeostasis and marked vulnerability to adverse health outcomes. [9]
2. METHODS
2.1. Study design
This is a longitudinal, prospective, single-center, cohort telephone follow-up (FU) study. All consecutive patients admitted at Careggi University Hospital for COVID-19 were prospectively enrolled in a telephone longitudinal FU program aimed at monitoring symptoms after 1, 3, 6, 9 and 12 months from hospital discharge.
2.2. Data collection
All patients aged >18 years discharged from a COVID-19 unit at Careggi University Hospital were included in the FU study. Only patients with 12 months FU data were included in the final analysis. Administrative data were acquired from electronic charts and data were stored on a password-protected digital platform provided by REDCap (Research Electronic Data Capture, REDCap 8.11.6 - © 2021 Vanderbilt University). Patients participating to the study were assigned a specific identification number and anonymized. Baseline clinical data of patients have been previously published [10].
On each telephone contact, patients received an appointment for the subsequent interview session. When not answering the call, patients were re-called twice at least 24 h apart and, only if unanswered on both occasions, they were considered lost to FU. For patients unable to answer, the interview was lead with the primary caregiver or attending nurse in case of admittance to healthcare facilities. Each interview was conducted by trained quality abstractors.
The study was conducted according to the guidelines of the Declaration of Helsinki and its protocol approved by the local Ethical Review Board (Comitato Area Vasta Centro, CARE-COVID19 AOU Careggi Protocol 00/08761 April 2020). Each patient provided an informed oral consent which was recorded during the 1-month telephone interview.
2.3. Study variables
The survey interview screened for somatic (fatigue, dyspnea at rest, exertional dyspnea, palpitations, cough, chest pain, abdominal pain, ageusia, anosmia, bowel symptoms) and emotional (insomnia, confusion, altered sense of reality, loss of appetite, fear, and depression) symptoms which were routinely assessed during hospitalization and at 1, 3, 6, 9 and 12 months after hospital discharge.
Symptoms already present before SARS-CoV-2 infection, which had been recorded on hospital admission [10], were identified and were not considered for the analysis. Main comorbidities were noted.
In addition, a series of questions were adapted to self-report and assess the frailty phenotype described by Fried and colleagues, which defines frailty as the presence of three or more out of five indicators: weakness, slowness/impaired mobility, weight loss, low physical activity, and exhaustion needing assistance [9,11]. Persons with one or two indicators were classified as pre-frail, while those with three or more as frail [9].
2.4. Study endpoints
Prevalence of COVID-19 symptoms at 12 months and their association with baseline, pre-infection, patients’ characteristics. To provide additional information regarding their temporal trends, we described also both somatic and emotional symptoms prevalence at 1, 3, 6, 9 and 12 months.
2.5. Statistical analysis
Continuous and categorical variables were reported as mean ± standard deviation or as counts and percentages, respectively. Categorical variables were compared between groups with χ2 test, or Fisher's exact test when the expected cell count was less than 5. For matched pairs at 1, 3, 6, 9 and 12 months the McNemar test was used.
Binary logistic multivariable regression analysis (with backward stepwise deletion) was used to assess determinants of all, somatic and emotional symptoms.
To describe baseline factors associated with persistence of symptoms at 12 months, the dependent variable was defined as follows: symptoms present in >1 standard deviation of the mean at 12 months were considered most frequent and chosen as dependent variable for regression analysis.
All variables collected at baseline (1-month interview) with p<.10 were entered into the multivariable models. A 2-sided p<.05 was considered statistically significant. Statistical analysis was performed using the SPSS, version 27.0, statistical package for Macintosh (IBM, Armonk, NY) and GraphPad Prism (Version 9.2.0 [283]) was used for graphs.
3. Results
3.1. Study population characteristics at first interview
As of July 30th 2021, a total of 1825 patients were enrolled in the FU program and successfully completed 1-month interviews. Of these, 951 (52.1%), 618 (33.4%), 296 (16.3%) and 254 (13.9%) completed the interviews at 3, 6, 9, and 12 months after discharge. The initial cohort of patients discharged 12 months before would have consisted of 273 individuals; of these, 19 (6.2%) were lost to FU either because they did not answer calls (N = 12) or refused to participate (N = 7). The characteristics of the remaining 254 patients with a 12-month complete follow-up are reported in Table 1 .
Table 1.
Baseline (month 1) characteristics of patients discharged after COVID-19 with a complete 12-month follow up.
| Overall N = 254 | No Symptoms N = 151 | Symptoms N = 103 | p | |
| Age (mean ± SD) | 62 ± 15 | 59 ± 15 | 66 ± 14 | <0.001 |
| <40 years, N (%) | 21 (8.3) | 15 (9.9) | 6 (5.8) | <0.001 |
| 40–60 years, N (%) | 94 (37.0) | 68 (45.0) | 26 (25.2) | |
| 61–75 years, N (%) | 82 (32.3) | 42 (27.8) | 40 (38.8) | |
| 76–90 years, N (%) | 57 (22.4) | 26 (17.2) | 31 (30.1) | |
| Women, N (%, yes vs no) | 102 (40.2) | 51 (33.8) | 51 (49.5) | 0.012 |
| Unintentional weight loss >5 kg, N (%) | 85 (33.5) | 53 (35.1) | 32 (31.1) | 0.588 |
| Frail Phenotype, N (%) | 88 (34.6) | 41 (27.2) | 47 (45.6) | 0.001 |
| Pre-frail Phenotype, N (%) | 93 (36.6) | 58 (38.4) | 35 (34.0) | |
| Discharged home, N (%) | 177 (69.7) | 106 (71.6) | 71 (70.3) | 0.887 |
| Living with family member (spouse and/or children), N (%) | 196 (77.2) | 120 (79.5) | 76 (73.8) | 0.551 |
| Living with caregiver, N (%) | 6 (2.4) | 2 (1.3) | 4 (3.9) | |
| Living in a nursing facility, N (%) | 21 (8.3) | 10 (6.6) | 11 (10.7) | |
| Needing assistance for shopping/managing finances, N (%) | 141 (55.5) | 75 (49.7) | 66 (64.0) | 0.043 |
| Needing assistance with therapy, N (%) | 95 (36.6) | 43 (28.5) | 52 (50.4) | <0.001 |
| More than 2 symptoms at first contact, N (%) | 161 (63.8) | 87 (57.6) | 75 (72.8) | 0.013 |
| Impaired mobility (confined to bed/limited mobility),N (%) | 68 (26.7) | 36 (23.8) | 32 (31.0) | 0.189 |
| Prior chronic comorbidities, N (%) | 180 (70.9) | 104 (70.3) | 76 (75.2) | 0.389 |
| Allergies, N (%) | 10 (3.9) | 6 (4.0) | 4 (3.9) | 0.971 |
| Chronic Obstructive Pulmonary disease, N (%) | 10 (3.9) | 1 (0.7) | 9 (8.7) | 0.002 |
| Diabetes Mellitus, N (%) | 42 (16.5) | 28 (18.5) | 14 (13.6) | 0.297 |
| History of Cancer, N (%) | 20 (7.9) | 9 (6.0) | 11 (10.7) | 0.170 |
| Hypertension, N (%) | 89 (35.0) | 56 (37.1) | 33 (32.0) | 0.408 |
| Ischemic Heart Disease, N (%) | 56 (22.0) | 28 (18.5) | 28 (27.2) | 0.103 |
For categorical variables, each line reports the absolute number and the percentage of patients presenting the indicated condition.
Mean age at first telephone contact was 6215 years (>75 years: 22.4%) and 102 (40.2%) were women. Overall, 88 (34.6%) patients were categorized as frail and 93 (36.6%) as pre-frail. Only 3 patients were re-admitted due to pulmonary symptoms (COVID-19 negative swab).
3.2. Persistence of symptoms
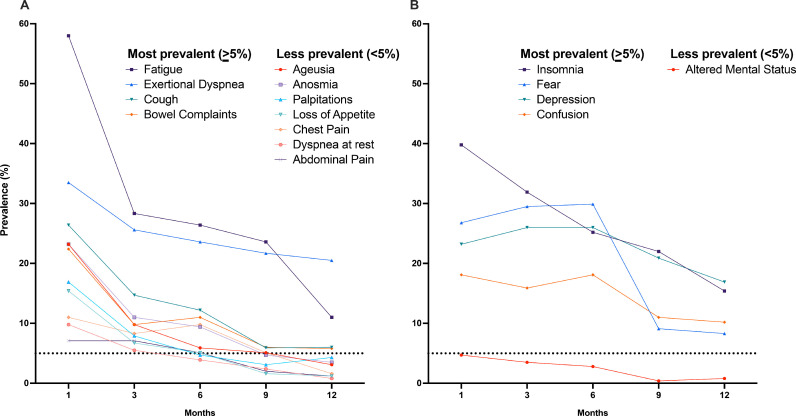
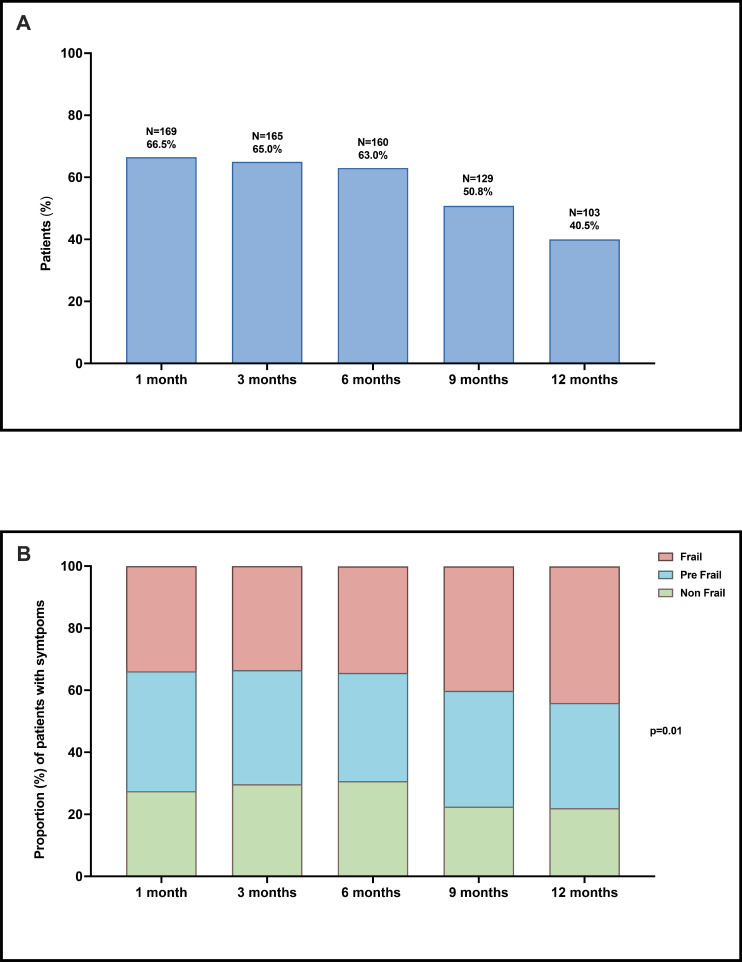
All symptoms decreased over time (Fig. 1 ). The most prevalent (>5% at 12 months) somatic (Panel A) and psycho-emotional (Panel B) symptoms were fatigue, exertional dyspnea, cough, bowel complaints, and insomnia, confusion, fear, and depression, respectively. Notably, emotional symptoms like fear increased up to month 6 and only afterwards decreased. At 12 months, 103 (40.5%) patients reported at least one of the most prevalent symptoms (Fig. 2 , Panel A). A total of 72 (28.3%) patients presented with somatic symptoms and 80 (31.4%) reported psycho-emotional ones, with 43 (17.0%) having symptoms of both classes. Notably, the proportion of frail symptomatic patients increased significantly over time, from 33.9% (N = 57/169) at 1 month to 45.6% (N = 47/103) at 12 months (p=.01, Fig. 2, Panel B).
Fig. 1.
Persistence of major symptoms at 1, 3, 6, 9 and 12 months by domain (Panel A, Somatic symptoms, Panel B, Emotional symptoms). The dotted line on the Y axis corresponds to the 5% cut-off value for most frequent symptoms.
Fig. 2.
Panel A: Patients (%) with one or more symptoms at 1, 3, 6, 9 and 12 months. Panel B: proportion (%) of patients with symptoms by frailty status.
3.3. Factors associated with symptoms at 12 months
At multivariable logistic regression analysis (Table 2 ; see also Supplementary Table 1 for crude associations), factors associated with persistent symptoms at 12 months were frailty, age, female gender, >2 symptoms at first interview, and chronic obstructive pulmonary disease (COPD). When considering symptoms by domain, frailty, COPD and >2 symptoms at first interview were associated with somatic symptoms while age, female gender and >2 symptoms at first interview were associated with psycho-emotional symptoms.
Table 2.
Multivariable logistic regression analysis of factors associated with major symptom prevalence at 12 months.
| Overall Symptoms | Somatic Symptoms | Emotional Symptoms | |||||||
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Frailty | 1.88 | 1.04–3.41 | 0.038 | 1.96 | 1.09–3.53 | 0.024 | |||
| >2 symptoms at baseline | 1.79 | 1.01–3.18 | 0.046 | 1.95 | 1.03–3.37 | 0.041 | 2.28 | 1.23–4.20 | 0.041 |
| Age (decade increase) | 1.25 | 1.02–1.53 | 0.029 | 1.21 | 1.02–1.46 | 0.048 | |||
| Female Gender | 1.90 | 1.10–3.28 | 0.021 | 2.37 | 1.36–4.12 | 0.002 | |||
| COPD | 10.74 | 1.28–59.33 | 0.028 | 3.96 | 1.05–15.04 | 0.043 | |||
OR: odds ratio, CI: confidence intervals, COPD: chronic obstructive pulmonary disease.
Variables excluded from the model (backward deletion, p out >0.10): Number of chronic comorbidities, allergies, diabetes mellitus, ischemic heart disease, history of cancer, hypertension.
4. Discussion
In this longitudinal, prospective cohort study of post-discharge COVID-19 patients, burden of symptoms not attributable to alternative, pre-existing, chronic diagnoses decreased over time but remained as high as 40% at 12 months. The most common (prevalence >5%) symptoms at 12-month contact were fatigue, exertional dyspnea, insomnia, confusion, fear, depression, cough, and bowel symptoms. Furthermore, different symptoms had different trends over time while somatic symptoms all decreased from month 1 to 12, emotional symptoms like fear increased up to month 6 and only afterwards decreased.
Age, female gender, COPD, >2 symptoms at first interview, and frailty were associated with a higher probability of reporting symptoms at one year. When considering separately the two symptom domains, frailty was associated with somatic symptoms, while female gender, age, and symptoms at first interview were associated with psycho-emotional ones.
The prevalence and persistence of symptoms reported by patients in our follow-up study is consistent with early evidence from United States [12], United Kingdom [13] and other international studies [14,15] of cohorts with much shorter follow-up, and proves that the effects of COVID-19 extend long beyond the acute phase. This strengthens the importance of specific and individually tailored care after COVID-195.
Advanced age and male gender have been associated with a more aggressive COVID-19 acute phenotype [10] and, therefore, a greater long-term persistence of symptoms might be expected in older males [16,17]. Conversely, in our study women had an almost two-fold higher probability of emotional symptoms persistence at 12 months, reinforcing the view that gender-related differences in coping with hospitalization and acute care may extend well beyond the previously reported three-month duration [18]. This unexpected finding represents a novelty in the increasing number of studies on long-COVID.
The close association of COPD with the overall and somatic symptoms was remarkable. COPD has been already linked with an increased risk of severe pneumonia and poorer outcomes in the acute phase [19]. Although our results should be considered with caution due to the small numbers of patients with a COPD diagnosis, we found that this condition may predispose to longer symptom persistence, likely due to poor underlying lung reserves. [20]
During the COVID-19 pandemic, the frail phenotype [21] has been considered as a potential marker of vulnerability, leading to prolonged hospital stay and increased acute mortality, even beyond the impact of older age [22], [23], [24]. In general, frailty has been linked to worse outcomes in both acute and chronic care settings. Although its assessment in individuals aged <65 years warrants caution, reports have shown pre-frailty to be closely linked to higher morbidity (like ischemic heart disease and COPD) and mortality in individuals of all ages and have suggested that its identification and management in older, as well as in middle aged patients may be an effective strategy to improve healthcare and clinical outcomes [24].
Given the clinical context of the COVID-19 pandemic, with a greater prevalence of patients >65 years particularly among those requiring hospitalization, assessment of frailty status could be implemented prior to discharge and in early follow-up, to identify those individuals at higher risk of long-term persisting symptoms and, possibly, of incident disability. As a case in point, patients discharged with COVID-19 with symptoms including mood disorders, fatigue, and perceived cognitive impairment at 3 months had more difficulties on resumption of functional and occupational activities [25]. Thus, assessment of functional status could be a proxy to further clinical and social evaluation.
Our study has some limitations. The results reflect a prospective single-center telephone follow-up which could have led to a bias in symptom recall and limit the representativeness of the cohort. However, our sample seems to have an adequate representation of Italian patients with an age range between 18 and 90 years and an almost balanced gender distribution. Moreover, some patients may have been too symptomatic to answer the call and potentially lead to a selection bias in the enrollment in the study. For this reason, when necessary, study operators interviewed also primary caregivers. In addition, assessment of possible associations of symptoms with instrumental/bio-humoral characteristics was unfeasible due to the lockdown restriction as well as further characterization of symptoms. Finally, frailty status, which represents a general functional framework, was assessed only at the baseline evaluation, and was not monitored afterwards.
To our knowledge, this is the first study to explicitly describe the health status in a large, real-life population of COVID-19 patients at 12 months that specifically investigated persistence of symptoms which were not present prior to SARS-CoV-2 infection.
5. Conclusions
In this prospective cohort study of COVID-19 patients, almost one-in-two patients reported COVID-19-related symptoms at 12 months after hospital discharge. Fatigue, exertional dyspnea, insomnia, confusion, fear, depression, cough, and bowel symptoms were the most common symptoms at 12-month contact. Age, gender, COPD, >2 symptoms at baseline and frailty at baseline were associated with a higher probability of symptoms, highlighting potential patient categories who may benefit from specific follow up strategies. Ongoing longitudinal follow-up is needed to better characterize the natural history and pathogenesis of the Long-COVID.
Declaration of Competing Interest
None to disclose for the present work.
References
- 1.World Health Organization. WHO coronavirus (COVID-19) emergency dashboard. https://covid19.who.int. Published, 2021.
- 2.Guan W., Ni Z., Hu Y., et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020:1–13. doi: 10.1056/nejmoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Docherty A.B., Harrison E.M., Green C.A., et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrilli C.M., Jones S.A., Yang J., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah W., Hillman T., Playford E.D., Hishmeh L. Managing the long term effects of covid-19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ. 2021;372 doi: 10.1136/bmj.n136. [DOI] [PubMed] [Google Scholar]
- 7.Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. JAMA - J Am Med Assoc. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carvalho-Schneider C., Laurent E., Lemaignen A., et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2021;27(2):258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fried L.P., Tangen C.M., Walston J., et al. Frailty in older adults: evidence for a phenotype. Journals Gerontol - Ser A Biol Sci Med Sci. 2001;56(3) doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 10.Fumagalli C., Rozzini R., Vannini M., et al. Clinical risk score to predict in-hospital mortality in COVID-19 patients: a retrospective cohort study. BMJ Open. 2020;10(9) doi: 10.1136/bmjopen-2020-040729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papachristou E., Wannamethee S.G., Lennon L.T., et al. Ability of Self-Reported Frailty Components to Predict Incident Disability, Falls, and All-Cause Mortality: results From a Population-Based Study of Older British Men. J Am Med Dir Assoc. 2017;18(2):152–157. doi: 10.1016/j.jamda.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chopra V., Flanders S.A., O'Malley M., Malani A.N., Prescott H.C. Sixty-Day Outcomes Among Patients Hospitalized With COVID-19. Ann Intern Med. 2021;174(4):576–578. doi: 10.7326/m20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halpin S.J., McIvor C., Whyatt G., et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2021;93(2):1013–1022. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- 14.Huang C., Huang L., Wang Y., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sudre C.H., Murray B., Varsavsky T., et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falagas M.E., Mourtzoukou E.G., Vardakas K.Z. Sex differences in the incidence and severity of respiratory tract infections. Respir Med. 2007;101(9):1845–1863. doi: 10.1016/J.RMED.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Peckham H., de Gruijter N.M., Raine C., et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun 2020 111. 2020;11(1):1–10. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai X., Hu X., Ekumi I.O., et al. Psychological Distress and Its Correlates Among COVID-19 Survivors During Early Convalescence Across Age Groups. Am J Geriatr Psychiatry. 2020;28(10):1030–1039. doi: 10.1016/j.jagp.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerayeli F.V., Milne S., Cheung C., et al. COPD and the risk of poor outcomes in COVID-19: a systematic review and meta-analysis. EClinicalMedicine. 2021;33 doi: 10.1016/j.eclinm.2021.100789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung J.M., Niikura M., CWT Yang, Sin D.D. COVID-19 and COPD. Eur Respir J. 2020;56(2) doi: 10.1183/13993003.02108-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fried L.P., Tangen C.M., Walston J., et al. Frailty in Older Adults: evidence for a Phenotype. J. Gerontol Ser A Biol Sci Med Sci. 2001;56(3):M146–M157. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 22.Hewitt J., Carter B., Vilches-Moraga A., et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Heal. 2020;5(8):e444–e451. doi: 10.1016/S2468-2667(20)30146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fumagalli C., Ungar A., Rozzini R., et al. Predicting Mortality Risk in Older Hospitalized Persons with COVID-19: a comparison of the COVID-19 Mortality Risk Score with Frailty and Disability. Journal of the American Medical Directors Association. 2021 doi: 10.1016/j.jamda.2021.05.028. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sablerolles R.S.G., Lafeber M., van Kempen J.A.L., et al. Association between Clinical Frailty Scale score and hospital mortality in adult patients with COVID-19 (COMET): an international, multicentre, retrospective, observational cohort study. Lancet Heal Longev. 2021;2(3):e163–e170. doi: 10.1016/s2666-7568(21)00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanichkachorn G., Newcomb R., Cowl C.T., et al. Post–COVID-19 Syndrome (Long Haul Syndrome): description of a Multidisciplinary Clinic at Mayo Clinic and Characteristics of the Initial Patient Cohort. Mayo Clin Proc. 2021;96(7):1782–1791. doi: 10.1016/J.MAYOCP.2021.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]