Abstract
Several Janus kinase (JAK) inhibitors (jakinibs) have recently been approved to treat inflammatory, autoimmune and hematological conditions. Despite emerging roles for JAKs and downstream signal transducer and activator of transcription (STAT) proteins in platelets, it remains unknown whether jakinibs affect platelet function. Here, we profile platelet biochemical and physiological responses in vitro in the presence of five different clinically relevant jakinibs, including ruxolitinib, upadacitinib, oclacitinib, baricitinib and tofacitinib. Flow cytometry, microscopy and other assays found that potent JAK1/2 inhibitors baricitinib and ruxolitinib reduced platelet adhesion to collagen, as well as platelet aggregation, secretion and integrin αIIbβ3 activation in response to the glycoprotein VI (GPVI) agonist collagen-related peptide (CRP-XL). Western blot analysis demonstrated that jakinibs reduced Akt phosphorylation and activation following GPVI activation, where ruxolitinib and baricitinib prevented DAPP1 phosphorylation. In contrast, jakinibs had no effects on platelet responses to thrombin. Inhibitors of GPVI and JAK signaling also abrogated platelet STAT5 phosphorylation following CRP-XL stimulation. Additional pharmacologic experiments supported roles for STAT5 in platelet secretion, integrin activation and cytoskeletal responses. Together, our results demonstrate that ruxolitinib and baricitinib have inhibitory effects on platelet function in vitro and support roles for JAK/STAT5 pathways in GPVI/ITAM mediated platelet function.
Keywords: Platelets, JAK, STAT5, ruxolitinib, baricitinib, GPVI
INTRODUCTION
Pleiotropic JAK/STAT pathways transduce receptor signaling events in response to a plethora of more than 50 different cytokine and growth factors to regulate innate and adaptive immune responses, as well as cell proliferation, differentiation, migration, apoptosis, and cell survival.1,2,3 While JAK/STAT pathways serve key roles in maintaining cellular hemostasis, their dysregulation is also tightly linked to the progression of hematopoietic and immune diseases and represent important pharmacologic targets. In the past decade, several JAK inhibitors (jakinibs) have been approved for a range of conditions.4 Ruxolitinib was the first jakinib approved for use in patients with polycythemia vera5 and myelofibrosis.6 Tofacitinib was later approved for the treatment of rheumatoid arthritis.7 More recently, the Food and Drug Administration (FDA) approved upadacitinib and baricitinib for rheumatoid arthritis, and oclacitinib for veterinary use.8,9,10
Despite safety and efficacy, some complications are apparent for jakinib therapies,11 including thrombotic12,13,14 as well as bleeding events,15 potentially involving platelets.16,17,18 For instance, tofacitinib has been found to increase the risk of blood clot formation in a dose-dependent manner.19 The recently approved JAK1/2 inhibitor baricitinib significantly increases the incidence of venous thromboembolisms (VTE) in patients with rheumatoid arthritis.20 Another JAK1/2 inhibitor, ruxolitinib, has been suggested to reduce the risk of thromboembolic events in patients with myeloproliferative neoplasms, and has also been associated with bleeding.21,22,23,24 Other classes of therapeutic tyrosine kinase inhibitors can also cause bleeding or thrombosis and are suspected of interfering with platelet function. Recent studies have profiled mechanisms of other tyrosine kinase therapies on platelet biology (i.e., the Bruton’s tyrosine-kinase (BTK) inhibitor ibrutinib, the BCR-Abl inhibitor ponatinib);25,26,27,28 however, the effects of therapeutic jakinibs on platelet function remain wholly unknown.
Platelets express several JAK and STAT proteins with the potential to be affected by jakinibs. For instance, the JAK2/STAT3 axis has been shown to play a role in collagen-mediated platelet activation,29,30 and studies with STAT3 inhibitors have found roles for STAT3 in platelets independent of transcription factor activities.31,32 Houck and colleagues demonstrated a physical proximity of GPVI and the interleukin (IL)-6 receptor gp130 in membrane lipid rafts in a manner supporting the cross activation of a JAK2/STAT3 axis in platelets stimulated with collagen.33,34 More recently, the Falet lab has detailed roles for JAK2 in platelet GPVI/ITAM responses using Jak2−/− mouse models.35 Roles for JAK2 and STAT proteins in platelet ITAM-mediated signaling are further suggested by recent proteomics studies of phosphorylation events following platelet GPVI as well as C-type lectin (CLEC)-2 activation.36,37 JAK3 is also expressed by platelets and phosphorylated following thrombin stimulation to mediate STAT1 and STAT3 activation.38,39 Other JAK and STAT family members, including tyrosine kinase 2 (TYK2) and STAT5, are phosphorylated and activated in platelets in response to thrombopoietin (TPO) stimulation.40,41,42 Other studies using Jak2 V617F mutant mice have demonstrated that gain-of-function Jak2 mutations impair platelet formation and upregulate platelet responses to classical agonists.43,44
While a number of studies demonstrate roles for JAK and STAT activation in platelet physiology29,31,38,45 – most recently GPVI mediated platelet function30,32,34,35 – the effects of therapeutic JAK inhibitors on platelets remain unknown. Here, we investigate whether jakinibs affect platelet activation in vitro in response to stimulation of the platelet immunoreceptor GPVI. We find that the JAK1/2 inhibitors ruxolitinib and baricitinib effectively inhibit GPVI-mediated platelet function, whereas JAK1 and JAK3 inhibitors upadacitinib, oclacitinib and tofacitinib have more limited effects on platelets. Additionally, we show that platelet GPVI pathways incorporate STAT5 phosphorylation in a manner associated with platelet functional responses in vitro.
MATERIALS AND METHODS
Reagents
Soluble collagen was from Corning (Corning, NY, USA). Prostaglandin I2 (PGI2) was from Cayman Chemical Company (Ann Arbor, MN, USA). Crosslinked collagen related peptide (CRP-XL) was from CambCol Laboratories (Cambridge, UK) and human fibrinogen (FIB3) was from Enzyme Research Labs (South Bend, IN, USA). Chrono-lume detection agent was from Chrono-Log Corporation (Havertown, PA, USA). TRITC-phalloidin (P1951) was from Sigma-Aldrich (St. Louis, MO, USA). Rhodocytin was kindly provided by Dr. Johannes Eble (University of Münster, Germany).
Antisera.
Flow cytometry antibodies CD62P-APC and PAC1-FITC were from BioLegend (San Diego, CA, USA) and Becton Dickinson (Franklin Lakes, NJ, USA) respectively. Akt S473 (9271), BTK Y551 (18805), DAPP1 Y139 (13703), JAK1 Y1034/1035 (3331), JAK2 Y1007/1008 (3771), JAK3 Y980 (5031), PAK1 S192S197 (2605), PLCγ2 Y1217 (3871), Syk Y525 (2711), STAT3 Y705 (9131), STAT5A/B Y694 (9351), TYK2 Y1054/1055 (9321), phosphorylated Akt substrate (9614) and phosphorylated PKC substrate (6967) antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-STAT5a (MAB2174-SP) and STAT5b (MAB15841-SP) were from R&D Systems (Minneapolis, MN, USA). Tubulin (T6199) antibody was from Sigma-Aldrich. Alexa Fluor 488 goat anti-mouse (A21042) and 594 goat anti-rabbit A32740) secondary antibodies were from Invitrogen (ThermoFisher Scientific; Carlsbad, CA, USA).
Inhibitors.
Ruxolitinib (TLRL-Rux) was from Invivogen and baricitinib (HY-15315) was from MedChem Express (Mounmouth Junction, NJ, USA). Tofacitinib (S2789), entospletinib (S7523), upadacitinib (S8162) and oclacitinib (S8195) were from Selleck Chemicals (Houston, TX, USA). STAT5 inhibitor (15784) was from Cayman Chemical Company. PP2 (1407) and Ro 31–8220 (2002) were from Tocris (Bristol, UK).
Platelet isolation
Human venous blood was drawn from healthy adult male and female volunteers by venipuncture into 3.8% sodium citrate (1:9, v/v) following an Institutional Review Board (IRB) protocol approved by Oregon Health & Science University. Blood was then centrifuged (200×g, 20 min) at room temperature (rt) and the platelet-rich plasma (PRP) was collected. PRP was then centrifuged at 1000×g for 10 min in the presence of PGI2 at a final concentration of 0.1 μg/ml. The supernatant was discarded, and the platelet pellet was resuspended in HEPES/Tyrode buffer (HT; 129 mM NaCl, 0.34 mM Na2HPO4, 2.9 mM KCl, 12 mM NaHCO3, 20 mM HEPES, 5 mM glucose, 1 mM MgCl2; pH 7.3) and 10% acid citrate dextrose buffer. Platelets were centrifuged in the presence of PGI2 at 1000g for 10 min, resuspended in HT, and diluted in HT at the indicated concentrations for each assay.
Static adhesion and spreading assays
Glass coverslips (12 mm round, #1.5 thickness, 12–545-102) were coated with human fibrinogen or soluble collagen at a final concentration of 50 μg/ml for 1 h at rt, followed by surface blocking with denatured, boiled and filtered fatty-acid free bovine serum albumin (BSA) at 5 mg/ml (1 h at rt). 500 μL of platelets at 2×107/ml were incubated with either jakinibs or DMSO vehicle for 10 min. Platelets were then incubated for 45 min on collagen or fibrinogen at 37ºC and washed 3 times with phosphate buffered saline (PBS). Platelet samples were fixed with 4% paraformaldehyde (PFA), washed 3 times with PBS and imaged using Kohler-illuminated Nomarski differential interference contrast (DIC) optics with a Zeiss 63× oil immersion lens.16 For fibrinogen experiments, platelets were stained for 15 min with Phalloidin-TRITC at 1:500 in the presence of sodium-dodecyl sulfate (SDS) 0.1%. Platelets were imaged using Slidebook 5.0 software (Intelligent Imaging Innovations). For collagen adhesion assays, data are expressed as the number of platelets per imaged field (14,587 μm2). For fibrinogen, the data are presented as area covered per platelet, which was manually calculated using ImageJ.
Platelet aggregation
Jakinibs were added to 300 μl of platelets (2×108/ml) for 10 min at 37ºC prior to stimulation with low dose of CRP-XL (1 μg/ml) and monitored under continuous stirring at 1200 rpm at 37°C by measuring changes in light transmission in a PAP-4 aggregometer. Data are presented as percentage of inhibition relative to CRP-XL.
Platelet flow cytometry
Washed platelets (5×107/ml) were pre-treated with the indicated inhibitors for 10 min before stimulation with CRP-XL (2–20 μg/ml), recombinant human thrombin (0.01–0.1 U/ml) or rhodocytin (100 and 200 nM) for 30 min in the presence of CD62P-APC and PAC-1-FITC antibodies (1:25). Samples were fixed with 2% PFA, diluted in PBS and analysed with a BD FACSCanto II flow cytometer. For F-actin content analysis, platelets were treated with jakinibs for 10 min at 37ºC prior to stimulation with CRP-XL for 20 min. Platelets were then fixed with 2% PFA and then permeabilized with 0.1% Triton X-100, and stained with phalloidin-TRITC (1:100), prior to flow cytometry analysis. Data were analysed using FlowJo software and presented as mean fluorescence intensity (MFI).
Western Blot
Platelets (1×109/ml) were pre-treated with inhibitors as indicated for 10 min at rt and subsequently activated with CRP-XL at 10 μg/ml. Platelet lysates were prepared directly in Laemmli sample buffer supplemented with 200 mM DTT. Platelet lysates were separated by SDS-PAGE and transferred to nitrocellulose membranes. Primary antibodies were incubated overnight at 4ºC and secondary antibodies for 1 h at rt. Films were scanned and densitometric analysis performed using QuantityOne analysis software (Bio-Rad). Data were normalized to tubulin and expressed as fold induction compared to vehicle.
Dense granule secretion assay
ATP release was used to measure dense granule secretion followed by an ATP-luciferin-luciferase luminescence reaction. Platelets (2×108/ml; 70 μl) were incubated with the inhibitors before stimulation with CRP-XL, thrombin or rhodocytin in a Corning Costar flat bottom 96-well plate. The Chrono-lume reagent (10 μl) was immediately added and luminescence measured using an Infinite M200 spectrophotometer (Tecan). Data are expressed as total luminescence arbitrary units (a.u.). Technical duplicates were performed for each experiment.
Fluorescence microscopy
Platelets were seeded onto fibrinogen-coated slides for 45 min and then fixed with 4% PFA. Cells were then permeabilized with a blocking solution containing 0.1% SDS in PBS and stained with primary antibodies for STAT5a and STAT5b (15 μg/ml) overnight at 4°C. Alexa Fluor secondary antibodies (1:500) were added in blocking buffer for 2h at rt. After washing with PBS, platelet samples were mounted and imaged with a Zeiss 63× oil immersion lens.
Statistical analysis
Two group data presented in the study followed normal distribution and were analysed performing a two-tailed Student’s t-test. For three or more groups, data were analysed by one-way ANOVA with a Tukey post-hoc test. Statistical significance was considered for p<0.05. Statistic calculations were performed using GraphPad PRISM 9 (San Diego, CA, USA).
RESULTS
JAK1/2 inhibitors ruxolitinib and baricitinib reduce platelet adhesion to collagen
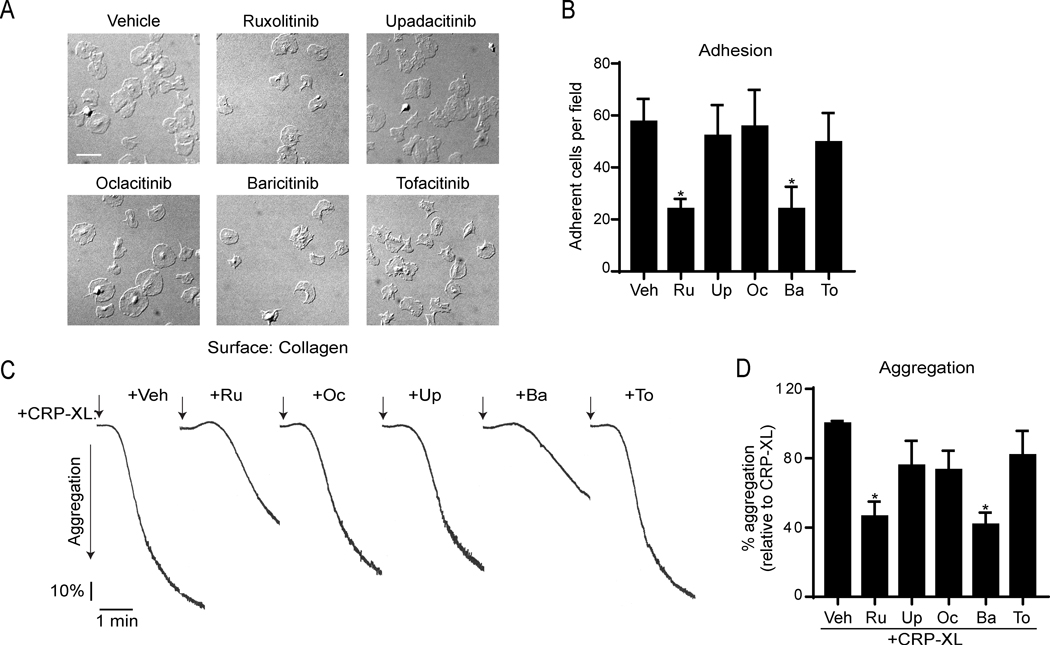
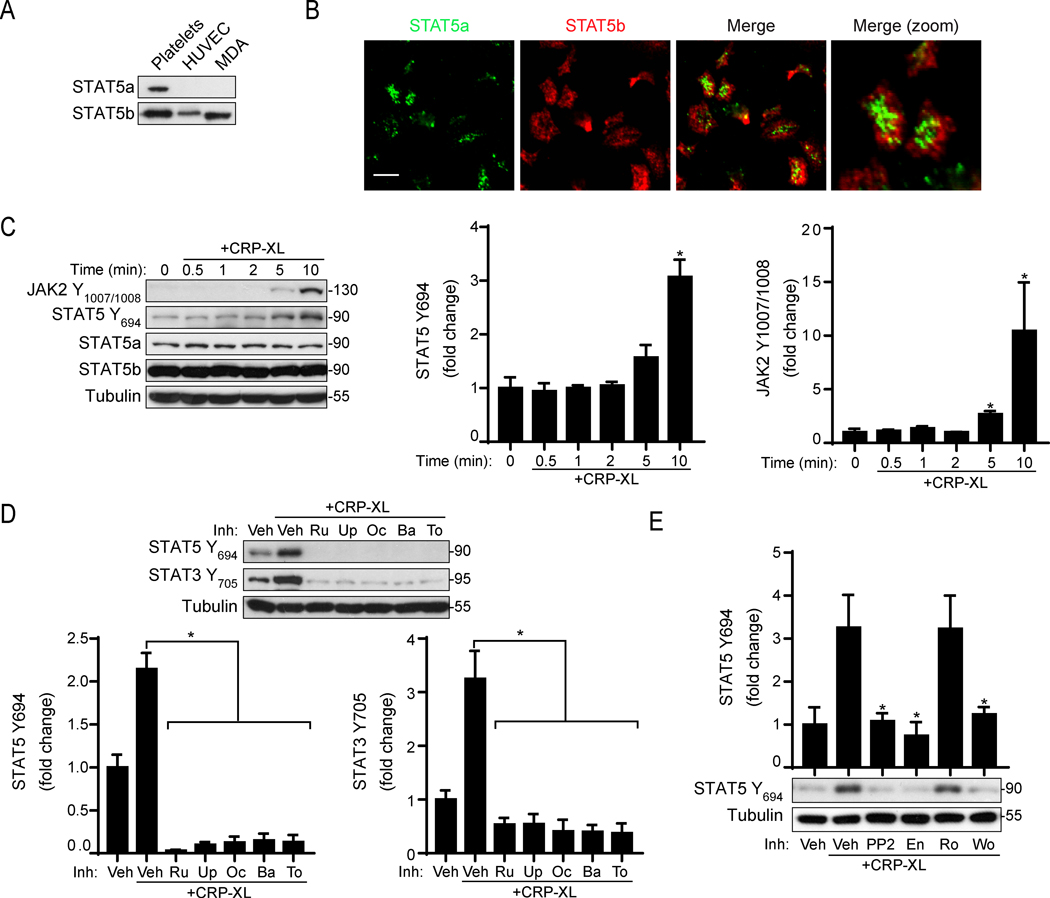
Following vascular injury, platelets rapidly engage collagen and activate via the immune receptor tyrosine activation motif (ITAM)-coupled receptor GPVI to trigger the formation of a hemostatic plug.46,47 First, we analyzed whether the adhesion of human platelets to collagen was affected by jakinibs, including ruxolitinib, baricitinib, upadacitinib, oclacitinib and tofacitinib. As shown in Figure 1A-B, only ruxolitinib (24 ± 7.8 platelets/field; p=0.0225) and baricitinib (24 ± 14.8 platelets/field; p=0.035) significantly impaired the number of platelets attached to collagen under static conditions (57 ± 21.5 platelets/field).
Figure 1. Ruxolitinib and baricitinib impair platelet adhesion to collagen and aggregation in response to the GPVI agonist CRP-XL.
(A) Glass coverslips were coated with soluble collagen (50 μg/ml), blocked with BSA, and incubated with replicate samples of washed human platelets (2×107/ml) pretreated with vehicle (0.1% DMSO) or jakinibs (10 μM each). After 45 min (37ºC), coverslips were washed with PBS, and adherent platelets were fixed with PFA and imaged with DIC microscopy. Representative images of adherent platelets for each condition are shown. Scale bar = 10 μm. (B) Mean adherent cell count per 63×-imaged field (14587 μm2); 3 images captured per condition; n=4 experiments. (C) Replicate samples of washed platelets (2×108/ml) were preincubated with jakinibs (10 μM each) or vehicle (DMSO 0.1%) for 10 min at 37ºC before stimulation with 1 μg/ml of CRP-XL. Platelet aggregation was monitored in a PAP4 aggregometer at a stirring rate of 1200 rpm. Representative aggregation traces and (D) % platelet aggregation in response to CRP-XL relative to vehicle pretreatment (n=3). * indicates statistical significance (p<0.05) compared to vehicle (B) or CRP-XL (D). Error bars indicate standard error of the mean (SEM).
Ruxolitinib and baricitinib inhibit GPVI/ITAM-mediated platelet activation
To determine whether JAK inhibition affects GPVI-mediated platelet responses, we next carried out aggregation experiments in response to crosslinked collagen-related peptide (CRP-XL), which specifically engages the GPVI and Fc receptor-γ chain complex.48 Preincubation of platelets with ruxolitinib and baricitinib blunted platelet aggregation in response to low doses of CRP-XL to 46% (p=0.013) and 41% (p=0.007), respectively (Figure 1C-D). Other jakinibs did not significantly inhibit aggregation. This data suggests that JAK inhibitors may target GPVI-mediated responses in human platelets.
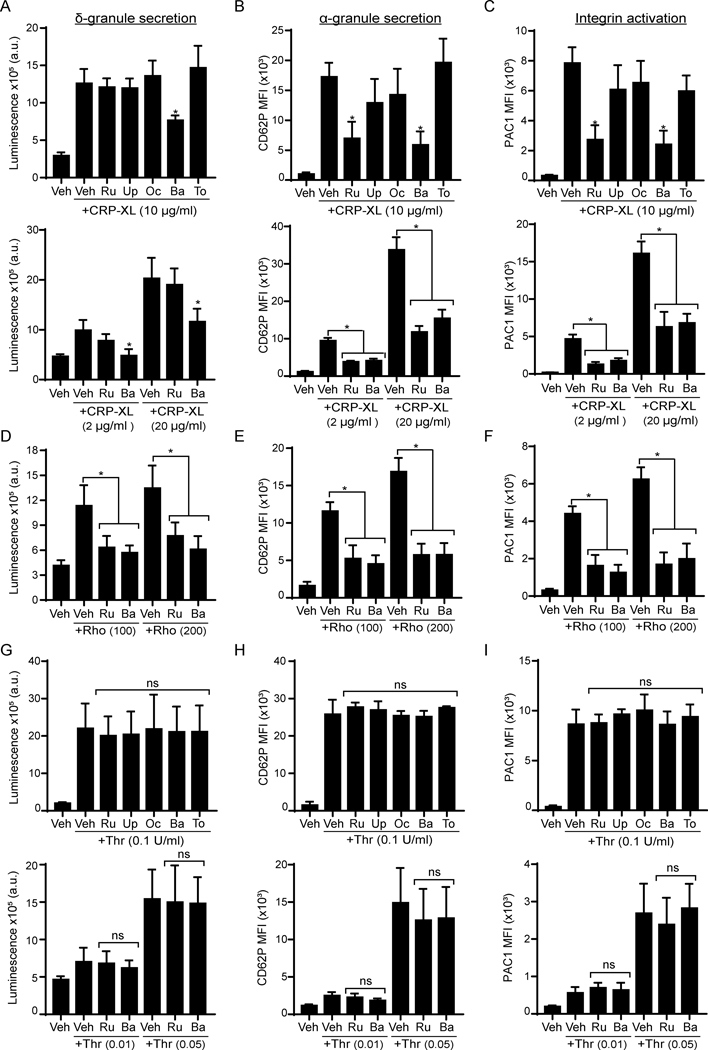
Platelets harbor bioactive molecules in dense- and α-granules, which are released upon activation to propagate responses maintaining vascular hemostasis.46,47 To analyze whether jakinibs affect platelet dense granule secretion, we used a luminescence-based reaction catalyzed by ATP secreted from dense granules. Preincubation of platelets with baricitinib reduced dense granule secretion by 40% in response to 10 μg/ml of CRP-XL, while other jakinibs had no significant effects (Figure 2A, upper panel). We next analyzed P-selectin (CD62P) levels on activating platelets to measure α-granule secretion. Flow cytometry analysis found that, relative to vehicle treated platelets (0.1% DMSO), P-selectin exposure in response to CRP-XL stimulation was inhibited by 72% and 76% in the presence of ruxolitinib or baricitinib, respectively. Upadacitinib and oclacitinib also reduced α-granule secretion in a minor, although not statistically significant manner (Figure 2B, upper panel). Baricitinib (p=0.008) and ruxolitinib (p=0.015) similarly inhibited platelet integrin αIIbβ3 activation in response to CRP-XL by more than 2-fold (Figure 2C, upper panel). The effects of jakinibs on platelet function were similar in response to a range of lower and higher doses of CRP-XL (Figure 2A-C, lower panel).
Figure 2. Baricitinib and ruxolitinib impair GPVI and CLEC-2 mediated platelet function.
Replicate preparations of washed human platelets were preincubated with jakinibs (10 μM) for 10 min prior to stimulation with CRP-XL (2–20 μg/ml; A-C), rhodocytin (Rho; 100–200 nM; D-F) or thrombin (0.01–0.1 U/ml; G-I) and analysis of platelet granule secretion and integrin activation. (A, D, G) Platelets (2×108/ml) were incubated with a luciferin luciferase reagent to measure ATP release and dense granule secretion. Data are expressed as raw mean luminescence (a.u.); n=4 experiments. (B-H) Platelets (5×107/ml) were pre-treated and activated as above in the presence of fluorophore-conjugated antibodies against P-selectin (CD62P-APC) (B) or the activated conformation of human integrin αIIbβ3 (PAC1-FITC) (C) and subsequent analysis by flow cytometry; n=5 experiments. Data are presented as total mean fluorescence intensity (MFI). * indicates statistical significance (p<0.05) compared to CRP-XL or rhodocytin (Veh); ns = not significant. Error bars indicate standard error of the mean (SEM).
To determine whether the effects of ruxolitinib and baricitinib on platelet function are common to ITAM-mediated signaling, we also analyzed their effects on CLEC-2 receptor activation. As seen in Figure 2D, stimulation of platelets with the CLEC-2 agonist rhodocytin (Rho) induced dense granule secretion, which was inhibited only by pretreatment with baricitinib (Figure 2D). In contrast, platelet α-granule secretion and integrin activation in response to rhodocytin were reduced by both ruxolitinib and baricitinib (Figure 2E-F). As shown in Figure 2G-I, platelet response to the G protein-coupled receptor (GPCR) and protease-activated receptor (PAR) agonist thrombin were not significantly affected by any of the JAK inhibitors. Together these data support a targetable role for JAK1/2 in platelet activation mediated by GPVI/ITAM pathways but not by GPCR agonists.
JAK inhibitors impair platelet actin cytoskeleton dynamics
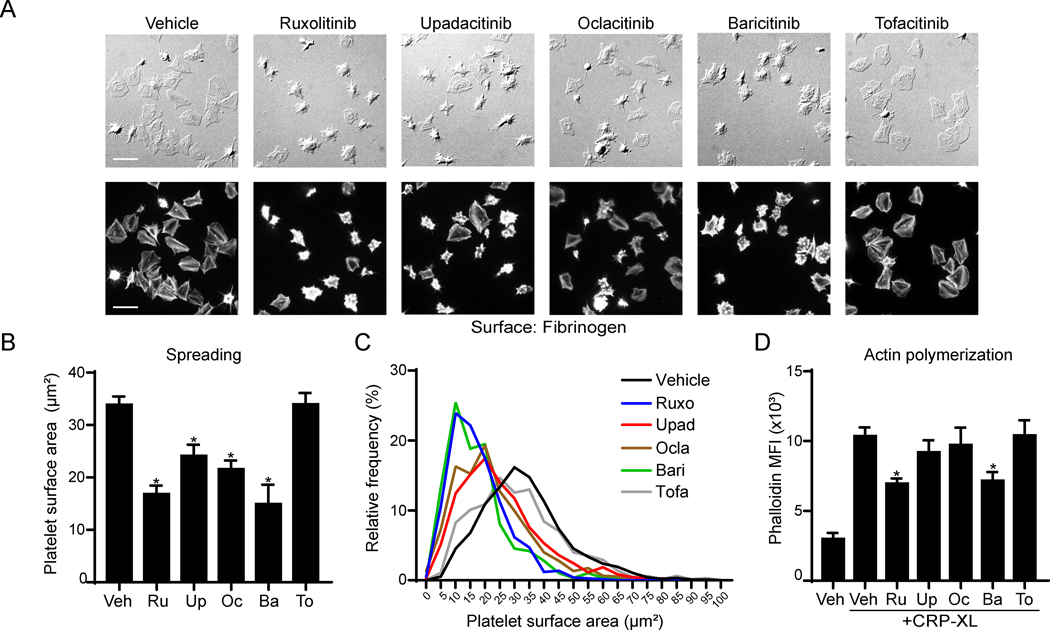
We next examined whether cytoskeletal rearrangements following platelet integrin activation are affected by JAK inhibition. Washed human platelets were incubated with fibrinogen-coated cover glass in the presence of jakinibs or vehicle alone, prior to fixation, imaging and analysis of platelet surface area. As seen in Figure 3A-C, platelet spreading on fibrinogen was significantly reduced by all jakinibs (p<0.005) except for tofacitinib (p=0.53). Flow cytometry analysis found that platelet filamentous actin (F-actin) nucleation in response to CRP-XL stimulation was significantly inhibited by ruxolitinib and baricitinib (Figure 3D). Together, these results suggest that integrin activation and cytoskeletal nucleation responses in platelet GPVI systems can be affected by JAK inhibitors in vitro.
Figure 3. Jakinibs inhibit platelet lamellipodia formation and F-actin nucleation.
(A) Cover glass was incubated with fibrinogen (50 μg/ml) and blocked with BSA for 1 h. Platelets (2×107/ml) were pretreated with jakinibs (10 μM) for 10 min and incubated on cover glass for 45 minutes under static conditions at 37ºC. Platelets were then fixed and stained for actin with phalloidin-TRITC at 1:500 and 3 images captured per condition. Representative images are shown for DIC or Cyt3 fluorescence channels. (B) Mean cell area calculation and statistical analysis and line plot showing cell size distribution (C) are shown; n=3 experiments. (D) Platelets were pretreated with jakinibs, stimulated with CRP-XL (10 μg/ml) and fixed with PFA. Platelets were then permeabilized with Triton-X100 (0.1%) and stained with phalloidin-TRITC (1:100). Mean fluorescence intensity (MFI) was analyzed by flow cytometry. * indicates statistical significance (p<0.05) compared to vehicle (B) or to CRP-XL (Veh) (D). n indicates the number of independent experiments using blood from different donors. Error bars indicate standard error of the mean (SEM).
Jakinibs inhibit PI3K/Akt signaling following GPVI activation
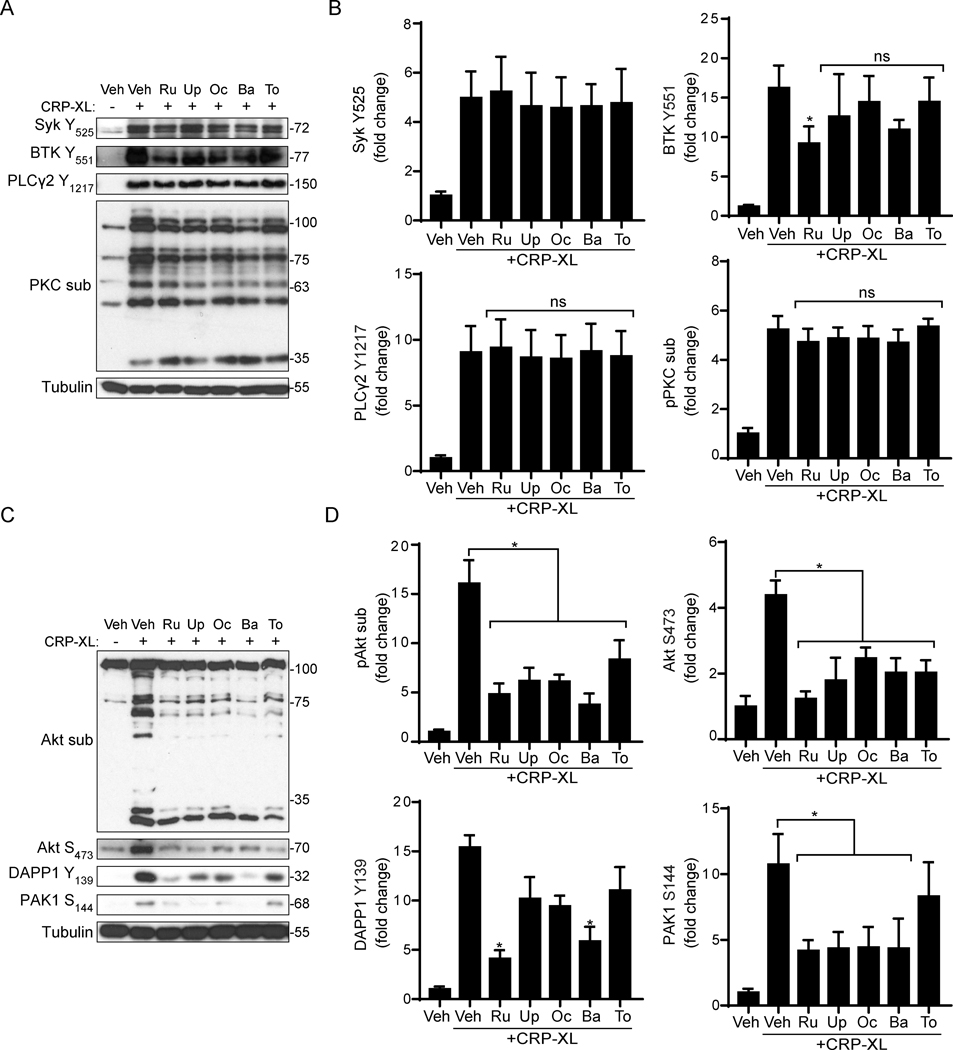
Next, we investigated the effects of jakinibs on platelet GPVI signaling events in response to CRP-XL stimulation. As seen in Figure 4A-B, CRP-XL stimulation of vehicle treated platelets upregulated the phosphorylation of key nodes in GPVI pathways, including Syk Y525, BTK Y551 and PLCγ2 Y1217 as well as the phosphorylation of protein kinase C (PKC) substrates. Pretreatment of platelets with jakinibs did not significantly affect the phosphorylation of these proteins in response to CRP-XL, with the exception of ruxolitinib, which had minor although significant effects on BTK phosphorylation (Figure 4A-B, Supplemental Figure S1). Similarly, CRP-XL stimulated phosphorylation of LAT Y171, Fyn Y530, Lyn Y507, Src Y416 and p38 MAPK T180/Y182 were all unaffected by jakinibs (data not shown).
Figure 4. Jakinibs impair Akt signaling in CRP-XL-activated platelets.
(A) Replicate samples of washed human platelets (1×109/ml) were pre-treated with jakinibs (10 μM) for 10 min and stimulated with CRP-XL (10 μg/ml, rt). Cell lysates were analyzed by immunoblot with indicated antibodies against key nodes in platelet GPVI signaling. Representative blots n=4 experiments are shown. (B) Densitometric quantification and statistical analysis of signal intensities. * indicates statistical significance (p<0.05) compared to CRP-XL (Veh); ns = not significant; Error bars indicate standard error of the mean (SEM). (C) Platelet lysates were similarly analyzed for Akt phosphorylation and phosphorylation of Akt substrates as well as DAPP1 and PAK1 phosphorylation. Representative blots n=4 experiments are shown and (D) quantified for statistical analysis.
In contrast, relative to vehicle controls, pretreatment of platelets with each jakinib significantly inhibited Akt S473 phosphorylation (p<0.02) and the phosphorylation of Akt substrates (p<0.01) in response to CRP-XL stimulation (Figure 4C-D). Akt phosphorylation following thrombin stimulation was not affected by any jakinibs (Supplemental Figure S2). Given associations between ITAM and PI3K/Akt signaling systems in other cell types,49,50 and emerging roles for Dual Adaptor of Phosphotyrosine and 3-Phosphoinositides 1 (DAPP1) in connecting platelet ITAM and PI3K/Akt pathways,37,51 we also examined the effects of jakinibs on DAPP1 phosphorylation. As seen in Figure 4C-D, baricitinib (p=0.001) and ruxolitinib (p=0.002) significantly inhibited DAPP1 phosphorylation. Similar experiments also suggest roles for jakinibs in as all jakinibs – with the exception of tofacitinib (p=0.63) – significantly (p<0.005) limited the phosphorylation of the Rho GTPase (Rac1/Cdc42) effector p21 Activated Kinase 1 (PAK1). Together, these data suggest that jakinibs – especially inhibitors with high specificity for JAK2 – can disrupt crosstalk between platelet ITAM and JAK-STAT pathways proximal to PI3K/Akt signaling.
JAK2/STAT5 signaling in GPVI/ITAM mediated platelet function
Previous studies have found that a JAK2/STAT3 axis is activated in human platelets in response to GPVI agonists.30,32,34,52 However, roles for other STAT proteins in platelet GPVI signaling remain unknown. We recently measured increased JAK2 Y1007/1008 and STAT5 Y694 phosphorylation following GPVI activation with quantitative mass spectrometry and phosphoproteomic analyses.37 Accordingly, we next sought to analyze whether STAT5 phosphorylation has roles in GPVI-mediated platelet responses. Western blot and fluorescence microscopy determined that human platelets express both STAT5 isoforms, STAT5a and STAT5b (Figure 5A-B). Consistent with our previous phosphoproteomics findings, Western blot analysis found that JAK2 (p=0.003) and STAT5 (p<0.001) were phosphorylated in a time-dependent manner in platelets in response to 10 μg/ml of CRP-XL (Figure 5C). Additional experiments found TYK2 phosphorylation but not JAK1 or JAK3 phosphorylation in response to CRP-XL (Supplemental Figure S3).
Figure 5. STAT5 expression and phosphorylation in CRP-XL stimulated platelets.
(A) Washed human platelet, HUVEC and MDA cell lysates were analyzed for STAT5a and STAT5b expression by Western Blot and (B) immunofluorescence microscopy. (C) Platelets (1×109/ml) collected over a 10 min time course following stimulation with 10 μg/ml CRP-XL for Western blot analysis with antibodies, as indicated. Representative Western blots and densitometric analysis are shown. Tubulin serves as a control for equal protein loading between samples. (D) Platelets were pretreated with jakinibs (10 μM) as indicated prior to CRP-XL stimulation Western blot analysis for STAT3 and STAT5 phosphorylation; n=4. (E) Platelets were pretreated with the Src kinase inhibitor PP2, the Syk inhibitor entospletinib (En), the PKC inhibitor Ro 31–8220 (Ro) and the PI3K inhibitor wortmannin (Wo) prior to stimulation with CRP-XL and Western blot analysis for STAT5 phosphorylation; n=4. * indicates statistical significance (p<0.05) compared to untreated (C) or to CRP-XL (Veh) (D-E). Error bars indicate standard error of the mean (SEM).
To further examine the role of JAK activity in GPVI-mediated STAT phosphorylation, we pretreated platelets with jakinibs prior to stimulation with CRP-XL. All JAK inhibitors abrogated phosphorylation of STAT5 as well as STAT3 in response to CRP-XL (Figure 5D). To further determine how JAK2/STAT5 signaling may place into GPVI pathways, we pretreated platelets with inhibitors of GPVI signaling prior to CRP-XL stimulation. The Src inhibitor PP2 (p=0.02) and the Syk inhibitor entospletinib (p=0.01) reverted STAT5 activation triggered by CRP-XL (Figure 5E). In addition, the PI3K inhibitor wortmannin (p=0.03) inhibited STAT5 phosphorylation (Figure 5E). The PKC inhibitor Ro-31–8820 had no effect on STAT phosphorylation. Together with data above (Figure 4), these experiments suggest that jakinibs may target a crosstalk between JAK/STAT5 and GPVI/ITAM systems in platelets through PI3K/Akt pathways independent of PLCγ2-PKC signaling.
Pharmacologic inhibition of STAT5 affects platelet function
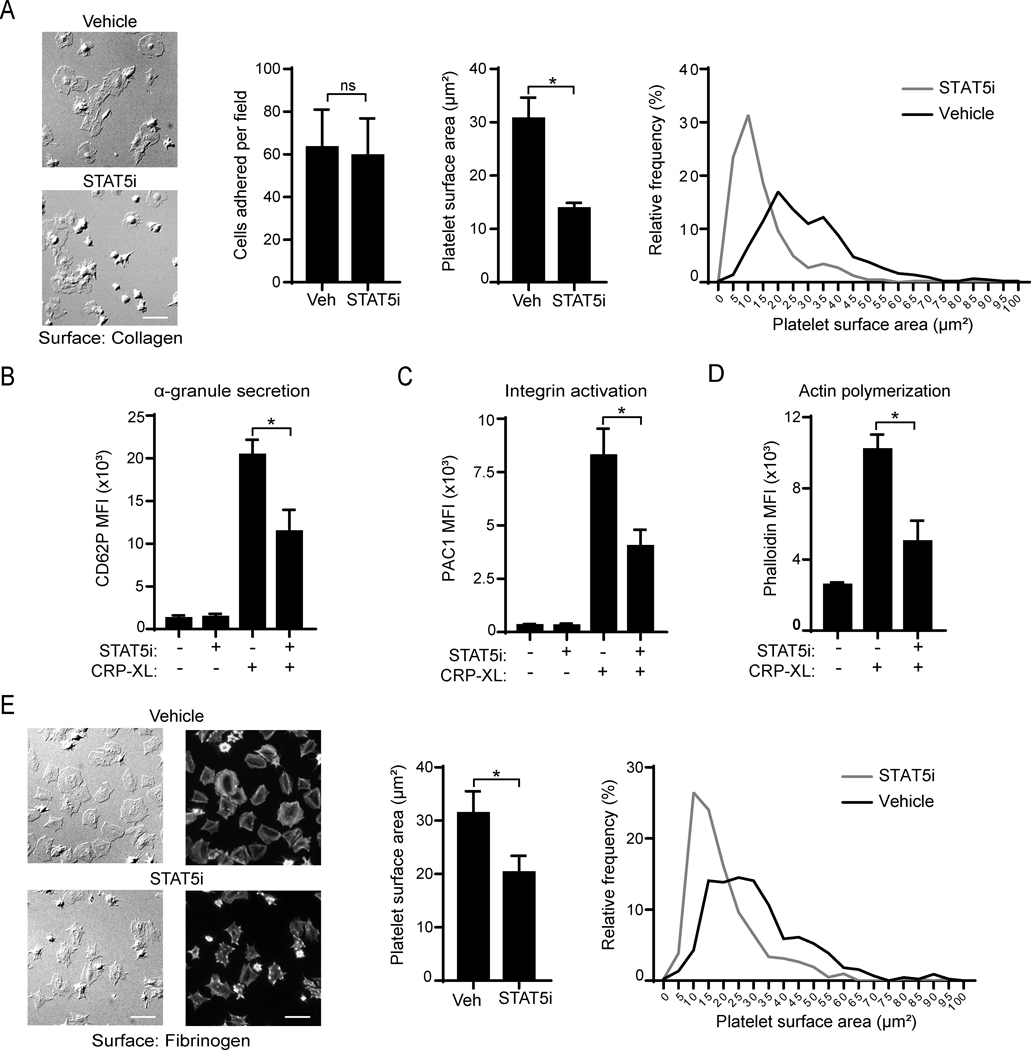
Finally, we assessed potential roles for STAT5 in GPVI-mediated platelet responses using a small molecule inhibitor (STAT5i) that blocks JAK-STAT5 SH2 domain interactions and STAT5 function.53 Incubation of platelets with STAT5i did not affect the number of platelets adherent to collagen under static conditions, but significantly reduced platelet spreading on collagen (30.6 ± 3.9 μm2 vs 13.8 ± 1.0 μm2; p=0.002) (Figure 6A). In addition, flow cytometry assays demonstrated that STAT5 inhibition reduced α-granule secretion (p=0.007) and integrin activation (p=0.006) in response to CRP-XL treatment (Figure 6B-C). As shown in Figure 6D, STAT5 inhibition also impaired actin nucleation in response to CRP-XL (p=0.004). Relative to vehicle control, STAT5i also reduced platelet spreading on fibrinogen (31.3 ± 4.1 μm2 vs 20.0 ± 2.6 μm2; p=0.036) (Figure 6E). Pretreatment of platelets with STAT5i did not affect PLCγ2 phosphorylation in response to CRP-XL stimulation (Supplemental Figure S4). Together, these experiments support a role for STAT5 in GPVI-mediated platelet function.
Figure 6. STAT5i inhibits platelet function following GPVI activation.
(A) Replicate samples of washed human platelets were incubated with a STAT5 inhibitor (STAT5i, 40 μM) or vehicle alone (0.2% DMSO) for 10 min before assessing platelet adhesion to and spreading on cover glass coated with soluble collagen (50 μg/ml) The mean cell count and surface area per cell was assessed and analyzed (middle graphs). A histogram showing population distribution is shown (right graph); n=4. Scale bar = 10 μm. (B) Platelets (5×107/ml) were activated with CRP-XL (10 μg/ml) in the presence of P-selectin (CD62P-APC) or (C) PAC1-FITC antibody and subsequently analyzed in a Canto II flow cytometer; n=4. (D) Platelets were pretreated with jakinibs, stimulated with CRP-XL (10 μg/ml) and fixed with PFA. Platelets were then permeabilized with Triton-X100 (0.1%) and stained with phalloidin-TRITC (1:100). Mean fluorescence intensity (MFI) was analyzed by flow cytometry. (E) Spreading on fibrinogen (50 μg/ml) was analyzed similarly to A. Platelets were then fixed and stained for actin with a phalloidin-TRITC and 3 random images taken per condition. Representative images of an experiment using a 63× objective for DIC (left images) or Cyt3 fluorescence channel (right images). The cell area, statistical analysis (middle graph) and a histogram showing cell size distribution (right graph) are shown; n=3. * indicates statistical significance (p<0.05) compared to vehicle or to CRP-XL (Veh). Error bars indicate standard error of the mean (SEM).
DISCUSSION
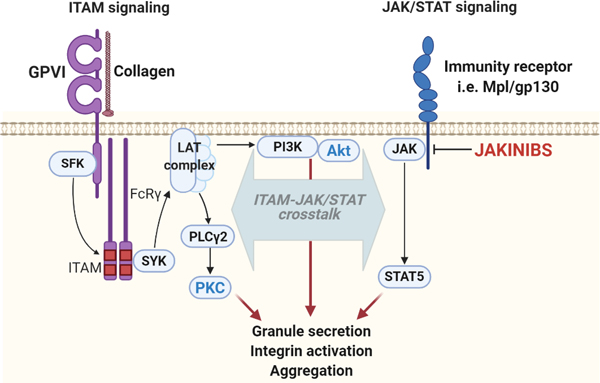
Here, we demonstrate that clinically relevant, small molecule inhibitors targeting Janus kinases impair JAK kinase activity in platelets in a manner that may affect platelet function mediated by the platelet ITAM-coupled collagen receptor, GPVI. In addition, we establish a previously unrecognized role for JAK-mediated STAT5 phosphorylation in platelet GPVI pathways and platelet function. Altogether, this study provides further insights into the emerging roles of the JAK/STAT pathways in regulating platelet function and suggests roles for a JAK2/STAT5 axis in GPVI in an interconnected manner associated with PI3K/Akt pathways (Figure 7).
Figure 7. Hypothesized model of platelet GPVI/ITAM and JAK/STAT crosstalk.
Following activation of GPVI, Src family kinases (SFKs) activate Syk to establish a LAT signalosome supporting. activation of PLCγ2, PKC activation and several platelet functional responses. Other PI3K signaling events around LAT also help to organize pleckstrin homology (PH) domain-containing proteins, including BTK, DAPP1 and Akt, to regulate platelet function. Chemokine receptors proximal to GPVI, such as c-Mpl or gp130, activate JAKs promote the recruitment and phosphorylation of STAT5 as well as feedback to PI3K/Akt pathways to regulate GPVI signaling and platelet function. Inhibition of JAKs by Jakinibs reduces the activation of PI3K/Akt pathways, thus affecting the extent of platelet functional responses. GPVI: glycoprotein VI; ITAM: immunoreceptor tyrosine-based activation motif; JAK: Janus kinases; LAT: linker for activation of T-cells; PI3K: phosphoinositide 3-kinase; PKC: protein kinase C; PLCγ2: phospholipase gamma 2; SFK: Src family of protein kinases; STAT: signal transducers and activators of transcription; SYK: spleen tyrosine kinase.
In addition to their physiologically vital functions in hemostasis, platelets have increasingly recognized roles in the regulation of the immunity and inflammation.17,54 As such, platelets express and utilize several components of cytokine and growth factor receptor signaling systems, where JAK/STAT pathways play major roles.55,56 JAK pathways are not only involved in signaling events triggered by immune mediators, but also in platelet responses triggered by classical hemostatic agonists, including collagen and thrombin.30,38,52 Phosphoproteomics36,37 as well as computational modeling approaches also suggest roles for JAKs in platelet activation programs.36,37 From a therapeutic perspective, JAK/STAT pathways have been attractive targets for conditions involving an aberrant immune response, such as rheumatoid arthritis and myeloproliferative neoplasms.57,58 In this study we report the effects of five clinically available drugs targeting JAK pathways in platelets isolated from healthy male and female volunteers. Our findings suggest that JAK2, rather than the other JAK members, is involved in GPVI-mediated platelet responses, which is consistent with our previously published phosphoproteomic data.37
Our data suggests that jakinibs modulate platelet activation in a manner specific to GPVI and ITAM-mediated activation. Biochemical experiments demonstrate that JAK inhibition impairs PI3K/Akt signaling, whereas PKC signaling (typically following PLCγ2 activation) appears unaffected. Consistent with our findings, Moore et al. have previously shown that TPO, whose upstream signaling is mediated by JAK/STAT, potentiates platelet activation via PI3K pathways.42 Potential candidates connecting JAK with the DAPP1/PI3K/Akt include Grb2 and Grb interacting proteins. In other cell types, JAK2 can directly phosphorylate GRB proteins,59 which act as adaptors connecting the JAK2 and DAPP1 pathways in a manner that regulates PI3K/Akt activation.49 Interestingly, the Hers group has demonstrated associations among DAPP1 and Grb2 adaptor proteins (GAB1, GAB2, GAB3 and GRAP2) in platelet PIP3 signalosomes.51 Cooperation between JAK/STAT and PI3K has also been reported in other cellular models,60,61,62 including other ITAM pathways (i.e., IgE-mediated mast cell activation).50 Together with these prior studies, our current findings suggest that PI3K/Akt and JAK/STAT pathways cooperatively modulate platelet function.
Comparatively, our results suggest that the JAK1/2 inhibitors ruxolitinib and baricitinib have the most potent effects of platelet activation in vitro, as demonstrated by biochemical, imaging, flow cytometry and other functional assays. Oclacitinib, upadacitinib and tofacitinib (JAK1 or JAK3 inhibitors) tended to decrease platelet adhesion, integrin activation and granule secretion induced by CRP-XL but not in a statistically significant manner. However, upadacitinib and oclacitinib significantly affected platelet spreading on fibrinogen. The main difference between ruxolitinib and baricitinib was that only baricitinib reduced platelet dense granule secretion, which may be explained by specific signaling events downstream of PI3K/Akt that remain to be explored. Interestingly, despite increased risk of thromboembolic events,14 baricitinib was recently approved as a therapy to target cytokine storm and immune dysregulation in severe acute respiratory syndrome-coronavirus disease-2 (SARS-CoV-2) infection and COVID-19 in combination with remdesivir.63 As platelets have roles in SARS-CoV-2 infection,64 how baricitinib treatment modulates hemostasis and platelet function in these patients may help understand the roles of JAK/STAT pathways in thromboinflammatory diseases.
Following JAK activation, the STATs are known to take on non-transcriptional roles to regulate platelet function. For instance, preincubation with STA-21, a novel STAT3 inhibitor, blunted collagen-driven platelet activation and aggregation.31 Similarly, piperlongumine – an alkyl amide STAT3 inhibitor from long pepper fruit – impairs platelet aggregation induced by collagen via blockade of STAT3.30 From a mechanistic point of view, STAT3 has been described to act as a protein scaffold between Syk and PLCγ2.52 Moreover, it has been shown that GPVI is in physical proximity with immunity receptors in lipid rafts in a manner that enhances platelet activation via the JAK2/STAT3 axis.34 Apart from STAT3, roles for other STAT family remained to be explored. In this study, we identify a potential role for STAT5 in GPVI-mediated platelet responses. Our recently published phosphoproteomic profiling of CRP-XL-triggered signaling events in platelets indicated that STAT5 is activated in response to high doses of CRP-XL,37 which is in contrast with the lack of STAT5 activation reported by other studies using lower doses of CRP-XL.65
With Western blot and immunofluorescence microscopy (Fig. 5), we demonstrate that human platelets express both STAT5 isoforms, namely STAT5a and STAT5b. These proteins participate in hematopoiesis and immunoregulation,66 and have been described to play differential roles in specific disease settings.67 Previous studies have reported the expression and activation of STAT5 in TPO- and thrombin-stimulated platelets, whereas the functional consequences as well as involvement in GPVI/ITAM pathways remained unexplored.41,42 To assess the potential role of STAT5 in platelet function, we used a novel inhibitor that targets the SH2 domain of STAT5, thus impeding STAT5 recruitment by upstream JAK.53 Our platelet function assays showed that inhibition of STAT5 partially impaired platelet spreading on collagen and fibrinogen, α-granule secretion and integrin activation. These finding adds novel insights into the functionality of STAT5 in platelets beyond its role in regulating TPO signaling.41,68
Conclusions
Our study demonstrates that the JAK1/2 inhibitors ruxolitinib and baricitinib partially inhibit GPVI-induced platelet activation in vitro. Moreover, we describe a role for JAK/STAT5 signaling in GPVI-mediated platelet function. Future studies based upon this mechanistic work will further advance an understanding of the effects of targeting JAK/STAT pathways in human platelets.
Supplementary Material
Table 1:
Pharmacology of the different JAK inhibitors used in the study.
| Inhibitor (Brand name) | Target IC50 enzymatic assays (nM)* | Indication (FDA approved) | |||
|---|---|---|---|---|---|
| JAK1 | JAK2 | JAK3 | TYK2 | ||
| Ruxolitinib (Jakafi®) | 3.3 | 2.2 | 428 | 19 | Polycythemia vera, acute graft-versus-host disease, myelofibrosis |
| Oclacitinib (Apoquel®) | 10 | 18 | 99 | 84 | Veterinary use |
| Upadacitinib (Rinvoq®) | 43 | 200 | 2300 | 4700 | Rheumatoid arthritis |
| Baricitinib (Olumiant®) | 5.9 | 5.7 | 560 | 53 | Rheumatoid arthritis |
| Tofacitinib (Xeljanz®) | 1 | 20 | 112 | 472 | Rheumatoid arthritis, ulcerative colitis, psoriatic arthritis |
IC50 values are from References as well as manufacturer data (Selleck Chemicals, Invivogen, MedChemExpress)
ACKNOWLEDGEMENTS
This work was supported by the American Heart Association (17SDG33350075 to J.E.A.), the American Society of Hematology (ASH Scholar Award to J.E.A.), the National Institutes of Health (R01HL146549 to J.E.A. and R01HL101972 to O.J.T.M.). and the Medical Research Foundation of Oregon.
Footnotes
Declaration of interest: The authors report no declarations of interest.
CONFLICT OF INTEREST
The authors declare no competing interests.
REFERENCES
- 1.Subramaniam PS, Torres BA & Johnson HM So many ligands, so few transcription factors: a new paradigm for signaling through the STAT transcription factors. Cytokine 15, 175–187 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Kiu H. & Nicholson SE Biology and significance of the JAK/STAT signalling pathways. Growth Factors Chur Switz. 30, 88–106 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aaronson DS & Horvath CM A road map for those who don’t know JAK-STAT. Science 296, 1653–1655 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Kontzias A, Kotlyar A, Laurence A, Changelian P. & O’Shea JJ Jakinibs: A New Class of Kinase Inhibitors in Cancer and Autoimmune Disease. Curr. Opin. Pharmacol 12, 464–470 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKeage K. Ruxolitinib: A Review in Polycythaemia Vera. Drugs 75, 1773–1781 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Plosker GL Ruxolitinib: a review of its use in patients with myelofibrosis. Drugs 75, 297–308 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Tanaka Y. Recent progress and perspective in JAK inhibitors for rheumatoid arthritis: from bench to bedside. J. Biochem. (Tokyo) 158, 173–179 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Genovese MC et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. The Lancet 391, 2513–2524 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Markham A. Baricitinib: First Global Approval. Drugs 77, 697–704 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Gonzales AJ et al. Oclacitinib (APOQUEL(®)) is a novel Janus kinase inhibitor with activity against cytokines involved in allergy. J. Vet. Pharmacol. Ther 37, 317–324 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Virtanen, A. T, Haikarainen T, Raivola J. & Silvennoinen O. Selective JAKinibs: Prospects in Inflammatory and Autoimmune Diseases. BioDrugs 33, 15–32 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajasimhan S, Pamuk O. & Katz JD Safety of Janus Kinase Inhibitors in Older Patients: A Focus on the Thromboembolic Risk. Drugs Aging 37, 551–558 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verden A, Dimbil M, Kyle R, Overstreet B. & Hoffman KB Analysis of Spontaneous Postmarket Case Reports Submitted to the FDA Regarding Thromboembolic Adverse Events and JAK Inhibitors. Drug Saf. 41, 357–361 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Mehta P, Ciurtin C, Scully M, Levi M. & Chambers RC JAK inhibitors in COVID-19: need for vigilance regarding increased inherent thrombotic risk. Eur. Respir. J (2020) doi: 10.1183/13993003.01919-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barraco F. et al. Real-world non-interventional long-term post-authorisation safety study of ruxolitinib in myelofibrosis. Br. J. Haematol (2020) doi: 10.1111/bjh.16729. [DOI] [PubMed] [Google Scholar]
- 16.Aslan JE Platelet Shape Change. in Platelets in Thrombotic and Non-Thrombotic Disorders: Pathophysiology, Pharmacology and Therapeutics: an Update (eds. Gresele P, Kleiman NS, Lopez JA & Page CP) 321–336 (Springer International Publishing, 2017). doi: 10.1007/978-3-319-47462-5_24. [DOI] [Google Scholar]
- 17.Koupenova M, Clancy L, Corkrey HA & Freedman JE Circulating Platelets as Mediators of Immunity, Inflammation, and Thrombosis. Circ. Res 122, 337–351 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aslan JE Platelet Proteomes, Pathways, and Phenotypes as Informants of Vascular Wellness and Disease. Arterioscler. Thromb. Vasc. Biol 41, 999–1011 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Research C. for D. E. and. FDA approves Boxed Warning about increased risk of blood clots and death with higher dose of arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR). FDA (2019). [Google Scholar]
- 20.Taylor PC et al. Cardiovascular Safety During Treatment With Baricitinib in Rheumatoid Arthritis. Arthritis Rheumatol. Hoboken NJ 71, 1042–1055 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samuelson BT et al. The impact of ruxolitinib on thrombosis in patients with polycythemia vera and myelofibrosis: a meta-analysis. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb 27, 648–652 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Marchetti M. et al. Which patients with myelofibrosis should receive ruxolitinib therapy? ELN-SIE evidence-based recommendations. Leukemia 31, 882–888 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Harrison CN et al. Momelotinib versus best available therapy in patients with myelofibrosis previously treated with ruxolitinib (SIMPLIFY 2): a randomised, open-label, phase 3 trial. Lancet Haematol. 5, e73–e81 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Verstovsek S. et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N. Engl. J. Med 366, 799–807 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shatzel JJ et al. Ibrutinib-associated bleeding: pathogenesis, management and risk reduction strategies. J. Thromb. Haemost. JTH 15, 835–847 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng TJ et al. Assessment of the effects of Syk and BTK inhibitors on GPVI-mediated platelet signaling and function. Am. J. Physiol. Cell Physiol (2021) doi: 10.1152/ajpcell.00296.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rigg RA et al. Oral administration of Bruton’s Tyrosine Kinase (Btk) inhibitors impairs GPVI-mediated platelet function. Am. J. Physiol.-Cell Physiol (2015) doi: 10.1152/ajpcell.00325.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loren CP et al. The BCR-ABL inhibitor ponatinib inhibits platelet immunoreceptor tyrosine-based activation motif (ITAM) signaling, platelet activation and aggregate formation under shear. Thromb. Res 135, 155–160 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu W-J et al. Role of a Janus kinase 2-dependent signaling pathway in platelet activation. Thromb. Res 133, 1088–1096 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Yuan H. et al. Piperlongumine Blocks JAK2-STAT3 to Inhibit Collagen-Induced Platelet Reactivity Independent of Reactive Oxygen Species†. PLOS ONE 10, e0143964 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Z. et al. A novel STAT3 inhibitor negatively modulates platelet activation and aggregation. Acta Pharmacol. Sin 38, 651–659 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Z. et al. STAT3 Regulates Collagen-Induced Platelet Aggregation Independent of its Transcription Factor Activity. Circulation 127, 476–485 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heinrich PC, Behrmann I, Müller-Newen G, Schaper F. & Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem. J 334 ( Pt 2), 297–314 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Houck KL et al. Physical proximity and functional cooperation of glycoprotein 130 and glycoprotein VI in platelet membrane lipid rafts. J. Thromb. Haemost 17, 1500–1510 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Eaton N. et al. Bleeding diathesis in mice lacking JAK2 in platelets. Blood Adv. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Izquierdo I. et al. A Comprehensive Tyrosine Phosphoproteomic Analysis Reveals Novel Components of the Platelet CLEC-2 Signaling Cascade. Thromb. Haemost 120, 262–276 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Babur Ö et al. Phosphoproteomic quantitation and causal analysis reveal pathways in GPVI/ITAM-mediated platelet activation programs. Blood 136, 2346–2358 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodríguez-Liñares B. & Watson SP Phosphorylation of JAK2 in thrombin-stimulated human platelets. FEBS Lett. 352, 335–338 (1994). [DOI] [PubMed] [Google Scholar]
- 39.Tibbles HE et al. Role of a JAK3-dependent biochemical signaling pathway in platelet activation and aggregation. J. Biol. Chem 276, 17815–17822 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Ezumi Y, Takayama H. & Okuma M. Thrombopoietin, c-Mpl ligand, induces tyrosine phosphorylation of Tyk2, JAK2, and STAT3, and enhances agonists-induced aggregation in platelets in vitro. FEBS Lett. 374, 48–52 (1995). [DOI] [PubMed] [Google Scholar]
- 41.Miyakawa Y. et al. Thrombopoietin induces tyrosine phosphorylation of Stat3 and Stat5 in human blood platelets. Blood 87, 439–446 (1996). [PubMed] [Google Scholar]
- 42.Moore SF, Smith NR, Blair TA, Durrant TN & Hers I. Critical roles for the phosphatidylinositide 3-kinase isoforms p110β and p110γ in thrombopoietin-mediated priming of platelet function. Sci. Rep 9, 1468 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hobbs CM et al. JAK2V617F leads to intrinsic changes in platelet formation and reactivity in a knock-in mouse model of essential thrombocythemia. Blood 122, 3787–3797 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shinobu Matsuura et al. Platelet Dysfunction and Thrombosis in JAK2V617F-Mutated Primary Myelofibrotic Mice. Arterioscler. Thromb. Vasc. Biol 40, e262–e272 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dellas C. et al. Leptin signalling and leptin-mediated activation of human platelets: importance of JAK2 and the phospholipases Cgamma2 and A2. Thromb. Haemost 98, 1063–1071 (2007). [PubMed] [Google Scholar]
- 46.Bye AP, Unsworth AJ & Gibbins JM Platelet signaling: a complex interplay between inhibitory and activatory networks. J. Thromb. Haemost 14, 918–930 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stalker TJ, Newman DK, Ma P, Wannemacher KM & Brass LF Platelet signaling. Handb. Exp. Pharmacol 59–85 (2012) doi: 10.1007/978-3-642-29423-5_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kehrel B. et al. Glycoprotein VI is a major collagen receptor for platelet activation: it recognizes the platelet-activating quaternary structure of collagen, whereas CD36, glycoprotein IIb/IIIa, and von Willebrand factor do not. Blood 91, 491–499 (1998). [PubMed] [Google Scholar]
- 49.Tt Z. et al. Phosphoinositide 3-kinase-regulated adapters in lymphocyte activation. Immunol. Rev 232, 255–272 (2009). [DOI] [PubMed] [Google Scholar]
- 50.Kiwanuka KN et al. Stat5B is required for IgE-Mediated mast cell function in vitro and in vivo. Cell. Immunol 364, 104344 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Durrant TN et al. In-depth PtdIns(3,4,5)P3 signalosome analysis identifies DAPP1 as a negative regulator of GPVI-driven platelet function. Blood Adv. 1, 918–932 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Z. et al. STAT3 Regulates Collagen-Induced Platelet Aggregation Independent of Its Transcription Factor Activity. Blood 116, 157–157 (2010).20233971 [Google Scholar]
- 53.Müller J, Sperl B, Reindl W, Kiessling A. & Berg T. Discovery of chromone-based inhibitors of the transcription factor STAT5. Chembiochem Eur. J. Chem. Biol 9, 723–727 (2008). [DOI] [PubMed] [Google Scholar]
- 54.Margraf A. & Zarbock A. Platelets in Inflammation and Resolution. J. Immunol 203, 2357–2367 (2019). [DOI] [PubMed] [Google Scholar]
- 55.Gear ARL & Camerini D. Platelet chemokines and chemokine receptors: linking hemostasis, inflammation, and host defense. Microcirc. N. Y. N 1994 10, 335–350 (2003). [DOI] [PubMed] [Google Scholar]
- 56.D’ Atri LP & Schattner M. Platelet toll-like receptors in thromboinflammation. Front. Biosci. Landmark Ed 22, 1867–1883 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Fragoulis GE, McInnes IB & Siebert S. JAK-inhibitors. New players in the field of immune-mediated diseases, beyond rheumatoid arthritis. Rheumatology 58, i43–i54 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Banerjee S, Biehl A, Gadina M, Hasni S. & Schwartz DM JAK–STAT Signaling as a Target for Inflammatory and Autoimmune Diseases: Current and Future Prospects. Drugs 77, 521–546 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haines E. et al. Tyrosine phosphorylation of Grb2: role in prolactin/epidermal growth factor cross talk in mammary epithelial cell growth and differentiation. Mol. Cell. Biol 29, 2505–2520 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abell K. & Watson CJ The Jak/Stat pathway: a novel way to regulate PI3K activity. Cell Cycle Georget. Tex 4, 897–900 (2005). [DOI] [PubMed] [Google Scholar]
- 61.Lu Y. et al. JAK/STAT and PI3K/AKT pathways form a mutual transactivation loop and afford resistance to oxidative stress-induced apoptosis in cardiomyocytes. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol 21, 305–314 (2008). [DOI] [PubMed] [Google Scholar]
- 62.Saxena NK et al. Concomitant Activation of the JAK/STAT, PI3K/AKT, and ERK Signaling Is Involved in Leptin-Mediated Promotion of Invasion and Migration of Hepatocellular Carcinoma Cells. Cancer Res. 67, 2497–2507 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Richardson P. et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. The Lancet 395, e30–e31 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parra-Izquierdo I. & Aslan JE Perspectives on Platelet Heterogeneity and Host Immune Response in Coronavirus Disease 2019 (COVID-19). Semin. Thromb. Hemost 46, 826–830 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blair TA et al. Phosphoinositide 3-kinase p110α negatively regulates thrombopoietin-mediated platelet activation and thrombus formation. Cell. Signal 50, 111–120 (2018). [DOI] [PubMed] [Google Scholar]
- 66.Grimley PM, Dong F. & Rui H. Stat5a and Stat5b: fraternal twins of signal transduction and transcriptional activation. Cytokine Growth Factor Rev. 10, 131–157 (1999). [DOI] [PubMed] [Google Scholar]
- 67.Kollmann S. et al. Twins with different personalities: STAT5B—but not STAT5A—has a key role in BCR/ABL-induced leukemia. Leukemia 33, 1583–1597 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Drayer AL, Boer A-K, Los EL, Esselink MT & Vellenga E. Stem cell factor synergistically enhances thrombopoietin-induced STAT5 signaling in megakaryocyte progenitors through JAK2 and Src kinase. Stem Cells Dayt. Ohio 23, 240–251 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.