Abstract
Objective:
Children living with HIV can experience cognitive difficulties. Most neuropsychological tests have been constructed in Western languages, meaning they may not be appropriate for use in non-Western settings. To address this, we used an entirely nonverbal measure of cognitive ability in a sub-Saharan African sample.
Methods:
For this cross-sectional analysis, 316 children (162 HIV+ and 154 HIV−, ages 3-8) completed the Leiter-3 as part of a larger study in Dar es Salaam, Tanzania. Statistical tests included analysis of covariance and multiple linear regression to account for environmental variables.
Results:
HIV+ children performed worse than HIV− controls on two composite scores: Nonverbal IQ (p < .001) and Processing Speed (p < 0.001). Similar trends were observed on core subtests. Multiple linear regression models revealed that age, socioeconomic status, and school attendance predicted all Leiter-3 test composites. Critically, the addition of HIV status to the models improved prediction of Nonverbal IQ (ΔR2 = 0.03, p = .001) and Processing Speed (ΔR2 = 0.06, p < .001).
Conclusions:
Children living with HIV performed worse than HIV− controls on most Leiter-3 measures. While age, SES, and school attendance predicted Leiter-3 performance, HIV status improved prediction capabilities when added to the model. The Leiter-3 may offer a viable measure of cognitive ability in non-Western settings that can be used in its original form without translation.
Introduction
HIV infection can damage the central nervous system (Saylor et al., 2016). Children living with HIV are at increased risks for cognitive deficits and developmental delays (Sherr et al., 2009). Thus, determining if thinking skills are maturing and growing appropriately is very important especially in young children with HIV. Early detection and intervention may result in better functional outcomes (Wedderburn et al., 2019). Assessing global cognitive functioning, however, has traditionally been conducted in Western cultures. Several studies have used cognitive testing with pediatric populations in Sub-Saharan African, by translating language-based Western tests from English into local languages (Boivin et al., 2019; Gruver et al., 2020; Ruel et al., 2012). While such methods are valid and reliable (Chernoff et al., 2018), they still involve significant alterations to standardized test administration procedures and require a unique translation for different populations.
When engaging in the assessment of cognitive abilities across cultures, verbal communication should be limited or nonexistent, answers should be selectable (e.g., multiple-choice) rather than open-ended, and factors such as time/speed must be assessed with cultural values incorporated, as not all cultures value speed as much as accuracy (Oakland, 2009). The use of nonverbal tests may be the best method for assessing intelligence in individuals from culturally diverse settings (McCallum, 2017). While nonverbal tests place less emphasis on language, just like all forms of assessment, they still have cultural biases (Flanagan, 2013). Nevertheless, nonverbal assessment helps reduce bias in traditional testing practices (McCallum, 2017). And as HIV in children is more common outside of the industrialized West, it is particularly important to have measures of cognitive functioning that can apply to non-Western settings.
The current study assessed the utility of an entirely nonverbal assessment to test cognitive development in African children with and without HIV. To our knowledge, no studies have used entirely language-free Western tests for the cognitive assessment of children in Africa. This testing method may be useful for comparing outcomes for children with different language and cultural backgrounds across Africa.
Methods
The Dartmouth College Committee for the Protection of Human Subjects and the Research Ethics Committee of the Muhimbili University of Health and Allied Sciences (MUHAS) approved the research. A parent or guardian provided consent for the children to participate.
Participants
Participants were part of a longitudinal study in Dar es Salaam, Tanzania. 162 HIV+ and 154 HIV− children between the ages of 3 and 9 (mean 5.6 ± 1.6 years) were included for this cross-sectional study. In the HIV+ group, all children, except one who was infected via blood transfusion, were perinatally-infected and had their status-verified by research study staff. All children with HIV were receiving antiretroviral treatment. The HIV− cohort was recruited in the same local communities in the city of Dar es Salaam as participants with HIV; 10% of these children had at least one parent with HIV. Across HIV+ and HIV− groups, school attendance and measures of socioeconomic status varied (see Table 1).
Table 1.
Characteristics of the participants
| HIV + | HIV − | Total | χ2/z/t | p | ||||
|---|---|---|---|---|---|---|---|---|
| (n) | (%) | (n) | (%) | (n) | (%) | |||
| Gender | ||||||||
| Female | 82 | 50.62% | 90 | 58.44% | 172 | 54.43% | ||
| Male | 80 | 49.38% | 64 | 41.56% | 144 | 45.57% | χ2=1.95 | 0.163 |
| Age | ||||||||
| 3 | 8 | 4.94% | 25 | 16.23% | 33 | 10.44% | ||
| 4 | 33 | 20.37% | 26 | 16.88% | 59 | 18.67% | ||
| 5 | 25 | 15.43% | 34 | 22.08% | 59 | 18.67% | ||
| 6 | 29 | 17.90% | 32 | 20.78% | 61 | 19.30% | ||
| 7 | 37 | 22.84% | 24 | 15.58% | 61 | 19.30% | ||
| 8 | 29 | 17.90% | 13 | 8.44% | 42 | 13.29% | ||
| 9 | 1 | 0.62% | 0 | 0.00% | 1 | 0.32% | ||
| Mean (S.D.) | 5.75 (1.51) | 6.39 (1.57) | 6.08 (1.57) | z = 3.4 | <0.001 | |||
| Ever attended school | ||||||||
| Yes | 145 | 89.51% | 130 | 84.41% | 275 | 87.03% | ||
| No | 17 | 10.49% | 24 | 15.58% | 41 | 12.97% | χ2=1.81 | 0.18 |
| HIV exposure (Mother/father HIV +) | ||||||||
| Yes | 156 | 96.30% | 16 | 10.39% | 172 | 54.43% | ||
| No/ unknown | 6 | 3.70% | 138 | 89.61% | 144 | 45.57% | ||
| Socioeconomic Status | ||||||||
| Mean (S.D.) | −0.079 (0.93) | 0.11 (1.08) | 0.014 (1.01) | t = −1.68 | 0.094 | |||
Note. Chi-square (χ2), Mann-Whitney U Test (z), and independent samples ttest (t) were used based on the level of measurement of the dependent variable.
Procedures & Measures
The Leiter International Performance Scale – Third Edition (Leiter-3)
The Leiter-3 assesses cognitive functioning in individuals from 3 years to 75+ years of age. The Leiter-3 measures intellectual functioning in the absence of language, through tasks of fluid and categorical reasoning, mental sequencing, and visual identification. Designed partially to meet the needs of children with communication difficulties, the Leiter-3 is a completely nonverbal testing instrument. All directions and task instructions are delivered in pantomime. This includes a variety of gestures (hand movements), head movements, facial expressions, and task demonstration/modeling. All responses are given by pointing, placement of blocks or manipulatives, and crossing items out on paper and pencil tasks.
The Leiter-3 was administered by five different clinical officers in Tanzania. All test administrators were trained in-person by a pediatric neuropsychologist. Ongoing training occurred via video conference, and videos were reviewed by the pediatric neuropsychologist to provide feedback. Additionally, each examiner completed a fidelity rating following testing; the pediatric neuropsychologist reviewed these as well, and testing was consistently of high fidelity. Our study team in Tanzania included medical professionals (doctors, nurses), who worked in close collaboration with American team members. Limited cultural adaptations were needed due to the Leiter-3’s entirely nonverbal nature. However, we followed guidelines for adapting Western tests to non-Western settings (Hambleton, 2005) whenever appropriate. For example, the gesture for “do this task quickly” in the Leiter-3 manual involves rotating the index fingers on each hand quickly around each other. This gesture did not resonate with our Tanzania team, so the gesture was changed to use whole hands, rather than index fingers. Similarly, when indicating consecutive sizes, the Leiter-3 manual uses fingers, and this was replaced with whole-hand open-palm gestures. Neither individual stimuli nor items were judged or evaluated for cross-cultural issues within the local community.
All Leiter-3 testing was completed in designated testing rooms within a single infectious disease clinic. Children being seen for care at 12 different infectious disease clinics and treatment centers in Dar es Salaam were invited to participate. These children made up our HIV+ cohort. Children without HIV were recruited from the same communities within Dar es Salaam. No other activities occurred in that space during testing, and any windows to the hallways were covered with material to reduce visual distractions. All study participants were evaluated prior to the outbreak of the COVID-19 pandemic. Thus, no personal protective equipment confounded pantomime gestures, such as facial expressions.
The Leiter-3 yields three composite scores (standard scores; M = 100, SD = 15), which are derived from subtest scores (scaled scores; M = 10, SD = 3). The Nonverbal IQ composite measures nonverbal global intelligence by testing aspects of problem solving and logical reasoning, using visualization (e.g., visual closure, pattern recognition, visual-spatial relationships). This composite includes Figure Ground (FG), Form Completion (FC), Classification/Analogies (CA), & Sequential Order (SO). Visual Patterns (VP) is an optional subtest, which can contribute to the Nonverbal IQ when one of the core four subtests is spoiled. The Nonverbal Memory composite measures attention, working memory, and retrieval. This composite includes the Forward Memory (FM) and Reverse Memory (RM) subtests. The Processing Speed composite measures attention and speed of information processing. This composite includes the Attention Sustained (AS) and Nonverbal Stroop (NS). See Table 2 for a list of composites and subtests.
Table 2.
Leiter-3 Index and Subtest Descriptions.
| Leiter- 3 Index / subtest | Task | Ability |
|---|---|---|
| Nonverbal IQ | Global intelligence | |
| Figure Ground (FG) | Identification of figure or designs within a stimulus | Visual recognition and spatial reasoning |
| Form Completion (FC) | Recognize a “whole object” from a random array of fragments | Visual closure and perceptual-organization |
| Classification/Analogies (CA) | Categorization of objects or designs; matrix analogies | Pattern recognition, categorical reasoning, and mental shifting |
| Sequential Order (SO) | Selection of stimuli that fit into a logical progression/order | Sequential reasoning and pattern completion |
| Visual Patterns (VP) | Matching of visual stimuli; completing figural object patterns | Visual matching and deductive reasoning |
| Nonverbal Memory | Attention and working memory | |
| Forward Memory (FM) | Recall the sequence of pictures to which the examiner pointed | Visual registration and immediate attention |
| Reverse Memory (RM) | Recall the sequence of pictures to which the examiner pointed, but in reverse order | Visual attention, working memory, and sequencing |
| Processing Speed | Speed of response | |
| Attention Sustained (AS) | Cross out objects with paper-and-paper under timed constraints | Attention and processing speed |
| Nonverbal Stroop (NS) | Paper-and-pencil matching under timed constraints | Processing speed and mental inhibition |
Socioeconomic Status (SES)
Cognitive functioning can be related to a variety of external and environmental factors. Socio-economic status (SES) in particular requires serious consideration due to the connection between SES and brain development (Farah, 2017; Hackman & Farah, 2009). To assess this, a medical history and demographic questionnairewas completed for each study participant. This form covered several topics potentially related to SES: education, health problems, early life experiences, developmental milestones, information on household members, caregiver/parent status, caregiver education and employment, quality of the home environment, income, and material possessions. Data were stored in a Research Electronic Data Capture (REDCap) database. We used the information from the demographic form to create an SES composite variable.
Socioeconomic status was calculated using principal component analysis (PCA) of relevant variables from the REDCap demographics questionnaire. PCA was used to reduce the dimensionality of the dataset by linearly transforming it into a non-correlated set of principal components (Dunteman, 1989; Filmer & Pritchett, 2001; Vyas & Kumaranayake, 2006). The eigenvalues of the principal components were used to represent the amount of variance that each principal component captured, and thus, the majority of the total variance in the data could be represented in relatively few principal components (Dunteman, 1989). Although PCA is not intended for use with binary variables, it was found to perform as well as other methods of dimension reduction in the creation of a socioeconomic index (Howe et al., 2008).
The PCA was based on questions that addressed three main categories of socioeconomic status: household assets and access to resources, measures of income and occupation, and measures of parental education (Table 3). Binary variables (“Refrigerator”, “Is the pooled household income sufficient for the child’s needs?”) and categorical variables (“Source of power for cooking/ boiling water”, “Source of Water”) were coded to 1 if yes and 0 if no, with the null category excluded in the case of categorical variables to avoid linear dependence. Ordinal variables (“Total household income per month”) were coded on a scale from 0 to 3, corresponding to four categories: “Less than 100,000 Tanzanian shillings (TSh)”, “100,000-300,000 TSh”, “300,000 TSh-500,000 TSh”, and “More than 500,000 TSh”. (Currently, 500,000 TSh corresponds to approximately 215 US dollars or 178 Euros.) Maternal and paternal years of education were continuous. Before conducting principal components analysis, all variables were centered to mean of zero and scaled to standard deviation of one (mean = 0, variance = 1).Missing values were replaced with mean values. The first principal component, explaining 32.7% of the variance in the data, was used to calculate a single value estimating socioeconomic status (SES) for each participant. The calculated SES score was centered to mean 0 and scaled to variance 1 before being used in analyses.
Table 3.
Summary statistics and item weights from the first principal coefficient.
| Question | Mean | SD | Missing | Item Weight |
|---|---|---|---|---|
| Refrigerator | 0.34 | 0.47 | 2 (0.58%) | 0.38 |
| Is the pooled household income sufficient for the child's family needs? | 0.37 | 0.48 | 4 (1.16%) | 0.28 |
| Total household income per month (TSh) | 1.18 | 0.72 | 3 (0.87%) | 0.27 |
| Source of power for cooking/ boiling water | ||||
| Electricity/ Gas | 0.39 | 0.49 | 0 | 0.46 |
| Fuel stove/ Firewood/ Charcoal | 0.53 | 0.50 | 0 | −0.43 |
| Source of Water | ||||
| Tap water inside house | 0.24 | 0.43 | 0 | 0.30 |
| Tap water on plot, single use | 0.28 | 0.45 | 0 | −0.02 |
| Communal Tap, multiple households | 0.32 | 0.47 | 0 | −0.19 |
| Mother/female guardian years of education | 7.85 | 3.06 | 13 (3.78%) | 0.33 |
| Father/male guardian years of education | 9.03 | 3.58 | 44 (12.79%) | 0.28 |
Statistical Analyses
Descriptive statistics were used to summarize demographic and SES variables for children in both HIV+ and HIV− cohorts (Table 1). As these groups differed significantly on age (where HIV+ was younger), a one-way ANCOVA was run to compare the effect of HIV on Leiter-3 test scores after controlling for age as a covariate. Additionally, to account for the effects of HIV-exposed children within the HIV− control group, we ran independent sample t-tests on Leiter-3 outcome variables (Table 4). Multiple linear regression analyses were performed to develop a model that accounted for variables expected to predict Leiter-3 test outcomes, including age, sex, SES, school attendance, and HIV status.
Table 4.
Results of independent samples t-test within HIV negative group.
| Test | HIV exposure (n = 16) |
No known HIV exposure (n = 138)* |
t | Sig. | Cohen’s d |
|---|---|---|---|---|---|
| Nonverbal IQ | 80.44 (12.50) | 78.23 (15.79) | −0.54 | 0.591 | 0.14 |
| Nonverbal Memory | 69.38 (13.22) | 75.38 (14.55) | 1.58 | 0.117 | 0.42 |
| Processing Speed † | 69.56 (16.18) | 71.73 (15.27) | 0.51 | 0.616 | 0.14 |
| FG Scaled † | 8.13 (1.89) | 7.49 (3.00) | −1.18 | 0.251 | 0.22 |
| FC Scaled | 8.81 (2.59) | 8.33 (3.03) | −0.62 | 0.538 | 0.16 |
| CA Scaled | 8.50 (3.76) | 8.09 (3.05) | −0.50 | 0.618 | 0.13 |
| SO Scaled | 5.00 (1.46) | 5.47 (2.22) | 0.83 | 0.407 | 0.22 |
| VP Scaled † | 5.44 (4.10) | 5.27 (4.38) | −0.15 | 0.880 | 0.04 |
Note. Data are presented as mean (standard deviation). The significance values are for two-tailed hypothesis tests.
n = 137 for SO and VP
Assumption of equal variances violated; t-test conducted using Satterthwaite’s Approximation.
All assumptions were met for each regression model: linearity was assessed by partial regression plots and plots of studentized residuals against predicted values; independence of residuals was assessed by Durbin-Watson statistic (near two for all regression models); homoscedasticity was assessed by plots of studentized residuals versus unstandardized predicted values; lack of multicollinearity was assessed by tolerance values (greater than 0.1 for all regression models). There were no leverage values greater than 0.2 or Cook's distance values above 1. In the case where potential outliers were identified (standardized residual more than ±3), these data points did not have high leverage values or Cook’s distance values and so the data points were left in. The assumption of normality was met, as assessed by a normal probability plot of residuals. MATLAB® R2020b was used for analysis and creation of plots and figures.
Results
Participant Characteristics and Demographics
Participants across HIV+ and HIV− groups did not significantly differ on gender, school attendance, or SES. They did significantly differ on age (see Table 1). Within the HIV− control group, independent samples t-tests revealed no significant differences between HIV-exposed and non-exposed children on Leiter-3 variables.
Leiter-3 Performance Across Groups: ANCOVA
After adjusting for age, HIV+ children performed significantly worse than HIV− controls on the Nonverbal IQ (p < .001, d = 0.27) and Processing Speed (p < .001, d = 0.52) composites, but not the Nonverbal Memory composite (p = .662, d = 0.23). On the core subtests, Figure Ground (d = 0.16), Form Completion (d = 0.29), Classifications and Analogies (d = 0.17), and Visual Patterns (d = 0.40) yielded significant differences between HIV+ and HIV− children (Table 5).
Table 5.
Results of ANCOVA with age as a covariate.
| Test | HIV− (n = 154)* |
HIV+ (n = 162)** |
F | Sig. | Cohen’s d |
|---|---|---|---|---|---|
| Nonverbal IQ | 77.9 (16.5) | 73.8 (14.8) | 12.908 | <0.001 | 0.27 |
| Nonverbal Memory | 77.2 (14.4) | 74 (13.4) | 0.191 | 0.662 | 0.23 |
| Processing Speed | 71.8 (14.9) | 65.1 (11.3) | 24.972 | <0.001 | 0.52 |
| FG Scaled | 7.5 (3) | 7 (2.6) | 7.200 | 0.008 | 0.16 |
| FC Scaled | 8.2 (3.2) | 7.3 (3) | 18.323 | <0.001 | 0.29 |
| CA Scaled | 8.1 (3.3) | 7.5 (3.2) | 5.918 | 0.016 | 0.17 |
| SO Scaled | 5.5 (2.4) | 5.1 (1.9) | 2.309 | 0.130 | 0.19 |
| VP Scaled | 5.7 (4.6) | 4.2 (3.0) | 9.476 | 0.002 | 0.40 |
Note. Data are presented as mean (standard deviation). The significance values are for the F statistic. Cohen’s dis calculated as () and represents the effect size (Cohen 1988).
n = 153 for SO and VP
n = 161 for VP
Leiter-3 Performance: Multiple Regressions
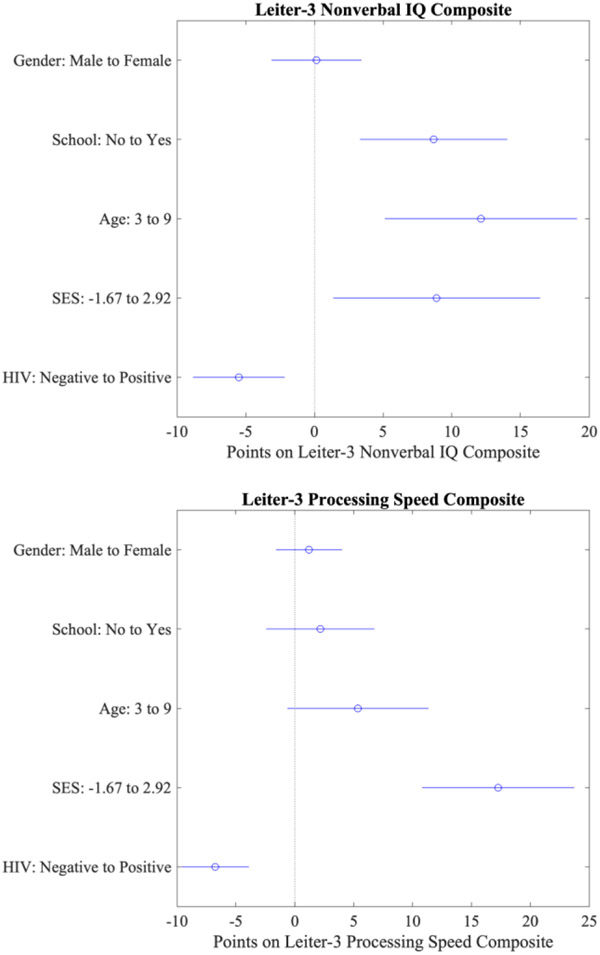
The first multiple regression model examined the effects of age, gender, socioeconomic status, and school attendance on the cognitive test scores. Model 1 was statistically significant for the following test scores: Nonverbal IQ, Nonverbal Memory, Processing Speed, Figure Ground, Form Completion, Classification/Analogies, Sequential Order, and Visual Patterns. In general, age and SES were significant predictors of cognitive outcomes. Gender was not a strong predictor.
For the second multiple regression model, HIV status was added as an independent variable. Model 2 was statistically significant for the following test scores: Nonverbal IQ, Nonverbal Memory, Processing Speed, Figure Ground, Form Completion, Classification/Analogies, Sequential Order, and Visual Patterns, HIV was a significant predictor of cognitive outcomes for Nonverbal IQ, Processing Speed, Figure Ground, Form Completion, Classification Analogies, and Visual Patterns (Table 6).
Table 6.
Hierarchical Multiple Regression Models.
| Independent Variables | ||||||||
|---|---|---|---|---|---|---|---|---|
| Gender | School | Age | SES | HIV | R 2 | Δ R2 | Δ F | |
| Nonverbal IQ | ||||||||
| Model 1 | 0.099*** | |||||||
| β | −0.017 | 0.186** | 0.171** | 0.139* | ||||
| Model 2 | 0.126*** | 0.027** | 10.567** | |||||
| β | −0.005 | 0.187** | 0.202** | 0.125* | −0.176** | |||
| Nonverbal Memory | ||||||||
| Model 1 | 0.208*** | |||||||
| β | 0.081 | 0.009 | −0.444*** | 0.106* | ||||
| Model 2 | 0.206*** | 0.002 | 0.203 | |||||
| β | 0.083 | 0.009 | −0.44*** | 0.104* | −0.023 | |||
| Processing Speed | ||||||||
| Model 1 | 0.091*** | |||||||
| β | −0.061 | 0.052 | 0.058 | 0.299*** | ||||
| Model 2 | 0.148*** | 0.057*** | 21.715*** | |||||
| β | −0.044 | 0.054 | 0.103 | 0.279*** | −0.249*** | |||
| Figure Ground | ||||||||
| Model 1 | 0.141*** | |||||||
| β | −0.058 | 0.211*** | 0.201** | 0.166** | ||||
| Model 2 | 0.152*** | 0.012* | 5.309* | |||||
| β | −0.05 | 0.211*** | 0.223*** | 0.156** | −0.123* | |||
| Form Completion | ||||||||
| Model 1 | 0.128*** | |||||||
| β | 0.064 | 0.144* | 0.261*** | 0.124* | ||||
| Model 2 | 0.169*** | 0.041*** | 16.182*** | |||||
| β | 0.078 | 0.146* | 0.3*** | 0.107* | −0.212*** | |||
| Classification/ Analogies | ||||||||
| Model 1 | 0.05*** | |||||||
| β | −0.011 | 0.059 | 0.189** | 0.125* | ||||
| Model 2 | 0.06*** | 0.01* | 4.155* | |||||
| β | −0.003 | 0.06 | 0.21** | 0.116* | −0.114* | |||
| Sequential Order | ||||||||
| Model 1 | 0.021* | |||||||
| β | −0.051 | 0.152* | −0.146* | 0.05 | ||||
| Model 2 | 0.024* | 0.003 | 2.051 | |||||
| β | −0.046 | 0.153* | −0.131* | 0.043 | −0.082 | |||
| Visual Patterns | ||||||||
| Model 1 | 0.078*** | |||||||
| β | 0.023 | 0.219*** | −0.238*** | 0.13* | ||||
| Model 2 | 0.102*** | 0.024** | 9.334** | |||||
| β | 0.035 | 0.221*** | −0.207** | 0.116* | −0.168** | |||
Note. β = standardized slope coefficient; R2 = coefficient of determination (adjusted).
p<0.05
p<0.01
p<0.001
Hierarchical multiple regression was performed to assess if HIV status improved the proportion of variance explained by the model above and beyond the variance explained by age, gender, school attendance, and socioeconomic status. Adding HIV to the regression model improved the prediction of Nonverbal IQ (ΔR2 = 0.029) and Processing Speed (ΔR2 = 0.005), but not Nonverbal Memory (ΔR2 = 0.001). The addition of HIV also improved the fit of the model for the Figure Ground (ΔR2 = 0.014), Form Completion (ΔR2 = 0.043), Classification/Analogies (ΔR2 = 0.012), and Visual Patterns (ΔR2 = 0.027) subtests. In general, accounting for HIV status significantly improved the ability to predict cognitive test performance (Table 6).
Discussion
This study examined cognitive performance of children living with and without HIV in Dar es Salaam, Tanzania using the Leiter-3, an entirely nonverbal, non-vocal, and language-free assessment. We found that children living with HIV performed worse than HIV− controls on the Nonverbal IQ and Processing Speed Leiter-3 composites when controlling for age. These differences were small to moderate in magnitude and remained significant when controlling for participants’ age, schooling, or SES. This replicates previous reports. Differences between HIV+ and HIV− children from Sub-Saharan Africa on cognitive and neuropsychological tests have been frequently reported in the literature (Boivin et al., 2019; Boivin & Giordani, 2009). Previous studies, however, used Western tests translated from English to the language spoken in the particular Sub-Saharan study sites. The current study found consistent differences between HIV+ and HIV− children using entirely nonverbal tests that did not need to be translated from the original language. Thus, the assessment of cognition remained faithful to the original test design.
As expected, age, socioeconomic status, and school attendance predicted Leiter-3 outcomes. This is consistent with the study by Ajayi et al. (Ajayi et al., 2017), which showed that factors such as SES, school attendance, and age were all significant predictors of cognitive outcomes in children in rural South Africa. In that study, however, HIV itself was not a significant predictor. In the current study, when controlling for sociodemographic predictor variables, HIV status was predictive of Nonverbal IQ and Processing Speed, as well as the Figure Ground, Form Completion, Classification/Analogies, and Visual Patterns subtests. Thus, in our cross-sectional study, HIV did prove to be a significant predictor of overall IQ, information processing speed, and many other key elements to intelligence (e.g., reasoning, problem-solving, pattern completion, visual-spatial relationships), even when the important SES and demographic variables were taken into account. These associations are important, but were mostly small (i.e., betas = 0.11 to 0.18) with the exception of processing speed (beta = 0.25). These results build upon our previous work (Niemczak et al., 2021; White-Schwoch et al., 2020), further establishing the difference in brain-based functional test results between individuals living with HIV and individuals without HIV.
On overall IQ, performance fell at the 4th and 7th percentile for the HIV+ and HIV− groups, respectively. Performance levels were similar compared to other published studies on HIV+ and HIV− cohorts in other Sub-Saharan African countries that used Western tests to assess cognitive functioning. HIV+ and control groups both performed well below age-based expectations on Western tests of global IQ and processing, using Western norms (Boivin et al., 2018; Boivin et al., 2019; Chernoff et al., 2018). This is likely because although the Leiter-3 is nonverbal, the assessment is not “culture-free.” Using Western norms on the Leiter-3 might not be the most appropriate measurement of performance when used in Africaas it may underestimate actual ability. Standardized scores should be interpreted cautiously from a clinical perspective. Nevertheless, since the Leiter-3 does not require unique translations to be used in non-Western settings it may offer a consistent measure of cognitive ability across a variety of settings.
There are some limitations to the current study. As a cross-sectional design was used, evidence cannot be offered about the temporal relationship between HIV and our cognitive outcome variables. Additionally, all data were collected at one site, making it unclear if our findings would generalize to different research sites or countries. While we accounted for a variety of neurodevelopmental risk factors, several potentially important elements were not included (e.g., psychosocial factors in the home environment, other nutritional and growth concerns). Similarly, while there was no significant difference between our SES variable between groups, standard deviations were very large and could have affected the lack of difference between groups. This study also did not differentiate how children living with HIV were infected, which may yield different risk profiles or developmental trajectories. Importantly, we used a Western test not validated for use in African cultures, and we did not perform a systematic judgment of each test item for cross-cultural issues within the community. Further examination of the Leiter-3 and its performance across other Sub-Saharan countries is needed. Admittedly, nonverbal assessment is challenging and requires training.But although the neuropsychologist was not onsite for all testing, the team received training from a neuropsychologist and had ongoing oversight via video review and regular team meetings. Finally, the HIV+ and HIV− groups differed in age.
There are also important strengths to this study. While our findings are not meant to be definitive and endorse one method of assessment over another, the results of this study do suggest that nonverbal assessment might offer an alternative to translated Western tests when conducting cognitive assessment of children in non-Western countries. We introduced an entirely nonverbal method for measuring cognition and found that performance was very similar to previous studies using translated tests on domains of global intelligence, aspects of attention and memory, and speed of processing. While performances found here may not predict future abilities, the fact that children living with HIV performed significantly worse than HIV− children does suggest that the Leiter-3 may be picking up on risks for cognitive and neuropsychological deficits in young children. Importantly, the Leiter-3 is the most recent version of this line of nonverbal instruments. When using Western tests in Sub-Saharan Africa, researchers interested in studying cognition and neuropsychological functioning are encouraged to use the most current version of these measures. Furthermore, this study offered an initial look at the feasibility of conducting nonverbal assessments in resource-limited settings. The use of technology (e.g., video conference, video recording) allowed for ongoing oversight of examiner reliability. Such a model of training and ongoing evaluation could certainly be carried over to other sites and studies, where collaboration is useful between Western and non-Western colleagues.
Figure 1.
Main Effect Plots of Multiple and Hierarchical Regression Models
Note: Points on Leiter-3 composites are in standard scores. SES: lower score = lower SES. Main effect x-axis represents the change in adjusted response due to changing a single predictor variable (y-axis). Horizontal lines represent the 95% confidence interval in test score change.
Acknowledgements
We would like to thank our Dar es Salaam-based Study Team Members: Joyce Machunda, FilmonAloyce, Pascal Maibe, Godfrey Njau, Matilda Kabeho, Joyce Joseph, and Claudia Gasana.
We also thank Dr. MeckyMatee for his help with sustaining the research environment in Dar es Salaam. Finally, we thank Phoebe Cunningham who conducted the Principal Component Analysis on the demographics survey to create the SES composite score.
Funding sources:
This study was funded by the National Institutes of Health (NIH), grant number 5R01HD095277 to principal investigator JCB.
References
- Ajayi OR, Matthews G, Taylor M, Kvalsvig J, Davidson LL, Kauchali S, & Mellins CA (2017). Factors associated with the health and cognition of 6-year-old to 8-year-old children in KwaZulu-Natal, South Africa. Tropical Medicine & International Health, 22(5), 631–637. doi: 10.1111/tmi.12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin MJ, Barlow-Mosha L, Chernoff MC, Laughton B, Zimmer B, Joyce C, Bwakura-Dangarembizi M, Ratswana M, Abrahams N, Fairlie L, Gous H, Kamthunzi P, McCarthy K, Familiar-Lopez I, Jean-Phillippe P, Coetzee J, Violari A, Cotton MF, Palumbo PE, & Team IPS (2018). Neuropsychological performance in African children with HIV enrolled in a multisite antiretroviral clinical trial. AIDS, 32(2), 189–204. doi: 10.1097/QAD.0000000000001683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin MJ, Chernoff M, Fairlie L, Laughton B, Zimmer B, Joyce C, Barlow-Mosha L, Bwakura-Dangarembizi M, Vhembo T, Ratswana M, Kamthunzi P, McCarthy K, Familiar-Lopez I, Jean-Philippe P, Coetzee J, Abrahams N, Gous H, Violari A, Cotton MF, & Palumbo PE (2019). African Multi-Site 2-Year Neuropsychological Study of School-Age Children Perinatally Infected, Exposed, and Unexposed to Human Immunodeficiency Virus. Clinical Infectious Diseases, doi: 10.1093/cid/ciz1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin MJ, & Giordani B (2009). Neuropsychological assessment of African children: evidence for a universal brain/behavior omnibus within a coconstructivist paradigm. Progress in Brain Research, 178, 113–135. doi: 10.1016/S0079-6123(09)17808-1 [DOI] [PubMed] [Google Scholar]
- Chernoff MC, Laughton B, Ratswana M, Familiar I, Fairlie L, Vhembo T, Kamthunzi P, Kabugho E, Joyce C, Zimmer B, Ariansen JL, Jean-Philippe P, & Boivin MJ (2018). Validity of Neuropsychological Testing in Young African Children Affected by HIV. The Journal of Pediatric Infectious Diseases, 13(3), 185–201. doi: 10.1055/s-0038-1637020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnett CW (1980). Pairwise Multiple Comparisons in the Homogeneous Variance, Unequal Sample-Size Case. Journal of the American Statistical Association, 75(372), 789–795. doi:Doi 10.2307/2287160 [DOI] [Google Scholar]
- Dunteman GH (1989). Principal Components Analysis. Newbury Park, California: SAGE Publications, Inc. [Google Scholar]
- Farah MJ (2017). The Neuroscience of Socioeconomic Status: Correlates, Causes, and Consequences. Neuron, 96(1), 56–71. doi: 10.1016/j.neuron.2017.08.034 [DOI] [PubMed] [Google Scholar]
- Filmer D, & Pritchett LH (2001). Estimating wealth effects without expenditure data--or tears: an application to educational enrollments in states of India. Demography, 38(1), 115–132. doi: 10.1353/dem.2001.0003 [DOI] [PubMed] [Google Scholar]
- Flanagan DP, Ortiz SO, & Alfonso SO . (2013). Essentials of cross-battery assessment (3rd ed.). Hoboken, NJ: Wiley. [Google Scholar]
- Gruver RS, Mall S, Kvalsvig JD, Knox JR, Mellins CA, Desmond C, Kauchali S, Arpadi SM, Taylor M, & Davidson LL (2020). Cognitive and Language Development at Age 4-6 Years in Children HIV-Exposed But Uninfected Compared to Those HIV-Unexposed and to Children Living With HIV. New Directions for Child and Adolescent Development, 171, 39–54. doi: 10.1002/cad.20351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, & Farah MJ (2009). Socioeconomic status and the developing brain. Trends in Cognitive Sciences, 13(2), 65–73. doi: 10.1016/j.tics.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambleton RK (2005). Issues, designs and technical guidelines for adapting tests into multiple languages and cultures. . In Hambleton RK, Merenda PF, & Spielberger CD (Ed.), Adapting psychological and educational tests for cross-cultural assessment (pp. 3–38). Mahwah, NJ: Lawrence Erlbaum. [Google Scholar]
- Howe LD, Hargreaves JR, & Huttly SR (2008). Issues in the construction of wealth indices for the measurement of socio-economic position in low-income countries. Emerging Themes in Epidemiology, 5, 3. doi: 10.1186/1742-7622-5-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum RS (2017). Handbook of Nonverbal Assessment (2nd ed., pp. 1 online resource (XII, 320 pages 315 illustrations, 328 illustrations in color). doi: 10.1007/978-3-319-50604-3 [DOI] [Google Scholar]
- Niemczak C, Fellows A, Lichtenstein J, White-Schwoch T, Magohe A, Gui J, Wilbur J, Clavier O, Massawe E, Moshi N, Boivin M, Kraus N, & Buckey J (2021). Central Auditory Tests to Track Cognitive Function in People With HIV: Longitudinal Cohort Study. JMIR Formative Research, 5(2), e26406. doi: 10.2196/26406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakland T (2009). How universal are test development and use? In Grigorenko EL (Ed.), Multicultural psychoeducational assessment (1st ed., pp. 1–40). New York, NY: Springer. [Google Scholar]
- Ruel TD, Boivin MJ, Boal HE, Bangirana P, Charlebois E, Havlir DV, Rosenthal PJ, Dorsey G, Achan J, Akello C, Kamya MR, & Wong JK (2012). Neurocognitive and motor deficits in HIV-infected Ugandan children with high CD4 cell counts. Clinical Infectious Diseases, 54(7), 1001–1009. doi: 10.1093/cid/cir1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A, Volsky DJ, & McArthur JC (2016). HIV-associated neurocognitive disorder - pathogenesis and prospects for treatment. Nature Reviews Neurology, 12(5), 309. doi: 10.1038/nrneurol.2016.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr L, Mueller J, & Varrall R (2009). A systematic review of cognitive development and child human immunodeficiency virus infection. Psychololgy, Health, & Medicine, 14(4), 387–404. doi: 10.1080/13548500903012897 [DOI] [PubMed] [Google Scholar]
- Vyas S, & Kumaranayake L (2006). Constructing socio-economic status indices: how to use principal components analysis. Health Policy and Planning, 21(6), 459–468. doi: 10.1093/heapol/czl029 [DOI] [PubMed] [Google Scholar]
- Wedderburn CJ, Evans C, Yeung S, Gibb DM, Donald KA, & Prendergast AJ (2019). Growth and Neurodevelopment of HIV-Exposed Uninfected Children: a Conceptual Framework. Current HIV/AIDS Reports, 16(6), 501–513. doi: 10.1007/s11904-019-00459-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White-Schwoch T, Magohe AK, Fellows AM, Rieke CC, Vilarello B, Nicol T, Massawe ER, Moshi N, Kraus N, & Buckey JC (2020). Auditory neurophysiology reveals central nervous system dysfunction in HIV-infected individuals. Clinical Neurophysiology, 131(8), 1827–1832. doi: 10.1016/j.clinph.2020.04.165 [DOI] [PMC free article] [PubMed] [Google Scholar]