Abstract
Introduction:
Symptoms ofirritable bowel syndrome (IBS) are common reasons for endoscopic procedures. We examined the yield of colonoscopy and upper endoscopy in IBS for several organic diseases.
Methods:
Matched population-based prevalence study in Sweden. We identified 21,944 participants diagnosed with IBS from 1987–2016 undergoing colonoscopy with a biopsy from all of Sweden’s 28 pathology departments within 6 months of diagnosis. We compared prevalence of histopathology-proven diagnoses of inflammatory bowel disease (IBD), colorectal cancer, precancerous polyps, and microscopic colitis between patients recently diagnosed with IBS and matched controls without IBS (n=81,101) undergoing colonoscopy. We also compared prevalence of celiac disease between patients diagnosed with IBS (n=9,965) and matched controls (n=45,584) undergoing upper endoscopy with biopsy. IBS patients were also compared to their siblings. Conditioned logistic regression estimated adjusted odds ratios (aORs).
Results:
Biopsy-proven IBD was seen in 1.6% of IBS and in 5.9% of controls (aOR=0.21; 95%CI=0.19–0.24). The prevalence of precancerous polyps was 4.1% vs. 13.0% (aOR=0.28; 95%CI=0.26–0.30), colorectal cancer 0.8% vs. 6.3% (aOR=0.17; 95%CI=0.14–0.20) and celiac disease 1.9% vs. 3.4% (aOR=0.54; 95%CI=0.47–0.63). Conversely, the prevalence of microscopic colitis was 2.9% vs. 1.7% (aOR=1.77; 95%CI=1.61–1.95), with higher prevalence in older patients and patients with IBS with diarrhea. Yield of colonoscopy for precancerous polyps, colorectal cancer, and microscopic colitis increased by age. Our findings were consistent using unaffected siblings as the comparator group.
Discussion:
The diagnostic yield of upper endoscopy and colonoscopy for organic disease is low in patients with a first-time diagnosis of IBS, though increases with age.
Keywords: colonoscopy, functional GI disease, healthcare utilization, histopathology, esophagogastroduodenoscopy
INTRODUCTION
Irritable bowel syndrome (IBS) affects 11.2% of the global population,[1] with significant impacts on patients’ quality of life despite no increased risk of mortality.[2] Among this sizeable population, there is a disproportionate number of patients <50 years[1] who would not traditionally meet criteria for endoscopy, still IBS patients accounted for up to 36% of gastroenterology consultations in one catchment area,[3] with most US patients undergoing at least one outpatient procedure the year of diagnosis,[4] with 28.7% of colonoscopies in those <50 performed in patients with IBS.[5] Procedure rates are similarly high in less procedure-incentivized payment systems. Between 63–84% of IBS patients in Europe will undergo a diagnostic procedure, with more than 1/3 undergoing colonoscopy.[6]
High endoscopic utilization persists despite some evidence that colonoscopy is unlikely to reveal structural disease. In a prospective trial of 466 non-constipated, suspected IBS patients undergoing colonoscopy, the rate of structural disease was no greater than controls.[7] Contrasting data, however, suggest an increased risk of structural disease on colonoscopy in IBS patients without alarm symptoms,[8–10] microscopic colitis being the most likely ‘missed’ diagnosis in some cohorts.[11–13]
Similarly disparate findings are found when examining the use of upper endoscopy to exclude celiac disease in patients with IBS symptoms, though a more recent meta-analysis showed an increased risk of biopsy-proven celiac disease[14]—though only one small, population-based study was included.[15] Limitations to the existing literature include retrospective or non-population-based design,[9, 12] limited numbers of IBS patients without alarm symptoms,[7, 8, 12, 15] and lack of inclusion of patients with IBS without diarrhea. Thus, the role of endoscopic evaluation of typical IBS remains uncertain.
We used a nationwide histopathology register to examine the overall risk of structural gastrointestinal disease (inflammatory bowel disease, microscopic colitis, precancerous colon polyps, colorectal cancer, celiac disease) in a large cohort of individuals with IBS undergoing upper endoscopy and colonoscopy with biopsy compared to matched comparators undergoing the same investigations for other reasons.
MATERIALS and METHODS
We compared the first gastrointestinal biopsy after IBS diagnosis to first gastrointestinal biopsy in individuals without a prior IBS diagnosis, and calculated the prevalence of inflammatory bowel disease, pre-cancerous polyps, colorectal cancer (CRC), microscopic colitis, and celiac disease according to IBS status.
Source database with outcome data
IBS patients were selected from the ESPRESSO study, a histopathology-based cohort consisting of gastrointestinal pathology reports collected across Sweden’s 28 pathology departments from 1965–2017.[16]
For this study, biopsy results from individuals with IBS and matched controls were categorized according to the Systematized Nomenclature of Medicine (SNOMED) pathology codes originating from their endoscopic procedure to define our outcomes: inflammatory bowel disease, colorectal cancer, microscopic colitis, and celiac disease (Table S1). The SNOMED codes for inflammatory bowel disease (in conjunction with ICD codes), microscopic colitis and celiac disease have all been validated and found to have positive predictive values ≥95%.[17–19] Precancerous polyps were categorized into non-advanced (tubular adenomas and sessile serrated polyps) or advanced (tubulovillous adenomas, villous adenomas, or any adenoma with cellular dysplasia) on the basis of histology only without reference to size (not available from ESPRESSO database). Our prior validation study indicated a high accuracy for identification of serrated polyps, with a positive predictive value of 95%.[20]
Definition of IBS
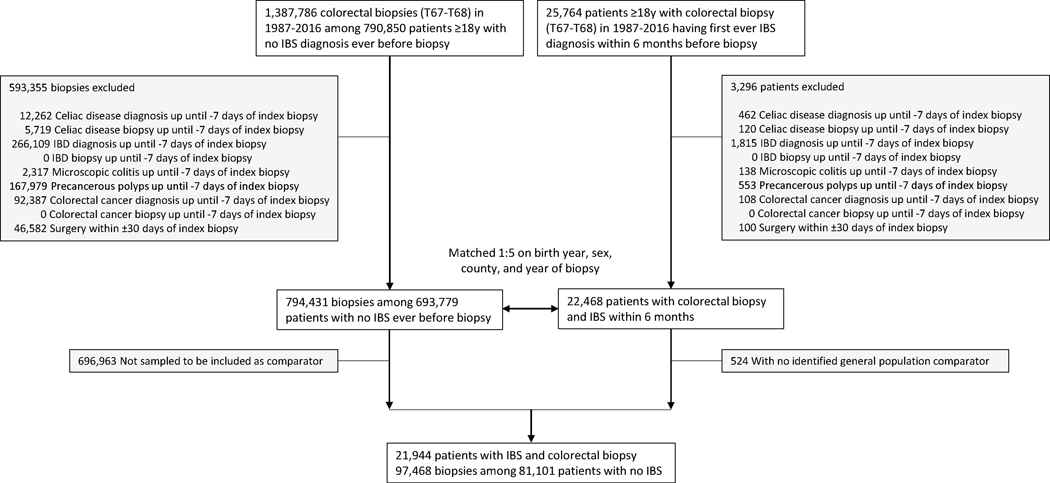
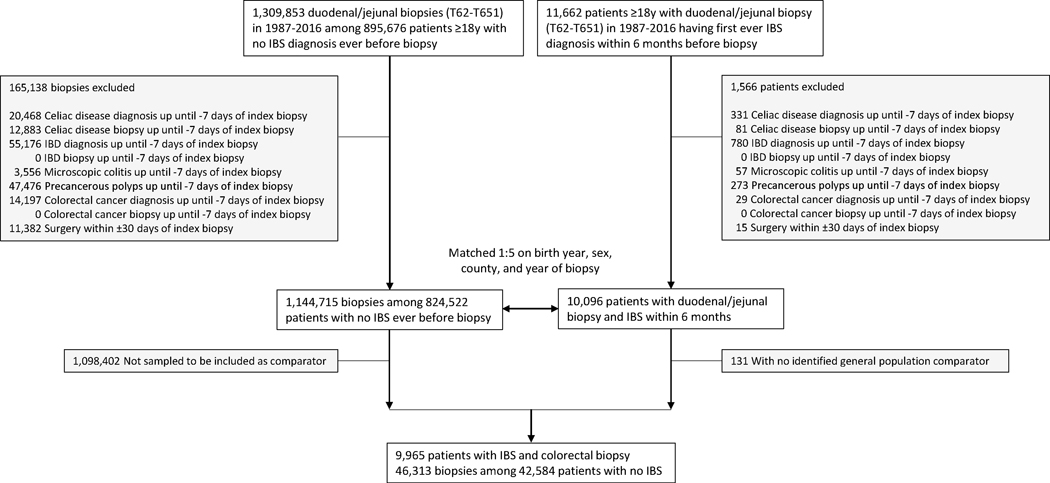
We first identified all individuals undergoing a colonoscopy with biopsy (Table S1) in the ESPRESSO cohort and excluded those whose histopathology samples came from a surgical resection (Table S2). We used ICD codes to identify individuals within this cohort with a diagnosis of IBS (ICD-9 564B; ICD-10 K58) in inpatient or non-primary outpatient care within 6 months before their upper endoscopy or colonoscopy with biopsy from 1987–2016 (1987 corresponding to the first year that the ICD-9 codes were introduced in Sweden). Both IBS patients and their matched controls (see below) were excluded from all analyses if at baseline they had an earlier diagnosis of celiac disease, microscopic colitis, precancerous polyps, or colorectal cancer (Table S1) up until −7 days from the index biopsy since these disorders may lead to false-positive IBS diagnoses (Figures 1 and 2). The 7-day window was selected because biopsy specimens may have been assigned a date a few days after the actual biopsy despite the diagnosis being coded a few days prior. For inflammatory bowel disease specifically, we required a concomitant ICD diagnosis of inflammatory bowel disease from −7 days to +365 days from the biopsy to ensure a true diagnosis. Using alternative inclusion criteria, we examined risk of a colorectal cancer diagnosis among patients in the ESPRESSO cohort regardless of previous history of precancerous polyps.
Figure 1.
Flow chart of identified IBS patients undergoing colonoscopy and their matched controls
IBS, irritable bowel syndrome; IBD, inflammatory bowel disease
Figure 2.
Flow chart of identified IBS patients undergoing upper endoscopy and their matched controls
IBS, irritable bowel syndrome; IBD, inflammatory bowel disease
Patients with IBS were divided into those with diarrhea predominance (IBS-D) and those without diarrhea predominance (IBS non-D)(see ICD codes in Table S1).
Registers and covariates
Demographic data (dates of birth and death, immigration/emigration, sex, age, county of residence, and education) from all study participants were retrieved from the Total Population Register[21] and the longitudinal integrated database for health insurance and labor market studies (LISA) using an individual’s PIN.[22] Data were linked to the Swedish Patient Register[23] to obtain data on inpatient and outpatient medical encounters. The diagnostic validity of the national patient register is generally 85–95%[23] with high validity seen for IBS.[24]
Controls
Index individuals with IBS were matched on age, sex, and county with up to five controls from the ESPRESSO cohort who also underwent upper endoscopy or colonoscopy with biopsy in the same calendar year.
Siblings
IBS patients were also compared to their siblings without IBS. Siblings were identified from the ESPRESSO cohort and underwent upper endoscopy or colonoscopy within 5 years of each other. Sibling comparisons allowed us to examine the influence of intrafamilial confounding.
Covariates
Within the study population we determined medical comorbidities related to overall health status in the last five years prior to study entry using ICD-8/9/10 codes prospectively recorded in routine clinical practice to determine presence of diabetes, cardiovascular disease, neurologic disease, and cancer (Table S3).
Statistical Analysis
We calculated crude prevalence for each histopathology-defined outcome and used conditional logistic regression to estimate multivariable-adjusted odds ratios (OR) and 95% confidence intervals (CI).
Because colonoscopy is more commonly used as a diagnostic for those with IBS-D, we also performed an analysis according to disease subtype (IBS-D and IBS non-D). Additionally, we performed stratified analyses according to age, quantifying the risk a traditional age-appropriate screening population (≥50 years old) vs those <50 years old. We also used a logistic regression model to estimate the prevalence of organic disease by age on a continuous scale, where age was included as a natural cubic spline function (with knots at percentiles 5%, 27.5%, 50%, 72.5%, and 95%). To account for possible temporal bias over time, we performed a subanalysis restricted to outcomes diagnosed in 2002 (the Swedish outpatient register was introduced in 2001) or later. To evaluate for increasing of a shift from right-sided to left-sided colorectal cancers,[25] we also evaluated the location of the CRC cases diagnosed in the cohort using location codes when available.
All analyses were conditioned on matching factors (sex, age, county, year of biopsy), except in siblings where we conditioned on matching set within family. We further adjusted for baseline medical comorbidities (cancer other than colorectal cancer, diabetes, cardiovascular disease including myocardial infarction, and neurologic disease) as well as for education. A sensitivity analysis additionally adjusted for presence of family history (in a parent or sibling) of inflammatory bowel disease, colorectal cancer, or celiac disease. Statistics were carried out using SAS statistical software v9.4. A p-value <0.05 was considered statistically significant. The study was approved by the Stockholm Ethics Review Board (Protocol 2014/1287–31/4). Informed consent was waived by the board since the study was strictly register-based.[26]
RESULTS
Yield of colonoscopy in IBS
We identified 25,764 patients diagnosed with IBS 1987–2016 followed by a colonoscopy within 6 months (Figure 1). After exclusions, there were 21,944 patients with IBS who were matched to 81,101 patients (with 97,468 biopsies; each IBS biopsy was linked to a non-IBS control biopsy) without IBS undergoing colonoscopy.
The average time interval between the IBS diagnosis and colonoscopy was 0.7 (SD 1.3) months. Table 1 shows the characteristics of study participants according to IBS status. Both IBS patients and matched controls were predominantly female with a mean age in the mid-40s. Compared to matched controls, IBS patients were less likely to have a preexisting diagnosis of cancer (excluding colorectal cancer) prior to colonoscopy. IBS was the primary diagnosis in the pre-colonoscopy clinical encounter almost 90% of the time, with IBS-D as the predominant subtype among the IBS cohort (62.4%). We also compared 1,240 IBS patients with their siblings (n=1,415).
Table 1.
Baseline characteristics of patients with irritable bowel syndrome (IBS) and matched controls
| Patients undergoing colonoscopy | Patients undergoing upper endoscopy | Patients undergoing colonoscopy compared to siblings | ||||
|---|---|---|---|---|---|---|
| Characteristic | IBS (n=21,944) | Matched controls (n=97,468) | IBS (n=9,965) | Matched controls (n=46,313) | IBS (n=1,240) | Siblings (n=1,415) |
| Women, no. (%) | 15 213 (69.3%) | 68 358 (70.1%) | 7 199 (72.2%) | 33 990 (73.4%) | 876 (70.6%) | 790 (55.8%) |
| Men, no (%) | 6 731 (30.7%) | 29 110 (29.9%) | 2 766 (27.8%) | 12 323 (26.6%) | 364 (29.4%) | 625 (44.2%) |
| Age, years | ||||||
| Mean (SD) | 44.7 (17.0) | 45.7 (17.0) | 41.9 (16.8) | 42.5 (16.8) | 48.3 (15.6) | 50.1 (16.2) |
| Median (IQR) | 43.2 (30.0–57.7) | 44.8 (30.9–58.9) | 39.3 (27.3–54.2) | 40.2 (27.6–55.1) | 50.4 (35.3–61.1) | 52.6 (36.3–63.8) |
| Range, min-max | 18.0–96.1 | 18.0–97.2 | 18.0–93.3 | 18.0–92.7 | 18.1–81.9 | 18.3–80.5 |
| Categories, no. (%) | ||||||
| 18y - <30y | 5 495 (25.0%) | 22 750 (23.3%) | 3 110 (31.2%) | 13 957 (30.1%) | 215 (17.3%) | 225 (15.9%) |
| 30y - <40y | 4 137 (18.9%) | 17 360 (17.8%) | 2 013 (20.2%) | 9 045 (19.5%) | 177 (14.3%) | 186 (13.1%) |
| 40y - <50y | 4 015 (18.3%) | 17 673 (18.1%) | 1 713 (17.2%) | 8 063 (17.4%) | 223 (18.0%) | 222 (15.7%) |
| 50y - <60y | 3 637 (16.6%) | 17 084 (17.5%) | 1 447 (14.5%) | 7 007 (15.1%) | 287 (23.1%) | 294 (20.8%) |
| 60y - <70y | 2 775 (12.6%) | 13 527 (13.9%) | 995 (10.0%) | 4 922 (10.6%) | 257 (20.7%) | 344 (24.3%) |
| ≥70y | 1 885 (8.6%) | 9 074 (9.3%) | 687 (6.9%) | 3 319 (7.2%) | 81 (6.5%) | 144 (10.2%) |
| Country of birth, no (%) | ||||||
| Nordic country | 19 982 (91.1%) | 86 388 (88.6%) | 8 953 (89.8%) | 38 520 (83.2%) | 1 213 (97.8%) | 1 378 (97.4%) |
| Other | 1 961 (8.9%) | 11 078 (11.4%) | 1 012 (10.2%) | 7 791 (16.8%) | 27 (2.2%) | 37 (2.6%) |
| Level of education, no (%) | ||||||
| ≤9 years | 4 159 (19.0%) | 19 632 (20.1%) | 1 803 (18.1%) | 10 097 (21.8%) | 249 (20.1%) | 356 (25.2%) |
| 10–12 years | 10 636 (48.5%) | 45 079 (46.3%) | 4 839 (48.6%) | 21 604 (46.6%) | 571 (46.0%) | 651 (46.0%) |
| >12 years | 7 032 (32.0%) | 32 051 (32.9%) | 3 255 (32.7%) | 14 168 (30.6%) | 419 (33.8%) | 403 (28.5%) |
| Missing | 117 (0.5%) | 706 (0.7%) | 68 (0.7%) | 444 (1.0%) | 1 (0.1%) | 5 (0.4%) |
| Year of index biopsy, no. (%) | ||||||
| 1987–1996 | 428 (2.0%) | 1 641 (1.7%) | 380 (3.8%) | 1 653 (3.6%) | 6 (0.5%) | 23 (1.6%) |
| 1997–2006 | 8 447 (38.5%) | 37 373 (38.3%) | 4 229 (42.4%) | 19 788 (42.7%) | 446 (36.0%) | 470 (33.2%) |
| 2007–2011 | 7 473 (34.1%) | 33 367 (34.2%) | 3 151 (31.6%) | 14 663 (31.7%) | 480 (38.7%) | 522 (36.9%) |
| 2012–2016 | 5 596 (25.5%) | 25 087 (25.7%) | 2 205 (22.1%) | 10 209 (22.0%) | 308 (24.8%) | 400 (28.3%) |
| ≥1997 | 21 516 (98.0%) | 95 827 (98.3%) | 9 585 (96.2%) | 44 660 (96.4%) | 1 234 (99.5%) | 1 392 (98.4%) |
| Months between IBS diagnosis and index biopsy | ||||||
| Mean (SD) | 0.7 (1.3) | 1.2 (1.5) | 0.8 (1.3) | |||
| Median (IQR) | 0.1 (0.0–0.9) | 0.4 (0.0–1.9) | 0.1 (0.0–0.9) | |||
| Range, min-max | 0.0–6.0 | 0.0–6.0 | 0.0–6.0 | |||
| Disease history within 5 years before biopsy, no (%) | ||||||
| Cardiovascular disease (CVD) | 3 635 (16.6%) | 17 424 (17.9%) | 1 440 (14.5%) | 6 517 (14.1%) | 225 (18.1%) | 313 (22.1%) |
| Cardiovascular disease in inpatient care | 2 051 (9.3%) | 10 032 (10.3%) | 846 (8.5%) | 4 326 (9.3%) | 125 (10.1%) | 190 (13.4%) |
| Cancer (excluding CRC) | 725 (3.3%) | 9 779 (10.0%) | 285 (2.9%) | 2 485 (5.4%) | 50 (4.0%) | 156 (11.0%) |
| Diabetes | 791 (3.6%) | 3 857 (4.0%) | 314 (3.2%) | 1 905 (4.1%) | 56 (4.5%) | 80 (5.7%) |
| Neurologic disease | 2 114 (9.6%) | 7 875 (8.1%) | 892 (9.0%) | 3 524 (7.6%) | 138 (11.1%) | 148 (10.5%) |
| IBS subtype (in biopsies ≥1997), no. (%) | ||||||
| IBS with diarrhea | 13 434 (62.4%) | 5 260 (54.9%) | 796 (64.5%) | |||
| IBS without diarrhea | 8 057 (37.4%) | 4 309 (45.0%) | 436 (35.3%) | |||
| IBS unspecified | 25 (0.1%) | 16 (0.2%) | 2 (0.2%) | |||
| IBS as main diagnosis, no. (%) | ||||||
| Yes | 19 615 (89.4%) | 8 363 (83.9%) | 1 086 (87.6%) | |||
| No | 2 329 (10.6%) | 1 602 (16.1%) | 154 (12.4%) | |||
| Deaths, no. (%) | ||||||
| ≤30 days after index date | 15 (0.1%) | 244 (0.3%) | 10 (0.1%) | 181 (0.4%) | 0 | 4 (0.3%) |
| ≤90 days after index date | 40 (0.2%) | 708 (0.7%) | 31 (0.3%) | 427 (0.9%) | 1 (0.1%) | 14 (1.0%) |
| ≤365 days after index date | 148 (0.7%) | 2 079 (2.1%) | 84 (0.8%) | 1 122 (2.4%) | 4 (0.3%) | 29 (2.0%) |
Both IBS patients and their matched controls were excluded from all analyses if at baseline they had an earlier diagnosis of inflammatory bowel disease, celiac disease, microscopic colitis, precancerous polyps or colorectal cancer up until −7 days from the index biopsy since these disorders may lead to false-positive IBS diagnoses.
Conditioned on matching set and further adjusted for age, sex, education and baseline medical comorbidities (cancer, diabetes, CVD, and neurologic disease)
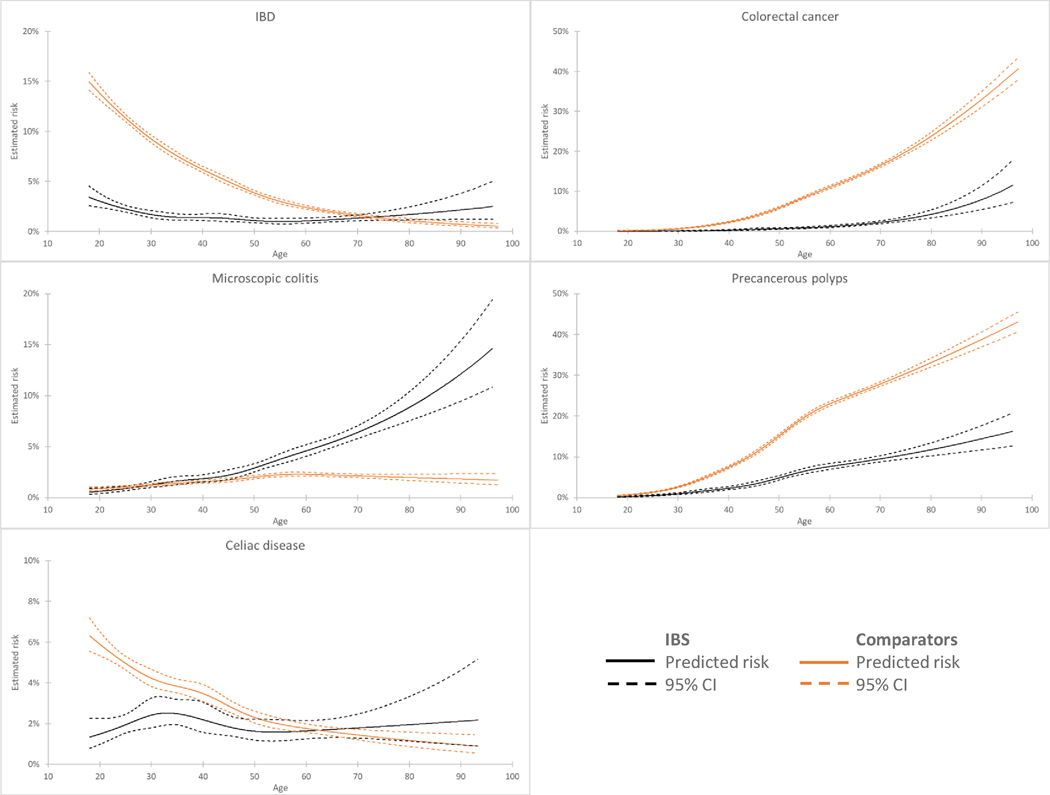
Among all IBS patients, the diagnostic yield for the specified organic diseases was 9.3%, falling to 5.1% among those <50 years old (16.3% for those ≥50 years old). A summary of the absolute yield of colonoscopy and upper endoscopy for the studied diseases in IBS by age is found in Figure 3. The odds of organic disease in IBS relative to comparators is found in Figure S1.
Figure 3.
Spline regressions showing estimated yield of organic disease diagnosis by colonoscopy (for inflammatory bowel disease, colorectal cancer, microscopic colitis, and precancerous polyps) or upper endoscopy (for celiac disease) by age
IBS, irritable bowel syndrome; IBD, inflammatory bowel disease
Inflammatory bowel disease
The crude prevalence of inflammatory bowel disease was lower in patients with IBS than in controls (1.6% vs. 5.9%)(Table 2). Conditioning on matching variables (age, sex, county, and calendar period), and adjusting for potential confounders including education and baseline medical comorbidities, patients with IBS were at 79% decreased risk of inflammatory bowel disease (aOR=0.21; 95% CI=0.19–0.24) (Table 2). Risk of inflammatory bowel disease did not vary significantly with sex or comorbidities (Table S4a).
Table 2.
Prevalence and odds ratio of inflammatory bowel disease (IBD), microscopic colitis, precancerous polyps, colorectal cancer and celiac disease in patients with IBS and matched controls as well as IBS and sibling comparators.
| Group | N events (%) IBS | Comparators | Odds ratio* (95%CI) | Odds ratio** (95%CI) | Sibling comparatOdds ratio*** (95%CI) |
|---|---|---|---|---|---|
| Overall | |||||
| IBD | 341 (1.6%) | 5 796 (5.9%) | 0.22 (0.20–0.25) | 0.21 (0.19–0.24) | 0.32 (0.19–0.53 |
| Colorectal cancer | 166 (0.8%) | 6 166 (6.3%) | 0.10 (0.09–0.12) | 0.17 (0.14–0.20) | 0.13 (0.05–0.37 |
| Microscopic colitis | 640 (2.9%) | 1 649 (1.7%) | 1.88 (1.71–2.06) | 1.77 (1.61–1.95) | 2.30 (1.30–4.08 |
| Precancerous polyps | 902 (4.1%) | 12 635 (13.0%) | 0.28 (0.26–0.30) | 0.28 (0.26–0.30) | 0.23 (0.16–0.33 |
| Non advanced | 669 (3.0%) | 7 995 (8.2%) | 0.35 (0.32–0.38) | 0.34 (0.32–0.37) | 0.23 (0.15–0.36 |
| Advanced | 249 (1.1%) | 5 042 (5.2%) | 0.21 (0.18–0.24) | 0.23 (0.20–0.26) | 0.34 (0.19–0.60 |
| Celiac disease | 192 (1.9%) | 1 568 (3.4%) | 0.54 (0.47–0.63) | 0.54 (0.47–0.63) | 0.47 (0.19–1.18 |
| Age <50y | |||||
| IBD | 243 (1.8%) | 4 902 (8.5%) | 0.17 (0.15–0.20) | 0.17 (0.15–0.20) | 0.22 (0.11–0.45 |
| Colorectal cancer | 24 (0.2%) | 981 (1.7%) | 0.10 (0.06–0.14) | 0.21 (0.13–0.34) | - |
| Microscopic colitis | 203 (1.5%) | 782 (1.4%) | 1.18 (1.01–1.38) | 1.16 (0.99–1.36) | 14.67 (0.37–578.5 |
| Precancerous polyps | 229 (1.7%) | 3 071 (5.3%) | 0.31 (0.27–0.35) | 0.32 (0.28–0.37) | 0.19 (0.07–0.50 |
| Non advanced | 177 (1.3%) | 2 171 (3.8%) | 0.35 (0.30–0.41) | 0.35 (0.30–0.41) | 0.17 (0.05–0.59 |
| Advanced | 54 (0.4%) | 961 (1.7%) | 0.23 (0.18–0.31) | 0.26 (0.19–0.34) | 0.83 (0.18–3.74 |
| Celiac disease | 136 (2.0%) | 1 300 (4.2%) | 0.45 (0.38–0.54) | 0.46 (0.38–0.55) | 0.27 (0.07–1.00 |
| Age ≥50y | |||||
| IBD | 98 (1.2%) | 894 (2.3%) | 0.51 (0.42–0.63) | 0.46 (0.38–0.58) | 0.55 (0.13–2.30 |
| Colorectal cancer | 142 (1.7%) | 5 185 (13.1%) | 0.10 (0.09–0.12) | 0.16 (0.13–0.20) | 0.14 (0.04–0.44 |
| Microscopic colitis | 437 (5.3%) | 867 (2.2%) | 2.58 (2.29–2.91) | 2.36 (2.09–2.66) | 2.24 (1.14–4.42 |
| Precancerous polyps | 673 (8.1%) | 9 564 (24.1%) | 0.27 (0.25–0.29) | 0.27 (0.25–0.29) | 0.20 (0.13–0.33 |
| Non advanced | 492 (5.9%) | 5 824 (14.7%) | 0.36 (0.32–0.39) | 0.34 (0.31–0.37) | 0.25 (0.15–0.42 |
| Advanced | 195 (2.4%) | 4 081 (10.3%) | 0.20 (0.18–0.24) | 0.22 (0.19–0.25) | 0.26 (0.13–0.55 |
| Celiac disease | 56 (1.8%) | 268 (1.8%) | 1.04 (0.78–1.39) | 1.00 (0.74–1.34) | 0.47 (0.04–5.29 |
Conditioned on matching set (age, sex, county, and calendar period)
Conditioned on matching set and further adjusted for education and baseline medical comorbidities (cancer, diabetes, CVD, and neurologic disease).
Conditioned on matching set and further adjusted for age, sex, education and baseline medical comorbidities (cancer, diabetes, CVD, and neurologic disease).
Colorectal cancer
The crude prevalence of colorectal cancer was 0.8% in patients with IBS compared to 6.3% in controls. This corresponded to an 83% decreased risk of colorectal cancer in IBS (aOR=0.17; 95% CI 0.14–0.20)(Table 2). Risk of diagnosing colorectal cancer in IBS patients similarly did not vary significantly by age or sex. (Table S4b). Also when restricting our cohort to IBS patients aged ≥50 years, we did see a lower prevalence of colorectal cancer compared to matched controls (Table 2). Age affected prevalence estimates significantly. The proportion of colorectal cancer in IBS patients was 0–0.1% for those <40 years old compared to 3.8% for those ≥70 (Table S4b). There was no clear trend toward increasing left-sided colon cancers in our cohort (Table S7), though ESPRESSO contains a high proportion of codes that do not specify left- or right-sided CRC location and are therefore listed as unspecified.
Microscopic colitis
The crude prevalence of microscopic colitis was increased in IBS patients in comparison to matched controls (2.9% vs. 1.7%), which corresponded to an adjusted OR of 1.77 (95% CI 1.61–1.95). Overall, older women with IBS had an increased prevalence of microscopic colitis as well as those diagnosed with IBS with diarrhea (IBS-D) prior to colonoscopy (Table S4c). Among those IBS patients ≥50 years old, the risk of microscopic colitis was greater (OR=2.36; 95% CI 2.09–2.66) compared to controls than in those <50 years old (OR=1.16; 95% CI 0.99–1.36)(P for interaction <0.001)(Table 2). Again, age affected prevalence estimates significantly: the proportion of IBS patients having microscopic colitis was 0.9–1.6% in those <40 years old compared to 8.2% in those ≥70 (Table S4c).
Precancerous polyps
IBS patients had a lower yield of precancerous polyps on colonoscopy than comparators (4.1% vs. 13.0%), including lower prevalence of non-advanced and advanced adenomas (Table 2). The adjusted risk of any precancerous polyps was 72% lower in IBS (aOR=0.28; 95% CI 0.26–0.30), with similar findings for non-advanced and advanced adenomas. The estimates were similar across subgroups divided by age, sex, years of biopsy, comorbidity, and IBS subtype (Table S4d). Similar trends were seen for non-advanced and advanced adenoma subgroups (Tables S4e and S4f).
Yield of upper endoscopy in celiac disease
We identified 11,662 patients diagnosed with IBS 1987–2016 who had an upper endoscopy within 6 months (Figure 2). After exclusions, there were 9,965 patients with IBS who were matched to 42,584 patients (with 46,313 biopsies) without IBS undergoing upper endoscopy.
The average time interval from IBS diagnosis to upper endoscopy was 1.2 (SD 1.5). Table 1 shows the characteristics of study participants according to IBS status. Similar to the patients undergoing colonoscopy, both IBS patients and matched controls were predominantly female with a mean age in the mid-40s. IBS was the primary diagnosis in the pre-colonoscopy clinical encounter 83.9% of the time, with IBS-D as the predominant subtype among the IBS cohort.
Celiac disease
The crude prevalence of celiac disease in IBS was 1.9% compared to 3.4% in matched controls, which translated to a 46% decreased risk of celiac disease (aOR=0.54; 95 CI 0.47–0.63) (Table 2). The prevalence of positive biopsies for celiac was about the same as the general population above age 50 and significantly lower below age 30. (Table S4g).
An IBS diagnosis prior to upper endoscopy was associated with a 53% decreased risk of celiac disease on biopsy compared to siblings (OR= 0.43; 95% CI 0.20–0.89), but this was attenuated by adjustment for confounders (including age and sex)(aOR=0.47; 95 CI 0.19–1.18) with a wide CI likely due to small patient number (Table 2).
Sibling analyses
Sibling analyses confirmed the main analyses above (Table 2).
Analysis by endoscopy date
A subanalysis accounting for date of colonoscopy or upper endoscopy (as a possible source of temporal bias) evaluating patients diagnosed in 2002 and later confirmed the main analyses (Table S5).
Role of family history
For diseases with a strong heritable component (inflammatory bowel disease, colorectal cancer, and celiac disease), additional adjustment for family history of these diseases in a parent or sibling did not materially alter our effect estimates (Table S6).
DISCUSSION
In this nationwide, population-based survey of over 21,000 newly-diagnosed IBS patients undergoing colonoscopy with biopsy and over 9,900 newly-diagnosed IBS patients undergoing upper endoscopy with biopsy, we found an overall low diagnostic yield of inflammatory bowel disease, precancerous polyps, colorectal cancer, and celiac disease. Practically, the lower risk translates to only 1 out of 132 individuals with IBS undergoing colonoscopy being diagnosed with colorectal cancer. Conversely, patients initially diagnosed with IBS who later underwent colonoscopy were more likely to be diagnosed with microscopic colitis (especially older patients with IBS-D) than comparators undergoing colonoscopy for other indications. However, the absolute diagnostic yield was similarly low.
To our knowledge, these results represent the largest, population-based analysis of the yield of endoscopy in IBS with the benefit of pathology confirmation of disease findings. The economic impacts of our finding are potentially substantial given the high prevalence of IBS across global societies with high direct healthcare costs.[6] In Europe, a third of IBS patients will undergo colonoscopy,[27] and almost 30% of colonoscopies in the US performed in patients under 50 are performed for IBS.[5]
Existing data have been mixed as to the yield of endoscopic procedures in IBS. In a community-based colon cancer screening cohort (n=9,160 with IBS) in Taiwan demonstrated an approximately 20% increased risk of colonic adenomas and colorectal cancer.[10] Two other Taiwan-based cohorts found an increased risk of colorectal cancer (compared to randomly-selected controls[28] and expected cancer rate[29]) among IBS patients in the first one to two years after diagnosis that attenuated afterward. Data from a Danish registry with large numbers of IBS patients (n=57,851) demonstrated a similar trend with increased risk of colorectal cancer early on after IBS diagnosis followed by a decreased risk in later years.[30]
Our findings are consistent with other studies looking at the diagnostic yield of colonoscopy. In their prospective study of 466 suspected IBS patients undergoing colonoscopy compared to controls undergoing screening or surveillance colonoscopy, Chey et al found decreased rates of colonic adenomas and colorectal cancers and adenomas compared to controls.[7] They also found no significantly-increased risk of inflammatory bowel disease or microscopic colitis, though there were few cases of each overall. In a random population of 1,220 Swedish patients undergoing colonoscopy, the prevalence of inflammatory bowel disease was 1.8% and 0.4% among those with and without IBS symptoms, respectively.[31]
A small, uncontrolled study did demonstrate a 4.6% risk of microscopic colitis among 87 IBS patients,[12] and meta-analyses incorporating larger numbers of patients have not suggested an increased risk of microscopic colitis in IBS.[11, 13] Our own data more definitively establish the low risk of a microscopic colitis diagnosis overall among patients with IBS undergoing colonoscopy, though the odds of diagnosis was increased compared to matched comparators—especially among those diagnosed with IBS-D and in older patients. The difference in diagnostic yield by age is striking: 1 in 80 IBS patients <40 years old undergoing colonoscopy will be diagnosed with microscopic colitis compared to 1 in 12 among those ≥70 years old.
Our data suggest a decreased yield of celiac disease among patients with IBS undergoing upper endoscopy with biopsy, though we would argue that the availability and excellent test characteristics of celiac serology testing may decrease the yield of patients with IBS undergoing upper endoscopy. Many of these patients would likely have undergone serologic testing prior to their clinicians making a diagnosis of IBS. However, our celiac disease prevalence of 1.9% is not substantially lower than the 3.3% celiac prevalence from a meta-analysis performed among those with positive celiac serologies undergoing duodenal biopsy.[14] Moreover, a multicenter study of 492 IBS patients without constipation undergoing serology and confirmatory endoscopy demonstrated a similar yield of celiac disease between the IBS population and controls.[32]
Our study had several strengths which bolster our conclusions. It is the largest study to date utilizing a population-based approach for IBS data with diagnoses of interest confirmed by pathology, minimizing the risk of misclassification bias. By including over 103,000 patients undergoing colonoscopy with biopsy and more than 52,000 patients undergoing upper endoscopy with biopsy, we were able to prospectively capture less common diagnoses in significant quantity including inflammatory bowel disease, colorectal cancer, and celiac disease. Well-validated Swedish registers[33] ensure minimal loss to follow up, and the availability of matched siblings also undergoing endoscopic evaluation confirms our main findings in those with similar genetic and environmental backgrounds.
We acknowledge several limitations. Sweden does not have a nationwide colorectal cancer screening program, and thus our IBS cohort may represent a more severe subset of disease with symptoms bothersome enough to merit endoscopic evaluation. The majority of IBS patients will not seek care for their disease.[34] We also acknowledge that some patients presenting with IBS-like symptoms would have avoided a clinical diagnosis of IBS by virtue of some historical or diagnostic feature such as fecal calprotectin for inflammatory bowel disease, or alarm symptoms for colorectal cancer. We also relied on a single diagnostic code for identification of IBS patients, which may introduce misclassification bias. Nevertheless, the ICD system is thought to have strong validity in IBS[24] and conditioning the IBS diagnosis on having an associated gastrointestinal biopsy also likely increases data validity and reduces the risk of transcription error in ICD coding. Finally, because cases of structural GI disease were matched to controls at the time of biopsy, we are unable to evaluate the yield of endoscopy in patients diagnosed with IBS after an endoscopic procedure or those with IBS who never underwent endoscopy.
In summary, we show that the diagnostic yield of upper endoscopy and colonoscopy is relatively low in patients with a first-time diagnosis of IBS. Age of IBS diagnosis is clearly an important factor, and we demonstrate an increasing yield of diagnostic colonoscopy for both colorectal cancer and microscopic colitis in older patients. Because IBS is a disease primarily diagnosed in the young, this data importantly highlights the low yield of endoscopic evaluations in this population. Our findings support a symptom-based diagnostic approach to IBS not involving endoscopy, saving healthcare expenditures and minimizing patient risk.
Supplementary Material
HIGHLIGHTS.
Symptoms of irritable bowel syndrome (IBS) are common reasons for endoscopic procedures
We examined the yield of colonoscopy and upper endoscopy in IBS in a large cohort
Inflammatory bowel disease, precancerous polyps, colorectal cancer, and celiac disease were all less common in IBS compared to controls
Microscopic colitis was more common in IBS patients
The yield of upper endoscopy and colonoscopy for organic disease is low in patients with a first-time diagnosis of IBS
Acknowledgments
Financial support
KS is supported by NIH K23DK120945. HK is supported by the Crohn’s and Colitis Foundation Senior Research Award, American Collage of Gastroenterology Clinical Research Award and the Beker Foundation Grant.
Abbreviations:
- CI
confidence interval
- OR
Odds ratio
- IBD
inflammatory bowel disease
- IBS
irritable bowel syndrome
Footnotes
Guarantor of the article
JFL
Declaration of interests
KS has received research support from AstraZeneca, Ironwood, and Urovant, has served as a speaker for Shire/Takeda, and has served as a consultant to Arena, Gelesis, Shire/Takeda, and Synergy. JFL coordinates a study on behalf of the Swedish IBD quality register (SWIBREG). This study has received funding from Janssen. OO has been PI for projects (unrelated to the current paper) at KI financed by grants from Janssen, Ferring, and Pfizer. HK has received consulting fees from Abbvie and Takeda. He has also received grant funding from Takeda and Pfizer.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–21 e4. [DOI] [PubMed] [Google Scholar]
- [2].Staller K, Olen O, Soderling J, Roelstraete B, Tornblom H, Khalili H, et al. Mortality Risk in Irritable Bowel Syndrome: Results From a Nationwide Prospective Cohort Study. Am J Gastroenterol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wells NE, Hahn BA, Whorwell PJ. Clinical economics review: irritable bowel syndrome. Aliment Pharmacol Ther. 1997;11:1019–30. [DOI] [PubMed] [Google Scholar]
- [4].Levy RL, Von Korff M, Whitehead WE, Stang P, Saunders K, Jhingran P, et al. Costs of care for irritable bowel syndrome patients in a health maintenance organization. Am J Gastroenterol. 2001;96:3122–9. [DOI] [PubMed] [Google Scholar]
- [5].Lieberman DA, Williams JL, Holub JL, Morris CD, Logan JR, Eisen GM, et al. Colonoscopy utilization and outcomes 2000 to 2011. Gastrointest Endosc. 2014;80:133–43. [DOI] [PubMed] [Google Scholar]
- [6].Canavan C, West J, Card T. Review article: the economic impact of the irritable bowel syndrome. Aliment Pharmacol Ther. 2014;40:1023–34. [DOI] [PubMed] [Google Scholar]
- [7].Chey WD, Nojkov B, Rubenstein JH, Dobhan RR, Greenson JK, Cash BD. The yield of colonoscopy in patients with non-constipated irritable bowel syndrome: results from a prospective, controlled US trial. Am J Gastroenterol. 2010;105:859–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Patel P, Bercik P, Morgan DG, Bolino C, Pintos-Sanchez MI, Moayyedi P, et al. Prevalence of organic disease at colonoscopy in patients with symptoms compatible with irritable bowel syndrome: cross-sectional survey. Scand J Gastroenterol. 2015;50:816–23. [DOI] [PubMed] [Google Scholar]
- [9].Gu HX, Zhang YL, Zhi FC, Jiang B, Huang Y. Organic colonic lesions in 3,332 patients with suspected irritable bowel syndrome and lacking warning signs, a retrospective case--control study. Int J Colorectal Dis. 2011;26:935–40. [DOI] [PubMed] [Google Scholar]
- [10].Chang HC, Yen AM, Fann JC, Chiu SY, Liao CS, Chen HH, et al. Irritable bowel syndrome and the incidence of colorectal neoplasia: a prospective cohort study with community-based screened population in Taiwan. Br J Cancer. 2015;112:171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Guagnozzi D, Arias A, Lucendo AJ. Systematic review with meta-analysis: diagnostic overlap of microscopic colitis and functional bowel disorders. Aliment Pharmacol Ther. 2016;43:851–62. [DOI] [PubMed] [Google Scholar]
- [12].Hilpusch F, Johnsen PH, Goll R, Valle PC, Sorbye SW, Abelsen B. Microscopic colitis: a missed diagnosis among patients with moderate to severe irritable bowel syndrome. Scand J Gastroenterol. 2017;52:173–7. [DOI] [PubMed] [Google Scholar]
- [13].Kamp EJ, Kane JS, Ford AC. Irritable Bowel Syndrome and Microscopic Colitis: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2016;14:659–68 e1; quiz e54–5. [DOI] [PubMed] [Google Scholar]
- [14].Irvine AJ, Chey WD, Ford AC. Screening for Celiac Disease in Irritable Bowel Syndrome: An Updated Systematic Review and Meta-analysis. Am J Gastroenterol. 2017;112:65–76. [DOI] [PubMed] [Google Scholar]
- [15].Sanders DS, Patel D, Stephenson TJ, Ward AM, McCloskey EV, Hadjivassiliou M, et al. A primary care cross-sectional study of undiagnosed adult coeliac disease. Eur J Gastroenterol Hepatol. 2003;15:407–13. [DOI] [PubMed] [Google Scholar]
- [16].Ludvigsson JF, Lashkariani M. Cohort profile: ESPRESSO (Epidemiology Strengthened by histoPathology Reports in Sweden). Clin Epidemiol. 2019;11:101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Svensson M, Bergman D, Olen O, Myrelid P, Bohr J, Wickbom A, et al. Validating microscopic colitis (MC) in Swedish pathology registers. Scand J Gastroenterol. 2018;53:1469–75. [DOI] [PubMed] [Google Scholar]
- [18].Ludvigsson JF, Brandt L, Montgomery SM, Granath F, Ekbom A. Validation study of villous atrophy and small intestinal inflammation in Swedish biopsy registers. BMC Gastroenterol. 2009;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nguyen LH, Ö AK, C Y, Simon TG, Roelstraete B, Song M, et al. Antibiotic use and the development of inflammatory bowel disease: a national case/control study in Sweden. Lancet Gastroenterol Hepatol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bozorg SR, Song M, Emilsson L, Ludvigsson JF. Validation of serrated polyps (SPs) in Swedish pathology registers. BMC Gastroenterol. 2019;20:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ludvigsson JF, Almqvist C, Bonamy AK, Ljung R, Michaelsson K, Neovius M, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31:125–36. [DOI] [PubMed] [Google Scholar]
- [22].Ludvigsson JF, Svedberg P, Olen O, Bruze G, Neovius M. The longitudinal integrated database for health insurance and labour market studies (LISA) and its use in medical research. Eur J Epidemiol. 2019;34:423–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jossan N, Backman AS, Linder M, Altman M, Simren M, O O, et al. Validation of the Use of the ICD-10 Diagnostic Code for Irritable Bowel Syndrome in the Swedish National Patient Register. Gastroenterology. 2014;146:S543. [Google Scholar]
- [25].Siegel RL, Fedewa SA, Anderson WF, Miller KD, Ma J, Rosenberg PS, et al. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. J Natl Cancer Inst. 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ludvigsson JF, Haberg SE, Knudsen GP, Lafolie P, Zoega H, Sarkkola C, et al. Ethical aspects of registry-based research in the Nordic countries. Clin Epidemiol. 2015;7:491–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pare P, Gray J, Lam S, Balshaw R, Khorasheh S, Barbeau M, et al. Health-related quality of life, work productivity, and health care resource utilization of subjects with irritable bowel syndrome: baseline results from LOGIC (Longitudinal Outcomes Study of Gastrointestinal Symptoms in Canada), a naturalistic study. Clin Ther. 2006;28:1726–35; discussion 10–1. [DOI] [PubMed] [Google Scholar]
- [28].Hsiao CW, Huang WY, Ke TW, Muo CH, Chen WT, Sung FC, et al. Association between irritable bowel syndrome and colorectal cancer: a nationwide population-based study. Eur J Intern Med. 2014;25:82–6. [DOI] [PubMed] [Google Scholar]
- [29].Hu LY, Ku FC, Lu T, Shen CC, Hu YW, Yeh CM, et al. Risk of cancer in patients with irritable bowel syndrome: a nationwide population-based study. Ann Epidemiol. 2015;25:924–8. [DOI] [PubMed] [Google Scholar]
- [30].Norgaard M, Farkas DK, Pedersen L, Erichsen R, de la Cour ZD, Gregersen H, et al. Irritable bowel syndrome and risk of colorectal cancer: a Danish nationwide cohort study. Br J Cancer. 2011;104:1202–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kjellstrom L, Molinder H, Agreus L, Nyhlin H, Talley NJ, Andreasson A. A randomly selected population sample undergoing colonoscopy: prevalence of the irritable bowel syndrome and the impact of selection factors. Eur J Gastroenterol Hepatol. 2014;26:268–75. [DOI] [PubMed] [Google Scholar]
- [32].Cash BD, Rubenstein JH, Young PE, Gentry A, Nojkov B, Lee D, et al. The prevalence of celiac disease among patients with nonconstipated irritable bowel syndrome is similar to controls. Gastroenterology. 2011;141:1187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24:659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol.2014;6:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.