Abstract
Objective:
To evaluate the antidiabetic effects of the senolytic agent dasatinib in older patients with type 2 diabetes mellitus (T2DM).
Patients and Methods:
This retrospective cohort study included enterprise-wide Mayo Clinic patients using Informatics for Integrating Biology at the Bedside (i2B2) from January 1994 through December 2019. The anti-diabetic outcomes (change in hemoglobin A1c, serum glucose, and diabetic medications) after one year of a strongly senolytic tyrosine kinase inhibitor (TKI), dasatinib (n=16), was compared to a weakly senolytic TKI, imatinib (n=32).
Results:
Relative to imatinib, patients treated with dasatinib had a mean 43.7 mg/dL (P=.005) reduction in serum glucose, required 28.8 less total daily insulin units (P=.08) in the setting of a 4.8 kg relative weight loss (5.3% of total body weight; P=.045). Linear regression analysis suggests that the relative difference in weight accounts for 8.4 mg/dL of the 43.7 mg/dL blood glucose value decrease, or 19.2%. Relative to imatinib, patients treated with dasatinib had a mean 0.80 absolute points (P=.05) reduction in hemoglobin A1c, required 18.2 less total daily insulin units (P=.16) in the setting of a 5.9 kg relative weight loss (6.3% of total body weight; P=.06).
Conclusion:
Dasatinib may have anti-diabetic effects comparable to contemporary diabetic treatments and may be considered for use as a novel diabetic therapy. Future studies are needed to determine if these results are translatable to patients with T2DM without underlying malignancies and to determine whether the anti-diabetic effects of dasatinib are due to its senolytic properties.
Introduction
Type 2 diabetes (T2DM) entails significant morbidity and mortality, affecting an estimated 31 and 425 million people in the United States and worldwide, respectively [1]. The prevalence of T2DM increases with aging, affecting approximately 18 million Americans over the age of 65 years [2]. Despite improved understanding of the pathophysiology of T2DM, current oral pharmacologic therapies are non-curative and limited in efficacy.
Tyrosine kinase inhibitors (TKIs) are used for the treatment of select malignancies. Imatinib was the first TKI approved for Philadelphia chromosome-positive chronic myelogenous leukemia (CML) in 2001 and was soon followed by other TKIs, such as dasatinib in 2006. Sporadic case reports suggest that TKIs, primarily imatinib, may improve glycemic control or even result in complete remission of T2DM [3–8]. A subsequent retrospective cohort study of patients with (n=17) and without T2DM (n=61) showed that treatment with various TKIs (sunitinib, sorafenib, dasatinib, and imatinib) was associated with lower serum glucose levels. This finding was limited by a small T2DM cohort, heterogenous malignancies, and possible confounding by malignancy progression and weight changes [9]. Currently, experts recommend close glucose level monitoring when initiating TKIs due to the potential concern for hypoglycemia [10]. However, other studies examining this issue have been inconsistent [11–12]. The literature is limited by retrospective observational studies with small number of diabetic patients (<10), and potential confounding by malignancy and alterations in weight.
While TKIs have been suggested as a novel therapy for T2DM, the mechanism of action to reduce hyperglycemia is unclear owing to numerous off-target effects. However, there is a growing body of evidence to suggest that cellular senescence is a cause and consequence of many age-related diseases, including T2DM [13]. In pre-clinical animal models, reducing senescent cell burden through treatment with senolytic drugs, such as the combination of dasatinib and quercetin, has been shown to improve glucose tolerance and to enhance insulin sensitivity [14].
Further studies are required to determine if there is an anti-diabetic effect of TKIs in humans with T2DM and if so, to determine if this effect is mediated by reducing senescent cell burden. The objective of this retrospective cohort study was to evaluate the anti-diabetic effect of the strongly senolytic TKI dasatinib as compared to the weakly senolytic agent imatinib [15].
Methods
Study Design and Participants
This study was a retrospective cohort design. The cohort was identified using Mayo Clinic’s Informatics for Integrating Biology at the Bedside (i2B2), an informatics framework that organizes and transforms patient clinic data into a research database. As interrogated, Mayo Clinic’s i2B2 database included patients from campuses in Arizona (1999–2020), Florida (2003–2020), Rochester (1994–2020), and the Mayo Clinic Health System in Minnesota (2006–2020). Inclusion criteria were continuous use of dasatinib or imatinib for at least 12 consecutive months; age >18 years old; and T2DM diagnosed prior to the initiation of dasatinib or imatinib. Exclusion criteria for both groups were a TKI indication of hypereosinophilia syndrome (treated with concurrent glucocorticoids), and for the imatinib group the use of any prior TKI. Since dasatinib is typically used as a second line agent, excluding subjects with prior TKI use was not always possible.
Data were obtained via medical record chart review. Hemoglobin A1c, serum blood glucose, weight, and information regarding diabetic medications were collected at the time of initiation of dasatinib or imatinib and again 12 months later. Individuals were included only if they had values recorded less than 3 months prior to the initiation of the tyrosine kinase inhibitor. Fasting outpatient serum glucose values were preferred and, if not specified, the lowest outpatient morning glucose was recorded. Total daily insulin dose was calculated by adding fixed long and short acting insulin units (sliding scale or carbohydrate correction insulin doses were not included).
Statistical Analysis
A two-sided unpaired Student t test was used to compare differences in continuous variables and Fisher’s exact test to compare differences in categorical variables between the dasatinib and imatinib groups. The association between the change in weight loss and the change in hemoglobin A1C or glucose was evaluated by group (dasatinib or imatinib) using a simple linear regression model, and was compared between groups using the model with weight loss, group, and their interaction for hemoglobin A1c or glucose. A significant interaction in the above model implies differential associations between the dasatinib and imatinib groups. A p-value of <0.05 was considered statically significant. All the statistical analyses were performed in Microsoft Excel 2010 and R version 3.6.1.
Compliance with Ethical Standards
The Mayo Clinic Institutional Review Board approved this study. This study was exempted from the need to obtain informed consent.
Results
Characteristics of Study Participants
A total of 9,311,258 individuals were screened for use of either dasatinib or imatinib. There were 279 and 118 individuals included in the imatinib and dasatinib groups, respectively (Figure 1). A total of 247 individuals were removed from the imatinib group due to failure to meet inclusion/exclusion criteria, resulting in a cohort of 32 individuals. Baseline and 1-year data were complete in 26/32 and 22/32 individuals for serum glucose and hemoglobin A1c, respectively. A total of 102 individuals were removed from the dasatinib group after application of inclusion/exclusion criteria, resulting in a cohort of 16 individuals. In the dasatinib group, baseline and 1-year data were complete in 12/16 and 13/16 individuals for serum glucose and hemoglobin A1c, respectively.
Figure 1.
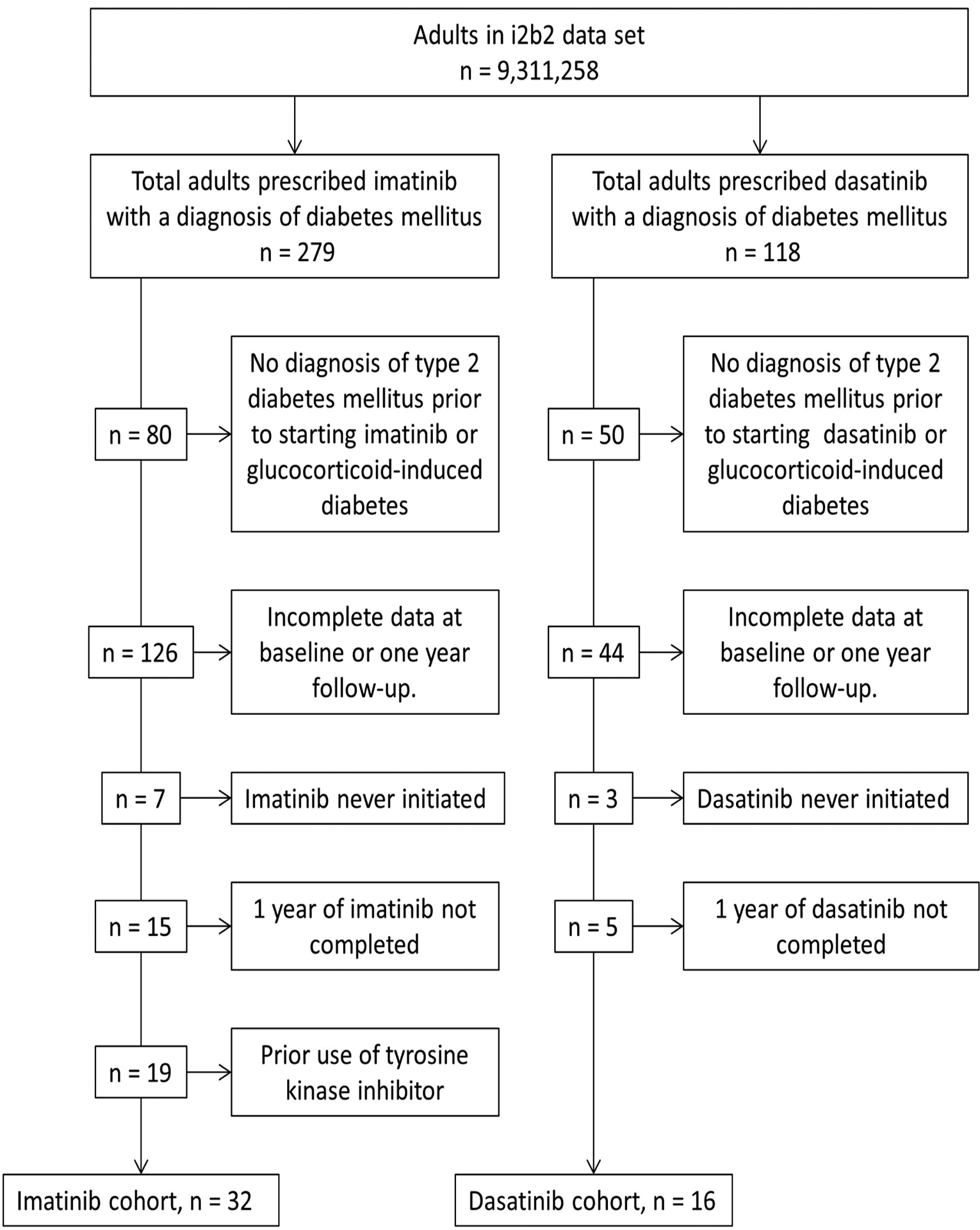
Selection of Study Participants and Identification of Dasatinib and Imatinib Cohorts
Table 1 and Table 2 provides baseline characteristics of the study participants with respect to changes in serum glucose and hemoglobin A1c, respectively. Baseline parameters, including mean age at malignancy diagnosis, gender, race, type of malignancy, prior use of TKI, serum glucose value, insulin dependency, total daily insulin requirement, oral anti-hyperglycemic agents, and weight were evaluated between the dasatinib and imatinib groups. When comparing serum glucose, there were no statistically significant differences between the groups other than prior TKI use (P=0.007). When comparing hemoglobin A1c, there were no statistically significant differences between the groups other than prior TKI use (P=.01) and gastrointestinal stromal tumor (GIST) diagnoses (P=.02).
Table 1.
Baseline Characteristics of Dasatinib vs. Imatinib: Serum Glucose Values
| Dasatinib | Imatinib | *P-value | |
|---|---|---|---|
| Number | 12 | 26 | |
| Male | 7 (58%) | 17 (65%) | 0.728 |
| Race, White | 10 (83%) | 24 (92%) | 0.577 |
| Malignancy | |||
| CML a | 9 (75%) | 13 (50% ) | 0.178 |
| ALL a | 2 (17%) | 1 (4%) | 0.230 |
| GIST a | 1 (8%) | 11 (42%) | 0.060 |
| Melanoma | 0 | 1 (4%) C | >0.99 |
| Age at Diagnosis | 61.7 ± 10.9 | 64.0 ± 11.0 | 0.304 |
| TKI prior to dasatinib/imatinib | 4/12 (33%) | 0 | 0.007 |
| Baseline Glucose (mg/dL) | 145 ± 48 | 132 ± 39 | 0.414 |
| On Insulin | 4 (33%) | 7 (25%) | 0.714 |
| Average Total Daily Units | 71 ± 47 | 67 ± 52 | 0.907 |
| On oral agents | 10 (83%) | 19 (73%) | 0.689 |
| Number of agents | 1.2 ± 0.8 | 1.0 ± 0.8 | 0.568 |
| Baseline Weight (kg) | 98.5 ± 23.5 | 95.6 ± 23.0 | 0.728 |
P-values obtained using a 2-tailed two-sample t-test assuming unequal variances for continuous variables, or Fisher’s exact test for categorical variables
CML, chronic myelogenous leukemia; ALL, Acute lymphoblastic leukemia; GIST, Gastrointestinal Stromal Tumor.
Table 2.
Baseline Characteristics of Dasatinib vs. Imatinib: Hemoglobin A1c Values
| Dasatinib | Imatinib | *P-value | |
|---|---|---|---|
| Number | 13 | 22 | |
| Male | 9 (69%) | 11 (50%) | 0.31 |
| Race, White | 11 (85%) | 20 (91%) | 0.18 |
| Malignancy | |||
| CML a | 11 (85%) | 13 (59%) | 0.15 |
| ALL a | 2 (15%) | 1 (5%) | 0.54 |
| GIST a | 0 | 8 (36%) | 0.02 |
| Melanoma | 0 | 0 | >0.99 |
| Age at Diagnosis | 62.6 ± 11.2 | 66.6 ± 10.3 | 0.31 |
| TKI prior to dasatinib/imatinib | 4/13 (31%) | 0 | 0.01 |
| Baseline A1c (%) | 6.9 ± 0.8 | 6.5 ± 0.8 | 0.24 |
| On Insulin | 6 (46%) | 7 (32%) | 0.48 |
| Average Total Daily Units | 69 ± 51 | 56 ± 49 | 0.65 |
| On oral agents | 9 (69%) | 19 (86%) | 0.38 |
| Number of agents | 1.1 ± 0.9 | 1.2 ± 0.7 | 0.71 |
| Baseline Weight (kg) | 102.8 ± 19.6 | 95.9 ± 24.1 | 0.36 |
P-values obtained using a 2-tailed two-sample t-test assuming unequal variances for continuous variables, or Fisher’s exact test for categorical variables
CML, chronic myelogenous leukemia; ALL, Acute lymphoblastic leukemia; GIST, Gastrointestinal Stromal Tumor.
Change in Glycemic Control After One Year of TKI Therapy
Table 3 displays the change in serum glucose after one year of either dasatinib or imatinib. Relative to imatinib, the dasatinib group had a decrease in serum glucose of 43.7 mg/dL (P=.005). Total daily insulin requirements decreased by 28.8 units (P=.08), while use of other anti-hyperglycemic agents was unchanged (decrease by 0.04; P=.66). Relative body weight decreased by −4.8 kg (P=.045), equivalent to a change of −5.3% (P=.03). Of note, 2/4 insulin-dependent patients in the dasatinib group did not require any insulin (weight decrease of 1.5 and 2.5 kg) compared to 1/9 insulin-dependent patients (weight decrease of 18 kg) in the imatinib group.
Table 3.
Serum Glucose and Other Glycemic Indices After One Year of TKI Therapy
| Dasatinib | Imatinib | Difference | * P-value | |
|---|---|---|---|---|
| Number | 12 | 26 | ||
| Serum Glucose (mg/dL) | −31.4 ± 34.2 | 12.3 ± 43.6 | −43.7 | 0.005 |
| Total daily insulin (Units) | −23.5 ± 22.2 | 5.3 ± 19.2 | −28.8 | 0.08 |
| Other antihyperglycemic medications | 0.0 ± 0.0 | 0.04 ± 0.45 | −0.04 | 0.66 |
| Weight (kg) | −1.62 ± 4.89 | 3.18 ± 9.30 | 4.80 | 0.045 |
| Body mass (%) | −1.7 ± 2.3 | 3.6 ± 8.9 | 5.3 | 0.03 |
P -values obtained using a 2-tailed two-sample t-test assuming unequal variances for continuous variables, or Fisher’s exact test for categorical variables
Table 4 displays the change in hemoglobin A1c after one year of either dasatinib or imatinib. Relative to imatinib, the dasatinib group had an absolute decrease in hemoglobin A1c value of 0.80% (P=.05). Total daily insulin requirements decreased by 18.2 units (P=.16), while use of other anti-hyperglycemic agents was unchanged (P=.93). Relative body weight decreased −5.9 kg (P=.06), equivalent to a change of −6.3% (P=.04).
Table 4.
Hemoglobin A1c and Other Glycemic Indices After One Year of TKI Therapy
| Dasatinib | Imatinib | Difference | * P-value | |
|---|---|---|---|---|
| Number | 13 | 22 | ||
| Hemoglobin A1c (%) | −0.74 ± 1.06 | 0.06 ± 1.25 | −0.80 | 0.05 |
| Total daily insulin (Units) | −18.8 ± 21.9 | −0.6 ± 15.3 | −18.2 | 0.16 |
| Other antihyperglycemic medications | −0.1 ± 0.3 | −0.1 ± 0.6 | 0.0 | 0.93 |
| Weight (kg) | −3.0 ± 7.6 | 2.8 ± 10.1 | 5.9 | 0.06 |
| Body mass (%) | −3.1 ± 3.06 | 3.2 ± 9.6 | 6.3 | 0.04 |
P-values obtained using a 2-tailed two-sample t-test assuming unequal variances for continuous variables, or Fisher’s exact test for categorical variables
A sensitivity analysis that excluded 5 subjects with prior TKI use in the dasatinib group is shown in Tables 5 and 6. This showed that relative to treatment with imatinib, patients treated with dasatinib had a larger magnitude of hypoglycemic effect for both serum glucose and hemoglobin A1c values, as well as a significant reduction in body weight.
Table 5.
Serum Glucose and Other Glycemic Indices After One Year of TKI Therapy, Excluding Prior TKI Therapy
| Dasatinib | Imatinib | Difference | * P-value | |
|---|---|---|---|---|
| Number | 8 | 26 | ||
| Serum Glucose | −33.6 ± 30.1 | 12.3 ± 43.6 | −45.9 | 0.007 |
| Total daily insulin (Units) | −33 ± 30.0 | 5.3 ± 19.2 | −38.3 | 0.30 |
| Other antihyperglycemic medications | 0 ± 0.0 | 0.04 ± 0.45 | −0.04 | 0.66 |
| Weight (kg) | −2.8 ± 4.71 | 3.2 ± 9.30 | 60 | 0.02 |
| Body mass (%) | −2.7 ± 3.8 | 3.6 ± 8.9 | 63 | 0.02 |
P-values obtained using a 2-tailed two-sample t-test assuming unequal variances for continuous variables, or Fisher’s exact test for categorical variables
Table 6.
Hemoglobin A1c and Other Glycemic Indices After One Year of TKI Therapy, Excluding Prior TKI Therapy
| Dasatinib | Imatinib | Difference | * P-value | |
|---|---|---|---|---|
| Number | 9 | 22 | ||
| Hemoglobin A1c (%) | −1.08 ± 1.09 | 0.06 ± 1.25 | −1.14 | 0.02 |
| Total daily insulin (Units) | −22.0 ± 28.4 | −0.6 ± 15.3 | −21.4 | 0.32 |
| Other antihyperglycemic medications | −0.1 ± 0.3 | −0.1 ± 0.5 | 0.0 | 0.91 |
| Weight (kg) | −5.0 ± 8.1 | 2.8 ± 10.1 | 7.9 | 0.04 |
| Body mass (%) | −5.0 ± 0.9 | 3.2 ± 9.6 | 8.3 | 0.03 |
P-values obtained using a 2-tailed two-sample t-test assuming unequal variances for continuous variables, or Fisher’s exact test for categorical variables
A simple linear regression (Figure 2) was used to evaluate the association between change in body weight with changes in either serum glucose or hemoglobin A1c for the dasatinib and imatinib groups separately. The mean glucose change was 0.42 mg/dL (95% CI: −5.85 to 6.69) per kg decrease in weight in the dasatinib group but was 2.44 mg/dL (95% CI: 0.75 to 4.13) per kg in the imatinib group. The between-group difference did not reach statistical difference (P=.49). The hemoglobin A1c decrease was 0.10% (95% CI: 0.04 to 0.17) per kg weight loss in the dasatinib group, which was similar to the value of 0.08% (95% CI: 0.04 to 0.13) seen in the imatinib group (P=.63).
Figure 2.
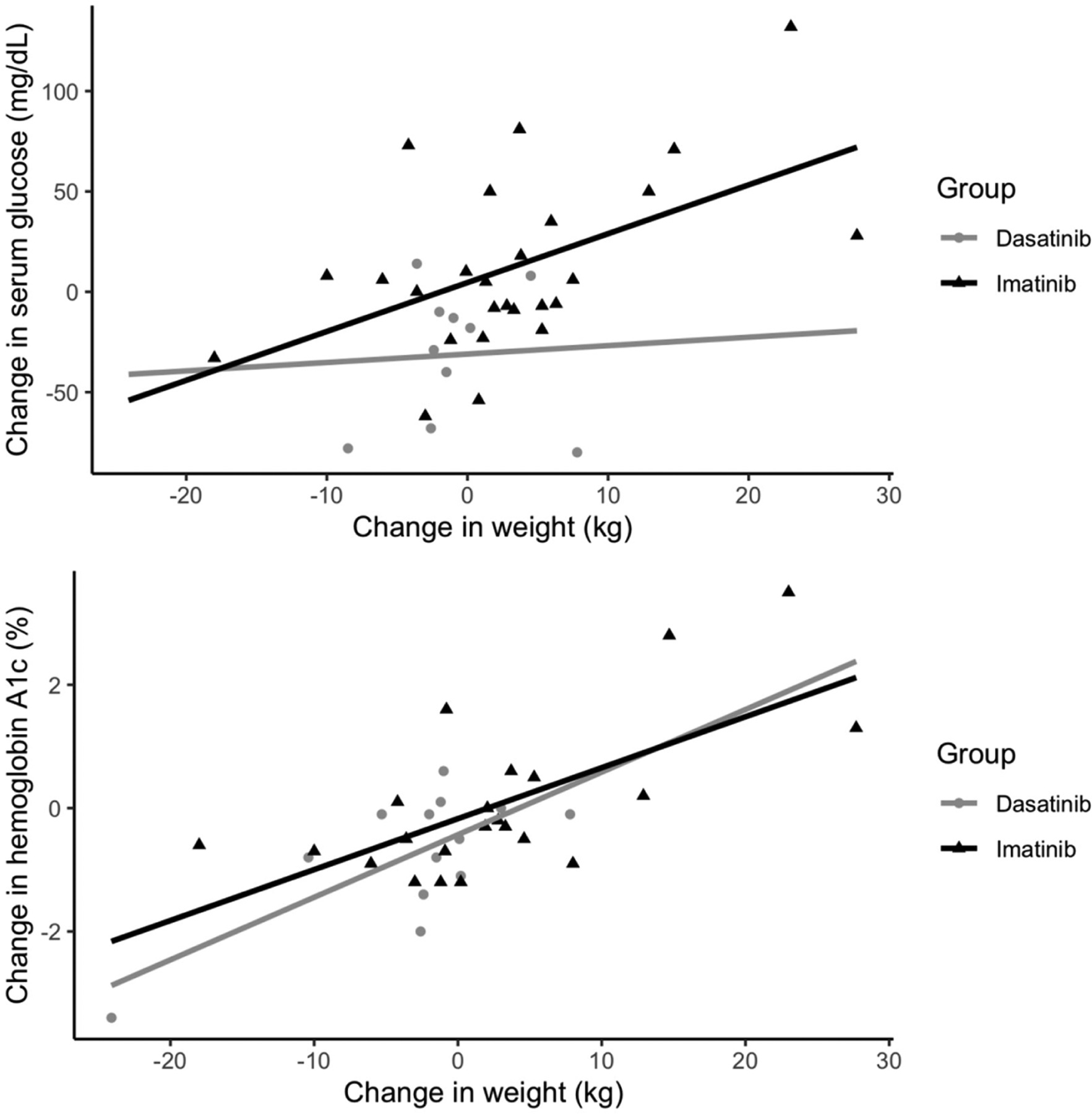
Association Between Change in Serum Glucose or Hemoglobin A1c and Change in Weight in the Dasatinib and Imatinib Cohorts
Discussion
In this study we show that dasatinib, when compared to imatinib, lowers serum glucose values in patients with pre-existing T2DM by almost 45 mg/dL, with perhaps a reduction of total daily insulin requirements (−28.2 daily units, P=0.08) and absolute reduction of hemoglobin A1c values (−0.80%, P=0.05). This hemoglobin A1c decrease is quantitatively comparable to that which occurs with the use of commonly used hypoglycemic agents when used as first-line agents such as metformin or sulfonylureas, which typically lower hemoglobin A1c values by 1–2%, thiazolidinediones by 0.5–1.4%, Glucagon-like peptide-1 receptor agonists by 0.5–1.5%, sodium-glucose co-transporter-2 receptor antagonists by 0.5–0.7%, and Dipeptidyl peptidase-4 inhibitors by 0.5–0.8% [16]. Imatinib was chosen to be the control group for the following reasons: (1) like dasatinib, it is a tyrosine kinase inhibitor, (2) it is used in the treatment of similar malignancies, and (3) compared to dasatinib, it represents the less efficacious end of the spectrum of senolytic agents.
The improved glycemic control in patients treated with dasatinib compared to imatinib was in the context of a relative −4.80 kg (P=.045) weight loss after one year, or −5.3% of total body weight. There was no statistically significant difference in relative weight change seen for the reduction in hemoglobin A1c. Weight loss is key to improving glycemic control and intentional weight loss of 10% has been shown to reduce hemoglobin A1c by 0.81% (or about 23 mg/dL) in patients with T2DM [17]. Our linear regression analysis (Figure 2) comparing change in glucose to change in weight for the dasatinib and imatinib groups suggests the relative difference in weight accounts for only 8.4 mg/dL of the 43.7 mg/dL blood glucose value decrease, or 19.2%.
The magnitude of improvement in glycemic control in response to treatment with TKIs is not well established in the current literature. A previous retrospective cohort study found that dasatinib treatment lowers mean serum glucose values by 52 mg/dL regardless of T2DM status [9], whereas prior case reports have shown improvements including remission of pre-existing T2DM [5, 6, 8, 17]. Our findings of an absolute improvement of serum glucose values by 31.4 mg/dL, including 2/4 patients who no longer required insulin within 12 months of dasatinib initiation, is consistent with prior literature on the anti-diabetic properties of dasatinib. Retrospective cohort studies are conflicting regarding the impact of imatinib on glycemic control, with some reporting a modest improvement in serum glucose [9 mg/dL] [9] and other studies showing no improvement [11, 12, 19]. These observations are consistent with our finding of an absolute increase in serum glucose of 12.3 mg/dL in patients treated with imatinib. It is also important to note that the few cohort studies that examined the impact of TKI treatment on glycemic control included heterogeneous malignancies, small patient populations with and without T2DM, followed patients for a variable duration, and did not control for longitudinal weight changes. Our study utilized the largest cohort of T2DM patients receiving TKI treatment and controlled for weight change.
There are several limitations to our study. First, included subjects had heterogeneous malignancy indications at baseline for the selection of two TKI therapies and underlying disease aggressiveness was not directly measured. This was mitigated by our requirement for one year of continuous TKI therapy, a requirement that excluded subjects who died within one year of TKI initiation or who developed disease progression that would have prompted a change in TKI therapy. Second, 5/16 individuals in the dasatinib group received prior TKI therapy. Given prior reports of a class anti-diabetic effect of TKIs [20], this may have underestimated the magnitude of a pure dasatinib effect and therefore increases the robustness of our findings regarding the potential anti-diabetic effects of dasatinib. This is supported in our sensitivity analysis, which excluded 5 subjects with prior TKI use. Third, non-pharmacologic interventions to improve glycemic control, such as exercise and dietary changes, could not be directly measured. Instead, weight was considered a reasonable proxy. Fourth, serum glucose and hemoglobin A1c levels were sometimes lacking, creating smaller sub-cohorts. Finally, this study was of a single health care system which limits external generalizability.
Potential confounders included changes in oral antihyperglycemic agent and management of malignancy. Changes in oral antihyperglycemic medications were negligible. Only 1/16 patients treated with dasatinib had a change (from 1 to 0 oral meds). 7/32 patients treated with imatinib had a change (3 patients discontinued medications, one of which discontinued 2 medications; 4 patients started new medication; with a net change of 0). Another potential confounder is management of malignancy that could affect weight, especially edema, which is a known adverse reaction to tyrosine kinase inhibitors (particularly dasatinib). In our cohort, we do not suspect it played a major role, since clinically significant edema is an indication for a change in therapy.
The potential mechanism(s) behind the anti-diabetic effects of TKIs remains an area of continuing investigation. Potential mechanisms based on in vivo and in vitro studies suggest the following: (1) Abelson tyrosine kinase (c-Abl) inhibition increases β-cell survival; (2) platelet-derived growth factor receptor (PDGFR) and epidermal growth factor receptor (EGFR) inhibition improves insulin sensitivity; and (3) inhibition of vascular endothelial growth factor receptor 2 (VEGFR2) reduces insulitis [20]. Anti-diabetic effects from multiple TKIs may be a result of influences on one or more of these various targets.
Apart from these potential roles for TKIs in modulating glycemia, increasing evidence from animal models suggests that cellular senescence may be a key driver of T2DM. Excessive caloric intake in mice to induce T2DM resulted in the generation of increased senescence factors, including upregulation of p53 and insulin resistance [21]. Likewise, hyperglycemia itself was found to cause cellular senescence in the renal tubules in a Type 1 Diabetes Mellitus mouse model [22]. Further, mice with T2DM that were treated with senolytic agents (dasatinib and quercetin) had reduced cellular senescence markers and improved glucose tolerance in addition to enhanced insulin sensitivity [14]. These findings suggest the anti-diabetic effects seen with certain TKIs may be due to their senolytic properties. Indeed, this would also explain why the strong senolytic agent dasatinib would produce greater glycemic control compared to the much weaker senolytic agent imatinib [15]. In addition, it would also suggest that the combination of two agents with senolytic activity, dasatinib and quercetin, might have even greater anti-diabetic effects in humans with T2DM than treatment with dasatinib alone. If determined to be true, this raises the intriguing possibility that senolytic treatment either alone or in combination might only need to be given intermittently, akin to a “hit and run” approach, since repopulation of senescent cells after clearance in response to senolytic exposure is expected to occur over the order of weeks [23].
While current senolytics such as dasatinib and quercetin are limited by cost and side effect profile, safer and improved drugs targeting senescence are needed. The natural flavonoid fisetin has been shown to have similar senolytic properties to dasatinib and quercetin in progeroid and aged mice [24]. If the glycemic benefit of dasatinib is indeed though senescence clearance, then fisetin is an attractive alternative senolytic in future clinical trials.
Conclusion
This retrospective cohort study shows that dasatinib used for the treatment of malignancy may have an anti-diabetic effect comparable to, or perhaps even greater than, contemporary anti-diabetic medications in patients with pre-existing T2DM. Our findings suggest that dasatinib or related senolytic drugs may become novel diabetic therapies. Future studies are needed to determine if these findings can be translated to patients with T2DM but without underlying malignancy. In addition, further investigation is also needed to determine whether the anti-diabetic effect of dasatinib is due primarily to its senolytic properties. If so, the effectiveness of combining senolytic drugs, such as dasatinib and quercetin, for the treatment of T2DM could be greater than treatment with dasatinib alone.
Acknowledgements
This publication was supported by the National Center for Advancing Translational Sciences (NCATS; UL1 TR002377) and the Robert and Arlene Kogod Professorship in Geriatric Medicine (to RJP), the National Institute on Aging (R37 AG13925), the Noaber Foundation Professorship in Aging, the Connor Group, and the Robert J. and Theresa W. Ryan Foundation (to JLK) as well as the Travelers Chair in Geriatrics and Gerontology (to GAK).
This publication was supported by the National Center for Advancing Translational Sciences (NCATS; UL1 TR002377) and the Robert and Arlene Kogod Professorship in Geriatric Medicine (to RJP), the National Institute on Aging (R37 AG13925), the Noaber Foundation Professorship in Aging, the Connor Group, and the Robert J. and Theresa W. Ryan Foundation (to JLK) as well as the Travelers Chair in Geriatrics and Gerontology (to GAK). This work was not supported in any way by pharmaceutical companies, private industry, or other for-profit agencies.
Abbreviations and Acronyms
- ALL
acute lymphocytic leukemia
- CML
chronic myelogenous leukemia
- GIST
gastrointestinal stromal tumor
- T2DM
Type 2 Diabetes Mellitus
- TKI
tyrosine kinase inhibitor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Per journal policy, all COIs were declared at the time of manuscript submission. JLK has the most relevant financial interest related to this paper: patents on senolytic drugs are held by Mayo Clinic. Research findings related to those patents that are cited in this paper were previously reviewed by the Mayo Clinic Conflict of Interest Review Board and are in compliance with Mayo Clinic Conflict of Interest policies.
References
- 1.IDF Diabetes Atlas http://www.diabetesatlas.org/ (Accessed on April 1, 2020).
- 2.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2020. [Google Scholar]
- 3.Breccia M, et al. Imatinib mesylate may improve fasting blood glucose in diabetic Ph+ chronic myelogenous leukemia patients responsive to treatment. J. Clin. Oncol, 22 (2004), pp. 4653–4655 [DOI] [PubMed] [Google Scholar]
- 4.Veneri D, et al. Imatinib and regression of type 2 diabetes. N. Engl. J. Med, 352 (2005), pp. 1049–1050 [DOI] [PubMed] [Google Scholar]
- 5.Breccia M, et al. Fasting glucose improvement under dasatinib treatment in an accelerated phase chronic myeloid leukemia patient unresponsive to imatinib and nilotinib. Leuk. Res, 32 (2008), pp. 1626–1628 [DOI] [PubMed] [Google Scholar]
- 6.Huda MS, et al. Tyrosine kinase inhibitor sunitinib allows insulin independence in long-standing type 1 diabetes. Diabetes Care, 37 (2014) pp. e87–e88 [DOI] [PubMed] [Google Scholar]
- 7.Brooks MB. Erlotinib appears to produce prolonged remission of insulin requiring type 2 diabetes associated with metabolic syndrome and chronic kidney disease. Br. J. Diabetes Vasc. Dis, 12(2012), pp. 87–90 [Google Scholar]
- 8.Iizuka K, et al. Dasatinib improves insulin sensitivity and affects lipid metabolism in a patient with chronic myeloid leukaemia. Case Reports 2016; 2016; bcr2015214284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agostino NM, et al. Effect of the tyrosine kinase inhibitors (sunitinib, sorafenib, dasatinib, and imatinib) on blood glucose levels in diabetic and nondiabetic patients in general clinical practice. J. Oncol. Pharm. Pract, 17 (2011), pp. 197–202 [DOI] [PubMed] [Google Scholar]
- 10.Breccia M, et al. How tyrosine kinase inhibitors impair metabolism and endocrine system function: a systematic updated review. Leuk Res, 38 (2014), pp. 1392–1398 [DOI] [PubMed] [Google Scholar]
- 11.Dingli D, et al. Imatinib and type 2 diabetes. Endocr. Pract, 2007; 13:126–30 [DOI] [PubMed] [Google Scholar]
- 12.Mariani S, et al. Imatinib does not substantially modify the glycemic profiles in patients with chronic myeloid leukoaemia. Lek. Res, 2010; 34:e5–7 [DOI] [PubMed] [Google Scholar]
- 13.Palmer A, et al. Cellular senescence in type 2 diabetes: a therapeutic opportunity. Diabetes 2015; 64:2289–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmer A, et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell. 2019; 18:e12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, et al. (2015). The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell, 14:644–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nathan DM, Buse JB, et al. Medical Management of Hyperglycemia in Type 2 Diabetes: A Consensus Algorithm for the Initiation and Adjustment of Therapy: A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32;193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shantha G, et al. (2012) Association between glycosylated hemoglobin and intentional weight loss in overweight and obese patients with type 2 diabetes mellitus: a retrospective cohort study. Diabetic Education. 38:417–426 [DOI] [PubMed] [Google Scholar]
- 18.Ono K et al. (2012) Rapid amelioration of hyperglycemia facilitated by dasatinib in chronic myeloid leukemia patient with type 2 diabetes mellitus. Intern. Med 51, 5763–2766 [DOI] [PubMed] [Google Scholar]
- 19.Chodorowski Z, et al. (2007) No influence of imatinib on type 2 diabetes. Przegl. Lek 64 (370–371) [PubMed] [Google Scholar]
- 20.Fountas A, et al. (2015) Tyrosine Kinase Inhibitors and Diabetes: A novel treatment paradigm? Trends in Endocrinology and Metabolism. 11: 643–656 [DOI] [PubMed] [Google Scholar]
- 21.Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, et al. (2009). A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat. Med 15 1082–1087. 10.1038/nm.2014 [DOI] [PubMed] [Google Scholar]
- 22.Kitada K, Nakano D, Ohsaki H, et al. Hyperglycemia causes cellular senescence via a SGLT2- and p21- dependent pathway in proximal tubules in the early stage of diabetic nephropathy. J Diabetes Complications. 2014;28:604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pignolo RJ, Passos JF, Khosla S, Tchkonia T, Kirkland JL (2020). Reducing senescent cell burden in aging and disease. Trends in Molecular Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yousefzadeh MJ, Zhu Y, McGowan SJ, et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018;36:18–28. doi: 10.1016/j.ebiom.2018.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]


