Abstract
Background:
Physical frailty is defined as a syndrome of decreased physiologic reserve conferring vulnerability to functional decline, mortality and other adverse outcomes upon experiencing stressors. Self-efficacy, which is confidence in one’s ability to perform well in a domain of life, is modifiable. Self-efficacy is associated with improved health behavior and decreased chronic disease burden. Its relationship to frailty is unknown. The purpose of this study was to evaluate whether a general self-efficacy proxy predicts incident frailty.
Methods:
A nationally-representative sample of 4,825 US older adults aged 65 and older living in the community or non-nursing home care setting enrolled in the National Health and Aging Trends Study from 2011–2018 was used. Self-efficacy was dichotomized into low and high groups using the one-item self-efficacy proxy measure. The Physical Frailty Phenotype was used to categorize participants as frail and non-frail. A discrete time hazard model using data from 8 rounds was used to obtain incident hazard ratios of frailty in two models. Model 1 was adjusted for age, race, sex, education and income. Model 2 contained Model 1 covariates and added disability and co-morbidities.
Results:
Among people without frailty at baseline, risk of developing frailty over 7 years was increased by 41% among those with low vs. high self-efficacy after adjustment for socio-demographics (P=0.002), and by 27% after further adjustment for disability and co-morbidities (P=0.032).
Conclusion:
This study generates a rationale to further explore self-efficacy in frailty research. Self-efficacy may be a key modifiable element to incorporate into multi-modal physical frailty interventions.
Keywords: Healthy Aging, Personal Mastery, Behavior Change, Stress Response
INTRODUCTION
Physical frailty is conceptualized as a syndrome of decreased physiologic reserve conferring vulnerability to adverse consequences from stressors,1,2 resulting in increased disability3 and increased all-cause mortality.4,5 Physical frailty is distinguished from other notable frailty constructs by its theorized underlying biological basis as opposed to a composite risk index of functional, medical and social deficits (in the deficit accumulation model of frailty).6 The construct of physical frailty is commonly operationalized using the Physical Frailty Phenotype which assesses frailty using five criteria: weakness, slowness, low activity, exhaustion, and weight loss.7 Approximately 15% of U.S. older adults living outside nursing homes are physically frail, with increased prevalence in women, racial and ethnic minority communities, older individuals, and those with lower socio-economic status.8,9
Although physical frailty focuses on the biomedical changes underlying decreased physiologic reserve, the cycle of frailty described by Fried et. al.9 also accounts for personal and environmental characteristics, like stressful life events, under-nutrition and decreased physical activity that contribute to the acquisition and/or perpetuation of its clinical state. Accordingly, self-efficacy, which is confidence in individual performance in specific domains, may interact with the cycle of frailty in two distinct ways. (Figure 1) First, self-efficacy may directly affect the body’s physiologic responses within the stress response networks.10–12 Second, self-efficacy beliefs influence the adoption and maintenance of healthy behaviors,13,14 making self-efficacy an instrumental part of behavior change. In older adults, increases in self-efficacy are associated with improved health behavior.15 General self-efficacy scales assess a global domain of beliefs to successfully achieve broad life goals despite obstacles.16 Of particular interest, self-efficacy is malleable, meaning that it can improve.17 This makes improvements in self-efficacy a potential target for physical frailty multi-modal interventional design and measurement.
Figure 1.
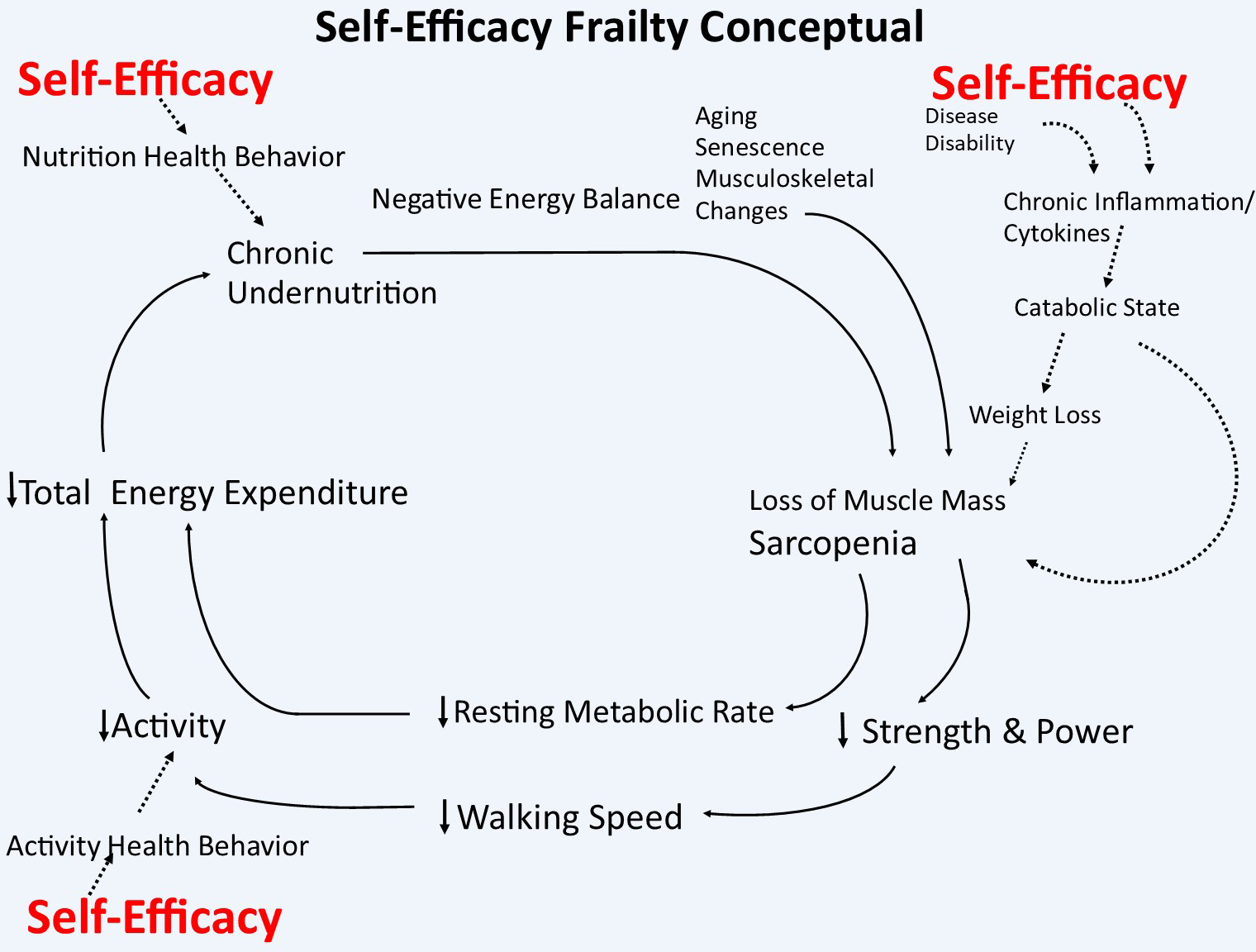
Self-Efficacy Contributions to the Cycle of Physical Frailty (adapted from the Fried Walston Physical Frailty Framework, used with permission).
There is limited research on the relationship between self-efficacy and frailty. Using other frailty constructs (like clinician rapid screening or a deficit accumulation index), higher self-efficacy was associated with greater physical functioning18 and lower odds of functional decline19 in those with frailty. Regarding the Fried physical frailty phenotype, some researchers have assessed the impact of exercise self-efficacy on the physical function of physically frail older adults.20,21 These studies, however, did not evaluate the direct effect of self-efficacy on physical frailty, apart from physical function. Our previous work showed an association between coping self-efficacy and physical frailty but was limited by a cross-sectional design, small sample size and, self-reported frailty measure.22 This current study overcomes previous limitations using a nationally representative sample, longitudinal design and a well-established measure of physical frailty to explore a possible prospective relationship between self-efficacy and physical frailty. Therefore, the purpose of this study was to evaluate the general self-efficacy proxy measure available in the National Health and Aging Trends Study (NHATS) as a potential predictor of physical frailty incidence over 7 years from 2011–2018.
METHODS
Sample and Design
Data were drawn from NHATS that enrolled 8,245 participants at baseline in 2011.23 Subsequent visits were conducted annually through 2018 with an average of 12.22 months between visits. Study participants were identified using a stratified probability sampling technique from Medicare beneficiaries ages 65 years and older to create a nationally representative sample by age group and race/ethnicity. All NHATS participants completed an informed consent, and the study was approved by the Institutional Review Board at Johns Hopkins University. Inclusion criteria for this analysis required baseline assessment of frailty and self-efficacy measures. Exclusion criteria were frailty at baseline and residence in a nursing home. Our initial study sample included all participants living in the community or non-nursing home residential care settings who completed an in-person assessment (n=7,609) at baseline. We removed 319 participants without self-efficacy measurement or who had no follow-up frailty data beyond baseline (n=1006) resulting in an analytic sample of 4,825. (Figure 2.) Supplementary Table S1 compares baseline characteristics of study sample (N=4,825) to those excluded (N=1,006) for no follow-up frailty information or death between rounds 1 and 2 of data collection. Excluded participants were more likely to have lower incomes and education and identify as non-white.
Figure 2.
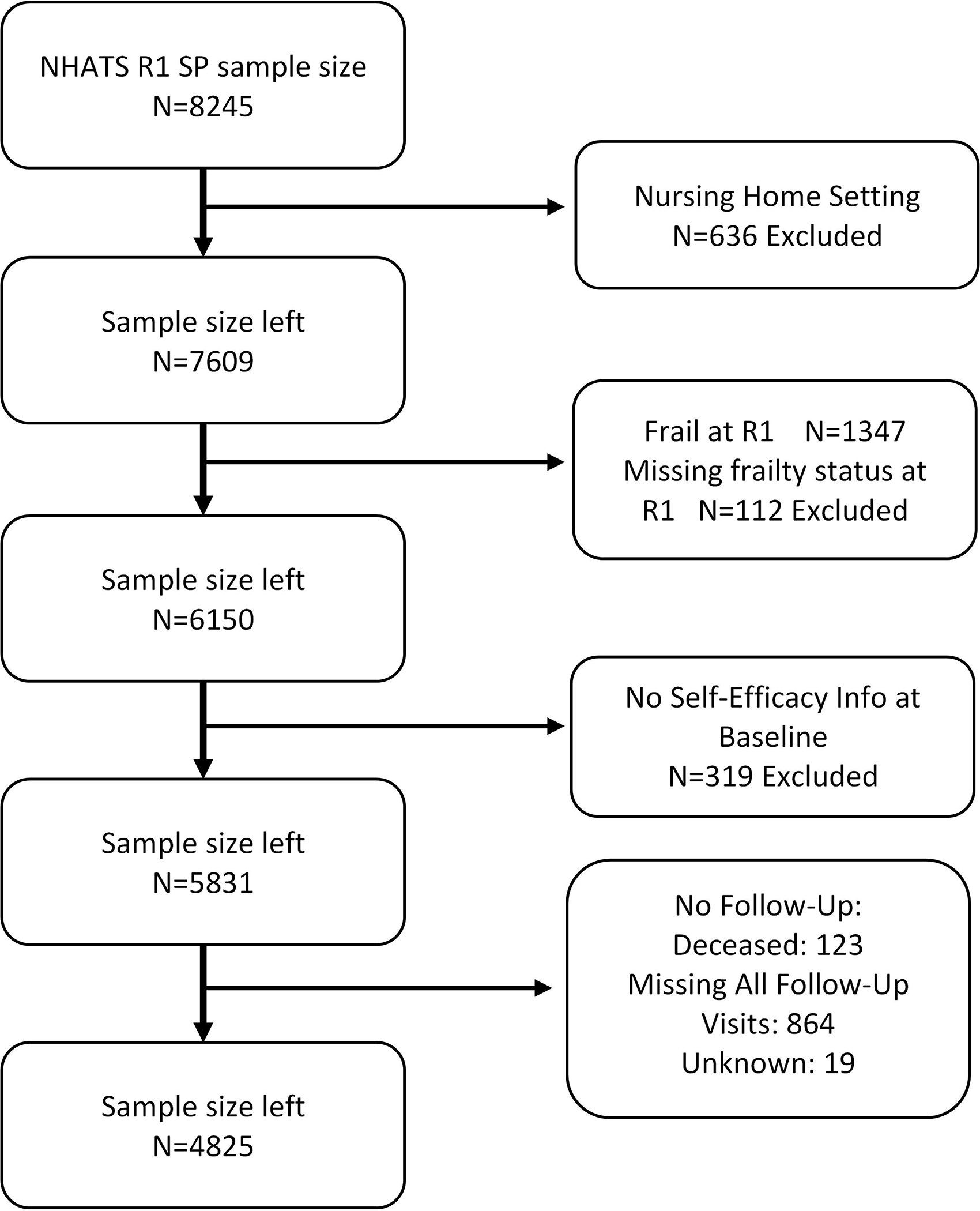
Flow Chart for Final Sample Size
Notes: R1: Round 1
Measures
Self-Efficacy Proxy:
We used the following self-reported statement as our self-efficacy proxy measure: “When I really want to do something, I usually find a way to do it.” This question was drawn from the NHATS Well-Being questionnaire. It was a general question left open to interpretation, not specific to any health behavior. Originally, there were three response options: agree a lot, agree a little and agree not at all. The measure was dichotomized into low and high general self-efficacy because only 1% of participants answered “agree not at all”, which made dichotomizing the sample to combine the “agree not at all” and “agree a little” groups appropriate for statistical analysis. Participants who “agree a lot” were categorized as having high self-efficacy; those who “agree a little” or “agree not at all” were categorized as having low self-efficacy. The NHATS Well-Being module, including the self-efficacy question, was not completed if the forms were completed by a proxy.
Frailty:
Frailty was assessed using the Physical Frailty Phenotype (PFP).1,2 The PFP includes five criteria: unintentional weight loss; slowness; weakness; low physical activity; and exhaustion. We used the same measures and cutoffs for PFP here as have been used in previous NHATS studies.8,9 In summary, unintentional weight loss was defined by self-reported unintentional loss of 10 pounds or more in the last year or by a body mass index (BMI) less than 18.5kg/m2. Participants met the low physical activity criterion by self-report of having not walked for exercise or engaged in vigorous activities recently. Exhaustion was defined by self-report of having low energy or being easily exhausted recently. Participants met the slowness criterion if their walking speed (m/s) was at or below the 20th percentile of the weighted study population using four sex and height categories. Slowness was measured over 3 meters, using the first of two trials as has been done in previous studies.8 The weakness criterion was defined as a grip strength (kg) at or below the 20th percentile using eight sex and BMI categories; a maximal value from two dynamometer tests of the dominant hand was used. Participants who did not complete walking speed or grip strength tests due to safety reasons, recent surgery or hand pain, or who attempted but could not complete these tests were coded as having met slowness or weakness criteria. We dichotomized our frailty measure such that participants who met 0–2/5 criteria were categorized as not-frail and those who met 3+/5 criteria were categorized as frail. We opted to dichotomize frailty to evaluate both the direct and indirect effect of self-efficacy status on incident frailty. 112 participants were missing 3 or more criteria for frailty measurement and were excluded (See Figure 2).
Demographics:
We considered age (categorized in 5-year increments between ages 65–89, and a category of 90+ years old); race/ethnicity (White, non-Hispanic; Black, non-Hispanic; Hispanic, Other); sex; education (less than 8th grade completed; 9th –12th grade but no diploma; high school diploma; greater than high school diploma); and income (categorized as <15k, 15k-30k, 30k-60k, >60k annually) at baseline.
Medical conditions:
Self-report of the following diseases were added together to create a comorbid disease count: arthritis, cancer, diabetes, heart disease, hip fracture, hypertension, lung disease, osteoporosis, and stroke. Co-morbidities were measured continuously.24,25
Disability:
A late-life hierarchical disability scale was used to assess functional limitations, drawing from self-care measures in the NHATS survey.26,27 Functional status was defined by self-reported activities of daily living (ADLs; including using the toilet, getting cleaned up, dressing and eating) and results were reported on an ordinal scale including: fully able; use of accommodations or reduce frequency of activities; difficulty with activities; or need of assistance with activities.
Depression:
The Patient Health Questionnaire (PHQ-2) 2-item screening measure was used to screen for depressive symptoms.28 Results were dichotomized with 3 or greater indicating a positive depression screen for our sensitivity analysis.
Statistical Analysis & Model Building
Baseline demographic and health characteristics were summarized for the entire sample and compared between those with low vs high self-efficacy using two-sample two-sided t-test for continuous variables and chi-square test for categorical variables. We used discrete-time Cox proportional hazards models to evaluate the association between self-efficacy and the first incidence of frailty during the seven-year follow-up (2011–2018) among study subjects who were not frail in 2011.29 Subjects were considered censored if they did not develop frailty, were lost to follow-up or died. The discrete-time Cox model was chosen instead of the conventional Cox proportional hazards model because frailty status was only ascertained at each annual visit in NHATS, resulting in incidence data being grouped into time intervals defined by the visits. Model parameters were estimated using standard generalized linear model with binomial distribution and a complementary log-log link. The interpretation of the parameters is analogous to that of a continuous-time Cox model. To adjust for confounding, we fit two nested models. Model 1 was adjusted for age, race/ethnicity, sex, education and income. Model 2 contained Model 1 covariates and disability and comorbid disease burden. We believe that disability may mediate the relationship between the general self-efficacy proxy measure and frailty. For that reason, Model 1 is the primary model in order to estimate the independent total effect of general self-efficacy on frailty incidence after accounting for sociodemographic confounders. We also evaluated potential mediation of pre-frailty in these models. Given the negative correlation between depressive symptoms and self-efficacy30,31 as well as the overlap in measurement between frailty and depression23, we chose to examine our findings with and without those with depressive symptoms as a sensitivity analysis. Likewise, we also performed a sensitivity analysis with the original self-efficacy variable with three response items in our model.
In the current study, we observed that 32% of subjects with high self-efficacy and 44% of subjects with low self-efficacy developed frailty during the 7-year follow-up. The total sample size of 4,825, of which 4,417 were in the high self-efficacy group and 408 were in the low self-efficacy group, was able to detect with at least 0.81 power of a hazard ratio of 1.29 or greater. This is based on a two-sided test at a 0.05 significance level using Cox proportional hazards regression.
RESULTS
At baseline, our study sample of 4,825 non-frail adults were older (mean 74.4 +/− 6.9), predominantly white (84%) women (55%) with a high school education or greater (56%) and an income of 30K or greater (58%). 10% reported difficulty or needing assistance with ADLs and the mean number of diseases was 2.2 (SD=1.4) Among our baseline non-frail participants, 48% were robust and 52% were pre-frail (Table 1).
Table 1.
Sample Demographic and Health Characteristics
| Variable | Total Observations N=4825 | High Self-Efficacy N=4417 (92%*) | Low Self-Efficacy N=408 (8%*) | P-value** | |
|---|---|---|---|---|---|
| Baseline Age | 0.003 | ||||
| 65–69 | 1011 (31%) | 937 (31%) | 74 (27%) | ||
| 70–74 | 1100 (26%) | 1026 (27%) | 74 (22%) | ||
| 75–79 | 1016 (19%) | 920 (19%) | 96 (23%) | ||
| 80–84 | 926 (14%) | 850 (14%) | 76 (14%) | ||
| 85–89 | 479 (7%) | 430 (7%) | 49 (9%) | ||
| 90+ | 293 (3%) | 254 (3%) | 39 (5%) | ||
| Race/ Ethnicity | 0.12 | ||||
| White, non-Hispanic | 3512 (84%) | 3224 (85%) | 288 (80%) | ||
| Black, non-Hispanic | 960 (7%) | 880 (7%) | 80 (8%) | ||
| Hispanic | 236 (6%) | 206 (5%) | 30 (9%) | ||
| Other | 117 (3%) | 107 (3%) | 10 (3%) | ||
| Education | <0.001 | ||||
| < 8th grade | 446 (7%) | 387 (7%) | 59 (11%) | ||
| 9–12th No diploma | 614 (10%) | 563 (10%) | 51 (9%) | ||
| High school diploma | 1319 (27%) | 1190 (26%) | 129 (31%) | ||
| > High school | 2444 (56%) | 2275 (56%) | 169 (49%) | ||
| Sex | 0.034 | ||||
| Male | 2085 (45%) | 1929 (45%) | 156 (39%) | ||
| Female | 2740 (55%) | 2488 (55%) | 252 (61%) | ||
| Baseline Income | <0.001 | ||||
| <15k | 1180 (20%) | 1037 (19%) | 143 (30%) | ||
| 15k–30k | 1175 (22%) | 1069 (22%) | 106 (24%) | ||
| 30k–60k | 1292 (28%) | 1202 (28%) | 90 (24%) | ||
| >60k | 1178 (30%) | 1109 (31%) | 69 (21%) | ||
| Baseline Disability | <0.001 | ||||
| Fully able | 2082 (47%) | 1944 (48%) | 138 (37%) | ||
| Accommodate/reduced frequency | 2186 (43%) | 2014 (43%) | 172 (43%) | ||
| Difficulty | |198 (4%) | 163 (3%) | 35 (8%) | ||
| Need assistance | 359 (6%) | 296 (6%) | 63 (12%) | ||
| Disease Count | Mean (SD) | 2.18 (1.4) | 2.16 (1.38) | 2.42 (1.54) | <0.001 |
| Baseline Frailty | <0.001 | ||||
| Robust | 4825 (48%) | 1970 (49%) | 110 (33%) | ||
| Pre-Frail | 2745 (52%) | 2447 (51%) | 298 (67%) | ||
Notes: 1. N=4825 is number of non-frail complete cases and self-efficacy information at baseline. 2. N for education variable is 4823. 3. Chi-squared test for categorical variables and t-test for continuous variables are examined by self-efficacy. 4. Percentages, means, and SDs are weighted measures.
Weighted prevalence
P-value is testing the overall association between self-efficacy and each demographic factor.
About 9% (n=408) of the sample reported low general self-efficacy. Those with low self-efficacy were older, more likely to be female, had lower education attainment, lower income, greater comorbid disease burden, higher prevalence of difficulty and dependency with ADLs and more likely to be pre-frail as opposed to robust (Table 1).
During the seven-year follow-up (median 5 years), 33% (n=1588) of the sample developed frailty with a corresponding incidence rate of 8.6 per 100 person-years. The incidence rate was 13.2 and 8.2 per 100 person-years in low and high self-efficacy groups, respectively. For those with low self-efficacy, the median number of months to frailty onset was 46 (Range 10–88 months) compared to 52 (Range 8–89) for those with high self-efficacy. Figure 3 shows the unadjusted Kaplan Meier curve for incident frailty showing a significant difference between the low and high efficacy groups based on log-rank test (p<0.01). After adjusting for age, race, education, sex, and income (Model 1), low self-efficacy was associated with a 41% increase in the risk of developing frailty (hazard ratio (HR)=1.41; 95% confidence interval (CI)=1.15–1.73). The association remained significant after further adjustment (Model 2) for disability and comorbidity disease burden (HR=1.27; 95% CI=1.02–1.58). Older age, black (vs. white) race, greater ADL disability, and greater comorbid disease burden were independently associated with greater risk of frailty onset. On the other hand, higher education (i.e., high school diploma or higher) and higher income were associated with lower risk of frailty onset (Table 2).
Figure 3.
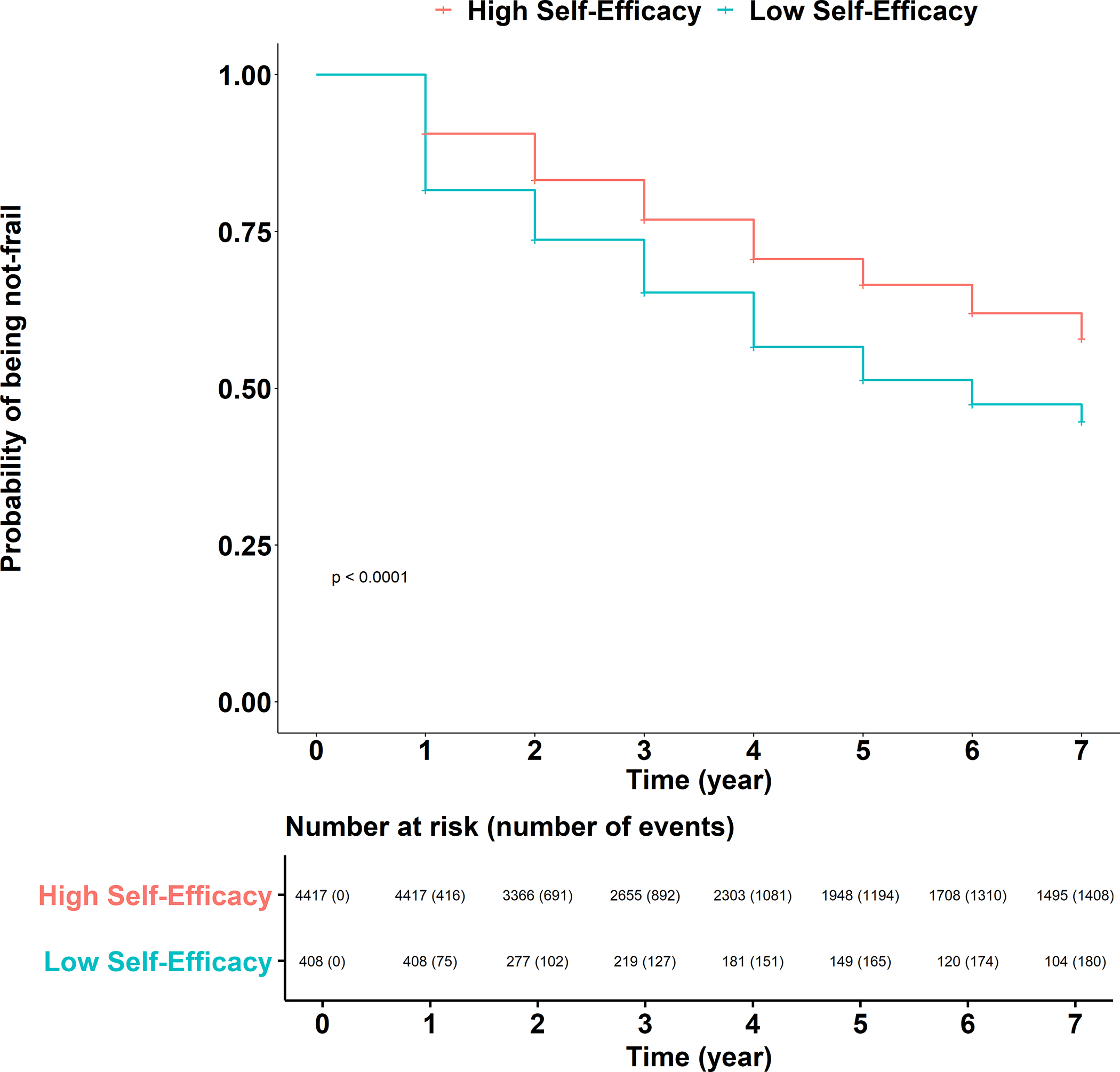
Kaplan Meyer curve for incident frailty by low versus high self-efficacy.
Table 2.
Frailty Incidence for Low vs High Self-Efficacy - weighted counts
| Model 1 (Primary) | Model 2 | ||||
|---|---|---|---|---|---|
| Variable | Hazard Ratios | P values | Hazard Ratios | P values | |
| Time intervals | 1 | ref | ref | ref | ref |
| 2 | 0.86 (0.71–1.03) | 0.103 | 0.89 (0.74–1.07) | 0.207 | |
| 3 | 0.86 (0.69–1.06) | 0.149 | 0.90 (0.72–1.12) | 0.336 | |
| 4 | 0.96 (0.79–1.16) | 0.636 | 1.03 (0.85–1.25) | 0.757 | |
| 5 | 0.67 (0.52–0.86) | 0.002 | 0.74 (0.58–0.96) | 0.024 | |
| 6 | 0.83 (0.68–1.02) | 0.082 | 0.94 (0.76–1.16) | 0.552 | |
| 7 | 0.83 (0.63–1.08) | 0.163 | 0.95 (0.72–1.25) | 0.708 | |
| Self-Efficacy | |||||
| Low | 1.41 (1.15–1.73) | 0.002 | 1.27 (1.02–1.58) | 0.032 | |
| Demographics | |||||
| Baseline Age | 65–69 | ref | ref | ref | ref |
| 70–74 | 1.65 (1.35–2.01) | <0.001 | 1.52 (1.23–1.88) | <0.001 | |
| 75–79 | 2.21 (1.83–2.66) | <0.001 | 1.93 (1.60–2.33) | <0.001 | |
| 80–84 | 2.95 (2.42–3.59) | <0.001 | 2.51 (2.03–3.10) | <0.001 | |
| 85–89 | 4.06 (3.29–5.02) | <0.001 | 3.31 (2.63–4.16) | <0.001 | |
| 90+ | 5.27 (4.13–6.72) | <0.001 | 4.01 (3.17–5.08) | <0.001 | |
| Race/ Ethnicity | White, non-Hispanic | ref | ref | ref | ref |
| Black, non-Hispanic | 1.28 (1.10–1.50) | 0.002 | 1.28 (1.09–1.49) | 0.003 | |
| Hispanic | 1.11 (0.87–1.41) | 0.383 | 1.21 (0.95–1.55) | 0.117 | |
| Other | 1.07 (0.66–1.73) | 0.780 | 1.13 (0.72–1.80) | 0.585 | |
| Education | < 8th grade | ref | ref | ref | ref |
| 9–12th No diploma | 0.84 (0.65–1.09) | 0.193 | 0.85 (0.66–1.11) | 0.238 | |
| High school diploma | 0.69 (0.57–0.83) | <0.001 | 0.77 (0.63–0.93) | 0.009 | |
| > High school | 0.60 (0.50–0.72) | <0.001 | 0.64 (0.52–0.79) | <0.001 | |
| Sex | Male | ref | ref | ref | ref |
| Female | 1.05 (0.92–1.20) | 0.457 | 0.90 (0.78–1.03) | 0.113 | |
| Baseline Income | <15k | ref | ref | ref | ref |
| 15k–30k | 0.89 (0.76–1.04) | 0.127 | 0.86 (0.73–1.03) | 0.094 | |
| 30k–60k | 0.70 (0.58–0.84) | <0.001 | 0.73 (0.59–0.89) | 0.003 | |
| >60k | 0.56 (0.46–0.69) | <0.001 | 0.61 (0.50–0.75) | <0.001 | |
| Baseline Disability | Fully able | ref | ref | ||
| Accommodate/reduced frequency | 1.40 (1.22–1.60) | <0.001 | |||
| Difficulty | 2.41 (1.86–3.12) | <0.001 | |||
| Need assistance | 2.74 (2.22–3.39) | <0.001 | |||
| Disease Count | 1.30 (1.25–1.35) | <0.001 | |||
Notes: Model 1 is adjusted for age, race/ethnicity, sex, education and income. Model 2 is further adjusted for chronic disease count and disability.
Mediation Analysis
A mediation analysis was performed by adding pre-frailty status as a binomial covariate to our primary and secondary models. Our self-efficacy hazard ratios decreased to 1.26 (95% CI= 1.02–1.57) in our primary model and 1.18 (95%CI= 0.94–1.48) in our secondary model. The association between self-efficacy and incident frailty remained. (Supplemental Table S2.)
Sensitivity Analyses
A sensitivity analysis of participants without depressive symptoms at baseline (N= 4294) was also performed. The hazard ratios for incidence frailty changed little in magnitude from our primary Model 1 (HR= 1.32; 95% CI= 1.03– 1.69). With wider confidence intervals commensurate a 15% reduction in sample size, the association fell short of conventional levels of significance for Model 2 (HR= 1.21; CI= 0.94–1.56). (Supplemental Table S3.) We also performed a second sensitivity analysis with all three categories of our self-efficacy proxy measure, rather than a dichotomized measure. Results showed incrementally increasing hazard ratios for both Model 1 (agree a little: HR=1.38, CI=1.10–1.73 and agree a lot: HR=1.75, CI=0.96–3.19) and Model 2 (agree a little: HR=1.25, CI=0.99–1.57 and agree a lot: 1.56, CI-0.80–3.02) with much larger confidence intervals and decreases in significance due to the changes in sample size. (Supplemental Table S4.)
DISCUSSION
We found that a proxy indicator of low general self-efficacy predicted a 41% increased risk of developing frailty over 7 years after adjustment for socio-demographics and a 27% increased risk of incident frailty after further adjustment for disability and co-morbidities. In other words, after accounting for physical function, disease burden, age, race/ethnicity, sex, education and income, low self-efficacy predicted a significant increase in risk of incident frailty. These results add preliminary evidence of a possible prospective association between low general self-efficacy and incidence frailty.
There are several ways to conceptualize frailty and its relationship to self-efficacy. Researchers like Rockwood, Gobbens, and Tilberg have conceptualized frailty as an accumulation of medical, psychological and psychosocial deficits.32–34 These conceptualizations of frailty account for some psychosocial factors as a part of the measurement of frailty itself. Accounting for the larger context (e.g. financial and social) beyond the physical domain as part of an accumulation of burden that affects health is one way to approach frailty measurement.
For our purposes, we conceptualize frailty as a clinical syndrome with specific biologic and physiologic underpinnings that can be influenced mechanistically by self-efficacy (and other psychosocial and environmental factors) both directly, through an effect on the immune system via the stress response network, and indirectly, through nutritional and physical activity related health behavior. (Figure 1). Self-efficacy beliefs play an integral role in the stress appraisal process. For example, if someone believes they have the internal and external resources needed to meet a presented challenge (i.e. new diagnosis or recent fall, etc.), they do not perceive the presented challenge as overwhelming and likely elicit less of a physiologic response to stress. The stress response networks directly feed into the cycle of frailty through the promotion of inflammation and hypothalamic pituitary adrenal axis dysregulation.35–38
Likewise, frailty development includes nutrition and physical activity as important contributing behaviors. Self-efficacy is an integral part of integrating and maintaining those behaviors.39–42 McAuley et.al. (2007) found that exercise self-efficacy predicted higher levels of physical activity at 2 years and 5 years following a 6 month RCT for exercise.43 Delahanty et.al. (2013) showed that self-efficacy associated with healthy diet predicted better long-term weight loss in the Diabetes Prevention Program RCT.44
Self-efficacy beliefs can be task or domain specific, like exercise self-efficacy,17 or broad, like coping self-efficacy.45 Although our study evaluated a general self-efficacy proxy measure, there are inter-domain relationships between perceived efficacies that share similar higher-order self-regulatory skills.46 In other words, increasing self-efficacy in one area (e.g. exercise self-efficacy) has the potential to influence self-efficacy in other domains (e.g. general self-efficacy). Evaluation of the mechanistic pathways through the stress response networks and health behavior as partial mediators of the self-efficacy frailty relationship was beyond the scope of this study but will be an important next step in this research.
Ultimately, our intent was to identify malleable risk factors for physical frailty to design tailored interventions. A strength of self-efficacy is its malleability. Self-efficacy is influenced by four distinct mechanisms including personal mastery of a skill, vicarious experience, verbal persuasion, and physiologic feedback.17 Each of these mechanisms can be influenced through interventions.
Our results extend earlier findings that frailty prevalence is higher in women, racial and ethnic minority populations, older age, greater disease and disability burden, lower education and lower income, by reporting that these same factors augur heightened frailty incidence.8,9 Our findings add to the literature that low general self-efficacy is associated with many of these same covariates such as older age, female sex, lower education and income, greater disability, disease burden and pre-frail status compared to robust status (Table 1).
Self-efficacy and depression have a bi-directional relationship.30,31 A depressed mood can result from a perception of low self-efficacy in a domain(s) of life, and therefore may mediate the relationship of self-efficacy and frailty. At the same time, a depressed mood can depress future self-efficacy gains due to possible overarching helplessness or hopelessness to change. Because self-efficacy and depression are related in these ways and because depression and physical frailty may share fatigue and other psychosomatic symptoms, we did not include depressive symptoms in our primary models. We performed a sensitivity analysis removing participants with depressive symptoms to evaluate the effect of self-efficacy on incident frailty apart from depressive symptoms. The influence of depressive symptoms on estimated associations of our general self-efficacy proxy measure with incident frailty were similar in both models.
This study has limitations. First, we used a general self-efficacy measure, which was the best available option in the NHATS dataset. Some argue that the best way to measure self-efficacy is within a specific domain, like coping or exercise because the action related to self-efficacy is usually related to a task or domain. There is precedence, however, in a more general self-efficacy. It is widely used in research and is predictive of multiple health outcomes as well as decreased mortality/ morbidity.47,48 We chose to conceptualize general self-efficacy as a type of global coping with life. A second limitation is that self-efficacy beliefs are optimally evaluated by measuring both the depth and range of the domain including emotional, cognitive and behavioral aspects of efficacy beliefs. In this study, we used a one item measure, which does not allow us to explore the complexity of the domain. This shortcoming of the measure led us to refer to it as a “proxy” of self-efficacy, approximating the content validity needed to more fully capture general self-efficacy. Though this one-item measure does not encompass the full-dimensionality of the concept of general self-efficacy,16 it provides an overall assessment of a person’s belief that they have the capacity to carry behavior(s) deemed needed to attain a specific goal. Lastly, those excluded from the study analysis due to death or lack of follow-up were more likely to have low self-efficacy, identify as non-White with lower education and income parameters (Supplemental Table S1). The attrition of non-White participants and those with lower socio-economic status has been well documented as an important problem in longitudinal research.49,50 These participants also may be at higher risk of low self-efficacy. Likewise, our decision to exclude those in a nursing home setting may have also led to a lower estimation of how self-efficacy influences frailty incidence. Lastly, there may also be unmeasured confounding factors that influenced the relationship between this self-efficacy proxy measure and frailty in this sample.
The strengths of this study are also worth noting. The sample size is large and generalizable to the U.S. older adult population. Our analysis was conducted over a 7-year time frame allowing us to capture incident frailty in one-year increments to further characterize the self-efficacy- physical frailty relationship. The frailty measure used is the current widely accepted measure of physical frailty.7 We present two nested models for covariate adjustment, i.e., with vs. without disability and disease burden, both of which identified the general self-efficacy proxy measure as a significant predictor of incident physical frailty in our overall sample.
In our model, we found that having this low self-efficacy proxy measure provided an increase in risk of developing frailty equivalent to one additional chronic disease. Those with low self-efficacy had the same level of incident frailty (by months) as those 85 years old and older. In other words, for a 65 year-old, having low self-efficacy was equivalent to the age-associated frailty incidence of an 85 year old. This result compels us to look more closely at the relationship between physical frailty and self-efficacy.
From a policy perspective, further exploration of how self-efficacy affects frailty progression is warranted because it offers the potential of a non-pharmacological tool in multi-modal physical frailty interventional design. These findings inform the physical frailty research agenda in four ways. First, validated self-efficacy measures should be considered for inclusion in longitudinal studies in order to more comprehensively understand the relationship between self-efficacy and physical frailty. Future analyses should look at modelling change in self-efficacy over time and associations with incident frailty. Second, researchers involved in preventing physical frailty should consider using a self-efficacy-based theoretical framework to inform design aspects, including a self-efficacy assessment and actions to increase self-efficacy. Third, potential mediators between self-efficacy and frailty such as immune function and health behavior (especially nutritional and exercise self-efficacy) should be explored to establish relationships. Fourth, covariates such as sex, race/ethnicity, income and education need to be carefully considered within analyses and intervention design in order to meet the heterogenous needs of the older adult community and minimize attrition bias in the research design.
Supplementary Material
Supplemental Table S1. Comparison of Demographic and Health Characteristics for Included and Excluded Sample
Supplemental Table S2. Frailty Incidence for Low vs High Self-Efficacy with Prefrail as Covariate
Supplemental Table S3. Frailty Incidence for Low vs High Self-Efficacy with No Baseline Depressive Symptoms Sample
Supplemental Table S4. Frailty Incidence for Three Categories of Self-Efficacy
Key Points:
Self-efficacy is a modifiable influencer of stress response and behavior change.
It may also be associated with frailty.
Our results show an increase in incident physical frailty by low vs. high self-efficacy.
Why does this matter?
Self-efficacy may be an important aspect of multi-modal frailty intervention design.
ACKNOWLEDGMENTS
Financial Support: This work was supported by: (1) NIH/NINR 3P30NR018093 for MDH (2) NIH/ NIA UH2UH3 AG056933-02 for MDH, JZ, BJB, KBR, JDW, QLX.
Sponsor’s Role: The funding body had no role in the design, data collection, implementation, analysis, or the decision to publish the results.
Footnotes
Conflict of Interest: The authors have declared no conflict of interest for this article.
Disclosure Statement: This work was presented as an abstract at the 2020 Gerontological Society of America Scientific Meeting.
References
- 1.Bandeen-Roche K, Xue Q-L, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61(3):262–266. http://www.ncbi.nlm.nih.gov/pubmed/16567375. Accessed April 5, 2016. [DOI] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. http://www.ncbi.nlm.nih.gov/pubmed/11253156. Accessed August 27, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Kojima G Frailty as a predictor of disabilities among community-dwelling older people: a systematic review and meta-analysis. Disabil Rehabil. 2017;39(19):1897–1908. doi: 10.1080/09638288.2016.1212282 [DOI] [PubMed] [Google Scholar]
- 4.Crow RS, Lohman MC, Titus AJ, et al. Mortality Risk Along the Frailty Spectrum: Data from the National Health and Nutrition Examination Survey 1999 to 2004. J Am Geriatr Soc. 2018;66(3):496–502. doi: 10.1111/jgs.15220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grabovac I, Haider S, Mogg C, et al. Frailty Status Predicts All-Cause and Cause-Specific Mortality in Community Dwelling Older Adults. J Am Med Dir Assoc. 2019;20(10):1230–1235.e2. doi: 10.1016/j.jamda.2019.06.007 [DOI] [PubMed] [Google Scholar]
- 6.Walston JD, Bandeen-Roche K. Frailty: a tale of two concepts. 2015. doi: 10.1186/s12916-015-0420-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. http://www.ncbi.nlm.nih.gov/pubmed/11253156. Accessed December 6, 2018. [DOI] [PubMed] [Google Scholar]
- 8.Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in Older Adults: A Nationally Representative Profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70(11):1427–1434. doi: 10.1093/gerona/glv133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Usher T, Buta B, Thorpe RJ, et al. Dissecting the Racial/Ethnic Disparity in Frailty in a Nationally Representative Cohort Study with Respect to Health, Income, and Measurement. Journals Gerontol Ser A 2020. doi: 10.1093/gerona/glaa061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiedenfeld SA, O’Leary A, Bandura A, Brown S, Levine S, Raska K. Impact of perceived self-efficacy in coping with stressors on components of the immune system. J Pers Soc Psychol. 1990;59(5):1082–1094. http://www.ncbi.nlm.nih.gov/pubmed/2148350. Accessed November 4, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Bandura A, Cioffi D, Taylor CB, Brouillard ME. Perceived Self-Efficacy in Coping With Cognitive Stressors and Opioid Activation. J Pers Soc Psychol. 1988;55(3):479–488. https://www.uky.edu/~eushe2/Bandura/Bandura1988JPSP.pdf. Accessed May 17, 2018. [DOI] [PubMed] [Google Scholar]
- 12.Bandura A, Taylor CB, Williams SL, Mefford IN, Barchas JD. Catecholamine secretion as a function of perceived coping self-efficacy. J Consult Clin Psychol. 1985;53(3):406–414. http://www.ncbi.nlm.nih.gov/pubmed/4008724. Accessed November 13, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Grembowski D, Patrick D, Diehr P, et al. Self-efficacy and health behavior among older adults. J Health Soc Behav. 1993;34(2):89–104. http://www.ncbi.nlm.nih.gov/pubmed/8277130. Accessed May 17, 2015. [PubMed] [Google Scholar]
- 14.Sheeran P, Maki A, Montanaro E, et al. The impact of changing attitudes, norms, and self-efficacy on health-related intentions and behavior: A meta-analysis. Heal Psychol. 2016;35(11):1178–1188. doi: 10.1037/hea0000387 [DOI] [PubMed] [Google Scholar]
- 15.Marks R, Allegrante JP, Lorig K. A review and synthesis of research evidence for self-efficacy-enhancing interventions for reducing chronic disability: implications for health education practice (part II). Health Promot Pract. 2005;6(1):148–156. http://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=15855284&site=ehost-live&scope=site. [DOI] [PubMed] [Google Scholar]
- 16.Schwarzer R, Jerusalem M. Generalized Self-Efficacy Scale. In: Weinman J, Wright S, Johnston M, eds. Measures in Health Psychology: A User’s Portfolio. Causal and Control Beliefs. Windsor, UK: NFER-NELSON; 1995:35–37. [Google Scholar]
- 17.Bandura A Self-Efficacy: The Exercise of Control. New York: W.H. Freeman and Company; 1997. [Google Scholar]
- 18.Stretton CM, Latham NK, Carter KN, Lee AC, Anderson CS. Determinants of physical health in frail older people: the importance of self-efficacy. Clin Rehabil. 2006;20(4):357–366. doi: 10.1191/0269215506cr946oa [DOI] [PubMed] [Google Scholar]
- 19.Hoogendijk EO, van Hout HPJ, van der Horst HE, et al. Do psychosocial resources modify the effects of frailty on functional decline and mortality? J Psychosom Res. 2014;77(6):547–551. doi: 10.1016/j.jpsychores.2014.09.017 [DOI] [PubMed] [Google Scholar]
- 20.Matsuda PN, Shumway-Cook A, Ciol MA. The effects of a home-based exercise program on physical function in frail older adults. J Geriatr Phys Ther. 2010;33(2):78–84. doi: 10.1097/JPT.0b013e3181deff9e [DOI] [PubMed] [Google Scholar]
- 21.McPhee JS, French DP, Jackson D, Nazroo J, Pendleton N, Degens H. Physical activity in older age: perspectives for healthy ageing and frailty. Biogerontology. 2016;17(3):567–580. doi: 10.1007/s10522-016-9641-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hladek MD, Gill J, Bandeen-Roche K, et al. High coping self-efficacy associated with lower odds of pre-frailty/frailty in older adults with chronic disease. Aging Ment Heal. 2020;24(12). doi: 10.1080/13607863.2019.1639136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasper JD, Freedman VA. Findings From the 1st Round of the National Health and Aging Trends Study (NHATS): Introduction to a Special Issue. Journals Gerontol Ser B Psychol Sci Soc Sci. 2014;69(Suppl 1):S1–S7. doi: 10.1093/geronb/gbu125 [DOI] [PubMed] [Google Scholar]
- 24.de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity. a critical review of available methods. J Clin Epidemiol. 2003;56(3):221–229. http://www.ncbi.nlm.nih.gov/pubmed/12725876. Accessed April 4, 2015. [DOI] [PubMed] [Google Scholar]
- 25.Huntley AL, Johnson R, Purdy S, Valderas JM, Salisbury C. Measures of multimorbidity and morbidity burden for use in primary care and community settings: a systematic review and guide. Ann Fam Med. 10(2):134–141. doi: 10.1370/afm.1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gill TM, Williams CS. Evaluating Distinctions in the Assessment of Late-Life Disability. Journals Gerontol - Ser A Biol Sci Med Sci. 2017;72(11):1538–1546. doi: 10.1093/gerona/glx022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freedman VA, Kasper JD, Cornman JC, et al. Validation of new measures of disability and functioning in the national health and aging trends study. Journals Gerontol - Ser A Biol Sci Med Sci. 2011;66 A(9):1013–1021. doi: 10.1093/gerona/glr087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroenke K, Spitzer RL, Williams JBW, Löwe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 32(4):345–359. doi: 10.1016/j.genhosppsych.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 29.Prentice R, Gloeckler L. Regression analysis of grouped survival data with application to breast cancer data - PubMed. Biometrics. 1978;34(1):57–67. https://pubmed.ncbi.nlm.nih.gov/630037/. Accessed July 24, 2020. [PubMed] [Google Scholar]
- 30.Maddux JE. Self-Efficacy, Adaptation, and Adjustment : Theory, Research, and Application. New York: Plenum Press; 1995. https://catalyst.library.jhu.edu/catalog/bib_1891576. Accessed December 14, 2018 [Google Scholar]
- 31.Schwarzer R Self-Efficacy : Thought Control of Action. Washington: Hemisphere Pub. Corp; 1992. https://catalyst.library.jhu.edu/catalog/bib_897056. Accessed December 14, 2018 [Google Scholar]
- 32.Gobbens RJJ, van Assen MALM, Luijkx KG, Wijnen-Sponselee MT, Schols JMGA. The tilburg frailty indicator: Psychometric properties. J Am Med Dir Assoc. 2010;11(5):344–355. doi: 10.1016/j.jamda.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 33.Gobbens RJJ, Luijkx KG, Wijnen-Sponselee MT, Schols JMGA. Towards an integral conceptual model of frailty. J Nutr Heal Aging. 2010;14(3):175–181. doi: 10.1007/s12603-010-0045-6 [DOI] [PubMed] [Google Scholar]
- 34.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–336. doi: 10.1100/tsw.2001.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le N, Varadhan R, Fried L, Cappola AR. Cortisol and Dehydroepiandrosterone Response to ACTH and Frailty in Older Women - PubMed. J Gerontol A Biol Sci Med Sci. 2020;June 5. https://pubmed-ncbi-nlm-nih-gov.proxy1.library.jhu.edu/32502234/. Accessed June 25, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collerton J, Martin-Ruiz C, Davies K, et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: Cross-sectional findings from the Newcastle 85+ Study. Mech Ageing Dev. 2012;133(6):456–466. doi: 10.1016/j.mad.2012.05.005 [DOI] [PubMed] [Google Scholar]
- 37.Hubbard RE, O’Mahony MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med. 2009;13(9 B):3103–3109. doi: 10.1111/j.1582-4934.2009.00733.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: Results from the Cardiovascular Health Study. Arch Intern Med. 2002;162(20):2333–2341. doi: 10.1001/archinte.162.20.2333 [DOI] [PubMed] [Google Scholar]
- 39.Rimal RN. Closing the knowledge-behavior gap in health promotion: The mediating role of self-efficacy. Health Commun. 2000;12(3). doi: 10.1207/S15327027HC1203_01 [DOI] [PubMed] [Google Scholar]
- 40.Du H, Everett B, Newton PJ, Salamonson Y, Davidson PM. Self-efficacy: A useful construct to promote physical activity in people with stable chronic heart failure. J Clin Nurs. 2012;21(3–4):301–310. doi: 10.1111/j.1365-2702.2011.03983.x [DOI] [PubMed] [Google Scholar]
- 41.Kekäläinen T, Kokko K, Tammelin T, Sipilä S, Walker S. Motivational characteristics and resistance training in older adults: A randomized controlled trial and 1-year follow-up. Scand J Med Sci Sport. 2018;28(11):2416–2426. doi: 10.1111/sms.13236 [DOI] [PubMed] [Google Scholar]
- 42.AbuSabha R, Achterberg C. Review of self-efficacy and locus of control for nutrition- and health- related behavior. J Am Diet Assoc. 1997;97(10):1122–1132. doi: 10.1016/S0002-8223(97)00273-3 [DOI] [PubMed] [Google Scholar]
- 43.McAuley E, Morris KS, Motl RW, Hu L, Konopack JF, Elavsky S. Long-term follow-up of physical activity behavior in older adults. Heal Psychol. 2007;26(3):375–380. doi: 10.1037/0278-6133.26.3.375 [DOI] [PubMed] [Google Scholar]
- 44.Delahanty LM, Peyrot M, Shrader PJ, Williamson DA, Meigs JB, Nathan DM. Pretreatment, psychological, and behavioral predictors of weight outcomes among lifestyle intervention participants in the diabetes prevention program (DPP). Diabetes Care. 2013;36(1):34–40. doi: 10.2337/dc12-0733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chesney MA, Neilands TB, Chambers DB, Taylor JM, Folkman S. A validity and reliability study of the coping self-efficacy scale. Br J Health Psychol. 2006;11(Pt 3):421–437. doi: 10.1348/135910705X53155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bandura A Guide for Constructing Self-Efficacy Scales. In: Self-Efficacy Beliefs of Adolescents. Information Age Publishing; 2006:307–337. [Google Scholar]
- 47.Assari S General Self-Efficacy and Mortality in the USA; Racial Differences. J Racial Ethn Heal Disparities. 2017;4(4):746–757. doi: 10.1007/s40615-016-0278-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luszczynska A, Scholz U, Schwarzer R. The general self-efficacy scale: multicultural validation studies. J Psychol. 2005;139(5):439–457. doi: 10.3200/JRLP.139.5.439-457 [DOI] [PubMed] [Google Scholar]
- 49.Ejiogu N, Norbeck JH, Mason MA, Cromwell BC, Zonderman AB, Evans MK. Recruitment and retention strategies for minority or poor clinical research participants: Lessons from the healthy aging in neighborhoods of diversity across the life span study. Gerontologist. 2011;51(SUPPL. 1). doi: 10.1093/geront/gnr027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolovou V, Moriarty Y, Gilbert S, et al. Recruitment and retention of participants from socioeconomically deprived communities: lessons from the Awareness and Beliefs About Cancer (ABACus3) Randomised Controlled Trial. BMC Med Res Methodol. 2020;20(1). doi: 10.1186/s12874-020-01149-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1. Comparison of Demographic and Health Characteristics for Included and Excluded Sample
Supplemental Table S2. Frailty Incidence for Low vs High Self-Efficacy with Prefrail as Covariate
Supplemental Table S3. Frailty Incidence for Low vs High Self-Efficacy with No Baseline Depressive Symptoms Sample
Supplemental Table S4. Frailty Incidence for Three Categories of Self-Efficacy


