Abstract
Purpose:
PGF2α analogs are commonly used to treat glaucoma and are associated with higher rates of meibomian gland dysfunction (MGD). The purpose of this study was to evaluate the physiological effects of PGF2α and PGE2 on immortalized human meibomian gland epithelial cells (HMGECs).
Methods:
HMGECs were immunostained for the four PGE2 receptors (EP1, EP2, EP3, EP4) and the one PGF2α receptor (FP) and imaged. Rosiglitazone-differentiated HMGECs were exposed to PGF2α and PGE2 (10−9 to 10−6 M) for three hours. Cell viability was assessed by an ATP-based luminescent assay, and lipid extracts were analyzed for cholesteryl esters (CEs), wax esters (WEs), and triacylglycerols (TAGs) by ESI-MSMSALL in positive ion mode by a Triple TOF 5600 Mass Spectrometer using SCIEX LipidView 1.3.
Results:
HMGECs express three PGE2 receptors (EP1, EP2, EP4) and the one PGF2α receptor (FP). Neither PGE2 nor PGF2α showed signs of cytotoxicity at any of the concentrations tested. WEs were not detected from any of the samples, but CEs and TAGs both exhibited a diverse and dynamic profile. PGE2 suppressed select CEs (CE 22:1, CE 26:0, CE 28:1, CE 30:1). PGF2α dose dependently increased several CEs (CE 20:2, CE 20:1, CE 22:1, CE 24:0) yet decreased others. Both prostaglandins led to nonspecific TAG remodeling.
Conclusion:
PGE2 and PGF2α have minimal effect on HMGEC viability. PGF2α influences lipid expression greater than PGE2 and may do so by interfering with meibocyte differentiation. This work may provide insight into the mechanism of MGD development in glaucoma patients treated with PGF2α analogs.
Keywords: meibomian gland, Prostaglandins, human meibomian gland epithelial cells, cholesteryl esters, triacylglycerols
Introduction
There are greater than 64.3 million people between the ages of 40 and 80 who suffer from glaucoma worldwide.1 Up to 80 percent, more than 50 million of these patients, may also have concurrent meibomian gland dysfunction (MGD).2 This number is even more alarming when considering that ocular surface diseases, such as MGD, may exacerbate glaucoma by interfering with intraocular pressure (IOP) reduction and/or surgical outcomes.3,4 Aggressive management of glaucoma-associated MGD with both oral and topical medications has shown to improve not only the signs and symptoms of MGD, but also IOP management.3 The mechanism that underlies the onset of MGD in glaucoma patients is not fully understood, but there is strong evidence that the medical management of glaucoma is culpable.2,3,5–13
Prostaglandin analogs (PGAs), which are primarily PGF2α analogs, are a common first-line treatment in glaucoma patients, owing to their efficacy, affordability, convenient dosing schedule, and favorable systemic side effect profile.14 Their therapeutic benefits are thought to be mediated through the PGF2α FP receptor, a seven transmembrane G-protein coupled receptor.15,16 Upon ligand binding, the FP receptor induces a calcium-dependent chloride current, as well as accumulation of inositol triphosphate.17,18 Signaling through FP receptors promotes upregulation of several matrix metalloproteinases capable of degrading the extracellular matrix.19 This mechanism is largely credited for the FP receptor’s favorable effect on IOP: a degraded extracellular matrix reduces aqueous humor outflow resistance, permits increased outflow, and ultimately lowers the IOP.15,19
Unlike the minimal adverse effects observed systemically, the ocular side effects of PGAs are numerous and may be partly mediated through crossover binding to other prostaglandin receptors, such as those for PGE2 (EP1, EP2, EP3, and EP4).14 The PGA side effects consist of redness, burning, itching, pigmentation changes, and eyelash growth, among several others.14 More recently, development and/or progression of MGD has also been associated with chronic PGA use.12 The mechanism, though currently unknown, is likely related to pathologic changes induced by the topical application of both the PGA and its preservative system. One hypothesis is that the increase in MGD may be due to an alteration in the secretions from the meibomian gland secondary to stimulation by PGAs.20
The meibomian glands produce a lipid-rich fluid, termed meibum, that spreads across the ocular surface to form the tear film lipid layer.21 Meibum is comprised mostly of nonpolar lipids with significant contributions provided by the hydrophobic wax esters (WEs, 48 percent) and cholesteryl esters (CEs, 40 percent).22,23 Another nonpolar lipid class, triacylglycerols (TAGs), is of less abundance, ranging between 0.05 to 6 percent,24–28 but preliminary work has suggested that several TAGs are upregulated in a preclinical model of MGD29 that uses the same human meibomian gland epithelial cell line (HMGEC) as in this study. We have previously described the CE and TAG profiles from differentiated HMGECs;30,31 WE expression from these cells, however, appears to be minimal or undetectable.32–35 As the only human meibocyte cell line available, this HMGEC model was used to better understand the outcomes provoked by PGE2 and PGF2α on the differential expression of CEs, WEs, and TAGs. We hypothesize that HMGECs undergo a shift in their lipid expression following supplementation with PGE2 and PGF2α and that these lipidomic alterations are likely mediated through prostaglandin receptors found on HMGECs.
Materials Methods
Reagents Materials
Rosiglitazone, PGE2, and PGF2α were purchased from Cayman Chemical (Ann Arbor, MI). Stock solutions were made by dissolving each compound in sterile-filtered dimethyl sulfoxide (DMSO, Hybri-Max™, Sigma-Aldrich, St. Louis, MO) and stored under nitrogen at −20°C. Rosiglitazone, PGE2, and PGF2α were added fresh to media preparations just prior to each experiment. DMSO concentration was maintained at 0.5% in all samples. Falcon 8-well chambered cell culture slides were used for immunocytochemistry and purchased from ThermoFisher (Waltham, MA). Clear-bottom, white-walled 96-well plates were used for luminescent cell viability assays and were also purchased from ThermoFisher (Waltham, MA). Glass petri dishes were used for lipidomics experiments and were purchased from Sigma Aldrich (St. Louis, MO).
Immunocytochemistry
HMGECs were cultured on 8-well chambered slides and maintained in proliferating conditions consisting of KSFM with 5 ng/ml epidermal growth factor (EGF) and 50 μg/ml bovine pituitary extract. Media changes were performed every other day until 80 to 90% confluence when the culture media was changed to DMEM/F12 with 10 ng/ml EGF, 2% fetal bovine serum (FBS), and 50 μM rosiglitazone. After 24 hours, HMGECs were fixed in freshly prepared PBS containing 4% paraformaldehyde for 10 mins at room temperature, followed by four consecutive wash cycles. Fixed HMGECs were blocked with PBS containing 10% goat serum (ThermoFisher, Waltham, MA), 1% bovine serum albumin (BSA), and 0.3% Triton-X 100 for 30 mins on ice, then washed four times. Blocked HMGECs were incubated at room temperature for one hour in a humidified chamber with a rabbit polyclonal antibody against one of the following receptors: EP1, EP2, EP3, EP4, or FP. All primary antibodies were purchased from ThermoFisher and diluted 1:50 in PBS with 1% BSA and 0.3% Triton-X 100. Following four wash cycles, HMGECs were incubated with goat anti-rabbit IgG (ThermoFisher, Waltham, MA) conjugated to Alexa Fluor Plus 555 for one hour in the dark (1:500 dilution in PBS with 1% BSA and 0.3% Triton-X 100). Each slide was mounted with mounting media consisting of DAPI counterstain, 70% glycerol, 10% n-propyl gallate, and 20% PBS. Slides were imaged with a Zeiss Axioplan 2 Fluorescent Microscope (Jena, Germany). Images were captured with a 40x objective at a z-step of 0.4 μm. To optimize signal-to-noise ratios, laser intensities were set at 25% and 75% and exposure times at 250 ms and 1500 ms for the channels exciting DAPI and Alexa Fluor Plus 555, respectively. Image stacks were corrected for chromatic aberration and crosstalk and deconvolved with Huygens Professional Software v19.10 (Scientific Volume Imaging, Hilversum, North Holland, Netherlands). All wash cycles consisted of a five-minute period on a shaker at 50 rpm. All experiments consisted of two experimental replicates and two technical replicates.
Cell viability
HMGECs were grown to 80 to 90% confluence and then split at a density of 30,000 cells per well in 96-well plates. All cells were incubated in DMEM/F12 with 10 ng/ml EGF, 2% FBS, and 50 μM rosiglitazone for two days to induce differentiation. PGE2- or PGF2α-containing media (10−9, 10−8, 10−7, 10−6 M) were added to HMGECs and allowed to incubate for three hours. Following incubation, cell viability was assessed using the Cell Titer-Glo Luminescent Cell Viability Assay (Promega, Madison, WI) according to the manufacturer’s instructions. Luminescence was quantified using the Wallac Perkin-Elmer 1420–041 Victor2 Multiplate Multifluorescence Reader (Mt. Waverly, Victoria, Australia) over an acquisition time of one second. All experiments were performed with two experimental replicates and three technical replicates.
Lipid extraction and analysis by mass spectrometry
HMGECs were seeded at one million cells per 6-cm glass petri dish in DMEM/F12 with 10 ng/ml EGF, 2% FBS, and 50 μM rosiglitazone for two days to induce differentiation. Following incubation, HMGECs were exposed to PGE2 or PGF2α (10−8, 10−7, or 10−6 M) for three hours. Lipids were extracted using a recently described modification of the Folch technique optimized to efficiently extract lipids from cultured HMGECs.32,36 Briefly, 3 ml of pre-chilled chloroform-methanol (2:1 v/v, −20°C) were added directly to the HMGECs, which were then scraped with a sterile stainless steel scraper. The suspension was then transferred to a glass vial where 0.75 ml of molecular biology-grade water containing ammonium acetate (50 mM) was added. The emulsion was shaken at 350 rpm for 20 mins on ice and centrifuged at 1600 xg for five mins to promote stratification. The lower nonpolar phase was withdrawn and stored at −80°C until analysis. All steps involving organic solvents were performed with glass, stainless steel, or polytetrafluoroethylene (PTFE). All experiments were performed with two experimental replicates and two technical replicates.
Untargeted lipidomics was performed on dried lipids using a SCIEX Triple TOF 5600 Mass Spectrometer (SCIEX, Framingham, MA) in positive ion mode via direct infusion. The direct infusion solvent was methanol-chloroform (2:1) with 5 mM ammonium acetate. Each sample was delivered to the source by isocratic flow at 7 μl/min using a 500 μl Hamilton Gas Tight Syringe (Reno, NV). Prior to and after each sample, the syringe was cleaned with two flushes each of 100% methanol, 100% acetonitrile, 10% isopropyl alcohol, and 100% direct infusion solvent. Calibration runs were performed in positive mode with the APCI Positive Calibration Solution (SCIEX, Framingham, MA). The analyte mass evaluation range was 200 to 1200 m/z. A high-resolution time-of-flight (TOF) scan was acquired initially for 250 ms, then a series of high-sensitivity product ion scans were acquired per one Dalton (1 m/z) mass starting at 200 m/z through 1200 m/z. The collision energy was fixed at 35 eV, curtain gas to 20.00, GS1 to 20.00, GS2 to 15.00, spray voltage to 5000 volts, and interface temperature to 400°C. The acquisition time per sample was six minutes.
The acquired mass spectrometry data were processed with SCIEX LipidView 1.3 software (Framingham, MA). Lipid identities were assigned by LipidView, which utilizes a database of known ion fragmentations. To confirm selected lipid identities, SCIEX PeakView 2.2 was used to further investigate fragments. The mass tolerance window was set to 5 mDa, and the peaks greater than a signal-to-noise ratio of three were considered for analysis. Identification of individual lipid species from LipidView assignments was based on mass accuracy (<5 ppm) and MS/MS spectra obtained from PeakView.
Data analysis
For mass spectrometry data, CEs, WEs, and TAGs were the predominant focus of this study. To be included in the analysis, a given lipid species had to be present in all replicates of all samples. Each lipid was normalized to the sum intensity per class and reported as percent of the overall class. For TAGs, only those that were present at a threshold concentration of 0.1% were included. All data for both cell viability and lipidomics were analyzed by one-way ANOVA with Tukey post-hoc testing (SPSS v26, Armonk, NY) when tests of normality (Kolmogorov-Smirnov) and homogeneity of variance (Levene’s Test) were satisfied. If the assumption of equal variance was violated, then Games-Howell post-hoc testing was used. If the assumption of normality was violated, then the non-parametric Kruskal Wallis test was used. A p-value of 0.05 was considered significant.
The labeling convention for CEs consists of two numbers, separated by a colon. The two numbers represent the numbers of carbons and double bonds, respectively, in the fatty acyl chain. For some species, a third number is present, which denotes that the CE has been oxidized with the specified number of oxygenations. Each parent TAG is identified similarly by the total numbers of carbons and double bonds in the three fatty acyl chains. Following each TAG, however, is a product ion, either a fatty acid (FA) or another TAG, which is labeled similarly. This notation represents that the LipidView software identified a neutral loss corresponding to that specific fatty acid or TAG. As an example, TAG 54:3 (FA 18:1) is a TAG that has 54 total carbons and three total double bonds among its fatty acyl chains, where one of those chains consists of 18 carbons and one double bond.
Results
Expression of FP- and EP-type receptors
HMGECs were immunostained against the four PGE2 receptors (EP1, EP2, EP3, and EP4) and the one PGF2α receptor (FP) and imaged by fluorescent microscopy. Positive signal (pseudocolored red) was observed for EP1, EP2, EP4, and FP receptors (Figure 1). There was no appreciable EP3 signal above background.
Figure 1:
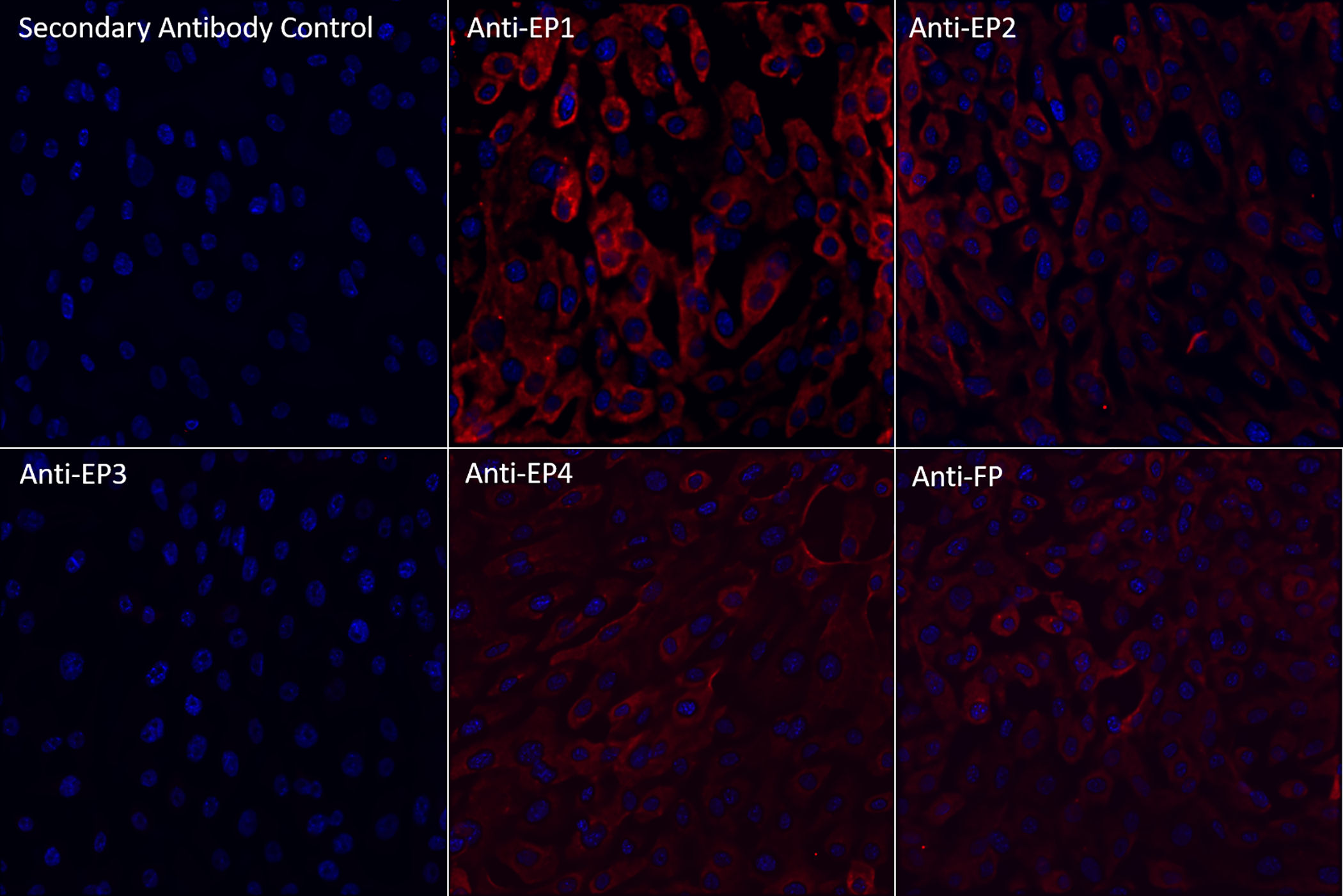
Fluorescent microscopy images (40x) of HMGECs stained with primary antibodies against PGE2 (EP1, EP2, EP3, or EP4) or PGF2α (FP) receptors and counterstained with DAPI (blue) after 24 hours of culture in differentiation media containing DMEM/F12, 10 ng/ml EGF, 2% FBS, and 50 μM rosiglitazone. Positive signal for each primary antibody (pseudocolored red) was detected for EP1, EP2, EP4, and FP receptors.
PGE2: prostaglandin E2
PGF2α: prostaglandin F2α
Influence of prostaglandins on cell viability
To determine whether PGE2 or PGF2α affects cell viability of differentiated HMGECs, ATP was quantitated from HMGECs exposed to 10−9, 10−8, 10−7, 10−6 M PGE2 or PGF2α for three hours. As shown in Figure 2, none of the PGE2 concentrations (p = 0.20) or PGF2α concentrations (p = 0.82) reduced viability compared to the vehicle control. Further, there were no differences in viability between any of the PGE2 and PGF2α concentrations (p = 0.32). The cytotoxic detergent Triton-X 100 (1%) served as a positive control and strongly reduced viability.
Figure 2:
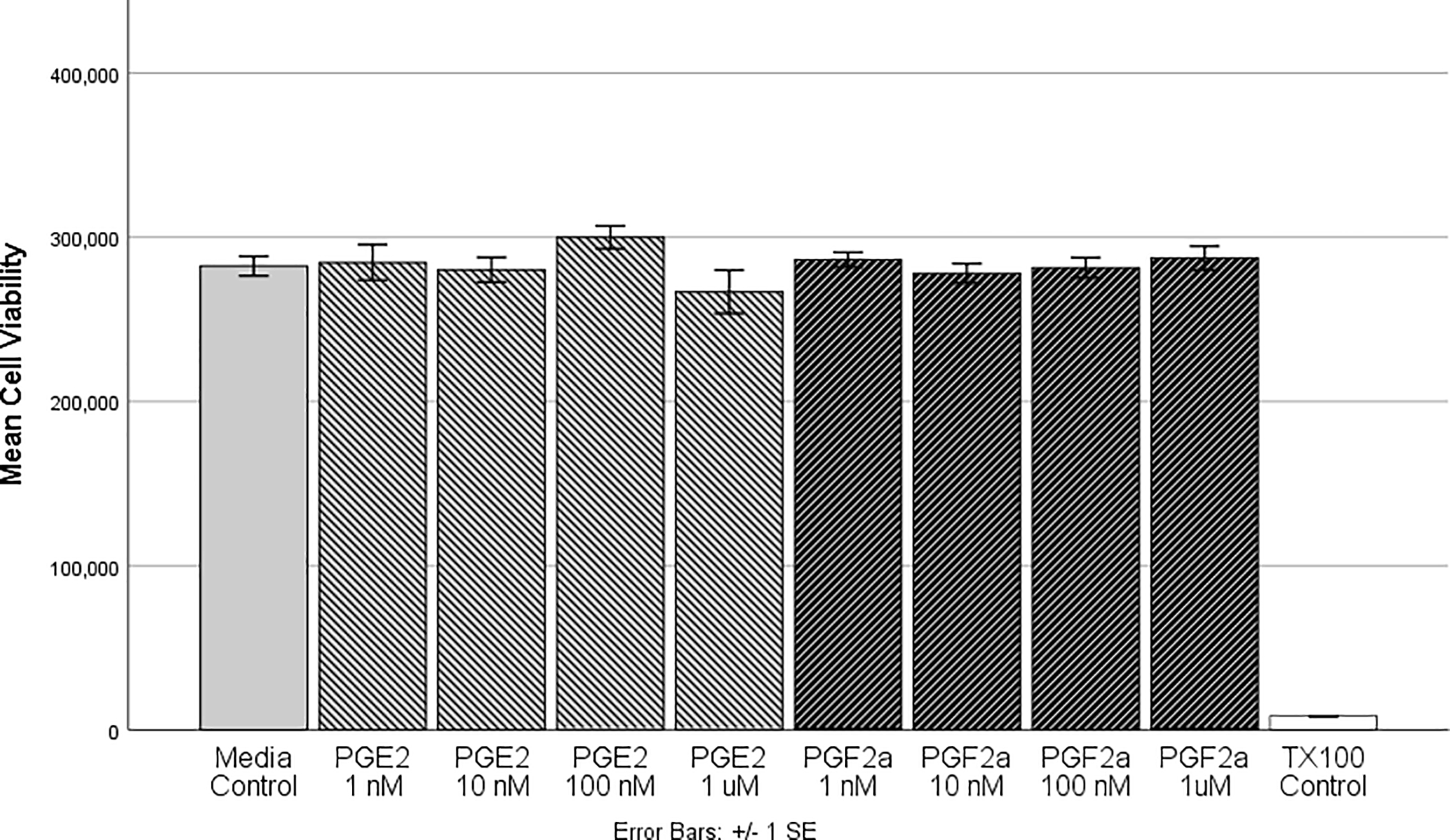
HMGECs were differentiated for two days (see Methods) prior to exposure to PGE2 or PGF2α for 3 hours. After incubation, cell viability was assessed with a luminescent ATP-based assay. There were no significant differences between the vehicle control and any of the PGE2 or PGF2α concentrations, suggesting that these prostaglandins at physiologic concentrations do not alter cell viability. Further there were no differences between PGE2 or PGF2α. Triton-X 100 (1%) was used as a positive control, which differed significantly from all other concentrations (p < 0.001). n = 6 per condition
HMGEC: human meibomian gland epithelial cell
PGE2: prostaglandin E2
PGF2α: prostaglandin F2α
Description of the CE and TAG profiles across all samples
This study focused on the expression of CEs, WEs, and TAGs. Although WEs were not detected among any of the samples in this study, CEs and TAGs were. There were 107 CEs detected across all samples; however, only 39 met the criteria for inclusion in the analysis (Figure 3A). The chain length varied from 11 carbons to 32 carbons, and the double-bond count varied from zero to five. Six CEs were found to be oxidized. The most abundant CE was CE 18:1. Very long-chain (20 ≤ carbon number [nc] ≤ 25) and ultra long-chain CEs (nc ≥ 26) were present from all conditions and comprised 26.2% and 6.9% of the overall CE pool, respectively. Of the 39 CEs, nine were saturated, 15 were monounsaturated, and 15 were polyunsaturated. Monounsaturated CEs were the most abundant (51.1%), followed by polyunsaturated (28.4%) and saturated (20.5%).
Figure 3:
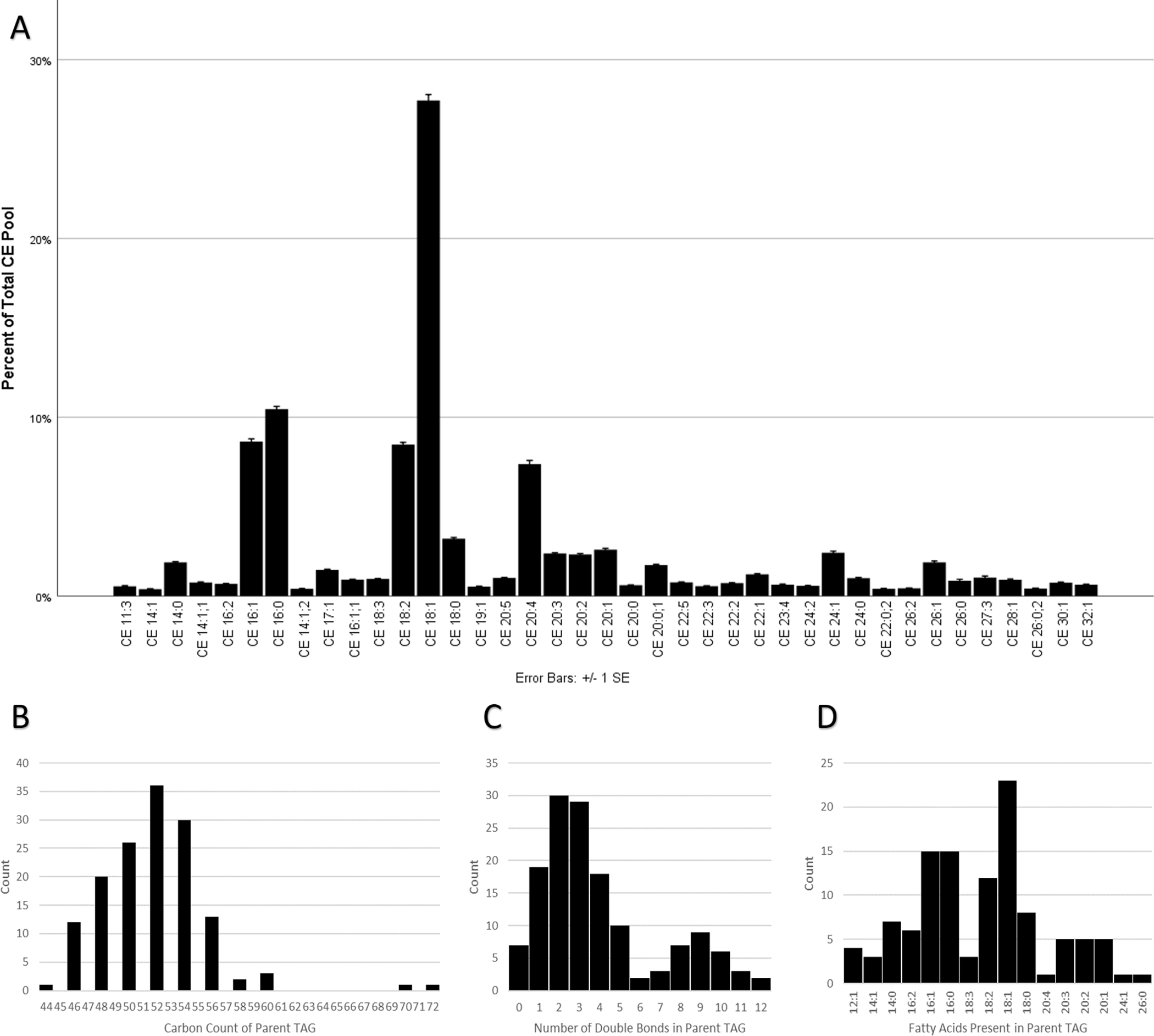
(A) HMGECs expressed 39 unique CEs, including 6 oxidized CEs (oxCEs). CE 18:1 was the most abundant, followed by CE 16:0. The chain length varied from 11 carbons to 32 carbons. The double-bond count ranged from 0 to 5. CEs are labeled by carbon number and double-bond count, respectively. When a third number is present, it denotes an oxCE with the corresponding number of oxygenations. n = 28
(B-D) HMGECs expressed 145 TAGs that met the criteria for analysis (see Methods). The carbon count varied from 44 to 72 (B), and the double-bond count varied from 0 to 12 (C). LipidView 1.3 detected the neutral loss of sixteen unique fatty acyl chains (D) from the parent TAGs. FA 18:1 was the most frequently observed. n = 28
HMGEC: human meibomian gland epithelial cell
CE: cholesteryl ester
TAG: triacylglycerol
FA: fatty acid
There were 3,706 TAGs detected across all samples; however, only 145 met the criteria for inclusion in the analysis. The total carbon count from the three acyl chains, excluding the glycerol backbone, ranged from 44 to 72 with the majority (137/145, 94.5%) falling within the range of 46 to 56 (Figure 3B). All expressed TAGs consisted of an even carbon count (145/145, 100%). The number of double bonds in the acyl chains of the TAGs varied from zero to 12. Very few TAGs were fully saturated (7/145, 4.8%). The degree of unsaturation followed a bimodal distribution (Figure 3C) that was heavily weighted toward a lower degree of unsaturation. TAGs were primarily of lower unsaturation (106/145, 73.1%, one to five double bonds) or of higher unsaturation (22/145, 15.2%, eight to ten double bonds). Only five of 145 (3.4%) had a moderate degree of unsaturation (six or seven double bonds) or a very high degree of unsaturation (11 or 12 double bonds). The LipidView 1.3 software identified the neutral loss of 16 unique fatty acyl chains from the 145 TAGs (Figure 3D). Their individual carbon counts varied from 12 to 26 with double bonds ranging from zero to 4. Similar to the parent TAG molecules, all fatty acyl chains consisted exclusively of even numbers of carbons. The most frequently observed fatty acyl group was oleic acid (FA 18:1), which was present in 23 of 145 TAGs (15.9%). The second most common was palmitic acid (FA 16:0) and palmitoleic acid (FA 16:1), which were each present in 15 (10.3%) TAGs. Several TAGs (18/145, 12.4%) consisted of very long-chain fatty acids (at least 20 carbons).
Influence of prostaglandins on CE and TAG expression
To determine the effects of PGF2α on CE expression, lipid extracts from differentiated HMGECs were exposed to 10−8, 10−7, 10−6 M PGF2α for three hours. Of the 39 CEs analyzed, expression of seven CEs was significantly different among the concentrations (Figure 4A). CE 14:1 and CE 26:0 were suppressed relative to control at all tested concentrations of PGF2α, though the 10−6 M concentration failed to reach significance for both CEs. One oxidized CE (CE 26:0;2) reached significance, showing a decrease in expression at higher PGF2α concentrations. The remaining 4 CEs (CE 20:2, CE 20:1, CE 22:1, and CE 24:0) demonstrated a dose-dependent increase in expression among the three PGF2α concentrations; however, the low-dose PGF2α suppressed expression relative to the vehicle control.
Figure 4:
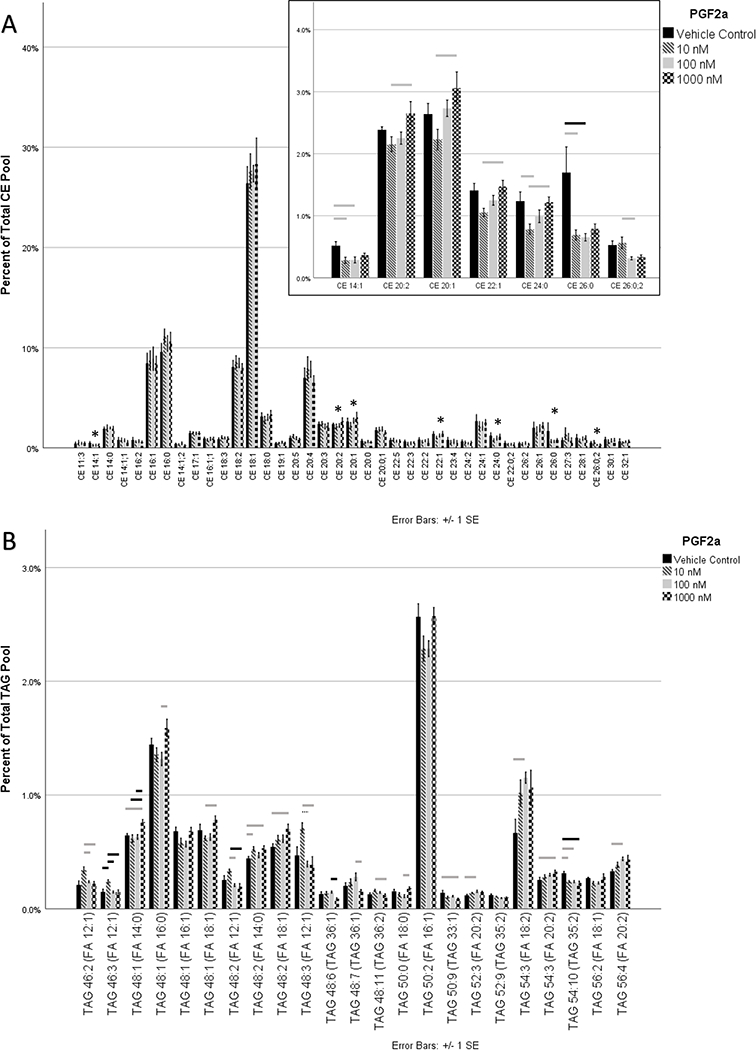
HMGECs were differentiated for two days (see Methods) prior to exposure to PGF2α for three hours. Lipid extracts were analyzed by mass spectrometry. (A) Thirty-nine CEs met the criteria for analysis. Seven of the 39 varied significantly with PGF2α supplementation (inset). All significant CEs were reduced relative to control with low-dose PGF2α; however, four of these CEs demonstrated a dose-dependent relationship with PGF2α. This low-dose suppression following by a dose-dependent upregulation suggests that multiple competing pathways may be activated (see Discussion). CEs are labeled by carbon number and double-bond count, respectively. When a third number is present, it denotes an oxCE with the corresponding number of oxygenations. (B) One hundred forty-five TAGs met the criteria for analysis. To aid legibility, only the 23 that varied significantly with PGF2α are displayed. Four of the 23 failed to reach significance in pairwise comparisons. PGF2α promoted generalized TAG remodeling, affecting 15.9% of all analyzed TAGs. TAGs are labeled by two numbers corresponding to the total number of carbons and the total number of double bonds, respectively. The fatty acid in parentheses represents one of the three fatty acids of the parent TAG molecule.
n = 4 per condition
* denotes significance
gray bar p ≤ 0.05, black bar p ≤ 0.01, dashed bar p ≤ 0.001
HMGEC: human meibomian gland epithelial cell
CE: cholesteryl ester
oxCE: oxidized cholesteryl ester
TAG: triacylglycerol
FA: fatty acid
Twenty-three of 145 TAGs showed statistically significant differences in expression among the different PGF2α concentrations (Figure 4B). Four of the 23, however, failed to reach significance in pairwise comparisons: TAG 48:1 (FA 16:1), TAG 50:2 (FA 16:1), TAG 52:9 (TAG 35:2), and TAG 56:2 (FA 18:1). Six of the 23 were upregulated across all concentrations: TAG 48:2 (FA 18:1), TAG 54:2 (FA 18:2), TAG 54:3 (FA 20:2), TAG 56:4 (FA 20:2), TAG 48:2 (FA 14:0), and TAG 52:3 (FA 20:2). Two were decreased across all concentrations: TAG 50:9 (TAG 33:1) and TAG 54:10 (TAG 35:2). Seven were upregulated at low-dose PGF2α but reduced at higher doses, including all of the FA 12:1-containing TAGs: TAG 46:2 (FA 12:1), TAG 46:3 (FA 12:1), TAG 48:2 (FA 12:1), TAG 48:3 (FA 12:1), TAG 48:6 (TAG 36:1), TAG 48:7 (TAG 36:1), and TAG 48:11 (TAG 36:2). The remaining four TAGs were reduced at low-dose PGF2α but upregulated at higher doses: TAG 48:1 (FA 14:0), TAG 48:1 (FA 16:0), TAG 48:1 (FA 18:1), and TAG 50:0 (FA 18:0).
To determine the effects of PGE2 on CE expression, lipid extracts from differentiated HMGECs exposed to 10−8, 10−7, or 10−6 M PGE2 for three hours were analyzed by mass spectrometry. Of the 39 CEs that met the inclusion criteria, four showed a statistically significant change in expression (Figure 5A). A dose-dependent decrease was observed in CE 22:1 and CE 26:0. For CE 22:1, significance was achieved between the media control lacking PGE2 and the 10−6 M concentration (p = 0.01). For CE 26:0, significance was achieved between the media control and both the 10−7 M 10−6 M concentrations (p = 0.01 for both). CE 28:1 and CE 30:1 also reached significance, where the 10−6 M (p = 0.02) and 10−7 M (p = 0.04) concentrations, respectively, were decreased relative to 10−8 M PGE2.
Figure 5:
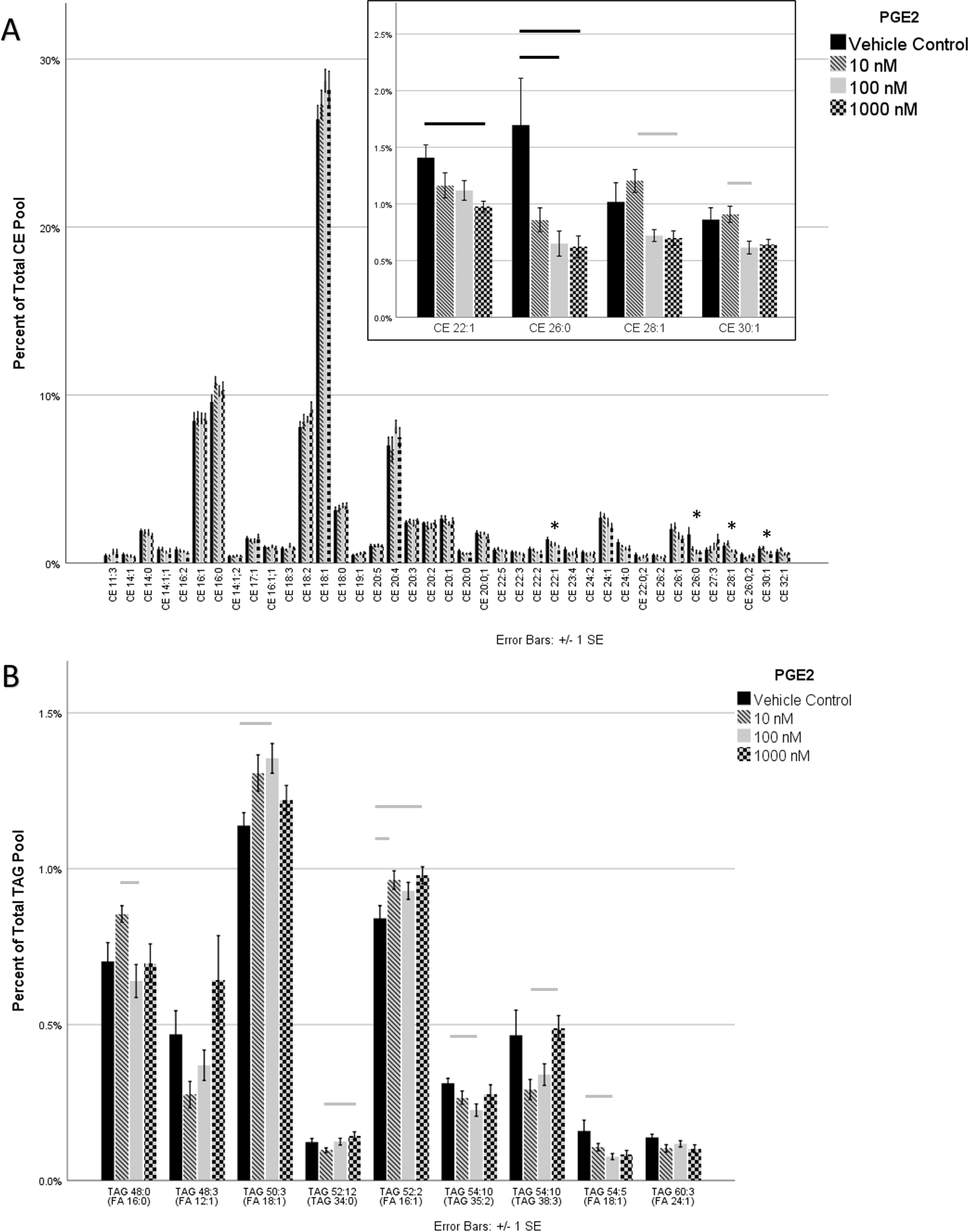
HMGECs were differentiated for two days (see Methods) prior to exposure to PGE2 for three hours. Lipid extracts were analyzed by mass spectrometry. (A) Thirty-nine CEs met the criteria for analysis. Four of the 39 varied significantly with PGE2 supplementation (inset), and all were reduced with increased PGE2 concentrations. CEs are labeled by carbon number and double-bond count, respectively. When a third number is present, it denotes an oxCE with the corresponding number of oxygenations. (B) One hundred forty-five TAGs met the criteria for analysis. To aid legibility, only the nine that varied significantly with PGE2 are displayed. Two of the 9 failed to reach significance in pairwise comparisons. PGE2 promoted generalized TAG remodeling. TAGs are labeled by two numbers corresponding to the total number of carbons and the total number of double bonds, respectively. The fatty acid in parentheses represents one of the three fatty acids of the parent TAG molecule.
n = 4 per condition
* denotes significance
gray bar p ≤ 0.05, black bar p ≤ 0.01, dashed bar p ≤ 0.001
HMGEC: human meibomian gland epithelial cell
CE: cholesteryl ester
oxCE: oxidized cholesteryl ester
TAG: triacylglycerol
FA: fatty acid
Only nine of 145 analyzed TAGs (6.2%) showed statistically significant differences in expression among the different PGE2 concentrations (Figure 5B). The remaining 136 (93.8%) did not vary significantly with respect to PGE2. Two of the nine that were statistically significant, however, failed to reach significance in pairwise comparisons: TAG 48:3 (FA 12:1) and TAG 60:3 (FA 24:1). Two of the remaining seven TAGs showed a dose-dependent increase in response to PGE2: TAG 52:12 (TAG 34:0) and TAG 54:10 (TAG 35:2). TAG 54:10 (FA 16:1) and TAG 50:3 (FA 18:1) were elevated across all PGE2 concentrations. TAG 54:5 (FA 18:1) appeared to have reduced expression across all PGE2 concentrations. The remaining two TAGs that reached significance had varying degrees of expression among the different concentrations of PGE2: TAG 48:0 (FA 16:0) and TAG 54:10 (TAG 38:3).
Discussion
In light of growing awareness of the association between chronic PGA use in glaucoma and development of MGD,2,12,20 we sought to identify the physiologic effects of PGE2 and PGF2α on HMGECs in culture. We report, for the first time, that HMGECs express three of the four PGE2 receptors (EP1, EP2, and EP4) and the one PGF2α receptor (FP). Across a broad range of physiologic concentrations (10−9 to 10−6 M), we found that neither PGE2 nor PGF2α altered cell viability. Though their effects were not cytotoxic, the observed changes in the HMGEC lipidome suggest that prostaglandins, particularly PGF2α, affect lipid expression and/or metabolism. Much of this variation is consistent with general lipidomic remodeling, though specific patterns following PGF2α exposure support the presence of multiple, competing pathways that appear to be concentration dependent. Taken together, these findings suggest that the meibomian glands express FP- and EP-type receptors, making them vulnerable to unwanted side effects associated with commercially available PGAs. Just one “dose” administered in this study, which was orders of magnitude less than commercially available formulations, modulated the lipid profile after only three hours. In vivo, lipidomic remodeling may translate into altered tear film lipid layer quality, accelerated tear evaporation, and/or poor tear film stability.
Expression of FP- and EP-type receptors
A notable advancement from this work is the discovery that HMGECs express FP receptors. This finding suggests that the meibomian gland could be added to the long list of other ocular and adnexal tissues known to express FP receptors: conjunctiva, cornea, sclera, iris, ciliary body, lens, retina, and optic nerve.37–39 The FP receptor’s role in meibomian gland physiology is largely unknown, but in adipocytes and sebocytes, other cells optimized for high lipid turnover, signal transduction cascades that are initiated by FP binding ultimately inactivate PPARγ,40 a known inducer of differentiation.41,42 Recently, Kim et al have shown that PPARγ regulates differentiation in HMGECs,43,44 and we have found that most of the CEs and TAGs produced by HMGECs are highly modulated in response to PPARγ agonism.30,31 Therefore, FP expression in the meibomian gland may have a direct yet adverse influence on cellular differentiation and nonpolar lipid production. Because PGF2α is capable of binding EP receptors at 10−8 to 10−6 M affinities,17 we were also interested in PGE2 and the EP-type receptors. We found that HMGECs express EP1, EP2, and EP4 but not EP3 receptors, similar to the expression pattern reported in human sebocytes.45 The effect of EP receptor engagement depends upon its exact EP subtype, the specific G protein it is coupled to, and its downstream signal transduction pathways,46 a topic further discussed below.
Influence of prostaglandins on cell viability
Across all the concentrations tested in this study, we found that neither PGE2 nor PGF2α affected HMGEC viability. Although some reports have linked PGE2 to apoptosis by promoting calcium influx, these responses are typically at much higher PGE2 concentrations—approximately 50 times greater than the highest concentration used in this study.47,48 At physiologic concentrations, PGE2 and PGF2α have been shown to promote cell survival and/or inhibit apoptosis,49–51 supporting our observation of sustained viability during the three-hour incubation with either prostaglandin. Although the exact concentrations of PGE2 and PGF2α are unknown in or near the meibomian glands, their concentrations have been measured in the tear film. PGE2 varies between 0.4 to 2.5 nM in normal patients and 0.5 to 7.7 nM in those with dry eye or MGD.52–54 PGF2α shows less disparity, averaging 1.0 nM in normals and 1.4 nM in MGD.52 To account for potential differences in fluid concentrations versus tissue concentrations,55 we chose to investigate PGE2 and PGF2α concentrations in 10x increments from 1 nM to 1 μM. Even after incubating with the upper limit of physiologic concentrations of either prostaglandin, cell viability was not affected.
Influence of prostaglandins on lipid expression
Our results support that PGF2α influences lipid expression, an outcome strongly influenced in HMGECs by cellular differentiation.56,57 Nearly 18% of all expressed CEs and 16% of all expressed TAGs varied in response to PGF2α supplementation. PGF2α was associated with nonspecific changes in several TAG species, suggesting that it induces generalized TAG remodeling. Regarding CEs, however, more consistent patterns emerged. Low-dose PGF2α (10−8 M) decreased expression of six of the seven statistically significant CEs compared to control. Despite this low-dose suppression, four of these six CEs (CE 20:2, CE 20:1, CE 22:1, and CE 24:0) increased thereafter in a dose-dependent fashion with PGF2α (Figure 4A). For this pattern to consistently emerge, we hypothesize that multiple, competing pathways that influence cellular differentiation and/or lipid synthesis are concurrently activated.
First, we believe that PGF2α may be inducing a relative suppression of differentiation and thus reducing meibum-relevant lipid expression. Previous reports have not only shown that PGF2α suppresses adipocyte differentiation but that it does so via the FP receptor, ultimately leading to phosphorylation and inactivation of PPARγ.40,58 In our experimental model, we differentiated our cells with rosiglitazone, a PPARγ agonist, for two days prior to introducing PGF2α for three hours. During that three-hour incubation, we believe that PGF2α led to a heterogeneous mixture of both phosphorylated (inactive) and unphosphorylated (active) forms of PPARγ, manifesting as a relative suppression of lipid expression compared to control. If this were the only pathway at play, however, a dose-dependent decrease would have been observed across the remaining PGF2α concentrations.
Instead, we observed an increase in four specific CEs and believe this effect may be related to the known opposing effects that different prostaglandins have on adipogenesis, all converging on PPARγ.40 PGF2α-induced upregulation of PGD2 and PGJ2 have been previously described in a variety of cell types.59–61 and PGD2 and PGJ2 have both been shown to potently activate PPARγ at a rate of approximately 80-fold and 20-fold, respectively.62
If these two pathways are occurring simultaneously, as hypothesized, a portion of PPARγ protein would be inactivated by PGF2α and another portion activated by PGD2 and PGJ2. For this latter mechanism to explain the dose-dependent increase observed in our study, then PPARγ activation by PGD2 and PGJ2 must dominate over the relative PPARγ inhibition by PGF2α. To better interrogate these pathways, additional work is needed to isolate these mechanisms using receptor antagonists, COX inhibitors, and quantitative methods for other prostaglandins, PPARγ, and associated gene products.
We also assessed the effects of PGE2 on lipid expression and found that PGE2 led to a significant decrease in 10% of meibum-relevant CEs (CE 22:1, CE 26:0, CE 28:1, and CE 30:1) and significant remodeling of just 6% of TAGs. The role of PGE2 on lipid synthesis is complex, yet its seemingly dichotomous effects may be explained by its diverse array of receptors. Some sources cite PGE2 as an inhibitor of lipogenesis, which is mediated through the EP3 receptor.63,64 Other sources credit PGE2 for promoting lipogenesis and fat accumulation, an outcome attributed to EP2 and EP4 signaling.65 Here, we did not detect EP3 expression on HMGECs, so the inhibitory effect of PGE2 on lipogenesis may not exist in these cells. In the absence of this mechanism, it is currently unknown how PGE2 induced the suppression of several meibum-relevant CEs in this study. Additional work is needed with receptor antagonists to determine which EPs are mediating this observation.
We detected the presence of six unique oxidized CEs (oxCEs) from our samples, only one of which (CE 26:0;2) varied significantly between low-dose and higher-dose PGF2α. None of the six oxCEs varied with respect to PGE2. Lipid oxidation of the tears has been previously investigated by Borchman et al, who found that the degree of lipid oxidation is greater in normal subjects compared to those with MGD.66 They further described these oxide moieties to be hydrophilic groups among hydrophobic regions, interfering with lipid-lipid interactions and conferring increased fluidity to the overall lipid compartment. In simpler terms, a greater degree of oxidation decreases lipid order and promotes a more fluid secretion. In MGD, a lesser degree of oxidation increases lipid order and, thus, increases viscosity. The origin, mechanism of production, and significance of the oxCEs that we discovered from HMGECs remains to be explored.
Potential for PGF2α’s effects to translate to the ocular surface with dosing of anti-glaucoma PGAs
Our experiments were designed to identify changes in the HMGEC lipidome and cell viability due to PGE2 and PGF2α after one exposure for three hours. Alteration in lipid production is just one mechanism that could lead to obstructive MGD as a result of PGA use in glaucoma.57 Other mechanisms almost definitely exist, such as prostaglandin-mediated inflammation and preservative-induced toxicity. It is currently unknown whether the changes we observed in lipid expression are also produced by anti-glaucoma PGAs, but other researchers have begun investigating similar outcomes. Kam et al first reported that bimatoprost adversely influences a marker of HMGEC survivability but that it has no effect on lipid production assessed by LipidTOX, a nonpolar lipid stain.67 Unfortunately, the use of bimatoprost in this study complicates generalizability to other PGAs, as bimatoprost is technically a prostamide analog whose functions may or may not be mediated through the FP receptor.14,68,69 More recently, Rath and colleagues evaluated several preserved and non-preserved formulations of PGAs on HMGECs.70 They found that only latanoprost affected cell viability and that bimatoprost upregulated cornulin and involucrin mRNA, two keratins that may be upregulated in MGD.70 This study reportedly used commercially available formulations of PGAs, though it was unclear how these eye drops were added to the cell culture media without affecting the original concentration of the PGAs and without diluting the components of the culture media itself. Regardless, it is important to note that antiglaucoma PGAs are significantly more concentrated than the physiologic concentrations of PGF2α that we used in this study. Specifically, commercially available latanoprost is approximately 100 times more concentrated than our highest dose of PGF2α; bimatoprost is about 240 to 720 times more concentrated. Ultimately, it remains unknown whether anti-glaucoma PGAs mimic the effects of PGF2α observed here, what concentrations of PGAs reach the meibomian glands, or whether there are cumulative effects with daily instillation.
In vivo, iatrogenic MGD that is associated with anti-glaucoma therapeutics is a problem compounded by long-term, daily instillation of preserved eye drops.12 One perceived limitation of our experiments, therefore, could be the short duration of exposure (three hours) to the prostaglandin challenge. This cell culture model, however, is supported by the pharmacokinetics of prostaglandins and prostaglandin analogs. Ishihara et al reported that the half-lives of PGE2 and PGF2α in cell culture experimentation are approximately 9 hours and 15 hours, respectively, and further asserted that incubations greater than a few hours would be subject to significant metabolism.71 Repeated daily introductions of PGE2 and PGF2α were considered; however, Sjöquist et al previously reported that the pharmacokinetics of latanoprost, a representative PGF2α analog used to treat glaucoma, were similar between single doses and repeated doses.72 Latanoprost showed no accumulation in tissues, suggesting that repeated dosing of prostaglandins specifically is an unlikely contributor to disease development. Therefore, we chose to perform a single challenge to PGE2 and PGF2α for three hours, a duration that is under the reported half-lives of PGE2 and PGF2α and equivalent to the known pharmacokinetics of latanoprost (half-life between two to four hours73,74). Guided by these literature sources, we found that even a short duration of exposure to prostaglandins is sufficient to alter the lipidomic expression from HMGECs.
Another perceived weakness of our study may be our decision to differentiate with rosiglitazone, a PPARγ agonist, considering that PGF2α is known to influence PPARγ function. Previous research has shown that PGF2α can block differentiation of adipocytes by inhibiting PPARγ if introduced in the first two days of treatment.58 We, however, did not introduce the prostaglandins until two days after the initial exposure to differentiating conditions, allowing the cells to reach a more mature, differentiated state. Further, the decision to utilize rosiglitazone-induced differentiation is well-supported by literature that has more comprehensively defined and characterized its mechanism30,31,43,44,75 compared to other differentiating agents,33,76,77 reinforcing its use as a preferred method for HMGEC differentiation. Lastly, terminally differentiated meibocytes in vivo are believed to be under the influence of PPARγ regulation, so any interference by prostaglandins would likely translate into a similar interference that would occur at the ocular surface.78
In conclusion, we have reported that HMGECs express FP receptors and three EP receptors (EP1, EP2, and EP4), potentially making them vulnerable to undesirable side effects caused by the PGF2α analogs used in clinical practice to treat glaucoma. Just one exposure to PGF2α, to an extent greater than PGE2, led to lipidomic remodeling of HMGECs with significant changes observed in the expression of both CEs and TAGs. Alterations to the lipid chemistry of the meibomian gland secretions could affect the biochemical and biophysical interactions of the tear film lipid layer, potentially altering tear film viscosity and tear film stability. Further work is needed to determine how these observations translate to commercially available PGAs on the ocular surface.
Acknowledgments:
The authors would like to thank Drs. Jose Luis Roig-Lopez and Steven Pittler for their assistance with immunocytochemistry, fluorescent microscopy, and image deconvolution.
Funding:
Career development support for the first author was provided by the National Eye Institute under K23 EY028629-01. This work was further supported by the Office of Research Infrastructure Programs of the National Institutes of Health under S10 RR027822-01 and the National Eye Institute under P30 EY003039.
Footnotes
Conflict of interest: The authors have no conflicts of interest to disclose.
References
- 1.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma projections of glaucoma burden through 2040: a systematic review meta-analysis. Ophthalmology. 2014;121(11):2081–2090. [DOI] [PubMed] [Google Scholar]
- 2.Uzunosmanoglu E, Mocan MC, Kocabeyoglu S, Karakaya J, Irkec M. Meibomian Gland Dysfunction in Patients Receiving Long-Term Glaucoma Medications. Cornea. 2016;35(8):1112–1116. [DOI] [PubMed] [Google Scholar]
- 3.Batra R, Tailor R, Mohamed S. Ocular surface disease exacerbated glaucoma: optimizing the ocular surface improves intraocular pressure control. J Glaucoma. 2014;23(1):56–60. [DOI] [PubMed] [Google Scholar]
- 4.Broadway DC, Grierson I, O’Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medication. II. The outcome of filtration surgery. Arch Ophthalmol. 1994;112(11):1446–1454. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh S, O’Hare F, Lamoureux E, Vajpayee RB, Crowston JG. Prevalence of signs symptoms of ocular surface disease in individuals treated not treated with glaucoma medication. Clin Exp Ophthalmol. 2012;40(7):675–681. [DOI] [PubMed] [Google Scholar]
- 6.Kim JH, Shin YU, Seong M, Cho HY, Kang MH. Eyelid Changes Related to Meibomian Gland Dysfunction in Early Middle-Aged Patients Using Topical Glaucoma Medications. Cornea. 2018;37(4):421–425. [DOI] [PubMed] [Google Scholar]
- 7.Agnifili L, Brescia L, Oddone F, Sacchi M, D’Ugo E, Di Marzio G, et al. The ocular surface after successful glaucoma filtration surgery: a clinical, in vivo confocal microscopy, immune-cytology study. Sci Rep. 2019;9(1):11299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SM, Lee JE, Kim SI, Jung JH, Shin J. Effect of topical glaucoma medication on tear lipid layer thickness in patients with unilateral glaucoma. Indian J Ophthalmol. 2019;67(8):1297–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwab IR, Linberg JV, Gioia VM, Benson WH, Chao GM. Foreshortening of the inferior conjunctival fornix associated with chronic glaucoma medications. Ophthalmology. 1992;99(2):197–202. [DOI] [PubMed] [Google Scholar]
- 10.Liesegang TJ. Conjunctival changes associated with glaucoma therapy: implications for the external disease consultant the treatment of glaucoma. Cornea. 1998;17(6):574–583. [DOI] [PubMed] [Google Scholar]
- 11.Tauber J, Melamed S, Foster CS. Glaucoma in patients with ocular cicatricial pemphigoid. Ophthalmology. 1989;96(1):33–37. [DOI] [PubMed] [Google Scholar]
- 12.Mocan MC, Uzunosmanoglu E, Kocabeyoglu S, Karakaya J, Irkec M. The Association of Chronic Topical Prostaglandin Analog Use With Meibomian Gland Dysfunction. J Glaucoma. 2016;25(9):770–774. [DOI] [PubMed] [Google Scholar]
- 13.Cho WH, Lai IC, Fang PC, Chien CC, Tseng SL, Lai YH, et al. Meibomian Gland Performance in Glaucomatous Patients With Long-term Instillation of IOP-lowering Medications. J Glaucoma. 2018;27(2):176–183. [DOI] [PubMed] [Google Scholar]
- 14.Hollo G The side effects of the Prostaglandin analogues. Expert Opin Drug Saf. 2007;6(1):45–52. [DOI] [PubMed] [Google Scholar]
- 15.Lindsey JD, Kashiwagi K, Boyle D, Kashiwagi F, Firestein GS, Weinreb RN. Prostaglandins increase proMMP-1 proMMP-3 secretion by human ciliary smooth muscle cells. Curr Eye Res. 1996;15(8):869–875. [DOI] [PubMed] [Google Scholar]
- 16.Breyer MD, Breyer RM. G protein-coupled prostanoid receptors the kidney. Annu Rev Physiol. 2001;63:579–605. [DOI] [PubMed] [Google Scholar]
- 17.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes signaling. Annu Rev Pharmacol Toxicol. 2001;41:661–690. [DOI] [PubMed] [Google Scholar]
- 18.Abramovitz M, Boie Y, Nguyen T, Rushmore TH, Bayne MA, Metters KM, et al. Cloning expression of a cDNA for the human prostanoid FP receptor. J Biol Chem. 1994;269(4):2632–2636. [PubMed] [Google Scholar]
- 19.Schachtschabel U, Lindsey JD, Weinreb RN. The mechanism of action of Prostaglandins on uveoscleral outflow. Curr Opin Ophthalmol. 2000;11(2):112–115. [DOI] [PubMed] [Google Scholar]
- 20.Cunniffe MG, Medel-Jimenez R, Gonzalez-Cial M. Topical antiglaucoma treatment with Prostaglandin analogues may precipitate meibomian gland disease. Ophthalmic Plast Reconstr Surg. 2011;27(5):e128–129. [DOI] [PubMed] [Google Scholar]
- 21.Green-Church KB, Butovich I, Willcox M, Borchman D, Paulsen F, Barabino S, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on tear film lipids lipid-protein interactions in health disease. Invest Ophthalmol Vis Sci. 2011;52(4):1979–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, Green KB, Nichols KK. Quantitative profiling of major neutral lipid classes in human meibum by direct infusion electrospray ionization mass spectrometry. Invest Ophthalmol Vis Sci. 2013;54(8):5730–5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Nichols KK. Comprehensive shotgun lipidomics of human meibomian gland secretions using MS/MS(all) with successive switching between acquisition polarity modes. J Lipid Res. 2018;59(11):2223–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, Green-Church KB, Nichols KK. Shotgun lipidomic analysis of human meibomian gland secretions with electrospray ionization tem mass spectrometry. Invest Ophthalmol Vis Sci. 2010;51(12):6220–6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicolaides N, Kaitaranta JK, Rawdah TN, Macy JI, Boswell FM, 3rd, Smith RE. meibomian gland studies: comparison of steer human lipids. Invest Ophthalmol Vis Sci. 1981;20(4):522–536. [PubMed] [Google Scholar]
- 26.McCulley JP, Shine W. A compositional based model for the tear film lipid layer. Trans Am Ophthalmol Soc. 1997;95:79–88; discussion 88–93. [PMC free article] [PubMed] [Google Scholar]
- 27.Cory CC, Hinks W, Burton JL, Shuster S. meibomian gland secretion in the red eyes of rosacea. Br J Dermatol. 1973;89(1):25–27. [DOI] [PubMed] [Google Scholar]
- 28.Mathers WD, Lane JA. meibomian gland lipids, evaporation, tear film stability. Adv Exp Med Biol. 1998;438:349–360. [DOI] [PubMed] [Google Scholar]
- 29.Ziemanski J, Chen J, Nichols KK. The effects of omega-6:omega-3 fatty acid ratios on the lipidome from human meibomian gland epithelial cells treated with without 13-cis retinoic acid. ARVO; 2018; Honolulu, HI. [Google Scholar]
- 30.Ziemanski JF, Wilson L, Barnes S, Nichols KK. Triacylglycerol lipidome from human meibomian gland epithelial cells: description, response to culture conditions, perspective on function. Exp Eye Res. 2020;207(June 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ziemanski JF, Wilson L, Barnes S, Nichols KK. Saturation of cholesteryl esters produced by human meibomian gland epithelial cells after treatment with rosiglitazone. Ocul Surf. 2020;20:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ziemanski JF, Chen J, Nichols KK. Evaluation of Cell Harvesting Techniques to Optimize Lipidomic Analysis from Human Meibomian Gland Epithelial Cells in Culture. Int J Mol Sci. 2020;21(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan DA, Liu Y, Kam WR, Ding J, Green KM, Shaffer SA, et al. Serum-induced differentiation of human meibomian gland epithelial cells. Invest Ophthalmol Vis Sci. 2014;55(6):3866–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu S, Hatton MP, Khelwal P, Sullivan DA. Culture, immortalization, characterization of human meibomian gland epithelial cells. Invest Ophthalmol Vis Sci. 2010;51(8):3993–4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hampel U, Schroder A, Mitchell T, Brown S, Snikeris P, Garreis F, et al. Serum-induced keratinization processes in an immortalized human meibomian gland epithelial cell line. PLoS One. 2015;10(6):e0128096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 37.Woodward DF, Regan JW, Lake S, Ocklind A. The molecular biology ocular distribution of prostanoid receptors. Surv Ophthalmol. 1997;41 Suppl 2:S15–21. [DOI] [PubMed] [Google Scholar]
- 38.Mukhopadhyay P, Bian L, Yin H, Bhattacherjee P, Paterson C. Localization of EP(1) FP receptors in human ocular tissues by in situ hybridization. Invest Ophthalmol Vis Sci. 2001;42(2):424–428. [PubMed] [Google Scholar]
- 39.Schlotzer-Schrehardt U, Zenkel M, Nusing RM. Expression localization of FP EP prostanoid receptor subtypes in human ocular tissues. Invest Ophthalmol Vis Sci. 2002;43(5):1475–1487. [PubMed] [Google Scholar]
- 40.Reginato MJ, Krakow SL, Bailey ST, Lazar MA. Prostaglandins promote block adipogenesis through opposing effects on peroxisome proliferator-activated receptor gamma. J Biol Chem. 1998;273(4):1855–1858. [DOI] [PubMed] [Google Scholar]
- 41.Rosen ED, Spiegelman BM. PPARgamma : a nuclear regulator of metabolism, differentiation, cell growth. J Biol Chem. 2001;276(41):37731–37734. [DOI] [PubMed] [Google Scholar]
- 42.Hammarstedt A, ersson CX, Rotter Sopasakis V, Smith U. The effect of PPARgamma ligs on the adipose tissue in insulin resistance. Prostaglandins Leukot Essent Fatty Acids. 2005;73(1):65–75. [DOI] [PubMed] [Google Scholar]
- 43.Kim SW, Xie Y, Nguyen PQ, Bui VT, Huynh K, Kang JS, et al. PPARgamma regulates meibocyte differentiation lipid synthesis of cultured human meibomian gland epithelial cells (hMGEC). Ocul Surf. 2018;16(4):463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim SW, Brown DJ, Jester JV. Transcriptome analysis after PPARgamma activation in human meibomian gland epithelial cells (hMGEC). Ocul Surf. 2019;17(4):809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen W, Tsai SJ, Wang CA, Tsai JC, Zouboulis CC. Human sebocytes express Prostaglandin E2 receptors EP2 EP4 but treatment with Prostaglandin E2 does not affect testosterone production. Br J Dermatol. 2009;161(3):674–677. [DOI] [PubMed] [Google Scholar]
- 46.Markovic T, Jakopin Z, Dolenc MS, Mlinaric-Rascan I. Structural features of subtype-selective EP receptor modulators. Drug Discov Today. 2017;22(1):57–71. [DOI] [PubMed] [Google Scholar]
- 47.Foller M, Kasinathan RS, Duranton C, Wieder T, Huber SM, Lang F. PGE2-induced apoptotic cell death in K562 human leukaemia cells. Cell Physiol Biochem. 2006;17(5–6):201–210. [DOI] [PubMed] [Google Scholar]
- 48.Lang PA, Kempe DS, Myssina S, Tanneur V, Birka C, Laufer S, et al. PGE(2) in the regulation of programmed erythrocyte death. Cell Death Differ. 2005;12(5):415–428. [DOI] [PubMed] [Google Scholar]
- 49.Turini ME, DuBois RN. Cyclooxygenase-2: a therapeutic target. Annu Rev Med. 2002;53:35–57. [DOI] [PubMed] [Google Scholar]
- 50.Tsujii M, DuBois RN. Alterations in cellular adhesion apoptosis in epithelial cells overexpressing Prostaglandin endoperoxide synthase 2. Cell. 1995;83(3):493–501. [DOI] [PubMed] [Google Scholar]
- 51.Jansen KM, Pavlath GK. Prostaglandin F2alpha promotes muscle cell survival growth through upregulation of the inhibitor of apoptosis protein BRUCE. Cell Death Differ. 2008;15(10):1619–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ambaw YA, Chao C, Ji S, Raida M, Torta F, Wenk MR, et al. Tear eicosanoids in healthy people ocular surface disease. Sci Rep. 2018;8(1):11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lekhanont K, Sathianvichitr K, Pisitpayat P, Anothaisintawee T, Soontrapa K, Udomsubpayakul U. Association between the levels of Prostaglandin E2 in tears severity of dry eye. Int J Ophthalmol. 2019;12(7):1127–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shim J, Park C, Lee HS, Park MS, Lim HT, Chauhan S, et al. Change in Prostaglandin expression levels synthesizing activities in dry eye disease. Ophthalmology. 2012;119(11):2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kroin JS, Buvanendran A, Watts DE, Saha C, Tuman KJ. Upregulation of cerebrospinal fluid peripheral Prostaglandin E2 in a rat postoperative pain model. Anesth Analg. 2006;103(2):334–343, table of contents. [DOI] [PubMed] [Google Scholar]
- 56.Liu S, Kam WR, Ding J, Hatton MP, Sullivan DA. Effect of growth factors on the proliferation gene expression of human meibomian gland epithelial cells. Invest Ophthalmol Vis Sci. 2013;54(4):2541–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52(4):1938–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Casimir DA, Miller CW, Ntambi JM. Preadipocyte differentiation blocked by Prostaglandin stimulation of prostanoid FP2 receptor in murine 3T3-L1 cells. Differentiation. 1996;60(4):203–210. [DOI] [PubMed] [Google Scholar]
- 59.Maldve RE, Kim Y, Muga SJ, Fischer SM. Prostaglandin E(2) regulation of cyclooxygenase expression in keratinocytes is mediated via cyclic nucleotide-linked Prostaglandin receptors. J Lipid Res. 2000;41(6):873–881. [PubMed] [Google Scholar]
- 60.Ueno T, Fujimori K. Novel suppression mechanism operating in early phase of adipogenesis by positive feedback loop for enhancement of cyclooxygenase-2 expression through Prostaglandin F2alpha receptor mediated activation of MEK/ERK-CREB cascade. FEBS J. 2011;278(16):2901–2912. [DOI] [PubMed] [Google Scholar]
- 61.Yousufzai SY, Ye Z, Abdel-Latif AA. Prostaglandin F2 alpha its analogs induce release of endogenous Prostaglandins in iris ciliary muscles isolated from cat other mammalian species. Exp Eye Res. 1996;63(3):305–310. [DOI] [PubMed] [Google Scholar]
- 62.Yu K, Bayona W, Kallen CB, Harding HP, Ravera CP, McMahon G, et al. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J Biol Chem. 1995;270(41):23975–23983. [DOI] [PubMed] [Google Scholar]
- 63.Neyrinck AM, Margagliotti S, Gomez C, Delzenne NM. Kupffer cell-derived Prostaglandin E2 is involved in regulation of lipid synthesis in rat liver tissue. Cell Biochem Funct. 2004;22(5):327–332. [DOI] [PubMed] [Google Scholar]
- 64.Mater MK, Thelen AP, Jump DB. Arachidonic acid PGE2 regulation of hepatic lipogenic gene expression. J Lipid Res. 1999;40(6):1045–1052. [PubMed] [Google Scholar]
- 65.Enomoto N, Ikejima K, Yamashina S, Enomoto A, Nishiura T, Nishimura T, et al. Kupffer cell-derived Prostaglandin E(2) is involved in alcohol-induced fat accumulation in rat liver. Am J Physiol Gastrointest Liver Physiol. 2000;279(1):G100–106. [DOI] [PubMed] [Google Scholar]
- 66.Borchman D, Foulks GN, Yappert MC, Milliner SE. Differences in human meibum lipid composition with meibomian gland dysfunction using NMR principal component analysis. Invest Ophthalmol Vis Sci. 2012;53(1):337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kam WR, Liu Y, Ding J, Sullivan DA. Do Cyclosporine A, an IL-1 Receptor Antagonist, Uridine Triphosphate, Rebamipide, /or Bimatoprost Regulate Human Meibomian Gland Epithelial Cells? Invest Ophthalmol Vis Sci. 2016;57(10):4287–4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Woodward DF, Liang Y, Krauss AH. Prostamides (Prostaglandin-ethanolamides) their pharmacology. Br J Pharmacol. 2008;153(3):410–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Camras CB, Toris CB, Sjoquist B, Milleson M, Thorngren JO, Hejkal TW, et al. Detection of the free acid of bimatoprost in aqueous humor samples from human eyes treated with bimatoprost before cataract surgery. Ophthalmology. 2004;111(12):2193–2198. [DOI] [PubMed] [Google Scholar]
- 70.Rath A, Eichhorn M, Trager K, Paulsen F, Hampel U. In vitro effects of benzalkonium chloride Prostaglandins on human meibomian gland epithelial cells. Ann Anat. 2019;222:129–138. [DOI] [PubMed] [Google Scholar]
- 71.Ishihara O, Sullivan MH, Elder MG. Differences of metabolism of Prostaglandin E2 F2 alpha by decidual stromal cells macrophages in culture. Eicosanoids. 1991;4(4):203–207. [PubMed] [Google Scholar]
- 72.Sjoquist B, Uhlin A, Byding P, Stjernschantz J. Pharmacokinetics of latanoprost in the cynomolgus monkey. 2nd communication: repeated topical administration on the eye. Arzneimittelforschung. 1999;49(3):234–239. [DOI] [PubMed] [Google Scholar]
- 73.Sjoquist B, Stjernschantz J. Ocular systemic pharmacokinetics of latanoprost in humans. Surv Ophthalmol. 2002;47 Suppl 1:S6–12. [DOI] [PubMed] [Google Scholar]
- 74.Sjoquist B, Johansson A, Stjernschantz J. Pharmacokinetics of latanoprost in the cynomolgus monkey. 3rd communication: tissue distribution after topical administration on the eye studied by whole body autoradiography. Glaucoma Research Laboratories. Arzneimittelforschung. 1999;49(3):240–249. [DOI] [PubMed] [Google Scholar]
- 75.Jester JV, Potma E, Brown DJ. PPARgamma Regulates Mouse Meibocyte Differentiation Lipid Synthesis. Ocul Surf. 2016;14(4):484–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Y, Kam WR, Ding J, Sullivan DA. One man’s poison is another man’s meat: using azithromycin-induced phospholipidosis to promote ocular surface health. Toxicology. 2014;320:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu Y, Kam WR, Ding J, Sullivan DA. Effect of azithromycin on lipid accumulation in immortalized human meibomian gland epithelial cells. JAMA Ophthalmol. 2014;132(2):226–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hwang HS, Parfitt GJ, Brown DJ, Jester JV. Meibocyte differentiation renewal: Insights into novel mechanisms of meibomian gland dysfunction (MGD). Exp Eye Res. 2017;163:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]


