Abstract
Bacillus Calmette–Guérin (BCG) vaccine is an attenuated live strain of Mycobacterium bovis. It may be the most widely used vaccine in human history and is the only licensed human tuberculosis (TB) vaccine available. Despite its excellent safety history, a century of use in global vaccination programs, and its significant contribution to reducing TB mortality among children, the efficacy of BCG continues to be disputed due to its incomplete protection against pulmonary TB in adults. Still vaccines offer the best chance to contain the ongoing spread of multi-drug resistance TB and disease dissemination. The development of improved vaccines against TB therefore remains a high global priority. Interestingly, recent studies indicate that genetically modified BCG, or administration of existing BCG through alternate routes, or revaccination, offers improved protection, suggesting that BCG is well poised to make a comeback.
Intravesical BCG is also the only approved microbial immunotherapy for any form of cancer, and is the first-line therapy for treatment-naïve non-muscle invasive bladder cancer (NMBIC), which represents a majority of the new bladder cancer cases diagnosed. However, almost a third of patients with NMIBC are either BCG unresponsive or have tumor recurrence, leading to a higher risk of disease progression to malignancy. With very few advances in intravesical therapy over the past two decades for early-stage disease, and a limited pipeline of therapeutics in Phase 3 or late Phase 2 development, there is a major unmet need for improved intravesical therapies for NMIBC. Indeed, genetically modified candidate BCG vaccines engineered to express molecules that confer stronger protection against pulmonary TB or induce potent anti-tumor immunity in NMIBC have shown great promise in both pre-clinical and clinical settings. This review discusses the development of second generation of genetically modified BCG candidates as TB vaccines and as anti-tumor adjuvant therapy for NMIBC.
Keywords: BCG, tuberculosis, bladder cancer, vaccine, immunotherapy, trained immunity
1.0. Introduction
BCG for TB prevention.
A hundred years ago, BCG, a vaccine developed by Albert Calmette and Camille Guérin through experimental attenuation of Mycobacterium bovis [1], was first administered to humans to protect against tuberculosis (TB) [2]. BCG may be the most widely administered of all vaccines with an estimated ~152 million doses administered to newborns annually in virtually all nations of the world except the US and Canada and certain countries in Western Europe [3]. Despite its remarkable safety profile, and effectiveness in the prevention of extra-pulmonary TB in children, BCG fails to provide adequate protection in preventing pulmonary TB in adolescents and adults, which represents the highest TB burden in the global fight against TB [4–6]. BCG’s incomplete protection against adult pulmonary TB has been attributed in part to pre-existing immune responses to antigens that are common to environmental mycobacteria and Mycobacterium tuberculosis (Mtb) and to waning protection (~10–20 years) following childhood vaccination [7,8]. These limitations of BCG suggest the need for either an improved neonatal vaccine which confers non-waning protection or development of a modified adult vaccine to boost failing BCG-induced immunity.
BCG for bladder cancer immunotherapy.
The use of microbes in cancer immunotherapy was pursued long before 1976 when Morales and others established the salutary effects of intravesical BCG on human NMIBC patients [9–11]. Now 45 years later, BCG remains the only bacterial agent approved by the FDA for cancer and remains the gold-standard treatment for patients with intermediate- and high-risk NMIBC [12]. Despite this, ~40% of NMIBC patients do not respond to BCG or show high-grade recurrence that typically require cystectomy or intravesical cytotoxic chemotherapy [12]. Although intravesical BCG induces a strong antitumor immune response, the absence of long-lasting immunity and strain-specific suboptimal immune responses are thought to contribute to limited BCG efficacy [12]. While enhancing the immunogenicity of BCG for bladder cancer may improve efficacy, such efforts must be tempered with the need to maintain safety. In order to decrease the mortality, morbidity, and the burden of health-care costs related to bladder cancer, development of novel treatment options for BCG-unresponsive NMIBC, including improving the efficacy of BCG, remains a high clinical priority.
BCG and trained immunity.
Recent clinical studies suggest that BCG vaccination may have heterologous protective effects against generalized viral infections including COVID-19 [13,14]. This BCG-induced mechanism of protection against heterologous pathogens has been termed ‘trained immunity’ and has been correlated with BCG-mediated epigenetic reprogramming of innate immune cells [15,16]. Detailed discussions of BCG-mediated ‘trained immunity’, a paradigm shift in our understanding of innate immune system, can be found in several recent reviews [17,18]. Briefly, it is believed that BCG-linked microbial ligands such as MDP2 activate NOD2-dependent metabolic, epigenetic and transcriptional reprogramming, leading to a heightened immune activation and enhanced production of pro-inflammatory cytokines and antigen-independent, enhanced immune responses to subsequent pathogen challenge [19,20]. A recent study indicating that BCG-mediated training of hematopoietic stem cells in the bone marrow may explain how trained immunity mechanisms enhance protective immunity against TB [21]. In light of this heterologous activity of BCG, it is possible that certain recombinant BCGs may also offer benefit in the non-traditional settings where BCG has been used in tumors aside from bladder cancer or infectious diseases other than TB such as viral infections [22].
2.0. Studies towards recombinant BCG vaccines with improved efficacy and safety for TB
In 2019 there were an estimated 10 million new cases of active TB disease and 1.4 million deaths were reported worldwide, fueled largely by lapses in drug adherence, the emergence of multi-drug resistance strains of Mtb, and high co-infection rates with HIV [23]. The target of WHO’s post 2015 End Global TB Strategy is to reduce global TB deaths by 95% and to reduce new cases of TB by 90% by the year 2035 [23]. Global TB control efforts are also likely to suffer substantial setbacks from COVID-19 pandemic-related disruption both in TB health services and in basic and clinical research [24].
BCG-mediated protective immunity against TB: helpful against childhood disseminated disease but little protection against adult pulmonary TB.
As discussed previously, BCG is currently the only licensed vaccine against TB. An estimated 154 of 180 countries recommend universal BCG vaccination, and >4 billion people worldwide have received BCG since 1921 [25,26]. In countries with a high prevalence of TB, WHO recommends administration of a single dose of BCG to all infants shortly after birth, except infants or persons known to have HIV or immunosuppressive conditions [23]. In countries with a low TB incidence, mainly in Europe, North America and Australia, immunization is currently restricted to those neonates and infants at increased risk [23]. While BCG immunization reaches a global coverage of more than 90% and is estimated to provide ~85% protection against disseminated TB in children, it fails to adequately protect against adult pulmonary TB probably due to waning cell-mediated immunity [23].
Despite the waning immunity hypothesis, most studies of BCG revaccination of adolescents or adults have not demonstrated additional preventive efficacy against adult pulmonary TB [27], although there have been some exceptions [28]. Multiple studies have tried to uncover the underlying causes of differential BCG efficacy, and factors that are most likely to contribute include geographical location, exposure to environmental mycobacteria, age at vaccination, host HLA allelic variation, and genetic differences in BCG strains in use [29]. One popular explanation for the lack of efficacy of BCG revaccination is that the combination of exposure to environmental mycobacteria as well as BCG in the neonatal period may prevent BCG proliferation at the time of revaccination and hence reduce immune exposure.
Needs and challenges in modifying BCG as an improved TB vaccine.
The WHO Preferred Product Characteristics (PPC) for New Tuberculosis Vaccines [30], is an important guideline for developing new vaccine candidates. Among the goals it sets forth are development of a safe, effective, and affordable TB vaccine to be used among neonates, infants, adolescents and adults with improved safety and efficacy as compared to existing BCG. Additional goals are that new TB vaccines for adolescents and adults should be greater than 50% efficacious in preventing confirmed pulmonary TB, and should remain protective in individuals with and without latent Mtb infections for at least more than 10 years after primary immunization. For neonates, new TB vaccines should protect from severe and disseminated TB, TB meningitis, as well as pulmonary TB with equal or greater than 80% efficacy as compared to BCG. Lastly, new TB vaccines should be safe enough to be administered to neonates and infants with innate or acquired immunodeficiencies, including HIV-infected infants.
Major challenges towards accomplishing these goals include: (i) lack of validated immune correlates of protection against disease that are known to reliably predict vaccine efficacy in humans (such correlates may include Mtb-antigen specific T cells, polyfunctional T cells, whole blood RNA signatures, and antibody responses [31]), and (ii) our poor understanding of how to balance BCG proliferation (thought important for protective efficacy) with vaccine safety particularly in risk groups such as HIV+ individuals, immunocompromised individuals and the elderly where the risk of severe local or disseminated BCG infection is elevated. Additionally, while small animal models for vaccine efficacy testing and safety testing such as the mouse and guinea pig models have been widely implemented, the degree to which they predict human efficacy is problematic, and there are clear examples of vaccine candidates such as MVA85A that showed small animal efficacy that subsequently failed in large human clinical trials [32]. Nevertheless, the non-human primate model, while expensive, is currently considered the closest human predictor for pre-clinical vaccine evaluation [33].
Alternative BCG vaccination routes for improved protection against TB:
Despite limited efficacy of BCG in protection against adult pulmonary TB, some recent animal studies testing alternative routes of vaccination suggest that the capabilities of the BCG vaccine have not been fully optimized. That changing the dose and routes of administration (from intradermal to the aerosol, intravenous (IV), intranasal, or intratracheal) significantly increases the protective efficacy of BCG has been demonstrated in both mice and non-human primates [34–38]. Two of the studies using non-human primates showed that animals vaccinated intravenously with BCG showed no evidence of any TB infection in challenged animals using PET/CT in life and CFU analysis at necropsy [35,37]. Although the safety of these routes is difficult to gauge in these animal models, the ability of IV BCG to prevent not only disease, but also infection has garnered significant attention.
Approaches in the development of novel recombinant BCG candidates:
As previously described, the limited protective immunity of BCG in adults against pulmonary TB after neonatal BCG vaccination may be due to waning immunity that eventually drops under a DTH immune threshold needed for immunological protection against TB, as documented the large southern Indian BCG vaccine trial [8,39]. Serious efforts have been made to address this potential limitation of the BCG vaccine by generating variants engineered to express desired foreign antigens that would improve priming of the immune system and hence generation of more sustained immunity against TB [8,40]. While BCG revaccination has been shown to be safe [40], repeated doses of traditional BCG have not been shown to confer improved protection against TB disease [40]. Re-engineered BCGs, in contrast, may offer the potential to (i) offer prolonged immunity after neonatal vaccination which is not subject to waning, (ii) enhance BCG-induced immunity in adults previously vaccinated with traditional BCG. It should also be mentioned that re-engineered forms of BCG may also have value among the non-traditional diseases (non-TB, non-bladder cancer) where BCG is being studied such as protection against viral diseases or solid tumors [22,40,41].
The last two decades have seen considerable efforts to develop safer and more efficacious recombinant BCG vaccine candidates. BCG remains a highly attractive choice as a microbe-based delivery vehicle for desired foreign antigens since it has (i) an excellent adjuvant profile, (ii) proven safety record and (iii) an ability to induce a durable and robust immune responses [42]. In addition, while the lack of an esx-1 locus makes BCG sufficiently attenuated (a favorable safety phenotype), it compromises penetration of the phagosomal membrane that would allow BCG-released products to make direct cytosolic contact and induce host cell responses following uptake by macrophages and dendritic cells (DCs) [43,44]. Approaches to address the existing limitations of BCG include overexpression of dominant antigens or facilitating antigenic escape from the phagosomal compartment into the cross-priming machinery [45]. Some of these approaches demonstrated success in improving the immunogenicity and antigenicity of rBCGs in preclinical models (Table 1). The generation of rBCGs has been fueled by the discovery of mycobacterial shuttle vectors carrying strong promoters (mycobacterial hsp60 and hsp70 promoters) and the extrachromosomal and integrative expression of desired foreign antigens in BCG [46]. A number of rBCG candidates secreting numerous foreign antigens have been developed, and in general these generate stronger immune responses and in some cases are safer than existing BCG in preclinical models (Table 1). However, the complex nature of the immune response following BCG vaccination and our lack of understanding of the correlates of protection against TB disease has made it challenging to identify Mtb antigens, human cytokines, and innate immune pathways which should be manipulated. Below, we discuss four strategies which have been explored by recombinant BCG research to date: (i) expression of Mtb antigens (alone or as fusion proteins), (ii) expression of human cytokines, (iii) alteration of phagosome permeability, and (iv) expression of small molecules with stimulate innate immunity. While there is also a significant literature on rBCGs for immune priming (where prior exposure to BCG serves as an adjuvant to enhance immunity to a subsequent vaccine) in two-vaccine prime-boost strategies, we refer readers to other review that address that topic [47,48].
Table. 1:
Recombinant BCG (rBCG) strains tested as TB vaccine candidates.
| Development of rBCG strains expressing bacterial antigens and immune mediators tested as TB vaccine candidates | |||
|---|---|---|---|
| Name | Foreign antigen | Immune response and safety | Therapeutic efficacy |
| rBCG30 | Mtb - 30kDa secretory protein | Cutaneous DTH response and increased antibody titer | Improved protective immunity (Guinea pig model of Mtb infection) |
| Horwitz et al., 2006 | |||
| Ref. 57. | |||
| PMID: 16257099 | |||
| rBCG (mbtB)30 | M. tb. - 30kDa secretory protein, deleted mbtB | More attenuated than BCG in SCID mice | Improved protective efficacy (Guinea pig model of Mtb infection) |
| Tullius et al., 2008 | |||
| Ref. 59. | |||
| PMID: 18725418 | |||
| rBCG (panCD)30 | M. tb. - 30kDa secretory protein and deleted panCD; pantothenate auxotroph | More attenuated than BCG in SCID mice | Improved protective efficacy (Guinea pig model of Mtb infection) |
| Tullius et al., 2008 | |||
| Ref. 59. | |||
| PMID: 18725418 | |||
| rBCG::Ag85A | Mtb-Ag85A antigen | Stronger antigen-specific IFN-γ responses and higher antibody titers | Improved protective efficacy in (C57BL/6 mouse model of Mtb infection) |
| Xu et al., 2016 | |||
| Ref. 60. | |||
| PMID: 26858942 | |||
| rBCG::AB | Mtb-Ag85A and Ag85B antigen | Increased antigen-specific IFN-γ response in vitro | Long-term protection (C57BL/6 mouse model of Mtb infection) |
| Wang et al., 2012 | |||
| Ref. 61 | |||
| PMID: 22570667 | |||
| rBCG::XB | Mtb-Ag85B and HspX latency antigen | Increased antigen specific CD4+ and CD8+ T cells. Increased CD4+ TEM and CD8+ TCM response | Long-term protection (C57BL/6 mouse model of Mtb infection) |
| Yuan et al., 2015 | |||
| Ref. 62. | |||
| PMID: 26363555 | |||
| BCG85B-ES | Mtb-Ag85B and ESAT-6 fusion protein | No evidence | Improved protective efficacy (Mouse model of Mtb infection) |
| Palendira et al., | |||
| PMID: 15705472 | |||
| rBCG-AE | Mtb-Ag85A-ESAT-6 fusion protein | No evidence | No improvement in protective efficacy (BALB/c mouse model of Mtb infection) |
| Deng et al., 2014 | |||
| Ref. 63. | |||
| PMID: 23357605 | |||
| rBCG::Ag85B-ESAT6-Rv2608 | Mtb-Ag85B-ESAT-6-Rv2608 fusion protein | Increased CD4+ and CD8+ T lymphocytes proliferative response, stronger TH1 cytokine response and stronger humoral response | No evidence of protective immunity |
| Lu et al., 2012 | |||
| Ref. 64. | |||
| PMID: 22671973 | |||
| rBCG::Ag85B-ESAT6-Rv3620c | Mtb-Ag85B-ESAT-6-Rv3620c fusion protein | Increased CD4+ and CD8+ T lymphocytes proliferative response, stronger TH1 cytokine response and stronger humoral response | No evidence of protective immunity |
| Yang et al., 2014 | |||
| Ref. 65. | |||
| PMID: 24726737 | |||
| rBCG-AEI | Mtb-Ag85B-ESAT-6 and murine IFN-γ fusion protein | Increased antibody titers and higher cellular immune response as compared to rBCG::AE and wild-type BCG | Improved or similar protective immune response as compared rBCG::AE |
| Xu et al., 2007 | |||
| Ref. 66. | |||
| PMID: 17919299 | |||
| rBCG-Ag85B-ESAT6-TNF- α | Mtb-Ag85B-ESAT-6 and murine TNF- α fusion protein | Stronger immune response (IFN-γ and TNF- α) as compared to rBCG-Ag85B-ESAT-6 or BCG | No evidence of protective immunity |
| Shen et al., 2010 | |||
| Ref. 67. | |||
| PMID: 20646207 | |||
| Ag85B-IL15 | Mtb-Ag85B and murine IL-15 fusion protein | Increased IFN-γ producing CD4+ T cells as compared to rBCG-AG85B or BCG | No evidence of protective immunity |
| Tang et al., 2008 | |||
| Ref. 68. | |||
| PMID: 18422438 | |||
| BCG::ESAT-L28A/L29S | Mutated version (modifications at residue Lue28-Leu29 of ESAT-6 of Mtb | Attenuated for virulence in SCID mice as compared to BCG-ESAT6 and BCG | Improved protective efficacy (C57BL/6 mouse model of Mtb infection) |
| Bottai et al., 2015 | |||
| Ref. 70. | |||
| PMID: 25869896 | |||
| (H)PE-ΔMPT64-BCG | Mtb-MPT64 fused to the PE domain of the PE_PGRS33 protein | Induction of MPT64-specific cytokine producing CD4 and CD8 cells; multifunctional CD4 cells specific for MPT64 | Improved protective efficacy (C57BL/6 mouse model of Mtb infection) |
| Sali et al., 2010 | |||
| PMID: 20921146 | |||
| BCG: ESX-1Mmar | ESX-1 region of M. marinum | Increased Type 1 IFN and infiammasomes, increased antigen specific CD8+ and polyfunctional CD4+ TH1 cells. Reduced virulence in SCID mice | Improved protective efficacy in (C57BL/6 mouse model of Mtb infection) |
| Gröschd et al., 2017 | |||
| Ref. 71 | |||
| PMID: 28297677 | |||
| VPM1002 | N-terminal region of Ag85B of Mtb. listeriolysin O (LLO) of L. monocytogenes and deletion of ureC gene | Increased antigen specific CD4+ and CD8+ T cells, increased autophagy, infiammasome activation and macrophage apoptosis. Reduced virulence in different rodent and non-human primates, safer in humans as compared to BCG | Improved protective efficacy in different animal models |
| (BCG ΔureC::hly+) | |||
| Grode et al. 2005 | |||
| PMID: 16110326 | |||
| VPM1002 ΔnuoG | Genetic modification ofVPM1002 vaccine with deletion of nuoG | Increased autophagic targeting of intracellular BCG, increased CD4+ T response as compared to VPM1002. Excellent safety profile in animal models | Improved protective efficacy as compared to VPM1002 as well as BCG in different animal models |
| Gengenbacher et al., 2016 | |||
| Ref. 76. | |||
| PMID: 27222470 | |||
| VPM1002 Δpdx1 | VPM1002 modified for deletion of pdx-1 (Vitamin B6 auxotrophy) | Increased innate immune response, increased CD4+ T- and B cell response. Increased safety in SCID mice as compared to VPM1002 and BCG | Improved protective efficacy (prime-boost model of Mtb infection) |
| Gengenbacher et al., 2014 | |||
| Ref. 78. | |||
| PMID: 24895310 | |||
| AERAS-401 (BCG1331 ΔureC::ΩpfoAG137Q) | BCG1331 expressing a mutant form of perfringolysin O (PfoAG137Q) from Clostridium perfringens and deleted ureC gene | Excellent safety profile in SCID mice | Improved protective efficacy (mouse model of Mtb infection) |
| Sun et al., 2009 | |||
| Ref. 79. | |||
| PMID: 19500523 | |||
| AFRO-1 or AERAS-422 | AERAS-401 expressing Ag85A, Ag85B, and TB10.4 of Mtb | Enhanced immune response in mice and guinea pigs | Improved protective efficacy (mouse model of hypervirulent Mtb infection) |
| Sun et al., 2009 | |||
| Ref. 79. | |||
| PMID: 19500523 | |||
| rBCG Δzmp1 | BCG zmp1 mutant | Increased safety profile in SCID mice | Improved protective efficacy (guinea pig model of Mtb infection) |
| Sander et al., 2015 | |||
| Ref. 81 | |||
| PMID: 25657094 | |||
| BCG ΔleuD/Ag85B | Mtb-Ag85B antigen expressed in BCG ΔleuD strain | Increased tumor cytotoxicity, strong induction pro-apoptotic genes in cancer cells as compared to wild-type BCG | No efficacy data |
| Begnini et al., 2013 | |||
| PMID: 23053076 | |||
| BCG-disA-OE | Mtb disA diadenylate cyclase gene overexpressed in BCG with excess release of the STING agonist, c-di-AMP | Increased IRF3 activation | Improved protective efficacy (guinea pig model of Mtb infection) |
| Dey et al., 2020 | |||
| Ref. 83 | |||
| PMID: 30901058 | |||
2.1. Recombinant BCGs expressing Mtb antigens alone or as fusion proteins:
BCG attenuation is believed to be a direct consequence of genetic deletions in 16 genomic regions of difference (known as RD1-RD16) in different BCG substrains [44]. The RD1 region contains a genetic segment that encodes immunodominant antigens such as ESAT-6, CFP-10, and several other T-cell antigens and components of the type VII ESAT- secretion system ESX-1 that facilitate release of these virulence factors [43]. Besides acting as a T-cell antigen, ESAT-6 strongly induces IFN-γ secretion by Mtb antigen-independent memory CD8+ T cells and NK cells [49,50]. Indeed, several rBCG candidates expressing Mtb ESAT-6 antigens have shown preclinical efficacy via boosting immune responses against shared mycobacterial antigens expressed under strong promoters [51] (Table. 1). While the ESX-1 secretion locus is may be considered an Mtb antigen, we will discuss rBCGs that utilize ESX-1 elements in section 2.3 under the topic of rBCGs with altered phagosome permeability.
BCG fbpB+ (rBCG30):
This rBCG30 overexpresses the secreted antigen, Ag85B (Rv1886c; mycolyl transferase 85B or fbpB), under its native promoter [52]. It showed improved protective efficacy over its parental strain in a preclinical challenge model and was found to be safe in adult volunteers [53–55]. In a double-blinded phase 1 trial of rBCG30 in 35 adults randomized to receive either rBCG30 or the parental strain, BCG Tice intradermally, rBCG30 induced significantly increased Ag85B-specific T cell lymphoproliferation, interferon-γ secretion, significantly enhanced CD4+ and CD8+ T cells specific to Ag85B that were capable of concurrent expansion and effector function [56]. Another variant of rBCG30 was made by deleting both the mbtB (mycobactin synthetase B) and panCD (pantothenate synthetase C and D) genes rendering it dependent on exogenous mycobactin for iron acquisition or conferring auxotrophy for pantothenate (vitamin B5), respectively [57] (Table. 1).
In a similar approach, an rBCG known as rBCG::Ag85A was designed to express the immunodominant antigen, Ag85A (diacylglycerol acyltransferase or mycolyltransferase Ag85A), and it also showed improved immunogenicity, but only a slight improvement in protective efficacy, over the parental BCG strain in a mouse model of Mtb challenge [58]. In an another rBCG version known as rBCG::AB, co-expression of Mtb antigens, Ag85A and Ag85B in BCG was found to confer a long-lasting protective immunity against Mtb as compared to parental BCG or those expressing either Ag85A or Ag85B in animal models [59].
BCG expressing Mtb antigens as fusion proteins:
Efforts were made to improve BCG protective immunity via co-expression of different Mtb antigens either alone or in combination with desired host cytokines or chemokines to selectively manipulate host anti-TB immunity. Since, BCG induced T cell immunity specifically targets secretory antigens of rapidly multiplying bacteria but not latency antigens in dormant Mtb, multistage fusion proteins of dormant and actively replicating bacteria were expressed to in an effort to extend the durability of protection. As a proof-of-concept, another recombinant strain known as rBCG::XB was developed to overexpress the multistage antigens Ag85B (mycolyl transferase 85B) and HspX (14 kDa latency antigen), which are both expressed in dormancy models of Mtb in vitro, and it was found to provide stronger and long-lasting protection against Mtb as compared to BCG alone [60]. Other multivalent BCG vaccines have been constructed and their safety and immunogenicity tested in animal models, including rBCG-AE (rBCG::Ag85B-ESAT6) [61], rBCG::Ag85B-ESAT6-Rv2608 [62] and rBCG::Ag85BESAT6-Rv3620c [63].
2.2. BCG expressing human cytokines:
A related approach towards multivalency has been to fuse mycobacterial antigens to host cytokines. rBCG strains expressing these fusion proteins such as Ag85B-ESAT6-IFN-γ (rBCG-AEI) [64] or rBCG-Ag85B-ESAT6-TNF-α [65] demonstrated improved cellular immune responses against Mtb thought their protective efficacy remained comparable to BCG. In this class of rBCGs, rBCG-Ag85B-IL15 designed to express Ag85B and the secreted form of IL-15 (a cytokine important for the proliferation and survival of memory CD8+ T cells) elicited increased numbers of IFN-γ producing CD44+ CD4+ and CD44+ CD8+T cells and decreased bacterial burdens after intratracheal Mtb challenge as compared to the monovalent BCG-Ag85B [66]. rBCG-Ag85B-IL15 also showed crossover activity against bladder cancer (discussed below in section 3.1).
2.3. Recombinant BCGs which alter phagosome permeability.
Several approaches have been taken to generate BCG strains which have enhanced phagosome permeability so that mycobacterial antigens have greater access to the cytosol and its immune detection and antigen processing systems.
2.3.a. rBCGs complemented with ESX-1 variants:
Although complementing BCG with the complete RD1 locus (BCG:RD1 or BCG:ESX-1) resulted in improved protective immunity against Mtb, it also caused increased virulence and prolonged persistence of this recombinant BCG in vivo raising safety concerns [51]. To address this issue, novel rBCGs containing mutated versions of the ESX-1-secreted antigen ESAT-6 were developed attempting to counterbalance the increased virulence resulting from integration of the complete, intact RD1 locus. The BCG::ESX-1 variant strain known as BCG::ESAT-L28A/L29S showed strong virulence attenuation and conferred high protective efficiency both in the mouse and guinea-pig models [67]. In a similar approach, expression of the M. marinum esx-1 locus by BCG (strain known as BCG: ESX-1Mmar) was also found to enhance safety and confer with improved protection against Mtb challenge in the mouse model [68]. Interestingly, this low-virulence, ESX-1-variant strain, BCG:ESX-1Mmar, induced type I IFN and inflammasome activity leading to significant increases in mycobacteria-specific cytokine-producing CD4+ TH1- and CD8+ T cells [68].
Other than overexpression of the immunodominant Mtb antigens (such as Ag85A, Ag85B, or variants of ESX-1) alone or as fusion proteins, efforts have been made to genetically modify BCG to make its own antigens more accessible to the cytosolic immune surveillance system in order to boost cellular immunity.
2.3.b. Recombinant BCGs which alter phagosome permeability using pore-forming cytolysins from other bacteria.
VPM1002 (BCG ΔureC::hly) is a rBCG candidate which expresses a recombinant fusion protein composed of the N-terminal region of Ag85B of Mtb and LLO (listeriolysin O) of Listeria monocytogenes under the transcriptional control of the constitutive mycobacterial hsp60 promoter [69]. Additionally, disruption of ureC (the gene encoding the mycobacterial urease C subunit) in VPM1002 improves secretion of Ag85B-LLO to the cytosol by yielding a phagosomal pH closer to the acidic optimum for native LLO expression. The pore-forming LLO protein in VPM1002 also induces autophagy, inflammasome activation, and apoptosis and expansion of antigen-specific CD4+ and CD8+ T cells [70]. Notably, VPM1002 also enhances TH1- and TH17 responses, and it demonstrates an improved safety profile in combination with enhanced protective immunity in several rodent models and in non-human primates (NHPs) [71]. This candidate vaccine has demonstrated safety and immunogenicity in adults and infants in South Africa and Germany though VPM1002 recipients showed non-statistically significantly higher rates of grade 3 or 4 blood laboratory abnormalities that those who received BCG [72]. Encouraged with these results, VM1002 is also being tested for its efficacy against pulmonary TB in a phase II/III clinical trial (NCT03152903) [70].
A derivative of VPM1002, VPM1002 ΔnuoG carries deletion of the nuoG gene the encodes NADH dehydrogenase 1 subunit G which is an antiapoptotic virulence gene. This genetic alteration led to increased autophagosome formation with elevated LC3 recruitment. Compared with VPM1002, VPM1002 ΔnuoG carries showed improved TB protective efficacy and reduced virulence in mice suggesting a route towards a second-generation VPM1002 [73]. Another modification of VPM1002 includes the addition of antigen diversity via expression of Rv2659c, Rv1733c and Rv3407 that led to increased long-term protective efficacy in mice with this construct known as rBCGΔureC:hly-pMPIIB01 as compared to canonical BCG [74]. Another second-generation construct, BCG ΔureC:hly Δpdx1 or VPM1002 Δpdx1 is deficient in pyridoxine synthase, an enzyme required for synthesis of the essential vitamin B6 [75]. This candidate was more attenuated in SCID mice than the parental strain and it showed protective efficacy against TB in mice in a pyridoxine-dependent manner. The authors suggested that the vitamin-dependence strategy may offer a vaccination approach potentially suitable for immunization of immunocompromised individuals including HIV+ infants and adults. Additionally, there have also been efforts to increase the immunogenicity of BCG ΔureC:hly by bacterial expression of IL-7 and IL-18. Nevertheless, VPM1002, VPM1002 ΔnuoG, and VPM1002 Δpdx1 are currently being tested further for safety and protective efficacy [45].
Another rBCG series designed to alter phagosome permeability using a pore-forming cytolysin from another microbe is comprised by AERAS-401 and AERAS-422. Both of these rBCG candidates express the cholesterol-binding cytolysin perfringolysin O (Pfo), a pore-forming protein secreted by Clostridium perfringens [45]. The derivation of AERAS 401 (also called BCG1331 ΔureC:ΩpfoAG137Q) was generated by replacing the mycobacterial ureC gene with the PfoAG137Q gene under the control of the Ag85B promoter. AERAS-401 secreted biologically active Pfo and had a good safety profile. AERAS-422 is a further modification of AERAS-401 which leads to the overexpression of Ag85A, Ag85B and TB10.4, each shared antigens present in both Mtb and BCG [76]. Later, TB10.4 was replaced by Rv3407, which has been associated with latent TB. This strain also expressed a pore-forming component Pfo (perfringolysin) of C. perfringens and its urease C gene was disrupted by integration of the Pfo-expressing cassette [69]. Altogether, AERAS-422 combines several vaccine strategies thought beneficial for VPM1002 and rBCG30. While it was highly promising in numerous preclinical models and advanced to human studies, adverse side-effects (varicella zoster in 2 of 8 recipients) led to termination of its development after a phase-I clinical trial [77].
Another rBCG in this category is BCG-Δzmp1 which lacks a zinc metalloprotease 1 (Zmp1) that facilitates delivery of the BCG bacilli to the terminal phagosome of the host cells thus causing increased antigen presentation and T cell response. This strain was found more immunogenic than traditional BCG in the guinea pig model and was as safe as conventional BCG in SCID mice [78].
2.4. Recombinant BCG expressing small molecules which stimulate innate immunity:
The cGAS-STING cytosolic surveillance system in an innate immune pathway which detects nucleic acids molecules including bacterial cyclic dinucleotides. Our group earlier reported that Mtb and BCG synthesize and secrete cyclic-di-AMP, a STING agonist closely related to the natural human STING ligand, cGAMP [79]. We showed that bacterial-derived c-di-AMP activates the IRF3 pathway and induces Type I IFN responses through STING-dependent signaling. This microbial pathogenesis study utilized a genetically engineered BCG strain called BCG-disA-OE or BCG-STING in which the endogenous microbial diadenylate cyclase gene, disA, was fused to a strong mycobacterial promoter, Phsp60, causing the overexpression of disA. Guinea pigs vaccinated with BCG-disA-OE via the aerosol route were strongly protected against virulent Mtb as compared to animals vaccinated with wild type BCG [80], a result subsequently confirmed by another group [81]. Our findings suggest that microbial delivery of a STING agonist may enhance BCG’s TB protection and bladder cancer therapeutic efficacy as described below. In both roles, our findings show that the mechanism of protective efficacy of BCG-overexpressing a STING agonist is most likely via enhanced trained immunity induced in myeloid cells characterized by epigenetic and metabolomic reprogramming, and altered polarization of myeloid and lymphoid cells [41].
3.0. Recombinant BCGs to address unmet needs in bladder cancer
During the mid-20th century BCG was used successfully as an intra-tumoral immunotherapy for a variety of solid tumors including melanoma, breast, and colon cancers. However its use fell out of favor as cytotoxic chemotherapy--with its concomitant immunosuppression--became more widely used [82]. As discussed earlier, in 1976 intravesical BCG was established as an effective immunotherapy for NMIBC and to date remains the gold-standard first-line treatment for NMIBC [10]. It is also the only live microbial agent approved by the FDA to treat any form for cancer [83].
Bladder cancer is the most common malignancy of the urinary tract and is the fifth most common malignancy in the USA. The majority of patients, ~70%, present with NMIBC, a disease that is associated with a significant risk of recurrence (31–70%), and progression to muscle invasive bladder cancer (MIBC) [12]. The treatment of NMIBC includes endoscopic surgical removal of all visible tumor, followed by repetitive intravesical instillations of BCG as an immunotherapy to eliminate residual tumor cells and reduce the potential for recurrence. The exact molecular mechanism of BCG-induced antitumor immunity is not yet fully understood, but findings from preclinical and clinical studies demonstrate that success of BCG immunotherapy is associated with robust local inflammatory responses followed by a strong TH1 cell biased adaptive immune response [12,84]. The immunological cascade that accompanies this antitumor response following intravesical BCG instillation is detailed elsewhere [12,85].
BCG immunotherapy induces initial complete response rates ranging from 55 to 75% depending on the initial disease stage of NMIBC. Despite the remarkable success of BCG, 20–45% of patients do not benefit from therapy, and an additional 40% of patients eventually have relapse following BCG immunotherapy despite initial success [12]. Patients with incomplete responses to BCG therapy can be classified as BCG refractory, BCG relapsing, or BCG intolerant, groups that are not prognostically equal [12]. Overall, ~30% of NMIBC is BCG-unresponsive, which represents a potentially life-threatening condition since there is a significant risk of muscle-invasive disease and dissemination that increases with time. Radical cystectomy remains the treatment of choice for NMIBC patients who have failed intravesical BCG immunotherapy, though this is not always feasible due to competing comorbidities or a patient’s wish for bladder preservation [12].
Undesirable off-target effects of BCG immunotherapy that contribute to BCG unresponsiveness include, a predominant TH2 response and increased levels of immunosuppressive tumor associated macrophages (TAMs), regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) [41,86,87]. Effective bladder-sparing strategies for BCG unresponsive patients have low success rates and mainly involve intravesical chemotherapy, checkpoint blockers and adenoviral gene therapies [88]. The management of NMIBC is further complicated by an ongoing BCG shortage in the US, effectively leading to rationing strategies which prioritize for full-strength BCG treatment for high-risk, but not low-risk patients with NMIBC [89].
3.1. Genetically modified BCGs for NMIBC.
To develop novel therapeutic options for BCG-unresponsive NMIBC, genetic modifications of BCG with the goal of enhancing TH1 immune responses in the bladder have been undertaken [90]. To accomplish this a number of bladder cancer-directed rBCGs were developed to express and secreting TH1 cytokines such as IL-2, IL-6, GM-CSF and IFN-γ as well as certain chemokines. Many of these were shown to potentiate cell-mediated immune response in vitro and demonstrated promising antitumor activity animal models of bladder cancer compared to existing BCG (reviewed in [91] see Table 2). More recently, rBCG for bladder cancer have been developed which express IFNα2β, IL-18, and fusion proteins such as IL-15-Ag85B (Table. 2). rBCG-Ag85B-IL15, which also showed anti-TB activity (see section 2.2) [66] induced antitumor efficacy of canonical BCG via strong infiltration of neutrophils in the bladder wall [92]. These recombinant strains exhibited anti-tumor efficacy and shown to evoke immune responses in preclinical studies and animal models of bladder cancer. Few, if any, however, have outperformed BCG therapy, underscoring the complexity of antitumor immune responses following BCG immunotherapy. It is thought that while these rBCGs augment levels of a particular cytokine or chemokine, the temporal and spatial release of these immunomodulators may not be optimized to adequately supplement the antitumor activity of traditional BCG immunotherapy alone. Additionally, it is not known whether these cytokine-secreting rBCGs alter the safety profile compared to traditional BCG. None of these cytokine constructs are currently being evaluated in clinical trials.
Table. 2:
Recombinant BCG strains expressing cytokines, chemokines, tumor antigens or other non-mycobacterial antigens
| Name | Cytokine/Chemokine | Immune responses and safety | Therapeutic efficacy |
|---|---|---|---|
| 2A. Recombinant BCG strains expressing cytokines and chemokines. Some tested as cancer immunotherapeutic agents. | |||
| rBCG-IL4, rBCG-IL6, rBCG-GM-CSF, rBCG-IFN-γ, rBCG-rIL-2 | Secretory murine IL-4, IL-6, GM-CSF, IFN-γ and IL-2 | Increased cell-mediated immune response ex-vivo (murine splenocytes following restimulation) | No efficacy data (tumor model) |
| Murray et al., 1996 | |||
| PMID: 8570663 | |||
| rBCG-IL2 | Secretory murine IL-2 | Enhanced IFN-γ release ex-vivo (murine splenocytes following restimulation) | No efficacy data (tumor model) |
| O’Donnell et al., 1994 | |||
| PMID: 8188376 | |||
| rBCG-mIL-18 | Secretory murine IL-18 | Enhanced TH1 cytokine release ex-vivo (murine splenocytes following restimulation), macrophage toxicity against cancer cells | No efficacy data (tumor model) |
| Luo et al., 2004 | |||
| PMID: 15196240 | |||
| rBCG-IL-18 | Secretory murine IL-18 | Enhanced TH1 cytokine release ex-vivo (murine splenocytes following restimulation) | No efficacy data (tumor model) |
| Biet et al., 2002 | |||
| PMID: 12438324 | |||
| rBCG/IL-18 | Secretory murine IL-18 | Diminished IFN-γ release ex-vivo (murine splenocytes following restimulation) | No efficacy data (tumor model), reduced protective efficacy (mouse model of M. bovis infection) |
| Young et al., 2002 | |||
| PMID: 12067407 | |||
| rBCG/IL-2 | Secretory murine IL-2 | Enhanced IFN-γ release ex-vivo (murine splenocytes following restimulation) | No efficacy data (tumor model), reduced protective efficacy (mouse model of M. bovis infection) |
| Young et al., 2002 | |||
| PMID: 12067407 | |||
| BCG-IFN-γ | Secretory murine IFN-γ | No evidence | No efficacy data (tumor model) |
| Moreira et al., 2000 | |||
| PMID: 10968949 | |||
| rBCG (MCP3) | Secretory chemokine monocyte chemotactic protein 3 (MCP3) | Increased lymphocyte migration in vivo, increased T-cell responses and increased safety in SCID mice | No efficacy data (tumor model) Comparable protective efficacy against Mtb infection |
| Ryan et al., 2007 | |||
| PMID: 17074853 | |||
| rBCG(α-Ag-IL-2) | Secretory murine IL-2 | Increased IL-12 and IFN-γ secretion in peritoneal macrophages (C3H/HeN immunized mice), increased toxicity against urothelial cells | No efficacy data (tumor model) |
| Yamada et al., 2000 | |||
| PMID: 10893638 | |||
| rhIFN-α | Secretory human IFNα-2β | Increased release of IFN-γ and IP-10 in PBMCs post challenge | No efficacy data (tumor model) |
| Luo et al., 2001 | |||
| PMID: 11207657 | |||
| rBCG IFNα-2β | Secretory human IFNα-2β | Increased TH1 cytokine release, Increased cytotoxicity of PBMC against urothelial cells | No efficacy data (tumor model) |
| Fan and Han, 2007 | |||
| PMID: 24573929 | |||
| rBCG-IFNα | Secretory human IFNα-2β | Increased cytotoxicity of PBMC against urothelial cells via NKT and CD8+ T cells | No efficacy data (tumor model) |
| Liu et al., 2009 | |||
| PMID: 19214503 | |||
| rBCG-IFN-γ and rBCG-IL-2 | Secretory murine IFN-γ and IL-2 | Increased IL-2 and IL-4, enhanced CD4+T infiltration in vivo, increased tumor cytotoxicity | Prolonged animal survival and antitumor activity in MB49 orthotopic bladder cancer model |
| Arnold et al., 2014 | |||
| PMID: 14770083 | |||
| rBCG-IL-15 | Fusion protein expressing murine IL-15 and Mtb-Ag85B | Increased chemokine MIP2 and MIP1α | Prolonged animal survival, increased antitumor response driven by neutrophils and prolonged in MB49 orthotopic bladder cancer tumor model |
| Takeuchi et al., 2016 | |||
| Ref. 69. | |||
| PMID: 27093372 | |||
| 2B. Recombinant BCG strains expressing tumor antigens or other non-mycobacterial antigens. Some tested as cancer immunotherapeutic agents. | |||
| rBCG-S1PT | Genetically detoxified S1 subunit of pertussis toxin (S1PT) | Increased TNF- α and IL-10 | Prolonged animal survival and increased antitumor response and in MB49 orthotopic bladder tumor model |
| Andrade et al., 2010 | |||
| PMID: 19272796 | |||
| rBCG-S1+S1i | Genetically detoxified S1 subunit of pertussis toxin 9K/129G (rBCG-S1PT) | Increased polyfunctional CD4+ T cells | No efficacy data |
| Kanno et al., 2019 | |||
| PMID: 30941374 | |||
| rBCG 0148 and rBCG 2108 | Pneumococcal PspA-PdT fusion protein | Induced IL-17 α and IFN-γ production | No efficacy data (tumor model) |
| Goulart et al., 2017 | |||
| PMID: 28242071 | |||
| BCG-hIL2MUC1 | Truncated MUC1 and hIL-2 fusion protein | Increased CD8+ T cell infiltration in hu-PBL-SCID mouse model | Increased antitumor activity and survival (Breast Cancer model) |
| Chung et al., 2003 | |||
| PMID: 12649188 | |||
| BCG-disA-OE | Mtb disA diadenylate cyclase gene overexpressed in BCG with excess release of the STING agonist, c-di-AMP | Increased pro-inflammatory cytokine responses, greater myeloid cell reprogramming (M1 shift), enhanced epigenetic and metabolomic changes, and enhanced Teff infiltration | Improved protective efficacy vs bladder cancer (rat MNU model and mouse MB49 orthotopic model of NMIBC) |
| Singh et al., 2020 | |||
| Ref. 85bioRxiv | |||
| 2020.04.25.061531 | |||
A genetically modified BCG that is currently advancing in human clinical trials for bladder cancer is VPM1002BC which is VPM1002 (rBCGΔureC::hly, currently being tested in as a TB vaccine as described above [72,93] formulated at a higher dose (Table 1 and Table 2). In a Phase I trial in six NMIBC patients with BCG failure VPM1002BC has recently shown to be safe, tolerated (although 4 of 6 subjects experienced grade 2 adverse events), and to evoke Th1 responses [94]. Results from a larger Phase II trial are anticipated by the end of 2022.
Recently, STING agonists have gained significant attention as cancer immunotherapies due their ability to promote Type I IFN and NF-κB-mediated anti-tumor responses (see Fig. 1) [95,96]. Novel anticancer immunotherapies based on recombinant Type I IFNs, Type I IFN-encoding vectors or expressing cells, and soluble, small-molecule STING agonists are therefore currently being developed. STING is activated endogenously by the cyclic dinucleotide cGAMP which is produced by activated cyclic GMP-AMP synthase (cGAS) or by exogenous cyclic dinucleotide PAMPs released by microbes such as cyclic-di-AMP [97]. We found that a BCG strain engineered in to overexpress the STING agonist c-di-AMP known as BCG-disA-OE demonstrated superior anti-tumor efficacy against bladder cancer in two animal models: the MNU-induced rat model (intravesical treatment) and the MB49 syngeneic heterotopic flank mouse model (intra-tumoral treatment). Also, the STING agonist overexpressing BCG elicited more potent pro-inflammatory cytokine responses in macrophage and bladder cancer cells, greater myeloid polarization with an M1 shift, enhanced phagocytosis, increased autophagy, a higher degree of proinflammatory epigenetic marks in human MDM and a greater metabolomic shift towards glycolysis in human MDM. In terms of safety, BCG-disA-OE displayed reduced pathogenicity compared with standard BCG in two mouse models [41]. These findings support the local induction by BCG-disA-OE of enhanced and remodeled innate immune responses, and ultimately enhanced T cell immunity. They reveal that STING pathway activation is a proximal trigger in trained immunity remodeling, which may be a central mechanism for both BCG and BCG-STING anti-tumor activity in NMIBC [41].
Figure 1. Drivers of anti-tumor STING signaling in tumor microenvironment.
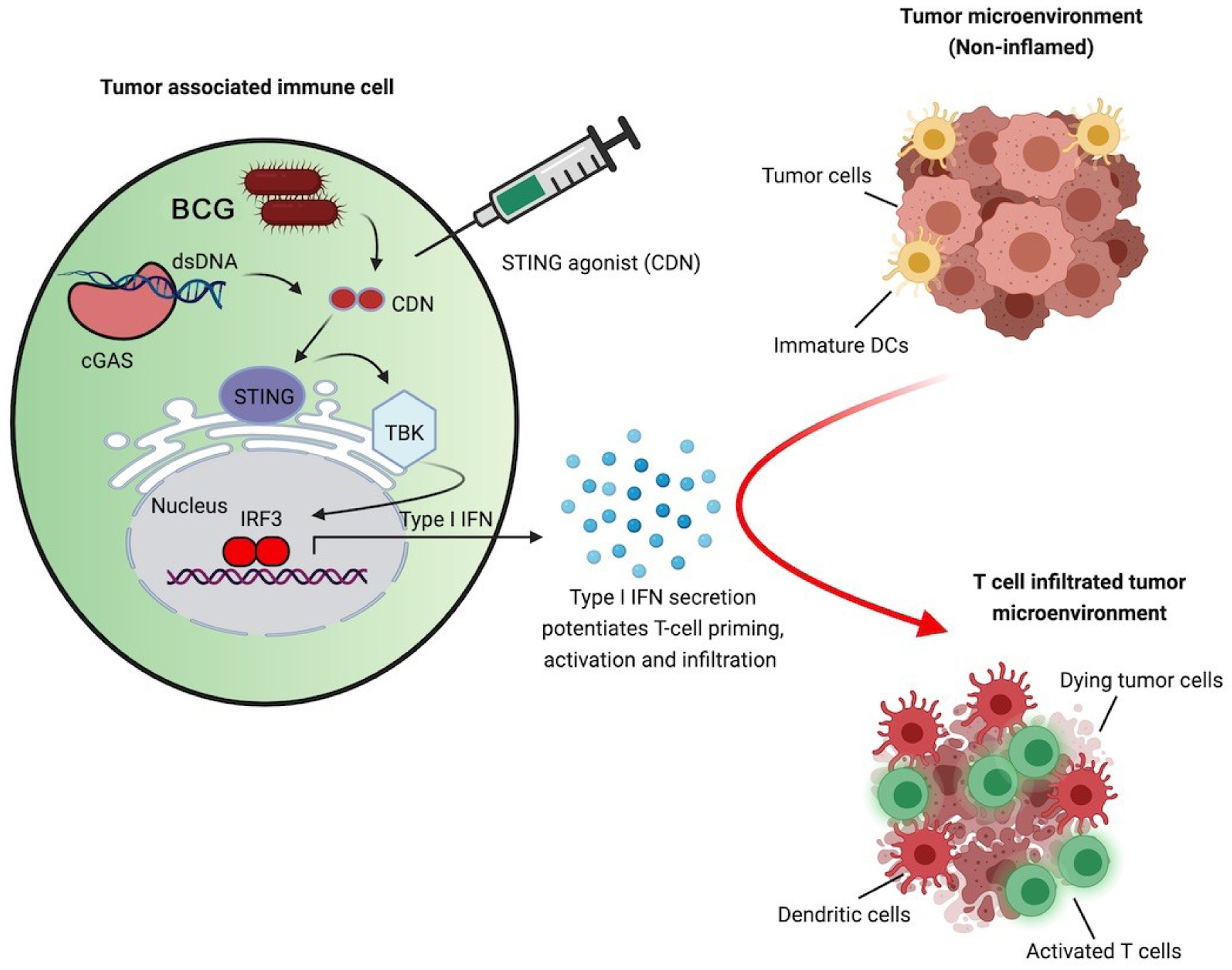
Activation of STING/IRF3/TBK1/IFN I in a myeloid cell is achieved (i) endogenously by cGAMP (produced via nucleic acid sensing by cGAS following immunogenic cell death), and exogenously through (ii) recombinant BCG secreting small molecule STING agonist cyclic dinucleotide (CDN) (e.g., c-di-AMP), or (iii) direct administration of CDNs (e.g., c-GAMP). Activated STING activates kinase TBK1 kinase leading to phosphorylation and dimerization of IRF3 which translocate to the nucleus and stimulates the production of Type I IFNs. Type I IFNs act on dendritic cells to promote the induction of tumor-specific T cells and reprogramming of the tumor microenvironment to facilitate anti-tumor responses.
4.0. Conclusions
Over the last century, BCG vaccine has protected millions of infants from disseminated forms of TB. Since its approval as an immunotherapeutic for bladder cancer in the 1970s, intravesical administration of BCG has also remained a gold standard of treatment for patients with NMIBC aimed at preventing recurrence or progression of the disease. Despite these successes, traditional BCG has not been fully effective in preventing pulmonary TB in adults and in controlling the resurgence of the disease globally, and a third of patients with NMIBC do not respond to BCG. This article reviews efforts to generate new recombinant BCG candidates that may improve either BCG efficacy, BCG safety, or both parameters for the indications of TB prevention and for bladder cancer immunotherapy. While much has been learned about the biology of BCG’s effects on the human immune system, certain fundamental questions remain such the need to identify validated biomarkers of efficacy. As some the rBCG candidates described herein advance through human clinical trials for both TB prevention and bladder cancer therapy, we can anticipate an expanded understanding of parameters that correlate with efficacy and safety that may ultimately lead to a next-generation version of BCG.
Acknowledgements
This work was supported by the National Institutes of Health grant AI155346.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
AKS and WRB are inventors for patents on recombinant forms of BCG. WRB is a co-founder of OncoSTING which has rights to commercialize BCG-disA-OE
Data statement
This is a review article. As such it does not contain new data. The authors confirm that data presented in the review are correct representations of the literature reviewed
Credit author statement
AKS, GS, and WRB conceived the overall concept for the review. AKS and GS wrote and edited the first draft. AKS, GS, and WRB edited subsequent drafts.
References:
- [1].Guérin C, Rosenthal SR. The history of BCG: early history. BCG Vaccination Against Tuberculosis, London, J&H Churchill; 1957, p. 48–57. [Google Scholar]
- [2].Calmette A Preventive Vaccination Against Tuberculosis with BCG. Proc R Soc Med 1931;24:1485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zwerling A, Behr MA, Verma A, Brewer TF, Menzies D, Pai M. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med 2011;8:e1001012. 10.1371/journal.pmed.1001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Roy A, Eisenhut M, Harris RJ, Rodrigues LC, Sridhar S, Habermann S, et al. Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis. BMJ 2014;349:g4643. 10.1136/bmj.g4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 2006;367:1173–80. 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- [6].Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PEM, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis 2014;58:470–80. 10.1093/cid/cit790. [DOI] [PubMed] [Google Scholar]
- [7].Dockrell HM, Smith SG. What Have We Learnt about BCG Vaccination in the Last 20 Years? Front Immunol 2017;8:1134. 10.3389/fimmu.2017.01134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Andersen P, Doherty TM. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nat Rev Microbiol 2005;3:656–62. 10.1038/nrmicro1211. [DOI] [PubMed] [Google Scholar]
- [9].Dobosz P, Dzieciątkowski T. The Intriguing History of Cancer Immunotherapy. Front Immunol 2019;10:2965. 10.3389/fimmu.2019.02965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol 1976;116:180–3. 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- [11].Gandhi NM, Morales A, Lamm DL. Bacillus Calmette-Guérin immunotherapy for genitourinary cancer. BJU Int 2013;112:288–97. 10.1111/j.1464-410X.2012.11754.x. [DOI] [PubMed] [Google Scholar]
- [12].Pettenati C, Ingersoll MA. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat Rev Urol 2018;15:615–25. 10.1038/s41585-018-0055-4. [DOI] [PubMed] [Google Scholar]
- [13].Giamarellos-Bourboulis EJ, Tsilika M, Moorlag S, Antonakos N, Kotsaki A, Domínguez-Andrés J, et al. Activate: Randomized Clinical Trial of BCG Vaccination against Infection in the Elderly. Cell 2020;183:315–323.e9. 10.1016/j.cell.2020.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tsilika M, Taks E, Dolianitis K, Kotsaki A, Leventogiannis K, Damoulari C, et al. ACTIVATE-2: A double-blind randomized trial of BCG vaccination against COVID-19 in individuals at risk. MedRxiv; 2021. 10.1101/2021.05.20.21257520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Butkeviciute E, Jones CE, Smith SG. Heterologous effects of infant BCG vaccination: potential mechanisms of immunity. Future Microbiology 2018;13:1193–208. 10.2217/fmb-2018-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol 2020;20:375–88. 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kleinnijenhuis J, van Crevel R, Netea MG. Trained immunity: consequences for the heterologous effects of BCG vaccination. Trans R Soc Trop Med Hyg 2015;109:29–35. 10.1093/trstmh/tru168. [DOI] [PubMed] [Google Scholar]
- [18].Netea MG, Joosten LAB, Latz E, Mills KHG, Natoli G, Stunnenberg HG, et al. Trained immunity: A program of innate immune memory in health and disease. Science 2016;352:aaf1098. 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kleinnijenhuis J, Quintin J, Preijers F, Joosten LAB, Ifrim DC, Saeed S, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A 2012;109:17537–42. 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Arts RJW, Carvalho A, La Rocca C, Palma C, Rodrigues F, Silvestre R, et al. Immunometabolic Pathways in BCG-Induced Trained Immunity. Cell Reports 2016;17:2562–71. 10.1016/j.celrep.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kaufmann E, Sanz J, Dunn JL, Khan N, Mendonça LE, Pacis A, et al. BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell 2018;172:176–190.e19. 10.1016/j.cell.2017.12.031. [DOI] [PubMed] [Google Scholar]
- [22].Singh AK, Netea MG, Bishai WR. BCG turns 100: its nontraditional uses against viruses, cancer, and immunologic diseases. J Clin Invest 2021;131:148291. 10.1172/JCI148291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Global tuberculosis report 2020. Geneva: World Health Organization; 2020. [Google Scholar]
- [24].WHO: Global TB progress at risk. World Health Organization, Geneva: 2020. [Google Scholar]
- [25].McShane H Tuberculosis vaccines: beyond bacille Calmette–Guérin. Phil Trans R Soc B 2011;366:2782–9. 10.1098/rstb.2011.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ottenhoff THM, Kaufmann SHE. Vaccines against Tuberculosis: Where Are We and Where Do We Need to Go? PLoS Pathog 2012;8:e1002607. 10.1371/journal.ppat.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].BCG vaccines: WHO position paper – February 2018. Wkly Epidemiol Rec 2018;93:73–96. [PubMed] [Google Scholar]
- [28].Nemes E, Geldenhuys H, Rozot V, Rutkowski KT, Ratangee F, Bilek N, et al. Prevention of M. tuberculosis Infection with H4:IC31 Vaccine or BCG Revaccination. N Engl J Med 2018;379:138–49. 10.1056/NEJMoa1714021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zimmermann P, Curtis N. Factors That Influence the Immune Response to Vaccination. Clin Microbiol Rev 2019;32. 10.1128/CMR.00084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Vekemans J, Brennan MJ, Hatherill M, Schrager L, Fritzell B, Rutkowski K, et al. Preferred product characteristics for therapeutic vaccines to improve tuberculosis treatment outcomes: Key considerations from World Health Organization consultations. Vaccine 2020;38:135–42. 10.1016/j.vaccine.2019.10.072. [DOI] [PubMed] [Google Scholar]
- [31].Scriba TJ, Netea MG, Ginsberg AM. Key recent advances in TB vaccine development and understanding of protective immune responses against Mycobacterium tuberculosis. Semin Immunol 2020;50:101431. 10.1016/j.smim.2020.101431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 2013;381:1021–8. 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cardona P-J, Williams A. Experimental animal modelling for TB vaccine development. Int J Infect Dis 2017;56:268–73. 10.1016/j.ijid.2017.01.030. [DOI] [PubMed] [Google Scholar]
- [34].Derrick SC, Kolibab K, Yang A, Morris SL. Intranasal administration of Mycobacterium bovis BCG induces superior protection against aerosol infection with Mycobacterium tuberculosis in mice. Clin Vaccine Immunol 2014;21:1443–51. 10.1128/CVI.00394-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Darrah PA, Zeppa JJ, Maiello P, Hackney JA, Wadsworth MH, Hughes TK, et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature 2020;577:95–102. 10.1038/s41586-019-1817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Scriba TJ, Nemes E. Protection against tuberculosis by mucosal BCG administration. Nat Med 2019;25:199–201. 10.1038/s41591-019-0347-0. [DOI] [PubMed] [Google Scholar]
- [37].Dijkman K, Sombroek CC, Vervenne RAW, Hofman SO, Boot C, Remarque EJ, et al. Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat Med 2019;25:255–62. 10.1038/s41591-018-0319-9. [DOI] [PubMed] [Google Scholar]
- [38].Sharpe S, White A, Sarfas C, Sibley L, Gleeson F, McIntyre A, et al. Alternative BCG delivery strategies improve protection against Mycobacterium tuberculosis in non-human primates: Protection associated with mycobacterial antigen-specific CD4 effector memory T-cell populations. Tuberculosis (Edinb) 2016;101:174–90. 10.1016/j.tube.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Baily GV. Tuberculosis prevention Trial, Madras. Indian J Med Res 1980;72 Suppl:1–74. [PubMed] [Google Scholar]
- [40].Bannister S, Sudbury E, Villanueva P, Perrett K, Curtis N. The safety of BCG revaccination: A systematic review. Vaccine 2021;39:2736–45. 10.1016/j.vaccine.2020.08.016. [DOI] [PubMed] [Google Scholar]
- [41].Singh AK, Praharaj M, Lombardo KA, Yoshida T, Matoso A, Baras AS, et al. Recombinant BCG overexpressing a STING agonist elicits trained immunity and improved antitumor efficacy in non-muscle invasive bladder cancer. BioRxiv; 2020. 10.1101/2020.04.25.061531. [DOI] [Google Scholar]
- [42].O’Donnell MA. The genetic reconstruction of BCG as a new immunotherapeutic tool. Trends Biotechnol 1997;15:512–7. 10.1016/S0167-7799(97)01134-7. [DOI] [PubMed] [Google Scholar]
- [43].Gröschel MI, Sayes F, Simeone R, Majlessi L, Brosch R. ESX secretion systems: mycobacterial evolution to counter host immunity. Nat Rev Microbiol 2016;14:677–91. 10.1038/nrmicro.2016.131. [DOI] [PubMed] [Google Scholar]
- [44].Etna MP, Giacomini E, Pardini M, Severa M, Bottai D, Cruciani M, et al. Impact of Mycobacterium tuberculosis RD1-locus on human primary dendritic cell immune functions. Sci Rep 2015;5:17078. 10.1038/srep17078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nieuwenhuizen NE, Kaufmann SHE. Next-Generation Vaccines Based on Bacille Calmette-Guérin. Front Immunol 2018;9:121. 10.3389/fimmu.2018.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Stover CK, de la Cruz VF, Fuerst TR, Burlein JE, Benson LA, Bennett LT, et al. New use of BCG for recombinant vaccines. Nature 1991;351:456–60. 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- [47].Dalmia N, Ramsay AJ. Prime-boost approaches to tuberculosis vaccine development. Expert Rev Vaccines 2012;11:1221–33. 10.1586/erv.12.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rouanet C, Locht C. Boosting BCG to protect against TB. Expert Rev Respir Med 2010;4:339–48. 10.1586/ers.10.25. [DOI] [PubMed] [Google Scholar]
- [49].Ravn P, Demissie A, Eguale T, Wondwosson H, Lein D, Amoudy HA, et al. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J Infect Dis 1999;179:637–45. 10.1086/314640. [DOI] [PubMed] [Google Scholar]
- [50].Kupz A, Zedler U, Stäber M, Perdomo C, Dorhoi A, Brosch R, et al. ESAT-6-dependent cytosolic pattern recognition drives noncognate tuberculosis control in vivo. J Clin Invest 2016;126:2109–22. 10.1172/JCI84978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Pym AS, Brodin P, Majlessi L, Brosch R, Demangel C, Williams A, et al. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat Med 2003;9:533–9. 10.1038/nm859. [DOI] [PubMed] [Google Scholar]
- [52].Horwitz MA, Harth G, Dillon BJ, Maslesa-Galic’ S. Recombinant bacillus calmette-guerin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc Natl Acad Sci U S A 2000;97:13853–8. 10.1073/pnas.250480397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Horwitz MA, Harth G. A new vaccine against tuberculosis affords greater survival after challenge than the current vaccine in the guinea pig model of pulmonary tuberculosis. Infect Immun 2003;71:1672–9. 10.1128/iai.71.4.1672-1679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Horwitz MA, Harth G, Dillon BJ, Maslesa-Galić S. Extraordinarily few organisms of a live recombinant BCG vaccine against tuberculosis induce maximal cell-mediated and protective immunity. Vaccine 2006;24:443–51. 10.1016/j.vaccine.2005.08.001. [DOI] [PubMed] [Google Scholar]
- [55].Horwitz MA, Harth G, Dillon BJ, Maslesa-Galić S. A novel live recombinant mycobacterial vaccine against bovine tuberculosis more potent than BCG. Vaccine 2006;24:1593–600. 10.1016/j.vaccine.2005.10.002. [DOI] [PubMed] [Google Scholar]
- [56].Hoft DF, Blazevic A, Abate G, Hanekom WA, Kaplan G, Soler JH, et al. A new recombinant bacille Calmette-Guérin vaccine safely induces significantly enhanced tuberculosis-specific immunity in human volunteers. J Infect Dis 2008;198:1491–501. 10.1086/592450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Tullius MV, Harth G, Maslesa-Galic S, Dillon BJ, Horwitz MA. A Replication-Limited Recombinant Mycobacterium bovis BCG vaccine against tuberculosis designed for human immunodeficiency virus-positive persons is safer and more efficacious than BCG. Infect Immun 2008;76:5200–14. 10.1128/IAI.00434-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Xu ZZ, Chen X, Hu T, Meng C, Wang XB, Rao Y, et al. Evaluation of Immunogenicity and Protective Efficacy Elicited by Mycobacterium bovis BCG Overexpressing Ag85A Protein against Mycobacterium tuberculosis Aerosol Infection. Front Cell Infect Microbiol 2016;6:3. 10.3389/fcimb.2016.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wang C, Fu R, Chen Z, Tan K, Chen L, Teng X, et al. Immunogenicity and protective efficacy of a novel recombinant BCG strain overexpressing antigens Ag85A and Ag85B. Clin Dev Immunol 2012;2012:563838. 10.1155/2012/563838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yuan X, Teng X, Jing Y, Ma J, Tian M, Yu Q, et al. A live attenuated BCG vaccine overexpressing multistage antigens Ag85B and HspX provides superior protection against Mycobacterium tuberculosis infection. Appl Microbiol Biotechnol 2015;99:10587–95. 10.1007/s00253-015-6962-x. [DOI] [PubMed] [Google Scholar]
- [61].Deng Y-H, He H-Y, Zhang B-S. Evaluation of protective efficacy conferred by a recombinant Mycobacterium bovis BCG expressing a fusion protein of Ag85A-ESAT-6. J Microbiol Immunol Infect 2014;47:48–56. 10.1016/j.jmii.2012.11.005. [DOI] [PubMed] [Google Scholar]
- [62].Lu Y, Xu Y, Yang E, Wang C, Wang H, Shen H. Novel recombinant BCG coexpressing Ag85B, ESAT-6 and Rv2608 elicits significantly enhanced cellular immune and antibody responses in C57BL/6 mice. Scand J Immunol 2012;76:271–7. 10.1111/j.1365-3083.2012.02726.x. [DOI] [PubMed] [Google Scholar]
- [63].Yang E, Lu Y, Xu Y, Liang Q, Wang C, Wang H, et al. Recombinant BCG coexpressing Ag85B, ESAT-6 and Rv3620c elicits specific Th1 immune responses in C57BL/6 mice. Microb Pathog 2014;69–70:53–9. 10.1016/j.micpath.2014.03.011. [DOI] [PubMed] [Google Scholar]
- [64].Xu Y, Zhu B, Wang Q, Chen J, Qie Y, Wang J, et al. Recombinant BCG coexpressing Ag85B, ESAT-6 and mouse-IFN-gamma confers effective protection against Mycobacterium tuberculosis in C57BL/6 mice. FEMS Immunol Med Microbiol 2007;51:480–7. 10.1111/j.1574-695X.2007.00322.x. [DOI] [PubMed] [Google Scholar]
- [65].Shen H, Wang C, Yang E, Xu Y, Liu W, Yan J, et al. Novel recombinant BCG coexpressing Ag85B, ESAT-6 and mouse TNF-alpha induces significantly enhanced cellular immune and antibody responses in C57BL/6 mice. Microbiol Immunol 2010;54:435–41. 10.1111/j.1348-0421.2010.00232.x. [DOI] [PubMed] [Google Scholar]
- [66].Tang C, Yamada H, Shibata K, Maeda N, Yoshida S, Wajjwalku W, et al. Efficacy of recombinant bacille Calmette-Guérin vaccine secreting interleukin-15/antigen 85B fusion protein in providing protection against Mycobacterium tuberculosis. J Infect Dis 2008;197:1263–74. 10.1086/586902. [DOI] [PubMed] [Google Scholar]
- [67].Bottai D, Frigui W, Clark S, Rayner E, Zelmer A, Andreu N, et al. Increased protective efficacy of recombinant BCG strains expressing virulence-neutral proteins of the ESX-1 secretion system. Vaccine 2015;33:2710–8. 10.1016/j.vaccine.2015.03.083. [DOI] [PubMed] [Google Scholar]
- [68].Gröschel MI, Sayes F, Shin SJ, Frigui W, Pawlik A, Orgeur M, et al. Recombinant BCG Expressing ESX-1 of Mycobacterium marinum Combines Low Virulence with Cytosolic Immune Signaling and Improved TB Protection. Cell Rep 2017;18:2752–65. 10.1016/j.celrep.2017.02.057. [DOI] [PubMed] [Google Scholar]
- [69].Velmurugan K, Grode L, Chang R, Fitzpatrick M, Laddy D, Hokey D, et al. Nonclinical Development of BCG Replacement Vaccine Candidates. Vaccines (Basel) 2013;1:120–38. 10.3390/vaccines1020120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Nieuwenhuizen NE, Kulkarni PS, Shaligram U, Cotton MF, Rentsch CA, Eisele B, et al. The Recombinant Bacille Calmette-Guérin Vaccine VPM1002: Ready for Clinical Efficacy Testing. Front Immunol 2017;8:1147. 10.3389/fimmu.2017.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kaufmann SH, Cotton MF, Eisele B, Gengenbacher M, Grode L, Hesseling AC, et al. The BCG replacement vaccine VPM1002: from drawing board to clinical trial. Expert Rev Vaccines 2014;13:619–30. 10.1586/14760584.2014.905746. [DOI] [PubMed] [Google Scholar]
- [72].Loxton AG, Knaul JK, Grode L, Gutschmidt A, Meller C, Eisele B, et al. Safety and Immunogenicity of the Recombinant Mycobacterium bovis BCG Vaccine VPM1002 in HIV-Unexposed Newborn Infants in South Africa. Clin Vaccine Immunol 2017;24. 10.1128/CVI.00439-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Gengenbacher M, Nieuwenhuizen N, Vogelzang A, Liu H, Kaiser P, Schuerer S, et al. Deletion of nuoG from the Vaccine Candidate Mycobacterium bovis BCG ΔureC::hly Improves Protection against Tuberculosis. MBio 2016;7. 10.1128/mBio.00679-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Reece ST, Nasser-Eddine A, Dietrich J, Stein M, Zedler U, Schommer-Leitner S, et al. Improved long-term protection against Mycobacterium tuberculosis Beijing/W in mice after intra-dermal inoculation of recombinant BCG expressing latency associated antigens. Vaccine 2011;29:8740–4. 10.1016/j.vaccine.2011.07.144. [DOI] [PubMed] [Google Scholar]
- [75].Gengenbacher M, Vogelzang A, Schuerer S, Lazar D, Kaiser P, Kaufmann SHE. Dietary pyridoxine controls efficacy of vitamin B6-auxotrophic tuberculosis vaccine bacillus Calmette-Guérin ΔureC::hly Δpdx1 in mice. MBio 2014;5:e01262–01214. 10.1128/mBio.01262-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Sun R, Skeiky YAW, Izzo A, Dheenadhayalan V, Imam Z, Penn E, et al. Novel recombinant BCG expressing perfringolysin O and the over-expression of key immunodominant antigens; pre-clinical characterization, safety and protection against challenge with Mycobacterium tuberculosis. Vaccine 2009;27:4412–23. 10.1016/j.vaccine.2009.05.048. [DOI] [PubMed] [Google Scholar]
- [77].Hoft DF, Blazevic A, Selimovic A, Turan A, Tennant J, Abate G, et al. Safety and Immunogenicity of the Recombinant BCG Vaccine AERAS-422 in Healthy BCG-naïve Adults: A Randomized, Active-controlled, First-in-human Phase 1 Trial. EBioMedicine 2016;7:278–86. 10.1016/j.ebiom.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Sander P, Clark S, Petrera A, Vilaplana C, Meuli M, Selchow P, et al. Deletion of zmp1 improves Mycobacterium bovis BCG-mediated protection in a guinea pig model of tuberculosis. Vaccine 2015;33:1353–9. 10.1016/j.vaccine.2015.01.058. [DOI] [PubMed] [Google Scholar]
- [79].Dey B, Dey RJ, Cheung LS, Pokkali S, Guo H, Lee J-H, et al. A bacterial cyclic dinucleotide activates the cytosolic surveillance pathway and mediates innate resistance to tuberculosis. Nat Med 2015;21:401–6. 10.1038/nm.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Dey RJ, Dey B, Singh AK, Praharaj M, Bishai W. Bacillus Calmette-Guérin Overexpressing an Endogenous Stimulator of Interferon Genes Agonist Provides Enhanced Protection Against Pulmonary Tuberculosis. J Infect Dis 2020;221:1048–56. 10.1093/infdis/jiz116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ning H, Wang L, Zhou J, Lu Y, Kang J, Ding T, et al. Recombinant BCG With Bacterial Signaling Molecule Cyclic di-AMP as Endogenous Adjuvant Induces Elevated Immune Responses After Mycobacterium tuberculosis Infection. Front Immunol 2019;10:1519. 10.3389/fimmu.2019.01519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Hersh EM, Gutterman JU, Mavligit GM. BCG as adjuvant immunotherapy for neoplasia. Annu Rev Med 1977;28:489–515. 10.1146/annurev.me.28.020177.002421. [DOI] [PubMed] [Google Scholar]
- [83].Bernardes N, Seruca R, Chakrabarty AM, Fialho AM. Microbial-based therapy of cancer: current progress and future prospects. Bioeng Bugs 2010;1:178–90. 10.4161/bbug.1.3.10903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Larsen ES, Joensen UN, Poulsen AM, Goletti D, Johansen IS. Bacillus Calmette-Guérin immunotherapy for bladder cancer: a review of immunological aspects, clinical effects and BCG infections. APMIS 2020;128:92–103. 10.1111/apm.13011. [DOI] [PubMed] [Google Scholar]
- [85].Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer--a current perspective. Nat Rev Urol 2014;11:153–62. 10.1038/nrurol.2014.15. [DOI] [PubMed] [Google Scholar]
- [86].Joseph M, Enting D. Immune Responses in Bladder Cancer-Role of Immune Cell Populations, Prognostic Factors and Therapeutic Implications. Front Oncol 2019;9:1270. 10.3389/fonc.2019.01270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Luo Y, Chen X, O’Donnell MA. Role of Th1 and Th2 cytokines in BCG-induced IFN-gamma production: cytokine promotion and simulation of BCG effect. Cytokine 2003;21:17–26. 10.1016/s1043-4666(02)00490-8. [DOI] [PubMed] [Google Scholar]
- [88].Rayn KN, Hale GR, Grave GP-L, Agarwal PK. New therapies in nonmuscle invasive bladder cancer treatment. Indian J Urol 2018;34:11–9. 10.4103/iju.IJU_296_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Barlow LJ. Non-muscle-invasive Bladder Cancer Management During BCG Shortages: NYU Case of the Month, February 2020. Rev Urol 2020;22:35–6. [PMC free article] [PubMed] [Google Scholar]
- [90].Luo Y, Henning J, O’Donnell MA. Th1 cytokine-secreting recombinant Mycobacterium bovis bacillus Calmette-Guérin and prospective use in immunotherapy of bladder cancer. Clin Dev Immunol 2011;2011:728930. 10.1155/2011/728930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Begnini KR, Buss JH, Collares T, Seixas FK. Recombinant Mycobacterium bovis BCG for immunotherapy in nonmuscle invasive bladder cancer. Appl Microbiol Biotechnol 2015;99:3741–54. 10.1007/s00253-015-6495-3. [DOI] [PubMed] [Google Scholar]
- [92].Takeuchi A, Eto M, Tatsugami K, Shiota M, Yamada H, Kamiryo Y, et al. Antitumor activity of recombinant Bacille Calmette-Guérin secreting interleukin-15-Ag85B fusion protein against bladder cancer. Int Immunopharmacol 2016;35:327–31. 10.1016/j.intimp.2016.03.007. [DOI] [PubMed] [Google Scholar]
- [93].Kaufmann SHE. Vaccination Against Tuberculosis: Revamping BCG by Molecular Genetics Guided by Immunology. Front Immunol 2020;11:316. 10.3389/fimmu.2020.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Rentsch CA, Bosshard P, Mayor G, Rieken M, Püschel H, Wirth G, et al. Results of the phase I open label clinical trial SAKK 06/14 assessing safety of intravesical instillation of VPM1002BC, a recombinant mycobacterium Bacillus Calmette Guérin (BCG), in patients with non-muscle invasive bladder cancer and previous failure of conventional BCG therapy. Oncoimmunology 2020;9:1748981. 10.1080/2162402X.2020.1748981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Wu J-J, Zhao L, Hu H-G, Li W-H, Li Y-M. Agonists and inhibitors of the STING pathway: Potential agents for immunotherapy. Med Res Rev 2020;40:1117–41. 10.1002/med.21649. [DOI] [PubMed] [Google Scholar]
- [96].Medrano RFV, Hunger A, Mendonça SA, Barbuto JAM, Strauss BE. Immunomodulatory and antitumor effects of type I interferons and their application in cancer therapy. Oncotarget 2017;8:71249–84. 10.18632/oncotarget.19531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Cai X, Chiu Y-H, Chen ZJ. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol Cell 2014;54:289–96. 10.1016/j.molcel.2014.03.040. [DOI] [PubMed] [Google Scholar]


