Abstract
Objective:
Tenofovir alafenamide (TAF) preferentially loads peripheral blood mononuclear cells (PBMC), resulting in higher PBMC tenofovir-diphosphate (TFV-DP) vs. tenofovir disoproxil fumarate (TDF). No studies have yet compared TFV-DP in PBMC from lower than daily dosing between prodrugs, which has potential implications for event-driven pre-exposure prophylaxis and pharmacologic forgiveness.
Design:
Two separate randomized, directly observed therapy (DOT) crossover studies (DOT-DBS and TAF-DBS) were conducted to mimic low, medium, and high adherence.
Methods:
HIV-negative adults were randomized to two 12-week DOT regimens of 33%, 67%, or 100% of daily dosing with emtricitabine (F)/TAF 200mg/25mg (TAF-DBS) or F/TDF 200mg/300mg (DOT-DBS), separated by a 12-week washout. PBMC steady-state concentrations (Css) of TFV-DP and FTC-TP were estimated using nonlinear mixed models and compared between F/TAF and F/TDF.
Results:
Thirty-five participants contributed to 33% (n=23), 67% (n=23), and 100% (n=23) of daily F/TAF regimens. Forty-four contributed to 33% (n=15), 67% (n=16), and 100% (n=32) of daily F/TDF regimens. PBMC TFV-DP Css were 7.3- (95% CI: 6.4–8.2), 7.1- (5.9–8.2), and 6.7- (4.4–8.9) fold higher (p<0.0001) following F/TAF vs. F/TDF; 593 vs. 81.7, 407 vs. 57.4, and 215 vs. 32.3 fmol/106 cells, respectively. TFV-DP was 2.6- (2.1–3.1) fold higher with 33% F/TAF vs. 100% F/TDF. Estimated half-lives (95% CI) of TFV-DP in PBMC were 2.9 (1.5– 5.5) days for F/TAF and 2.1 (1.5–2.9) days for F/TDF. FTC-TP was similar in both studies (p=0.119).
Conclusions:
F/TAF produced 6.7- to 7.3-fold higher TFV-DP in PBMC vs. F/TDF across adherence levels, supporting increased potency and pharmacologic forgiveness with F/TAF in the PBMC compartment.
Keywords: HIV, PrEP, tenofovir, emtricitabine, pharmacology
Introduction
There are two prodrugs of tenofovir (TFV) commercially available in the US, tenofovir alafenamide (TAF) and tenofovir disoproxil (TDF). Both prodrugs, in combination with other antiretroviral agents, are indicated for the treatment and prevention of HIV (although the F/TAF HIV prevention indication excludes risk from vaginal sex at this time).
TFV, its prodrugs, or their intermediate metabolites enter peripheral blood mononuclear cells (PBMC), where they are phosphorylated to the active anabolite, tenofovir-diphosphate (TFV-DP).[1–3] The first available prodrug of TFV, TDF, produced effective TFV-DP in PBMC but resulted in high TFV plasma exposures, which were associated with nephrotoxicity and decreased bone mineral density.[4, 5] TAF is a newer prodrug of TFV which undergoes selective cleavage by carboxylesterase-1 in hepatocytes, and cathepsin-A in lymphatic tissue, resulting in the preferential loading of PBMC and lymphoid tissues that highly express this enzyme.[6, 7] This results in lower doses of TFV equivalents, lower plasma TFV levels, and higher PBMC TFV-DP concentrations with its use;[8–10] therefore, TAF has been shown to have lower rates of both renal and bone toxicity compared to TDF.[3]
PBMC include target cells for HIV, such as CD4+ T cells, which traffic back and forth between blood and tissues.[11, 12] Therefore, higher TFV-DP in PBMC with the use of TAF compared to TDF has important clinical implications for both HIV treatment and pre-exposure prophylaxis (PrEP). For example, PBMC TFV-DP concentrations have been a strong correlate of PrEP efficacy in clinical trials, with concentrations of 40 and 83 fmol/106 cells being associated with 90% and 99% efficacy in the iPrEx study, respectively.[13] Higher TFV-DP concentrations were also associated with greater HIV RNA declines on antiretroviral therapy (ART).[14]
Variable adherence is expected to influence TFV-DP in PBMC, and thus PrEP and treatment outcomes, and event driven PrEP uses less than daily dosing. However, studies have not characterized differences in TFV-DP in PBMC between F/TAF and F/TDF with low, medium, and high levels of adherence/dosing. In this study, we compared TFV-DP in PBMC following mimicked low, medium and high adherence to F/TAF vs. F/TDF using directly observed therapy (DOT) in HIV negative volunteers.
Methods
Two separate randomized, controlled studies were conducted to assess intracellular TFV-DP concentrations in DBS and PBMC in participants receiving F/TDF 200mg/300mg (DOT-DBS) or F/TAF 200mg/25mg (TAF-DBS). TAF-DBS was conducted at the University of Colorado Anschutz Medical Campus, and DOT-DBS was conducted at the University of Colorado Anschutz Medical Campus and the San Francisco Department of Public Health. Studies were approved by the local institutional review boards and registered at clinicaltrials.gov (NCT02022657 and NCT02962739). All participants provided written informed consent.
Participants were adults ages 18–59 (TAF-DBS) or 18–50 (DOT-DBS) without HIV who were at low risk of HIV infection and able to comply with study procedures, including DOT. Full inclusion and exclusion criteria for these studies have been published elsewhere.[15, 16]
DOT-DBS and TAF-DBS were 36-week, prospective, randomized, crossover studies (Supplemental Figure 1). Participants were randomized to two different 12-week dosing regimens of either 33%, 67%, or 100% daily dosing, separated by a 12-week washout period. Dosing regimens were defined as follows: 33% was dosing on day 1, followed by no dosing on days 2–3, repeated for a total of 12 weeks; 67% was dosing on days 1 and 2, followed by no dosing on day 3, repeated for 12 weeks; 100% was daily dosing for 12 weeks. All doses were directly observed either in person or via video streaming. The DOT-DBS study consisted of intermittent and holiday arms for 33% and 67% dosing. Holiday arms consisted of doses missed by weeks rather than by days (e.g., one week on followed by two weeks off for 33% dosing). In order to directly compare concentrations between the two studies, only intermittent dosing regimens from DOT-DBS were included for this analysis. The primary analyses for both studies was TFV-DP in red blood cells measured with dried blood spots, which were previously reported.[15, 16] This communication describes secondary analyses for both studies, TFV-DP and FTC-TP in PBMC.
For TAF-DBS, blood was collected weekly throughout the dosing periods, as well as through the washout. For DOT-DBS, blood was collected every other week during the 12-week dosing periods and every three weeks during washout. To characterize steady-state and washout kinetics, concentrations in PBMC were quantified at weeks 2, 4, 10, 12, 15, 26, 28, 34, and 36 in DOT-DBS and the same plus 4 hours after the first dose, and weeks 1, 3, 13, 14, 16, 17, 25, and 27 in TAF-DBS. For PBMC, blood from an EDTA tube was centrifuged with lymphocyte separation medium. The PBMC buffy layer was removed into a separate tube, followed by washes, red cell lysis, and PBMC counting via an automated hemocytometer. Cells were lysed and suspended in 500 μL cold 70% methanol/30% water and stored at −80°C prior to analysis.
TFV-DP and FTC-TP concentrations were quantified using a previously validated LC/MS-MS method[17] and reported as fmol (TFV-DP) or pmol (FTC-TP) per 106 cells. The quantifiable linear range of the assay is 2.5 – 6,000 fmol/sample for TFV-DP and 0.1 – 200 pmol/sample for FTC-TP. Two million cells were typically assayed. TFV-DP concentrations below the limit of quantification (BLQ) were included in analyses as half of the lower limit of quantification (LLOQ). Samples in both studies were processed and analyzed using the same procedures and in the same laboratory.
Steady-state TFV-DP concentrations (Css) and half-lives for TFV-DP and FTC-TP were estimated using nonlinear mixed models. Significance tests and confidence Intervals for Css ratios between F/TAF and F/TDF utilized a delta method approximation, assuming no covariance between studies. Mixed-effect models were used to assess dose proportionality during steady state and model TFV-DP dynamics in PBMC. Dose proportionality was assumed if the 90% confidence interval for the log(dose) coefficient was contained in 0.8–1.25. For TFV-DP dynamics, log-transformed TFV-DP concentrations were modeled with results back-transformed for interpretability. During the accumulation phase, natural b-spline transformations of study day and dose were used, with subsequent piecewise transitions to a constant steady-state, and exponential decay during the washout period. Covariates, including body mass index (BMI), weight, creatinine clearance (CrCl), race, and sex were added separately to assess for predictors of TFV-DP in PBMC.
Results
Thirty-five participants contributed to 69 F/TAF dosing regimens and 44 participants contributed to 63 F/TDF regimens for this analysis (participants received two different dosing regimens). Breakdown of dosing regimens and participant baseline clinical and demographic characteristics are shown in Table 1.
Table 1.
Baseline clinical and demographic characteristics
| F/TAF (N=35) | F/TDF (N=44) | |
|---|---|---|
| Dosing breakdown (n) | 33% (23), 67% (23), 100% (23) | 33% (15), 67% (16), 100% (32) |
|
| ||
| Male at birth, n (%) | 18 (51.4%) | 21 (47.7%) |
|
| ||
| Race | ||
| Caucasian | 29 (82.9%) | 25 (56.8%) |
| Black | 5 (14.3%) | 8 (18.2%) |
| Asian | 0 (0%) | 3 (6.8%) |
| Native Hawaiian | 0 (0%) | 1 (2.3%) |
| Unknown | 1 (2.9%) | 7 (15.9%) |
|
| ||
| Hispanic | 6 (17.1%) | 12 (27.3%) |
|
| ||
| Age (years) | 29 (18, 41) | 29 (21, 49) |
|
| ||
| Weight (kg) | 72.8 (45.9, 118.2) | 75.4 (51.2, 155.3) |
|
| ||
| CrCl (mL/min) | 120.3 (85.9, 243.7) | 121.4 (77.6, 256.6) |
All values expressed as n (%) or median (range). CrCl = Creatinine clearance, calculated using Cockcroft-Gault equation and actual body weight.
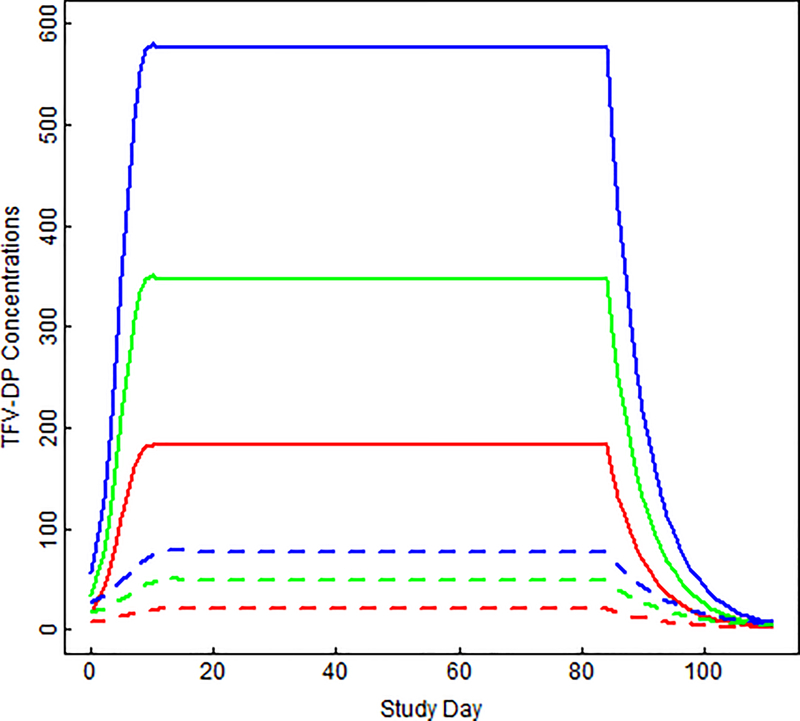
In the nonlinear mixed model, estimated Css of TFV-DP in PBMC were 7.3-, 7.1-, and 6.7-fold higher (p<0.0001) on F/TAF vs. F/TDF (Figure 1): 593 vs. 81.7 fmol/106 cells for 100%, 407 vs. 57.4 fmol/106 cells for 67%, and 215 vs. 32.3 fmol/106 cells for 33%, respectively (Table 2). Low (33%) adherence to F/TAF produced 2.6 (2.1, 3.1)-fold higher TFV-DP in PBMC vs. high (100%) adherence to F/TDF (p<0.0001). Observed and predicted (95% CI) concentrations by study day from F/TAF and F/TDF are shown in Supplemental Figures 2 and 3. Predicted (95% CI) half-lives of TFV-DP in PBMC were 2.1 (1.5, 2.9) days for F/TAF and 2.9 (1.5, 5.5) days for F/TDF, and did not significantly vary by dosing frequency (F/TDF p=0.22, F/TAF p=0.42).
Figure 1. Predicted TFV-DP concentrations in PBMC following F/TDF and F/TAF.
Model-fitted TFV-DP in PBMC (fmol/106 cells) by study day for 33% dosing (red), 67% dosing (green) and 100% dosing (blue). Solid lines are concentrations following F/TAF dosing; dashed lines are concentrations following F/TDF dosing. Prior to steady state, a nonlinear mixed effect model with tensor product of natural b-spline transformation of study day and study arm was used to model concentrations. The estimate was constant over time at steady state, and then an exponential decay was modeled during washout.
Table 2.
Predicted steady-state TFV-DP concentrations in PBMC
| Dosing Regimen | F/TAF | F/TDF | Fold-difference |
|---|---|---|---|
| 33% | 214.8 (182.2, 247.4) |
32.3 (22.5, 42.1) |
6.7 |
| 67% | 407.0 (378.7, 435.3) |
57.4 (48.9, 65.8) |
7.1 |
| 100% | 593.5 (560.3, 626.7) |
81.7 (72.8, 90.5) |
7.3 |
All values are model estimate (95% confidence interval) and concentrations are expressed in fmol/106 cells.
TFV-DP was dose proportional for TAF (90% CI for log[dose] 0.94, 1.10), but not F/TDF (90% CI 1.08, 1.32).
Weight, BMI, and CrCl, when assessed separately, were not significantly associated with TFV-DP in PBMC following F/TAF dosing. However, males had 13.2% lower (−1.0%, −23.7%) TFV-DP than females (p=0.043). For F/TDF, non-white participants had 27.6% lower concentrations (95% CI: −11.0, −41.2%, p=0.0039) vs. white participants, a CrCl decrease of 10 mL/min was associated with a 4.2% increase in TFV-DP in PBMC (95% CI: 2.0, 6.4%, p=0.0006), and there was a trend toward an association between a 10 kg greater body weight and 4.7% lower TFV-DP in PBMC (95% CI: −9.0, 0.2%, p=0.056).
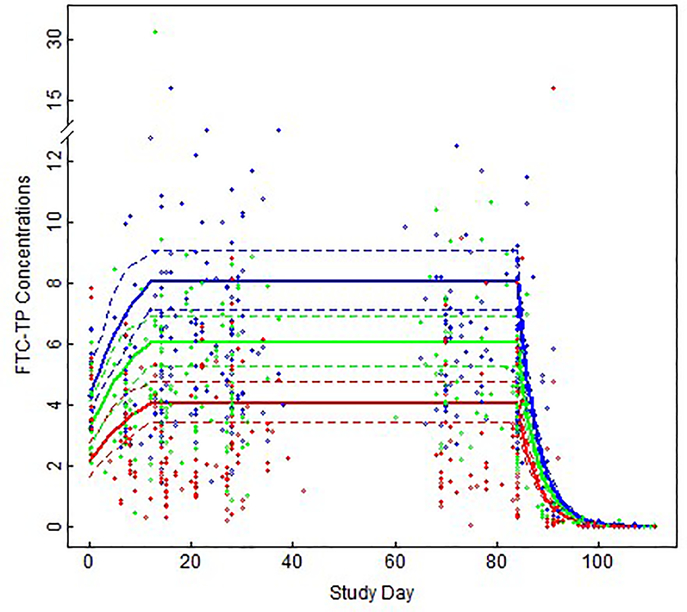
The estimated half-life (95% CI) of FTC-TP in PBMC was 54.5 (36.5, 81.4) hours. There was no significant difference in FTC-TP concentrations on F/TAF vs. F/TDF dosing after adjusting for time since last dose (F/TDF vs. F/TAF percent difference [95% CI]: 11.1% [−2.5%, 26.8%]; p=0.119). The estimated (95% CI) Css of FTC-TP following F/TAF were 4.0 (3.4, 4.8), 6.1 (5.3, 7.0), and 8.0 (7.1, 9.1) pmol/106 cells with 33%, 67%, and 100% daily dosing, respectively. On F/TDF, the estimated FTC-TP Css were 3.6 (3.0, 4.4), 5.5 (4.6, 6.4), and 7.2 (6.4, 8.2) pmol/106 cells with 33%, 67%, and 100% daily dosing, respectively. Figure 2 shows observed and estimated FTC-TP in PBMC by dose.
Figure 2. Observed and predicted FTC-TP concentrations in PBMC following F/TAF and F/TDF.
Observed (open circles) and model-fitted [95% CI] (solid [dashed] lines) FTC-TP in PBMC (pmol/106 cells) by study day for 33% dosing (red), 67% dosing (green), and 100% dosing (blue), after adjusting for time since last dose. Prior to steady state, a nonlinear mixed effect model with tensor product of natural b-spline transformation of study day and study arm was used to model concentrations. The estimate was constant over time at steady state, and then an exponential decay was modeled during washout.
When assessed separately, CrCl was significantly associated with FTC-TP concentrations after adjustment for formulation and time since last dose, and a trend was seen with race (white vs. non-white). A 10 mL/min lower CrCl was associated with a 2.2% higher FTC-TP in PBMC (95% CI: 0.4, 4.0%, p=0.02). Non-white participants had 13.3% lower FTC-TP in PBMC vs. white participants (95% CI: −25.8, 0.6%, p=0.054).
Discussion
This was an analysis of two randomized, cross over, directly observed dosing studies among adult participants without HIV comparing PBMC TFV-DP and FTC-TP concentrations following mimicked low, medium, and high adherence to F/TAF vs. F/TDF. Fitted Css TFV-DP in PBMC were 6.7- to 7.3-fold higher following F/TAF vs. F/TDF across all levels of adherence. This is consistent with preferential PBMC loading with TAF vs TDF[8–10] but differs from TFV-DP in red blood cells (measured with DBS), which lack cathepsin-A, where TFV-DP concentrations were 11-fold lower following F/TAF vs. F/TDF.[15, 16] The PBMC advantage for TAF was so great that the lowest F/TAF dosing group (33%) had 2.6-fold higher TFV-DP concentrations compared with the highest (100%) F/TDF dosing group. These data show that TAF has substantially increased pharmacologic forgiveness in the PBMC compartment compared to TDF, which may be relevant for both treatment and prevention, including less than daily PrEP dosing. For example, the model-estimated Css TFV-DP in PBMC with the lowest (33%) F/TAF dosing group, 215 fmol/106 cells, was more than five times the 90% effective concentration (EC90) for PrEP (i.e., 40 fmol/106 cells).[13] While an analogous EC90 does not exist for ART, to our knowledge, dose-ranging studies with TAF and TDF showed greater declines in HIV RNA with higher TFV-DP in PBMC.[14] These observations suggest clinical relevance for our findings.
Because TFV is renally eliminated, it was not surprising that a decrease in CrCl of 10 ml/min was associated with a ~5% increase in TFV-DP in PBMC with F/TDF dosing. However, this same relationship was not observed for F/TAF. Race was also found to be associated with TFV-DP in PBMC with F/TDF, but not F/TAF, with non-white participants having ~28% lower concentrations vs. white participants. Although these lower concentrations were observed in this analysis, it should be noted that there was no difference in the efficacy of F/TDF as PrEP in the prespecified subgroup analyses of the iPrEx study by race.[18] The reason for this difference is unclear and our non-white sample sizes were low, so this finding should be confirmed in future studies.
The estimated half-life of TFV-DP in PBMC was similar following F/TAF and F/TDF dosing (2.1 vs. 2.9 days, respectively), and are consistent with previously reported values ranging from 48 to 180 hours.[19–24]
FTC-TP in PBMC were in the range of previous studies, and did not significantly differ following F/TAF vs. F/TDF dosing.[13, 19] As expected, since FTC is renally eliminated, significantly lower concentrations were seen among those with increased CrCl.[3] Concentrations were about half in the 33% arm vs 100% arms which must be factored in when considering pharmacological forgiveness.
Strengths of these studies included the prospective and controlled designs and the use of directly observed dosing at varied levels of adherence. Further, both studies used the same processing and analytical procedures for TFV-DP and FTC-TP measurements. However, there were also some limitations, including the lack of mucosal or lymph node tissue concentration measurements, and therefore the inability to compare concentrations following F/TAF vs. F/TDF in these compartments. It is still unclear if mucosal concentrations at the site of transmission are associated with greater PrEP efficacy, and past studies have found concentrations with TAF are similar to or less than that with TDF at these sites.[25, 26] Thus, the relationship between these concentrations and PrEP efficacy should be evaluated further. Similarly, our studies did not measure prevention efficacy, as the study participants were at low risk of infection, so direct correlations with outcomes were not possible. Lastly, racial balance was not quite achieved among the groups, which limits our ability to generalize these findings to diverse populations.
In conclusion, F/TAF produced significantly higher concentrations in PBMC vs. F/TDF across low, medium, and high levels of adherence, with the lowest adherence on F/TAF resulting in more than 2-fold higher concentrations than the highest level of adherence on F/TDF. Future studies should assess the implications of these findings as they relate to increased potency and pharmacologic forgiveness for HIV prevention and treatment.
Supplementary Material
Supplemental Figure 1. Study design for DOT-DBS and TAF-DBS Studies. DOT-DBS and TAF-DBS were separate randomized, crossover studies, where participants received two different 12-week dosing regimens of 33%, 67% or 100% daily dosing, separated by a 12-week washout period. Blood was collected at weekly in TAF-DBS and bi-weekly in DOT-DBS. Concentrations in PBMC were quantified to characterize the steady-state and washout kinetics, at weeks 2, 4, 10, 12, 15, 26, 28, 34, and 36 in DOT-DBS and the same plus 4 hours after the first dose, then weeks 1, 3, 13, 14, 16, 17, 25, and 27 in TAF-DBS.
Supplemental Figure 2. Observed and predicted TFV-DP concentrations in PBMC following F/TAF. Observed (open circles) and model-fitted [95% CI] (solid [dashed] lines) TFV-DP in PBMC (fmol/106 cells) by study day for 33% dosing (red), 67% dosing (green), and 100% dosing (blue). Prior to steady state, a nonlinear mixed effect model with tensor product of natural b-spline transformation of study day and study arm was used to model concentrations. The estimate was constant over time at steady state, and then an exponential decay was modeled during washout.
Supplemental Figure 3. Observed and predicted TFV-DP concentrations in PBMC following F/TDF. Observed (open circles) and model-fitted [95% CI] (solid [dashed] lines) TFV-DP in PBMC (fmol/106 cells) by study day for 33% dosing (red), 67% dosing (green), and 100% dosing (blue). Prior to steady state, a nonlinear mixed effect model with tensor product of natural b-spline transformation of study day and study arm was used to model concentrations. The estimate was constant over time at steady state, and then an exponential decay was modeled during washout.
Acknowledgements
We would like to thank the study participants, the staff at the University of Colorado Clinical and Translational Research Center (CTRC), and the personnel at the Colorado Antiviral Pharmacology Laboratory for their support and contributions to this work. The University of Colorado is a Certara Center of Excellence school. The Center of Excellence program supports leading institutions with Certara’s state of the art model-informed drug development software.
Funding:
This work was supported by Gilead Sciences (CO-US-412-3992 [TAF-DBS Study, P.L.A]) and the NIH (U01 AI106499 [DOT-DBS Study, P.L.A] and UL1 TR002535 [University of Colorado Clinical and Translational Sciences Institute]). Gilead Sciences provided study drug.
Transparency Declarations: P. L. A. received consulting fees from Gilead, Merck, and ViiV, and research funding paid to his institution from Gilead Sciences.
References
- 1.Kearney BP, Flaherty JF, Shah J. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin Pharmacokinet 2004; 43(9):595–612. [DOI] [PubMed] [Google Scholar]
- 2.Brooks KM, Ibrahim ME, Castillo-Mancilla JR, MaWhinney S, Alexander K, Tilden S, et al. Pharmacokinetics of tenofovir monoester and association with intracellular tenofovir diphosphate following single-dose tenofovir disoproxil fumarate. J Antimicrob Chemother 2019; 74(8):2352–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wassner C, Bradley N, Lee Y. A Review and Clinical Understanding of Tenofovir: Tenofovir Disoproxil Fumarate versus Tenofovir Alafenamide. J Int Assoc Provid AIDS Care 2020; 19:2325958220919231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Havens PL, Kiser JJ, Stephensen CB, Hazra R, Flynn PM, Wilson CM, et al. Association of higher plasma vitamin D binding protein and lower free calcitriol levels with tenofovir disoproxil fumarate use and plasma and intracellular tenofovir pharmacokinetics: cause of a functional vitamin D deficiency? Antimicrob Agents Chemother 2013; 57(11):5619–5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulligan K, Glidden DV, Anderson PL, Liu A, McMahan V, Gonzales P, et al. Effects of Emtricitabine/Tenofovir on Bone Mineral Density in HIV-Negative Persons in a Randomized, Double-Blind, Placebo-Controlled Trial. Clin Infect Dis 2015; 61(4):572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babusis D, Phan TK, Lee WA, Watkins WJ, Ray AS. Mechanism for effective lymphoid cell and tissue loading following oral administration of nucleotide prodrug GS-7340. Mol Pharm 2013; 10(2):459–466. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher CV, Podany AT, Thorkelson A, Winchester LC, Mykris T, Anderson J, et al. The Lymphoid Tissue Pharmacokinetics of Tenofovir Disoproxil Fumarate and Tenofovir Alafenamide in HIV-Infected Persons. Clin Pharmacol Ther 2020; 108(5):971–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sax PE, Wohl D, Yin MT, Post F, DeJesus E, Saag M, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet 2015; 385(9987):2606–2615. [DOI] [PubMed] [Google Scholar]
- 9.Sax PE, Zolopa A, Brar I, Elion R, Ortiz R, Post F, et al. Tenofovir alafenamide vs. tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndr 2014; 67(1):52–58. [DOI] [PubMed] [Google Scholar]
- 10.Podany AT, Bares SH, Havens J, Dyavar SR, O’Neill J, Lee S, et al. Plasma and intracellular pharmacokinetics of tenofovir in patients switched from tenofovir disoproxil fumarate to tenofovir alafenamide. AIDS 2018; 32(6):761–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunter MC, Teijeira A, Halin C. T Cell Trafficking through Lymphatic Vessels. Front Immunol 2016; 7:613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bucy RP, Hockett RD, Derdeyn CA, Saag MS, Squires K, Sillers M, et al. Initial increase in blood CD4(+) lymphocytes after HIV antiretroviral therapy reflects redistribution from lymphoid tissues. J Clin Invest 1999; 103(10):1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012; 4(151):151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruane PJ, DeJesus E, Berger D, Markowitz M, Bredeek UF, Callebaut C, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J Acquir Immune Defic Syndr 2013; 63(4):449–455. [DOI] [PubMed] [Google Scholar]
- 15.Anderson PL, Liu AY, Castillo-Mancilla JR, Gardner EM, Seifert SM, McHugh C, et al. Intracellular Tenofovir-Diphosphate and Emtricitabine-Triphosphate in Dried Blood Spots following Directly Observed Therapy. Antimicrob Agents Chemother 2018; 62(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yager J, Castillo-Mancilla J, Ibrahim ME, Brooks KM, McHugh C, Morrow M, et al. Intracellular Tenofovir-Diphosphate and Emtricitabine-Triphosphate in Dried Blood Spots Following Tenofovir Alafenamide: The TAF-DBS Study. J Acquir Immune Defic Syndr 2020; 84(3):323–330. [DOI] [PubMed] [Google Scholar]
- 17.Zheng JH, Rower C, McAllister K, Castillo-Mancilla J, Klein B, Meditz A, et al. Application of an intracellular assay for determination of tenofovir-diphosphate and emtricitabine-triphosphate from erythrocytes using dried blood spots. J Pharm Biomed Anal 2016; 122:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363(27):2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seifert SM, Chen X, Meditz AL, Castillo-Mancilla JR, Gardner EM, Predhomme JA, et al. Intracellular Tenofovir and Emtricitabine Anabolites in Genital, Rectal, and Blood Compartments from First Dose to Steady State. AIDS Res Hum Retroviruses 2016; 32(10–11):981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burns RN, Hendrix CW, Chaturvedula A. Population pharmacokinetics of tenofovir and tenofovir-diphosphate in healthy women. J Clin Pharmacol 2015; 55(6):629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hendrix CW, Andrade A, Bumpus NN, Kashuba AD, Marzinke MA, Moore A, et al. Dose Frequency Ranging Pharmacokinetic Study of Tenofovir-Emtricitabine After Directly Observed Dosing in Healthy Volunteers to Establish Adherence Benchmarks (HPTN 066). AIDS Res Hum Retroviruses 2016; 32(1):32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louissaint NA, Cao YJ, Skipper PL, Liberman RG, Tannenbaum SR, Nimmagadda S, et al. Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue. AIDS Res Hum Retroviruses 2013; 29(11):1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawkins T, Veikley W, St Claire RL 3rd, Guyer B, Clark N, Kearney BP. Intracellular pharmacokinetics of tenofovir diphosphate, carbovir triphosphate, and lamivudine triphosphate in patients receiving triple-nucleoside regimens. J Acquir Immune Defic Syndr 2005; 39(4):406–411. [DOI] [PubMed] [Google Scholar]
- 24.Pruvost A, Negredo E, Benech H, Theodoro F, Puig J, Grau E, et al. Measurement of intracellular didanosine and tenofovir phosphorylated metabolites and possible interaction of the two drugs in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother 2005; 49(5):1907–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thurman AR, Schwartz JL, Cottrell ML, Brache V, Chen BA, Cochon L, et al. Safety and Pharmacokinetics of a Tenofovir Alafenamide Fumarate-Emtricitabine based Oral Antiretroviral Regimen for Prevention of HIV Acquisition in Women: A Randomized Controlled Trial. EClinicalMedicine 2021; 36:100893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cottrell ML, Garrett KL, Prince HMA, Sykes C, Schauer A, Emerson CW, et al. Single-dose pharmacokinetics of tenofovir alafenamide and its active metabolite in the mucosal tissues. J Antimicrob Chemother 2017; 72(6):1731–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Study design for DOT-DBS and TAF-DBS Studies. DOT-DBS and TAF-DBS were separate randomized, crossover studies, where participants received two different 12-week dosing regimens of 33%, 67% or 100% daily dosing, separated by a 12-week washout period. Blood was collected at weekly in TAF-DBS and bi-weekly in DOT-DBS. Concentrations in PBMC were quantified to characterize the steady-state and washout kinetics, at weeks 2, 4, 10, 12, 15, 26, 28, 34, and 36 in DOT-DBS and the same plus 4 hours after the first dose, then weeks 1, 3, 13, 14, 16, 17, 25, and 27 in TAF-DBS.
Supplemental Figure 2. Observed and predicted TFV-DP concentrations in PBMC following F/TAF. Observed (open circles) and model-fitted [95% CI] (solid [dashed] lines) TFV-DP in PBMC (fmol/106 cells) by study day for 33% dosing (red), 67% dosing (green), and 100% dosing (blue). Prior to steady state, a nonlinear mixed effect model with tensor product of natural b-spline transformation of study day and study arm was used to model concentrations. The estimate was constant over time at steady state, and then an exponential decay was modeled during washout.
Supplemental Figure 3. Observed and predicted TFV-DP concentrations in PBMC following F/TDF. Observed (open circles) and model-fitted [95% CI] (solid [dashed] lines) TFV-DP in PBMC (fmol/106 cells) by study day for 33% dosing (red), 67% dosing (green), and 100% dosing (blue). Prior to steady state, a nonlinear mixed effect model with tensor product of natural b-spline transformation of study day and study arm was used to model concentrations. The estimate was constant over time at steady state, and then an exponential decay was modeled during washout.