Abstract
Purpose:
This goal of this review is to help providers recognize, diagnose and manage gastrointestinal (GI) polyposis syndromes.
Recent findings:
Intestinal polyps include a number of histological sub-types such as adenomas, serrated, hamartomas among others. Over a quarter of individuals undergoing screening colonoscopy are expected to have colonic adenomas. While it is not uncommon for adults to have a few GI polyps in their lifetime, some individuals are found to have multiple polyps of varying histology throughout the GI tract. In these individuals, depending on polyp histology, number, location and size as well as extra-intestinal features and/or family history, a polyposis syndrome should be considered with appropriate testing and management.
Summary:
Diagnosis and management of polyposis syndromes has evolved with advent of multi-gene panel testing and new data on optimal surveillance strategies. Evidence-based recommendations and current practice guidelines for polyposis syndromes are reviewed here. Areas of uncertainty and future research are also highlighted.
Keywords: polyposis, genetics, adenomas, serrated polyps, hamartomas
Introduction
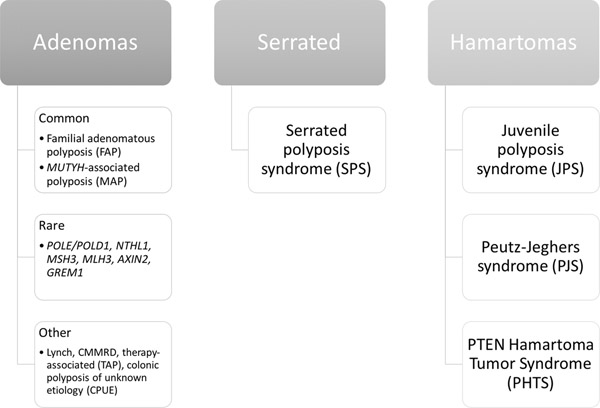
Performing risk assessment for polyposis syndromes can be complicated, as there are many genes that, when mutated, can increase the risk of polyps. Polyp histology, in particular, as well as polyp number, size and anatomic location of polyps can help narrow down the differential diagnosis (Figure 1). Testing for an adenomatous polyposis syndrome should be performed in an individual with 10 or more cumulative adenomas (1)(2). Adenomatous polyposis syndromes include familial adenomatous polyposis (FAP), MUTYH-associated polyposis (MAP) as well as a number of rare genetic or other non-genetic causes. Serrated polyposis syndrome (SPS) is defined by World Health Organization (WHO) criteria based on number, size and location of colonic serrated polyps(3); no definitive genetic basis for SPS has been identified. Hamartomatous polyposis syndromes include juvenile polyposis syndrome (JPS), Peutz-Jeghers syndrome (PJS) and PTEN hamartoma tumor syndromes (PHTS). Depending on the syndrome, hamartomas are found in the stomach, small intestine and/or colon with a number of extra-intestinal manifestations.
Figure 1: Gastrointestinal polyposis syndromes.
Gastrointestinal polyposis syndromes can be classified based on the predominant polyp histology: adenomas, serrated polyps and hamartomas. The most common and best characterized adenomatous polyposis syndromes include familial adenomatous polyposis (FAP) and MUTYH-associated polyposis (MAP). A number of additional genes have been implicated in adenomatous polyposis including: POLE/POLD1, NTHL1, MSH3, MLH3, AXIN2 and GREM1 (associated with hereditary mixed polyposis syndrome). Other conditions that present with adenomatous polyposis include Lynch syndrome (especially MSH2 and MSH6), constitutional mismatch repair deficiency (CMMRD), therapy-associated polyposis (TAP) in childhood and young adult cancer survivors and colonic polyposis of unknown etiology (CPUE) which includes a heterogeneous group of patients with unexplained polyposis ranging from phenotypes similar to FAP to more attenuated phenotypes likely related to low-penetrance genetic and environmental factors (tobacco, alcohol, obesity and poor diet). Serrated polyposis syndrome is defined clinically by World Health Organization criteria based on number, size, and location of serrated lesions. Hamartomas are overgrowth of normal gastrointestinal tissue. Hamartomatous polyposis syndromes are rare and include Juvenile polyposis syndrome (JPS), Peutz-Jeghers syndrome (PJS) and PTEN Hamartoma Tumor Syndrome (PHTS) of which Cowden syndrome is the most commonly encountered.
Currently, multi-gene panels are the preferred method of genetic testing (2)(4). Panel testing is cost effective, has a reasonable turnaround time, allows testing of many genes that may have overlapping phenotypes and is not dependent on family history knowledge. Identification of a pathogenic/likely pathogenic (P/LP) variant allows for appropriate management. A study of multigene panel testing in individuals with 10 or more polyps (5) showed that the likelihood of detecting a P/LP variant increases with the number of adenomatous polyps present, but not with the number of hamartomatous polyps. In total, 10.4% of patients with 10 or more polyps had a P/LP variant identified in a polyposis gene, while an additional 3.6% had a P/LP variant in a non-polyposis colorectal cancer gene for which surveillance guidelines exist. Once a P/LP variant has been identified, the patient should be counseled to inform family members so they can be tested (termed cascade testing).
Adenomatous polyposis syndromes
Familial adenomatous polyposis.
FAP results from P/LP variants in the APC gene and is inherited in an autosomal dominant fashion with nearly complete penetrance (6). The estimated live birth incidence of FAP is 1 in 8,000 to 1 to 10,000 (7). Nearly one third of FAP cases occur in individuals without a family history of the disease (6) due to de novo mutations or, as more recently reported, mosaicism (8)(9).
In classic FAP, 100s-1000s of synchronous adenomas carpet the colon. Attenuated FAP (AFAP) is characterized by oligopolyposis with polyp burden of 10s-100 adenomatous polyps (10). Gastric fundic gland polyps and duodenal adenomas are common in FAP, occurring in about 50% of cases (11), while adenomatous gastric polyps are less common. Other extracolonic manifestations of both FAP and AFAP can include papillary thyroid cancer, epidermal cysts, bone osteomas, desmoid tumors, adrenal gland adenomas, supernumerary teeth, and congenital hypertrophy of the retinal pigment epithelium (CHRPE) (12).
The lifetime risk of colon cancer in classic FAP approaches 100% with average age of onset 40 years, whereas in AFAP the lifetime risk is lower at 70% with average onset age closer to age 55 (13). Risk of duodenal cancer is increased and associated with duodenal polyposis burden. There is also a rising incidence of gastric cancer (14). For patients with the classic FAP phenotype, colonoscopy is recommended starting at age 10–12 and should be repeated every 1–2 years rated as having moderate level of evidence by the American Society of Gastrointestinal Endoscopy (ASGE) (15). In AFAP, colonoscopy should begin at age 18 unless symptoms warrant earlier screening (6) for which quality of evidence was deemed low (15). Upper endoscopy with a clear cap or side viewing endoscope is recommended starting at around age 20–25 for the detection of dysplastic gastric polyps (with special attention to carpeted or polyp mounds (16)), duodenal adenomas, or ampullary adenomas due to risk of gastric and duodenal cancers. These recommendations are based on low quality of evidence per ASGE (15). Additionally, due the papillary thyroid cancer risk, thyroid ultrasound is recommended for FAP patients starting in the late teenage years, with repeat every 2–5 years if negative (6).
In cases where colon polyp burden becomes unmanageable endoscopically (either due to number or morphology), colectomy should be considered. Additionally, development of multiple large adenomas (>6mm), dysplasia, or suspected carcinoma are indications for consideration of surgery (6,13). Patients with classic FAP should be counseled early on eventual need for colectomy, though elective colectomy can be deferred to the second decade of life in those with low and resectable polyp burden. For patients undergoing colectomy, shared decision making should be utilized in determining the optical surgical approach based on patient specific factors. Patients with a resultant ileoanal anastomosis or ileal pouch anal anastomosis (IPAA) should undergo yearly endoscopic surveillance due to the continued risk of cancer development in these areas. Those with an end ileostomy should have surveillance every 2 years (17). The role of chemoprevention in management of FAP is actively being studied (18) with promising results for sulindac and erlotinib (19),(20),(21).
MUTYH polyposis.
MUTYH-associated polyposis (MAP) is an oligopolyposis syndrome characterized by the development of 10s to 100 colon polyps. MAP results from germline P/LP variants in the DNA base excision repair gene MUTYH. In contrast to FAP, inheritance is autosomal recessive and requires a homozygous or compound heterozygous mutation phenotype for expression of the disease (22). Colon polyps in MAP are most commonly adenomas, however a mixed polyposis with sessile serrated lesions/polyps and hyperplastic polyps can also be seen. Duodenal polyposis also occurs in approximately 15–21% of MAP cases (6, 23) and, based on results from a recent international study of nearly 400 MAP patients, duodenal adenoma burden was lower than in FAP but the Spigelman staging system failed to identify high risk individuals and duodenal cancers (23).
The lifetime risk of colorectal cancer in MAP is estimated at 80% without surveillance (24). Management of MAP includes colonoscopy beginning at age 20–25, repeated every 1–2 years if negative based on low quality of evidence (6, 15). Similar to FAP and AFAP, colectomy is recommended if polyp burden cannot be managed with endoscopic surveillance. Due to the 4% lifetime risk of duodenal adenomas, EGD (with assessment of the ampulla) is recommended starting at age 30–35 (25) which is also based on low quality evidence (15).
Monoallelic P/LP variants in MUTYH are found in 1–2% of the population and do not present with a polyposis phenotype (26). This is especially important to recognize because monoallelic MUTYH variants are one of the most common findings on multigene panel tests. The colon cancer risk in these cases is estimated to be slightly higher than the general population especially with family history of colorectal cancer, though the absolute risk level is not well established (24). ACG guidelines recommend colonoscopy starting at age 40 in these patients with repeat screening exam every 5 years (6), while NCCN and ASGE recommend earlier surveillance only in individuals with a first degree relative with colorectal cancer (1,15).
Other polyposis syndromes.
A summary of other genes that lead to adenomatous polyposis (POLE, POLD1, NTHL1, MSH3, MLH3, AXIN2 and GREM1) is shown in Table 1. In addition to these, patients with P/LP variants in Lynch syndrome genes, especially MSH2 and MSH6 (27), can present with an attenuated polyposis phenotype. Individuals with biallelic MMR mutations have a condition called Constitutional Mismatch Repair Deficiency syndrome (CMMRD), characterized by young onset malignancies of the GI tract, gliomas and lymphomas/leukemias. A study of 24 individuals with CMMRD identified 9 individuals with adenomas (one with high-grade dysplasia), in addition to 7 with colorectal cancer at initial colonoscopy (28).
Table 1:
Rare adenomatous polyposis syndromes
| Gene(s)/ function |
Inheritance | Clinical features | References |
|---|---|---|---|
|
POLE/POLD1 Polymerase proofreading |
AD | • Adenomas (10s-100) • Colorectal, endometrial & brain cancers • Overlap features with Lynch and CMMRD |
(47–61) |
|
NTHL1 Base excision repair |
AR | • Adenomas (10s-100) • Colorectal cancer • Breast cancer • Possibly other cancers |
(62–76) |
|
MSH3 Mismatch repair |
AR | • Adenomas (10s-100) • Colorectal cancer |
(76, 77) |
|
MLH3 Mismatch repair |
AR | • Adenomas (10s-100) • Colorectal cancer |
(79,80) |
|
AXIN2 Wnt-signaling |
AD | • Adenomas (10s-100) • Colorectal cancer • Oligodontia |
(81–89) |
|
GREM1 TGFß-signaling |
AD | • Duplication found in AJ families • Mixed polyps (called Hereditary Mixed Polyposis Syndrome, HMPS) • Colorectal cancer |
(90–96) |
AD, autosomal dominant; AR, autosomal recessive; CMMRD, constitutional mismatch repair deficiency
Survivors of childhood or young adult cancers are at risk for therapy-associated polyposis (TAP). Clinical manifestations include polyposis with adenomas, serrated polyps, inflammatory/hamartomatous polyps and mixed polyp types (29). A recent multi-center study of 34 patients with TAP reported diagnosis an average of 27 years after initial treatment (30). The average number of polyps was 32, 94% of TAP patients had more than one histologic type, and 74% of patients who had an EGD had polyps in the upper GI tract. In total, 41% of patients had at least some of their colon resected; 50% for cancer and 50% for management of polyps. The authors propose current oncology specific colorectal cancer guidelines be expanded to include individuals receiving chemotherapy (without abdominopelvic radiation); colonoscopy every 5 years, beginning at age 35 (with modifications for age at time of chemotherapy or radiation) (31). They also recommend baseline EGD when colon polyposis is found.
Only 13.5% individuals with >10 adenomas had a P/LP variant on multigene panel testing, meaning that the cause of polyposis is not identified in most cases (5). An individual with a personal history of ≥10 polyps in the absence of an identifiable P/LP variant has a diagnosis of colonic polyposis of uncertain etiology (CPUE). It is likely that CPUE is due to low penetrance genetic and/or environmental factors (e.g., tobacco, alcohol, obesity, diet). NCCN recommends managing CPUE patients based on their personal and family history. Individuals with >100 adenomas should be followed as if they have FAP, while those with 20–99 polyps should have colonoscopy every 1–2 years. Patients with CPUE might also benefit of baseline EGD, with follow up based on findings. Patients with 11–20 adenomas should be managed based on clinical judgment and consider size, number, and type of polyp and family history.
Serrated Polyposis Syndrome
Serrated polyposis syndrome (SPS) is a clinical syndrome characterized by numerous cumulative colonic serrated lesions including traditional serrated adenomas, sessile serrated polyps, and hyperplastic polyps. SPS is the most common colonic polyposis syndrome with primary screening cohort studies finding 1:239 (0.42%) of patients meet criteria for SPS (32), though it is likely underdiagnosed (33). Updated WHO criteria for SPS (34) are listed in Table 2. A definitive genetic cause for SPS has not been established, though P/LP variants in RNF43 have been reported (35) but appear to be rare and not found in other cohorts (35, 36).
Table 2:
WHO criteria for Serrated Polyposis Syndrome (SPS)
| Criterion | Number | Location | Size |
|---|---|---|---|
| 1 | ≥5 serrated polyps | Proximal to rectum | All ≥5mm in size with at least 2 polyps ≥10mm |
| 2 | ≥ 20 serrated polyps | Distributed throughout colon with ≥5 polyps proximal to the rectum | Any size |
In SPS, colorectal cancer incidence is reported at 15–30% (32, 37). Once a diagnosis of SPS has been established, colonoscopy with polypectomy should be performed at short intervals to clear the initial polyp burden. Following this, surveillance colonoscopy is recommended every 1–3 years for polyp management (39–41) due to ongoing risk (42). Like other polyposis syndromes, surgical management should be considered when endoscopic management is no longer feasible.
Hamartomatous polyposis syndromes (Table 3)
Table 3:
Hamartomatous polyposis syndromes
| Syndrome | Gene(s) | Clinical manifestations |
|---|---|---|
|
| ||
| Juvenile polyposis syndrome (JPS) | BMPR1A SMAD4 | • Colonic juvenile polyps |
| • Gastric juvenile polyps (SMAD4 > BMPR1A) | ||
| • Hereditary Hemorrhagic Telangiectasia, HHT (only SMAD4) | ||
| • Congenital heart defects | ||
|
| ||
| Peutz-Jeghers syndrome (PJS) | STK11 | • Small bowel Peutz-Jeghers polyps |
| - bleeding & obstructions (in children) | ||
| • Mucocutaneous pigmentation | ||
| • GI and non-GI cancer risks (in adults) | ||
|
| ||
| PTEN Hamartoma Tumor Syndrome (PHTS) | PTEN | • Spectrum of condition with PTEN pathogenic variants |
| • Cowden syndrome: | ||
| - GI hamartomas & ganglioneuromas | ||
| - esophageal glycogenic acanthosis | ||
| - cancer risks (breast, endometrial, thyroid, kidney, colorectal) | ||
| - macrocephaly | ||
| - dermatological lesions | ||
Juvenile polyposis syndrome.
JPS has an estimated incidence of 1:16,000–1:100,000 live births. A clinical diagnosis is made when any one of the following is met: 5 or more juvenile polyps in the colon, multiple juvenile polyps in the GI tract or any number of juvenile polyps with family history of JPS (1). It should be noted that about 1% of children will develop a single juvenile polyp in their lifetime but should not be tested for JPS unless other clinical criteria are met. About 50% of individuals who meet clinical criteria are found to have P/LP variants in one of two genes involved in TGFß-signaling, SMAD4 and BMPR1A, and is inherited in an autosomal dominant pattern. The primary clinical manifestations are juvenile polyps and cancers in the colon and stomach. Carriers of SMAD4 P/LP variants are also at risk for hereditary hemorrhagic telangiectasia (HHT). A recent European multi-center study (43) characterized a large cohort of JPS patients (n=221 patients from 126 kindreds; 57% with P/LP variants in SMAD4 or BMPR1A) (43). Compared to BMPR1A carriers, SMAD4 carriers had higher prevalence of anemia (58% vs. 26%), HHT (32% vs. 0%) and gastric polyps (39% vs. 13%). Colonic polyps were found in both BMPR1A and SMAD4 index carriers (91% and 86%) with more proximal distribution and numbering between 5–100. Overall cancer rate was 15% with 78% being identified before or at the time of JPS diagnosis. Colorectal cancer was found in 12% and 7% of SMAD4 and BMPR1A carriers, respectively. Current US management guidelines recommend colonoscopy and upper endoscopy every 2–3 years (or more frequently based on polyps or symptoms) beginning at age 15; carriers of SMAD4 P/LP variants should also be screened for HHT (1).
Peutz-Jeghers syndrome.
PJS has an estimated incidence of 1:50,000–1:200,000 live births. A clinical diagnosis is based on 2 or more of the following: 2 or more PJ hamartomas in the GI tract, characteristic mucocutaneous pigmentation and/or family history of PJS (1). The primary clinical manifestations vary by age. In children, complications related to small bowel hamartomas such as bleeding or intussusception are most common, while, in adults, cancer risks predominate. Given the rarity of PJS, accurate cancer risk estimates are challenging due to small numbers and ascertainment bias. Within these limitations, lifetime estimates of GI cancer risks are: colorectal (36–39%), gastric (24–29%), small bowel (10–14%), and pancreatic (11–36%) (1, 43). Non-GI cancer risks include: breast (19–54%), sex cord/Sertoli cell ovarian (10–21%), cervical (10%), uterine (9%), testicular (9%) and lung (7–17%) (1, 43). About 80–94% of individuals with PJS are found to have a P/LP variant in STK11 (also known as LKB1), and it is inherited in an autosomal dominant pattern.
Current US (1) and European guidelines (44) recommend upper and lower endoscopy starting at age 8 and repeating every 1–3 years if polyps are present; if no polyps present at index examinations, can start regular surveillance at age 18. Small bowel surveillance in asymptomatic patients is also recommended using MRI or video capsule endoscopy at age 8 and repeating every 1–3 years if polyps are present. If no small bowel polyps are seen on index exam, regular surveillance should begin at age 18 or earlier if symptoms develop. Surveillance for pancreatic and breast cancers is also advocated.
PTEN Hamartoma Tumor Syndrome.
PHTS refers to a spectrum of syndromes due to germline P/LP in the tumor suppressor gene PTEN of which Cowden syndrome is the most common. The estimated incidence of Cowden syndrome is 1:200,000, though this is likely an underestimate. The clinical manifestations include features such as cancers (breast, endometrial, follicular thyroid, colorectal and renal cell), hamartomas in the GI tract, large head circumference and various dermatological lesions. The lifetime risk of colorectal cancer is estimated to be 9–32% with median age of diagnosis of 46–58 years (45), though studies are limited by small size and ascertainment bias. Current US guidelines list major and minor criteria for establishing a clinical diagnosis. The GI manifestations can include glycogenic acanthosis of the esophagus as well as a number of polyp types such as hamartomas, ganglioneuromas, hyperplastic, inflammatory, and adenomas.
Management guidelines for the GI tract include colonoscopy beginning at age 35 (or 5–10 years before the earliest colorectal cancer in the family) with surveillance every 5 years or earlier based on symptoms or polyps. A recent study (46) evaluated outcomes of surveillance in 70 patients with PTEN P/LP variants who had at least 2 upper or lower endoscopies at a single center. There was no difference in size of number of GI polyps during surveillance. One colon cancer and one gastric cancer were diagnosed during surveillance, both of which were associated with large polyp phenotypes underscoring the importance of close surveillance based on polyp size.
Conclusion
The title of this review posed the question “How many is too many?” when it comes to polyps in the GI tract. As discussed in this review, the answer depends on polyp histology, anatomic location, size and other features. For adenomatous polyposis syndromes, guidelines recommend multigene panel testing for 10 or more adenomas. For serrated polyposis syndrome, clinical criteria are based on number, size and location of serrated lesions. For hamartomatous polyposis syndromes, clinical criteria depend on hamartoma sub-type and anatomic location along with a host of other intestinal and extra-intestinal manifestations. Once a polyposis syndrome is suspected, the best next step is to refer to a specialized professional, such a genetic counselor, for further evaluation and multi-gene panel testing. Based on clinical criteria and genetic test results, management and cascade testing should follow current guidelines in order to reduce the burden of cancer in high-risk families. More research is needed to understand unexplained polyposis cases, to increase recognition and testing for polyposis syndromes, to develop robust evidence-based management guidelines and to study new treatment strategies such as chemoprevention.
Key points:
Identification of a polyposis syndrome depends on polyp histology, number, size, anatomic location, non-gastrointestinal manifestations and/or family history.
Multi-gene panel testing is recommended when a polyposis syndrome is suspected given the number of genes implicated and cost-effectiveness of testing multiple genes simultaneously.
An adenomatous polyposis syndrome, such as familial adenomatous polyposis, MUTYH-associated polyposis or a number of rare syndromes, should be suspected with 10 or more cumulative adenomas.
Serrated polyposis syndrome, the most common polyposis syndrome for which a genetic basis has not been definitively established, should be suspected based on the number, size and anatomic location of serrated lesions in the colon; once identified, patients should undergo regular surveillance given increased risk of colorectal cancer.
Hamartomatous polyposis syndromes, Juvenile Polyposis syndrome, Peutz-Jeghers syndrome and PTEN Hamartoma Tumor syndrome, are rare and manifest with multiple malignant and benign features that should be managed by a multi-disciplinary team.
Funding:
NIH/NCI R01 CA220329 (to S.S.K.)
Footnotes
3. Conflict of interest: SSK has performed collaborative research with Invitae in the last 12 months but did not receive financial compensation.
Disclosures:
Sonia S. Kupfer has performed collaborative research with Invitae but has not received financial compensation.
References:
- 1. National Comprehensive Cancer Network, Inc. 2021. Genetic/Familial High Risk Assessment: Colorectal v.1.2021 Available at https://www.nccn.org/guidelines/guidelines-detail?category=2&id=1436. ** NCCN summarizes their management recommendations for individuals with polyposis, which differs by mutated gene. NCCN guidelines are updated on a regular basis when new evidence becomes available.
- 2. Heald B, Hampel H, Church J, Dudley B, Hall MJ, Mork ME, et al. Collaborative Group of the Americas on Inherited Gastrointestinal Cancer Position statement on multigene panel testing for patients with colorectal cancer and/or polyposis. Fam Cancer. 2020. July;19(3):223–39. ** Collaborative Group of the Americas on Inherited Gastrointestinal Cancers published their position statement on genetic testing in polyposis, who recommend multi-gene panel testing given the number of polyposis syndromes (which can have overlapping features), and improvements in sequencing technology. They recommend that individuals with 10+ adenomas or 3+ hamartomatous polyps should undergo multi-gene panel testing. This statement also includes review of the minimum gene set to be used when testing individuals for a polyposis syndrome.
- 3.Serrated polyposis syndrome [Internet]. InSiGHT. [cited 2021 Aug 18]. Available from: https://www.insight-group.org/syndromes/serrated-polyposis-syndrome/
- 4.Stoll J, Kupfer SS. Risk Assessment and Genetic Testing for Inherited Gastrointestinal Syndromes. Gastroenterol Hepatol. 2019. September;15(9):462–70. [PMC free article] [PubMed] [Google Scholar]
- 5. Stanich PP, Pearlman R, Hinton A, Gutierrez S, LaDuca H, Hampel H, et al. Prevalence of Germline Mutations in Polyposis and Colorectal Cancer–Associated Genes in Patients With Multiple Colorectal Polyps. Clin Gastroenterol Hepatol. 2019. September 1;17(10):2008–2015.e3. ** Stanich et al summarizes genetic findings of 3789 patients and found that the chance to find a P/LP variant increases with age in adenomatous polyposis. With the exception of APC and MUTYH, pathogenic/likely pathogenic variants in adenomatous polyposis genes are rare. This study also showed that adenoma count is also associated with finding a pathogenic or likely pathogenic variant.
- 6. Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW. ACG Clinical Guideline: Genetic Testing and Management of Hereditary Gastrointestinal Cancer Syndromes. Am J Gastroenterol. 2015. February;110(2):223–63. * The American College of Gastroenterology (ACG) provides consensus recommendations for the identification and management of hereditary gastrointestinal cancer syndromes, including various polyposis syndromes. Strength of recommendation and level of evidence are provided as well.
- 7.Bisgaard ML, Fenger K, Bülow S, Niebuhr E, Mohr J. Familial adenomatous polyposis (FAP): frequency, penetrance, and mutation rate. Hum Mutat. 1994;3(2):121–5. [DOI] [PubMed] [Google Scholar]
- 8.Hes FJ, Nielsen M, Bik EC, Konvalinka D, Wijnen JT, Bakker E, et al. Somatic APC mosaicism: an underestimated cause of polyposis coli. Gut. 2008. January;57(1):71–6. [DOI] [PubMed] [Google Scholar]
- 9.Aretz S, Stienen D, Friedrichs N, Stemmler S, Uhlhaas S, Rahner N, et al. Somatic APC mosaicism: a frequent cause of familial adenomatous polyposis (FAP). Hum Mutat. 2007. October;28(10):985–92. [DOI] [PubMed] [Google Scholar]
- 10.Samadder NJ, Baffy N, Giridhar KV, Couch FJ, Riegert-Johnson D. Hereditary Cancer Syndromes-A Primer on Diagnosis and Management, Part 2: Gastrointestinal Cancer Syndromes. Mayo Clin Proc. 2019. June;94(6):1099–116. [DOI] [PubMed] [Google Scholar]
- 11.Bülow S, Björk J, Christensen IJ, Fausa O, Järvinen H, Moesgaard F, et al. Duodenal adenomatosis in familial adenomatous polyposis. Gut. 2004. March;53(3):381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anaya DA, Chang GJ, Rodriguez-Bigas MA. Extracolonic manifestations of hereditary colorectal cancer syndromes. Clin Colon Rectal Surg. 2008. November;21(4):263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarvepalli S, Burke CA, Monachese M, Leach BH, Laguardia L, O’Malley M, et al. Natural history of colonic polyposis in young patients with familial adenomatous polyposis. Gastrointest Endosc. 2018. October;88(4):726–33. [DOI] [PubMed] [Google Scholar]
- 14.Mankaney G, Leone P, Cruise M, LaGuardia L, O’Malley M, Bhatt A, et al. Gastric cancer in FAP: a concerning rise in incidence. Fam Cancer. 2017. July;16(3):371–6. [DOI] [PubMed] [Google Scholar]
- 15. Yang J, Gurudu SR, Koptiuch C, Agrawal D, Buxbaum JL, Abbas Fehmi SM, et al. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in familial adenomatous polyposis syndromes. Gastrointest Endosc. 2020. May;91(5):963–982.e2. ** The American Society of Gastrointestinal Endoscopy (ASGE) provides evidence-based recommendations for endoscopic screening and surveillance in adenomatous polyposis syndromes including FAP, AFAP, and MAP.
- 16.Leone PJ, Mankaney G, Sarvapelli S, Abushamma S, Lopez R, Cruise M, et al. Endoscopic and histologic features associated with gastric cancer in familial adenomatous polyposis. Gastrointest Endosc. 2019. May;89(5):961–8. [DOI] [PubMed] [Google Scholar]
- 17.Vasen HFA, Möslein G, Alonso A, Aretz S, Bernstein I, Bertario L, et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut. 2008. May;57(5):704–13. [DOI] [PubMed] [Google Scholar]
- 18.Kemp Bohan PM, Mankaney G, Vreeland TJ, Chick RC, Hale DF, Cindass JL, et al. Chemoprevention in familial adenomatous polyposis: past, present and future. Fam Cancer. 2021. January;20(1):23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ulusan AM, Rajendran P, Dashwood WM, Yavuz OF, Kapoor S, Gustafson TA, et al. Optimization of Erlotinib Plus Sulindac Dosing Regimens for Intestinal Cancer Prevention in an Apc-Mutant Model of Familial Adenomatous Polyposis (FAP). Cancer Prev Res Phila Pa. 2021. March;14(3):325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samadder NJ, Kuwada SK, Boucher KM, Byrne K, Kanth P, Samowitz W, et al. Association of Sulindac and Erlotinib vs Placebo With Colorectal Neoplasia in Familial Adenomatous Polyposis: Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018. May 1;4(5):671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samadder NJ, Neklason DW, Boucher KM, Byrne KR, Kanth P, Samowitz W, et al. Effect of Sulindac and Erlotinib vs Placebo on Duodenal Neoplasia in Familial Adenomatous Polyposis: A Randomized Clinical Trial. JAMA. 2016. March 22;315(12):1266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010. June;138(6):2044–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collaborative Group on Duodenal Polyposis in MAP, Thomas LE, Hurley JJ, Sanchez AA, Aznárez MR, Backman A-S, et al. Duodenal Adenomas and Cancer in MUTYH-associated Polyposis: An International Cohort Study. Gastroenterology. 2021. February;160(3):952–954.e4. [DOI] [PubMed] [Google Scholar]
- 24.Win AK, Dowty JG, Cleary SP, Kim H, Buchanan DD, Young JP, et al. Risk of colorectal cancer for carriers of mutations in MUTYH, with and without a family history of cancer. Gastroenterology. 2014. May;146(5):1208–1211.e1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta S, Provenzale D, Llor X, Halverson AL, Grady W, Chung DC, et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Colorectal, Version 2.2019. J Natl Compr Cancer Netw JNCCN. 2019. September 1;17(9):1032–41. [DOI] [PubMed] [Google Scholar]
- 26.Cleary SP, Cotterchio M, Jenkins MA, Kim H, Bristow R, Green R, et al. Germline MutY human homologue mutations and colorectal cancer: a multisite case-control study. Gastroenterology. 2009. April;136(4):1251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Engel C, Ahadova A, Seppälä TT, Aretz S, Bigirwamungu-Bargeman M, Bläker H, et al. Associations of Pathogenic Variants in MLH1, MSH2, and MSH6 With Risk of Colorectal Adenomas and Tumors and With Somatic Mutations in Patients With Lynch Syndrome. Gastroenterology. 2020. April;158(5):1326–33. * Engel et al discuss prevalence of adenomas in Lynch syndrome carriers, and showed that MSH2 and MSH6 carriers have a higher risk of adenomas than MLH1 carriers, however MLH1 carriers and MSH2 carriers have a similar rate of CRC. This indicates that CRC likely develops through different molecular pathways in Lynch syndrome and provides an opportunity to tailor CRC screening based on gene that has a P/LP variant.
- 28.Aronson M, Gallinger S, Cohen Z, Cohen S, Dvir R, Elhasid R, et al. Gastrointestinal Findings in the Largest Series of Patients With Hereditary Biallelic Mismatch Repair Deficiency Syndrome: Report from the International Consortium. Off J Am Coll Gastroenterol ACG. 2016. February;111(2):275–84. [DOI] [PubMed] [Google Scholar]
- 29.Yurgelun MB, Hornick JL, Curry VK, Ukaegbu CI, Brown EK, Hiller E, et al. Therapy-associated polyposis – a novel form of acquired gastrointestinal polyposis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2014. June;12(6):1046–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Biller LH, Ukaegbu C, Dhingra TG, Burke CA, Chertock Y, Chittenden A, et al. A multi-institutional cohort of therapy-associated polyposis in childhood and young adulthood cancer survivors. Cancer Prev Res Phila Pa. 2020. March;13(3):291–8. * Biller et al report findings on individuals who received treatment for cancer in childhood/young adulthood. Polyps were identified at a median of 27 years following treatment. Most people had more than one type of polyp and 35% had more than 50 polyps. Roughly a 75% of these patients had features that were suggestive of one or more hereditary predisposition syndrome, including some with extracolonic features.
- 31.COG_LTFU_Guidelines_v5.pdf [Internet]. [cited 2021 Aug 5]. Available from: http://www.survivorshipguidelines.org/pdf/2018/COG_LTFU_Guidelines_v5.pdf
- 32.IJspeert JEG, Bevan R, Senore C, Kaminski MF, Kuipers EJ, Mroz A, et al. Detection rate of serrated polyps and serrated polyposis syndrome in colorectal cancer screening cohorts: a European overview. Gut. 2017. July;66(7):1225–32. [DOI] [PubMed] [Google Scholar]
- 33.IJspeert JEG, Rana S a. Q, Atkinson NSS, van Herwaarden YJ, Bastiaansen B a. J, van Leerdam ME, et al. Clinical risk factors of colorectal cancer in patients with serrated polyposis syndrome: a multicentre cohort analysis. Gut. 2017. February;66(2):278–84. [DOI] [PubMed] [Google Scholar]
- 34.Rosty C, Brosens LAA, Dekker E. WHO Classification of Tumours. Digestive System Tumors. 5th ed. Lyon: International Agency for Research on Cancer; 2019. 532–534 p. [Google Scholar]
- 35.Yan HHN, Lai JCW, Ho SL, Leung WK, Law WL, Lee JFY, et al. RNF43 germline and somatic mutation in serrated neoplasia pathway and its association with BRAF mutation. Gut. 2017. September;66(9):1645–56. [DOI] [PubMed] [Google Scholar]
- 36.Buchanan DD, Clendenning M, Zhuoer L, Stewart JR, Joseland S, Woodall S, et al. Lack of evidence for germline RNF43 mutations in patients with serrated polyposis syndrome from a large multinational study. Gut. 2017. June;66(6):1170–2. [DOI] [PubMed] [Google Scholar]
- 37.van Herwaarden YJ, Koggel LM, Simmer F, Vink-Börger EM, Dura P, Meijer GA, et al. RNF43 mutation analysis in serrated polyposis, sporadic serrated polyps and Lynch syndrome polyps. Histopathology. 2021. April;78(5):749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carballal S, Rodríguez-Alcalde D, Moreira L, Hernández L, Rodríguez L, Rodríguez-Moranta F, et al. Colorectal cancer risk factors in patients with serrated polyposis syndrome: a large multicentre study. Gut. 2016. November;65(11):1829–37. [DOI] [PubMed] [Google Scholar]
- 39. Bleijenberg AG, IJspeert JE, van Herwaarden YJ, Carballal S, Pellisé M, Jung G, et al. Personalised surveillance for serrated polyposis syndrome: results from a prospective 5-year international cohort study. Gut. 2020. January;69(1):112–21. * In a multi-center prospective European trial, Belijenberg et al determine colon cancer and advanced neoplasia risk rates for SPS patients undergoing routine surveillance. Based on the results, the authors advocate for personalized surveillance recommendations and consideration of slightly extended surveillance internal (2 yrs vs. 1 yr) in cases of low polyp burden and other low risk factors.
- 40.Mankaney G, Rouphael C, Burke CA. Serrated Polyposis Syndrome. Clin Gastroenterol Hepatol. 2020. April 1;18(4):777–9. [DOI] [PubMed] [Google Scholar]
- 41.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012. September;143(3):844–57. [DOI] [PubMed] [Google Scholar]
- 42.Bleijenberg AGC, IJspeert JEG, Hazewinkel Y, Boparai KS, Oppeneer SC, Bastiaansen BAJ, et al. The long-term outcomes and natural disease course of serrated polyposis syndrome: over 10 years of prospective follow-up in a specialized center. Gastrointest Endosc. 2020. November;92(5):1098–1107.e1. [DOI] [PubMed] [Google Scholar]
- 43. Blatter R, Tschupp B, Aretz S, Bernstein I, Colas C, Evans DG, et al. Disease expression in juvenile polyposis syndrome: a retrospective survey on a cohort of 221 European patients and comparison with a literature-derived cohort of 473 SMAD4/BMPR1A pathogenic variant carriers. Genet Med Off J Am Coll Med Genet. 2020. September;22(9):1524–32. * Blatter R et al describe phenotypic features in a cohort of 221 European patients with juvenile polyposis syndrome and compare features in SMAD4 vs. BMPR1A carriers. Compared to BMPR1A carriers, SMAD4 carriers had higher prevalence of anemia, HHT and gastric polyps. Colonic polyps were found in both BMPR1A and SMAD4 index carriers with more proximal distribution and numbering between 5–100. Colorectal cancer was found in 12% and 7% of SMAD4 and BMPR1A carriers, respectively.
- 44. Wagner A, Aretz S, Auranen A, Bruno MJ, Cavestro GM, Crosbie EJ, et al. The Management of Peutz-Jeghers Syndrome: European Hereditary Tumour Group (EHTG) Guideline. J Clin Med. 2021. January 27;10(3):473. * Wagner A et al provide guidelines on management of Peutz-Jeghers Syndrome and provide an evaluation of the quality of evidence to support their recommendations. Overall, the recommendations are similar to the current US NCCN guidelines with some minor differences regarding surveillance intervals.
- 45. Hendricks LAJ, Hoogerbrugge N, Schuurs-Hoeijmakers JHM, Vos JR. A review on age-related cancer risks in PTEN hamartoma tumor syndrome. Clin Genet. 2021. February;99(2):219–25. * Hendricks LAJ et al performed a review of the literature to provide cancer risks in PTEN Hamartoma Tumor Syndrome based on age. Based on this review, lifetime risk of colorectal cancer is estimated to be 9–32% with median age of diagnosis of 46–58 years. Additional cancer risks are also provided and underscore the importance of regular surveillance by a multidisciplinary team.
- 46. Khare A, Burke CA, Heald B, O’Malley M, LaGuardia L, Milicia S, et al. Endoscopic Findings in Patients With PTEN Hamartoma Tumor Syndrome Undergoing Surveillance. J Clin Gastroenterol. 2021. July 7; * Khare A et al review endoscopic findings in 70 patients with PTEN pathogenic/likely pathogenic variants who were in surveillance with at least 2 EGD or colonoscopies at a single academic center. There was no difference in size of number of GI polyps during surveillance. One colon cancer and one gastric cancer were diagnosed during surveillance, both of which were associated with large polyp phenotypes underscoring the importance of close surveillance based on polyp size.
- 47.Palles C, Cazier J-B, Howarth KM, Domingo E, Jones AM, Broderick P, et al. Germline mutations in the proof-reading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat Genet. 2013. February;45(2):136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valle L, Hernández-Illán E, Bellido F, Aiza G, Castillejo A, Castillejo M-I, et al. New insights into POLE and POLD1 germline mutations in familial colorectal cancer and polyposis. Hum Mol Genet. 2014. July 1;23(13):3506–12. [DOI] [PubMed] [Google Scholar]
- 49.Church JM. Polymerase proofreading-associated polyposis: a new, dominantly inherited syndrome of hereditary colorectal cancer predisposition. Dis Colon Rectum. 2014. March;57(3):396–7. [DOI] [PubMed] [Google Scholar]
- 50.Hamzaoui N, Alarcon F, Leulliot N, Guimbaud R, Buecher B, Colas C, et al. Genetic, structural, and functional characterization of POLE polymerase proofreading variants allows cancer risk prediction. Genet Med. 2020. September;22(9):1533–41. [DOI] [PubMed] [Google Scholar]
- 51.Palles C, Martin L, Domingo E, Chegwidden L, McGuire J, Cuthill V, et al. The clinical features of polymerase proof-reading associated polyposis (PPAP) and recommendations for patient management. Fam Cancer [Internet]. 2021. May 5 [cited 2021 Aug 9]; Available from: 10.1007/s10689-021-00256-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elsayed FA, Kets CM, Ruano D, van den Akker B, Mensenkamp AR, Schrumpf M, et al. Germline variants in POLE are associated with early onset mismatch repair deficient colorectal cancer. Eur J Hum Genet. 2015. August;23(8):1080–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bellido F, Pineda M, Aiza G, Valdés-Mas R, Navarro M, Puente DA, et al. POLE and POLD1 mutations in 529 kindred with familial colorectal cancer and/or polyposis: review of reported cases and recommendations for genetic testing and surveillance. Genet Med. 2016. April;18(4):325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Esteban-Jurado C, Giménez-Zaragoza D, Muñoz J, Franch-Expósito S, Álvarez-Barona M, Ocaña T, et al. POLE and POLD1 screening in 155 patients with multiple polyps and early-onset colorectal cancer. Oncotarget. 2017. March 1;8(16):26732–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lorca V, Rueda D, Martín-Morales L, Fernández-Aceñero MJ, Grolleman J, Poves C, et al. Contribution of New Adenomatous Polyposis Predisposition Genes in an Unexplained Attenuated Spanish Cohort by Multigene Panel Testing. Sci Rep. 2019. July 8;9:9814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spier I, Holzapfel S, Altmüller J, Zhao B, Horpaopan S, Vogt S, et al. Frequency and phenotypic spectrum of germline mutations in POLE and seven other polymerase genes in 266 patients with colorectal adenomas and carcinomas. Int J Cancer. 2015;137(2):320–31. [DOI] [PubMed] [Google Scholar]
- 57.Elsayed FA, Tops CMJ, Nielsen M, Ruano D, Vasen HFA, Morreau H, et al. Low frequency of POLD1 and POLE exonuclease domain variants in patients with multiple colorectal polyps. Mol Genet Genomic Med. 2019. March 2;7(4):e00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mur P, García-Mulero S, del Valle J, Magraner-Pardo L, Vidal A, Pineda M, et al. Role of POLE and POLD1 in familial cancer. Genet Med. 2020;22(12):2089–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamaguchi K, Shimizu E, Yamaguchi R, Imoto S, Komura M, Hatakeyama S, et al. Development of an MSI-positive colon tumor with aberrant DNA methylation in a PPAP patient. J Hum Genet. 2019. August;64(8):729–40. [DOI] [PubMed] [Google Scholar]
- 60.Wimmer K, Beilken A, Nustede R, Ripperger T, Lamottke B, Ure B, et al. A novel germline POLE mutation causes an early onset cancer prone syndrome mimicking constitutional mismatch repair deficiency. Fam Cancer. 2017;16(1):67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buchanan DD, Stewart JR, Clendenning M, Rosty C, Mahmood K, Pope BJ, et al. Risk of colorectal cancer for carriers of a germline mutation in POLE or POLD1. Genet Med Off J Am Coll Med Genet. 2018. August;20(8):890–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Terradas M, Munoz-Torres PM, Belhadj S, Aiza G, Navarro M, Brunet J, et al. Contribution to colonic polyposis of recently proposed predisposing genes and assessment of the prevalence of NTHL1- and MSH3-associated polyposes. Hum Mutat. 2019. November;40(11):1910–23. * Terradas et al examines the prevalence of rare polyposis genes, and determined that biallelic MSH3 mutations are rare in European populations, and that biallelic pathogenic/likely pathogenic variants in NTHL1 accounts for an estimated 2% of unexplained polyposis.
- 63.Kuiper RP, Hoogerbrugge N. NTHL1 defines novel cancer syndrome. Oncotarget. 2015. October 27;6(33):34069–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weren RDA, Ligtenberg MJL, Kets CM, de Voer RM, Verwiel ETP, Spruijt L, et al. A germline homozygous mutation in the base-excision repair gene NTHL1 causes adenomatous polyposis and colorectal cancer. Nat Genet. 2015. June;47(6):668–71. [DOI] [PubMed] [Google Scholar]
- 65.Belhadj S, Mur P, Navarro M, González S, Moreno V, Capellá G, et al. Delineating the Phenotypic Spectrum of the NTHL1-Associated Polyposis. Clin Gastroenterol Hepatol. 2017. March;15(3):461–2. [DOI] [PubMed] [Google Scholar]
- 66.Fostira F, Kontopodis E, Apostolou P, Fragkaki M, Androulakis N, Yannoukakos D, et al. Extending the clinical phenotype associated with biallelic NTHL1 germline mutations. Clin Genet. 2018. December;94(6):588–9. [DOI] [PubMed] [Google Scholar]
- 67.Rivera B, Castellsagué E, Bah I, van Kempen LC, Foulkes WD. Biallelic NTHL1 Mutations in a Woman with Multiple Primary Tumors. N Engl J Med. 2015. November 12;373(20):1985–6. [DOI] [PubMed] [Google Scholar]
- 68.Belhadj S, Quintana I, Mur P, Munoz-Torres PM, Alonso MH, Navarro M, et al. NTHL1 biallelic mutations seldom cause colorectal cancer, serrated polyposis or a multi-tumor phenotype, in absence of colorectal adenomas. Sci Rep. 2019. June 21;9(1):9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weren RD, Ligtenberg MJ, Geurts van Kessel A, De Voer RM, Hoogerbrugge N, Kuiper RP. NTHL1 and MUTYH polyposis syndromes: two sides of the same coin?: Base excision repair polyposis syndromes. J Pathol. 2018. February;244(2):135–42. [DOI] [PubMed] [Google Scholar]
- 70.Khan N, Lipsa A, Arunachal G, Ramadwar M, Sarin R. Novel mutations and phenotypic associations identified through APC, MUTYH, NTHL1, POLD1, POLE gene analysis in Indian Familial Adenomatous Polyposis cohort. Sci Rep. 2017. May 22;7(1):2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Salo-Mullen EE, Maio A, Mukherjee S, Bandlamudi C, Shia J, Kemel Y, et al. Prevalence and Characterization of Biallelic and Monoallelic NTHL1 and MSH3 Variant Carriers From a Pan-Cancer Patient Population. JCO Precis Oncol. 2021;5:PO.20.00443. * Salo-Mullen et al describe prevalence of monoallelic NTHL1 and MSH3 pathogenic/likely pathogenic variants in a cancer cohort to determine cancer risks associated with a heterozygous variant. Pathogenic/likely pathogenic variants were not enriched in these genes compared to the general population, indicating that carriers of these variants likely do not have an increased risk of cancer, but there is some evidence to suggest that heterozygous variants may contribute to tumorigenesis.
- 72.Grolleman JE, de Voer RM, Elsayed FA, Nielsen M, Weren RDA, Palles C, et al. Mutational Signature Analysis Reveals NTHL1 Deficiency to Cause a Multi-tumor Phenotype. Cancer Cell. 2019. February;35(2):256–266.e5. [DOI] [PubMed] [Google Scholar]
- 73.Altaraihi M, Gerdes A-M, Wadt K. A new family with a homozygous nonsense variant in NTHL1 further delineated the clinical phenotype of NTHL1-associated polyposis. Hum Genome Var. 2019;6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li N, Zethoven M, McInerny S, Devereux L, Huang Y-K, Thio N, et al. Evaluation of the association of heterozygous germline variants in NTHL1 with breast cancer predisposition: an international multi-center study of 47,180 subjects. NPJ Breast Cancer. 2021. May 12;7(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kumpula T, Tervasmäki A, Mantere T, Koivuluoma S, Huilaja L, Tasanen K, et al. Evaluating the role of NTHL1 p.Q90* allele in inherited breast cancer predisposition. Mol Genet Genomic Med. 2020. November;8(11):e1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Groves A, Gleeson M, Spigelman AD. NTHL1-associate polyposis: first Australian case report. Fam Cancer. 2019. April;18(2):179–82. [DOI] [PubMed] [Google Scholar]
- 77.Adam R, Spier I, Zhao B, Kloth M, Marquez J, Hinrichsen I, et al. Exome Sequencing Identifies Biallelic MSH3 Germline Mutations as a Recessive Subtype of Colorectal Adenomatous Polyposis. Am J Hum Genet. 2016. August 4;99(2):337–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rohlin A, Rambech E, Kvist A, Törngren T, Eiengård F, Lundstam U, et al. Expanding the genotype–phenotype spectrum in hereditary colorectal cancer by gene panel testing. Fam Cancer. 2017. April;16(2):195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu H-X, Zhou X-L, Liu T, Werelius B, Lindmark G, Dahl N, et al. The Role of hMLH3 in Familial Colorectal Cancer. Cancer Res. 2003. April 15;63(8):1894–9. [PubMed] [Google Scholar]
- 80.Olkinuora A, Nieminen TT, Mårtensson E, Rohlin A, Ristimäki A, Koskenvuo L, et al. Biallelic germline nonsense variant of MLH3 underlies polyposis predisposition. Genet Med. 2019. August;21(8):1868–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lejeune S, Guillemot F, Triboulet J-P, Cattan S, Mouton C, PAFNORD Group, et al. Low frequency of AXIN2 mutations and high frequency of MUTYH mutations in patients with multiple polyposis. Hum Mutat. 2006. October;27(10):1064. [DOI] [PubMed] [Google Scholar]
- 82.Peterlongo P, Howe LR, Radice P, Sala P, Hong Y-J, Hong S-I, et al. Germline mutations of AXIN2 are not associated with nonsyndromic colorectal cancer. Hum Mutat. 2005. May;25(5):498–500. [DOI] [PubMed] [Google Scholar]
- 83.Lammi L, Arte S, Somer M, Järvinen H, Lahermo P, Thesleff I, et al. Mutations in AXIN2 Cause Familial Tooth Agenesis and Predispose to Colorectal Cancer. Am J Hum Genet. 2004. May;74(5):1043–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Renkonen ET, Nieminen P, Abdel-Rahman WM, Moisio A-L, Järvelä I, Arte S, et al. Adenomatous Polyposis Families That Screen APC Mutation–Negative by Conventional Methods Are Genetically Heterogeneous. J Clin Oncol. 2005. August 20;23(24):5651–9. [DOI] [PubMed] [Google Scholar]
- 85.Marvin ML, Mazzoni S, Herron CM, Edwards S, Gruber SB, Petty EM. AXIN2-Associated Autosomal Dominant Ectodermal Dysplasia and Neoplastic Syndrome. Am J Med Genet A. 2011. April;155(4):898–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rivera B, Perea J, Sánchez E, Villapún M, Sánchez-Tomé E, Mercadillo F, et al. A novel AXIN2 germline variant associated with attenuated FAP without signs of oligondontia or ectodermal dysplasia. Eur J Hum Genet. 2014. March;22(3):423–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Macklin- Mantia SK, Hines SL, Chaichana KL, Donaldson AM, Ko SL, Zhai Q, et al. Case report expanding the germline AXIN2- related phenotype to include olfactory neuroblastoma and gastric adenoma. BMC Med Genet. 2020. August 17;21:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Macklin-Mantia SK, Riegert-Johnson DL. An American patient with polyposis carrying a Scandinavian AXIN2 pathogenic variant. Hered Cancer Clin Pract. 2020. July 30;18:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Beard C, Purvis R, Winship IM, Macrae FA, Buchanan DD. Phenotypic confirmation of oligodontia, colorectal polyposis and cancer in a family carrying an exon 7 nonsense variant in the AXIN2 gene. Fam Cancer. 2019. July 1;18(3):311–5. [DOI] [PubMed] [Google Scholar]
- 90.Jaeger E, Leedham S, Lewis A, Segditsas S, Becker M, Cuadrado PR, et al. Hereditary mixed polyposis syndrome is caused by a 40kb upstream duplication that leads to increased and ectopic expression of the BMP antagonist GREM1. Nat Genet. 2012. May 6;44(6):699–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.ZIAI J, MATLOFF E, CHOI J, KOMBO N, MATERIN M, BALE AE. Defining the polyposis/colorectal cancer phenotype associated with the Ashkenazi GREM1 duplication: counselling and management recommendations. Genet Res. 2016. March 7;98:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Plesec T, Brown K, Allen C,A, Burke C, Church J, Kalady M, et al. Clinicopathological features of a kindred with SCG5-GREM1–associated hereditary mixed polyposis syndrome. Hum Pathol. 2017. February 1;60:75–81. [DOI] [PubMed] [Google Scholar]
- 93.Rohlin A, Eiengård F, Lundstam U, Zagoras T, Nilsson S, Edsjö A, et al. GREM 1 and POLE variants in hereditary colorectal cancer syndromes. Genes Chromosomes Cancer. 2016. January;55(1):95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McKenna DB, Van Den Akker J, Zhou AY, Ryan L, Leon A, O’Connor R, et al. Identification of a novel GREM1 duplication in a patient with multiple colon polyps. Fam Cancer. 2019. January;18(1):63–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.LAITMAN Y, JAEGER E, KATZ L, TOMLINSON I, FRIEDMAN E. GREM1 germline mutation screening in Ashkenazi Jewish patients with familial colorectal cancer. Genet Res. 2015. May 20;97:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lieberman S, Walsh T, Schechter M, Adar T, Goldin E, Beeri R, et al. Features of Patients With Hereditary Mixed Polyposis Syndrome Caused by Duplication of GREM1 and Implications for Screening and Surveillance. Gastroenterology. 2017. June 1;152(8):1876–1880.e1. [DOI] [PubMed] [Google Scholar]