Abstract
Mature CD4+ and CD8+ T cells constitutively experience weak T cell receptor (TCR) stimulation in response to self-antigens, termed tonic (or basal) signaling. How tonic TCR signal strength impacts T cell responses to foreign antigens is an active area of investigation. Such studies rely on surrogate markers of tonic signal strength, including CD5, Ly6C, and transgenic reporters of Nr4a genes. Recent research indicates that strong tonic TCR signal strength influences basal T cell metabolism, effector differentiation, and TCR signal transduction. T cells that experience the strongest tonic TCR signaling exhibit features of T cell activation and negative regulation. These data suggest a model whereby adaptation to tonic signaling has lasting effects that alter T cell activation and differentiation.
Introduction
T cells are positively selected in the thymus for weak reactivity to self-peptide antigens presented by Major Histocompatibility Complex (self-pMHC). Naive CD4+ and CD8+ T cells continue to experience low-level T cell receptor (TCR) signaling in response to self-pMHC in the periphery, termed basal or tonic signaling [1,2]. Tonic TCR signaling is sufficient to induce constitutive tyrosine phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) in the TCR complex and ZAP-70 recruitment to phosphorylated ζ chains, but not the production of IL-2 or clonal expansion [3–5]. A growing body of evidence suggests that tonic TCR signals experienced prior to cognate antigen exposure influence primary and secondary responses of CD4+ T cells. Here, we discuss recent advances in our understanding of how tonic TCR signaling is detected, how naive T cells adapt to varying tonic TCR signal strengths, and the impact on effector responses.
Markers of tonic signaling
CD5
The surface glycoprotein CD5 is a well-established surrogate marker of tonic signaling [6]. Naive CD4+ and CD8+ T cells expressing the highest levels of CD5 exhibit increased ζ-chain phosphorylation compared to CD5LO cells [7]. The magnitude of CD5 expression can vary between T cells with different TCR specificities, as demonstrated by comparing TCR transgenic populations [7]. However, CD5 expression and TCR specificity are not strictly linked, as two TCR transgenic strains can recognize the same Listeria monocytogenes epitope with similar affinity but exhibit different surface levels of CD5 [8,9]. In sum, CD5 has been a useful marker to identify T cells that experience relatively weak or strong tonic signaling.
Reporters of Nr4a
The immediate early gene Nr4a1 (encoding Nur77) is an orphan nuclear receptor in the same family as Nr4a2 and Nr4a3 [10]. Antigen-receptor stimulation, but not cytokine stimulation, induces expression of Nr4a1 in T and B cells [11,12]. Two Nur77-GFP reporter transgenes have been independently generated [11,12]. Like CD5, Nur77-GFP expression is initiated during thymic development and maintained in mature peripheral T cells [11,13]. The level of basal Nur77-GFP in naive T cells is relatively stable, as the majority of sorted cells retain similar Nur77-GFP intensity after adoptive transfer into WT recipients, but not in MHC II-deficient hosts [11,13]. Stimulation with cognate pMHC or TCR crosslinking antibodies leads to rapid upregulation of Nur77-GFP expression [11,14]. While Nur77-GFP expression is sensitive to TCR stimulation induced by self-pMHC interactions, Bending and colleagues recently showed that Nr4a3 reporter expression is two- to threefold less sensitive to TCR stimulation and is selectively activated by cognate pMHC stimulation [15].
Ly6C
Ly6C is a GPI-linked receptor with unknown function [16] that is upregulated on a subpopulation of naive CD4+ T cells shortly after thymic egress [17]. In contrast to CD5 and Nur77-GFP, Ly6C expression inversely correlates with reactivity to self-pMHC, as demonstrated by decreased ζ-chain phosphorylation in Ly6C+ naive CD4+ T cells (Figure 1) [17]. Within the regulatory T cell (Treg) population, Ly6C expression also marks a subset of Foxp3+ cells that experience weaker tonic signaling and exhibit decreased suppressive activity [18,19]. Mechanistically, the downregulation of Ly6C expression is dependent on TCR-induced Ca2+ signaling [20].
Figure 1.
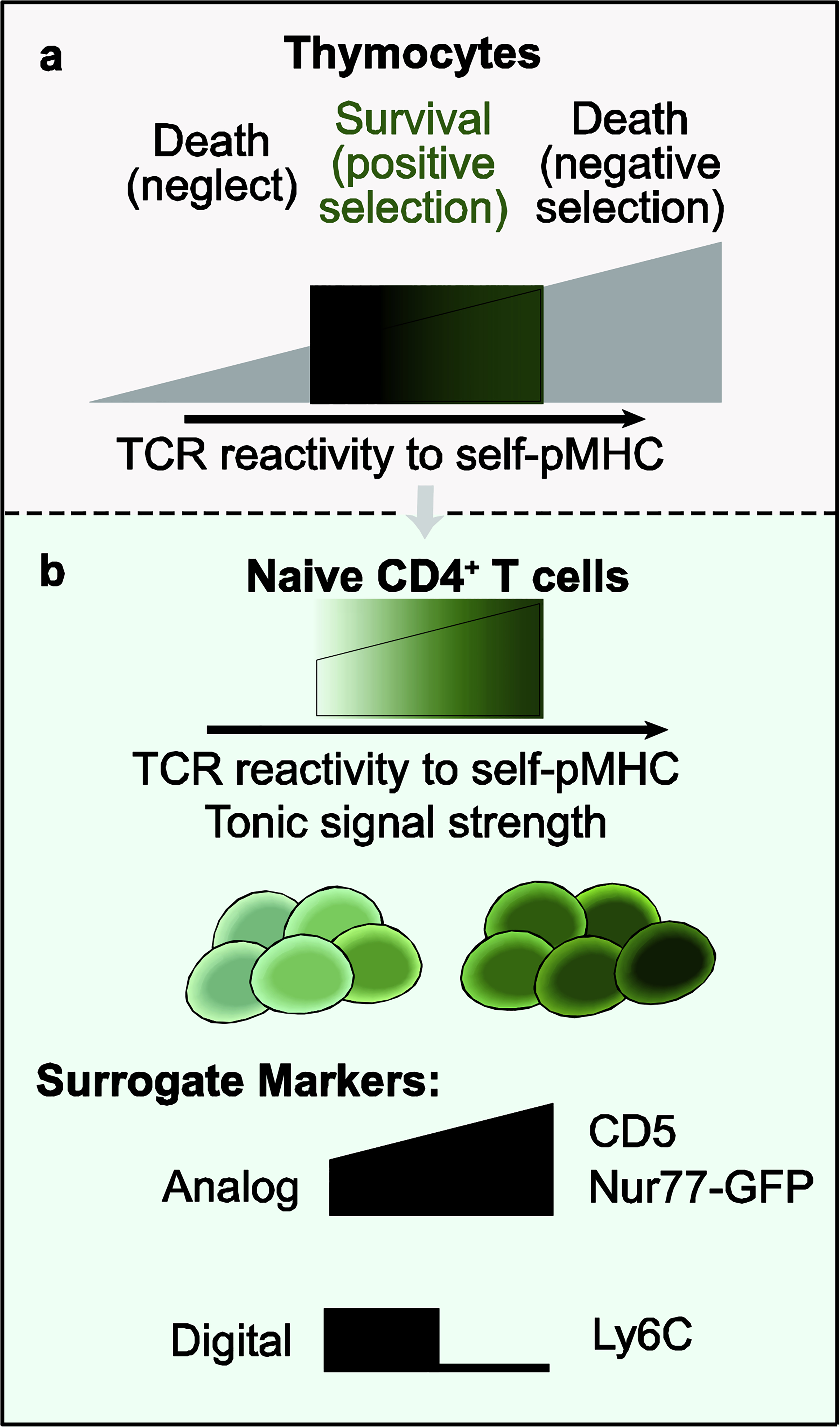
TCR reactivity to self-pMHC during development and in the periphery. a) There is a broad range of self-reactivity in the immature CD4+ CD8+ double-positive population. Positively selected thymocytes (in green) exhibit self-reactivity that is neither too weak nor too strong. b) Self-reactivity persists in the periphery, and naive CD4+ T cells experience varying strengths of tonic TCR signaling. Surrogate markers of tonic signal strength in mice include CD5, Ly6C, and the Nur77-GFP transgene.
Combination of markers
Our laboratory investigated whether a combination of markers could improve the dynamic range of tonic signaling that can be detected [13]. The combination of Nur77-GFP plus Ly6C exhibited a broader dynamic range compared to GFP plus CD5 or Ly6C plus CD5. In this scheme, Nur77-GFPLO Ly6C+ cells experience the weakest tonic signaling, and Nur77-GFPHI Ly6C− cells experience the strongest tonic signals, as shown by ζ-chain phosphorylation [13]. While Nur77-GFPHI Ly6C− cells express high levels of CD5, high CD5 expression alone does not solely mark the Nur77-GFPHI Ly6C− subset. These data raise the possibility that the range of tonic signal strength extends further than previously thought. Future studies with new markers or combinations of markers may improve the “resolution” to detect tonic signal strength.
Role of tonic signaling in CD4+ T cells
Our recent studies revealed that weak tonic signal strength, experienced by naive CD4+ T cells with a Nur77-GFPLO Ly6C+ phenotype, consistently correlated with the most robust activation, as reflected by IL-2 secretion, cell division, and ERK phosphorylation [13]. Nur77-GFPMED Ly6C+ and Nur77-GFPMED Ly6C− cells, which experience moderate tonic signal strength, mounted IL-2 responses comparable to Nur77-GFPLO Ly6C+ cells early (4hr post-stimulation), but the IL-2 responses of GFPLO Ly6C+ cells were consistently higher at later time points. These findings are compatible with the concept that strong tonic signaling correlates with short-lived acute responses. However, Nur77-GFPHI Ly6C− cells, which experience extensive tonic signaling, consistently exhibited decreased responsiveness to stimulation. This result is congruent with a “tunable” model where lymphocytes adapt to the amount of tonic signaling they experience [21]. Consequently, cells that experience strong tonic TCR signaling shift their activation threshold and effectively become de-sensitized to subsequent TCR stimulation (Figure 2).
Figure 2.
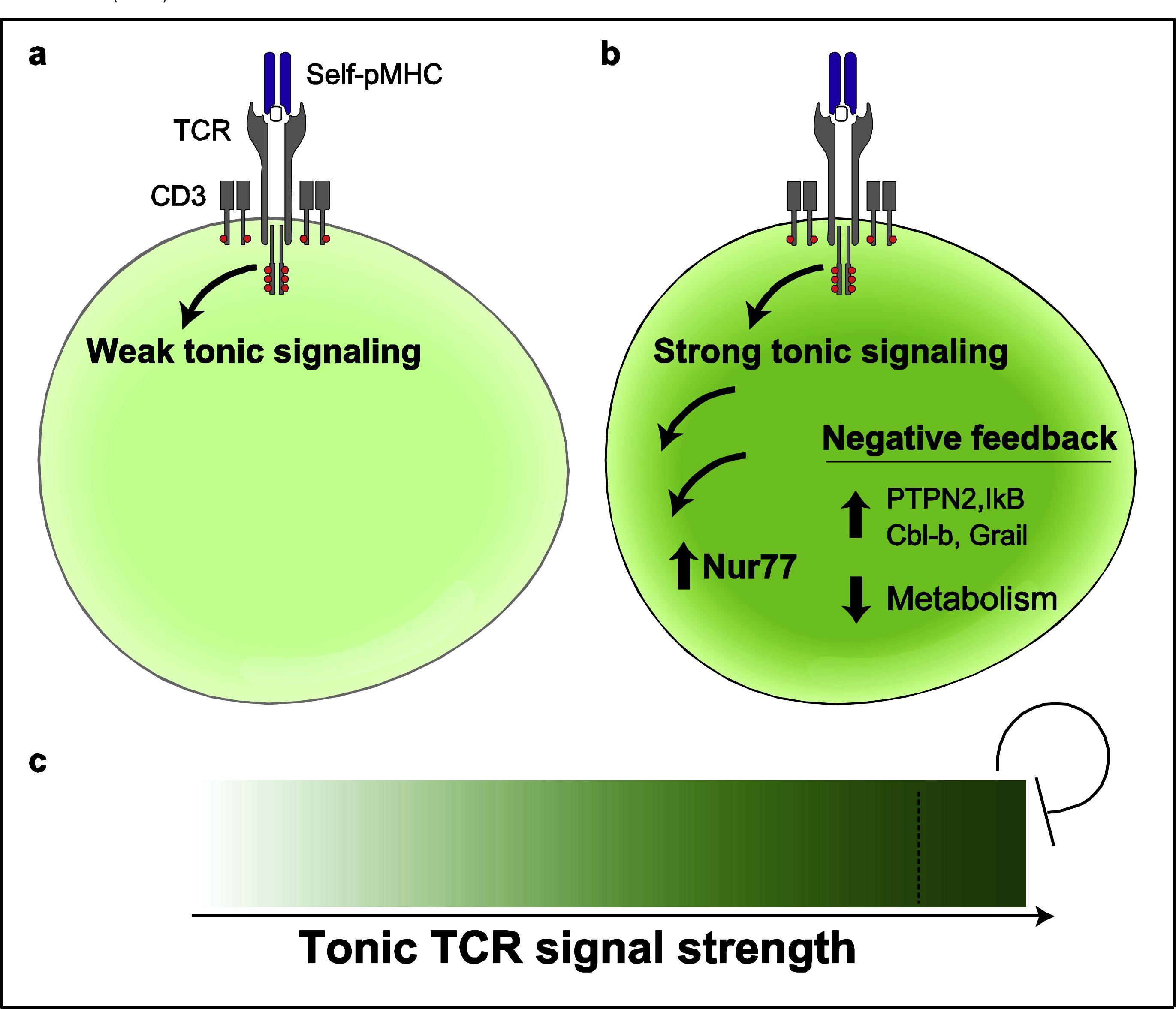
Adaptation to tonic signals through negative feedback. a) Individual naive T cells that exhibit relatively weak reactivity to self-pMHC induce weak tonic TCR signals. b) CD4+ T cells that exhibit strong reactivity to self-pMHC induce more extensive tonic TCR signaling, which results in higher expression of Nur77, and correlates with higher expression of negative regulators of TCR signaling and decreased basal metabolism. c) Naive CD4+ T cells that experience the most extensive tonic signal strength have attenuated responsiveness.
In experimental systems where CD5 was used to mark basal TCR signaling strength, CD5HI cells exhibit greater ERK phosphorylation and IL-2 production in response to acute stimulation [9]. However, at the late stages of the primary response, higher percentages of CD5HI TCR transgenic cells undergo apoptosis than CD5LO cells [8]. A model based on these studies suggests that strong tonic signaling correlates with a robust acute response that is not sustained due to increased cell death [22]. Hence, one potential consequence of naive CD4+ T cell heterogeneity is that different clones may engage in primary responses to foreign antigens with different kinetics. Finally, while some of the correlations identified using CD5 and Nur77-GFP as correlate markers of tonic TCR signaling strength overlap, differences remain. Further research is needed to clarify how tonic TCR signaling impacts CD4+ T cells at various stages of primary responses.
The molecular pathways activated by tonic TCR signaling remain incompletely understood [1]. A major challenge in studying the basal TCR signaling machinery has been the lack of an in vitro model. However, studies have highlighted how tonic signal strength can impact CD4+ T cells. For instance, in a mouse model with impaired NF-κβ signaling, naive T cells express lower levels of the IL-7 receptor α-subunit and exhibit reduced cell survival compared to WT cells [23], suggesting that the downstream effects of basal TCR signaling may affect cell survival. More recent studies add to the complexity and suggest that tonic TCR signaling could have both positive and negative effects on T cell effector function and influence T cell differentiation.
Tonic signal strength influences effector functions and cell fate decisions
Th1-polarized CD5HI cells express lower levels of Tbet and produce less IFNγ relative to CD5LO cells upon stimulation [24]. Similarly, strong tonic signaling correlates with impaired T follicular helper cell differentiation [25]. In contrast, naive CD4+ T cells that experience increased tonic signaling, such as CD5HI, Ly6C–, or Nur77-GFPHI Ly6C– populations have a higher propensity for Foxp3 expression under induced Treg differentiation conditions [13,17,26]. This functional heterogeneity may reflect mechanisms to attenuate highly self-reactive cells or divert them from an inflammatory effector state. However, in a lymphopenic environment, strong tonic signal strength correlates with increased autoreactive potential, as Ly6C– naive CD4+ cells induce more severe disease in an adoptive transfer model of colitis compared to Ly6C+ cells [17]. A correlation between basal TCR signaling and immunopathology can also be observed in mice that harbor mutations in the TCR signaling pathway [27–29]. The ZAP-70 mutation in SKG mice renders ZAP-70 hyporesponsive and thus allows positive thymic selection of T cell clones that otherwise would have undergone negative selection, resulting in an arthritis-like disease [30]. In the SKG mouse model of rheumatoid arthritis, Nur77-GFPHI naive CD4+ cells have increased arthritogenic potential compared to Nur77-GFPLO cells [27]. Likewise, T cells expressing a point mutation in LAT Tyrosine 136 experience weaker tonic signaling but paradoxically induce a Th2 lymphoproliferative disorder [28]. More specifically, weaker tonic signaling reduces the constitutive nuclear export of histone deacetylase 7, a transcriptional repressor of Nr4a1 and Irf4 [31]. Furthermore, a point-mutation in Rasgrp1 increases tonic mTORC1 signaling, which skews CD4+ T cells toward Th2 differentiation and instigates immunopathology in mice [29]. Together, these studies underscore that (i) TCR signaling can influence T helper effector function and cell fate decisions and (ii) strong tonic TCR signals correlate with increased autoimmune pathology if tolerance is compromised.
Potential mechanisms of negative regulation
An elegant study by Trefzer et al. investigated the effects of chronic antigen stimulation on CD4+ T cells in the absence of infection by utilizing a TCR transgenic mouse model in which cognate antigen expression is inducible [32]. In contrast to acute cognate antigen exposure, chronic exposure impaired cytokine production and induced gene expression signatures that bear similarities with gene expression patterns in anergic and exhausted T cells. Although constitutive cognate antigen stimulation differs from the constitutive low-level TCR stimulation T cells experience from self-pMHC interactions, T cells may similarly adapt to strong self-pMHC signals.
CD5 expression positively correlates with higher expression of IκB (a negative regulator of NFκB) [33], suggesting that self-reactive naive T cells potentially counterbalance an increased capacity of tonic signaling by expressing negative regulators of TCR signaling. A negative regulator of strong tonic signaling may also be CD5 itself. CD5 deficiency results in hyperresponsive TCR signaling in thymocytes, and mature CD5HI T cells exhibit a decreased TCR-induced calcium flux, consistent with CD5 as an inhibitor of TCR signaling [34–36]. Recent analyses of the CD5 interactome by mass spectrometry have highlighted several potential binding partners in mouse CD4+ T cells. One analysis identified negative regulators such as the E3 ubiquitin ligase Cbl-b and the phosphatase Ubash3a in the CD5 signalosome [37]. An independent analysis identified a required role for Tyrosine 429 of CD5 in the recruitment of c-Cbl, Cin85, and CrkL, which assemble molecular complexes that included both negative regulators (phosphatases SHIP-1 and Ubash3a) and positive regulators (PI3K) [38]. Moreover, CD5 also has a reported pro-survival role in mature T cells [39]. Hence, CD5 may have both positive and negative regulatory roles in TCR signaling, although further research is necessary to define the underlying mechanisms.
Tolerized and anergic T cells express high levels of Nur77 [40–42], and Nur77-deficiency impairs the induction of tolerance and exhaustion [40,41]. Furthermore, Nur77 deficient CD4+ T cells exhibit enhanced basal and maximal respiration and glycolytic capacity [43] in addition to enhanced IL-2 secretion upon stimulation [44], consistent with a role for Nur77 as a negative regulator of T cell activation. Moreover, extensive tonic signaling results in elevated levels of CBL-b and GRAIL, E3 ubiquitin ligases that negatively regulate TCR signal transduction and are associated with T cell anergy [13,45,46].
Increasing tonic signal strength attenuates metabolism
Ectopic expression of Scn5a, the pore-forming subunit of a voltage-gated sodium channel, enhances tonic TCR signal strength as reflected by elevated CD5 expression [47,48]. Increasing tonic signal strength by ectopic expression of Scn5a resulted in impaired cell expansion during a primary response to L. monocytogenes infection [48]. Furthermore, Scn5a-expressing cells have a decreased basal and maximal respiration rate and glycolytic rate [49]. These results suggest that strong tonic signaling limits the basal metabolism of naive T cells, perhaps to limit the autoimmune potential of self-reactive T cells.
Role of tonic signaling in CD8+ T cells
High CD5 expression positively correlates with increased persistence of antigen-specific CD8+ T cells during a primary response [50], suggesting a positive correlation between tonic signal strength and the magnitude of naive CD8+ T cell responses to foreign/agonist pMHC. Jameson’s group compared CD8+ cells specific to the self-antigen tyrosinase-related protein 2 (Trp2) harvested from WT and Trp2-deficient mice [51]. Although the Trp2-specific T cells were phenotypically and transcriptionally similar, Trp2-specific T cells from Trp2-deficient mice induced greater pathology in an adoptive transfer model of vitiligo. Moreover, a positive correlation between the expression of CD5 and the protein tyrosine phosphatase non-receptor type 2 (PTPN2), a negative regulator of TCR-proximal signal transduction, was detected in naive CD8+ T cells [52]. These findings are consistent with the concept that negative feedback from strong self-pMHC interactions reduces the pathogenic potential of the most self-reactive naive CD8+ T cells.
Strong tonic signaling is associated with virtual memory cells
Strong tonic signaling in CD8+ T cells positively correlates with the conversion of naive cells into antigen-inexperienced CD44HI memory phenotype cells [53], so-called virtual memory (VM) cells. Mouse models that enhance tonic signaling, such as Dock2 mutant mice and mice expressing a chimeric CD8 that couples with Lck at superphysiological stoichiometry, illustrate this correlation [54,55]. Furthermore, TCR sequencing of VM cells revealed enrichment of distinct clonotypes that, upon re-expression, possessed higher self-reactivity compared to TCRs isolated from the naive repertoire [56].
Tonic signaling in human T cells
Transcriptional analysis of human CD5LO vs. CD5HI naive CD4+ T cells revealed upregulation of genes associated with TCR signaling in CD5HI cells [57], consistent with a previous study that demonstrated increased CD5 staining intensity on antigen-experienced CD4+ T cells compared to naive cells [7]. The transcriptional profile of naive human CD8+ CXCR3+ cells was more similar to naive murine CD5HI than CD5LO CD8+ T cells [58]. Consistent with this finding, CXCR3 expression in the murine naive CD8+ population is limited to the CD5HI compartment [50]. Further studies are needed to build on our understanding of the functional implications of tonic signaling in naive human T cells. There appears to be some similarity in the functional capacities of human and mouse CD5LO and CD5HI CD4+ cells. Re-stimulation of activated human naive CD4+ T cells revealed differences in cytokine production; CD5LO cells produced higher levels of IFNγ under Th1 conditions [57], consistent with previous results in mice [24].
Tonic signaling strength and adoptive cell therapy (ACT)
Chimeric antigen receptor (CAR) T cell therapy is an individualized treatment strategy that relies on harvesting a patient’s T cells, expanding them in vitro, and transducing them with a synthetic T cell receptor that can recognize and eliminate tumor cells upon reinfusion into the patient [59]. Some degree of tonic signaling mediated by the endogenous TCR seems beneficial for CAR T cell therapy since deletion of the TCR negatively affected CAR T cell persistence in vivo [60]. However, too much basal signaling may be detrimental since tonic signals through the synthetic CAR T cell receptor are associated with T cell exhaustion [61,62]. Furthermore, TCRs that were engineered to have increased affinity for self-MHC resulted in diminished responsiveness upon stimulation [63]. Minguet and colleagues recently demonstrated that mutating a previously unknown Lck binding motif in CD3ε impaired the recruitment of Lck to the TCR complex and attenuated T cell activation [64]. CARs incorporating this mutated binding motif induced enhanced anti-tumor responses, possibly due to reduced CAR tonic signals [64]. Hence, determining the “optimum” amount of tonic signaling for T cells used in immunotherapy may further improve the therapeutic efficacy of ACT.
Concluding remarks
The view of naive T cells as a functionally homogenous group of cells is under revision as increasing evidence reveals further heterogeneity. How the effects of tonic TCR signal strength influence T cell responses in different contexts (i.e., autoimmunity, infection, cancer) remain incompletely understood. Further studies are also needed to identify the molecular mechanisms that regulate adaptations to varying strengths of tonic TCR signaling, including at the signaling, transcriptional, and epigenetic levels.
Highlights.
Surrogate markers of tonic TCR signaling include CD5, Ly6C, and Nur77-GFP
Tonic (or basal) TCR signaling in response to self-antigens can vary in intensity
Naive CD4+ T cells adapt to the strength of tonic TCR signals they experience
Extensive tonic TCR signals may induce negative feedback
Acknowledgments
This was supported in part by NIAMS K01 AR06548 (to B.A.) and Winship Cancer Institute #IRG-17-181-06 from the American Cancer Society (to B.A.). We thank Wendy Zinzow-Kramer and Wan-Lin Lo for critical reading of the manuscript.
Footnotes
Conflict of interest statement
Declarations of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- [1].Myers DR, Zikherman J, Roose JP: Tonic Signals: Why Do Lymphocytes Bother? Trends Immunol 2017, 38:844–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Paprckova D, Stepanek O: Narcissistic T cells: reactivity to self makes a difference. FEBS J 2020. [DOI] [PubMed] [Google Scholar]
- [3].van Oers NS, Tao W, Watts JD, Johnson P, Aebersold R, Teh HS: Constitutive tyrosine phosphorylation of the T-cell receptor (TCR) zeta subunit: regulation of TCR-associated protein tyrosine kinase activity by TCR zeta. Mol Cell Biol 1993, 13:5771–5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Stefanova I, Dorfman JR, Germain RN: Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature 2002, 420:429–434. [DOI] [PubMed] [Google Scholar]
- [5].Madrenas J, Wange RL, Wang JL, Isakov N, Samelson LE, Germain RN: Zeta phosphorylation without ZAP-70 activation induced by TCR antagonists or partial agonists. Science 1995, 267:515–518. [DOI] [PubMed] [Google Scholar]
- [6].Voisinne G, Gonzalez de Peredo A, Roncagalli R: CD5, an Undercover Regulator of TCR Signaling. Front Immunol 2018, 9:2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mandl JN, Monteiro JP, Vrisekoop N, Germain RN: T cell-positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity 2013, 38:263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Weber KS, Li QJ, Persaud SP, Campbell JD, Davis MM, Allen PM: Distinct CD4+ helper T cells involved in primary and secondary responses to infection. Proc Natl Acad Sci U S A 2012, 109:9511–9516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Persaud SP, Parker CR, Lo WL, Weber KS, Allen PM: Intrinsic CD4+ T cell sensitivity and response to a pathogen are set and sustained by avidity for thymic and peripheral complexes of self peptide and MHC. Nat Immunol 2014, 15:266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Odagiu L, May J, Boulet S, Baldwin TA, Labrecque N: Role of the Orphan Nuclear Receptor NR4A Family in T-Cell Biology. Front Endocrinol (Lausanne) 2020, 11:624122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA: T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med 2011, 208:1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zikherman J, Parameswaran R, Weiss A: Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature 2012, 489:160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Zinzow-Kramer WM, Weiss A, Au-Yeung BB: Adaptation by naive CD4(+) T cells to self-antigen-dependent TCR signaling induces functional heterogeneity and tolerance. Proc Natl Acad Sci U S A 2019, 116:15160–15169. • Our laboratory demonstrated that the combination of Nur77-GFP reporter expression and the surface marker Ly6C revealed a broader dynamic range of tonic signal strength in naive CD4+ T cells than previously shown. Furthermore, the cells that experienced the most extensive tonic signaling exhibited impaired responsiveness.
- [14].Au-Yeung BB, Zikherman J, Mueller JL, Ashouri JF, Matloubian M, Cheng DA, Chen Y, Shokat KM, Weiss A: A sharp T-cell antigen receptor signaling threshold for T-cell proliferation. Proc Natl Acad Sci U S A 2014, 111:E3679–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jennings E, Elliot TAE, Thawait N, Kanabar S, Yam-Puc JC, Ono M, Toellner KM, Wraith DC, Anderson G, Bending D: Nr4a1 and Nr4a3 Reporter Mice Are Differentially Sensitive to T Cell Receptor Signal Strength and Duration. Cell Rep 2020, 33:108328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lee PY, Wang JX, Parisini E, Dascher CC, Nigrovic PA: Ly6 family proteins in neutrophil biology. J Leukoc Biol 2013, 94:585–594. [DOI] [PubMed] [Google Scholar]
- [17].Martin B, Auffray C, Delpoux A, Pommier A, Durand A, Charvet C, Yakonowsky P, de Boysson H, Bonilla N, Audemard A, et al. : Highly self-reactive naive CD4 T cells are prone to differentiate into regulatory T cells. Nat Commun 2013, 4:2209. [DOI] [PubMed] [Google Scholar]
- [18].Delpoux A, Yakonowsky P, Durand A, Charvet C, Valente M, Pommier A, Bonilla N, Martin B, Auffray C, Lucas B: TCR signaling events are required for maintaining CD4 regulatory T cell numbers and suppressive capacities in the periphery. J Immunol 2014, 193:5914–5923. [DOI] [PubMed] [Google Scholar]
- [19].Lee JY, Kim J, Yi J, Kim D, Kim HO, Han D, Sprent J, Lee YJ, Surh CD, Cho JH: Phenotypic and Functional Changes of Peripheral Ly6C(+) T Regulatory Cells Driven by Conventional Effector T Cells. Front Immunol 2018, 9:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Guichard V, Bonilla N, Durand A, Audemard-Verger A, Guilbert T, Martin B, Lucas B, Auffray C: Calcium-mediated shaping of naive CD4 T-cell phenotype and function. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Grossman Z: Immunological Paradigms, Mechanisms, and Models: Conceptual Understanding Is a Prerequisite to Effective Modeling. Front Immunol 2019, 10:2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Milam AV, Allen PM: Functional Heterogeneity in CD4(+) T Cell Responses Against a Bacterial Pathogen. Front Immunol 2015, 6:621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Miller ML, Mashayekhi M, Chen L, Zhou P, Liu X, Michelotti M, Tramontini Gunn N, Powers S, Zhu X, Evaristo C, et al. : Basal NF-kappaB controls IL-7 responsiveness of quiescent naive T cells. Proc Natl Acad Sci U S A 2014, 111:7397–7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sood A, Lebel ME, Fournier M, Rogers D, Mandl JN, Melichar HJ: Differential interferon-gamma production potential among naive CD4(+) T cells exists prior to antigen encounter. Immunol Cell Biol 2019, 97:931–940. [DOI] [PubMed] [Google Scholar]
- [25]. Bartleson JM, Viehmann Milam AA, Donermeyer DL, Horvath S, Xia Y, Egawa T, Allen PM: Strength of tonic T cell receptor signaling instructs T follicular helper cell-fate decisions. Nat Immunol 2020. • By utilizing two different CD4+ TCR transgenic mice that recognize the same epitope with comparable affinity but display different levels of CD5 surface levels, this paper demonstrated that CD5HI cells are less prone to differentiate into TFH cells compared to CD5LO cells.
- [26].Henderson JG, Opejin A, Jones A, Gross C, Hawiger D: CD5 instructs extrathymic regulatory T cell development in response to self and tolerizing antigens. Immunity 2015, 42:471–483. [DOI] [PubMed] [Google Scholar]
- [27].Ashouri JF, Hsu LY, Yu S, Rychkov D, Chen Y, Cheng DA, Sirota M, Hansen E, Lattanza L, Zikherman J, et al. : Reporters of TCR signaling identify arthritogenic T cells in murine and human autoimmune arthritis. Proc Natl Acad Sci U S A 2019, 116:18517–18527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Roncagalli R, Mingueneau M, Gregoire C, Malissen M, Malissen B: LAT signaling pathology: an “autoimmune” condition without T cell self-reactivity. Trends Immunol 2010, 31:253–259. [DOI] [PubMed] [Google Scholar]
- [29].Myers DR, Norlin E, Vercoulen Y, Roose JP: Active Tonic mTORC1 Signals Shape Baseline Translation in Naive T Cells. Cell Rep 2019, 27:1858–1874 e1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sakaguchi N, Takahashi T, Hata H, Nomura T, Tagami T, Yamazaki S, Sakihama T, Matsutani T, Negishi I, Nakatsuru S, et al. : Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature 2003, 426:454–460. [DOI] [PubMed] [Google Scholar]
- [31].Myers DR, Lau T, Markegard E, Lim HW, Kasler H, Zhu M, Barczak A, Huizar JP, Zikherman J, Erle DJ, et al. : Tonic LAT-HDAC7 Signals Sustain Nur77 and Irf4 Expression to Tune Naive CD4 T Cells. Cell Rep 2017, 19:1558–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Trefzer A, Kadam P, Wang SH, Pennavaria S, Lober B, Akcabozan B, Kranich J, Brocker T, Nakano N, Irmler M, et al. : Dynamic adoption of anergy by antigen-exhausted CD4(+) T cells. Cell Rep 2021, 34:108748. •• Constitutive cognate antigen stimulation renders CD4+ TCR transgenic T cells functionally impaired, even in the absence of an infection. Furthermore, gene expression signatures of these T cells resemble anergic and exhausted T cells.
- [33].Matson CA, Choi S, Livak F, Zhao B, Mitra A, Love PE, Singh NJ: CD5 dynamically calibrates basal NF-kappaB signaling in T cells during thymic development and peripheral activation. Proc Natl Acad Sci U S A 2020, 117:14342–14353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tarakhovsky A, Kanner SB, Hombach J, Ledbetter JA, Muller W, Killeen N, Rajewsky K: A role for CD5 in TCR-mediated signal transduction and thymocyte selection. Science 1995, 269:535–537. [DOI] [PubMed] [Google Scholar]
- [35].Pena-Rossi C, Zuckerman LA, Strong J, Kwan J, Ferris W, Chan S, Tarakhovsky A, Beyers AD, Killeen N: Negative regulation of CD4 lineage development and responses by CD5. J Immunol 1999, 163:6494–6501. [PubMed] [Google Scholar]
- [36].Smith K, Seddon B, Purbhoo MA, Zamoyska R, Fisher AG, Merkenschlager M: Sensory adaptation in naive peripheral CD4 T cells. J Exp Med 2001, 194:1253–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mori D, Gregoire C, Voisinne G, Celis-Gutierrez J, Aussel R, Girard L, Camus M, Marcellin M, Argenty J, Burlet-Schiltz O, et al. : The T cell CD6 receptor operates a multitask signalosome with opposite functions in T cell activation. J Exp Med 2021, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Blaize G, Daniels-Treffandier H, Aloulou M, Rouquie N, Yang C, Marcellin M, Gador M, Benamar M, Ducatez M, Song KD, et al. : CD5 signalosome coordinates antagonist TCR signals to control the generation of Treg cells induced by foreign antigens. Proc Natl Acad Sci U S A 2020, 117:12969–12979. • Mass spectrometry analysis of CD5 binding partners in murine naive CD4+ T cells revealed that CD5 recruits both negative and positive regulators of TCR signaling. This study suggests that regarding CD5 solely as a negative regulator of T cell activation may be an oversimplification.
- [39].Burgueno-Bucio E, Mier-Aguilar CA, Soldevila G: The multiple faces of CD5. J Leukoc Biol 2019, 105:891–904. [DOI] [PubMed] [Google Scholar]
- [40].Liu X, Wang Y, Lu H, Li J, Yan X, Xiao M, Hao J, Alekseev A, Khong H, Chen T, et al. : Genome-wide analysis identifies NR4A1 as a key mediator of T cell dysfunction. Nature 2019, 567:525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chen J, Lopez-Moyado IF, Seo H, Lio CJ, Hempleman LJ, Sekiya T, Yoshimura A, Scott-Browne JP, Rao A: NR4A transcription factors limit CAR T cell function in solid tumours. Nature 2019, 567:530–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tuncel J, Benoist C, Mathis D: T cell anergy in perinatal mice is promoted by T reg cells and prevented by IL-33. J Exp Med 2019, 216:1328–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Liebmann M, Hucke S, Koch K, Eschborn M, Ghelman J, Chasan AI, Glander S, Schadlich M, Kuhlencord M, Daber NM, et al. : Nur77 serves as a molecular brake of the metabolic switch during T cell activation to restrict autoimmunity. Proc Natl Acad Sci U S A 2018, 115:E8017–E8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hiwa R, Nielsen HV, Mueller JL, Mandla R, Zikherman J: NR4A family members regulate T cell tolerance to preserve immune homeostasis and suppress autoimmunity. JCI Insight 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mueller DL: E3 ubiquitin ligases as T cell anergy factors. Nat Immunol 2004, 5:883–890. [DOI] [PubMed] [Google Scholar]
- [46].Nguyen TTT, Wang ZE, Shen L, Schroeder A, Eckalbar W, Weiss A: Cbl-b deficiency prevents functional but not phenotypic T cell anergy. J Exp Med 2021, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lo WL, Donermeyer DL, Allen PM: A voltage-gated sodium channel is essential for the positive selection of CD4(+) T cells. Nat Immunol 2012, 13:880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Milam AAV, Bartleson JM, Donermeyer DL, Horvath S, Durai V, Raju S, Yu H, Redmann V, Zinselmeyer B, White JM, et al. : Tuning T Cell Signaling Sensitivity Alters the Behavior of CD4(+) T Cells during an Immune Response. J Immunol 2018, 200:3429–3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Milam AAV, Bartleson JM, Buck MD, Chang CH, Sergushichev A, Donermeyer DL, Lam WY, Pearce EL, Artyomov MN, Allen PM: Tonic TCR Signaling Inversely Regulates the Basal Metabolism of CD4(+) T Cells. Immunohorizons 2020, 4:485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Fulton RB, Hamilton SE, Xing Y, Best JA, Goldrath AW, Hogquist KA, Jameson SC: The TCR’s sensitivity to self peptide-MHC dictates the ability of naive CD8(+) T cells to respond to foreign antigens. Nat Immunol 2015, 16:107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Truckenbrod EN, Burrack KS, Knutson TP, Borges da Silva H, Block KE, O’Flanagan SD, Stagliano KR, Hurwitz AA, Fulton RB, Renkema KR, et al. : CD8(+) T cell self-tolerance permits responsiveness but limits tissue damage. Elife 2021, 10. • CD8+ T cells specific for the self-antigen tyrosinase-related protein 2 (Trp2) were phenotypically and transcriptionally similar regardless if they were harvested from WT or Trp2-deficient mice. However, CD8+ T cells from WT mice had reduced autoreactive potential in a mouse model of vitiligo.
- [52].Wiede F, La Gruta NL, Tiganis T: PTPN2 attenuates T-cell lymphopenia-induced proliferation. Nat Commun 2014, 5:3073. [DOI] [PubMed] [Google Scholar]
- [53].White JT, Cross EW, Burchill MA, Danhorn T, McCarter MD, Rosen HR, O’Connor B, Kedl RM: Virtual memory T cells develop and mediate bystander protective immunity in an IL-15-dependent manner. Nat Commun 2016, 7:11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Mahajan VS, Demissie E, Alsufyani F, Kumari S, Yuen GJ, Viswanadham V, Huang A, Tran JQ, Moon JJ, Irvine DJ, et al. : DOCK2 Sets the Threshold for Entry into the Virtual Memory CD8(+) T Cell Compartment by Negatively Regulating Tonic TCR Triggering. J Immunol 2020, 204:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Drobek A, Moudra A, Mueller D, Huranova M, Horkova V, Pribikova M, Ivanek R, Oberle S, Zehn D, McCoy KD, et al. : Strong homeostatic TCR signals induce formation of self-tolerant virtual memory CD8 T cells. EMBO J 2018, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Miller CH, Klawon DEJ, Zeng S, Lee V, Socci ND, Savage PA: Eomes identifies thymic precursors of self-specific memory-phenotype CD8(+) T cells. Nat Immunol 2020, 21:567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57]. Sood A, Lebel ME, Dong M, Fournier M, Vobecky SJ, Haddad E, Delisle JS, Mandl JN, Vrisekoop N, Melichar HJ: CD5 levels define functionally heterogeneous populations of naive human CD4(+) T cells. Eur J Immunol 2021, 51:1365–1376. • This study demonstrated transcriptional differences between CD5LO and CD5HI naive human CD4+ T cells. Moreover, decreased percentages of naive CD5HI cells produced IFNγ under Th1 conditions, compared to CD5LO cells.
- [58].De Simone G, Mazza EMC, Cassotta A, Davydov AN, Kuka M, Zanon V, De Paoli F, Scamardella E, Metsger M, Roberto A, et al. : CXCR3 Identifies Human Naive CD8(+) T Cells with Enhanced Effector Differentiation Potential. J Immunol 2019, 203:3179–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Guedan S, Ruella M, June CH: Emerging Cellular Therapies for Cancer. Annu Rev Immunol 2019, 37:145–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Stenger D, Stief TA, Kauferle T, Willier S, Rataj F, Schober K, Vick B, Lotfi R, Wagner B, Grunewald TGP, et al. : Endogenous TCR promotes in vivo persistence of CD19-CAR-T cells compared to a CRISPR/Cas9-mediated TCR knockout CAR. Blood 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, Smith JP, Walker AJ, Kohler ME, Venkateshwara VR, et al. : 4–1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med 2015, 21:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lynn RC, Weber EW, Sotillo E, Gennert D, Xu P, Good Z, Anbunathan H, Lattin J, Jones R, Tieu V, et al. : c-Jun overexpression in CAR T cells induces exhaustion resistance. Nature 2019, 576:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Duong MN, Erdes E, Hebeisen M, Rufer N: Chronic TCR-MHC (self)-interactions limit the functional potential of TCR affinity-increased CD8 T lymphocytes. J Immunother Cancer 2019, 7:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64]. Hartl FA, Beck-Garcia E, Woessner NM, Flachsmann LJ, Cardenas RMV, Brandl SM, Taromi S, Fiala GJ, Morath A, Mishra P, et al. : Noncanonical binding of Lck to CD3epsilon promotes TCR signaling and CAR function. Nat Immunol 2020, 21:902–913. • Deleting a novel Lck-Sh3 domain binding site in CD3ε attenuates T cell activation and abrogates Lck recruitment to the TCR complex. Moreover, CAR T cells harboring the mutated CD3ε binding site experienced weaker tonic CAR signals and exhibited increased tumor control in a mouse model.


