Abstract
Lessons Learned
Long‐term safety of radium‐223 with enzalutamide was confirmed in this clinical trial.
PSA‐PFS2 was prolonged with the combination compared with enzalutamide alone.
Background
Previously, we showed the combination of radium‐223 and enzalutamide to be safe and associated with improved efficacy based on a concomitant decline in serum bone metabolism markers compared with enzalutamide alone in a phase II trial of men with metastatic castration‐resistant prostate cancer (mCRPC) [1].
Methods
Secondary endpoints were not included in our initial report, and we include them herein, after a median follow‐up of 22 months. These objectives included long‐term safety, prostate‐specific antigen (PSA)–progression‐free survival (PFS), and radiographic progression‐free survival; PSA‐PFS2 (time from start of protocol therapy to PSA progression on subsequent therapy); time to next therapy (TTNT); and overall survival (OS). Survival analysis and log‐rank tests were performed using the R statistical package v.4.0.2 (https://www.r-project.org). Statistical significance was defined as p < .05.
Results
Of 47 patients (median age, 68 years), 35 received the combination and 12 enzalutamide alone. After a median follow‐up of 22 months, final safety results did not show any increase in fractures or other adverse events in the combination arm. PSA‐PFS2 was significantly improved, and other efficacy parameters were numerically improved in the combination over the enzalutamide arm.
Conclusion
The combination of enzalutamide and radium‐223 was found to be safe and associated with promising efficacy in men with mCRPC. These hypothesis‐generating results portend well for the ongoing phase III PEACE III trial in this setting.
Keywords: Radium‐223, Enzalutamide, Metastatic castration‐refractory prostate cancer
Discussion
After an initial safety lead‐in cohort of 8 patients treated with the combination, 39 patients were randomized (2:1) to the combination of radium‐223 plus enzalutamide versus enzalutamide monotherapy [1] at approved doses (Fig. 1). Receipt of prior abiraterone and docetaxel was allowed and was balanced between the two groups [1]. Primary endpoints of safety and decline in bone metabolism markers were reported earlier and only included the randomized patients [1]. Herein, we report on the prespecified secondary endpoints of PSA‐PFS, radiographic PFS, and OS, which included all 47 eligible patients (35 received the combination and 12 received enzalutamide alone). In addition, we report a post hoc analysis on PSA‐PFS2 and TTNT, as well as long‐term safety of the combination (Fig. 2).
Figure 1.
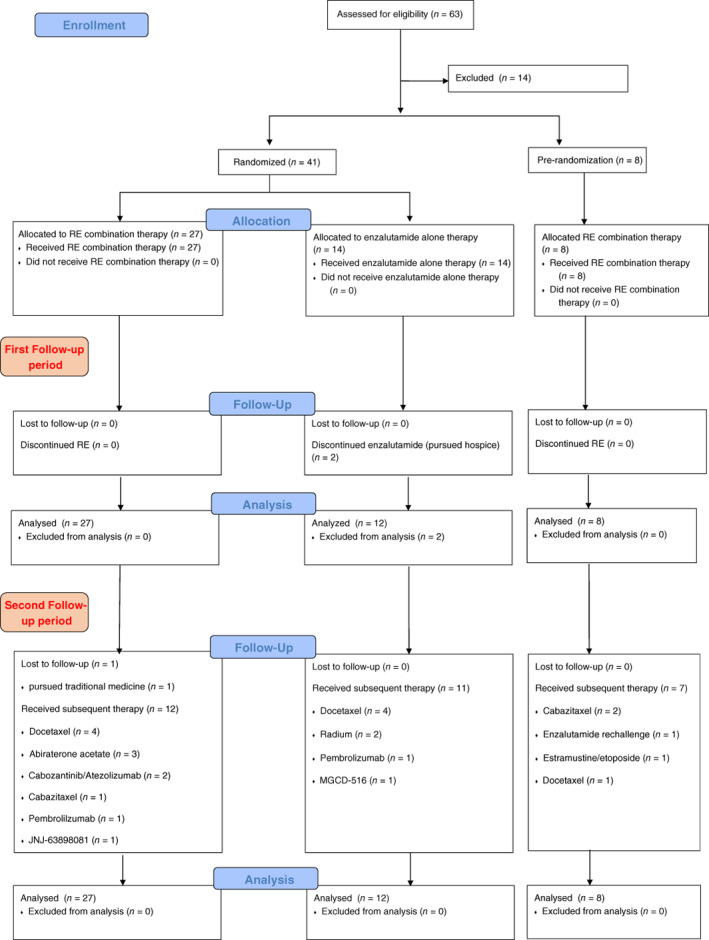
CONSORT flow diagram.Abbreviation: RE, radium‐223 plus enzalutamide.
Figure 2.
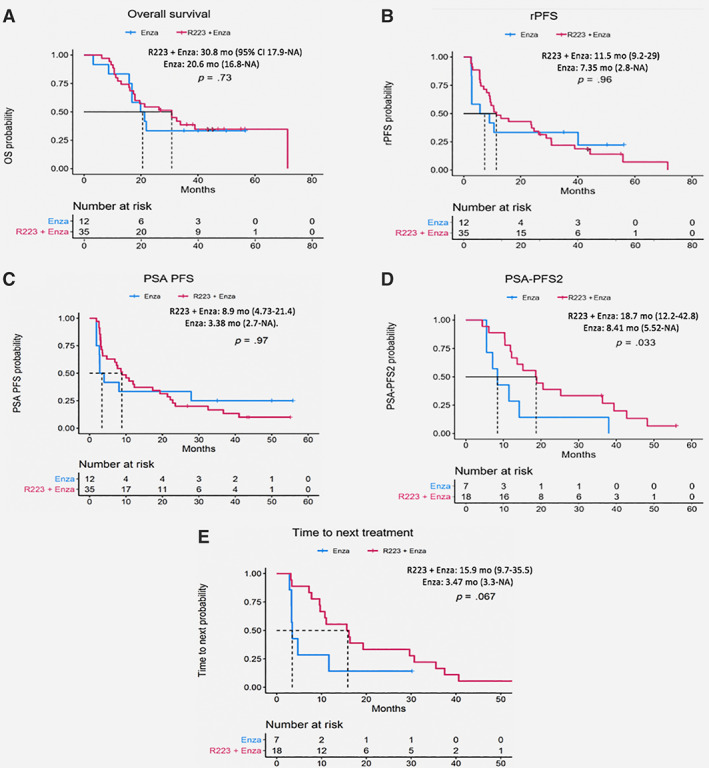
Kaplan‐Meier plots for trial secondary endpoints and post hoc clinical endpoints. Secondary endpoints: overall survival (A), radiographic progression‐free survival (B), PSA progression‐free survival (C). Post hoc clinical endpoints: time to PSA progression on subsequent therapy (D), time to next treatment (E).Abbreviations: CI, confidence interval; Enza, enzalutamide; PFS, progression‐free survival; PSA, prostate‐specific antigen; PSA‐PFS2, time from start of protocol therapy to PSA progression on subsequent therapy; NE, not evaluable; R223, radium‐223; rPFS, radiographic PFS.
Regarding safety, no patients on the enzalutamide arm and 2 of 35 patients on the combination arm developed fractures, both incidentally detected on imaging: one had rib fractures after a fall; the second had a vertebral fracture at the site of bone metastasis. There was no difference in any adverse events or any incidence of bone marrow disorders. These results are consistent with those seen with radium‐223 monotherapy [2, 3, 4, 5].
Secondary efficacy endpoints were numerically but not statistically improved in the combination arm: median OS (30.8 vs. 20.6 months; p = .73), PSA‐PFS (8.9 vs. 3.38 months; p = .97), and radiographic PFS (11.5 vs. 7.35 months; p = .96). The significant improvement in PSA‐PFS2 (18.7 vs. 8.41 months; p = .033) and near‐significant improvement in TTNT (15.9 vs. 3.47 months; p = .067) suggest delayed effect of radium‐223 on the disease trajectory, which was also evident in the pivotal ALSYMPCA trial, in which OS was significantly improved with radium‐223 without improvement in PFS [4]. The small sample size and the post hoc nature of some of the endpoints reported here are the limitations of this report.
Trial Information
| Disease | Advanced cancer, prostate cancer |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | No designated number of regimens |
| Type of Study | Phase II, randomized |
| Primary Endpoint | Safety, bone metabolism markers |
| Secondary Endpoint | Overall survival, PSA–progression‐free survival, radiographic progression‐free survival |
| Additional Details of Endpoints or Study Design | Post hoc analysis values of PSA‐PFS2 (time from start of protocol therapy to PSA progression on subsequent therapy) and TTNT |
| Investigator's Analysis | Active and should be pursued further |
Drug Information: Combination
| Generic Name | Radium‐223 |
| Drug Type | Bone targeting radiotherapeutic |
| Dose | 55 kBq per kg |
| Route | Intravenous (IV) |
| Schedule of Administration | 55 kBq/kg IV every 4 weeks for six doses |
| Generic Name | Enzalutamide |
| Drug Type | Nonsteroidal antiandrogen |
| Drug Class | Androgen receptor |
| Dose | 160 mg per flat dose |
| Route | p.o. |
| Schedule of Administration | 160 mg p.o. daily |
Drug Information: Control
| Generic Name | Enzalutamide |
| Drug Type | Nonsteroidal antiandrogen |
| Drug Class | Androgen receptor |
| Dose | 160 mg per flat dose |
| Route | p.o. |
| Schedule of Administration | 160 mg p.o. daily |
Patient Characteristics: Combination
| Number of Patients, Male | 35 |
| Number of Patients, Female | 0 |
| Age | Median: 71 years |
| Number of Prior Systemic Therapies | None |
| Performance Status: ECOG |
0 — 17 1 — 18 2 — 0 3 — 0 Unknown — 0 |
| Other | Prior therapies: nine of the patients in the enzalutamide‐only arm had prior progression on abiraterone; three of the patients in the enzalutamide‐only arm had prior progression on docetaxel. |
Patient Characteristics: Control
| Number of Patients, Male | 14 |
| Number of Patients, Female | 0 |
| Age | Median: 71 years |
| Number of Prior Systemic Therapies | None |
| Performance Status: ECOG |
0 — 7 1 — 7 2 — 0 3 — 0 Unknown — 0 |
| Other | Prior therapies: nine of the patients in the enzalutamide‐only arm had prior progression on abiraterone; three of the patients in the enzalutamide‐only arm had prior progression on docetaxel. |
Secondary Assessment Method: Combination
| Title | Overall survival |
| Number of Patients Screened | 35 |
| Number of Patients Enrolled | 35 |
| Number of Patients Evaluable for Toxicity | 35 |
| Number of Patients Evaluated for Efficacy | 35 |
| (Median) Duration Assessments OS | 30.8 months, CI: 17.9–not evaluable |
| Title | PSA‐PFS |
| Number of Patients Screened | 35 |
| Number of Patients Enrolled | 35 |
| Number of Patients Evaluable for Toxicity | 35 |
| Number of Patients Evaluated for Efficacy | 35 |
| Evaluation Method | Prostate Cancer Working Group 2 (PCWG2) |
| (Median) Duration Assessments PFS | 8.9 months, CI: 4.73–21.4 |
| Title | Radiographic PFS |
| Number of Patients Screened | 35 |
| Number of Patients Enrolled | 35 |
| Number of Patients Evaluable for Toxicity | 35 |
| Number of Patients Evaluated for Efficacy | 35 |
| Evaluation Method | Progressive disease on imaging |
| (Median) Duration Assessments PFS | 11.5 months, CI: 9.2–29 |
| Title | Radiographic objective response rate |
| Number of Patients Screened | 35 |
| Number of Patients Enrolled | 35 |
| Number of Patients Evaluable for Toxicity | 35 |
| Number of Patients Evaluated for Efficacy | 35 |
| Evaluation Method | Radiographic Response |
| Response Assessment CR | n = 0 (0%) |
| Response Assessment PR | n = 3 (9%) |
| Response Assessment SD | n = 28 (80%) |
| Response Assessment PD | n = 4 (11%) |
| Title | Post hoc: PSA‐PFS2 |
| Number of Patients Screened | 35 |
| Number of Patients Enrolled | 35 |
| Number of Patients Evaluable for Toxicity | 35 |
| Number of Patients Evaluated for Efficacy | 35 |
| Evaluation Method | PCWG2—time from start of protocol therapy to PSA progression on subsequent therapy |
| (Median) Duration Assessments PFS | 18.7 months, CI: 12.2–42.8 |
| Title | Post hoc: TTNT |
| Number of Patients Screened | 35 |
| Number of Patients Enrolled | 35 |
| Number of Patients Evaluable for Toxicity | 35 |
| Number of Patients Evaluated for Efficacy | 35 |
| Evaluation Method | time to subsequent therapy |
| (Median) Duration Assessments PFS | 15.9 months, CI: 9.7–35.5 |
Primary Assessment Method: Combination
| Title | Safety |
| Number of Patients Screened | 35 |
| Number of Patients Enrolled | 35 |
| Number of Patients Evaluable for Toxicity | 35 |
| Number of Patients Evaluated for Efficacy | 35 |
| Evaluation Method | Occurrences of adverse events |
| Outcome Notes | Fracture rate: 2 out of 35 participants (5.7%) in the combination and 0% for enzalutamide alone. |
Secondary Assessment Method: Control
| Title | Overall survival |
| Number of Patients Screened | 14 |
| Number of Patients Enrolled | 14 |
| Number of Patients Evaluable for Toxicity | 12 |
| Number of Patients Evaluated for Efficacy | 12 |
| Evaluation Method | Overall survival of patient |
| (Median) Duration Assessments OS | 20.6 months, CI: 16.8–NA |
| Title | PSA‐PFS |
| Number of Patients Screened | 14 |
| Number of Patients Enrolled | 14 |
| Number of Patients Evaluable for Toxicity | 12 |
| Number of Patients Evaluated for Efficacy | 12 |
| Evaluation Method | PCWG2 |
| (Median) Duration Assessments PFS | 3.38 months, CI: 2.7–NA |
| Title | Radiographic PFS |
| Number of Patients Screened | 14 |
| Number of Patients Enrolled | 14 |
| Number of Patients Evaluable for Toxicity | 12 |
| Number of Patients Evaluated for Efficacy | 12 |
| Evaluation Method | Progression on imaging |
| (Median) Duration Assessments PFS | 7.35 months, CI: 2.8–NA |
| Title | Radiographic objective response rate |
| Number of Patients Screened | 14 |
| Number of Patients Enrolled | 14 |
| Number of Patients Evaluable for Toxicity | 12 |
| Number of Patients Evaluated for Efficacy | 12 |
| Evaluation Method | Radiographic progression |
| Response Assessment CR | n = 0 (0%) |
| Response Assessment PR | n = 0 (0%) |
| Response Assessment SD | n = 8 (67%) |
| Response Assessment PD | n = 4 (33%) |
| Title | Post hoc: PSA‐PFS2 |
| Number of Patients Screened | 14 |
| Number of Patients Enrolled | 14 |
| Number of Patients Evaluable for Toxicity | 12 |
| Number of Patients Evaluated for Efficacy | 12 |
| Evaluation Method | PCWG2—time from start of protocol therapy to PSA progression on subsequent therapy |
| (Median) Duration Assessments PFS | 8.41 months, CI: 5.52–NA |
| Title | Post hoc: TTNT |
| Number of Patients Screened | 14 |
| Number of Patients Enrolled | 14 |
| Number of Patients Evaluable for Toxicity | 12 |
| Number of Patients Evaluated for Efficacy | 12 |
| Evaluation Method | Time to next subsequent therapy |
| (Median) Duration Assessments PFS | 3.47 months, CI: 3.3–NA |
Primary Assessment Method: Control
| Title | Safety |
| Number of Patients Screened | 14 |
| Number of Patients Enrolled | 14 |
| Number of Patients Evaluable for Toxicity | 12 |
| Number of Patients Evaluated for Efficacy | 12 |
| Evaluation Method | Occurrences of adverse events |
| Outcome Notes | Fractures rates were 0 out of 12 participants |
Adverse Events: Combination, All Cycles (percentages)
| Name | NC/NA | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | All Grades |
|---|---|---|---|---|---|---|---|
| Fracture | 94 | 6 | 0 | 0 | 0 | 0 | 6 |
| Bone marrow hypocellular | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Abdominal pain | 91 | 9 | 0 | 0 | 0 | 0 | 9 |
| Alkaline phosphatase increased | 97 | 0 | 3 | 0 | 0 | 0 | 3 |
| Allergic reaction | 97 | 3 | 0 | 0 | 0 | 0 | 3 |
| Anaphylaxis | 97 | 0 | 0 | 3 | 0 | 0 | 3 |
| Anemia | 74 | 17 | 9 | 0 | 0 | 0 | 26 |
| Anorexia | 66 | 17 | 17 | 0 | 0 | 0 | 34 |
| Anxiety | 97 | 0 | 3 | 0 | 0 | 0 | 3 |
| Arthralgia | 80 | 17 | 3 | 0 | 0 | 0 | 20 |
| Arthritis | 97 | 3 | 0 | 0 | 0 | 0 | 3 |
| Atrial fibrillation | 97 | 3 | 0 | 0 | 0 | 0 | 3 |
| Back pain | 80 | 3 | 14 | 3 | 0 | 0 | 20 |
| Bloating | 97 | 3 | 0 | 0 | 0 | 0 | 3 |
| Bone pain | 89 | 3 | 9 | 0 | 0 | 0 | 11 |
| Bronchial infection | 97 | 0 | 3 | 0 | 0 | 0 | 3 |
| Bruising | 97 | 3 | 0 | 0 | 0 | 0 | 3 |
| Chest wall pain | 97 | 0 | 3 | 0 | 0 | 0 | 3 |
| Chills | 94 | 6 | 0 | 0 | 0 | 0 | 6 |
| Constipation | 71 | 23 | 6 | 0 | 0 | 0 | 29 |
| Cough | 83 | 14 | 3 | 0 | 0 | 0 | 17 |
| Creatinine increased | 97 | 0 | 3 | 0 | 0 | 0 | 3 |
| Depression | 97 | 0 | 3 | 0 | 0 | 0 | 3 |
| Diarrhea | 46 | 40 | 11 | 3 | 0 | 0 | 54 |
| Dizziness | 89 | 11 | 0 | 0 | 0 | 0 | 11 |
| Dysesthesia | 97 | 3 | 0 | 0 | 0 | 0 | 3 |
| Dysgeusia | 86 | 3 | 11 | 0 | 0 | 0 | 14 |
| Dysphagia | 97 | 0 | 3 | 0 | 0 | 0 | 3 |
| Dyspnea | 89 | 11 | 0 | 0 | 0 | 0 | 11 |
| Ear and labyrinth disorders | 94 | 6 | 0 | 0 | 0 | 0 | 6 |
| Edema limbs | 83 | 11 | 6 | 0 | 0 | 0 | 17 |
| Epistaxis | 97 | 3 | 0 | 0 | 0 | 0 | 3 |
| Fall | 97 | 0 | 3 | 0 | 0 | 0 | 3 |
| Fatigue | 54 | 6 | 37 | 3 | 0 | 0 | 46 |
| Flatulence | 97 | 0 | 3 | 0 | 0 | 0 | 3 |
| Flu like symptoms | 83 | 9 | 9 | 0 | 0 | 0 | 17 |
| Gastroesophageal reflux disease | 97 | 3 | 0 | 0 | 0 | 0 | 3 |
| Gastrointestinal disorders | 97 | 0 | 0 | 3 | 0 | 0 | 3 |
| Generalized muscle weakness | 91 | 3 | 6 | 0 | 0 | 0 | 9 |
| Gum infection | 94 | 0 | 3 | 3 | 0 | 0 | 6 |
| Headache | 86 | 11 | 3 | 0 | 0 | 0 | 14 |
| Hemorrhoidal hemorrhage | 97 | 3 | 0 | 0 | 0 | 0 | 3 |
| Hoarseness | 97 | 3 | 0 | 0 | 0 | 0 | 3 |
| Hot flashes | 89 | 9 | 3 | 0 | 0 | 0 | 11 |
| Hyperglycemia | 94 | 0 | 0 | 3 | 3 | 0 | 6 |
| Hypertension | 91 | 3 | 6 | 0 | 0 | 0 | 9 |
| Hypoalbuminemia | 94 | 3 | 3 | 0 | 0 | 0 | 6 |
| Hypocalcemia | 91 | 9 | 0 | 0 | 0 | 0 | 9 |
| Hypokalemia | 94 | 6 | 0 | 0 | 0 | 0 | 6 |
| Hyponatremia | 89 | 11 | 0 | 0 | 0 | 0 | 11 |
| Hypotension | 97 | 0 | 0 | 3 | 0 | 0 | 3 |
| Insomnia | 94 | 6 | 0 | 0 | 0 | 0 | 6 |
| Laryngeal inflammation | 97 | 3 | 0 | 0 | 0 | 0 | 3 |
| Lip infection | 97 | 3 | 0 | 0 | 0 | 0 | 3 |
| Localized edema | 97 | 3 | 0 | 0 | 0 | 0 | 3 |
| Lung infection | 97 | 0 | 0 | 3 | 0 | 0 | 3 |
| Lymphocyte count decreased | 49 | 11 | 20 | 20 | 0 | 0 | 51 |
| Memory impairment | 97 | 3 | 0 | 0 | 0 | 0 | 3 |
| Musculoskeletal and connective tissue disorder | 97 | 0 | 3 | 0 | 0 | 0 | 3 |
| Myalgia | 77 | 17 | 6 | 0 | 0 | 0 | 23 |
| Nail discoloration | 97 | 3 | 0 | 0 | 0 | 0 | 3 |
| Nasal congestion | 91 | 3 | 6 | 0 | 0 | 0 | 9 |
| Nausea | 54 | 26 | 20 | 0 | 0 | 0 | 46 |
| Neck pain | 94 | 3 | 3 | 0 | 0 | 0 | 6 |
| Neoplasms benign, malignant, and unspecified (incl cysts and polyps) | 97 | 0 | 3 | 0 | 0 | 0 | 3 |
| Nervous system disorders | 97 | 3 | 0 | 0 | 0 | 0 | 3 |
| Neutrophil count decreased | 60 | 20 | 11 | 3 | 6 | 0 | 40 |
| Non‐cardiac chest pain | 94 | 3 | 0 | 3 | 0 | 0 | 6 |
| Oral pain | 97 | 3 | 0 | 0 | 0 | 0 | 3 |
| Osteonecrosis of jaw | 97 | 3 | 0 | 0 | 0 | 0 | 3 |
| Pain | 97 | 0 | 3 | 0 | 0 | 0 | 3 |
| Pain in extremity | 89 | 6 | 6 | 0 | 0 | 0 | 11 |
| Paresthesia | 89 | 11 | 0 | 0 | 0 | 0 | 11 |
| Peripheral motor neuropathy | 97 | 0 | 3 | 0 | 0 | 0 | 3 |
| Peripheral sensory neuropathy | 97 | 0 | 3 | 0 | 0 | 0 | 3 |
| Platelet count decreased | 80 | 17 | 0 | 0 | 3 | 0 | 20 |
| Pleural effusion | 94 | 3 | 3 | 0 | 0 | 0 | 6 |
| Productive cough | 97 | 3 | 0 | 0 | 0 | 0 | 3 |
| Pruritus | 86 | 14 | 0 | 0 | 0 | 0 | 14 |
| Rash acneiform | 97 | 3 | 0 | 0 | 0 | 0 | 3 |
| Rash maculo‐papular | 94 | 6 | 0 | 0 | 0 | 0 | 6 |
| Renal calculi | 97 | 3 | 0 | 0 | 0 | 0 | 3 |
| Rhinitis infective | 97 | 3 | 0 | 0 | 0 | 0 | 3 |
| Skin and subcutaneous tissue disorders | 94 | 3 | 3 | 0 | 0 | 0 | 6 |
| Sneezing | 97 | 3 | 0 | 0 | 0 | 0 | 3 |
| Stomach pain | 97 | 3 | 0 | 0 | 0 | 0 | 3 |
| Syncope | 94 | 0 | 0 | 6 | 0 | 0 | 6 |
| Testicular pain | 94 | 3 | 3 | 0 | 0 | 0 | 6 |
| Thromboembolic event | 97 | 0 | 3 | 0 | 0 | 0 | 3 |
| Toothache | 97 | 0 | 3 | 0 | 0 | 0 | 3 |
| Transient ischemic attacks | 97 | 3 | 0 | 0 | 0 | 0 | 3 |
| Tremor | 97 | 3 | 0 | 0 | 0 | 0 | 3 |
| Urinary frequency | 94 | 3 | 3 | 0 | 0 | 0 | 6 |
| Urinary tract infection | 97 | 0 | 0 | 3 | 0 | 0 | 3 |
| Urinary tract obstruction | 97 | 0 | 0 | 3 | 0 | 0 | 3 |
| Urinary tract pain | 97 | 3 | 0 | 0 | 0 | 0 | 3 |
| Urinary urgency | 94 | 3 | 3 | 0 | 0 | 0 | 6 |
| Vertigo | 97 | 0 | 3 | 0 | 0 | 0 | 3 |
| Vomiting | 89 | 11 | 0 | 0 | 0 | 0 | 11 |
| Weight loss | 89 | 9 | 3 | 0 | 0 | 0 | 11 |
| White blood cell decreased | 43 | 26 | 23 | 6 | 3 | 0 | 57 |
| Wound complication | 97 | 0 | 3 | 0 | 0 | 0 | 3 |
| Wound infection | 97 | 0 | 3 | 0 | 0 | 0 | 3 |
Abbreviation: NC/NA, no change from baseline/no adverse event.
Serious Adverse Events
| Name | Grade | Attribution |
|---|---|---|
| Anaphylaxis | 3 | Unrelated |
| Back pain | 3 | Unrelated |
| Gastrointestinal disorders ‐ Other, specify | 3 | Unrelated |
| Lung Infection | 3 | Unrelated |
| Neutrophil count decreased | 2 | Possible |
| Syncope | 3 | Unlikely |
| Urinary tract infection | 3 | Unrelated |
After a median follow‐up of 22 months, adverse events for radium‐223 plus enzalutamide versus enzalutamide alone are reported.
Adverse Events: Control, All Cycles (percentages)
| Name | NC/NA | 1 | 2 | 3 | 4 | 5 | All grades |
|---|---|---|---|---|---|---|---|
| Fracture | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bone marrow hypocellular | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Abdominal pain | 86 | 7 | 0 | 7 | 0 | 0 | 14 |
| Alkaline phosphatase increased | 79 | 0 | 21 | 0 | 0 | 0 | 21 |
| Alopecia | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Anemia | 93 | 0 | 7 | 0 | 0 | 0 | 7 |
| Anxiety | 93 | 0 | 7 | 0 | 0 | 0 | 7 |
| Arthralgia | 71 | 0 | 21 | 7 | 0 | 0 | 29 |
| Back pain | 93 | 0 | 7 | 0 | 0 | 0 | 7 |
| Bloating | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Blood bilirubin increased | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Bone pain | 86 | 14 | 0 | 0 | 0 | 0 | 14 |
| Chills | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Cough | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Creatinine increased | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Depression | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Diarrhea | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Dysgeusia | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Dyspnea | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Facial pain | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Fatigue | 79 | 7 | 7 | 7 | 0 | 0 | 21 |
| Flatulence | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Gastroesophageal reflux disease | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Gastrointestinal disorders | 93 | 0 | 0 | 7 | 0 | 0 | 7 |
| Generalized muscle weakness | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Gynecomastia | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Hypoalbuminemia | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Lymphocyte count decreased | 71 | 14 | 14 | 0 | 0 | 0 | 29 |
| Muscle weakness lower limb | 86 | 14 | 0 | 0 | 0 | 0 | 14 |
| Muscle weakness upper limb | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Musculoskeletal and connective tissue disorder | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Myalgia | 79 | 7 | 14 | 0 | 0 | 0 | 21 |
| Nasal congestion | 86 | 14 | 0 | 0 | 0 | 0 | 14 |
| Nausea | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | 86 | 7 | 0 | 0 | 0 | 7 | 14 |
| Non‐cardiac chest pain | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Pain | 93 | 0 | 0 | 7 | 0 | 0 | 7 |
| Pain in extremity | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Palpitations | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Peripheral sensory neuropathy | 93 | 0 | 7 | 0 | 0 | 0 | 7 |
| Productive cough | 86 | 7 | 7 | 0 | 0 | 0 | 14 |
| Psychiatric disorders | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Skin ulceration | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Sore throat | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Urinary frequency | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Vertigo | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
After a median follow‐up of 22 months, adverse events for radium‐223 plus enzalutamide versus enzalutamide alone are reported.
Abbreviation: NC/NA, no change from baseline/no adverse event.
Serious Adverse Events
| Name | Grade | Attribution |
|---|---|---|
| Neoplasms benign, malignant, and unspecified (incl cysts and polyps) ‐ Other, specify | 5 | Unrelated |
| Pain | 3 | Unrelated |
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator's Assessment | Active and should be pursued further |
We previously reported that in men with progressive metastatic castration‐resistant prostate cancer (mCRPC), treatment with the combination of radium‐223 plus enzalutamide is safe and associated with a significant decrease in bone metabolism markers, such as N‐terminal propeptide of type 1 collagen, compared with enzalutamide [1]. The relative change in serum bone metabolism marker N‐telopeptide levels from baseline to 6 months between the two arms and the safety and feasibility of the combination were coprimary endpoints for the study. Decline in bone markers directly correlated with prostate‐specific antigen (PSA) response, objective response rate, and radiographic progression‐free survival (PFS). These results were supported by a previously published trial—Morris et al. [7] conducted a randomized phase II clinical trial of radium‐223 (55 kBq/kg every 6 weeks × five doses) plus docetaxel (60 mg/m2 every 3 weeks) versus docetaxel (75 mg/m2) and reported improved bone metabolism markers with the combination compared with docetaxel alone in the setting of a more robust PSA response and longer median radiographic PFS.
In the current report, we present updated results on safety and efficacy with a median follow‐up of 22 months and provide the findings on the secondary endpoints not previously reported. The fracture rates observed in our trial of 5.7% (2 out of 35 patients) for the combination and 0% for enzalutamide are comparable with rates previously reported with radium‐223. It should be noted that only 2 of 47 patients in our study did not receive bone strengthening agents per their wishes. This further emphasizes the value of concurrent bisphosphonate or denosumab use in preventing bone fractures. The fracture rate reported in the PEACE III clinical trial [8], which did not initially mandate bone protective therapy, was 13% for enzalutamide and 33% for the radium‐223 plus enzalutamide arm. Following an amendment mandating use of a bone protective agent in response to published ERA 223 data, the fracture risk was almost abolished with bone protective therapy, 0% and 3%, respectively [6]. The fracture rate in the ALSYMPCA trial, 5% (32/614 patients) for the patients treated with radium‐223 [9], is also similar to our study.
There are previous reports of increased rates of bone marrow failure after radium‐223 treatment. Huynh‐Le et al. [2] reported an overall incidence of pancytopenia or bone marrow failure of 7.1% (154/2,182 patients) of patients undergoing radium‐223 therapy. Etchebehere et al. [3] reported even higher rates of bone marrow failure of 35% (32/92 patients) of patients treated with radium‐223. The ALSYMPCA trial [4] did not report any events of bone marrow failure. We did not observe any bone marrow failure events. It is possible that our sample size was insufficient to detect this uncommon side effect. Of particular importance is the early access study [5] of radium‐223 (n = 696), in which 27% of patients received concurrent treatment with abiraterone or enzalutamide or both, and only one patient developed bone marrow failure (1/696, <1%). The larger PEACE III clinical trial may further clarify the magnitude of this risk, specifically in combination with enzalutamide.
In this clinical trial, despite numerical improvement, no significant difference was observed between treatment arms regarding median overall survival (OS), PSA‐PFS, and radiographic PFS even with longer follow‐up. The statistically significant improvement in time from start of protocol therapy to PSA progression on subsequent therapy (PSA‐PFS2) and strong numerical trend for delay of subsequent therapy with radium‐223 are suggestive of a favorable effect of radium‐223 on the disease trajectory, especially in combination with enzalutamide. The lack of significant OS benefit possibly is explained by the small sample size of this trial, which was not designed to have sufficient power to detect a true difference in any of the secondary endpoints. In the ERA 223 clinical trial [6], no improvement in OS was demonstrated with radium‐223 plus abiraterone compared with abiraterone alone with a median OS of 30.7 months (95% confidence interval [CI], 25.8 months–NE) and 33.3 months (95% CI, 30.2–41.1 months), respectively (hazard ratio, 1.20; 95% CI, 0.95–1.51; p = .13). Similarly, there was no difference in the primary endpoint of symptomatic skeletal event–free survival, reported as 22.3 months (95% CI, 20.4–24.8) for radium‐223 plus abiraterone and 26.0 months (21.8–28.3) for abiraterone alone (hazard ratio, 1.122; 95% CI, 0.92–1.37; p = .26). However, the efficacy results are currently awaited from the ongoing phase III trial of combination of radium‐223 plus enzalutamide (PEACE III trial) in mCRPC.
Although there was a statistically significant improvement in PSA‐PFS2 in our clinical trial, it is important to emphasize that this is a post hoc analysis and may be the result of α error.
The results of our trial are hypothesis generating, and routine use of radium‐223 concurrently with enzalutamide may not be recommended based on these results. The ongoing phase III clinical trial (PEACE III trial) randomizing patients to radium‐223 plus enzalutamide versus enzalutamide alone in men with mCRPC will conclusively determine the therapeutic role of this combination for our patients in the clinic.
In summary, treatment with radium‐223 plus enzalutamide combination appears to be safe and has promising efficacy. As seen with the ERA 223 and PEACE III clinical trials, we observed few bone fractures in patients concurrently receiving bone modifying therapy. These data portend well for the ongoing phase III trial (PEACE III trial) investigating the role of this combination in men with mCRPC.
Disclosures
Benjamin L Maughan: Roche/Genentech, Pfizer, AVEO Oncology, Janssen Oncology, Astellas, Bristol‐Myers Squibb, Clovis, Tempu, Merck, Exelixis, Bayer Oncology, Peloton Therapeutics (C/A), Exelixis, Bavarian‐Nordic, Clovis,Genentech, Bristol‐Myers Squibb (FR–institutional); John M. Hoffman: NexEos Dx (OI); Umang Swami: Seattle Genetics (C/A), Janssen (RF); Neeraj Agarwal: Astellas, Argos Therapeutics, AstraZeneca, Aveo, Bayer, Bristol Myers Squibb, Calithera, Clovis, Eisai, Eli Lilly, EMDSerono, Exelixis, Foundation Medicine, Genentech, Gilead, Janssen, Merck, MEIPharma, Nektar, Novartis, Pfizer, Pharmacyclics, Seattle Genetics (C/A), AstraZeneca,Bavarian Nordic, Bayer, Bristol‐Myers Squibb, Calithera, Celldex, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Genentech, Glaxo Smith Kline, Immunomedics, Janssen, Medivation, Merck, Nektar, New Link Genetics, Novartis, Pfizer, Prometheus, Rexahn, Roche, Sanofi, Seattle Genetics, Takeda, Tracon (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
- ClinicalTrials.gov Identifier: NCT02199197
- Sponsor: University of Utah
- Principal Investigator: Neeraj Agarwal
- IRB Approved: Yes
Contributor Information
Benjamin L. Maughan, Email: benjamin.maughan@hci.utah.edu.
Adam Kessel, Email: adam.kessel@hsc.utah.edu.
References
- 1. Agarwal N, Nussenzveig R, Hahn AW et al. Prospective evaluation of bone metabolic markers as surrogate markers of response to radium‐223 therapy in metastatic castration‐resistant prostate cancer. Clin Cancer Res 2020;26:2104–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huynh‐Le MP, Shults RC, Connor MJ et al. Adverse events associated with radium‐223 in metastatic prostate cancer: Disproportionality analysis of FDA data reflecting worldwide utilization. Clin Genitourin Cancer 2020;18:192–200.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Etchebehere EC, Milton DR, Araujo JC et al. Factors affecting (223) Ra therapy: Clinical experience after 532 cycles from a single institution. Eur J Nucl Med Mol Imaging 2016;43:8–20. [DOI] [PubMed] [Google Scholar]
- 4. Parker C, Nilsson S, Heinrich D et al. Alpha emitter radium‐223 and survival in metastatic prostate cancer. N Engl J Med 2013;369:213–223. [DOI] [PubMed] [Google Scholar]
- 5. Saad F, Carles J, Gillessen S et al. Radium‐223 and concomitant therapies in patients with metastatic castration‐resistant prostate cancer: An international, early access, open‐label, single‐arm phase 3b trial. Lancet Oncol 2016;17:1306–1316. [DOI] [PubMed] [Google Scholar]
- 6. Smith M, Parker C, Saad F et al. Addition of radium‐223 to abiraterone acetate and prednisone or prednisolone in patients with castration‐resistant prostate cancer and bone metastases (ERA 223): A randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol 2019;20:408–419. [DOI] [PubMed] [Google Scholar]
- 7. Morris M, Loriot Y, Sweeney CJ et al. Radium‐223 in combination with docetaxel in patients with castration‐resistant prostate cancer and bone metastases: A phase 1 dose escalation/randomised phase 2a trial. Eur J Cancer 2019;114:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tombal BF, Loriot Y, Saad F et al. Decreased fracture rate by mandating bone‐protecting agents in the EORTC 1333/PEACE III trial comparing enzalutamide and Ra223 versus enzalutamide alone: An interim safety analysis. J Clin Oncol 2020;37(suppl 15):5007. [Google Scholar]
- 9. Sartor O, Coleman R, Nilsson S et al. Effect of radium‐223 dichloride on symptomatic skeletal events in patients with castration‐resistant prostate cancer and bone metastases: Results from a phase 3, double‐blind, randomised trial. Lancet Oncol 2014;15:738–746. [DOI] [PubMed] [Google Scholar]


