Abstract
Background
Outcomes of patients with metastatic melanoma discontinuing BRAF‐targeted therapy for cumulative toxicity after sustained response are unknown.
Materials and Methods
This retrospective case series analysis conducted at a single Cancer Center in Italy included patients with BRAF mutated metastatic melanoma treated with a BRAF inhibitor as a single agent or in combination with a MEK inhibitor between June 1, 2011 and January 1, 2020 and interrupted treatment due to cumulative toxicity after achieving complete response (CR) or long‐lasting partial response (PR; i.e. >12 months).
Results
We included 24 patients with a median treatment duration of 59.4 months (95% confidence interval [CI], 55.4–63.4; range, 12–88). CR and PR were achieved in 71% and 29% of patients, respectively. At a median follow‐up after treatment discontinuation of 37.8 months (95% CI, 33.7–41.9), the 12‐month progression‐free survival after discontinuation (dPFS) rate was 70.8% (95% CI 54.8–91.6) and 24‐month dPFS rate was 58.3% (95% CI, 41.6–81.8). Baseline patient and tumor characteristics as well as treatment duration and best response did not significantly impact on dPFS. Patients with CR and negative circulating tumor DNA (ctDNA) at time of discontinuation had a significantly improved dPFS compared with patients with either radiological residual disease or ctDNA positivity (p = .007). No patient in CR with undetectable ctDNA experienced progression.
Conclusion
The risk of progression is high even in patients with sustained sensitivity to BRAF/MEK inhibitors. Integration of liquid biopsy in clinical trials investigating the optimal management of patients with sustained sensitivity to BRAF/MEK inhibitors is warranted.
Implications for Practice
Outcomes of patients with metastatic melanoma discontinuing BRAF‐targeted therapy for cumulative toxicity are unknown. This study analyzed patients with sustained responses (median treatment duration 59.4 months). Twelve‐ and 24‐month progression‐free survival following discontinuation were 70.8% and 58.3%, respectively. Complete response and negative circulating tumor DNA at time of discontinuation are promising prognostic biomarkers in this setting.
Keywords: Melanoma, BRAF, Target therapy
Short abstract
This article reports clinical outcomes of patients with metastatic melanoma treated with BRAF/MEK inhibitors who interrupted the treatment after achieving a long‐lasting objective response, exploring the role of circulating tumor DNA assessed at time of discontinuation as a biomarker for post‐discontinuation progression‐free survival.
Introduction
BRAF inhibitors alone or in combination with MEK inhibitors are the standard of care in patients with metastatic melanoma harboring BRAF V600E/K mutations [1]. Long‐term follow‐up analysis of trials with upfront dabrafenib as a single agent or in combination with trametinib showed that 49% and 16% of patients experiencing complete (CR) and partial response (PR)respectively, are free of disease progression (PD) at 5 years [2]. Continuation of targeted therapy until PD or unacceptable toxicity is recommended because it is unknown if patients might be safely taken off targeted therapy after achieving tumor response. The outcomes following discontinuation of BRAF/MEK inhibitors in patients achieving a long‐term response are currently unknown, and no biomarkers are available to guide the decision‐making process in this setting. Interestingly, in patients with stage III melanoma, detectable baseline and post‐surgery circulating tumor DNA (ctDNA) strongly predicts relapse [3], suggesting that the evaluation of ctDNA by means of liquid biopsy might be an exploitable biomarker in melanoma in both the adjuvant and metastatic settings [4]. In the present study, we report on the clinical outcomes of patients with metastatic melanoma treated with BRAF/MEK inhibitors who interrupted the treatment after achieving a long‐lasting objective response, exploring the role of ctDNA assessed at time of discontinuation as a biomarker for post‐discontinuation progression‐free survival PFS (dPFS).
Materials and Methods
Patient Population
We retrieved data of patients with metastatic/unresectable melanoma harboring BRAF V600E/K mutations, treated with a BRAF V600 inhibitor alone or in combination with an MEK inhibitor, who discontinued treatment for cumulative toxicities after achieving long‐lasting (i.e. >12 months) CR or PR. Baseline clinicopathological and treatment characteristics were collected retrospectively. dPFS was defined as the time from treatment discontinuation to PD or death. The primary outcome measure was dPFS at 12 and 24 months. Exploratory outcome measures were dPFS according to RECIST best response and liquid biopsy. The study was approved by the Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Nazionale dei Tumori di Milano Institutional Review Board (study ID: INT 23/20) and conducted according to the ethical principles for medical research involving human subjects adopted in the Declaration of Helsinki. All patients signed a written Informed Consent.
Liquid Biopsy
Liquid biopsies were performed at time of targeted therapy discontinuation and at each further radiological evaluation until confirmed PD in patients with melanoma bearing the BRAF V600E mutation. CtDNA was isolated by QIAamp Circulating Nucleic Acid kit (Qiagen, Hilden, Germany) from 2 ml plasma, obtained from peripheral blood collected in EDTA Vacutainer tubes (BD) and stored at −80°C. CtDNA was eluted in 45 μl buffer AVE and 7.5 μl ctDNA were used for digital polymerase chain reaction (dPCR) analysis using QuantStudio 3D Digital PCR System (ThermoFisher Scientific, Waltham, MA). Quantification of the abundance of BRAF V600E mutated allele (c.1799T > A) and the corresponding wild type allele was determined by using commercially available TaqMan dPCR Liquid Biopsy Assay Hs000000004_rm (ThermoFisher, Waltham, Massachusetts, U.S.A) and by analyzing dPCR data with QuantStudio 3D Analysis Suite Cloud Software. The mutation cutoff threshold was set above background signal by the analysis of plasma samples from healthy controls (n = 21) and set to be zero copies of mutated allele. Positivity in patients ranged between 3 and 100 copies/mL. PCR specificity and sensitivity were defined in titration experiments by assaying dilutions of BRAF mutated DNA from a heterozygous melanoma cell line in wild‐type DNA, and dilutions in water of BRAF mutated DNA. The limit of detection was 0.1% mutated allele fraction given a DNA input of 5 ng. Levels of circulating BRAF V600E variants are expressed as % mutant allele frequency, calculated as (copies mutant/copies mutant + copies wild‐type) × 100.
Statistical Analysis
Continuous data were summarized as mean or median values. Categorical data were summarized as frequency and percentage. Event‐free survival was evaluated by the Kaplan‐Meier method. The log‐rank test was used to compare survival functions. Hazard ratios were calculated using univariate Cox's proportional hazards regression model. All statistical tests were two‐tailed, and p < .05 was considered to be statistically significant.
Results
Patient Population
Among 453 patients with BRAF mutated metastatic‐melanoma referring to the Fondazione IRCCS Istituto Nazionale dei Tumori Hospital between June 1, 2011, and January 1, 2020; 24 fulfilled the inclusion criteria. Patients' baseline demographics and disease characteristics and treatment received are detailed in Table 1.
Table 1.
Patient's characteristics
| Characteristic | n = 24, n (%) |
|---|---|
| Age, median (IQR), yr | 56 (43–63) |
| Gender | |
| Female | 12 (50) |
| Male | 12 (50) |
| ECOG PS | |
| 0 | 24 (100) |
| ≥1 | 0 |
| Synchronous metastases | |
| Yes | 3 (13) |
| No | 21 (87) |
| Stage | |
| M1a | 7 (29) |
| M1b | 7 (29) |
| M1c | 8 (33) |
| M1d | 2 (8) |
| Number of metastatic sites | |
| 1 | 9 (37) |
| ≥2 | 15 (63) |
| Baseline LDH | |
| Low | 21 (87) |
| High | 3 (13) |
| Previous lines of treatment for metastatic disease | |
| 0 | 21 (87) |
| 1 | 3 (13) |
| Treatment received | |
| Vemurafenib | 14 (58) |
| Dabrafenib | 1 (4) |
| Vemurafenib‐cobimetinib | 1 (4) |
| Dabrafenib‐trametinib | 8 (33) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; IQR, interquartile range; LDH, lactate dehydrogenase.
Outcomes Following Discontinuation
Median treatment duration was 59.4 months (95% CI, 55.4–63.4; range, 12–88). Best response (either CR or PR) was achieved after a median time of 5.3 (95% CI, 2.4–8.2) months. At the time of discontinuation, 17 (71%) and 7 (29%) patients had achieved a CR and PR, respectively. At a median follow up of 37.8 months (95% CI, 33.7–41.9) after treatment discontinuation, a total of 11 (45.8%) patients experienced PD. Clinical information and patterns of disease progression are detailed in supplemental online Table 1. No patient experienced symptomatic PD. All patients were alive at time of the present analysis. Two out of 11 patients displayed involvement of new organ sites. Twelve‐month dPFS rate was 70.8% (95% CI, 54.8%–91.6%) and 24‐month dPFS rate was 58.3% (95% CI, 41.6%–81.8%). Median time to PD after treatment discontinuation was 10.4 months (95% CI, 6.9–13.8). Neither baseline clinicopathological characteristics nor treatment duration influenced the risk of PD; time to best response was significantly associated with dPFS at univariate analysis (HR, 1.087; 95% CI, 1.005–1.175; p = .037; Table 2). We found a nonsignificant trend toward a worse dPFS for patients interrupting treatment with residual disease (12‐month dPFS rate 42.9%; 95%CI, 18.2%–100%) compared with those who interrupted treatment after achieving RECIST‐defined CR (12‐month dPFS rate 82.4%; 95% CI, 66.1%–100%; log‐rank p = .063; Fig. 1A).
Table 2.
Univariate analysis for dPFS
| Variable | dPFS, HR (95% CI) | p value |
|---|---|---|
| Gender | .727 | |
| Female | Ref | |
| Male | 0.807 (0.245–2.660) | |
| Age | 0.997 (0.951–1.045) | .889 |
| Synchronous metastases | .287 | |
| No | Ref | |
| Yes | 2.318 (0.493–10.900) | |
| Stage | .076 | |
| M1a | Ref | |
| M1b | 9.792 (1.127–85.090) | |
| M1c | 2.709 (0.279–26.269) | |
| M1d | 11.715 (1.003–136.738) | |
| Number of metastatic sites | .752 | |
| 1 | Ref | |
| ≥2 | 1.081 (0.667–1.752) | |
| Baseline LDH | .571 | |
| Normal | Ref | |
| High | 0.552 (0.070–4.320) | |
| Previous lines of treatment for metastatic disease | .853 | |
| None | Ref | |
| One or more | 1.157 (0.249–5.363) | |
| Treatment | .583 | |
| Mono | Ref | |
| Combo | 1.394 (0.425–4.571) | |
| Dose reduction | .301 | |
| No | Ref | |
| Yes | 1.914 (0.560–6.546) | |
| Treatment duration | 1.014 (0.982–1.0479 | .392 |
| Time to best response | 1.087 (1.005–1.175) | .037 |
| Best response | .063 | |
| CR | Ref | |
| PR | 3.118 (0.886–10.979) |
Abbreviations: CR, complete response; dPFS, post‐discontinuation progression‐free survival; LDH, lactate dehydrogenase; PR, partial response; Ref, reference.
Figure 1.
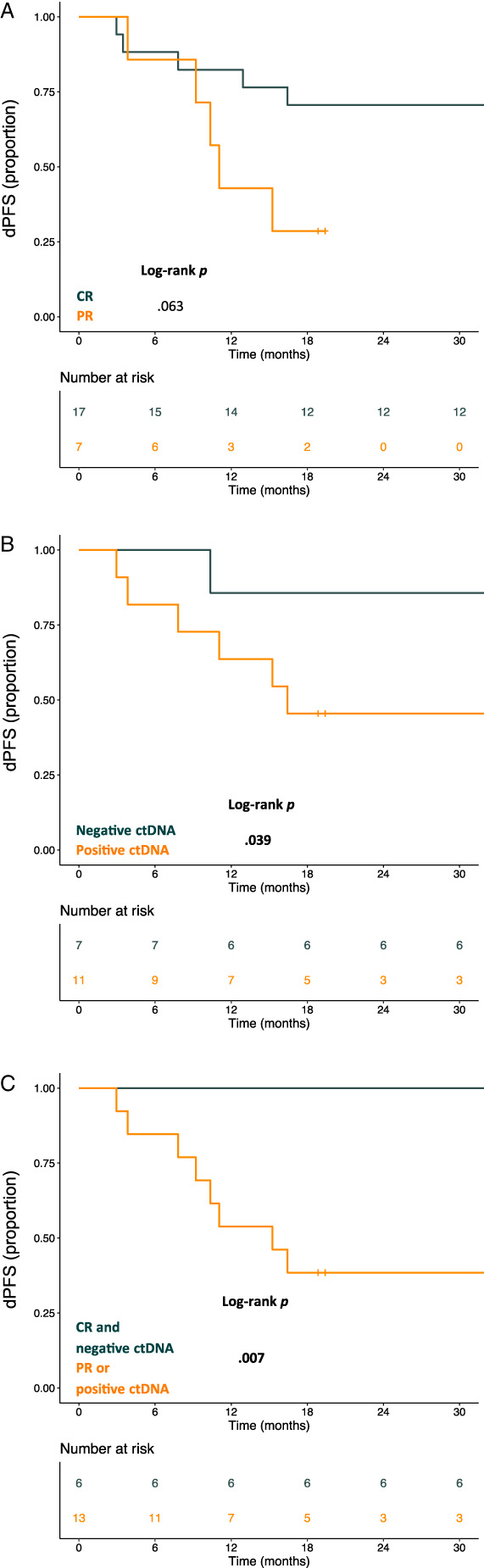
Kaplan‐Meier curve for dPFS according to radiological best response (A). Kaplan‐Meier curve for dPFS according to ctDNA at time of discontinuation of target therapy (B). Kaplan‐Meier curve for dPFS according to radiological best response and ctDNA at time of discontinuation of targeted therapy. Dark green indicates patients in CR with undetectable ctDNA at time of discontinuation. Orange line indicates all patients in PR and patients in CR with detectable ctDNA (C). Abbreviations: CR, complete response; ctDNA, circulating tumor DNA; dPFS, post‐discontinuation progression‐free survival; PR, partial response.
Prognostic Implications of Liquid Biopsy at Time of Discontinuation
Plasma samples of 18 patients with BRAF V600E melanoma were available at treatment discontinuation and assessable for ctDNA analysis. Six patients denied consent for plasma sampling for translational studies including two patients with tissue BRAF V600K mutation. Individual clinical course of patients with available ctDNA at time of discontinuation is shown in Figure 2. Longitudinal monitoring of ctDNA in patients experiencing disease progression after therapy discontinuation is shown in Figure 3. Out of six evaluable patients with positive ctDNA at time of discontinuation, five patients maintained detectable ctDNA during follow‐up. Patient ID9 had undetectable ctDNA at time of discontinuation and interrupted targeted therapy with residual brain metastases.
Figure 2.
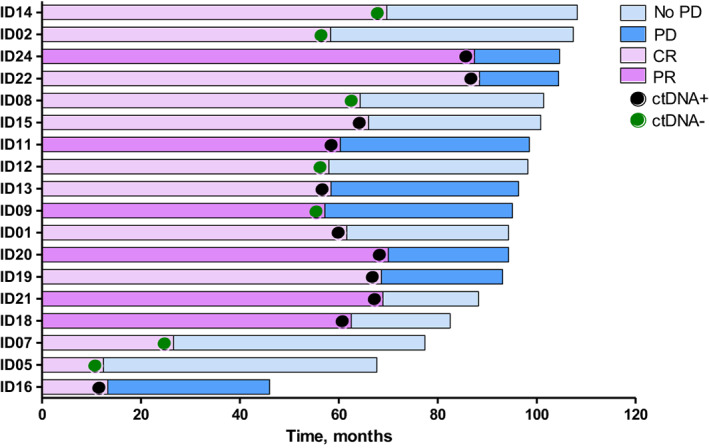
Swimmer plot for targeted therapy treatment duration and follow‐up in ctDNA‐evaluable patients. Dark and light violet rows indicate patients in PR and CR, respectively. Black and green dots indicate patients with ctDNA positive and negative at treatment discontinuation, respectively. Dark and light blue rows indicate patients who experienced PD and patients free of progression at the data cut‐off, respectively. Abbreviations: CR, complete response; ctDNA, circulating tumor DNA; PD, progressive disease; PR, partial response.
Figure 3.
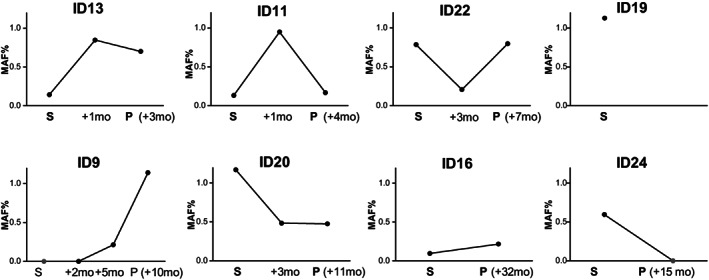
Longitudinal monitoring of ctDNA until disease progression after therapy discontinuation. The latter timepoint of ctDNA assessment matches with radiological disease progression (P, followed by individual progression‐free survival after discontinuation) except for patient ID19 whose liquid biopsy was unevaluable at time of disease progression. Black dots represent positive ctDNA, green dots represent negative ctDNA. Abbreviation: ctDNA, circulating tumor DNA; MAF, mutant allele frequency; P, time of radiological disease progression; S, time of discontinuation.
There was a significant worse dPFS in the group of patients with positive ctDNA (12‐month dPFS rate, 63.6%; 95% CI, 40.7%–99.5%) compared with patients with negative ctDNA at the time of treatment discontinuation (12‐month dPFS rate, 85.7%; 95% CI, 63.3%–100%; log‐rank p = .039; Fig. 1B). Of note, none of the patients with CR and negative ctDNA at time of discontinuation experienced a PD during the study period with a 12‐month dPFS of 100% compared with patients with either radiological residual disease or ctDNA positivity (12‐month dPFS rate, 53.8%; 95% CI, 32.6%–89.1%; log‐rank p = .007; Fig. 1C).
Activity of Retreatment with BRAF/MEK Inhibitors
Among patients who relapsed, clinical follow‐up was available for 10 patients. Six patients received a combination of BRAF and MEK inhibitors, and one patient was treated with nivolumab. All patients resuming targeted therapy achieved RECIST objective response, with three out of six patients achieving CR.
Discussion
In patients with sustained sensitivity to BRAF and MEK targeted therapy, cumulative toxicity burden might impair quality of life requiring dose reductions/drug holidays. However, even after complete radiological response, clinically undetectable minimal residual disease encompassed by heterogeneous cellular populations could drive relapse [5]. Several retrospective studies (summarized in [6]) addressed the outcomes of patients discontinuing targeted therapy for reasons other than PD after achieving CR or without evidence of disease following combined treatments (e.g. surgical resection of metastases) [6, 7, 8, 9, 10]. Collectively, these studies highlighted a heterogeneous but clinically meaningful risk of progression ranging from 20% to 100% following a median time of 1 to 3 years of treatment.
Herein, we report that risk of progression is as high as 35% and 71% after CR and PR, respectively.
Differently from reported studies, we focused on a hyper‐selected population including patients with a clearly retained sensitivity to BRAF/MEK inhibitors as outlined by the highest reported median treatment duration (i.e., ~5 years) and follow‐up since discontinuation (i.e., ~38 months). Baseline factors such as Eastern Cooperative Oncology Group performance status, lactate dehydrogenase level, and tumor burden were not associated with dPFS; however, it is unlikely that they might retain their prognostic impact for dPFS in patients who had reached deep and durable responses. Moreover, duration of drug exposure could interfere with post‐discontinuation risk of PD because it might reflect an underlying less aggressive biology of disease. However, we report that length of exposure to targeted therapy after achieving RECIST response is not associated with reduced risk of progression following discontinuation. Therefore, the optimal duration of targeted therapy for clearing minimal residual disease in patients who experience CR deserves further investigation. The achievement of RECIST CR seems a crucial determinant of a prolonged PFS after treatment discontinuation. Even if the small sample size prevented a formal demonstration of the impact of best response on post‐discontinuation progression, 6 out of 17 (35%) patients achieving CR eventually experienced PD during the study period compared with 5 out of 7 (71%) patients achieving PR. Recently, negative ctDNA at the time of treatment discontinuation emerged as a promising biomarker of dPFS in patients interrupting targeted therapy after sustained CR and might refine the identification of those patients at lower risk of relapse [6]. Therefore, we assessed whether the combination of complete radiological response and ctDNA negativity could more reliably predict dPFS than RECIST response alone. Intriguingly, none of the patients who achieved radiological CR and tested negative for ctDNA at treatment discontinuation relapsed. Importantly, combination of RECIST CR and negative ctDNA at time of discontinuation displayed high sensitivity, which represents a main goal for biomarkers informing de‐escalation strategies. In fact, low amounts of ctDNA in responder patients as well as presence of nonshedding tumors (e.g. central nervous system [CNS] metastases) might generate false negative results, thus preventing incorporation of liquid biopsy into clinical practice because of the risk of omission of therapy with a meaningful impact on survival such as targeted therapy in patients with sustained response to BRAF/MEK inhibitors. Accordingly, we report on a patient who discontinued therapy with brain metastases after achieving partial response and tested negative for ctDNA at time of discontinuation, highlighting potential suboptimal sensitivity of liquid biopsy in patients with CNS involvement [11]. Limitations of this study are related to the retrospective design, small sample size and lack of clinical validation of liquid biopsy in this setting.
Conclusion
The development of clinical grade monitoring of ctDNA in liquid biopsies appears of importance for the studies in this particular clinical setting. Therefore, the translation of these results into daily clinical practice is not recommended. Prospective trials aimed to define the role of liquid biopsy in informing the clinical decision‐making for patients with sustained responses to BRAF/MEK inhibitors are warranted.
Author Contributions
Conception/design: Lorenza Di Guardo, Giovanni Randon, Francesca Corti, Filippo De Braud, Michele Del Vecchio
Provision of study material or patients: Giovanni Randon, Francesca Corti, Vivianna Vallacchi, Marta Bini, Andrea Maurichi, Roberto Patuzzo, Gianfrancesco Gallino, Ilaria Mattavelli, Roberta Ruggeri, Martina Angi, Mara Cossa, Barbara Valeri, Carolina Cimminiello, Mario Santinami, Licia Rivoltini, Monica Rodolfo
Collection and/or assembly of data: Giovanni Randon, Francesca Corti, Viviana Vallacchi, Marta Bini, Andrea Maurichi, Roberto Patuzzo, Gianfrancesco Gallino, Ilaria Mattavelli, Roberta Ruggeri, Martina Angi, Mara Cossa, Barbara Valeri, Carolina Cimminello, Mario Santinami, Licia Rivoltini, Monica Rodolfo
Data analysis and interpretation: Lorenza Di Guardo, Giovanni Randon, Francesca Corti, Alessandra Raimondi, Giovanni Fucà
Manuscript writing: Lorenza Di Guardo, Giovanni Randon, Francesca Corti, Alessandra Raimondi, Giovanni Fucà, Marta Bini
Final approval of manuscript: Lorenza Di Guardo, Giovanni Randon, Francesca Corti, Viviana Vallacchi, Alessandra Raimondi, Giovanni Fucà, Marta Bini, Andrea Maurichi, Roberto Patuzzo, Gianfrancesco Gallino, Ilaria Mattavelli, Roberta Ruggeri, Martina Angi, Mara Cossa, Barbara Valeri, Carolina Cimminiello, Mario Santinami, Licia Rivoltini, Filippo De Braud, Monica Rodolfo, Michele Del Vecchio
Disclosures
Filippo De Braud: Ignyta, Pfizer, Amgen, Novartis, Daiichi Sankyo, Bristol‐Myers Squibb, Dompee, Pierre Fabre, Roche, Octimet, Incyte, Teofarma, EMD Serono, Merck Sharp & Dohme, Menarini (H), Novartis, Roche, Merck Sharp & Dohme, Ignyta, Medimmune, Nektar, Bristol‐Myers Squibb, Merck Serono, Bayer, Celgene, GlaxoSmithKline, Boeringher Ingelheim, Eli Lilly & Co., Pfizer, Servier (RF); Michele Del Vecchio: Novartis, Bristol‐Myers Squibb, Merck Sharp & Dohme, Sanofi, Pierre Fabre (C/A, H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Table S1 Clinical characteristics and ctDNA data of patients experiencing disease progression after discontinuation of BRAF inhibitorbased therapy.
Acknowledgments
We acknowledge the technical contribution of Paola Deho.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Michielin O, van Akkooi ACJ, Ascierto PA et al. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up†. Ann Oncol 2019;30:1884–1901. [DOI] [PubMed] [Google Scholar]
- 2. Robert C, Grob JJ, Stroyakovskiy D et al. Five‐year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med 2019;381:626–636. [DOI] [PubMed] [Google Scholar]
- 3. Tan L, Sandhu S, Lee RJ et al. Prediction and monitoring of relapse in stage III melanoma using circulating tumor DNA. Ann Oncol 2019;30:804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee JH, Long GV, Boyd S et al. Circulating tumour DNA predicts response to anti‐PD1 antibodies in metastatic melanoma. Ann Oncol 2017;28:1130–1136. [DOI] [PubMed] [Google Scholar]
- 5. Rambow F, Rogiers A, Marin‐Bejar O et al. Toward minimal residual disease‐directed therapy in melanoma. Cell 2018;174:843–855.e819. [DOI] [PubMed] [Google Scholar]
- 6. Warburton L, Meniawy TM, Calapre L et al. Stopping targeted therapy for complete responders in advanced BRAF mutant melanoma. Sci Rep 2020;10:18878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tolk H, Satzger I, Mohr P et al. Complete remission of metastatic melanoma upon BRAF inhibitor treatment ‐ what happens after discontinuation? Melanoma Res 2015;25:362–366. [DOI] [PubMed] [Google Scholar]
- 8. Carlino MS, Vanella V, Girgis C et al. Cessation of targeted therapy after a complete response in BRAF‐mutant advanced melanoma: A case series. Br J Cancer 2016;115:1280–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Desvignes C, Abi Rached H, Templier C et al. BRAF inhibitor discontinuation and rechallenge in advanced melanoma patients with a complete initial treatment response. Melanoma Res 2017;27:281–287. [DOI] [PubMed] [Google Scholar]
- 10. Vanhaecke C, Deilhes F, Chanal J et al. BRAF V600 inhibitor discontinuation after complete response in advanced melanoma: A retrospective analysis of 16 patients. Br J Dermatol 2017;177:e94–e95. [DOI] [PubMed] [Google Scholar]
- 11. Lee JH, Menzies AM, Carlino MS et al. Longitudinal monitoring of ctDNA in patients with melanoma and brain metastases treated with immune checkpoint inhibitors. Clin Cancer Res 2020;26:4064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Table S1 Clinical characteristics and ctDNA data of patients experiencing disease progression after discontinuation of BRAF inhibitorbased therapy.


