Abstract
Background
Fit patients with metastatic urothelial carcinoma (mUC) receive first‐line platinum‐based combination chemotherapy (fPBC) as standard of care and may receive additional later‐line chemotherapy after progression. Our study compares outcomes with subsequent platinum‐based chemotherapy (sPBC) versus subsequent non‐platinum‐based chemotherapy (sNPBC).
Materials and Methods
Patients from 27 international centers in the Retrospective International Study of Cancers of the Urothelium (RISC) who received fPBC for mUC and at least two cycles of subsequent chemotherapy were included in this study. A multivariable Cox proportional hazards model compared overall survival (OS) and progression‐free survival (PFS).
Results
One hundred thirty‐five patients received sPBC and 161 received sNPBC. Baseline characteristics were similar between groups, except patients who received sPBC had higher baseline hemoglobin, higher disease control rate with fPBC, and longer time since fPBC. OS was superior in the sPBC group (median 7.9 vs 5.5 months) in a model adjusting for comorbidity burden, performance status, liver metastases, number of fPBC cycles received, best response to fPBC, and time since fPBC (hazard ratio, 0.72; 95% confidence interval, 0.53–0.98; p = .035). There was no difference in PFS. More patients in the sPBC group achieved disease control than in the sNPBC group (57.4% vs 44.8%; p = .041). Factors associated with achieving disease control in the sPBC group but not the sNPBC group included longer time since fPBC, achieving disease control with fPBC, and absence of liver metastases.
Conclusion
After receiving fPBC for mUC, patients who received sPBC had better OS and disease control. This may help inform the choice of subsequent chemotherapy in patients with mUC.
Implications for Practice
Patients with progressive metastatic urothelial carcinoma after first‐line platinum‐based combination chemotherapy may now receive immuno‐oncology agents, erdafitinib, enfortumab vedotin, or sacituzumab govitecan‐hziy; however, those ineligible for these later‐line therapies or who progress after receiving them may be considered for subsequent chemotherapy. In this retrospective study of 296 patients, survival outcomes and disease control rates were better in those receiving subsequent platinum‐based rechallenge compared with non–platinum‐based chemotherapy, suggesting that patients should receive platinum rechallenge if clinically able. Disease control with platinum rechallenge was more likely with prior first‐line platinum having achieved disease control, longer time since first‐line platinum, and absence of liver metastases.
Keywords: Urologic neoplasms, Urinary bladder neoplasms, Platinum compounds, Cisplatin, carboplatin, Drug therapy, combination, Antineoplastic agents
Short abstract
This article examines differences in outcomes for patients treated with platinum‐based combination chemotherapy for metastatic urothelial carcinoma who later received subsequent platinum‐based chemotherapy or subsequent non‐platinum‐based chemotherapy. Clinical factors associated with better outcomes are reported.
Introduction
The standard of care for fit patients with metastatic urothelial carcinoma (mUC) is first‐line platinum‐based combination chemotherapy (fPBC) with gemcitabine and cisplatin or the dose‐dense combination of methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC), followed by maintenance avelumab therapy for those patients who achieve at least stable disease. Cisplatin‐ineligible patients may receive gemcitabine and carboplatin followed by maintenance avelumab therapy or immune checkpoint inhibitors atezolizumab or pembrolizumab [1]. Until recent years, options for subsequent therapies that improved survival after progression on a platinum‐based therapy were limited and generally consisted of further cytotoxic chemotherapy with either platinum‐based or non–platinum‐based regimens. Lacking evidence to inform the choice between rechallenging with platinum‐based chemotherapy or switching to other cytotoxic chemotherapy agents associated with different mechanisms of action, providers often considered clinical features such as prior response to platinum and time from prior platinum chemotherapy to help guide the choice of subsequent regimen [2, 3, 4].
Fortunately, since 2016, the U.S. Food and Drug Administration (FDA) has approved immune checkpoint inhibitors targeting programmed death‐one (PD‐1) or programmed death–ligand one (PD‐L1) for the post‐platinum mUC setting, including the drugs avelumab, nivolumab, and pembrolizumab [5, 6, 7]. Avelumab, used for maintenance therapy after fPBC for patients who have not progressed, has demonstrated an overall survival benefit over placebo [8]. Patients with fibroblast growth factor receptor (FGFR) 2 or 3 alterations are now also eligible for erdafitinib as post‐platinum therapy [9]. Recently, enfortumab vedotin, an antibody‐drug conjugate targeting Nectin‐4, and sacituzumab govitecan‐hziy, a Trop‐2‐directed antibody and topoisomerase inhibitor conjugate, were approved in the post‐platinum and post‐anti–PD‐1 or –PD‐L1 setting [10, 11]. However, despite these vast improvements in the landscape of treatment for mUC, patients who are ineligible for these therapies or who subsequently progress may still be considered for further cytotoxic chemotherapy. Therefore, the question of optimal subsequent chemotherapy regimen after fPBC remains a clinically relevant one.
To help address this gap in knowledge, we examined differences in outcomes for patients treated with fPBC for mUC who later received subsequent platinum‐based chemotherapy (sPBC) or subsequent non‐platinum‐based chemotherapy (sNPBC). We also sought to identify clinical factors that were associated with better outcomes with sPBC or sNPBC.
Materials and Methods
Data were abstracted from the Retrospective International Study of Cancers of the Urothelium (RISC), comprising 3,025 patients with muscle‐invasive or advanced urothelial cancers treated at 27 international centers between 2005 and 2012. Patients were included for this analysis if they had mUC (either at diagnosis or after progression of localized disease), had received fPBC in the metastatic setting, and had received at least two cycles of later‐line chemotherapy after fPBC. Patients who received prior platinum‐based chemotherapy in the nonmetastatic setting were excluded, as were patients with missing or incomplete data for treatment dates or survival status. Additional exclusion criteria are detailed in Figure 1. The primary endpoint of our study was overall survival (OS), with secondary endpoints including investigator‐designated progression‐free survival (PFS) and investigator‐designated best response to subsequent chemotherapy. Disease control was defined as having an investigator‐designated best response of stable disease, partial response, or complete response.
Figure 1.
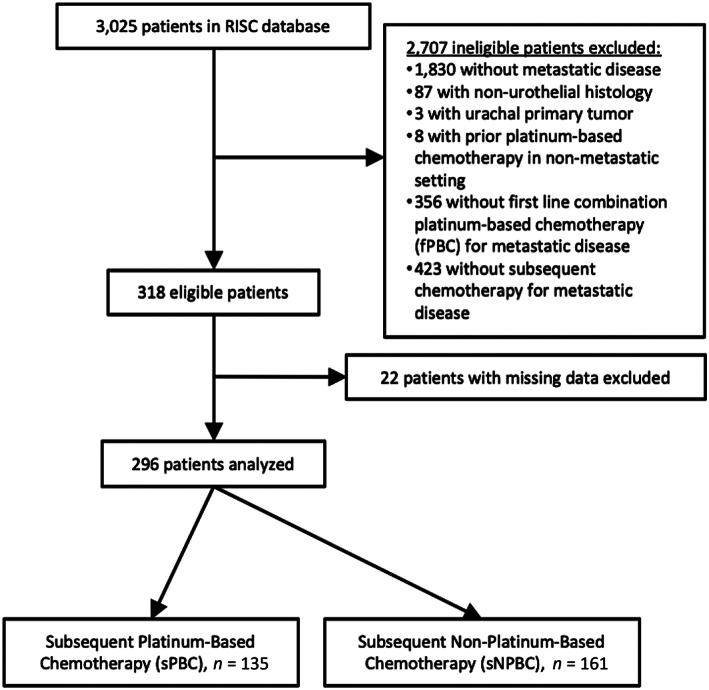
CONSORT diagram demonstrating how patients from the RISC database were selected for analysis in our study. Abbreviations: RISC, Retrospective International Study of Cancers of the Urothelium.
Statistical Analysis
Descriptive statistics, χ2, student's t‐test, and Mann‐Whitney U test were used to report and compare patient characteristics by sPBC versus sNPBC group. Kaplan‐Meier curves for OS and PFS were performed, with time‐to‐event calculations starting from the first day of the first cycle of sPBC or sNPBC. A multivariable Cox proportional hazards model was used to adjust for Eastern Cooperative Oncology Group performance status (ECOG PS), Charlson Comorbidity Index (CCI), presence of liver metastases, number of fPBC cycles received, investigator‐designated best response to fPBC, and time since last dose of fPBC. Hemoglobin and albumin levels were excluded from the multivariable model because of a high proportion of missing values for these parameters. Analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC). A two‐sided p value of ≤.05 was considered statistically significant.
Results
From 2005 to 2012, a total of 3,025 patients were enrolled in the RISC database. After exclusion criteria were applied, 296 patients were eligible for analysis (Fig. 1). With regards to fPBC, 200 patients were initially treated with cisplatin‐based combination chemotherapy (74.0% of them with gemcitabine and cisplatin, 20.5% with MVAC), and the remaining 96 patients were initially treated with carboplatin‐based combination chemotherapy (88.5% of them with gemcitabine and carboplatin). Five patients switched from cisplatin‐based chemotherapy to carboplatin‐based chemotherapy in the first line. With regards to subsequent chemotherapy, 135 patients were treated with sPBC and 161 patients received sNPBC; the most common sNPBC regimens included taxanes (71.4%), gemcitabine (11.8%), or pemetrexed (5.0%). In the sPBC group, almost all patients (97.0%) received a doublet or combination chemotherapy regimen. Ninety‐nine patients in this group had initially been treated with cisplatin in the first‐line setting, and 36 were again treated with cisplatin in the subsequent chemotherapy setting, though most of these patients (80.6%) were treated with a different combination regimen than in the first line. Similarly, 36 patients who received sPBC had initially been treated with carboplatin in the first‐line setting, and 24 were again treated with carboplatin in the subsequent chemotherapy setting, though most of these patients (58.3%) were treated with a different combination regimen.
Patient characteristics for the sPBC and sNPBC groups are compared in Table 1. The majority of baseline characteristics were similar between cohorts, including ECOG PS and comorbidity burden as assessed by the CCI. However, for the 251 patients for whom baseline hemoglobin at the start of fPBC was recorded, those who received sPBC tended to have higher baseline hemoglobin values (median 11.9 vs. 11.1 g/dL, p = .004). Patients who received sPBC also tended to have better responses to fPBC compared with patients who received sNPBC (p = .030) and to have experienced longer time between receipt of fPBC and initiation of subsequent chemotherapy (median 4.4 vs. 2.2 months, p = .010).
Table 1.
Patient characteristics
| Variable | sPBC (N = 135), a n (%) | sNPBC (N = 161), a n (%) | p value |
|---|---|---|---|
| Age at diagnosis, yr, median (IQR) | 64 (57–70) | 65 (58–73) | .144 |
| Female gender | 27 (20.1) | 31 (19.2) | .847 |
| Former/current smoker | 80 (69.6) | 110 (74.3) | .129 |
| CCI | .292 | ||
| 0 | 60 (44.4) | 70 (43.5) | |
| 1 | 14 (10.4) | 9 (5.6) | |
| 2 | 23 (17.0) | 38 (23.6) | |
| ≥3 | 38 (28.1) | 44 (27.3) | |
| ECOG PS | .141 | ||
| 0 | 31 (29.8) | 25 (19.5) | |
| 1 | 57 (54.8) | 73 (57.0) | |
| ≥2 | 16 (15.4) | 30 (23.5) | |
| Metastatic disease at diagnosis | 80 (59.3) | 95 (59.0) | .965 |
| Brain metastases b | 1 (0.8) | 5 (3.1) | .065 |
| Liver metastases b | 34 (26.2) | 50 (31.3) | .110 |
| Hemoglobin, g/dL, median (IQR) c | 11.9 (10.7–13.1) | 11.1 (10.1–12.5) | .004 |
| Albumin, g/dL, median (IQR) c | 3.8 (3.4–4.1) | 3.6 (3.3–3.9) | .431 |
| Creatinine, mg/dL, median (IQR) c | 1.2 (1.0–1.5) | 1.2 (0.9–1.5) | .448 |
| Number of fPBC cycles received | .621 | ||
| 2 | 13 (9.6) | 14 (8.7) | |
| 3–4 | 42 (31.1) | 47 (29.2) | |
| 5–6 | 68 (50.4) | 76 (47.2) | |
| ≥7 | 12 (8.9) | 24 (14.9) | |
| Best response to fPBC | .030 | ||
| Complete response | 16 (12.7) | 7 (4.7) | |
| Partial response | 50 (39.7) | 47 (31.3) | |
| Stable disease | 29 (23.0) | 41 (27.3) | |
| Progressive disease | 31 (24.6) | 55 (36.7) | |
| Received non‐chemotherapy treatment between fPBC and sPBC/sNPBC d | 6 (4.4) | 6 (3.7) | .755 |
| Months elapsed since fPBC, median (IQR) | 4.4 (1.2–7.9) | 2.2 (0.9–5.6) | .010 e |
Total number of patients for some variables may be less than “N” because of missing values.
Presence of brain or liver metastases assessed at time of diagnosis with metastatic disease.
Hemoglobin, albumin, and creatinine lab values assessed at time of initiation of fPBC.
Treatment in between fPBC and sPBC/sNPBC included the drugs sunitinib, pazopanib, erlotinib, and cetuximab.
Mann‐Whitney U test used to compare nonparametric data.
Abbreviations: CCI, Charlson Comorbidity Index; ECOG PS, Eastern Cooperative Oncology Group performance status; fPBC, first‐line platinum‐based chemotherapy; IQR, interquartile range; sNPBC, subsequent non–platinum‐based chemotherapy; sPBC, subsequent platinum‐based chemotherapy.
OS curves for patients who received sPBC or sNPBC are displayed in Figure 2. OS was superior for patients receiving sPBC (median 7.9 months) compared with sNPBC (median 5.5 months) in a multivariable model adjusting for CCI, ECOG PS, presence of liver metastases, number of fPBC cycles received, best response to fPBC, and time since fPBC (hazard ratio [HR], 0.72; 95% confidence interval [CI], 0.53–0.98; p = .035; Table 2). A similar analysis was performed for PFS (Fig. 3; Table 2). In the multivariable model, there was no statistical difference in PFS for sPBC versus sNPBC (HR, 0.83; 95% CI, 0.64–1.08; p = .159). Median PFS was 4.1 and 2.6 months for sPBC and sNPBC, respectively.
Figure 2.
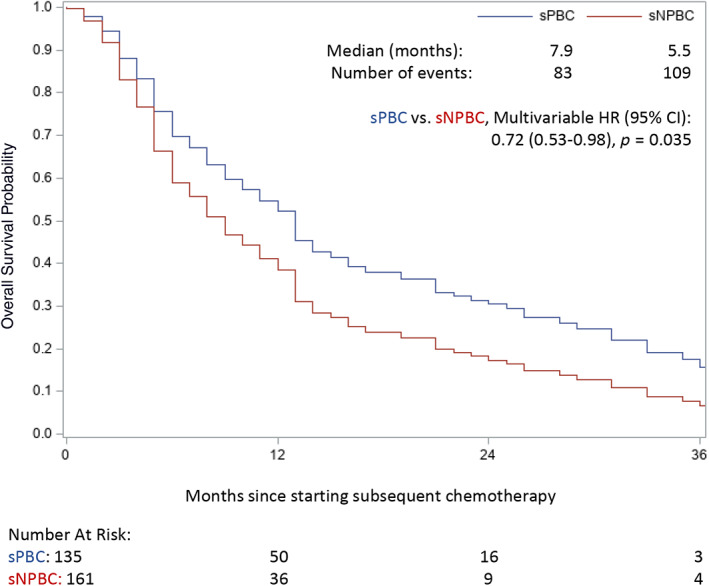
Kaplan‐Meier curves of overall survival (OS) by sPBC versus sNPBC, with median survival times reported. OS was superior for patients receiving sPBC in a multivariable model adjusting for several baseline factors, as detailed in Table 2. Abbreviations: CI, confidence interval; HR, hazard ratio; sNPBC, subsequent non–platinum‐based chemotherapy; sPBC, subsequent platinum‐based chemotherapy.
Table 2.
Factors associated with overall and progression‐free survival, multivariable model
| Variable | Risk of death | Risk of progression | |||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | Standard error | χ2 | HR (95% CI) | Standard error | χ2 | ||
| sPBC vs. sNPBC | 0.72 (0.53–0.98) | 0.16 | 0.035 | 0.83 (0.64–1.08) | 0.13 | 0.159 | |
| ECOG PS | |||||||
| 1 vs. 0 | 1.52 (0.99–2.33) | 0.22 | 0.055 | 0.99 (0.73–1.34) | 0.16 | 0.931 | |
| ≥2 vs. 0 | 2.02 (1.21–3.37) | 0.26 | 0.007 | 1.57 (1.01–2.44) | 0.23 | 0.048 | |
| Unknown vs. 0 | 1.87 (1.16–2.96) | 0.24 | 0.010 | 1.15 (0.81–1.64) | 0.18 | 0.423 | |
| CCI | |||||||
| 1 vs. 0 | 1.04 (0.59–1.83) | 0.29 | 0.904 | 1.31 (0.86–1.99) | 0.22 | 0.216 | |
| 2 vs. 0 | 0.79 (0.52–1.19) | 0.21 | 0.259 | 0.87 (0.61–1.25) | 0.18 | 0.458 | |
| ≥3 vs. 0 | 1.16 (0.80–1.67) | 0.19 | 0.437 | 1.11 (0.81–1.53) | 0.16 | 0.511 | |
| Liver metastases | |||||||
| Yes vs. no | 1.39 (1.00–1.93) | 0.17 | 0.053 | 1.19 (0.90–1.59) | 0.15 | 0.229 | |
| Unknown vs. no | 1.45 (0.57–3.70) | 0.48 | 0.434 | 0.56 (0.16–2.03) | 0.66 | 0.378 | |
| Number of fPBC cycles | 1.07 (0.96–1.19) | 0.06 | 0.217 | 1.15 (1.04–1.27) | 0.05 | 0.007 | |
| Best response to fPBC | |||||||
| PR vs. CR | 0.83 (0.45–1.19) | 0.32 | 0.565 | 1.21 (0.73–1.99) | 0.26 | 0.467 | |
| SD vs. CR | 0.92 (0.48–1.55) | 0.33 | 0.806 | 1.47 (0.87–2.49) | 0.27 | 0.149 | |
| PD vs. CR | 1.02 (0.52–1.77) | 0.34 | 0.946 | 1.76 (0.98–3.19) | 0.30 | 0.060 | |
| Unknown vs. CR | 0.84 (0.37–2.00) | 0.41 | 0.676 | 1.64 (0.90–3.01) | 0.31 | 0.108 | |
| Months elapsed since fPBC | 0.99 (0.96–1.01) | 0.01 | 0.270 | 0.98 (0.97–1.00) | 0.01 | 0.082 | |
Abbreviations: CI, confidence interval; CCI, Charlson Comorbidity Index; CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; fPBC, first‐line platinum‐based chemotherapy; HR, hazard ratio; PD, progressive disease; PR, partial response; SD, stable disease; sNPBC, subsequent non–platinum‐based chemotherapy; sPBC, subsequent platinum‐based chemotherapy.
Figure 3.
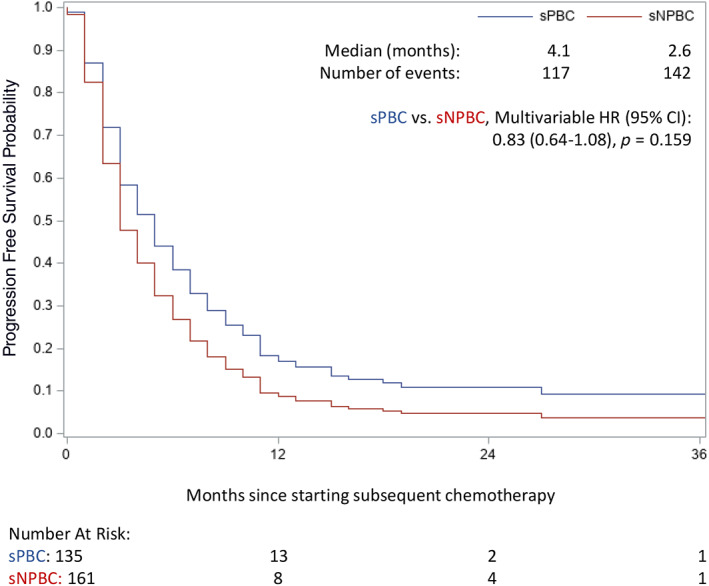
Kaplan‐Meier curves of progression‐free survival (PFS) by sPBC versus sNPBC, with median survival times reported. There was no statistical difference in PFS in a multivariable model adjusting for several baseline factors, as detailed in Table 2. Abbreviations: CI, confidence interval; HR, hazard ratio; sNPBC, subsequent non–platinum‐based chemotherapy; sPBC, subsequent platinum‐based chemotherapy.
In terms of disease control rate with subsequent chemotherapy, 70 patients (57.4%) who received sPBC achieved disease control compared with 65 patients (44.8%) who received sNPBC (p = .041). In the sPBC group, 33 patients (27.0%) achieved stable disease, 29 (23.8%) achieved partial response, and 8 (6.6%) achieved complete response; for sNPBC, 37 (25.5%), 21 (14.5%), and 7 (4.8%) patients achieved stable disease, partial response, and complete response, respectively.
A subgroup analysis was undertaken to compare factors associated with OS (data not shown) and PFS (Table 3) by type of subsequent chemotherapy. There were no unique associations with OS for either the sPBC or sNPBC group. However, for PFS, presence of liver metastases was associated with a higher likelihood of progression in the sPBC group (HR, 1.78; 95% CI, 1.14–2.79; p = .011), as was having a best response to fPBC of stable disease as opposed to complete response (HR, 2.75; 95% CI, 1.32–5.72; p = .007). For the sNPBC group, higher likelihood of progression was associated with having ECOG PS ≥2 compared with PS 0 (HR, 2.03; 95% CI, 1.28–3.22; p = .003), CCI 1 compared with 0 (HR, 2.22; 95% CI, 1.19–4.14; p = .013), and a higher number of fPBC cycles (HR, 1.21; 95% CI, 1.07–1.38; p = .003).
Table 3.
Factors associated with progression‐free survival, subgroup analysis by type of subsequent chemotherapy
| Variable | sPBC, risk of progression | sNPBC, risk of progression | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | Standard error | χ2 | HR (95% CI) | Standard error | χ2 | |
| ECOG PS | ||||||
| 1 vs. 0 | 0.97 (0.61–1.52) | 0.23 | 0.883 | 1.01 (0.67–1.51) | 0.20 | 0.970 |
| ≥2 vs. 0 | 1.18 (0.53–2.65) | 0.41 | 0.683 | 2.03 (1.28–3.22) | 0.24 | 0.003 |
| Unknown vs. 0 | 1.19 (0.69–2.06) | 0.28 | 0.530 | 1.06 (0.66–1.72) | 0.25 | 0.802 |
| CCI | ||||||
| 1 vs. 0 | 0.98 (0.52–1.83) | 0.32 | 0.937 | 2.22 (1.19–4.14) | 0.32 | 0.013 |
| 2 vs. 0 | 0.98 (0.52–1.82) | 0.32 | 0.942 | 0.84 (0.53–1.33) | 0.23 | 0.464 |
| ≥3 vs. 0 | 1.01 (0.67–1.53) | 0.21 | 0.947 | 1.39 (0.88–2.17) | 0.23 | 0.155 |
| Liver metastases | ||||||
| Yes vs. no | 1.78 (1.14–2.79) | 0.23 | 0.011 | 0.97 (0.68–1.39) | 0.18 | 0.859 |
| Unknown vs. no | 0.35 (0.07–1.84) | 0.85 | 0.214 | 1.07 (0.57–1.99) | 0.32 | 0.839 |
| Number of fPBC cycles | 1.10 (0.94–1.29) | 0.08 | 0.248 | 1.21 (1.07–1.38) | 0.07 | 0.003 |
| Best response to fPBC | ||||||
| PR vs. CR | 1.42 (0.72–2.81) | 0.35 | 0.313 | 0.82 (0.41–1.66) | 0.36 | 0.580 |
| SD vs. CR | 2.75 (1.32–5.72) | 0.37 | 0.007 | 0.88 (0.42–1.86) | 0.38 | 0.738 |
| PD vs. CR | 2.15 (0.95–4.87) | 0.42 | 0.066 | 1.49 (0.68–3.27) | 0.40 | 0.320 |
| Unknown vs. CR | 1.17 (0.46–3.00) | 0.48 | 0.747 | 1.76 (0.75–4.09) | 0.43 | 0.193 |
| Months elapsed since fPBC | 0.98 (0.94–1.02) | 0.02 | 0.295 | 0.99 (0.97–1.01) | 0.01 | 0.381 |
Abbreviations: CI, confidence interval; CCI, Charlson Comorbidity Index; CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; fPBC, first‐line platinum‐based chemotherapy; HR, hazard ratio; PD, progressive disease; PR, partial response; SD, stable disease; sNPBC, subsequent non–platinum‐based chemotherapy; sPBC, subsequent platinum‐based chemotherapy.
Subgroup analysis was also performed for factors associated with achieving disease control with subsequent chemotherapy (Table 4). Within the sPBC group, achieving disease control with fPBC was associated with a higher likelihood of achieving disease control with sPBC; 55 of 87 patients (63.2%) who achieved disease control with fPBC also achieved disease control with sPBC, whereas only 8 of the 27 patients (29.6%) who did not achieve disease control with fPBC achieved disease control with sPBC (p = .002). The same association was not seen within the sNPBC group; 44 of 87 patients (50.5%) who achieved disease control with fPBC also achieved disease control with sNPBC, and 19 of 30 (38.8%) patients who did not achieve disease control with fPBC achieved disease control with sNPBC, a difference that did not reach statistical significance (p = .185). Achieving disease control with fPBC was also associated with longer time since fPBC for patients who received sPBC (median 6.0 vs. 2.9 months, p = .008), but not for patients who received sNPBC (median 2.2 vs. 2.6 months, p = .769). Finally, in the sPBC group, liver metastases were negatively associated with the likelihood of achieving disease control with sPBC; only 14 of 32 patients (43.8%) with liver metastases achieved disease control with sPBC compared with 56 of 88 patients (63.6%) without liver metastases (p = .038). Again, this association was not significant with patients who received sNPBC (36.2% vs. 49.0% respectively, p = .147).
Table 4.
Factors associated with achieving disease control with subsequent chemotherapy
| Variable | Disease control with subsequent chemotherapy (all patients) | Disease control with sPBC | Disease control with sNPBC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes (N = 135), a n (%) | No (N = 132),a n (%) | p value | Yes (N = 70), a n (%) | No (N = 52), a n (%) | p value | Yes (N = 65),a n (%) | No (N = 80),a n (%) | p value | |
| ECOG PS | .065 | .384 | .334 | ||||||
| 0 | 32 (29.4) | 20 (19.2) | 19 (32.8) | 10 (24.4) | 13 (25.5) | 10 (15.9) | |||
| 1 | 63 (57.8) | 57 (54.8) | 33 (56.9) | 22 (53.7) | 30 (58.8) | 35 (55.6) | |||
| ≥2 | 14 (12.8) | 27 (26.0) | 6 (10.3) | 9 (22.0) | 8 (15.7) | 18 (28.6) | |||
| CCI | .345 | .403 | .910 | ||||||
| 0 | 60 (44.4) | 61 (46.2) | 32 (45.7) | 25 (48.1) | 28 (43.1) | 36 (45.0) | |||
| 1 | 14 (10.4) | 6 (4.5) | 10 (14.3) | 3 (5.8) | 4 (6.2) | 3 (3.8) | |||
| 2 | 26 (19.3) | 27 (20.5) | 11 (15.7) | 7 (13.5) | 15 (23.1) | 20 (25.0) | |||
| ≥3 | 35 (25.9) | 38 (28.8) | 17 (24.3) | 17 (32.7) | 18 (27.7) | 21 (26.3) | |||
| Liver metastases | .016 | .038 | .147 | ||||||
| Yes | 31 (23.0) | 48 (36.9) | 14 (20.0) | 18 (36.0) | 17 (26.2) | 30 (37.5) | |||
| No | 104 (77.0) | 82 (63.1) | 56 (80.0) | 32 (64.0) | 48 (73.9) | 50 (62.5) | |||
| Number of fPBC cycles | .340 | .193 | .703 | ||||||
| 2 | 14 (10.4) | 11 (8.3) | 7 (10.0) | 5 (9.6) | 7 (10.8) | 6 (7.5) | |||
| 3–4 | 43 (31.9) | 31 (23.5) | 24 (34.3) | 11 (21.1) | 19 (29.2) | 20 (25.0) | |||
| 5–6 | 63 (46.7) | 70 (53.0) | 35 (50.0) | 28 (53.8) | 28 (43.1) | 42 (52.5) | |||
| ≥7 | 15 (11.1) | 20 (15.2) | 4 (5.7) | 8 (15.4) | 11 (16.9) | 12 (15.0) | |||
| Best response with fPBC | .002 | .002 | .185 | ||||||
| Disease control | 99 (78.6) | 75 (60.5) | 55 (87.3) | 32 (62.7) | 44 (69.8) | 43 (58.9) | |||
| Progressive disease | 27 (21.4) | 49 (39.5) | 8 (12.7) | 19 (37.3) | 19 (30.2) | 30 (41.1) | |||
| Months elapsed since fPBC, median (IQR) | 3.9 (1.0–8.9) | 2.8 (1.1–5.5) | .051 b | 6.0 (1.5–10.1) | 2.9 (1.1–5.4) | .008 b | 2.2 (0.9–6.6) | 2.6 (1.0–5.6) | .769 b |
Total number of patients for some variables may be less than “N” because of missing values. Due to rounding, some columns may not add up to 100.0%.
Mann‐Whitney U test used to compare nonparametric data.
Abbreviations: CCI, Charlson Comorbidity Index; ECOG PS, Eastern Cooperative Oncology Group performance status; fPBC, first‐line platinum‐based chemotherapy; IQR, interquartile range; sNPBC, subsequent non–platinum‐based chemotherapy; sPBC, subsequent platinum‐based chemotherapy.
Discussion
In this retrospective study of 296 patients with mUC treated with further chemotherapy after having received fPBC for metastatic disease, OS and investigator‐designated best response to subsequent chemotherapy was superior for patients who received sPBC compared with those who received sNPBC. These findings support the use of platinum rechallenge in patients who are fit enough to receive additional platinum‐based chemotherapy, particularly if disease response is clinically desirable because of symptom burden. That being said, median OS was on the order of several months and median PFS was on the order of a few months (corresponding to timing of first imaging reassessment) in both groups; even in the sPBC group, disease control rate was only 57.4%. Based on our subgroup analysis, achieving disease control with platinum rechallenge may be more likely in patients who achieved disease control with fPBC (as opposed to progressive disease) and was associated with a longer time between receipt of fPBC and initiation of subsequent chemotherapy, as those who achieved disease control with platinum rechallenge experienced a median of 6.0 months between first‐line and subsequent chemotherapy compared with 2.9 months in patients who did not have disease control with platinum rechallenge. These data support clinical intuition that patients who achieve a deeper or more durable response to platinum‐based chemotherapy in the first‐line setting may also respond better to platinum rechallenge in later lines of therapy. Achieving disease control with sPBC was less likely if liver metastases were present, consistent with prior studies reporting visceral metastases as a risk factor associated with lower likelihood of response to platinum‐based chemotherapy regimens [12, 13, 14, 15]. In contrast to the sPBC group, patients who received additional non–platinum‐based chemotherapy had a higher likelihood of progression with greater ECOG PS and CCI, both indicators of the underlying “fitness” of the patient. In this group, progression was also more likely with a higher number of fPBC cycles received, which could possibly be explained by higher resistance to cytotoxic chemotherapy drugs or greater cumulative toxicity associated with prolonged prior chemotherapy administration.
To our knowledge, this study is unique in analyzing outcomes with platinum rechallenge in mUC after receipt of platinum‐based combination chemotherapy for first‐line treatment in the metastatic setting. Prior studies have examined the use of additional platinum chemotherapy after prior receipt of platinum‐based chemotherapy in the nonmetastatic setting, given perioperatively for localized disease to patients who ultimately developed advanced urothelial carcinoma, and largely showed that outcomes were better with additional platinum chemotherapy if at least 1 year had passed since receipt of prior platinum in the localized disease setting. For example, in a study of 41 patients with urothelial carcinoma who had received perioperative cisplatin‐based chemotherapy and later cisplatin‐based first‐line chemotherapy for advanced disease, Necchi et al. found that time from previous perioperative chemotherapy of 78 weeks or longer was an independent prognostic factor associated with better survival [16]. Similarly, Locke et al. performed a study of 145 patients who had received previous perioperative cisplatin‐based chemotherapy and later chemotherapy for advanced disease and found that cisplatin‐based first‐line chemotherapy for advanced disease was associated with worse survival for patients when less than 12 months had passed since their prior perioperative chemotherapy [17]. In a study of 132 patients from the RISC database who had received perioperative or radiation cisplatin‐containing chemotherapy and later cytotoxic chemotherapy in the first‐line metastatic setting, Do et al. found that survival was superior with first‐line platinum‐based cytotoxic chemotherapy in patients when more than a year had passed since their chemotherapy for localized disease [18]. Together, these findings that longer time since prior platinum was associated with better outcomes after platinum rechallenge is concordant with our results. Not surprisingly, in our study, in which fPBC was received by patients for metastatic and not localized disease, median time since prior platinum was much shorter and on the order of a few months.
This retrospective and nonrandomized study has several limitations. First, baseline differences between patients almost certainly influenced whether clinicians recommended sPBC versus sNPBC, and so caution must be used when comparing the two groups; for example, baseline hemoglobin values were slightly higher and patients appeared to have a higher initial disease control rate and time since fPBC in the sPBC group. Although performance status and comorbidity burden as estimated by the CCI were similar, there may have been other prognostic differences between the two groups not measured by the study that influenced OS and other outcomes. We were unable to include hemoglobin and albumin levels in our multivariable model because of the number of missing values for these parameters in our data, and there may be other factors not explicitly accounted for in our model that may be relevant to clinicians and patients selecting their next treatment approach, such as treatment toxicity. Nevertheless, despite these confounders, our data do support giving additional platinum‐based chemotherapy over non–platinum‐based chemotherapy to fit patients and suggest that the superior activity of platinum agents in urothelial carcinoma supersedes potential concerns that re‐exposing patients to platinum may result in decreased effect the second time around. Next, designations of disease response or progression were investigator‐defined without formally mandated criteria such as RECIST 1.1 or standardized criteria for imaging frequency, affecting both recorded best response to therapy and date of progression; unfortunately, this reflects the variability in real‐world practice patterns. These study data were collected prior to the advent of FDA‐approved immuno‐oncology agents, erdafitinib, enfortumab vedotin, and sacituzumab govitecan‐hziy for mUC; therefore, although we believe our findings are still helpful to clinicians choosing between sPBC and sNPBC for patients with mUC, patients in the modern era will differ from our study population in that they will have likely received some of these newer agents prior to consideration of subsequent chemotherapy. Finally, although the RISC database includes patients treated at 27 international sites over a number of years, our sample size does limit the power of our analyses, particularly in our multivariable model.
Conclusion
Patients with mUC who have progressed after treatment with platinum‐based combination chemotherapy in the first‐line setting now may receive immuno‐oncology agents, erdafitinib, enfortumab vedotin, or sacituzumab govitecan‐hziy as later‐line therapy; however, patients ineligible for these therapies or who progress after receiving them may still be considered for subsequent chemotherapy. In our study, patients who were rechallenged with platinum‐based chemotherapy experienced longer survival and better disease response than those who received non–platinum‐based chemotherapy, supporting the use of platinum rechallenge in fit patients who are clinical candidates for this treatment. Achieving disease control with platinum rechallenge appears more likely when patients have achieved disease control with prior platinum, have experienced a longer time since prior platinum, and do not have liver metastases.
Author Contributions
Conception/design: Risa L. Wong, Lorin A. Ferris, Evan Y. Yu
Provision of study material or patients: Jorge D. Ramos, Simon J. Crabb, Cora N. Sternberg, Joaquim Bellmunt, Sylvain Ladoire, Ugo De Giorgi, Lauren C. Harshman, Ulka N. Vaishampayan, Andrea Necchi, Sandy Srinivas, Sumanta K. Pal, Guenter Niegisch, Tanya B. Dorff, Matthew D. Galsky, Evan Y. Yu
Collection and/or assembly of data: Lorin A. Ferris, Olivia A. Do, Sarah K. Holt, Jorge D. Ramos
Data analysis and interpretation: Risa L. Wong, Lorin A. Ferris, Sarah K. Holt, Evan Y. Yu
Manuscript writing: Risa L. Wong, Lorin A. Ferris, Olivia A. Do, Sarah K. Holt, Jorge D. Ramos, Simon J. Crabb, Cora N. Sternberg, Joaquim Bellmunt, Sylvain Ladoire, Ugo De Giorgi, Lauren C. Harshman, Ulka N. Vaishampayan, Andrea Necchi, Sandy Srinivas, Sumanta K. Pal, Guenter Niegisch, Tanya B. Dorff, Matthew D. Galsky, Evan Y. Yu
Final approval of manuscript: Risa L. Wong, Lorin A. Ferris, Olivia A. Do, Sarah K. Holt, Jorge D. Ramos, Simon J. Crabb, Cora N. Sternberg, Joaquim Bellmunt, Sylvain Ladoire, Ugo De Giorgi, Lauren C. Harshman, Ulka N. Vaishampayan, Andrea Necchi, Sandy Srinivas, Sumanta K. Pal, Guenter Niegisch, Tanya B. Dorff, Matthew D. Galsky, Evan Y. Yu
Disclosures
Jorge D. Ramos: Seagen (E); Simon J. Crabb: Astex, Clovis Oncology, Roche, AstraZeneca (RF), Astellas, Bayer, Janssen‐Cilag, Merck Sharp & Dohme, Roche, AstraZeneca (C/A); Ugo De Giorgi: AstraZeneca, Sanofi, Roche (RF), Astellas, Bayer, Merck Sharp & Dohme, Novartis, Pharmamar, Sanofi, Bristol‐Myers Squibb, Ipsen, Janssen‐Cilag, Pfizer(C/A), Roche, Bristol‐Myers Squibb, Ipsen, Janssen‐Cilag, Pfizer (non‐financial support); Lauren C. Harshman: Surface Oncology (E), Dendreon/Valeant, Janssen, Medivation/Astellas, SOTIO, Takeda, Bristol‐Myers Squibb, Corvus, Endocyte, Genentech, Merck, Pfizer, Bayer (RF), EMD Serono, Exelixis, Jounce, Michael J. Hennessy Associates, Novartis, Ology Medical Education, Bristol‐Myers Squibb, Corvus, Endocyte, Genentech, Merck, Pfizer, Bayer (C/A), Bayer, Genentech (other); Ulka N. Vaishampayan: Aveo, Bayer, Pfizer, Sanofi, Alkermes, Bristol‐Myers Squibb, Exelixis, Merck, (C/A), Alkermes, Bristol‐Myers Squibb, Exelixis, Merck (RF); Guenter Niegisch: Bristol‐Myers Squibb, EMD Serono, Medac GmbH, Roche, Sanofi‐Aventis (C/A); Tanya B. Dorff: Seagen (C/A); Evan Y. Yu: Daiichi‐Sankyo, Taiho, Blue Earth, Bayer, Dendreon, Merck, Seagen, Advanced Accelerator Applications (RF), Abbvie, Clovis, Exelixis, Janssen, Sanofi, Bayer, Merck, Advanced Accelerator Applications (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Table 1 List of contributing institutions in the RISC database
Acknowledgments
This study was funded by the Fred Hutchinson Cancer Research Center. J.D.R. is currently affiliated with Seagen Inc, Bothell, WA; L.C.H. is currently affiliated with Surface Oncology Inc, Cambridge, MA; U.N.V. is currently affiliated with the University of Michigan, Ann Arbor, MI; T.B.D. is currently affiliated with the City of Hope Comprehensive Cancer Center, Duarte, CA.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Bladder Cancer. Version 2.2021. Plymouth Meeting, PA: National Comprehensive Cancer Center, 2021. https://www.nccn.org/professionals/physician_gls/PDF/bladder.pdf. Accessed March 31, 2021.
- 2. Raggi D, Miceli R, Sonpavde G et al. Second‐line single‐agent versus doublet chemotherapy as salvage therapy for metastatic urothelial cancer: A systematic review and meta‐analysis. Ann Oncol 2016;27:49–61. [DOI] [PubMed] [Google Scholar]
- 3. Sonpavde G, Pond GR, Fougeray R et al. Time from prior chemotherapy enhances prognostic risk grouping in the second‐line setting of advanced urothelial carcinoma: A retrospective analysis of pooled, prospective phase 2 trials. Eur Urol 2013;63:717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sonpavde G, Pond GR, Rosenberg JE et al. Improved 5‐factor prognostic classification of patients receiving salvage systemic therapy for advanced urothelial carcinoma. J Urol 2016;195:277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim HS, Seo HK. Immune checkpoint inhibitors for urothelial carcinoma. Investig Clin Urol 2018;59:285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramos JD, Yu EY. Immuno‐oncology in urothelial carcinoma: Who or what will ultimately sit on the iron throne? Immunotherapy 2017;9:951–954. [DOI] [PubMed] [Google Scholar]
- 7. Bellmunt J, de Wit R, Vaughn DJ et al. Pembrolizumab as second‐line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Powles T, Park SH, Voog E et al. Avelumab maintenance therapy for advanced or metastastic urothelial carcinoma. N Engl J Med 2020;383:1218–1230. [DOI] [PubMed] [Google Scholar]
- 9. Loriot Y, Necchi A, Park SH et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med 2019;381:338–348. [DOI] [PubMed] [Google Scholar]
- 10. Rosenberg JE, O'Donnell PH, Balar AV et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti‐programmed death 1/programmed death ligand 1 therapy. J Clin Oncol 2019;37:2592–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tagawa ST, Balar AV, Petrylak DP et al. TROPHY‐U‐01: A phase II open‐label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum‐based chemotherapy and checkpoint inhibitors. J Clin Oncol 2021;39:2474–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Von Der Maase H, Sengelov L, Roberts JT et al. Long‐term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 2005;23:4602–4608. [DOI] [PubMed] [Google Scholar]
- 13. Bellmunt J, Choueiri TK, Fougeray R et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum‐containing regimens. J Clin Oncol 2010;28:1850–1855. [DOI] [PubMed] [Google Scholar]
- 14. Lin CC, Hsu CH, Huang CY et al. Prognostic factors for metastatic urothelial carcinoma treated with cisplatin and 5‐fluorouracil‐based regimens. Urology 2007;69:479–484. [DOI] [PubMed] [Google Scholar]
- 15. Bajorin DF, Dodd PM, Mazumdar M et al. Long‐term survival in metastatic transitional‐cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol 1999;17:3173–3181. [DOI] [PubMed] [Google Scholar]
- 16. Do OA, Ferris LA, Holt SK et al. Treatment of metastatic urothelial carcinoma after previous cisplatin‐based chemotherapy for localized disease: A retrospective comparison of different chemotherapy regimens. Clin Genitourin Cancer 2020;19:125–134. [DOI] [PubMed] [Google Scholar]
- 17. Locke JA, Pond GR, Sonpavde G et al. Cisplatin‐ versus non‐cisplatin‐based first‐line chemotherapy for advanced urothelial carcinoma previously treated with perioperative cisplatin. Clin Genitourin Cancer 2016;14:331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Necchi A, Pond GR, Giannatempo P et al. Cisplatin‐based first‐line therapy for advanced urothelial carcinoma after previous perioperative cisplatin‐based therapy. Clin Genitourin Cancer 2015;13:178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Table 1 List of contributing institutions in the RISC database


