Abstract
Background
Endometrial cancer (EC) is the most common gynecologic cancer in the U.S. The objective of this cohort study was to characterize the clinical and pathologic features that are associated with endometrial cancer–specific death for women cared for at a single National Cancer Institute–designated comprehensive cancer center.
Patients, Materials, and Methods
This is a retrospective cohort from 2014 to 2017 including all women who had a hysterectomy for EC. Charts were reviewed for clinical and pathologic data, focusing on survival outcomes.
Results
Seven hundred seventy‐one patients with EC underwent hysterectomy with 760 informative for outcomes. Seventy‐six (10%) deaths were related to their EC; 62 women died from recurrent EC. Nonendometrioid histology and advanced stage were predictors of recurrence and EC death. Among patients with endometrioid ECs, mismatch repair status was significantly associated with EC‐specific survival (relative risk = 4.8; 95% confidence interval, 2.3–10.3; p < .0001). Most patients with EC who recurred died of their disease 62/83 (74.7%). Nearly half of the patients that recurred (27/62) had no additional therapy at the time of recurrence. Overall survival was significantly longer for those women who had additional treatment at the time of recurrence; however, the improvement in overall survival with therapy at recurrence was largely attributable to effects in those women who were adjuvant therapy naïve.
Conclusion
Although there is benefit of treatment at the time of recurrence for treatment‐naïve women; only approximately half of patients were able to receive therapy. There is an urgent need for continued efforts for more effective EC therapy in both the front‐line and recurrent setting as well as early identification of cancer diagnosis and recurrence.
Implications for Practice
Approximately 10% of patients died of their endometrial cancer. Most deaths were from recurrent disease; however, almost 20% of endometrial cancer deaths were within 120 days of surgery. Although treatment at the time of recurrence improves overall survival, only approximately half of patients will receive therapy at the time of recurrence. Traditional prognostic features like histology and stage remain important to predict risk of recurrence, and newer biomarkers, such as mismatch repair status, may improve risk stratification and targeted therapy. There remains an urgent need for improved therapy and early detection of diagnosis and recurrence.
Keywords: Endometrial cancer, Mismatch repair defect, Immunotherapy, Cancer death, Mortality
Short abstract
This article evaluates the clinical and pathologic data from a contemporary cohort of women who had a hysterectomy for endometrial cancer, to better understand factors that are associated with recurrences and deaths.
Introduction
Endometrial cancer (EC) is the most common gynecologic cancer in the U.S., with more than 60,000 women diagnosed each year [1]. The majority of endometrial cancers present with abnormal uterine bleeding leading to endometrial sampling and diagnosis at an early stage. For the majority of patients with EC, surgery remains the cornerstone of management and usually includes total hysterectomy, removal of both tubes and ovaries as well as a lymph node assessment. Decision making for use of adjuvant therapy after surgery is based on a variety of factors, the most important of which are histology and stage [2]. Adjuvant therapy may consist of radiation, chemotherapy (carboplatin and paclitaxel), or a combination of modalities.
Most ECs are diagnosed at an early stage, and the vast majority of patients are cured with primary therapy. Despite the overall favorable outcomes for patients with EC, there remain more than 11,000 EC‐specific deaths annually, making EC the sixth leading cause of cancer death in women [1]. Stage as well as histology are the most important determinants of recurrence and death [3, 4]. Whereas early‐stage EC has been classically associated with recurrence at the vaginal cuff and will have the opportunity for curative salvage therapy, distant recurrences universally have a worse prognosis and thus treatment is for the most part palliative [5, 6].
Recognizing that recurrences from EC may be both local or distant, much of the recent focus on adjuvant therapy has been in evaluating the role for multimodality treatment including systemic therapy for distant recurrence reduction and radiation therapy for locoregional recurrence reduction [7, 8, 9]. Along with refining the roles of both chemotherapy and radiation in EC, there has been increasing interest in evaluation of predictive biomarkers for targeted therapy both in the primary and recurrent settings. Finally, there is an emerging interest in molecular classification of EC as a prognostic marker for patient counseling, improved risk stratification as well as reproducibility for adjuvant therapy decision making [10].
The Ohio State University Comprehensive Cancer Center (OSUCCC) James Cancer Hospital and Solove Research Institute is 1 of 51 National Cancer Institute (NCI)–designated Comprehensive Cancer Centers (CCCs) and 1 of the 31 National Comprehensive Cancer Network (NCCN) institutions. The objective of this study was to evaluate the clinical and pathologic data from a contemporary cohort to better understand the current state of EC recurrences and deaths from a single, high‐volume institution. In line with the NCI‐CCC designation, recommendations for treatment often follow the NCCN Clinical Practice Guidelines in Oncology for Uterine Neoplasms and reflect the impact of those recommendations on outcomes in this large cohort.
Patients, Materials, and Methods
This is an institutional review board approved retrospective cohort from OSUCCC from June 1, 2014, to May 31, 2017. All patients who underwent a hysterectomy at OSUCCC were included. Clinical and demographic data were abstracted from the medical records. All subjects’ medical records were last reviewed with a minimum follow‐up time of 3 years. A portion of this cohort were included in a previous publication [11]. Patients with EC who had their surgery at an outside institution or did not have a hysterectomy were excluded.
All hysterectomy specimens were tested for mismatch repair (MMR) proteins using immunohistochemistry (IHC) as part of routine clinical practice unless there was inadequate sample available. IHC results were then reflexed for MLH1 methylation in cases of loss of staining for MLH1 and/or PMS2 as previously described [12].
Clinical‐pathologic relationships were assessed using χ2, Fisher's exact test, and t‐test. EC overall survival was defined as time from surgery until death from EC. The Kaplan‐Meier product limit was used to estimate survival. The log‐rank test was used to test for differences in survival. Significance was set at a p value of .05. Patients who underwent surgery and died as a result of postoperative‐, therapy‐, or EC‐related causes less than or equal to 120 days after surgery were included as part of the short‐term death group. Median follow‐up was calculated using the reverse Kaplan‐Meier method [13].
Results
In total, there were 771 patients included in this cohort. The median follow‐up time was 3.4 years. The clinical and pathologic features of the entire cohort are presented in Table 1. As expected, the majority of the cohort was endometrioid histology, obese, and stage I at presentation. The clinical‐pathologic features of endometrioid EC (EEC) versus nonendometrioid EC (non‐EEC) are presented in Table 2. The non‐EEC group was older, less obese, less likely to be White, more advanced stage, and more likely to receive adjuvant therapy. There was no significant difference in MMR status between EEC and non‐EEC (23.7% vs. 19%). For the entire cohort, 22.6% of tumors demonstrated an MMR defect, of which the majority were explained by MLH1 methylation (data not shown).
Table 1.
Demographic and clinical‐pathologic features for women with endometrial cancer
| Clinicopathologic factor | n (%) |
|---|---|
| Age at surgery, yr | |
| Mean (SD) | 61.59 (10.92) |
| Median | 62 |
| <60 | 316 (41) |
| ≥60 | 455 (59) |
| BMI | |
| Mean (SD) | 38.02 (10.38) |
| Median | 36.9 |
| <25 | 68 (8.8) |
| ≥25–30 | 106 (13.7) |
| ≥30–35 | 157 (20.4) |
| ≥35 | 440 (57.1) |
| Race | |
| White | 725 (94) |
| Black | 30 (3.9) |
| Other | 16 (2.1) |
| Histology | |
| Endometrioid | 632 (82) |
| Serous | 55 (7.1) |
| Mixed | 40 (5.2) |
| Carcinosarcoma | 22 (2.9) |
| Undifferentiated/dedifferentiated | 12 (1.6) |
| Mucinous | 6 a (0.8) |
| Clear cell | 4 (0.5) |
| FIGO grade, endometrioid only | |
| 1 | 517 (81.8) |
| 2 | 78 (12.3) |
| 3 | 37 (5.9) |
| Stage | |
| I | 613 (79.5) |
| II | 22 (2.9) |
| III | 100 (13) |
| IV | 36 (4.7) |
| Adjuvant therapy received | |
| Yes | 229 (29.7) |
| No | 542 (70.3) |
| MMR status | |
| Intact | 588 (76.3) |
| Deficient | 174 (22.6) |
| Unknown | 9 (1.2) |
All mucinous histologies were grade 1.
Abbreviations: BMI, body mass index; FIGO, International Federation of Gynecology and Obstetrics; MMR, mismatch repair.
Table 2.
Demographic and clinical‐pathologic features for women EEC vs. non‐EEC
| Clinicopathologic factor | EEC, n = 632 | Non‐EEC, n = 139 | p value |
|---|---|---|---|
| Age at surgery | |||
| Mean (SD) | 60.56 (10.91) | 66.25 (9.76) | <.0001 |
| Median | 61 | 66 | |
| BMI | |||
| Mean (SD) | 39.1 (10.54) | 33.28 (8.1) | <.0001 |
| Median | 38.01 | 31.99 | |
| Race, n (%) | |||
| White | 600 (94.9) | 125 (89.9) | .005 |
| Black | 18 (2.8) | 12 (8.6) | |
| Other | 14 (2.2) | 2 (1.4) | |
| Stage, n (%) | |||
| I | 545 (86.2) | 68 (48.9) | <.0001 |
| II | 17 (2.7) | 5 (3.6) | |
| III | 57 (9) | 43 (30.9) | |
| IV | 13 (2) | 23 (16.5) | |
| Adjuvant therapy received a , n (%) | |||
| Yes | 117 (18.9) | 110 (80.9) | <.0001 |
| No | 503 (81.1) | 26 (19.1) | |
| ND | 1 | 3 | |
| MMR status, n (%) | |||
| Intact | 477 (76.3) | 111 (81) | NS |
| Deficient | 148 (23.7) | 26 (19) | |
| ND | 7 | 2 |
Other malignancy n = 11.
Abbreviations: BMI, body mass index; EEC, endometrioid endometrial cancer; MMR, mismatch repair; ND, no data; NS, not significant.
Who Lives, Who Dies…
The outcomes of this cohort are shown in Figure 1. Eleven patients (eight EEC and three non‐EEC) had inadequate follow‐up data and were excluded from outcomes analysis below. There were 15 (2%) non‐EC–related deaths during the follow‐up time. Fourteen of these patients did not have recurrence and died of either medical comorbidities (n = 6), acute causes (n = 5) or other malignancy (n = 3). There was one patient that had an EC recurrence but likely died of a secondary malignancy. At the time of data analysis 649/760 (85.4%) patients were with no evidence of disease (NED) and had no recurrence of their EC. There were a total of 83 (10.9%) patients with EC recurrence, most of which (74.6%) succumbed to their disease. Thirteen (15.7%) of the 83 patients are currently NED following therapy for their recurrence (median follow‐up, 4.6 years). Seven patients are currently alive with disease. Clinical and pathologic features of those patients who recurred or died are presented in Table 3.
Figure 1.
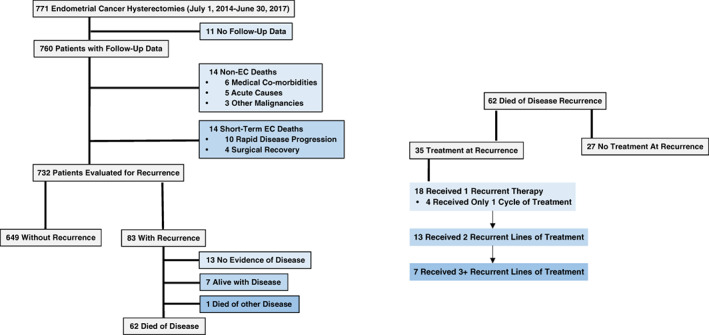
Endometrial cancer outcomes. Abbreviation: EC, endometrial cancer.
Table 3.
Clinical pathologic features for EC recurrences and deaths
| Clinicopathologic factor | DOD recurrence, n = 62 | NED after recurrence, n = 13 | AWD, n = 7 | Short‐term EC death, n = 14 |
|---|---|---|---|---|
| Age at surgery | ||||
| Mean (SD) | 66.95 (9.42) | 70.8 (8.28) | 59.86 (10.71) | 74.43 (8.75) |
| Median | 67.5 | 72 | 66 | 74 |
| <60 | 18 | 1 | 3 | 0 |
| ≥60 | 44 | 12 | 4 | 14 |
| Race | ||||
| White | 60 | 13 | 6 | 11 |
| Black | 2 | 0 | 1 | 3 |
| Other | 0 | 0 | 0 | 0 |
| Histology | ||||
| Endometrioid | 25 | 12 | 3 | 3 |
| Serous | 20 | 0 | 3 | 1 |
| Mixed | 5 | 0 | 1 | 2 |
| Carcinosarcoma | 10 | 1 | 0 | 2 |
| Undifferentiated/dedifferentiated | 1 | 0 | 0 | 3 |
| Mucinous | 0 | 0 | 0 | 1 |
| Clear cell | 1 | 0 | 0 | 0 |
| Stage | ||||
| I | 16 | 11 | 2 | 4 |
| II | 2 | 1 | 1 | 1 |
| III | 27 | 1 | 2 | 4 |
| IV | 17 | 0 | 2 | 5 |
| Adjuvant therapy received | ||||
| Yes | 51 | 4 | 6 | 5 |
| No | 11 | 9 | 1 | 9 |
| MMR status | ||||
| Intact | 42 | 7 | 4 | 10 |
| Deficient | 20 | 6 | 3 | 4 |
| Site of recurrence | ||||
| Vaginal | 3 | 8 | 1 | |
| Other | 59 | 5 | 6 |
Abbreviations: AWD, alive with disease; DOD, died of disease; EC, endometrial cancer; MMR, mismatch repair; NED, no evidence of disease.
Recurrences were more likely in non‐EEC histology 43/136 (31.6%) versus EEC histology 40/624 (6.4%); p < .0001. Of the 13 recurrences that are currently NED; 12 were EEC. Most of these were early‐stage and vaginal recurrences. There is only one recurrent patient with non‐EEC that is currently NED. This patient had carcinosarcoma with a retroperitoneal recurrence and was salvaged with chemoradiation.
Who Dies of Endometrial Cancer?
Seventy‐six patients (10%) in this cohort died from their EC or EC therapy. Fourteen (18.4%) of these patients had short‐term deaths directly related to their EC. For these 14 patients, 10 died directly related to EC (rapid disease progression) and 4 died from medical comorbidities exacerbated by surgery recovery. These patients with short‐term deaths were all over the age of 60, mostly non‐EEC and advanced stage (Table 3).
The remaining 62 patients died of their EC recurrence. When evaluating EC recurrence death rates by histologic subtype, women with EEC had the lowest death rate overall 25/624 (4%). The low EEC death rate is in stark contrast to carcinosarcoma and serous histologies (45.5% and 36.4% death rate by histology respectively). By absolute numbers, carcinosarcoma and serous carcinoma had more overall deaths combined compared with EEC histology although comprising only a fraction of total cases. Despite the low overall risk of death in the patients with EEC, EEC deaths accounted for 40% of the recurrent EC deaths.
Along with histology, stage was another predictor of death from EC recurrence in this cohort. Overall, only 16/608 (2.6%) of patients with stage I EC died of their disease across all histologies. There were eight EEC deaths (1.5%) in the stage I group, and of note, four had an MMR defect. As expected, advanced‐stage (stage III and IV) cases accounted for the majority of recurrent EC deaths. For stage III, 27/97 (27.8%) patients with ECs died of their disease recurrence. Of stage IV recurrences 17/33 (51.5%) died of their disease across all histologies.
MMR status was an important predictor of death in women with EEC. Among patients with EEC 15/146 (10.3%) of women with MMR deficient tumor died compared with 10/471 (2.1%) with intact MMR (relative risk = 4.8; 95% confidence interval, 2.3–10.3; p < .0001). Sixty percent of EEC deaths (15/25) were women with MMR defects, despite only 23.7% of all EEC demonstrating MMR deficiency. Unlike EEC, MMR status was not significantly associated with death in the non‐EEC group.
Of the 62 EC recurrence deaths, 51 (82.3%) had received adjuvant therapy as part of their initial treatment; 46 of whom received chemotherapy ± radiation. At the time of recurrence 35/62 (56.5%) received additional treatment. Table 4 presents the clinical‐pathologic features of those women who recurred and died of EC. Women that did not receive therapy at the time of recurrence were older at the time of initial surgery (mean, 70 vs. 65 years; p = .03). Between the two groups there was no significant difference in rate of EEC versus non‐EEC histology, stage, or adjuvant therapy use. For those patients with EC that were able to receive therapy EC‐specific survival was significantly longer 28.8 versus 17.5 months; p = .006 (Fig. 2A). However, when removing those patients that did not receive adjuvant therapy after their hysterectomy (n = 11) the EC‐specific survival was similar among the two groups 17.6 vs 19.8 months; p = .14. Despite EC‐specific survival observations, the time to recurrence for the two groups was similar; 11.4 versus 12.9 months (Fig. 2B).
Table 4.
Recurrent endometrial cancer deaths by therapy at time of recurrence
| Clinicopathologic factor | No additional treatment n = 27 | Treatment at time of recurrence n = 35 | p value |
|---|---|---|---|
| Age at surgery | |||
| Mean (SD) | 70.04 (8.074) | 64.57 (9.799) | .03 |
| Median | 71 | 66 | |
| Histology | |||
| Endometrioid | 11 | 14 | NS |
| Serous | 7 | 13 | |
| Mixed | 2 | 3 | |
| Carcinosarcoma | 5 | 5 | |
| Undifferentiated/dedifferentiated | 1 | 0 | |
| Mucinous | 0 | 0 | |
| Clear cell | 1 | 0 | |
| FIGO grade, endometrioid only | |||
| 1 | 4 | 9 | NS |
| 2 | 4 | 1 | |
| 3 | 3 | 4 | |
| Stage | NS | ||
| I | 7 | 9 | |
| II | 0 | 2 | |
| III | 17 | 10 | |
| IV | 3 | 14 | |
| Adjuvant therapy received | NS | ||
| Yes | 22 | 29 | |
| No | 5 | 6 | |
| MMR status | NS | ||
| Intact | 16 | 26 | |
| Deficient | 11 | 9 |
Abbreviations: FIGO, International Federation of Gynecology and Obstetrics; MMR, mismatch repair; NS, not significant.
Figure 2.
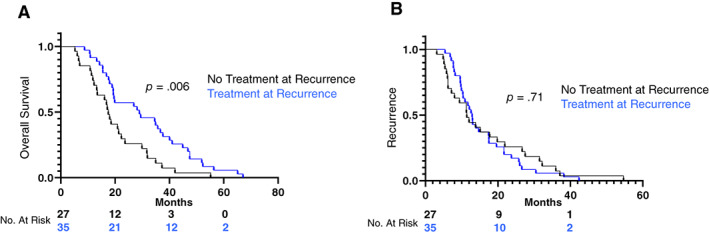
Overall survival by treatment at recurrence (A) and recurrence‐free survival by treatment at recurrence (B).
Discussion
Here we report a large contemporary EC cohort from an NCI‐designated CCC that represents patients who are recommended guidelines‐based therapy following their hysterectomy for EC. Our data support previous observations that histology and stage are important predictors of recurrence and EC death [1, 2, 3, 6]. Furthermore, our data continue to highlight the importance of tumor MMR status in prognosis and highlight the need for improved upfront therapy in EC, specifically the potential opportunities with immunotherapy in patients with MMR deficiency.
As expected, the patients with non‐EEC were more likely to die of their disease, most notably those women with serous carcinoma or carcinosarcoma. It is noteworthy that the patients with non‐EEC were almost never salvaged at the time of recurrence. One of the most important findings from this cohort study was that 72.6% of recurrent EC deaths received one or fewer lines of therapy at the time of recurrence. Taken together, the low rates of non‐EEC recurrence salvage and the relatively high likelihood of receiving limited therapy at the time of recurrence underscore the critical importance of maximizing adjuvant therapy in the upfront setting.
There have been several recent trials that have evaluated important questions for upfront management of EC including optimal chemotherapy regimen for advanced and recurrent EC patients (NCT00063999 ‐ GOG209), as well as chemotherapy for carcinosarcomas, which have often been excluded from other EC clinical trials (NCT00954174 ‐ GOG261) [14, 15]. Additionally, there have been three recent clinical trials evaluating multimodality therapy (PORTEC3, GOG258, and GOG249), all of which have failed to demonstrate significantly improved survival with multimodality therapy [7, 8, 9].
The most promising strategy in improving patient outcomes is likely biomarker driven therapy highlighted by the use of trastuzumab for advanced stage or recurrent uterine papillary serous carcinoma overexpressing human epidermal growth factor 2/neu [16]. In a prospective randomized phase II study, there was impressive improvement in progression‐free survival (8 vs. 12.6 months) with the addition of trastuzumab to carboplatin and paclitaxel leading to its inclusion in the NCCN guidelines [2]. The benefit of trastuzumab in progression‐free survival was most pronounced in the upfront setting (9.3 vs. 17.9 months). Although this study demonstrates the potential impact of biomarker directed therapy in the upfront setting, it only includes advanced or recurrent cases of one histologic subtype, highlighting the need for continued efforts in this area.
In addition to therapeutic trials, there has also been increasing interest in improving prognostication and reproducibility with molecular classification. Clinically accessible testing strategies have been proposed by several groups to improve reproducibility and prognostic counseling; these have been heavily guided by work from The Cancer Genome Atlas [17, 18, 19, 20]. As observed from our data, “traditional” methods of prognostic counseling, including histology and stage, perform well in identifying those women that may die of their EC, and although addition of molecular markers may further refine determination of adjuvant therapy and or counseling, the overall impact of additional molecular testing remains unclear. For the first time, the 2021 NCCN guidelines included a section highlighting molecular classification [2]. In fact, the NCCN guidelines state that “ancillary studies for POLE mutations, MMR/microsatellite instability (MSI) and aberrant p53 expression are encouraged to complement morphologic assessment of histologic tumor type.” Evaluation with these markers in this cohort will be separately reported.
Currently, MMR testing could provide the most meaningful information out of the molecular classes for clinicians treating EC. MMR or MSI testing was originally used in EC specifically for Lynch syndrome screening. Subsequent studies have identified MMR status as a potential important prognostic biomarker including our previous report from OSUCCC [11]. Our group, as well as others, has reported that patients with MMR deficiency, specifically MLH1 methylated tumors, have higher rates of recurrence [11, 21]. This remained consistent in this updated/expanded cohort. Furthermore, MMR status is an important predictive biomarker for use of immunotherapy, including the U.S. Food and Drug Administration approved programmed death‐one inhibitor pembrolizumab.
With the increasing evidence of poorer outcomes in MMR deficient EC there are several phase III clinical trials evaluating the use of immunotherapy in the upfront/adjuvant setting (NCT03981796, NCT03914612, NCT04214067). Previous reports have noted the excellent response rates in the recurrent setting, and thus, we suspect that immune therapy in the front‐line setting may be beneficial [22]. Our data establish the crucial importance of sequencing treatment as 11/20 recurrent EC deaths with MMR defects did not receive therapy at the time of recurrence. Thus, by waiting until recurrence to use these therapies, we may be missing the opportunity for benefit as these patients may not be well enough to proceed with additional treatment. For those patients with tumors that do not demonstrate an MMR deficiency, response rates to single agent pembrolizumab are much lower. Interestingly however, Makker et al. have demonstrated the impressive response rates with the combination of pembrolizumab and lenvatinib [23, 24]. This combination was not used in our cohort and certainly has become increasingly used in the recurrent setting. With many of the patients in this cohort receiving one line or less of therapy at recurrence, the importance of managing toxicities of regimens like pembrolizumab and lenvatinib will be an area of continued importance.
Another area to consider is increasing surveillance in patients with EC. In our cohort, surveillance methods were not standardized. The NCCN guidelines currently state that imaging should be guided by symptoms, risk assessment, and concern for recurrence. Although routine visits and clinical exams were routinely performed, imaging was used by provider preference. It is unclear if earlier detection of a recurrence would have led to improved outcomes. Earlier detection may allow for increased therapy utilization because many patients were too ill to receive therapy at recurrence; however, the improvements in survival we observed with treatment were limited to those that were treatment naïve, which was the minority of patients. Finally, aside from therapeutics, additional considerations to improve EC outcomes must address prevention and earlier diagnosis.
The major limitation of this study is that it is retrospective. There is also the possibility that some recurrences and/or deaths may not have been captured. We assumed that most recurrences would re‐present to OSUCCC if being followed locally, which is the case for the vast majority of patients treated at our center. We also did not include the small number of patients that had endometrial cancers that were treated without hysterectomy. Finally, the population treated at our tertiary care and comprehensive cancer center may not be representative of all populations with EC. The cohort does have strengths though in that it provides a large number of consecutive patients who would have received counseling and therapy based on NCCN guidelines after receiving their hysterectomy at an NCI‐CCC.
Conclusion
EC‐related death occurred in 10% of women, and although most deaths are from EC recurrence, 15.8% of EC deaths were ≤120 days from rapid disease‐related issues or inability to recover from surgery secondary to medical comorbidities. Most women who did experience recurrence died of their EC, and although there is benefit of treatment at the time of recurrence for treatment‐naïve women, only approximately half of patients were able to receive therapy. There is an urgent need for continued efforts for more effective EC therapy in both the front‐line and recurrent setting as well as early identification of cancer diagnosis and recurrence.
Author Contributions
Conception/design: Casey M. Cosgrove, Paul J. Goodfellow, David E. Cohn
Provision of study material or patients: Casey M. Cosgrove, Floor J. Backes, David O'Malley, Kristin L. Bixel, Adrian A. Suarez, Jeffrey M. Fowler, Larry J. Copeland, Paul J. Goodfellow, David E. Cohn
Collection and/or assembly of data: Casey M. Cosgrove, Floor J. Backes, David O'Malley, Kristin L. Bixel, Adrian A. Suarez, Jeffrey M. Fowler, Larry J. Copeland, Paul J. Goodfellow, David E. Cohn
Data analysis and interpretation: Casey M. Cosgrove, Floor J. Backes, David O'Malley, Kristin L. Bixel, Adrian A. Suarez, Jeffrey M. Fowler, Larry J. Copeland, Paul J. Goodfellow, David E. Cohn
Manuscript writing: Casey M. Cosgrove, Paul J. Goodfellow, David E. Cohn
Final approval of manuscript: Casey M. Cosgrove, Floor J. Backes, David O'Malley, Kristin L. Bixel, Adrian A. Suarez, Jeffrey M. Fowler, Larry J. Copeland, Paul J. Goodfellow, David E. Cohn
Disclosures
Floor J. Backes: Merck, Clovis, GlaxoSmithKline, Eisai, Genentech, Agenus, AstraZeneca (C/A), Merck, Clovis, Eisai, Immunogen (RF), Clovis (SAB); David O'Malley: AstraZeneca, Tesaro/GlaxoSmithKline, Immunogen, Ambry, Janssen/Johnson & Johnson, AbbVie, Regeneron, Amgen, Novocure, Genentech/Roche, GOG Foundation (C/A, SAB), AstraZeneca, Tesaro/GlaxoSmithKline, Immunogen, Ambry, Janssen/Johnson & Johnson, AbbVie, Regeneron, Amgen, Novcure, Genentech/Roche, GOG Foundation VentiRx, Array Biopharma, EMD Serono, Ergomed, Ajinomoto Inc., Ludwig Cancer Research, Stemcentrx, Inc., Cerulean Pharma, Bristol‐Myers Squibb, Serono Inc, TRACON Pharmaceuticals, Yale University, New Mexico Cancer Care Alliance, Iovance, INC Research, Inc., inVentiv Health Clinical, GenMab, Eisai, Agenus, Merck, SeaGen, Mersana, SDP Oncology (BBI), PRA Intl. (RF), Eisai, Agenus, Merck, SeaGen, Mersana, SDP Oncology (BBI), Myriad Genetics, Tarveda, Novartis, Rubis, Elevar Takeda, Toray, INXMED, Arquer Diagnostics, Roche Diagnostics, MSA Sorrento (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
This work was supported by the National Cancer Institute (P30 CA016058 supporting the Biostatistics shared resources at The Ohio State University Comprehensive Cancer Center) as well as a grant from the Kay Yow Cancer Fund.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 2. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Uterine Neoplasms Version 1.2021. Plymouth Meeting, PA: National Comprehensive Cancer Center, 2021. Available at https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf. Accessed July 4, 2021.
- 3. Lewin SN, Herzog TJ, Barrena Medel NI et al. Comparative performance of the 2009 International Federation of Gynecology and Obstetrics’ staging system for uterine corpus cancer. Obstet Gynecol 2010;116:1141–1149. [DOI] [PubMed] [Google Scholar]
- 4. Felix AS, Weissfeld JL, Stone RA et al. Factors associated with type I and type II endometrial cancer. Cancer Causes Control 2010;21:1851–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Creutzberg CL, van Putten WL, Koper PC et al. Survival after relapse in patients with endometrial cancer: Results from a randomized trial. Gynecol Oncol 2003;89:201. [DOI] [PubMed] [Google Scholar]
- 6. Surveillance, Epidemiology, and End Results Program . Cancer stat facts: Uterine cancer. National Cancer Institute Web site. Available at http://seer.cancer.gov/statfacts/html/corp.html. Accessed August 2,2021.
- 7. Randall ME, Filiaci V, McMeekin DS et al. Phase III trial: Adjuvant pelvic radiation therapy versus vaginal brachytherapy plus paclitaxel/carboplatin in high‐intermediate and high risk early stage endometrial cancer. J Clin Oncol 2019;37:1810–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Boer SM, Powell ME, Mileshkin L et al. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high risk endometrial cancer (PORTEC‐3): Patterns of recurrence and post hoc survival analysis of a randomized phase 3 trial. Lancet Oncol 2019;20:1273–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matei D, Filiaci V, Randall ME et al. Adjuvant chemotherapy plus radiation for locally advanced endometrial cancer. N Engl J Med 2019;380:2317–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arend RC, Jones BA, Martinez A et al. Endometrial cancer: Molecular markers and management of advanced stage disease. Gynecol Oncol 2018;150:569–580. [DOI] [PubMed] [Google Scholar]
- 11. Cosgrove CM, Cohn DE, Hampel H et al. Epigenetic silencing of MLH1 in endometrial cancer is associated with larger tumor volume, increased rate of lymph node positivity and reduced recurrence free survival. Gynecol Oncol 2017;146:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pearlman R, Frankel WL, Swanson B et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early onset colorectal cancer. JAMA Oncol 2017;3:464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schemper M, Smith TL. A note on quantifying follow‐up in studies of failure time. Control Clin Trials 1996;17:343–346. [DOI] [PubMed] [Google Scholar]
- 14. Miller DS, Filiaci VL, Mannel RS et al. Carboplatin and paclitaxel for advanced endometrial cancer: Final overall survival and adverse event analysis of a phase III trial (NRG Oncology/GOG209). J Clin Oncol 2020;38:3841–3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Powell MA, Filiaci VL, Hensley ML et al. A randomized phase 3 trial of paclitaxel (P) plus carboplatin versus paclitaxel plus ifosfamide in chemotherapy‐naïve patients with stage I‐IV, persistent or recurrent carcinosarcoma of the uterus or ovary: An NRG Oncology trial. J Clin Oncol 2019;37(suppl 15):5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fader AN, Roque DM, Siegel E et al. Randomized phase II trial of carboplatin‐paclitaxel versus carboplatin‐paclitaxel‐trastuzumab in uterine serous carcinomas that overexpress human epidermal growth factor receptor 2/neu. J Clin Oncol 2018;36:2044–2051. [DOI] [PubMed] [Google Scholar]
- 17. Kandoth C, Schultz N, Cherniack AD et al.; Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature 2013;497:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Talhouk A, McConechy MK, Leung S et al. A clinically applicable molecular‐based classification for endometrial cancers. Br J Cancer 2015;113:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stelloo E, Nout RA, Osse EM et al. Improved risk assessment by integrating molecular and clinicopathological factors in early‐stage endometrial cancer‐combined analysis of the PORTEC cohorts. Clin Cancer Res 2016;22:4215–4224. [DOI] [PubMed] [Google Scholar]
- 20. Cosgrove CM, Tritchler DL, Cohn DE et al. An NRG Oncology/GOG study of molecular classification for risk prediction in endometrioid endometrial cancer. Gynecol Oncol 2018:148;174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carr C, Son J, Yao M et al. Clinicopathologic characteristics and outcomes of endometrial cancer patients with mismatch repair deficiency in the era of universal Lynch syndrome screening. Gynecol Oncol 2020;159:712–720. [DOI] [PubMed] [Google Scholar]
- 22. Le DT, Uram JN, Wang H et al. PD‐1 blockade in tumors with mismatch‐repair deficiency. N Engl J Med 2015;372:2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Makker V, Rasco D, Vogelzang NJ et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: An interim analysis of a multicentre, open‐label, single‐arm, phase 2 trial. Lancet Oncol 2019;20:711–718. [DOI] [PubMed] [Google Scholar]
- 24. Makker V, Taylor MH, Aghajanian et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol 2020;38:2981–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]


