Abstract
Introduction
Systemic treatment for breast cancer in sub‐Saharan Africa (SSA) is cost effective. However, there are limited real‐world data on the translation of breast cancer treatment guidelines into clinical practice in SSA. The study aimed to identify provider factors associated with adherence to breast cancer guideline‐concordant care at Princess Marina Hospital (PMH) in Botswana.
Materials and Methods
The Consolidated Framework for Implementation Research was used to conduct one‐on‐one semistructured interviews with breast cancer providers at PMH. Purposive sampling was used, and sample size was determined by thematic saturation. Transcribed interviews were double‐coded and analyzed in NVivo using an integrated analysis approach.
Results
Forty‐one providers across eight departments were interviewed. There were variations in breast cancer guidelines used. Facilitators included a strong tension for change and a government‐funded comprehensive cancer care plan. Common provider and health system barriers were lack of available resources, staff shortages and poor skills retention, lack of relative priority compared with HIV/AIDS, suboptimal interdepartmental communication, and lack of a clearly defined national cancer control policy. Community‐level barriers included accessibility and associated transportation costs. Participants recommended the formal implementation of future guidelines that involved key stakeholders in all phases of planning and implementation, strategic government buy‐in, expansion of multidisciplinary tumor boards, leveraging nongovernmental and academic partnerships, and setting up monitoring, evaluation, and feedback processes.
Discussion
The study identified complex, multilevel factors affecting breast cancer treatment delivery in Botswana. These results and recommendations will inform strategies to overcome specific barriers in order to promote standardized breast cancer care delivery and improve survival outcomes.
Implications for Practice
To address the increasing cancer burden in low‐ and middle‐income countries, resource‐stratified guidelines have been developed by multiple international organizations to promote high‐quality guideline‐concordant care. However, these guidelines still require adaptation in order to be successfully translated into clinical practice in the countries where they are intended to be used. This study highlights a systematic approach of evaluating important contextual factors associated with the successful adaptation and implementation of resource‐stratified guidelines in sub‐Saharan Africa. In Botswana, there is a critical need for local stakeholder input to inform country‐level and facility‐level resources, cancer care accessibility, and community‐level barriers and facilitators.
Keywords: Resource‐stratified guidelines in sub‐Saharan Africa, Breast cancer, Implementation science methods, Barriers and facilitators, Consolidated framework for implementation research
Short abstract
To address an increasing mortality burden, resource‐stratified guidelines have been developed to ensure that breast cancer care is matched to specific resources in low‐ and middle‐income countries. This article identifies facilitators and barriers to the use of breast cancer guidelines by oncology providers at Princess Marina Hospital in Botswana using the Consolidated Framework for Implementation Research (CFIR).
Introduction
Breast cancer is the most common cause of cancer death among women globally [1]. Of the 1.7 million breast cancer cases diagnosed in 2012, slightly more than half were diagnosed in low‐ and middle‐ income countries (LMICs), with similar mortality trends due to breast cancer [2, 3]. Although the incidence of breast cancer is on the rise globally, the breast cancer mortality gap is more pronounced in sub‐Saharan Africa (SSA), which has the highest age‐standardized breast cancer mortality rate globally [4, 5, 6], where the mortality to incidence ratio is up to 0.57 compared with 0.15 in countries in North America [3, 7]. In contrast the breast cancer mortality rate in high‐income countries has continued to decline, with a 40% decline in the last three decades attributable to screening and evidence‐based highly effective treatment for nonmetastatic breast cancer [8, 9].
To address the increasing mortality burden, resource‐stratified guidelines have been developed to ensure that breast cancer care is matched to specific resources in LMICs [10, 11, 12]. The World Health Organization (WHO) Essential Medicines List has also been expanded to include more than 40 cancer medicines [13]. Subsequent mathematical modeling and cost‐effectiveness analysis by the WHO‐CHOICE (Choosing Interventions that are Cost‐Effective) team have shown that breast cancer systemic treatment delivery up to 95% coverage in SSA is cost effective [14, 15, 16]. However, real‐world data are lacking on factors associated with the delivery of guideline‐concordant care in the sub‐Saharan Africa.
Botswana is an upper–middle‐income country in SSA, and the government currently provides free access to all cancer care including essential cytotoxic chemotherapy and targeted medicines [17], as well as radiation therapy, to its citizens [18]. Despite these efforts, breast cancer still remains the one of the five leading causes of cancer mortality in the country, with an age‐standardized mortality rate of 7 per 100,000 [19, 20, 21]. As part of its breast cancer response, the Botswana Ministry of Health and Wellness (MoHW), together with its partners, developed national guidelines for breast cancer management, which were introduced at the Botswana National Cancer Symposium in 2016 but, for unknown reasons, not disseminated. There have been no studies evaluating the use of any breast cancer guidelines in clinical practice by oncology providers in the public health care sector in Botswana. This knowledge is critical for future implementation of national guidelines and the subsequent development and implementation of interventions to optimize breast cancer therapy delivery and improve clinical outcomes.
The objective of this qualitative study was to identify facilitators and barriers to the use of breast cancer guidelines by oncology providers at Princess Marina Hospital, which is the largest cancer referral center in the public sector in Botswana, using the Consolidated Framework for Implementation Research (CFIR) [22, 23]
Materials and Methods
The Intervention—Breast Cancer Guidelines
Botswana does not currently have any formally disseminated breast cancer national guidelines in clinical practice. Current treatment recommendations by providers in Botswana are largely in accordance with the National Comprehensive Cancer Network (NCCN) stratified guidelines for countries with “Enhanced Resources” as opposed to Basic or Core resources [24]. This classification is based on diagnostic and therapeutic modalities and essential cancer medicines available for cancer treatment within the country. For instance, the Enhanced Resources guidelines recommend human epidermal growth factor receptor 2 (HER2) testing and the use of trastuzumab (HER2‐targeted therapy), whereas that is not recommended in the Basic and Core guidelines, in which HER2 testing is not routinely available. The Enhanced Resources guidelines do not recommend pertuzumab, which is a newer HER2‐targeted therapy. This medicine is also not available on Botswana's national essential medicines list for cancer. Given the lack of formal national guidelines in current use, we therefore assessed the intervention as provider‐preferred breast cancer guidelines currently in use. Questions regarding processes were modified to understand preimplementation barriers and facilitators.
CFIR Framework
This study used the CFIR, which is a well‐established framework that has been used in the design and evaluation of contextual factors influencing the implementation success of health care initiatives [23]. Furthermore, the CFIR provides a framework for classifying multilevel factors as barriers or facilitators into five domains with associated constructs. The list of CFIR domains and potential factors related to breast cancer guideline adherence are described in Table 1 [25].
Table 1.
Factors that may affect breast cancer guideline‐concordant treatment implementation organized by Consolidated Framework for Implementation Research domains [23]
| Domain | Description |
|---|---|
| Intervention characteristics | Knowledge of respective breast cancer treatment guideline; the source of the guideline and strength of the evidence supporting the recommendations; the relative advantage of the identified guideline and how well adapted it is to the Botswana setting; complexity of the guideline, design quality and packaging as well as the cost of implementing all elements of the respective guideline. |
| Outer setting | Policies that support breast cancer guideline implementation or provide free cancer diagnostics, medicines, and other treatment modalities for breast cancer; staff contact with other cancer treatment facilities; patient perceptions, cultural pressures, barriers, and facilitators faced by patients and families while undergoing treatment for breast cancer. |
| Inner setting | Adequacy of oncology clinical staff; communication and networks between staff members across different specialties, hospital climate; tension of change and prioritization of breast cancer treatment and implementation of guideline‐concordant care; existence of goals and feedback to providers regarding therapy delivery; leadership within the hospital system for breast cancer care delivery; and availability and location of education materials. |
| Characteristics of individuals | Knowledge and skills of providers providing breast cancer care and communication with patients and families; self‐efficacy related to breast cancer management, counseling patients and families; attitudes and beliefs about breast cancer and care and about educating patients about breast cancer treatment. |
| Implementation process | Engagement of end‐users, that is cancer care providers at the hospital in the planning, designing, and introduction of breast cancer treatment guidelines; appointed internal health care system, team leaders and champions to increase implementation dissemination and adherence to guidelines; input from national policy advisors; collaboration with external partners; development of stepwise implementation plan that includes timeline, benchmarks, monitoring and evaluation and feedback to staff. |
Setting
In Botswana, cancer diagnosis and treatment in the public sector for Botswana citizens are fully funded by the national government. Essential cancer medicines are procured by the government through the Central Medical Stores, a semiautonomous agency responsible for the procurement and distribution of all medicines in the public sector. Additional resources for cancer care delivery such as pathology, radiology, palliative care, and surgical services are all funded by the government and available at PMH. Patients requiring radiation therapy are referred to the Gaborone Private Hospital (GPH), with public funds. We chose PMH as the study site, and it is referenced as the “Inner Setting” domain in this study.
Most of the cancer care in the public sector in Botswana is delivered by medical officers, who are the equivalent of hospitalists and internists. There is one fulltime oncologist in the public sector, four chemotherapy nurses, one surgical oncologist, and no oncology pharmacists at PMH. Oncology specialty training is currently not available in Botswana for all subspecialties, and length of training varies based on where providers obtain their specialty training. In Southern Africa, clinical oncology training, which combines radiation oncology and limited aspects of medical oncology, is 4 years long, and oncology nursing training is a minimum of 1 year.
Study Design and Participants
Purposive sampling was used to identify health care professionals involved in the direct management of cancer care at PMH. Consenting providers were enrolled to be part of the qualitative study, which involved one‐to‐one in‐depth interviews. Participants included personnel from oncology, surgery, pharmacy, radiology, pathology, and palliative care as well as biomedical engineers with a minimum work experience of 6 months. No patients or patient data were used in this study.
Interview Procedures
A semistructured interview guide was developed by modifying the CFIR to fit the health care and cultural context [25]. The following modifications were made. Intervention characteristics included national breast cancer guidelines and other guidelines used by the provider in the delivery of breast cancer care. Inner setting referred to departments at PMH that offered care to patients with a breast cancer diagnosis. Outer setting referred to patient needs and resources, most of which are best classified as community‐level resources in our study, for example, transportation and/or distance to treatment factors, external nongovernmental organizations (NGOs), policy‐making agencies, including MoHW, and private hospital networks. Characteristics of individuals were in reference to any provider at PMH delivering cancer care. Finally, because there was no formal process for dissemination and implementation of the guidelines, processes address both gaps in current systems and recommendations for formal implementation processes in the future. An initial pilot was conducted with three providers to inform the final interview guide. Subsequent revisions were made through an iterative process. All interviews were conducted in English or Setswana based on provider preference, by a bilingual English and Setswana‐fluent interviewer (L.M.).
Data Analysis
All interviews were transcribed in English, with any portions or phrases in Setswana translated and transcribed in English prior to analysis. Seventy‐five percent of the interviews were transcribed by at least two individuals to ensure the accuracy of the transcripts. Transcribed interviews were deidentified and imported into NVivo 12 software (QSR International, Melbourne, Australia) for coding and content analysis. Domains and constructs in the CFIR framework were used to develop the initial codebook. To maintain both inter‐ and intra‐coder reliability, each interview was coded independently by two team members (L.M. and Y.M.M.). Any discordant coding was discussed with an additional team member until an accepted agreement was reached on the final classification of the coded data. The aim for at least 80% agreement level for all codes was maintained. After all coding was completed, each code was summarized and examined for patterns.
Ethical Clearance
The University of Botswana, Health Research Development Committee—MoHW, and the University of Pennsylvania reviewed the study protocol and procedures and provided ethical clearance to proceed with the study. Verbal consent was obtained to allow for complete anonymity and allow respondents to be as honest as possible.
Results
Forty‐one interviews were conducted with health care providers in eight different departments and units, between April and September 2019. Interviews were stopped when thematic saturation was reached. Participant characteristics are summarized in Table 2.
Table 2.
Participant characteristics
| Participant characteristics | n (Total = 41) |
|---|---|
| Age, yr | |
| Median (IQR) | 36 (25–47) |
| Range | 21–58 |
| Sex | |
| Female | 21 |
| Male | 20 |
| Departments | |
| Oncology | 21 |
| Surgery | 4 |
| Pharmacy | 4 |
| Radiology | 3 |
| Pathology | 3 |
| Palliative care | 2 |
| Medical equipment management services | 2 |
| Interim home | 2 |
| Work experience, yr | |
| Median | 3.5 |
| Range | 0.5–23 |
| Interview Length, min | |
| Median | 38.40 |
| Range | 22–68 |
Abbreviation: IQR, interquartile range.
The next section presents factors associated with breast cancer guideline‐concordant care delivery organized by CFIR domains and constructs. The number of participants citing specific domains and constructs are highlighted in Figure 1. Constructs that were not referenced (n = 6) and ones that were referenced by only one provider were excluded from the analysis (n = 4). Descriptive summaries of relevant CFIR domains and constructs highlighted in our participant interviews are presented below, accompanied by illustrative quotations.
Figure 1.
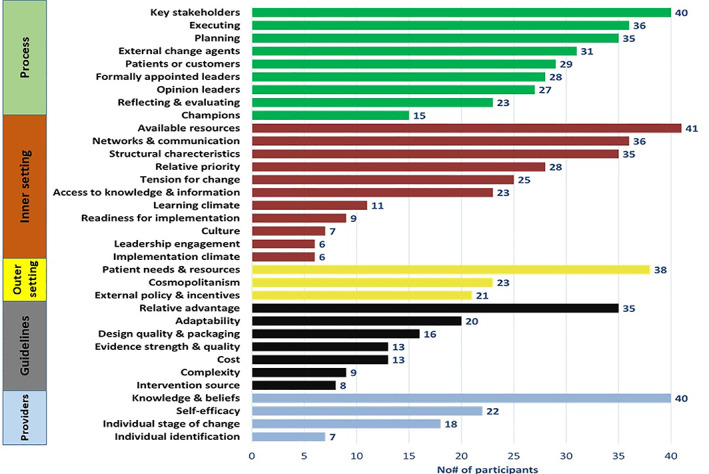
Frequency of factors associated with breast cancer treatment delivery.
Domain 1: Intervention Characteristics
The most common breast cancer treatment guidelines referenced by providers was the NCCN breast cancer guidelines (n = 13). Specifically, providers did not reference the NCCN harmonized guidelines for SSA and the questions were not designed to inquire about knowledge of these specific guidelines. Additional guidelines referenced but not used as commonly were the European Society for Medical Oncology breast cancer guidelines (n = 7), the Botswana palliative guidelines (n = 2), College of American Pathologists guidelines (n = 1), the National Health Policy for equipment (n = 1), and most frequently among nurses, the standard operating procedures for nurses in the public sector (n = 7). There was a variable and limited level of awareness of development of Botswana national breast cancer guidelines.
Facilitators included seeing the guidelines as useful in promoting standardized care, minimizing variations between providers, and improving the quality of care. Furthermore, guidelines were thought to help with budgeting and planning.
“[W]hen you look at… budgeting or planning, and you have guidelines it's a lot easier even …because when you have proper guidelines its easier for even those people at Central Medical Stores because they are the ones who bring medications for us, it would be easier for them because they would be able to prioritize” – [Participant PX03]
Providers preferred the accessibility of the NCCN guidelines and found flowcharts, phone apps, PDFs, and pocket‐size book designs most useful. Lack of adaptability to the Botswana context was seen as a barrier to guideline adherence:
“[T]he HIV guidelines are produced by Batswana [the name given to people from Botswana] for Batswana… they take into consideration the Botswana specificities… By using the NCCN sometimes we miss the reality in the field. …so if we could have also have [breast cancer] guidelines produced by Batswana it means the Ministry of Health is the one driving it …for the interest of Batswana people living in Botswana” – [Participant CX17]
Domain 2: Outer Setting
Barriers pertaining to community‐level resources were the most referenced construct highlighted by study participants (n = 38). These included long‐distance travel to access care and associated financial costs. The burden and fatigue of long‐distance travel were exacerbated by long delays at the clinic once a patient arrives, or appointment rescheduling in the event of drug stock‐out or toxicity. Other patient needs and resources identified were the need for psychosocial support, formal professional psychotherapy and counseling services at PMH and patient education on disease, treatment and prognosis.
“[I] believe one of the challenges they face is distance…most of our patients come from far places like Hukuntsi [~310 miles], Ghantsi [~450 miles], Kasane [~580 miles]… just to come and get this cancer treatment from PMH” – [Participant CX18]
Facilitators also included the current network with private hospitals (GPH, Bokamoso Private Hospital) for referral for services that are currently not available within the public sector, for example, radiation therapy. Majority of participants (n = 21) listed external policies and incentives as barriers to implementation. Few acknowledged that MoHW provision of comprehensive cancer care was a facilitator of implementation; however, barriers included lack of MoHW breast cancer or other cancer care policies. Other barriers included prolonged government processes for the procurement of equipment and medical products acquisitions.
“[F]rom a policy standpoint there is still a gap… [A]s a clinician on the ground yes …you would understand cancer is a problem and these are the issues for us to fix this situation, these are the measures we need to put in place… but then there hasn't been that escalation to Ministry… they [MoHw] will understand there is a problem, but they don't necessarily appreciate the challenges on the ground… I think the reason why there is no action is that there is no policy” – [Participant CX21]
Domain 3: Inner Setting
The most common barrier mentioned across all departments (n = 41) was lack of available resources, including drug stock‐out, lack of oncology space (isolation for neutropenic patients, male and female privacy), lack of equipment and servicing (mammograms), lack of pathology reagents, surgical theater space and time, and lack of decentralized oncology services in the country.
“[W]e have had patients who wait a long time expecting pathology results which take almost forever to come back”…– [Participant CX10]
The shortage of staff and specialty services was highlighted as a barrier by the majority of participants (n = 35). Staff shortages included nurses, doctors, oncologists, surgeons, pathologists, radiologists, and pharmacists. This led to providers being overburdened, which impeded therapy delivery. Providers, however, noted that communication within departments was facilitated by “WhatsApp” groups and standing meetings, through which notices and updates were shared. However, there was a general lack of interdepartmental communication between specialists working closely in breast cancer care.
“[R]ight now everybody… does what he knows, she does what she knows, and it is all silos and there is no cross‐communication of information which is sad”…[Participant CX35]
The majority of the study participants (n = 25) stressed a strong tension for change, the perception that the current situation was intolerable or needing change, in order to improve breast cancer therapy delivery for patients with breast cancer at PMH. Specific barriers raised include the lack of priority assigned to breast cancer compared with other disease areas like HIV/AIDS in Botswana (n = 28).
“[HIV AIDS] patients are given special treatment of care, but cancer patients are really struggling. Sometimes they can go for months being told that the drug you are supposed to take is finished/not available. So you tell me that if you were taking chemo which is supposed to be taken consecutively and then you are told for some months that it's not available, can we say treatment delivery is going well under such circumstances?”
“We really need to have something formalized, it's high time we do that, it will help in terms of saving money, and also making sure that there is no disparity in terms of patients getting treatment and the others not getting” ‐ [Participant CX28]
Domain 4: Characteristics of Individuals
Most of the physician participants were aware of some cancer‐specific guidelines; however, few nurses were not aware of any breast cancer guidelines. Participants expressed their confidence in implementing treatment care plans; however, lack of available resources and national‐specific guidelines and policies hampered their ability to deliver high‐quality care. Across different departments and specialties there was a lack of oncology training and specialists, which were mentioned as barriers to implementation.
“But because we are not specialized, like you don't have someone with specialty in HIV drugs, and somebody else with specialty in Oncology medications, because of that I don't have time to make sure that Oncology medications are available, and so forth. So there is nobody advocating for Oncology medications”. ‐[Participant PX01]
Domain 5: Process
Several suggestions were listed to facilitate processes for the future dissemination and implementation of Botswana national breast cancer guidelines. Providers recommended the formulation of an overarching national policy on breast cancer control and treatment that included formal dissemination of national breast cancer guidelines. It was, however, emphasized that this process should include all provider key stakeholders with strategic buy‐in and support from the Non‐Communicable Disease office at MoHW for successful implementation. Participants advised that guidelines should be brief and clear to everyone. Existing NGOs and academic partnerships were encouraged to be leveraged and streamlined to promote guideline‐concordant therapy delivery. Furthermore, key stakeholders recommended that the MoHW prioritize oncology skills retention in the public sector.
“[F]or guidelines to be developed, you need to really involve people who are treating the patient… because I always get worried when people develop guidelines for us but they have never contacted us… so if you develop a guideline there at Ministry or wherever but you are not the service provider… you are wasting your time… because what you are doing might not work.. and then we don't know what to do… so, I think the best thing [is to] engage the relevant people” – [Participant CX14]
Participants recommended that the implementation of future guidelines include training and established referral processes between oncology providers at PMH and providers in the peripheral hospitals. Providers recommended continuous medical education and training in oncology for providers treating patients with breast cancer.
“[Need for] basic training of medical officers …on suspecting cancers and how to expedite the diagnosis… and also, when the patients are on treatment, because they live in a community so they should have enough information on how to handle them [manage patients] when they live far from Princess Marina or Nyangabgwe [a cancer care referral center in Francistown, the second‐largest city in Botswana]” – [Participant CX17]
Currently, patients are seen in a multidisciplinary clinic with one surgeon, one medical oncologist, and occasionally a pathologist. Providers recommended a more diverse team to develop a weekly tumor board attended by more specialists involved in breast cancer therapy delivery, including more than one surgeon, radiologists, palliative care specialists, clinical psychologists, pathologists, and oncologists. Participants discouraged parallel implementation processes and recommended implementation of guidelines within the existing flow of care delivery using existing department managers, leaders, and champions within the various divisions and departments. Figure 2 shows the flow diagram for suggested processes for implementation.
Figure 2.
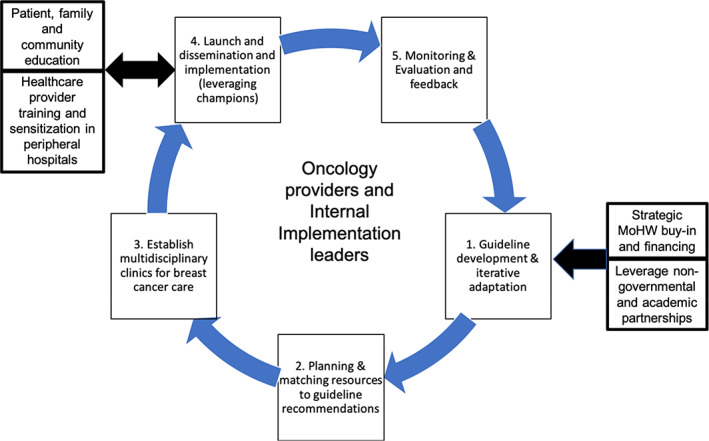
Participant suggested processes and workflow for future formal guideline implementation. Abbreviation: MoHW, Ministry of Health and Wellness.
Discussion
This is the first in‐depth study conducted in Botswana that examines the provider factors associated with breast cancer therapy delivery in the public sector using the CFIR approach. Although the CFIR has been used most commonly in formal postimplementation evaluation processes of HIV‐related interventions in sub‐Saharan Africa [26, 27, 28], in this study we adapted the CFIR in a qualitative study to examine provider barriers and facilitators to breast cancer therapy delivery, referencing any clinical guidelines that providers currently use in providing care to patients with cancer. In the processes domain we used questions to identify preimplementation factors that may promote effective dissemination processes and formal implementation of national breast cancer guidelines in Botswana in the near future.
The CFIR model enabled an appreciation of complex multilevel and multiple key stakeholder factors associated with guideline‐concordant breast cancer therapy delivery at Princess Marina Hospital. The study demonstrates significant heterogeneity of guidelines used by providers in the same hospital. These guidelines, predominantly developed to be used in high‐income settings, have been variably adapted by providers to fit the Botswana clinical context. The use of multiple guidelines by providers in the same facility may pose a barrier to the successful delivery of guideline‐concordant care [29, 30]. It is important to note that although the NCCN has developed harmonized guidelines for sub‐Saharan Africa [31], providers’ references to NCCN guideline use were to the North American version of the guidelines, which may indicate decreased awareness of the harmonized guidelines; however, our interview guide did not specifically probe for usage of the harmonized guidelines. Botswana has more government‐funded testing resources and systemic treatment options available than the standard of care recommendations in the harmonized guidelines for SSA. Subsequently, providers commonly reference those treatment guidelines developed for North America and Europe and adapt on a per case basis to patients being seen, either through multidisciplinary provider meetings or individual provider decision‐making. The lack of adaptability of these guidelines to clinical care in Botswana was listed as a barrier to implementation. There was uniformity in outlining advantages to guideline therapy delivery including transparency in clinical decision‐making, facilitating care delivery, and promoting health outcomes, similar to what has been achieved with HIV/AIDS.
Providers highlighted the lack of resources and shortages of staff across all departments as a significant barrier to therapy delivery, which is consistent with reports of inadequate resources and significantly higher clinical care workload of the oncology workforce in SSA compared with other countries [32, 33, 34]. Interactions between prioritization of breast cancer in relation to other diseases like HIV/AIDS by external policy agencies were thought to influence the response to these barriers in breast cancer care delivery. Several providers highlighted that breast cancer is not prioritized on the national level like HIV/AIDS, with a majority of providers not being aware of any national policies for national breast cancer control and management. The data, however, suggest that there might be an opportunity to leverage the health infrastructure established for HIV care in addressing noncommunicable diseases, including cancer [35].
The majority of providers highlighted community‐level factors that significantly impede the delivery of guideline‐concordant therapy, some of which were intercorrelated with inner setting barriers. These barriers included long‐distance travel to care and associated travel fare, foregone income, lack of psychosocial support and patient education and counseling. These results are similar to the results of the South African Breast Cancer and HIV Outcomes, which showed that systemic therapy and radiotherapy guideline‐concordant care for breast cancer was less common for women living >20 km from the treatment facility [36]. Furthermore, the lack of adequate patient education and shared decision‐making was thought to further exacerbate the problem. There was the added futility on the part of providers that there was no formal follow‐up system for following up on patients who become lost to follow‐up or miss their appointments.
Several participants highlighted a tension for change. They felt strongly that the current situation was not tenable and needed to change in order to improve breast cancer outcomes. They also emphasized the need to increase the momentum to implement national breast cancer guidelines [37]. Participants highlighted specific facilitators including the provision of comprehensive cancer care free of charge for all Botswana citizens. There was a mixed response about self‐efficacy, defined as the providers’ belief in their own capabilities to execute courses of action to achieve implementation goals [25], but few providers who expressed low self‐efficacy felt that this was correlated with health system limitations and inadequate access to knowledge. Additionally, cancer‐specific knowledge was thought to be suboptimal among providers in nonspecialist peripheral hospitals. Similar gaps were reported in cancer‐specific knowledge among general practitioners at District Hospitals in Rwanda [38].
To address knowledge gaps in breast cancer care among the multidisciplinary team, especially among general nurses on the wards, providers recommended oncology specialty training and continuous medical education. There have been prior initiatives to establish North‐South collaborations to facilitate oncology training programs for providers taking care of patients with cancer in Botswana. These are in different stages of curriculum development and pilot implementation, and there are currently no formalized processes for disseminating breast cancer knowledge for the interdisciplinary team that provides cancer care and for general practitioners in peripheral hospitals where most patients usually first present with symptoms. This research highlights that this remains an important challenge that needs to be addressed, especially given the context that the majority of providers who deliver cancer care are nonspecialists. There are currently come requirements for continuing professional development for some health professionals including nurses and laboratory personnel in Botswana. Potential initiatives could leverage some of the knowledge gaps and preferred teaching modalities highlighted by participants, such as multidisciplinary tumor boards and departmental meetings, to strengthen breast cancer education. Furthermore, basic breast cancer education should be integrated into the existing nursing and health provider curriculum and requirements for continuing professional development for health professionals.
Participants also provided very detailed assessment of other current gaps and suggested multistep processes within the CFIR “Processes” domain that will facilitate the dissemination of national breast cancer guidelines and effective implementation into routine clinical care.
The results of this study must be considered in light of several limitations. The participants interviewed were all care providers at PMH in Gaborone, and so these findings might not be applicable to the other cancer facilities in more remote areas such as Francistown. However, the vast majority of patients in the public sector are treated at PMH. Another limitation of the study is that although the majority of providers highlighted several patient barriers, this would be best informed by eliciting these perspectives directly from patients. Although the study captures barriers and facilitators, the findings are not directly mapped onto specific core elements of the guidelines. Given the heterogeneity of guideline usage, the study was focused more on multilevel systems and processes rather than detailed elements of the guidelines used themselves. Although this should be addressed in subsequent studies following formal Botswana‐specific breast cancer guidelines dissemination and implementation.
In spite of these limitations, the study had several strengths; by using the CFIR framework we were able to identify key elements to guideline‐concordant care implementation that are pertinent to current clinical practice in Botswana. The process allows for transparency and replication in other clinical settings that are in various stages of preimplementation or midimplementation of resource‐stratified breast cancer guidelines via NCCN or breast cancer global health initiative guidelines [10, 39].
Conclusion
As several countries in low‐resource settings begin to develop national cancer control plans [40], this study highlights the importance of evaluating implementation factors to ensure that high‐quality policies and national control plans translate into high‐quality therapy delivery and improved patient outcomes. Ultimately, by identifying some of these barriers in our study, targeted interventions can be developed to minimize random variability and optimize treatment, so targeted outcomes can be achieved.
Author Contributions
Conception/design: Tlotlo B. Ralefala, Anthony A. Oyekunle, Lawrence N. Shulman, Yehoda M. Martei
Provision of study material or patients: Tlotlo B. Ralefala, Lebogang Mokokwe, Yehoda M. Martei
Collection and/or assembly of data: Lebogang Mokokwe, Swetha Jammalamadugu, Dumelang Legobere, Warona S. Motlhwa, Yehoda M. Martei
Data analysis and interpretation: Tlotlo B. Ralefala, Lebogang Mokokwe, Anthony A. Oyekunle, Surbhi Grover, Frances K. Barg, Lawrence N. Shulman, Yehoda M. Martei
Manuscript writing: Tlotlo B. Ralefala, Lebogang Mokokwe, Swetha Jammalamadugu, Dumelang Legobere, Warona S. Motlhwa, Anthony A. Oyekunle, Surbhi Grover, Frances K. Barg, Lawrence N. Shulman, Yehoda M. Martei
Final approval of manuscript: Tlotlo B. Ralefala, Lebogang Mokokwe, Swetha Jammalamadugu, Dumelang Legobere, Warona S. Motlhwa, Anthony A. Oyekunle, Surbhi Grover, Frances K. Barg, Lawrence N. Shulman, Yehoda M. Martei
Disclosures
The authors indicated no financial relationships.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians 2021;71(3):209–249. 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2. Cumber SN, Nchanji KN, Tsoka‐Gwegweni JM. Breast cancer among women in sub‐Saharan Africa: Prevalence and a situational analysis. South African J Gynaecol Oncol 2017;9:35–37. [Google Scholar]
- 3. GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. International Agency for Research on Cancer Web site. Available at http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed May 7, 2018.
- 4. Azubuike SO, Muirhead C, Hayes L et al. Rising global burden of breast cancer: The case of sub‐Saharan Africa (with emphasis on Nigeria) and implications for regional development: A review. World J Surg Oncol 2018;16:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kantelhardt EJ, Zerche P, Mathewos A et al. Breast cancer survival in Ethiopia: A cohort study of 1,070 women. Int J Cancer 2014;135:702–709. [DOI] [PubMed] [Google Scholar]
- 6. Makanjuola SBL, Popoola AO, Oludara MA. Radiation therapy: A major factor in the five‐year survival analysis of women with breast cancer in Lagos, Nigeria. Radiother Oncol 2014;111:321–326. [DOI] [PubMed] [Google Scholar]
- 7. Choi E, Lee S, Nhung BC et al. Cancer mortality‐to‐incidence ratio as an indicator of cancer management outcomes in Organization for Economic Cooperation and Development countries. Epidemiol Health 2017;39:e2017006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berry DA, Cronin KA, Plevritis SK et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 2005;353:1784–1792. [DOI] [PubMed] [Google Scholar]
- 9. Plevritis SK, Munoz D, Kurian AW et al. Association of screening and treatment with breast cancer mortality by molecular subtype in US women, 2000–2012. JAMA. 2018;319:154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carlson RW, Scavone JL, Koh WJ et al. NCCN framework for resource stratification: A framework for providing and improving global quality oncology care. J Natl Compr Canc Netw 2016;14:961–969. [DOI] [PubMed] [Google Scholar]
- 11. Birnbaum JK, Duggan C, Anderson BO et al. Early detection and treatment strategies for breast cancer in low‐income and upper middle‐income countries: A modelling study. Lancet Glob Heal 2018;6:e885–e893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anderson BO, Yip CH, Smith RA et al. Guideline implementation for breast healthcare in low‐income and middle‐income countries. Cancer 2008;113:2221–2243. [DOI] [PubMed] [Google Scholar]
- 13. Shulman LN, Wagner CM, Barr R et al. Proposing essential medicines to treat cancer: Methodologies, processes, and outcomes. J Clin Oncol 2016;34:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ralaidovy AH, Gopalappa C, Ilbawi A et al. Cost‐effective interventions for breast cancer, cervical cancer, and colorectal cancer: New results from WHO‐CHOICE. Cost Eff Resour Alloc 2018;16:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zelle SG, Nyarko KM, Bosu WK et al. Costs, effects and cost‐effectiveness of breast cancer control in Ghana. Trop Med Int Heal 2012;17:1031–1043. [DOI] [PubMed] [Google Scholar]
- 16. Ginsberg GM, Lauer JA, Zelle S et al. Cost effectiveness of strategies to combat breast, cervical, and colorectal cancer in sub‐Saharan Africa and South East Asia: Mathematical modelling study. BMJ 2012;344:e614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martei YM, Chiyapo S, Grover S et al. Availability of WHO essential medicines for cancer treatment in Botswana. J Glob Oncol 2018;4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Suneja G, Ramogola‐Masire D, Medhin HG et al. Cancer in Botswana: Resources and opportunities. Lancet Oncol 2013;14:e290–e291. [DOI] [PubMed] [Google Scholar]
- 19. Bhatia RK, Narasimhamurthy M, Martei YM et al. Report of clinico‐pathological features of breast cancer in HIV‐infected and uninfected women in Botswana. Infect Agent Cancer 2019;14:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Age Standardized (World) Incidence Rates, Breast, All Ages International Agency for Research on Cancer Web site, 2018. Available at https://gco.iarc.fr/today/data/factsheets/populations/72-botswana-fact-sheets.pdf. Accessed February 7, 2019.
- 21. Sadigh KS, Hodgeman RM, Neo T et al. HIV is associated with decreased breast cancer survival: A prospective cohort study. Presented at Conference on Retroviruses and Opportunistic Infection; March 4–7, 2019; Seattle, WA. Available at http://www.croiconference.org/sessions/hiv‐associated‐decreased‐breast‐cancer‐survival‐prospective‐cohort‐study. Accessed February 4, 2020.
- 22. Keith RE, Crosson JC, O'Malley AS et al. Using the Consolidated Framework for Implementation Research (CFIR) to produce actionable findings: A rapid‐cycle evaluation approach to improving implementation. Implement Sci 2017;12:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Damschroder LJ, Aron DC, Keith RE et al. Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implement Sci 2009;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.NCCN Framework for Resource Stratification of NCCN Guidelines (NCCN Framework™). https://www.nccn.org/guidelines/nccn‐framework‐for‐resource‐stratification‐of‐nccn‐guidelines Accessed August 20, 2021.
- 25.Qualitative data. The Consolidated Framework for Implementation Research Web site. Available at https://cfirguide.org/evaluation‐design/qualitative‐data/. Accessed February 12, 2020.
- 26. Gimbel S, Rustagi AS, Robinson J et al. Evaluation of a systems analysis and improvement approach to optimize prevention of mother‐to‐child transmission of HIV using the consolidated framework for implementation research. J Acquir Immune Defic Syndr 2016;72(suppl 2):S108–S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Naidoo N, Zuma N, Khosa NS et al. Qualitative assessment of facilitators and barriers to HIV programme implementation by community health workers in Mopani district, South Africa. PLoS One 2018;13:e0203081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Means AR, Kemp CG, Gwayi‐Chore MC et al. Evaluating and optimizing the consolidated framework for implementation research (CFIR) for use in low‐ and middle‐income countries: A systematic review. Implement Sci 2020;15:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Niño de Guzmán E, Song Y, Alonso‐Coello P et al. Healthcare providers’ adherence to breast cancer guidelines in Europe: A systematic literature review. Breast Cancer Res Treat 2020;181:499–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Foster JA, Abdolrasulnia M, Doroodchi H et al. Practice patterns and guideline adherence of medical oncologists in managing patients with early breast cancer. J Natl Compr Canc Netw 2009;7:697–706. [DOI] [PubMed] [Google Scholar]
- 31. NCCN Harmonized Guidelines (Sub‐Saharan Africa): Breast Cancer. Version 1. 2019. Plymouth Meeting, PA: National Comprehensive Cancer Center, 2019. https://www.nccn.org/professionals/physician_gls/pdf/breast_harmonized‐africa.pdf. Accessed July 13, 2020. [Google Scholar]
- 32. Martei YM, Pace LE, Brock JE et al. Breast cancer in low‐ and middle‐income countries. Clin Lab Med 2018;38:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stefan DC. Cancer care in Africa: An overview of resources. J Glob Oncol 2015;1:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vanderpuye V, Hammad N, Martei Y et al. Cancer care workforce in Africa: Perspectives from a global survey. Infect Agent Cancer 2019;14:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vorkoper S, Kupfer LE, Anand N et al. Building on the HIV chronic care platform to address noncommunicable diseases in sub‐Saharan Africa: A research agenda. AIDS 2018;32(suppl 1):S107–S113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. O'Neil DS, Chen WC, Ayeni O et al. Breast cancer care quality in South Africa's public health system: An evaluation using American Society of Clinical Oncology/National Quality Forum measures. J Glob Oncol 2019;5:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liang S, Kegler MC, Cotter M et al. Integrating evidence‐based practices for increasing cancer screenings in safety net health systems: A multiple case study using the Consolidated Framework for Implementation Research. Implement Sci 2016;11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martin AN, Kaneza KM, Kulkarni A et al. Cancer control at the district hospital level in sub‐Saharan Africa: An educational and resource needs assessment of general practitioners. J Glob Oncol 2019;5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Breast Health Global Initiative (BHGI). Fred Hutch Web site. Available at https://www.fredhutch.org/en/research/divisions/public-health-sciences-division/research/epidemiology/breast-health-global-initiative.html. Accessed July 13, 2020.
- 40. Romero Y, Trapani D, Johnson S et al. National cancer control plans: A global analysis. Lancet Oncol 2018;19:e546–e555. [DOI] [PubMed] [Google Scholar]


