Abstract
Background
The development of immune checkpoint inhibitors (ICIs) represents a paradigm shift in the treatment of cancers. Despite showing remarkable efficacy, these agents can be associated with life‐threatening immune‐related adverse events. In recent years, several cases of myocarditis with myositis and/or myasthenia gravis overlap syndrome (IM3OS) have been reported. However, given the rarity, the clinical features and outcomes of these cases remain poorly understood. We, therefore, attempted to systematically review and summarize all cases of IM3OS reported in the literature.
Materials and Methods
Studies reporting IM3OS were identified in Embase and MEDLINE. Only case reports and case series published in journals or presented at conferences were included. We conducted a systematic review according to the PRISMA Harms guidelines.
Results
A total of 60 cases were eligible. The patients’ median age was 71 years, and the majority (67%) were males; melanoma was the most common indication for ICIs (38%). The most‐reported symptoms were fatigue (80%) and muscle weakness (78%). The median number of doses to the development of IM3OS was one. The average creatine kinase level was 9,645 IU/L. Cardiac arrhythmias occurred in 67% of patients, and 18% had depressed ejection fraction. Initial treatment consisted of immunosuppression with high‐dose steroids and supportive therapies. Sixty percent of the patients died in hospital because of acute complications.
Conclusion
IM3OS can be associated with significant mortality and morbidity. Prospective studies are needed to understand the optimal approach to diagnose and manage these patients and to develop biomarkers to predict the occurrence and severity of this rare but serious condition.
Implications for Practice
Clinicians should suspect coexisting myositis and/or myasthenia gravis in all patients with immune checkpoint inhibitor‐induced myocarditis, given their propensity to occur together. Early recognition and prompt treatment with the help of a multidisciplinary team might help improve the outcomes of this life‐threatening condition.
Keywords: Immune checkpoint inhibitors, Myositis, Myasthenia gravis, Myocarditis, Immune‐related adverse events, Immune checkpoint inhibitor, Induced myocarditis, Overlap syndrome
Short abstract
This review summarizes the reported cases of immune checkpoint inhibitor–induced myocarditis with myositis and/or myasthenia gravis overlap syndrome (IM3OS).
Introduction
Immune checkpoint inhibitors (ICIs) represent a paradigm shift in cancer therapy and have revolutionized cancer care. ICIs targeting cytotoxic T‐lymphocyte‐associated antigen 4, programmed cell death protein 1 (PD‐1) on T cells, or its ligand on tumor cells have been U.S. Food and Drug Administration approved for various solid tumors and hematologic malignancies. Despite showing impressive antitumor efficacy, these agents can, however, be associated with wide‐ranging life‐threatening inflammatory and autoimmune immune‐related adverse events (irAEs). ICI‐induced myocarditis is one such rare yet severe adverse event and has been reported in <1% of irAEs in clinical trials and institutional series [1].
Although most ICI‐induced myocarditis cases tend to occur by themselves, several cases of overlap syndrome with myositis and/or myasthenia gravis have been described, primarily as case reports or small case series. Some researchers have reported the incidence of concurrent myositis and myasthenia gravis in up to 30%–40% and 10% patients with immune‐related myocarditis, respectively [2]. However, given the rarity of concurrent myositis and myasthenia gravis, clinical presentation, treatment, and outcomes of these patients remain less clear. We, therefore, attempted to systematically review and summarize all cases of ICI‐induced myocarditis with myositis and/or myasthenia gravis overlap syndrome (IM3OS) reported in the literature.
Materials and Methods
Protocol and Registration
The protocol was registered in PROSPERO International Prospective Register of Systematic Reviews (CRD42021242304).
Study Design
We conducted a systematic review according to the PRISMA Harms guidelines (supplemental online Table 1) [3].
Search Strategy
We searched MEDLINE and Embase on July 14, 2021, to identify studies reporting concurrent myocarditis with myositis and/or myasthenia gravis associated with ICIs. We limited our search to studies conducted in humans and published after the first ICI's approval in the U.S. in 2011. We did not apply any language restrictions. We searched the reference section of included articles for additional reports. Our search strategy is described in supplemental online Table 2.
Eligibility Criteria
We included case reports and case series published in journals or presented in conferences. We excluded pharmacokinetic/pharmacodynamic studies and randomized controlled trials. We also excluded case series without detailed patient‐level data. The included studies reported patients who received an approved ICI (ipilimumab, nivolumab, pembrolizumab, cemiplimab, atezolizumab, avelumab, and durvalumab) either as a single‐agent or in combination with other ICIs and developed concurrent ICI‐induced myocarditis with myositis and/or myasthenia gravis.
Study Selection
Two reviewers (A.K. and R.P.) initially screened the titles and abstracts for eligibility using Microsoft Excel (Microsoft, Redmond, WA). Full texts of citations deemed eligible were retrieved and further assessed for eligibility. All disagreements were resolved through consensus. We achieved complete consensus before inclusion.
Data Extraction
Study and patient characteristics, description of the ICI‐induced myocarditis with myositis and/or myasthenia gravis, work‐up, and outcomes were extracted by one author (A.K.) and checked for accuracy by a second author (R.P.).
Data Synthesis
We report aggregated data from case reports and case series. No inferential or predictive statistics were computed given limitations in sample size.
Results
After a review of 1,615 citations in MEDLINE and Embase, we included 36 case reports and 6 case series; this resulted in a total of 60 cases of IM3OS (Fig. 1).
Figure 1.
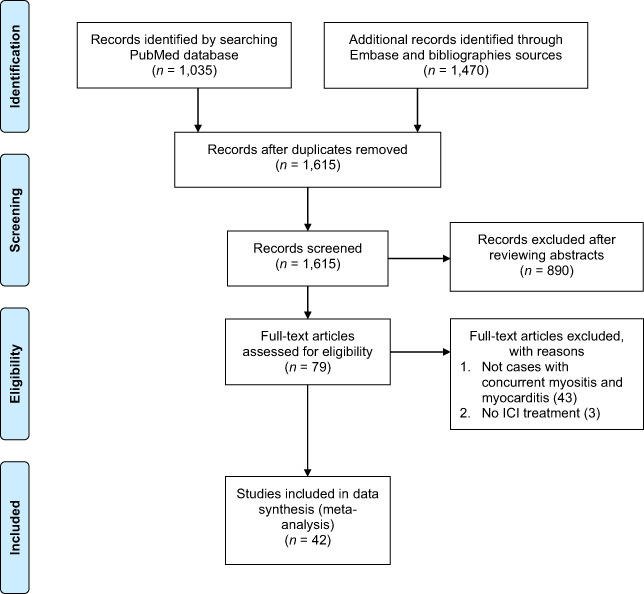
Flow diagram describing the systematic search and study selection process.Abbreviation: ICI, immune checkpoint inhibitor.
Completeness of Reporting Elements and Risk of Bias
All the included case reports and case series provided basic demographic information such as age and sex. Only 11 cases commented on the presence or absence of autoimmune disease at baseline; one patient has chronic lymphocytic lymphoma [4]. Laboratory data and outcome data were incomplete in most of the cases. Survival information was available for 48 cases. The risk of bias assessment is shown in supplemental online Table 3.
Clinical Characteristics
The characteristics of the included patients are described in Table 1. The median age of the patients was 71, and 67% (n = 40) of them were men. The most‐reported symptom was fatigue (80%) and muscle weakness (78%). There was no consistent pattern of preexisting autoimmune conditions—only one patient had a prior autoimmune disease [5]. Twenty‐two patients were reported to have concomitant myasthenia gravis [4, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18]. The median number of doses to the development of IM3OS was one. The mean creatine kinase (CK) level was 9,645 IU/L. Forty patients developed arrhythmias due to myocarditis and 11 patients had depressed ejection fraction (defined as less than 50% by echocardiography; Table 2).
Table 1.
Demographic parameters, presenting features, and outcomes of included cases
| Author, year | Age | Sex | Indication | ICI | Presenting signs/symptoms | Other irAEs | In‐hospital outcome | Best response on ICI? |
|---|---|---|---|---|---|---|---|---|
| Ang, 2021 | 74 | F | Melanoma | anti‐PD‐L1 (agent unclear) | Bilateral shoulder girdle pain/stiffness, fatigue, subjective reduction in effort tolerance | — | Alive | Complete response |
| Arangalage, 2017 | 35 | F | Melanoma | Nivolumab plus ipilimumab | Dyspnea | Thyroiditis | Alive | — |
| Arora, 2020 | 70 | M | Melanoma | Nivolumab plus ipilimumab | Palpitations, double vision, right ptosis, presyncope | — | Dead | — |
| Arora, 2020 | 79 | M | Melanoma | Pembrolizumab | Blurred vision, diplopia, fatigue, lower extremity weakness, diffuse pain | — | Dead | — |
| Arora, 2020 | 61 | F | Breast cancer | Durvalumab plus tremelimumab | Right eyelid ptosis and hepatitis. During hospital stay, patient developed episodes of chest pain, dizziness, dyspnea | — | Dead | — |
| Arora, 2020 | 69 | M | Bladder cancer | Pembrolizumab | Diffuse body pain and weakness | — | Dead | — |
| Arora, 2020 | 67 | F | Melanoma | Nivolumab plus ipilimumab | Diffuse body weakness, dyspnea, dysphagia, hepatitis | Hepatitis | Dead | — |
| Arora, 2020 | 83 | M | Melanoma | Nivolumab | Marked fatigue, weakness, chest pain and orthopnea. Initially diagnosed as pericarditis and colchicine and naproxen started but presented in a few days with chest tightness, dysphagia, and left eye ptosis | — | Dead | — |
| Arora, 2020 | 89 | M | Lung cancer | Pembrolizumab | Disconjugate gaze, dysphagia, blurred vision, and imbalance. | — | Dead | — |
| Behling, 2017 | 63 | M | Melanoma (uveal) | Nivolumab | Moderate pain in the proximal muscle groups of the upper limbs and a slight worsening of a pre‐existing dyspnea under chronic obstructive pulmonary disease that had started 10 days after the first infusion of nivolumab | — | Dead | — |
| Bukamur, 2019 | 88 | F | Lung cancer | Nivolumab | Muscle aches and proximal weakness | — | Alive | — |
| Charles, 2019 | 33 | M | Hodgkin's lymphoma | Nivolumab | Patient was admitted for an acute interstitial pneumonia associated with hepatitis and cutaneous eruption. He progressed to multiorgan failure. | Pneumonitis, hepatitis, rash | Dead | — |
| Chen, 2018 | 43 | M | Thymoma | Nivolumab | Moderate chest pain, dyspnea and generalized myalgias without fever, rash, diplopia, or dysphagia. | — | Dead | — |
| Chen, 2020 | 69 | F | Lung cancer | Camrelizumab | Shortness of breath and weakness of muscle. | Thyroiditis | Dead | — |
| Fazal, 2020 | 82 | M | Melanoma | Nivolumab | Two weeks of neck stiffness, head drop and gradually increasing fatigue with orthopnea, hypoxia, and dysarthric speech. On examination a unilateral left‐sided ptosis, with normal extra‐ocular muscle movements. Weakness of orbicularis oculi, neck extensors and flexors. | — | Dead | — |
| Fazel, 2019 | 78 | F | Melanoma | Nivolumab plus ipilimumab | Diplopia and bilateral proximal muscle weakness/myalgias (greater in the lower extremities), and decreased vibratory sensation in the distal extremities | — | Dead | — |
| Fuentes‐Antras, 2020 | 75 | M | Lung cancer | Pembrolizumab | Severe asthenia, myalgia, profuse sweating, and palpitations which aggravated with diplopia and blurred vision. | Thyroiditis | Dead | Complete response |
| Fukasawa, 2017 | 69 | F | Lung cancer | Nivolumab | General malaise and double vision | — | Alive | — |
| Hellman, 2019 | 84 | M | Bladder cancer | Pembrolizumab (with epacadostat) | Drooping of both of eyes, difficulty walking, feeling “imbalanced,” generalized weakness, and mild dysphagia | — | Dead | — |
| Imai, 2019 | 70 | M | Lung cancer | Pembrolizumab | Fever 2 weeks after receiving the second dose, followed by syncope and muscle weakness and tenderness | — | Dead | — |
| Jeyakumar, 2020 | 86 | M | Cutaneous SCC | Cemiplimab | Decreased vision in the left eye and a 48‐hour history of severe fatigue accompanied by lower back and bilateral hip pain. | — | Dead | — |
| Johnson, 2016 | 65 | F | Melanoma | Nivolumab plus ipilimumab | Atypical chest pain, dyspnea, and fatigue 12 days after receiving the first dose of nivolumab. | — | Dead | — |
| Johnson, 2016 | 63 | M | Melanoma | Nivolumab plus ipilimumab | Fatigue and myalgias | — | Dead | — |
| Kadota, 2019 | 78 | M | Melanoma | Pembrolizumab | — | — | Dead | Partial response |
| Kadota, 2019 | 80 | M | Melanoma | Nivolumab | — | MG | Alive | Stable disease |
| Kadota, 2019 | 63 | M | Melanoma | Nivolumab | — | — | Dead | — |
| Kadota, 2019 | 65 | F | Melanoma | Nivolumab plus ipilimumab | — | — | Dead | — |
| Kadota, 2019 | 63 | M | Melanoma | Nivolumab plus ipilimumab | — | — | Dead | — |
| Konstantina, 2019 | 58 | F | Thymoma | Pembrolizumab | Fever, rash, and oral ulcers | — | Dead | — |
| Konstantina, 2019 | 30 | F | Thymoma | Pembrolizumab | Chest pain and proximal muscle weakness | MG | Dead | |
| Liang, 2021 | 77 | M | Chordoma | Sintilimab and anlotinib. | Acute chest tightness, shortness of breath, and profuse sweating. Bilateral droopy eyelids 5 days later. | — | Alive | — |
| Lie, 2019 | 79 | M | Mesothelioma | Nivolumab | Severe proximal limb and truncal weakness, dyspnea, and generalized fatigue. | Alive | Progressive disease | |
| Lipe, 2020 | 49 | F | Thymoma | Pembrolizumab | The most common presenting symptoms were dyspnea, ptosis, diplopia, and fatigue | — | Alive | — |
| Lipe, 2020 | 67 | M | Lung SCC | Durvalumab | — | — | Alive | — |
| Lipe, 2020 | 77 | M | Urinary bladder cancer | Pembrolizumab | — | — | Dead | — |
| Lipe, 2020 | 81 | F | Renal cell carcinoma | Nivolumab and ipilimumab | — | — | Alive | — |
| Lipe, 2020 | 75 | M | Chondroma | Pembrolizumab | — | — | Alive | — |
| Lipe, 2020 | 66 | F | Renal cell cancer | Nivolumab plus ipilimumab | — | — | Alive | — |
| Lipe, 2020 | 74 | F | Melanoma | Nivolumab plus ipilimumab | — | — | Dead | — |
| Liu, 2020 | 71 | M | Melanoma | Nivolumab | Exertional dyspnea and diplopia. | — | Alive | |
| Martinez‐Calle, 2018 | 67 | F | Multiple myeloma | Pembrolizumab | Malaise and dyspnea on minimal exertion. | — | Dead | — |
| Matsui, 2020 | 69 | M | Bladder cancer | Pembrolizumab | Myalgia with slight elevation of CK after first dose which was believed to be due to exercise. Five days after the second dose, patient presented with bilateral diplopia, ptosis, and decreased deep tendon reflexes. Dysesthesia in bilateral hands and feet. Weakness in bilateral iliopsoas and neck muscles | — | Dead | — |
| Mehta, 2016 | 79 | M | Lung cancer | Nivolumab | Back pain and generalized weakness causing gait instability for two weeks and dyspnea for three days. Patient was hypotensive on presentation and had S3 gallop. | — | N/A | — |
| Monge, 2018 | 79 | M | Prostate cancer | Nivolumab | Blurred vision and pain and stiffness in the upper back. On examination, Eye was within normal limits and there was tenderness in trapezius muscles and decreased motor strength in arms and neck | — | Alive | — |
| Nasr, 2018 | 79 | M | Gastric adenocarcinoma | Pembrolizumab | Ten‐day history of increasing left ptosis, blurry vision and diplopia. Physical examination revealed complete bilateral ptosis and bilateral external ophthalmoplegia | — | Dead | CT showed partial response |
| Rota, 2019 | 71 | M | Renal cancer | Nivolumab | Dropped head, limb weakness progressing to inability to walk. | — | Dead | — |
| Saibil, 2019 | 67 | M | Melanoma | Nivolumab plus ipilimumab | Three‐day history of increasing fatigue, weakness, and dyspnea. On presentation, he also complained of feeling presyncopal. In the emergency room, he was bradycardic. | — | Dead | — |
| Sessums, 2020 | 74 | M | Bladder cancer | Atezolizumab | Patient initially presented to an outside institution with dyspnea and underwent cardiac catheterization with stent placement to the left anterior descending artery but dyspnea continued. Dysphonia, diplopia, dysphagia, ptosis and proximal muscle weakness | — | Dead | — |
| Shah, 2019 | 73 | M | Bladder cancer | Nivolumab plus ipilimumab | Mild jaw and throat discomfort when swallowing, followed by significant generalized weakness and myalgia, with bilateral ptosis and extraocular muscle weakness | — | Alive | — |
| Shirai, 2018 | 83 | M | Melanoma | Pembrolizumab | Fatigable weakness and muscle pain 25 days after receiving pembrolizumab. Physical examination revealed bilateral ptosis, diplopia, weakness of neck flexor and extensor, and bilateral thigh myalgia. | MG | Alive | CT after 5 weeks showed stable disease |
| So, 2019 | 55 | F | Melanoma | Nivolumab | Stiff neck, myalgia, ptosis, dysphagia, dyspnea, progressive ophthalmoplegia, and limb weakness | — | Alive | — |
| Swali, 2020 | 77 | M | Melanoma | Pembrolizumab | Acute weakness, fatigue, and drooping eyelids. | MG | Alive | Disease progression in brain after few months |
| Szuchan, 2019 | 70 | F | Thymic cancer | Pembrolizumab | 1st admission: Exertional dyspnea. 2nd admission: Dyspnea, orthopnea, and weakness | MG | Alive | improvement with significant decrease or resolution of all measurable sites of metastatic disease in the lungs |
| Todo, 2020 | 63 | M | Bladder cancer | Pembrolizumab | Grade 3‐diarrhea, Grade 3‐erythema multiforme with pruritus and left ptosis with diplopia. | MG, colitis, rash | Alive | Complete remission after a single dose of pembrolizumab |
| Tomoaia, 2020 | 63 | F | Lung cancer | Nivolumab | Dyspnea, progressive fatigue and lethargy six days after the dose of nivolumab. Cardiac arrest on the day of admission. | MG | Dead | — |
| Valenti‐Azcarate, 2019 | 66 | M | Lung cancer | Nivolumab plus ipilimumab | Binocular diplopia, fatigue, mild dyspnea, and upper back pain. | MG | Alive | — |
| Veccia, 2016 | 65 | M | Lung cancer | Nivolumab | Horizontal diplopia and mild ptosis of the right eye, without other neurologic signs 27 days after the dose of nivolumab. Ten days later, patient developed symmetric proximal muscle weakness, especially to the upper limbs. | MG | Dead | — |
| Witham, 2017 | 74 | M | Melanoma | Nivolumab plus ipilimumab | Exertional dyspnea, fever, diplopic images, and muscle weakness. Neurological examination revealed bulbar deviation and ptosis on the left side. | — | N/A | — |
| Xing, 2017 | 66 | M | Lung cancer | Sintilimab | Fatigue, myalgia, and tender muscles in both the upper and lower extremities four days after second dose of sintilimab followed by shortness of breath and progressive muscle weakness eight days later. | Myasthenic crisis | Alive | CT scan done after 2 months of sintilimab treatment showed disease progression |
| Yanase, 2020 | 59 | M | Renal cell cancer | Nivolumab plus ipilimumab | Bilateral ptosis and malaise, and left eyeball adduction, descent, and taste were also impaired. | — | Alive | Partial response of metastatic lung disease |
Note: —, unavailable/unreported data.
Abbreviations: CK, creatine kinase; CT, computed tomography; F, female; ICI, immune checkpoint inhibitor; irAE, immune‐related adverse event; M, male; MG, myasthenia gravis; N/A, not available; SCC, squamous cell cancer.
Table 2.
Summary of patients described in the case reports/case series
| Parameter | Single‐agent ICI therapy (n = 47) | Dual‐agent ICI therapy (n = 13) | Overall (n = 60) |
|---|---|---|---|
| Age, years, median (range) | 72 (30–89) | 65 (35–78) | 71 (33–89) |
| Male | 34 | 6 | 40 |
| Female | 13 | 7 | 20 |
| Indication for ICI | |||
| Melanoma | 14 | 9 | 23 |
| Lung | 10 | 2 | 12 |
| Bladder | 6 | 1 | 7 |
| Other | 17 a | 1 b | 18 c |
| Prior autoimmune disease | |||
| Yes | 1 | 0 | 1 |
| No | 8 | 2 | 10 |
| Unknown | 38 | 11 | 49 |
| Concomitant myasthenia gravis | 13 | 3 | 16 |
| Median number of doses to development of symptoms | 1 | 1 | 1 |
| Average creatine kinase (IU/L) | 7,662.3 | 11,628.3 | 9,645.3 |
| Arrhythmias | |||
| Yes | 31 | 9 | 40 |
| No | 3 | 0 | 3 |
| Unknown | 13 | 4 | 17 |
| Depressed ejection fraction | |||
| Yes | 8 | 3 | 11 |
| Treatment strategies | |||
| Steroids | 47 | 13 | 60 |
| Infliximab | 2 | 3 | 5 |
| Tacrolimus | 0 | 1 | 1 |
| Mycophenolate | 6 | 2 | 8 |
| Plasmapheresis | 4 | 3 | 7 |
| ATG | 1 | 2 | 3 |
| IVIG | 13 | 5 | 18 |
| Rituximab | 1 | 0 | 1 |
| In‐hospital outcomes | |||
| Alive | 21 | 2 | 23 |
| Died | 25 | 10 | 35 |
| Unknown | 1 | 1 | 2 |
Single‐agent ICI therapy refers to single agent anti‐programed cell death 1 (ligand 1) therapy such as pembrolizumab or nivolumab. Dual‐agent ICI therapy refers to ipilimumab + nivolumab or durvalumab + tremelimumab.
Cutaneous SCC 1, gastric 1, Hodgkin's lymphoma 1, mesothelioma 1, multiple myeloma 1, prostate 1, renal 4, thymoma 4, thymic carcinoma 1, chondroma 1, chordoma 1.
Breast 1.
Cutaneous SCC 1, gastric 1, Hodgkin's lymphoma 1, mesothelioma 1, multiple myeloma 1, prostate 1, renal 4, thymoma 4, thymic carcinoma 1, chondroma 1, chordoma 1, breast 1.
Abbreviations: ATG, antithymocyte globulin; ICI, immune checkpoint inhibitors; IVIG, intravenous immunoglobulin; SCC, squamous cell cancer.
Electrodiagnostic Tests
Electromyography (EMG) and/or nerve conduction studies were reported in 11 cases [4, 7, 9, 11, 19, 20, 21, 22, 23, 24, 25]. Most cases reported proximal muscle myopathic patterns with moderate to severe muscle injury. EMG was reported as within normal limits in three cases [7, 23, 24]. Repetitive nerve stimulation (RNS) studies are the most frequently used electrodiagnostic test for myasthenia gravis and is considered to be positive (i.e., abnormal) if the decrement in the compound muscle action potential amplitude with electrical stimulation 6 to 10 times at low rates (2 or 3 Hz) is greater than 10 percent. Single‐fiber electromyography is less widely available but is the most sensitive diagnostic test for myasthenia gravis [26]. Electrodiagnostic test results were not consistently described in the case reports; only three cases detailed the electrodiagnostic test findings in which the diagnosis was considered to be myasthenia gravis [4, 11, 21].
Serologic Testing for Myasthenia Gravis
Although the majority of patients with myasthenia gravis have detectable autoantibodies against the acetylcholine receptor (AChR) or against another target on the surface of the muscle membrane (muscle‐specific receptor tyrosine kinase [MuSK] or low‐density lipoprotein receptor‐related protein 4), some have antibodies against other skeletal muscle proteins such as titin and/or ryanodine (so‐called “anti‐striated muscle antibodies”) [26]. Less than 10% of patients can have seronegative myasthenia gravis, in which patients have negative standard assays for both AChR antibodies and MuSK antibodies. Results of the antibody testing were not consistently described in the case reports. All three cases in which electrodiagnostic testing suggested myasthenia gravis had positive anti‐AChR antibodies [4, 11, 21]. Todo et al. described a case of seronegative myasthenia gravis, although electrodiagnostic testing was reported to be negative [27]. Anti‐AChR antibodies were reported to be positive in the majority of cases in which concurrent myasthenia gravis was diagnosed [4, 6, 7, 10, 11, 12, 21, 28].
Cardiac Imaging
Eleven cases had cardiac magnetic resonance imaging (MRI) with features suggestive of myocarditis [4, 14, 20, 29, 30, 31, 32, 33]. Although a full description of MRI findings was not available in all cases, Arora et al. described evidence of myocardial edema and late gadolinium enhancement in the subepicardial midinferior wall consistent with acute myocarditis in one case. Only a slight myocardial gadolinium uptake was described by Witham et al. [33]. Two cases did not show any evidence of myocarditis: one of the cases had cardiac MRI performed 8 days after starting immunosuppression when cardiac biomarkers had already improved [4].
Histological Findings
Eighteen cases reported muscle biopsy results, with most cases describing myofiber necrosis and atrophy with lymphocytic (predominantly CD8 + T cell) and macrophage infiltrates [5, 9, 11, 12, 20, 21, 23, 24, 34, 35, 36, 37, 38, 39]. One case reported negative inflammatory cell infiltration or necrosis [24]. Fifteen cases had cardiac biopsies [10, 12, 13, 18, 22, 24, 31, 33, 34, 35, 36, 39, 40]. Most cases described lymphocytic infiltrate (which was positive for T‐cell markers, both CD4 and CD8) with CD68 positive macrophages. Variable extent of myocyte injury was described; only six cases [10, 12, 13, 24, 34, 35] described both myocyte injury, and inflammatory infiltrate consistent with the Dallas criteria for myositis and the World Heart Federation criteria for the diagnosis of acute myocarditis [41, 42]. Fukasawa et al. reported the expression of human leukocyte antigen (HLA)‐ABC and HLA‐DR on myocardial cells in addition to the CD4 and CD8 T‐cell infiltrations [13]. Some cases also described early interstitial fibrosis and endocardial fibrosis in areas of inflammatory infiltration, likely representing healing response [18, 33, 35]. Two case reports specifically described negative staining for CD20 expressing B lymphocytes [35, 39].
Treatment
Treatment consisted of two strategies: immunosuppression and supportive therapy. Corticosteroids were the most used primary immunosuppressive therapy (100%), with mycophenolate and cyclophosphamide being the most common steroid‐sparing agent. Although upfront plasmapheresis and intravenous immunoglobulin (IVIG) were used in most patients with concurrent myasthenia gravis, these autoantibodies directed therapies were not limited to patients with myasthenia gravis (Table 2; supplemental online Table 4). Adjunctive immunosuppressive therapies were used primarily after failing initial steroids (82%, 18 out of 22 patients in which the case reports described the sequence of drug therapy).
Clinical Outcomes and Follow‐up
Of the 58 patients with known survival, 35 (60%) patients died in the hospital because of acute complications. None of the survivors (n = 23) were rechallenged with ICIs in our series.
Discussion
We report the most extensive case series of IM3OS to date. In our study, most cases presented early, melanoma was the most common underlying malignancy, and the case fatality rate was high.
Although rare, ICI‐related myocarditis is a life‐threatening complication of ICI therapy, with mortality ranging from 25% to 50%. [2, 43] Although most cases of ICI‐related myocarditis occur in isolation, concurrent development of myositis and/or myasthenia gravis has been reported in up to 30%–40% of cases [44]. Although the underlying mechanisms for the overlap of myositis/myasthenia gravis and myocarditis in ICI‐treated patients remain unclear, molecular mimicry and the critical role of PD‐1 signaling pathways in regulating autoimmune responses in these tissues might be responsible [45, 46]. Given the high rates of co‐occurrence of these three conditions, it is essential to screen patients for the other two adverse events when one of these overlapping adverse events is seen. Work‐up for ICI‐induced myocarditis involves a high index of suspicion, electrocardiographs, and cardiac cytolysis biomarkers such as troponins and CK‐MB [47]. Diagnosis is based on excluding acute coronary syndrome with a negative coronary angiography and tissue characterization with cardiac MRI and/or endomyocardial biopsy [45, 47]. Although the diagnosis of myocarditis has been historically based on histologic demonstration of myocardial inflammation and myocyte injury, these criteria might not be applicable in the immunotherapy era [48, 49]. Furthermore, because many cases of myocarditis in our series only reported inflammatory infiltrate without myocyte injury, the question remains whether the demonstration of myocyte injury is essential for ICI‐treated patients. Further research is needed to refine the diagnostic criteria for myocarditis in ICI‐treated patients and to develop and validate noninvasive imaging techniques such as cardiac MRI in these patients [49].
ICI‐induced myositis tends to have a broad spectrum ranging from mild symptoms to life‐threatening complications [50]. In a study based on the World Health Organization pharmacovigilance database, Allenbach et al. identified 465 cases of myositis with an overall incidence of <1%. Most patients were elderly with a median age of 70, slightly favoring males (56.4%). Median onset for myositis was the shortest among rheumatic irAEs (arthritis, myositis, sarcoidosis, Sjogren's syndrome, scleroderma, and polymyalgia rheumatica) (median 31 days; range 19.2–57.8), with the highest fatality rate (24%), especially when associated with myocarditis (57%) [50]. In our study, most of the cases developed symptoms of myositis within a median of one ICI dose. The most common presenting symptoms included myalgia, proximal limb weakness, and myasthenia symptoms. Most cases were rapidly progressive, contrasting the relatively indolent onset in primary autoimmune polymyositis [51]. All cases reported elevated CK levels. There have been several cases of CK‐negative myositis with ICIs [52, 53]. Aldolase/CK discordance has been hypothesized to be due to high aldolase levels in regenerating myocytes, which tend to be preferentially involved in myositis [54]—aldolase level, therefore, should be checked even if CK levels are normal in the right setting.
ICI‐induced myositis can be associated with myasthenia gravis in up to 40% of patients, which can present with visual, bulbar, or respiratory symptoms [44]. Given the relatively high incidence, patients presenting with immune‐related myositis or myocarditis should be screened early for myasthenia gravis, given the risk of myasthenic crisis and adverse outcomes. In contrast to classical myasthenia gravis, immune‐related myasthenia gravis tends to be life‐threatening with higher rates of respiratory paralysis and death [55]. Despite the high morbidity and mortality, the diagnosis of immune‐related myasthenia gravis is challenging given the lower positivity rates of RNS and anti‐AChR autoantibodies (both approximating 60%) and a higher incidence of seronegativity [55]. Although the presence of thymoma is a crucial component of the pathogenesis of classical myasthenia gravis (and a potential diagnostic clue), it is not relevant to immune‐related myasthenia gravis [55].
ICI‐induced myositis has been described to be associated with CD8+ T lymphocytes and macrophage infiltration (some resembling granulomas) with myofiber necrosis mimicking necrotizing myositis [56]. Matas et al. reported marked necrosis, macrophagy, muscle regeneration with perivascular inflammatory infiltrates, and a significant component of macrophagic cells on a pathologic review of muscle biopsy from nine patients [56]. ICI‐induced myocarditis shares similar features with a predominance of T cells (especially CD8+ T cells). These findings have led to the use of T‐cell targeted therapies with significant benefit in patients with steroid‐refractory ICI‐induced myocarditis [57, 58, 59]. However, Balanescu et al. recently reported complement deposition within capillaries (pericapillary C4d) in two cases of ICI‐induced myocarditis, suggesting a component of antibody‐mediated injury [60]. This perhaps explains successes of using therapies directed against antibody‐mediated autoimmunity such as IVIG and plasmapheresis in a few case reports of IM3OS [61, 62].
All cases of IM3OS in our series were treated with high‐dose corticosteroids as per the current guidelines for immune‐related myocarditis [63]. In addition to the steroids, most patients with concurrent myocarditis and myasthenia gravis received upfront IVIG and plasmapheresis, given concerns regarding paradoxical exacerbation of myasthenia symptoms with steroids alone [28, 64]. However, some case reports employed IVIG and plasmapheresis even without myasthenia, highlighting the uncertainty in our understanding of the pathogenesis of myocarditis and myositis overlap syndromes. In addition to steroids and IVIG/plasmapheresis, other immunosuppressive drugs such as tacrolimus, infliximab, mycophenolate mofetil, or antithymocyte globulin were used as adjunctive therapies in patients without rapid improvement on steroids.
Finally, we found significant in‐hospital mortality in cases with IM3OS, with mortality rates approaching 60%. This underscores the importance of a high index of suspicion for concomitant myocarditis in patients presenting with ICI‐induced myositis and/or myasthenia gravis (or vice versa) to avoid delays in the diagnosis and treatment. High morbidity and mortality associated with IM3OS probably explain why none of the included patients were rechallenged with ICIs. Additionally, given the high mortality with IM3OS, patients might benefit from an upfront initiation of adjunctive immunomodulatory treatments, although this needs to be studied in prospective studies.
Limitations
Our study has several limitations. Owing to variability in the data available from case reports, there were missing data on clinical features, diagnostic studies, hospital course, and outcomes of some patients, subjecting our results to reporting bias. Given the reasons mentioned above, we relied on the case reports’ authors to adjudicate the diagnosis of concurrent myasthenia gravis. Some laboratory data parameters could not distinguish whether the lack of reporting reflected normal results versus the test not being conducted. Because our sample size was potentially nonrepresentative, we did not estimate any accuracy parameters.
Conclusion
Our study shows that IM3OS tends to develop early with ICI treatment and can be associated with significant morbidity and mortality. Prospective studies are needed to determine the biomarkers to predict the occurrence and severity of IM3OS and the optimal approach to diagnose and manage ICI‐treated patients with this potentially life‐threatening complication.
Author Contributions
Conception/design: Ranjan Pathak, Anjan Katel
Administrative Support: Anjan Katel
Collection and/or assembly of data: Ranjan Pathak, Anjan Katel, Erminia Massarelli, Victoria M Villaflor, Virginia Sun, Ravi Salgia
Data analysis and interpretation: Ranjan Pathak, Anjan Katel, Erminia Massarelli, Victoria M Villaflor, Virginia Sun, Ravi Salgia
Manuscript writing: Ranjan Pathak, Anjan Katel
Critical revision and final approval of manuscript: Ranjan Pathak, Anjan Katel, Erminia Massarelli, Victoria M Villaflor, Virginia Sun, Ravi Salgia
Disclosures
Erminia Massarelli: Merck, AstraZeneca, Eli Lilly & Co. (C/A); Vicky Villaflor: AstraZeneca, Bristol‐Myers Squibb, Genentech (C/A), Takeda (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplementary table 1 Compliance to PRISMA Harm guidelines.
Supplementary table 2. Search strategy
Supplementary table 3. Completeness of reporting and risk of bias.
Supplementary Table 4. Summary of adverse events and immunosuppressive therapies used.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Kostine M, Rouxel L, Barnetche T et al. Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer—clinical aspects and relationship with tumour response: A single‐centre prospective cohort study. Ann Rheum Dis 2018;77:393–398. [DOI] [PubMed] [Google Scholar]
- 2. Moslehi JJ, Salem JE, Sosman JA et al. Increased reporting of fatal immune checkpoint inhibitor‐associated myocarditis. Lancet 2018;391:933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zorzela L, Loke YK, Ioannidis JP et al. PRISMA harms checklist: Improving harms reporting in systematic reviews. BMJ 2016;352:i157. [DOI] [PubMed] [Google Scholar]
- 4. Arora P, Talamo L, Dillon P et al. Severe combined cardiac and neuromuscular toxicity from immune checkpoint blockade: An institutional case series. Cardiooncology 2020;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Swali R. Pembrolizumab‐induced myositis in the setting of metastatic melanoma: An increasingly common phenomenon. J Clin Aesthetic Dermatol 2020;13:44–45. [PMC free article] [PubMed] [Google Scholar]
- 6. Xing Q, Zhang ZW, Lin QH et al. Myositis‐myasthenia gravis overlap syndrome complicated with myasthenia crisis and myocarditis associated with anti‐programmed cell death‐1 (sintilimab) therapy for lung adenocarcinoma. Ann Transl Med 2020;8:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shirai T, Kiniwa Y, Sato R et al. Presence of antibodies to striated muscle and acetylcholine receptor in association with occurrence of myasthenia gravis with myositis and myocarditis in a patient with melanoma treated with an anti‐programmed death 1 antibody. Eur J Cancer 2019;106:193–195. [DOI] [PubMed] [Google Scholar]
- 8. Rota E, Varese P, Agosti S et al. Concomitant myasthenia gravis, myositis, myocarditis and polyneuropathy, induced by immune‐checkpoint inhibitors: A life‐threatening continuum of neuromuscular and cardiac toxicity. eNeurologicalSci 2019. Mar;14:4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kadota H, Gono T, Shirai Y et al. Immune checkpoint inhibitor‐induced myositis: A case report and literature review. Curr Rheumatol Rep 2019;21:10. [DOI] [PubMed] [Google Scholar]
- 10. Jeyakumar N, Etchegaray M, Henry J et al. The terrible triad of checkpoint inhibition: A case report of myasthenia gravis, myocarditis, and myositis induced by cemiplimab in a patient with metastatic cutaneous squamous cell carcinoma. Case Rep Immunol 2020;2020:5126717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. So H, Ikeguchi R, Kobayashi M et al. PD‐1 inhibitor‐associated severe myasthenia gravis with necrotizing myopathy and myocarditis. J Neurol Sci 2019;399:97–100. [DOI] [PubMed] [Google Scholar]
- 12. Fuentes‐Antrás J, Peinado P, Guevara‐Hoyer K et al. Fatal autoimmune storm after a single cycle of anti‐PD‐1 therapy: A case of lethal toxicity but pathological complete response in metastatic lung adenocarcinoma. Hematol Oncol Stem Cell Ther 2020; S1658‐3876(20)30098‐4 [DOI] [PubMed] [Google Scholar]
- 13. Fukasawa Y, Sasaki K, Natsume M et al. Nivolumab‐induced myocarditis concomitant with myasthenia gravis. Case Rep Oncol 2017;10:809–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liang S, Yang J, Lin Y et al. Immune myocarditis overlapping with myasthenia gravis due to anti‐PD‐1 treatment for a chordoma patient: A case report and literature review. Front Immunol 2021;12:682262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lipe DN, Galvis‐Carvajal E, Rajha E et al. Immune checkpoint inhibitor‐associated myasthenia gravis, myositis, and myocarditis overlap syndrome. Am J Emerg Med 2021;46:51–55. [DOI] [PubMed] [Google Scholar]
- 16. Yanase T, Moritoki Y, Kondo H et al. Myocarditis and myasthenia gravis by combined nivolumab and ipilimumab immunotherapy for renal cell carcinoma: A case report of successful management. Urol Case Rep 2021;34:101508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Konstantina T, Konstantinos R, Anastasios K et al. Fatal adverse events in two thymoma patients treated with anti‐PD‐1 immune check point inhibitor and literature review. Lung Cancer 2019;135:29–32. [DOI] [PubMed] [Google Scholar]
- 18. Szuchan C, Elson L, Alley E et al. Checkpoint inhibitor‐induced myocarditis and myasthenia gravis in a recurrent/metastatic thymic carcinoma patient: A case report. Eur Heart J Case Rep 2020;4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Veccia A, Kinspergher S, Grego E et al. Myositis and myasthenia during nivolumab administration for advanced lung cancer: A case report and review of the literature. Anticancer Drugs 2020;31:540–544. [DOI] [PubMed] [Google Scholar]
- 20. Valenti‐Azcarate R, Esparragosa Vazquez I, Toledano Illan C et al. Nivolumab and ipilimumab‐induced myositis and myocarditis mimicking a myasthenia gravis presentation. Neuromuscul Disord 2020;30:67–69. [DOI] [PubMed] [Google Scholar]
- 21. Shah M, Tayar JH, Abdel‐Wahab N et al. Myositis as an adverse event of immune checkpoint blockade for cancer therapy. Semin Arthritis Rheum 2019;48:736–740. [DOI] [PubMed] [Google Scholar]
- 22. Sessums M, Yarrarapu S, Guru PK et al. Atezolizumab‐induced myositis and myocarditis in a patient with metastatic urothelial carcinoma. BMJ Case Rep 2020;13:e236357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nasr F, El Rassy E, Maalouf G et al. Severe ophthalmoplegia and myocarditis following the administration of pembrolizumab. Eur J Cancer 2018;91:171–173. [DOI] [PubMed] [Google Scholar]
- 24. Matsui H, Kawai T, Sato Y et al. A fatal case of myocarditis following myositis induced by pembrolizumab treatment for metastatic upper urinary tract urothelial carcinoma. Int Heart J. 2020;61:1070–1074. [DOI] [PubMed] [Google Scholar]
- 25. Hellman JB, Traynis I, Lin LK. Pembrolizumab and epacadostat induced fatal myocarditis and myositis presenting as a case of ptosis and ophthalmoplegia. Orbit 2019;38:244–247. [DOI] [PubMed] [Google Scholar]
- 26. Melzer N, Ruck T, Fuhr P et al. Clinical features, pathogenesis, and treatment of myasthenia gravis: A supplement to the Guidelines of the German Neurological Society. J Neurol 2016;263:1473–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Todo M, Kaneko G, Shirotake S et al. Pembrolizumab‐induced myasthenia gravis with myositis and presumable myocarditis in a patient with bladder cancer. IJU Case Rep. 2020;3:17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Safa H, Johnson DH, Trinh VA et al. Immune checkpoint inhibitor related myasthenia gravis: Single center experience and systematic review of the literature. J Immunother Cancer 2019;7:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Monge C, Maeng H, Brofferio A et al. Myocarditis in a patient treated with Nivolumab and PROSTVAC: A case report. J Immunother Cancer 2018;6:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lie G, Weickhardt A, Kearney L et al. Nivolumab resulting in persistently elevated troponin levels despite clinical remission of myocarditis and myositis in a patient with malignant pleural mesothelioma: Case report. Transl Lung Cancer Res 2020;9:360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ang E, Mweempwa A, Heron C et al. Cardiac troponin I and T in checkpoint inhibitor–associated myositis and myocarditis. J Immunother 2021;44:162–163. [DOI] [PubMed] [Google Scholar]
- 32. Arangalage D, Delyon J, Lermuzeaux M et al. Survival after fulminant myocarditis induced by immune‐checkpoint inhibitors. Ann Intern Med 2017;167:683–684. [DOI] [PubMed] [Google Scholar]
- 33. Witham D, Knauss S, Marek A et al. Acute myocarditis and myositis after immune checkpoint inhibition. Eur J Heart Fail 2017;19(suppl 1):16a. [Google Scholar]
- 34. Tomoaia R, Beyer RȘ, Pop D et al. Fatal association of fulminant myocarditis and rhabdomyolysis after immune checkpoint blockade. Eur J Cancer 2020;132:224–227. [DOI] [PubMed] [Google Scholar]
- 35. Saibil SD, Bonilla L, Majeed H et al. Fatal myocarditis and rhabdomyositis in a patient with stage IV melanoma treated with combined ipilimumab and nivolumab. Curr Oncol 2019;26:e418–e421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Martinez‐Calle N, Rodriguez‐Otero P, Villar S et al. Anti‐PD1 associated fulminant myocarditis after a single pembrolizumab dose: The role of occult pre‐existing autoimmunity. Haematologica 2018;103:e318–e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Charles J, Giovannini D, Terzi N et al. Multi‐organ failure induced by Nivolumab in the context of allo‐stem cell transplantation. Exp Hematol Oncol 2019;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen Q, Huang DS, Zhang LW et al. Fatal myocarditis and rhabdomyolysis induced by nivolumab during the treatment of type B3 thymoma. Clin Toxicol (Phila) 2018;56:667–671. [DOI] [PubMed] [Google Scholar]
- 39. Johnson DB, Balko JM, Compton ML et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016;375:1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Imai R, Ono M, Nishimura N et al. Fulminant myocarditis caused by an immune checkpoint inhibitor: A case report with pathologic findings. J Thorac Oncol 2019;14:e36–e38. [DOI] [PubMed] [Google Scholar]
- 41. Aretz HT. Myocarditis: The Dallas criteria. Hum Pathol 1987;18:619–624. [DOI] [PubMed] [Google Scholar]
- 42. Caforio ALP, Pankuweit S, Arbustini E et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636–2648, 2648a–2648d. [DOI] [PubMed] [Google Scholar]
- 43. Mahmood SS, Fradley MG, Cohen JV et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018;71:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aldrich J, Pundole X, Tummala S et al. Inflammatory myositis in cancer patients receiving immune checkpoint inhibitors. Arthritis Rheumatol 2021;73:866–874. [DOI] [PubMed] [Google Scholar]
- 45. Palaskas N, Lopez‐Mattei J, Durand JB et al. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc 2020;9:e013757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tarrio ML, Grabie N, Bu D et al. PD‐1 protects against inflammation and myocyte damage in T cell‐mediated myocarditis. J Immunol 2012;188:4876–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pradhan R, Nautiyal A, Singh S. Diagnosis of immune checkpoint inhibitor‐associated myocarditis: A systematic review. Int J Cardiol 2019;296:113–121. [DOI] [PubMed] [Google Scholar]
- 48. Baughman KL. Diagnosis of myocarditis: Death of Dallas criteria. Circulation 2006;113:593–595. [DOI] [PubMed] [Google Scholar]
- 49. Bonaca MP, Olenchock BA, Salem JE et al. Myocarditis in the setting of cancer therapeutics: Proposed case definitions for emerging clinical syndromes in cardio‐oncology. Circulation 2019;140:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Allenbach Y, Anquetil C, Manouchehri A et al. Immune checkpoint inhibitor‐induced myositis, the earliest and most lethal complication among rheumatic and musculoskeletal toxicities. Autoimmun Rev 2020;19:102586. [DOI] [PubMed] [Google Scholar]
- 51. Dalakas MC. Polymyositis, dermatomyositis, and inclusion‐body myositis. N Engl J Med 1991;325:1487–1498. [DOI] [PubMed] [Google Scholar]
- 52. Liewluck T, Kao JC, Mauermann ML. PD‐1 inhibitor‐associated myopathies: Emerging immune‐mediated myopathies. J Immunother 2018;41:208–211. [DOI] [PubMed] [Google Scholar]
- 53. Moreira A, Loquai C, Pföhler C et al. Myositis and neuromuscular side‐effects induced by immune checkpoint inhibitors. Eur J Cancer 2019;106:12–23. [DOI] [PubMed] [Google Scholar]
- 54. Casciola‐Rosen L, Hall JC, Mammen AL et al. Isolated elevation of aldolase in the serum of myositis patients: A potential biomarker of damaged early regenerating muscle cells. Clin Exp Rheumatol 2012;30:548–553. [PMC free article] [PubMed] [Google Scholar]
- 55. Huang YT, Chen YP, Lin WC et al. Immune checkpoint inhibitor‐induced myasthenia gravis. Front Neurol 2020;11:634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Matas‐García A, Milisenda JC, Selva‐O'Callaghan A et al. Emerging PD‐1 and PD‐1L inhibitors‐associated myopathy with a characteristic histopathological pattern. Autoimmun Rev 2020;19:102455. [DOI] [PubMed] [Google Scholar]
- 57. Esfahani K, Buhlaiga N, Thébault P et al. Alemtuzumab for immune‐related myocarditis due to PD‐1 therapy. New Engl J Med 2019;380:2375–2376. [DOI] [PubMed] [Google Scholar]
- 58. Salem JE, Allenbach Y, Vozy A et al. Abatacept for severe immune checkpoint inhibitor–associated myocarditis. New Engl J Med 2019;380:2377–2379. [DOI] [PubMed] [Google Scholar]
- 59. Palaskas N, Lopez‐Mattei J, Durand JB et al. Immune checkpoint inhibitor myocarditis: Pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc 2020;9:e013757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Balanescu DV, Donisan T, Palaskas N et al. Immunomodulatory treatment of immune checkpoint inhibitor‐induced myocarditis: Pathway toward precision‐based therapy. Cardiovasc Pathol 2020;47:107211. [DOI] [PubMed] [Google Scholar]
- 61. Yamaguchi S, Morimoto R, Okumura T et al. Late‐onset fulminant myocarditis with immune checkpoint inhibitor nivolumab. Can J Cardiol 2018;34:812.e1‐812.e3. [DOI] [PubMed] [Google Scholar]
- 62. Yogasundaram H, Alhumaid W, Chen JW et al. Plasma exchange for immune checkpoint inhibitor–induced myocarditis. CJC Open 2021;3:379–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Thompson JA, Schneider BJ, Brahmer J et al. NCCN guidelines insights: Management of immunotherapy‐related toxicities, version 1.2020. J Natl Compr Cancer Netw 2020;18:230–241. [DOI] [PubMed] [Google Scholar]
- 64. Díez‐Porras L, Homedes C, Alberti MA et al. Intravenous immunoglobulins may prevent prednisone‐exacerbation in myasthenia gravis. Sci Rep 2020;10:13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplementary table 1 Compliance to PRISMA Harm guidelines.
Supplementary table 2. Search strategy
Supplementary table 3. Completeness of reporting and risk of bias.
Supplementary Table 4. Summary of adverse events and immunosuppressive therapies used.


