Abstract
Loss-of-function mutations in the synaptic protein neurexin1α (NRXN1α) are associated with several neurodevelopmental disorders, including ASD, schizophrenia and ADHD, and many of these disorders are defined by core deficits in social cognition. Mouse models of Nrxn1α deficiency are not amenable to studying aspects of social cognition because in general, mice do not engage in complex social interactions such as social play or prosocial helping behaviors. Rats, on the other hand, engage in these complex, well-characterized social behaviors. Using the Nrxn1tm1Sage Sprague Dawley rat, we tested a range of cognitive and social behaviors in juveniles with haplo- or biallelic Nrxn1α mutation. We found a deficit in ultrasonic vocalizations of male and female neonatal rats with Nrxn1α deficiency. A male-specific deficit in social play was observed in Nrxn1α-deficient juveniles, although sociability and social discrimination were unaltered. Nurturing behavior induced by exposure to pups was enhanced in male and female juveniles with biallelic Nrxn1α mutation. Performance in tasks of prosocial helping behavior and food retrieval indicated severe deficits in learning and cognition in juveniles with biallelic Nrxn1α mutation, and a less severe deficit in haploinsufficient rats, although Pavlovian learning was altered only in haploinsufficient males. We also observed a male-specific increase in mobility and object investigation in juveniles with complete Nrxn1α deficiency. Together, these observations more fully characterize the Nrxn1tm1Sage Sprague Dawley rat as a model for Nrxn1α-related neurodevelopmental disorders, and support a rationale for the juvenile rat as a more appropriate model for disorders that involve core deficits in complex social behaviors.
The quest to understand the genomic contributions to neuropsychiatric disorders is confounded by the large number of gene candidates and the high degree of overlap in genetic risk across disorders, particularly those with origins in development (Cristino et al., 2014). A large percentage of relatively rare single-nucleotide polymorphisms and copy-number variations associated with neurodevelopmental disorders, including autism spectrum disorder (ASD) schizophrenia, ADHD and intellectual disability, are found in genes coding for proteins involved in synapse formation and function. Among these are genes encoding the neurexins, a family of presynaptic cell adhesion molecules which are essential for assembly and organization of synapses (Sudhof, 2008; Li et al., 2015; Uemura et al., 2010; Matsuda and Yuzaki, 2011), and have critical roles in regulating neurotransmitter release (Missler et al., 2003; Kattenstroth et al., 2004; Zhang et al., 2005; Dudanova et al., 2006; Li et al., 2015; Tong et al., 2016) and the postsynaptic response (Uemura et al., 2010; Matsuda and Yuzaki, 2011). Mammalian neurexins are encoded by three genes (Nrxn1-3), each of which give rise to numerous splice variants expressed from two distinct promoters, α and β (Reissner et al., 2013). Nrxn1 in particular is one of the most robust candidate genes for neurodevelopmental disorders identified to date, and mutations in Nrxn1 are associated with intellectual disability (Dabell, 2013; Ching, 2010), schizophrenia (Kirov et al., 2009; Rujescu et al., 2009), attention-deficit hyperactivity disorder (ADHD), Tourette Syndrome (Bradley et al., 2010; Gudmundsson et al., 2019; Huang et al., 2017), bipolar disorder (Zhang et al., 2009), and ASD (Ching et al., 2010; Reissner et al., 2013; Hu et al., 2019). Aside from intellectual disability, ASD is the most common disorder associated with deletions in the Nrxn1 gene, and deletions or copy-number variations in Nrxn1 are found in roughly 0.5% of ASD cases, making it one of the most-studied gene candidates for ASD (Dabell et al., 2013; Bena et al., 2013; Schaaf et al., 2012). Both loss of function and copy number variants of Nrxn1 have been identified as risk factors, but in many cases penetrance is incomplete (Al Shehhi et al., 2019; Dabell et al., 2013; Castronovo et al., 2020), and this includes a significant muting of negative effects in female carriers (Wisniowiecka-Kowalnik et al., 2010). Interestingly, many of the neurodevelopmental disorders associated with Nrxn1 dysfunction, including schizophrenia, ADHD, Tourette syndrome, and ASD exhibit a male-biased sex difference in prevalence (Abel et al., 2010; Eldridge et al., 1977; Gaub and Carlson, 1997; Werling and Geschwind, 2013).
In addition to cognitive deficits, core symptomologies of Nrxn1-associated disorders involve deficits in executive function and social interaction that arise early in life, and many of these have been successfully modeled in rodents. For example, speech and language deficits are seen in most children with Nrxn1α deletions, with more than half of these cases characterized as social language difficulties (Brignell et al., 2018). Language deficits associated with genetic mutation are well modeled in rodents, which have a complex repertoire of calls that they emit during social interactions that are easily quantified and characterized (Portfors, 2007; Wohr, 2017). Deficits in social behaviors that involve reciprocal interactions are a hallmark of both autism and schizophrenia which begin to manifest in early childhood, in the case of ASD, or during late childhood to adolescence in schizophrenia (Jalbrzikowski et al., 2013; Wagner et al., 2019). Children with ASD engage in less social play, the most notable and conserved form of social interaction in young mammals (Jordan, 2003). Organization of the neural circuits of play during the sensitive period of early brain sexual differentiation is well established in animal models (Auger and Olesen, 2009) and considerable evidence suggests the same is true for humans (Auyeung et al., 2009; Hines and Kaufman, 1994; Cohen-Bendahan et al., 2005). Impaired social cognition is found in patients with schizophrenia and ASD (Brune, 2005; Pinkham et al, 2008), and ASD in particular is characterized by deficits in cognitive empathy, also referred to as “theory of mind” mentalizing (Hill and Frith, 2003; Jones et al., 2010; Lombardo et al., 2007). Modeling aspects of cognitive empathy outside of primates is difficult, although a significant body of evidence demonstrates that rodents, particularly rats, are sensitive to the affective state of conspecific social partners and strangers (Keum and Shin, 2016).
In general, mouse models of Nrxn1 deficiency have been criticized for lack of a robust phenotype and inconsistencies in observed behavioral changes (reviewed in Pak et al., 2015). In the homozygous Nrxn1 knockout mouse mild impairments are seen in tests of social interaction in some studies (Grayton et al., 2013; Armstrong et al., 2020), but not in others (Etherton et al., 2009). In addition, with the exception of intellectual disability, NRXN1-associated disorders generally involve haploinsufficiency, rather than biallelic mutation (Schaaf et al., 2012; Bena et al., 2013; Dabell et al., 2013), but few studies have utilized Nrxn1α heterozygous mice (Armstrong et al., 2020; Dachtler et al., 2015; Laarakker et al., 2012). More importantly, complex social interactions are not readily modeled in mice, making them a poor model for interrogating many of the core deficits in Nrxn1α-associated disorders. For example, while juvenile social play is observed across a wide spectrum of mammalian species with a striking degree of commonality (Siviy, 2016), mice are a rare exception in that they do not engage in play per se but instead display social affiliation with side-by-side contact. In contrast, rats engage in extensive play that is rich in complexity with discrete behavioral components that include boxing, pinning, chasing and pouncing, all of which are easily quantified and provide a far more nuanced analysis of juvenile social play behavior (Argue and McCarthy, 2015a,b; VanRyzin et al., 2019). Rats also display a nurturing behavior exemplified by care of unfamiliar pups that can be induced outside of maternal experience, in adults and juveniles of either sex, by repeated exposure to the pups (Bridges et al., 1974, Mayer and Rosenblatt, 1979; Mayer et al., 1979; Stolzenberg and Champagne, 2016). In contrast, mice have been artificially selected for spontaneous maternal behavior, divorcing the nurturing response from pup experience and limiting the usefulness of mice as a model for nurturing behavior (Dulac et al., 2014). In addition to nurturing, rats also exhibit helping behaviors towards conspecifics of the same age (Mason et al., 2021). They will free a social partner or stranger of a socially-familiar strain from a distressing situation, such as confinement in a box or tank of water, and this behavior is associated with affect arousal and not motivated by subsequent social interaction, indicating an empathy-like motivation (Ben-Ami Bartal et al., 2011; 2014; 2016; Cox and Reichel, 2020; Sato et al., 2015).Thus, in contrast to mice, rats engage in complex social behaviors that can be quantified and manipulated, and in the case of social play and pup-induced nurturing these behaviors are displayed by young animals, prior to puberty, thereby allowing for assessment at a life phase that coincides with behavioral changes seen in children with Nrxn1α-related disorders. Given the lack of robust genetic models for probing complex social interactions, we characterized a variety of social and cognitive behaviors in rats that carried heterozygous and homozygous mutations in Nrxn1α, with a particular focus on the juvenile lifestage. We found a range of effects on social behaviors in juvenile rats, as well as evidence of cognitive impairment, suggesting that Nrxn1α mutations exert diffuse but impactful influences on early life behavior.
Materials and Methods
Animals
Sprague Dawley (SD)-Nrxn1tm1sage heterozygous (Het) rats were purchased from Horizon Discovery Group, mated as Het X Het pairs in our facility, and allowed to deliver normally under standard husbandry conditions. Offspring from these crosses were used for experiments. Rats were housed in polycarbonate cages (20 × 40 × 20cm) with corncob bedding under a reverse 12:12h light/dark cycle. Food and water ad libitum. Pups were weaned on postnatal day 21 (PN21) into same-sex pairs or groups of 3. Behavioral tests were performed during the animals’ dark period, between the hours of 10:00 and 15:00. All breeding and experimental procedures were approved by the Institutional Care and Use Committee at the University of Maryland School of Medicine and performed in accordance with national animal care and use guidelines.
Genotyping
Tail snips were collected from pups within the first week of life or when rats were euthanized. Genomic DNA was extracted using MyTaq™ Extract-PCR Kit (Bioline). We used forward (5’ – GCAGCTCAGCTTCTCCATCT – 3’) and reverse (5’ – ACTTGACCTCCACCCACTTG – 3’) primers (Integrated DNA Technologies) which flanked the deleted region in exon 2 of the Nrxn1α gene and were suggested by Horizon Discovery Group. PCR products were resolved on a 4% agarose gel with a PCR marker (New England Biolabs) to identify the 158bp WT band and the 142bp KO band (see Figure 1A, B).
Figure 1: Nrxn1α mRNA is decreased in SD-Nrxn1tm1Sage HET pups and is not detectable in SD-Nrxn1tm1Sage KO pups.
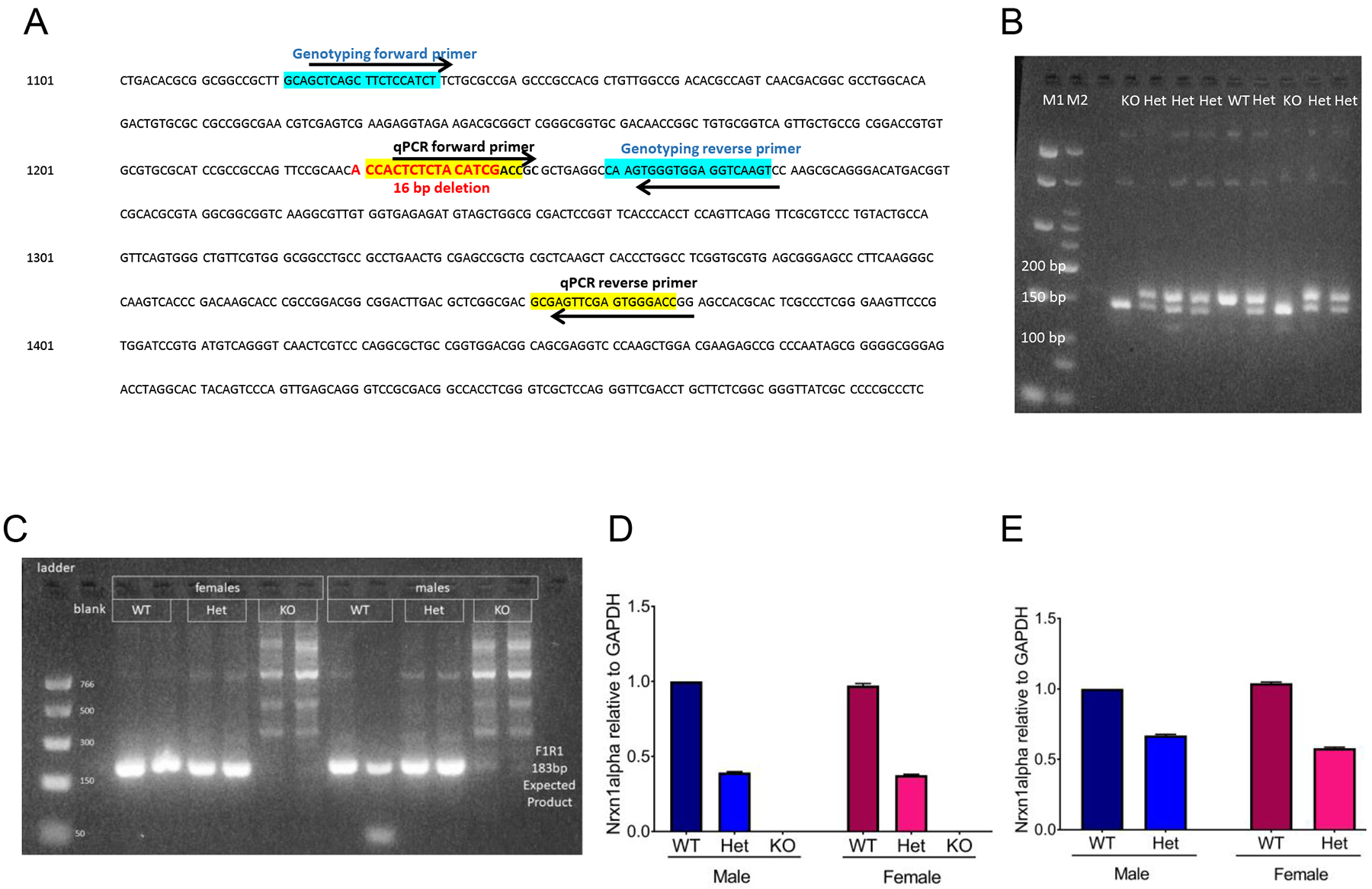
(A) Nrxn1α coding sequence. The region of exon 1 within the Nrxn1α gene that is deleted in the SD-Nrxn1tm1Sage rats is in bolded red text. The genotyping forward and reverse primers are highlighted in blue. The forward and reverse primers used for real-time quantitative PCR (qPCR) are highlighted in yellow. (B) Example agarose gel with products from a genotyping PCR reaction. (C–E) Nrxn1α expression in SD-Nrxn1tm1Sage pups containing wild type (WT), heterozygous (Het) and homozygous (KO) mutant Nrxn1α alleles as shown by qPCR. (C) Genomic DNA from tailsnips of rats of known genotype was used to validate qPCR primers for Nrxn1α expression. An 183bp product was expected for the WT Nrxn1α, which was detected in WT and Het males and females, but not in KOs of either sex. (D,E) Expression of Nrxn1α in cerebellum (D) and anterior cingulate cortex (E) of P2 pups, as determined by qPCR. Nrxn1α expression is normalized to Gapdh expression using the ΔΔCt method and expressed as mean fold expression relative to WT males +/− 95% confidence interval of mean fold expression. N = 6/group.
Quantitative PCR
Brain tissue was collected from PN2, quick frozen in 2-methylbutane on dry ice, placed in tubes chilled on dry ice, and stored at −80°C. Total RNA was isolated from the tissues using an RNeasy Kit according to the manufacturer’s instructions (Qiagen). Single-stranded complementary DNA (cDNA) was generated using the ABI High-Capacity cDNA reverse Transcription Kit (ThermoFisher Scientific). cDNA products were diluted 1:90 and 5μl was used for fluorescence-based, real-time PCR in a reaction mixture containing SYBR© Green PCR Master Mix (LifeTechnologies) and 250nM primers. PCR reactions were performed for 40 cycles on a ViiA™7 Real-Time PCR System (ABI). Nrxn1alpha expression was normalized to Gapdh as a proxy for total cellular RNA content, and expression among groups was calculated using the ΔΔCt method. Data were expressed as mean fold expression relative to WT males +/− 95% confidence interval of mean fold expression. Primer sequences were: Nrxn1alpha F: 5’-ACCACTCTCTACATCGACC −3’, Nrxn1alpha R: 5’-CATCACGGATCCAGCCCTT −3’, Gapdh F: 5’ – TGGTGAAGGTCGGTGTGAACGG – 3’, Gapdh R: 5’ – TCACAAGAGAAGGCAGCCCTGGT – 3’.
Behavioral Analyses
Neonatal USVs
Isolation-induced ultrasonic vocalizations were recorded from pups on postnatal (PN) days 4, 8 and 10. Pups were removed from the home cage and individually placed into a Styrofoam box for 5 minutes of recording and then returned to the home cage. Recordings were collected using an UltraSoundGate condenser microphone (CM16; Avisoft Bioacoustics) placed at a fixed height of 20 cm above the pup and connected via an Avisoft Ultra-SoundGate 416 USB audio device (Avisoft Bioacoustics) to a computer. Acoustic data were recorded with a sampling rate of 300 000 Hz in 16-bit format. Recordings were analyzed with SASLab Pro (version 5.10; Avisoft Bioacoustics) using an automated whistle-tracking algorithm with a hold time of 5 msec. A high pass filter was used to eliminate background noise below 20 kHz. Spectrograms were manually inspected to ensure that all calls detected using the algorithm were legitimate calls. The number of calls at 40 kHz, which is the characteristic frequency of distress calls for neonatal rat pups, were tabulated in 30 sec. time bins. (N = 3 WT males, 12 Het males, 6 KO males, 6 WT females, 5 Het females, 4 KO females. 1 KO male died after PN4 and 1 Het male was excluded from the analysis at PN8 because the recording failed).
Juvenile Rough-and-Tumble Play Behavior
Rough-and-tumble play behavior was assessed according to VanRyzin et al., (2020b). At weaning on PN21, an independent cohort of juveniles were housed in same-sex/genotype pairs or groups of 3. Beginning on PN27 and continuing daily for 4 days (PN27-PN30), juveniles were placed in 49 × 37 × 24cm enclosure with TekFresh bedding with a same-sex/genotype, non-littermate/cagemate play partner. The pair were allowed to acclimate for 2 min and were then video recorded for 10 min. Videos were manually scored for instances of pouncing, pinning, and boxing. For each individual, scores were averaged over the 4 days to generate a single data point. (N = 8 WT males, 8 Het males, 6 KO males, 8 WT females, 8 Het females, 8 KO females).
Open Field Behavior
The same animals used for the play behavior paradigm were tested a single time on PN26 in a standard open field arena measuring 90 × 90 cm. Locomotion, including distance traveled and line crossings, was tracked for 10 minutes using an overhead camera and AnyMaze tracking software. One WT male was removed from analysis because the tracking software did not accurately follow the rat.
Prosocial/Helping Behavior
Prosocial/helping behavior was assessed using a paradigm modified from Ben-Ami Bartal et al., (2011). Upon weaning on PN21, an independent cohort of experimental rats were housed with a same-sex WT littermate. On PN23 the cagemate pairs were placed in a 49 × 37 × 24cm enclosure with TekFresh bedding (the behavior box). The behavior box contained an empty and open 14 × 8 × 9cm box with a hinged door that is blocked by a lever when closed and requires combined actions by an animal outside and an animal inside the chamber to be opened (hereafter referred to as the confinement chamber). Cagemate pairs explored the behavior box and the confinement chamber together for 10 min. Testing began on PN24 and continued daily for 12 days. On each day, the WT sex-matched sibling of the experimental animal was placed inside the confinement chamber, which was placed inside the behavior box. A funnel filled with ice was positioned to drip into the confinement chamber as the ice melted, thereby motivating the animal in the confinement chamber to seek release. Video recording and a count-up timer began once the experimental rat was placed inside the behavior box. If at anytime the experimental rat released their cagemate from the confinement chamber, the time was recorded and the pair returned to their homecage. To facilitate learning, if 15 minutes elapsed without release, the lever blocking the door on the confinement chamber was loosened slightly, but not enough to allow for a gap in the door. After 5 additional min (20 min total testing time), the lever was opened further to allow for a gap in the door. At this point it was possible for the rat inside the confinement chamber to exit without any assistance from the experimental rat. The time at which the rat inside the confinement chamber exited was recorded (a 20 min maximum score was allowed). Release from the confinement chamber before assistance was provided at the 15min mark was considered a success. Test rats that released their cagemate from the confinement chamber before the 15 min mark 2 days in a row were considered successful, however they were still tested for the remainder of the days in the 12 day paradigm and the latency to open the door was recorded daily. The respective role of the animals in the pair did not vary, and the rat outside the chamber was considered the test subject (N = 6 WT males, 8 Het males, 8 KO males, 8 WT females, 7 Het females, 3 KO females).
Food Treat Retrieval Learning
This behavioral assessment was modified from Ben-Ami Bartal et al., (2011). A separate cohort of juveniles underwent a variation of the prosocial/helping protocol in which they were tasked with retrieving a cereal treat from the confinement chamber. Experimental rats were weaned on PN21 and housed in same-sex pairs or groups of 3 of random genotype. On PN23 cagemate pairs or groups were placed into the behavior box, containing an empty and open confinement chamber, and allowed to explore together for 10 min. Testing began on PN24 and continued daily for 12 days. On each day of testing, 3 cereal treats (orange Apple Jacks) were placed directly inside the door of a closed confinement chamber located inside the behavior box. Testing time began as soon as the experimental rat was placed inside the behavior box. If at any time the rat pushed the lever blocking the door to the confinement chamber completely to the side, the door was opened slightly for them to allow access to the cereal treats. This was done because the doors were designed to be opened with the assistance of the rat inside the confinement chamber once the lever was moved by the rat on the outside. If 15 min passed without the cereal treat being retrieved, the lever blocking the door was loosened slightly. After an additional 15 min had passed (30 min total testing time), the door to the confinement chamber was propped open to allow easy access to the cereal. Rats remained in the behavioral box until the 30 min total testing time mark before being placed back in their homecage. Rats that obtained a cereal treat before the 15 min mark 2 days in a row were considered successful learners (N = 3 WT males, 8 Het males, 8 KO males, 8 WT females, 8 Het females, 8 KO females).
Odor Sensing and Ability to Find a Buried Food Treat
Adolescent (PN38–40) SD-Nrxn1tm1sage KO, Het, and WT siblings that had been used for the prosocial/helping behavior paradigm were tested for olfactory ability to uncover a food reward. Test rats were placed in a novel 20 × 40 × 20cm polycarbonate cage with corncob bedding and a cereal treat buried ~1cm underneath the bedding in the center of the cage. The latency for the rats to uncover the treat and begin eating it was recorded. Rats were returned to their homecage for ~10min. They were then placed in the same 20 × 40 × 20cm polycarbonate cage with corncob bedding and a cereal treat buried ~6cm underneath a pile of TekFresh bedding in a corner. The latency for the rats to uncover the treat and begin eating it was recorded. N = 2 animals per sex/genotype. Males and females were combined for each genotype.
Juvenile Sensitization/Nurturing Behavior
Nurturing behavior in juvenile rats was assessed using a sensitization paradigm as described by Mayer and Rosenblatt (1979) and Oxley and Fleming (2000). This simple test involves exposing test subjects to newborn pups for a limited time each day for a series of days. Initially ignoring or avoiding the pups, rats eventually develop a nurturing behavior involving nest building, retrieval of pups to the nest and huddling with them to generate warmth (Rosenblatt, 1967). Beginning on PN24, an independent cohort of SD-Nrxn1tm1sage KO, Het, and WT juveniles were placed in a novel 20 × 40 × 20cm polycarbonate cage with corncob bedding, a large handful of TekFresh bedding in one corner, and 3 pups age 1 to 5 days old in the corner opposite the TekFresh bedding. The test rats were allowed to remain in the testing cage containing pups and bedding for 2 hours undisturbed, and then returned to the home cage. This was repeated daily for 14 days. At the end of each daily 2 hour testing period, the juveniles were scored for nurturing behavior, which was defined as moving pups to the TekFresh nesting material, moving the TekFresh to the pups and arranging it in a nest around the pups, or huddling with the pups. The first day that test rats showed nurturing behavior and the total number of days exhibiting nurturing behavior throughout the 14 day testing period were tabulated. Because there was no effect of sex, males and females were combined for analysis. (N = 5 WT Males, 6 Het males, 6 KO males, 5 WT females, 6 Het females, 6 KO females).
Eyeblink conditioning
An independent cohort of rats was used for the delay eyeblink conditioning, as adapted from the procedure described in Wood and Shors (1998). On PN47–48, SD-Nrxn1tm1sage Het or WT SD rats were anesthetized and surgically fitted with a headstage to permit delivery of the unconditioned stimulus (US) and recording of the electromyographic (EMG) activity from the right eye. Headstages were fitted onto the skull with dental cement anchored by screws. Four electrodes (insulated stainless steel wire with a diameter of 0.005 inches) were implanted through the upper eyelid muscle. Two electrodes delivered the US of periorbital stimulation (1 mA, 100 msec), and two recorded electromyographic (EMG) activity as a measure of blinks. Pavlovian eyeblink conditioning was initiated between 54–55 of age. The conditioned stimulus (CS) was a 2.8 kHz tone, at 85 dB, and 380 msec in duration. The CS and US were presented in a delay conditioning stimulus arrangement. The CS and US overlapped and co-terminated. Conditioned responses (CRs) were EMG signals that were at least 7 msec in duration and 4 standard deviations above a 280 msec baseline period immediately preceding the US. All rats received an acclimation session, in which the animal was attached to the stimulus delivery and data acquisition cable and placed in the conditioning chambers with no stimulus delivery. The following 4 days, 100 trials were presented each day with an intertrial interval of 30±5 sec. Ninety of these trials were CS+US trials, and 10 were CS alone trials to permit detection of the CR without contamination for the US. Only CRs expressed during the 100 msec prior to the onset of the US were considered CRs on paired trials, and CRs during the last 200 msec of the tone on non-reinforced trials were considered CRs (N = 6 WT males, 6 Het males, 4 KO males, 6 WT females, 5 Het females, 3 KO females).
Sociability and Social Discrimination
A separate cohort of juvenile SD-Nrxn1tm1sage KO, Het, and WT rats were used to assess sociability and preference for social novelty in a 3-chamber social interaction test, as described by Smith et al., 2015. The testing arena measured 40 × 90 cm and was divided into 3 equal-sized internal chambers with 2 clear plexiglass dividers, each with 15 × 15 cm openings to allow free passage between all 3 chambers. On PN28, test rats were individually placed in the center chamber of the arena and allowed to explore all chambers freely for 5 mins. At the end of the habituation phase, the test rat was removed to a holding cage. An empty wire containment cage was placed in one end chamber of the testing arena, and a wire containment cage containing an unfamiliar WT Sprague Dawley rat of similar age was placed in the other side chamber. The test rat was placed in the center chamber of the testing arena and allowed to explore freely for 5 mins. At the end of the sociability test phase, the test rat was removed to a holding cage while a novel WT Sprague Dawley juvenile was placed in the empty containment cage in the arena. The test rat was placed in the center chamber and allowed to explore freely for 5 mins, and movement was tracked from above using AnyMaze software. The amount of time spent in each chamber, determined by the presence of all four paws of the rat in the chamber, was tabulated. Sociability was calculated from the second 5 minute test stage, as the amount of time the test rat spent in the social chamber as a percentage of total time spent in either the social or non-social chamber. A measurement of preference for a novel social stimulus, often referred to as social discrimination, was calculated from the third 5 minute testing stage as the amount of time the rat spent in the novel social chamber as a percentage of total time spent in either the left or right social chambers (N = 4 WT males, 8 Het males, 5 KO males, 6 WT females, 10 Het females, 5 KO females).
Novel Object Investigation
The same rats used for the 3-chamber social interaction test were tested in a one-trial novel object recognition task, as described by Ennaceur and Delacour (1988). On PN30, individual rats were place in an arena measuring 90 × 90 cm and allowed to explore freely for 5 minutes. The rat was removed to a holding cage while two identical objects were placed in the arena, each approximately 20 cm equidistant from the arena walls. The rat was returned to the arena and allowed to freely explore for 10 minutes. The rat was then removed to a holding cage and the objects in the arena were replaced with a third object, identical to the first two, and another object with different shape and color properties but made of the same material. The objects used were small plastic tub toys, approximately 7 × 8 × 6 cm. The rat was then allowed to explore the arena freely for 10 minutes. In each stage of the test, movement was tracked from above using AnyMaze software. The resulting videos were scored for the amount of time spent exploring each object. Object investigation time as presented was scored from the first 5 minutes of the last stage of the test. 2 WT, 4 Het and 1 KO females in this cohort were used in the 3-chamber social interaction test but not used in the novel object recognition test due to equipment issues (N = 4 WT males, 8 Het males, 5 KO males, 4 WT females, 7 Het females, 4 KO females).
Statistical Analyses
Data were tested for normality using the Kolmogorov-Smirnov test. All parametric data were analyzed by multivariate ANOVA’s that included sex and genotype as factors (# of USVs, play events, locomotion), within-sex ANOVA with genotype as a factor (nurturing onset, total nurturing, average median latencies to open door, object investigation), or repeated measures ANOVA with sex and genotype as between subjects variables (conditioned eyeblink response). Post-hoc analyses, as indicated, were conducted following significant main effects or interactions and were based on whether there were multiple comparisons or a priori matched groups. Non-parametric categorical data (% successful door openers) were analyzed by Chi-Square. Kruskal-Wallis was used to test for significance of non-Gaussian distributed data. Post-hoc analyses, as indicated, were conducted following significant main effects or interactions and were based on whether there were multiple comparisons or a priori matched groups. Significance was established at p < 0.05.
Results
Confirmation of Haploinsufficiency and Complete Knockout in SD-Nrxn1tm1sage Rats.
Primers were designed to the region of Nrxn1α deleted in SD-Nrxn1tm1sage rats and were tested using genomic DNA from tail snips of rats of known genotype (Figure 1, A and B). Fresh cerebellum and anterior cingulate cortex tissue was collected at PN2 from pups resulting from SD-Nrxn1tm1sage Het X Het pairings. RT-PCR, normalized to GAPDH, confirmed a complete absence of Nrxn1alpha mRNA in full knockouts and a 50% decrease in heterozygous pups and no impact of sex (Figure 1, C–E).
Neonatal SD-Nrxn1tm1sage KO and HET Pups Have Reduced Ultrasonic Vocalizations When Separated from the Dam.
Pups from 4 separate litters were recorded for isolation-induced USVs at postnatal days 4, 8 and 10 (Figure 2). There was a main effect of genotype on the amount of these calls at all three ages. At 4 days of age, both male and female pups with biallelic mutations in the Nrxn1α gene made fewer USVs than WT pups throughout the 5 min recording session (Figure 2A; 2way ANOVA; main effect of genotype: F(2,30) = 10.5, p = .0004, η2p = .213). There was no effect of sex on the number of USVs at this age (F(1,30) = 1.1, p = .3015, η2p = .035). This pattern continued at PN8 where a main effect of genotype (2way ANOVA: F(2,28) = 19.6, p = .0001, η2p = .583) but not sex (2way ANOVA: F(1,28) = 1.32, p = .9091, η2p = .045) was seen, driven mainly by a significant decrease in the number of USVs by KO males and females (Figure 2B). At PN10 the effect of genotype on calls continued (2way ANOVA: F(2,29) = 29.1, p < .0001, η2p = .667), with KO pups producing fewer USVs (Figure 2C). At PN10 there was a main effect of sex, with females producing fewer calls than males, regardless of genotype (2way ANOVA: F(1,29) = 7.7, p = .0095, η2p = .347). At all three postnatal ages, there was a trend toward fewer USVs in Het males and females, compared to WT pups, however within-sex ANOVA did not detect significant differences between WT and Het pups. The maximum length of separation-induced USVs was also determined (Figure 2D–F). KO females had shorter call lengths at PN8 and PN10, compared to WT and Het females, accounting for the main effect of genotype on call length at these ages (Figure 2E,F: 2Way ANOVA at PN8, F(1,29) = 8.3, p = 0.0073, η2p = .364; at PN10, F(1,29) = 17.7, p = 0.0002, η2p = .549). Post-hoc analyses revealed no difference in call length among genotypes for females at PN4, or for males at any of these ages.
Figure 2: SD-Nrxn1tm1Sage KO pups emit fewer ultrasonic vocalizations when isolated from the dam.
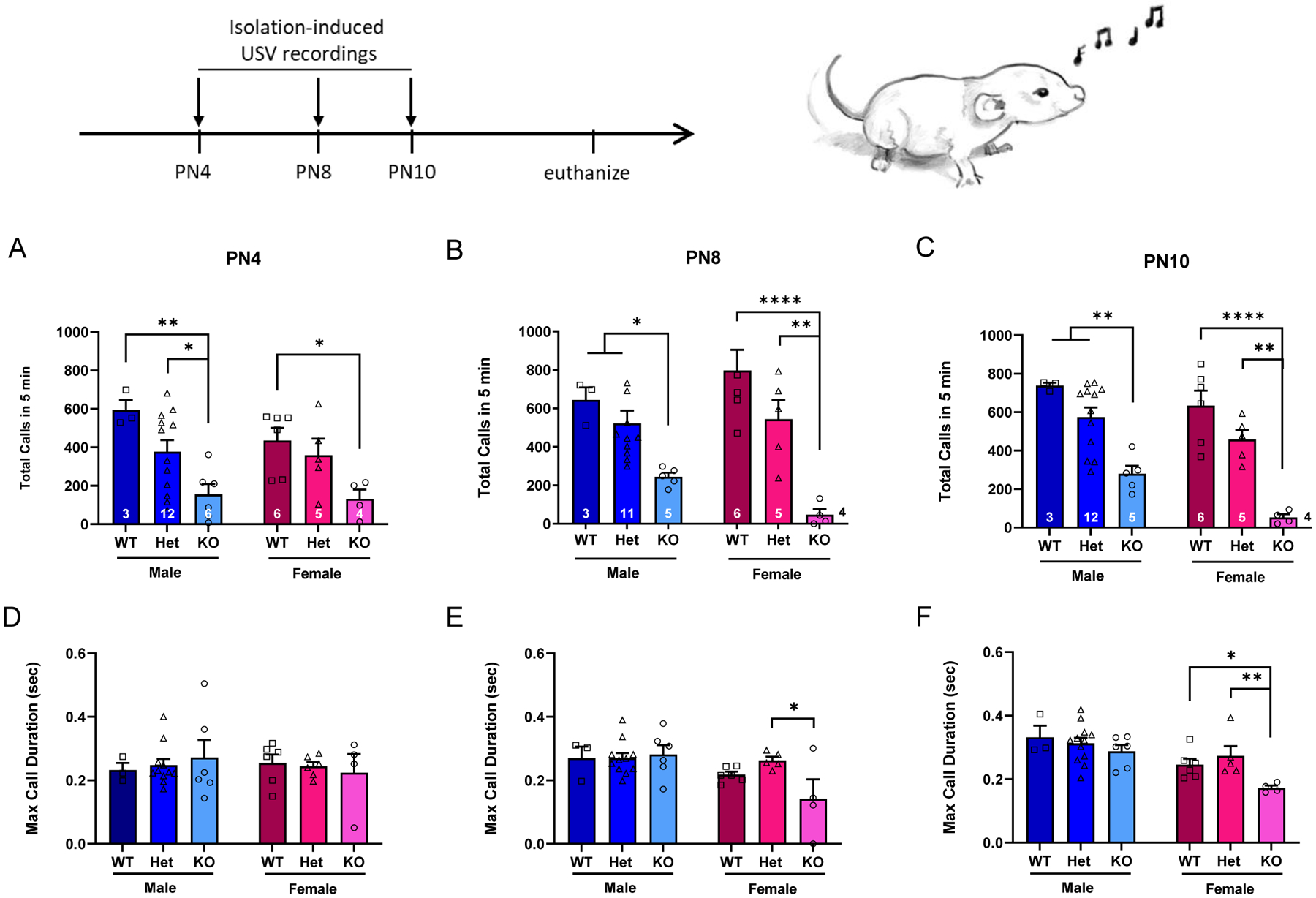
SD-Nrxn1tm1Sage Het, KO, and WT siblings from 5 litters were individually removed from the home cage and their ultrasonic calls were recorded for 5 minutes during the first two postnatal weeks. (A–C) Total number of calls emitted in 5 minutes. (D–F) Maximum duration of individual calls emitted. Both male and female pups with biallelic mutations in Nrxn1α emitted fewer calls than their WT and Het littermates when isolated on PN4 (A), PN8 (B) and PN10 (C). The maximum duration of individual calls was shorter in KO females on PN8 (E) and PN10 (F), but not on PN4 (D), compared to WT and Het females. 2Way ANOVA with Tukey’s posthoc within sex: *= p<0.05, ** = p < 0.005, **** = p < 0.0001. (N = 3 WT males, 12 Het males, 6 KO males, 6 WT females, 5 Het females, 4 KO females. 1 KO male died after PN4 and 1 Het male was excluded from the analysis at PN8 because the recording failed).
SD-Nrxn1tm1sage KO Males Show Increased Locomotion in the Open Field.
There was a significant effect of genotype on locomotive behavior, as determined by distance traveled in the open field (Figure 3; 2way ANOVA: F(2,41) = 5.5, p = .0075, η2p = .212). Males with biallelic Nrxn1α were significantly more mobile in the open field, compared to WT and Het males. There was no difference between WT and Het males in locomotive behavior. There was no effect of Nrxn1α deficiency on locomotive behavior in females, and no effect of sex (2way ANOVA: F(1,41) = 0.2, p = .6386, η2p = .005). There was no effect of genotype or sex on time spent in the center of the open field (2Way ANOVA, genotype: F(2,42) = 1.3, p = .2941; sex: F (1,41) = 0.67, p = .4191, η2p = .016).
Figure 3: Juvenile SD-Nrxn1tm1Sage KO males display increased locomotion in the open field.
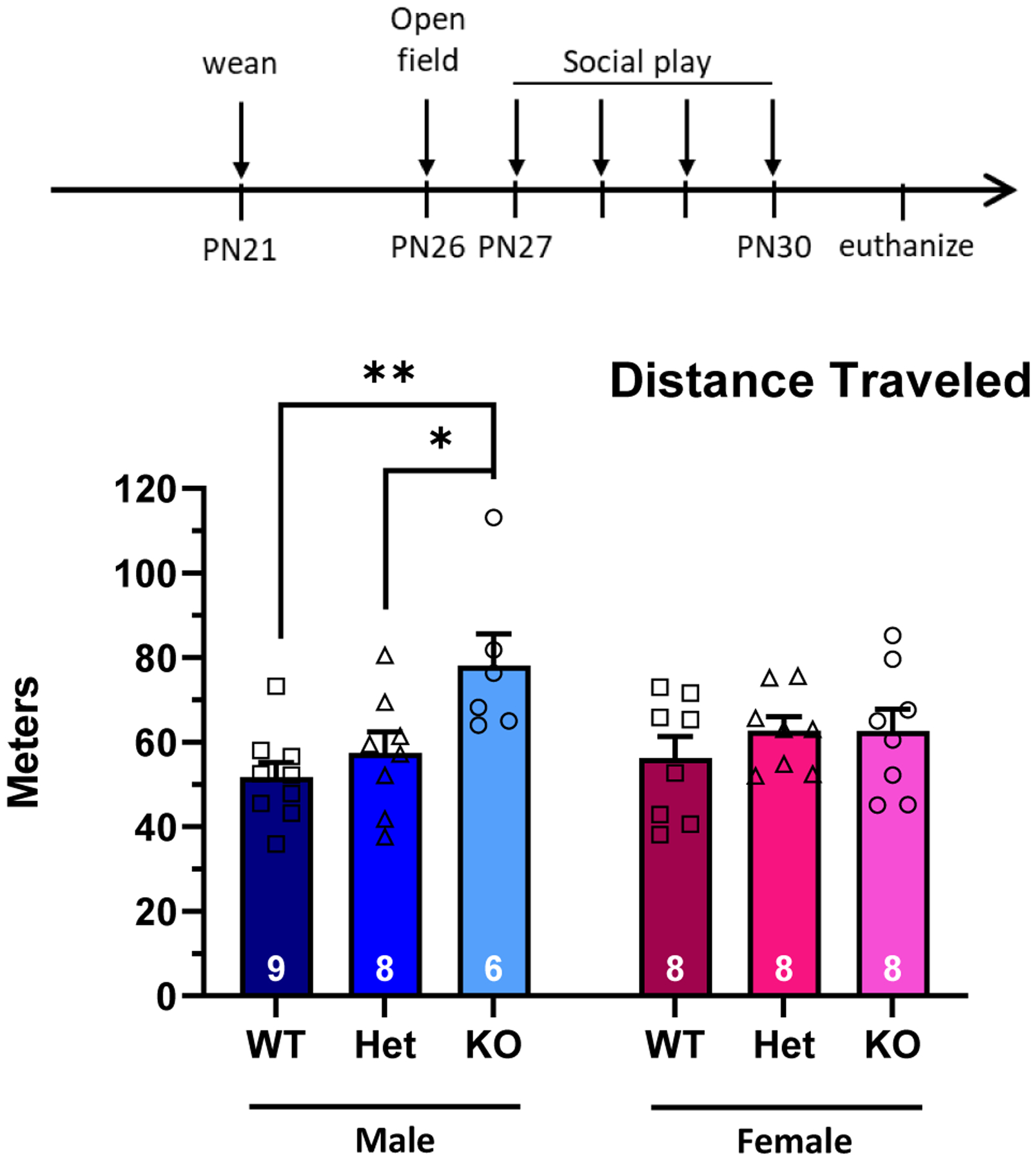
Biallelic mutation of Nrxn1α results in increased locomotive behavior in males, but not females, as shown by distance traveled in the open field. 2Way ANOVA with Tukey’s posthoc within sex: * = p < 0.05, ** = p < 0.01 (N = 8 WT males, 8 Het males, 6 KO males, 8 WT females, 8 Het females, 8 KO females).
Juvenile Rough-and-Tumble Play is Reduced in SD-Nrxn1tm1sage KO Males.
Juveniles were assessed for play behavior daily for 4 days, and events of pouncing, pinning, and boxing were scored (Figure 4). There was a main effect of genotype on the total number of play events, driven by decreased total play in KO males, compared to WT and Het males (Figure 4A; 2Way ANOVA, F(2,40) = 6.8, p = .0028, η2p = .254). There was no effect of sex on total play behavior (2Way ANOVA, F(1,40) = 0.3, p = .6029, η2p = .007). Play initiation, indicated by the number of pounces, was decreased in KO but not Het males, compared to WT males, and did not differ by genotype in females (Figure 4B; main effect of genotype: F(2,40) = 5.8, p = .0062, η2p = .225; sex: F(1,40) = 0.1, p = .7923, η2p = .002). The number of boxing bouts was significantly impacted by genotype but not sex (Figure 4C; main effect of genotype: F(2,40) = 6.2, p = .0045, η2p = .237; sex: F(1,40) = 0.02, p = .8924, η2p = .0004). However, there was a significant sex X genotype interaction in the number of boxing bouts (F(2,40) = 8.8, p = .0007, η2p = .306). Both KO and Het males engaged in fewer boxing bouts than WT males. In females, Het juveniles engaged in more boxing bouts than either WTs or KOs, which did not differ from each other. Similar to the deficit in play initiation seen in KO males, there was a main effect of genotype but not sex on play reciprocity, indicated by the number of pins (Figure 4D; 2Way ANOVA, main effect of genotype: F(2,40) = 7.4, p = .0019, η2p = .270; sex F(1,40) = 0.6, p = .4425, η2p = .014). KO males, but not females, engaged in fewer pins compared to WT and Het rats.
Figure 4: Juvenile SD-Nrxn1tm1Sage KO males have decreased juvenile rough-and-tumble social play behavior.
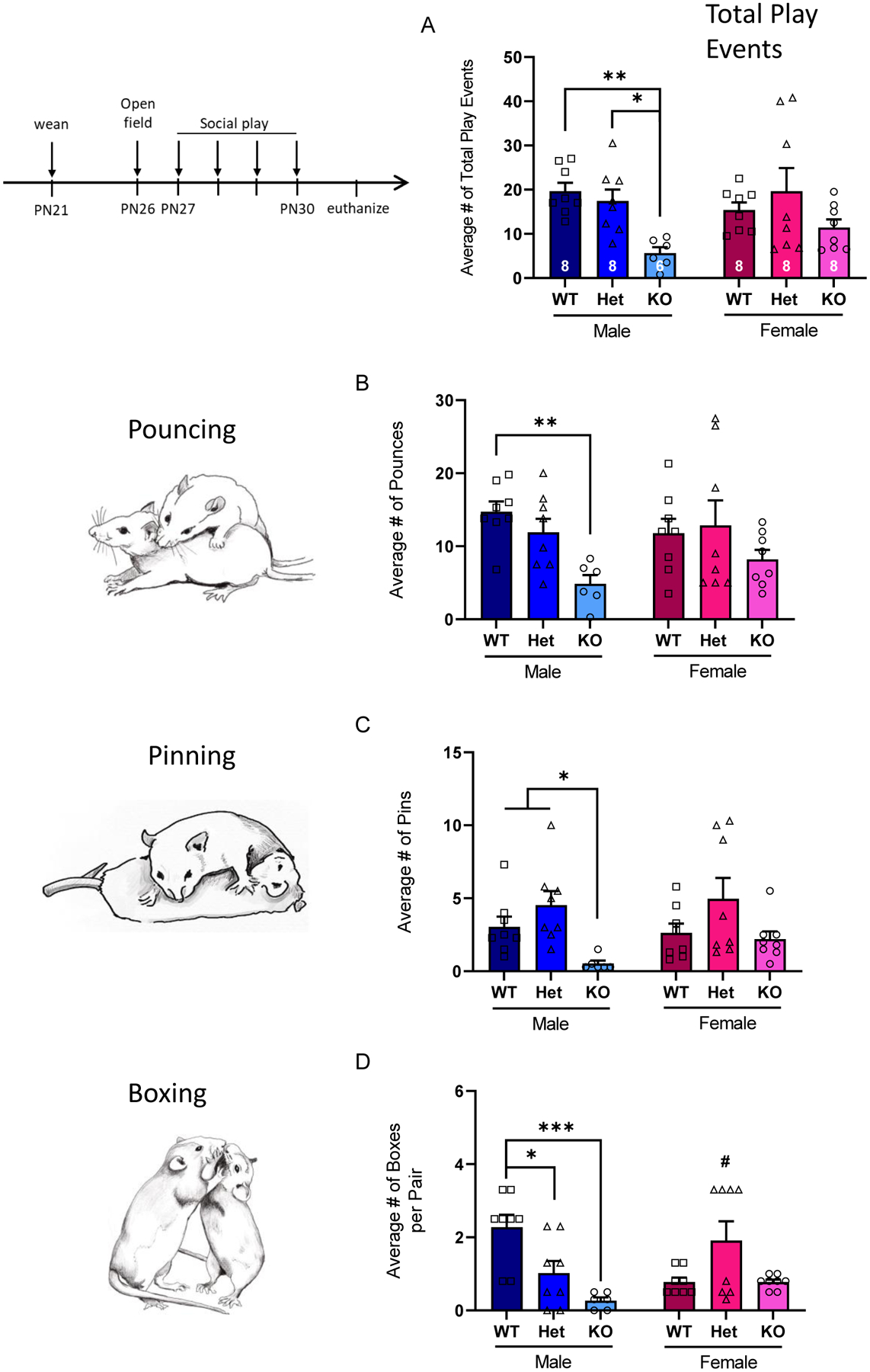
(A) The average number of total play events over 4 days. Juvenile KO males have decreased total play (combination of pouncing, pinning, and boxing) relative to WT and Het siblings. (B) The average number of pouncing events over 4 days. KO males pounced less compared to WT males. (C) The number of boxing events over 4 days. Both KO and Het males engaged in fewer boxing events. (D) The average number of pinning events over 4 days. Juvenile KO males did not pin as frequently as their sex-matched WT and Het siblings. 2Way ANOVA with Tukey’s posthoc within sex: *= p<0.05, ** = p < 0.01, *** = p < 0.001, # = p < 0.05 compared to WT and Het females (N = 8 WT males, 8 Het males, 6 KO males, 8 WT females, 8 Het females, 8 KO females).
Performance in a Test of Prosocial Helping Behavior is Impaired in SD-Nrxn1tm1sage HET and KO Juveniles of Both Sexes.
To investigate the social bond that SD-Nrxn1tm1sage rats form with conspecifics, we used a prosocial behavioral paradigm in which experimental rats were tasked with releasing their WT sex-matched sibling cagemate from a confinement box (Figure 5A), a task which takes multiple days to learn. Figure 5B shows the group median latency to open the confinement box door and release the cagemate on each day, and illustrates that Nrxn deficient males and females required more days to learn to open the door without assistance from the experimenter. As a group, even after 12 days of testing KO males and females did not successfully open the door unaided. Median door opening latencies averaged over the first 6 days of testing, when all groups were learning the task and required assistance to release the cagemate, shows no difference among genotypes in performing this task (Figure 5C) (Within-sex Kruskal-Wallis ranks test; Males: H (3) = 3.9, P = .1441, η2(H) = .1; females: H (3) = 1.7, P = .4381, η2(H) = .02). From day 7 onward, WT and Het rats of both sexes learned the task and required less time to open the door and release their cagemate, but KO males did not open the door prior to 15 mins of testing time, when the experimenter moved the door latch to facilitate door opening. The performance of KO females trended toward a deficit as well (Figure 5D) (Within-sex Kruskal-Wallis ranks test. Males: H (3) = 9.3, P = .0053, η2(H) = .384; females: H (3) = 5.4, P = .0619, η2(H) = .227). Juveniles are considered successful at this paradigm after two consecutive days of releasing their sibling. There was a sex difference in the acquisition of success in that more females were successful in releasing the confined social partner by the end of the paradigm (Figure 5E; X2 (11) = 23.73, p = 0.0139, V = 1.04). Among the SD-Nrxn1tm1sage Het juveniles there were fewer successful rats over the 12-day test period (3/8 or 38% of male Hets; 2/7 or 26% of female Hets), relative to sex-matched WTs (5/6 or 88% of male WTs; 7/8 or 88% of female WTs; Males X2 (11) = 66.55, p < 0.001, V = 1.74; Females X2 (11) = 41.13, p < 0.001, V = 1.37). SD-Nrxn1tm1sage KO juveniles also had a lower success rate relative to WTs. None of the female KO rats (0/3) successfully completed the task and only 2 of 7 (28%) male KO’s successfully released their cagemate siblings from the confinement box without assistance from the experimenter, within the 12-day period (Females X2(11) = 50.79, p < 0.001; males X2(11) = 25.61, p = 0.0074; (Figure 5E).
Figure 5: SD-Nrxn1tm1Sage Het and KO juveniles do not perform as well as WTs in a test of prosocial helping behavior.
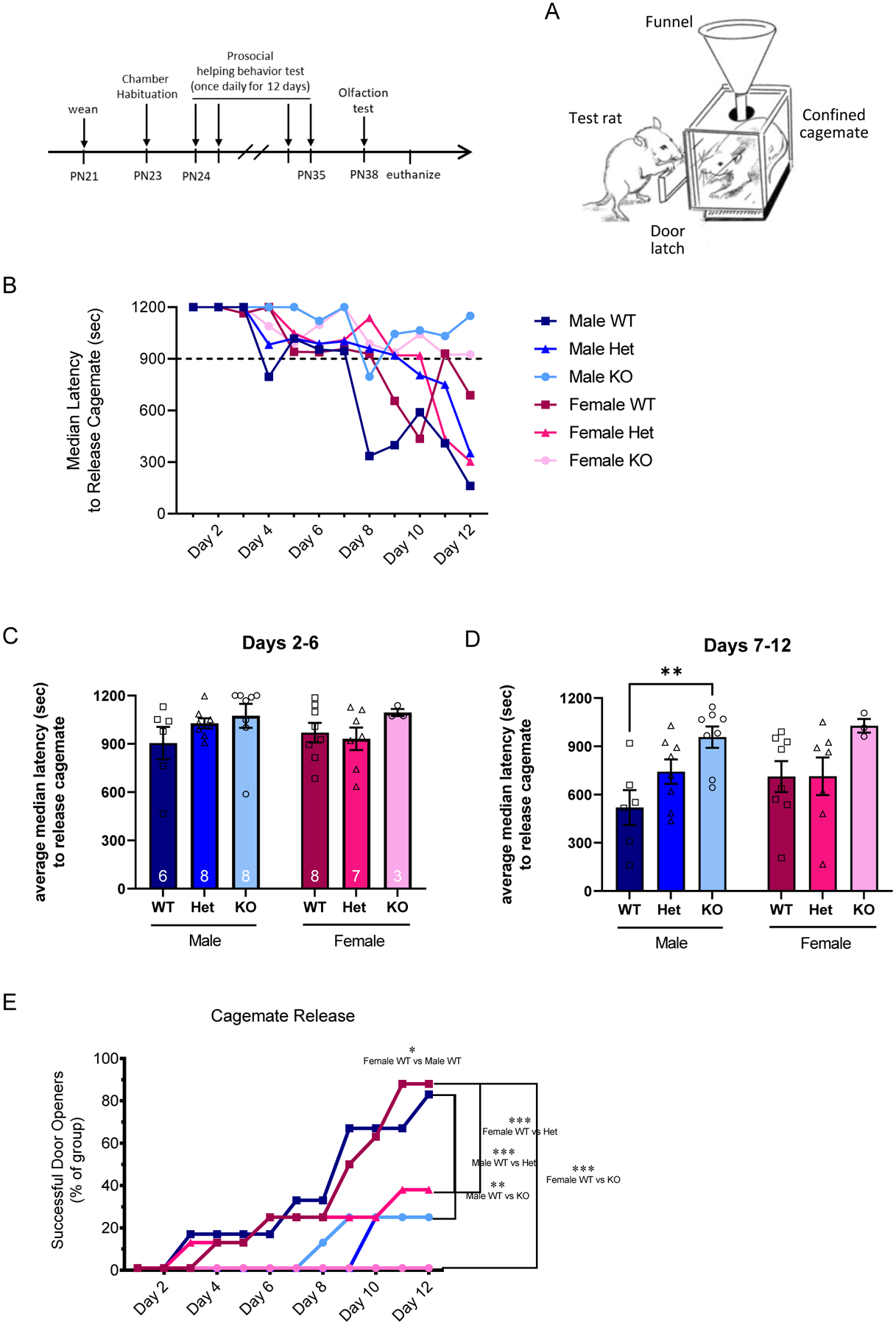
(A) Prosocial behavior test apparatus, showing the test animal successfully opening the latch on the confinement box door to release a confined sibling cagemate. (B) Median group latencies to release cagemate from confinement in the prosocial behavior test. The median time, in seconds, to release a confined sibling cagemate in a single trial each day over 12 consecutive days of testing is plotted for each group. Dashed line at 900 seconds indicates the time at which the experimenter released the door latch if the test animals had not already released the confined cagemate. As a group, KO males and females were not able to open the door and release the confined cagemate without assistance from the experimenter at any time within the 12 day trial. (C) Median group latencies to release the confined cagemate, averaged over days 2–6 of the trial. There were no differences among groups. (D) Median group latencies to release the confined cagemate, averaged over days 7–12 of the trial. KO males demonstrated increased latency to perform this task, compared to WT males. Kruskal-Wallis, ** = p < 0.01. (E) The percent of test rats per group that successfully released a confined sibling cagemate each day of testing. Fewer KO and Het males and females become successful door openers relative to sex-matched WT sibling control rats. Chi-Square * = p < 0.05, ** = p < 0.01, *** = p < 0.001 (N = 6 WT males, 8 Het males, 8 KO males, 8 WT females, 7 Het females, 3 KO females).
SD-Nrxn1tm1sage HETs and KO Do Not Learn to Retrieve a Food Treat as Well as WT Juveniles in a Complex Task.
To test whether deficits in the prosocial paradigm were due to an inability to learn the door-opening task, rather than an altered motivation to release their sibling cagemate, the prosocial helping paradigm was modified to involve retrieving a food treat from the same apparatus instead of freeing a social partner, and a separate cohort of rats was tested. Similar to the performance of SD-Nrxn1tm1Sage rats when releasing a confined cagemate, KO males and females did not successfully open the door to release a food treat throughout the 12 day test period without help from the experimenter (Figure 6A). Median door opening latencies averaged over the first 6 days of testing shows no difference among genotypes in performing this task (Figure 6B) (Within-sex Kruskal-Wallis test; Males: H (3) = 3.1, P = .2197; females: H (3) = 1.6, P = .4554). When averaged over days 7–12, KO males and females took significantly longer to perform this task (Figure 6C) (Within-sex Kruskal-Wallis test. Males: H (3) = 5.4, P = .0408, η2(H) = .213; females: H (3) = 6.0, P = .0391, η2(H) = .19). As also observed in the prosocial helping task, 100% of both male and female WT juveniles successfully learned to open latch of the confinement box door to retrieve a food treat within 12 days of testing (Figure 6D; 3/3 males and 8/8 females), with males learning faster than females (X2 (11) = 49.09, p < 0.001). Het juveniles were less successful than WTs, on average, with only 38% of each sex (3/8 each, males and females) successfully learning the task within 12 days (females X2 (11) = 19.56, p = 0.0518; males X2 (11) = 47.08, p < 0.001). The KO juveniles were the most impaired, with only 25% of female (2/8) and 13% of male (1/8) KOs successfully retrieving a food treat from the apparatus within 12 days of testing (females X2 (11) = 35.58, p = 0.0002; males X2 (11) = 50.43, p < 0.001). These results demonstrate that full or partial deletion of Nrxn1α results in learning deficits in this complex task. They further suggest that the deficits in the prosocial helping task exhibited by Nrxn deficient rats is at least partly due to a deficit in learning or cognition.
Figure 6: SD-Nrxn1tm1Sage Het and KO juveniles do not perform as well as WTs in a complex food-reward task.
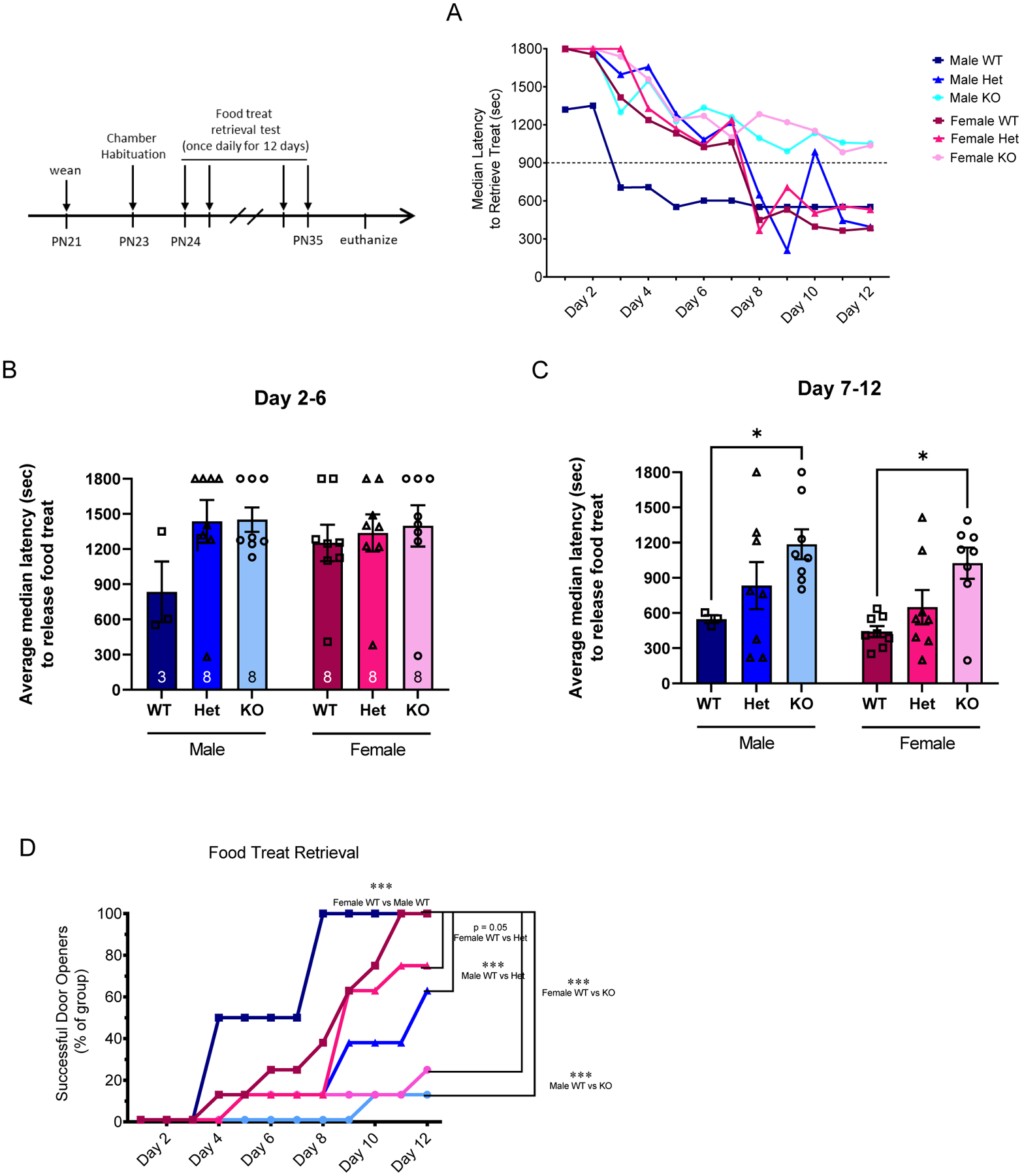
(A) Median group latencies to retrieve a food reward from the confinement box. The median time, in seconds, to retrieve a food reward in a single trial each day over 12 consecutive days of testing is plotted for each group. Dashed line at 900 seconds indicates the time at which the experimenter released the door latch if the test animals had not already opened the confinement box door. As a group, KO males and females were not able to open the door and access the food reward without assistance from the experimenter at any time within the 12 day trial. (B) Median group latencies to open the door and retrieve a food reward, averaged over days 2–6 of the trial. There were no differences among groups. (C) Median group latencies to retrieve the food reward, averaged over days 7–12 of the trial. KO males and females demonstrated increased latency to perform this task, compared to WT rats. Kruskal-Wallis, p < 0.05. (D) The percent of test rats per group that successfully retrieved the food reward on each day of testing. Fewer KO and Het males and females become successful door openers relative to sex-matched WT sibling control rats. Chi-Square *** = p < 0.001 (N = 3 WT males, 8 Het males, 8 KO males, 8 WT females, 8 Het females, 8 KO females).
Nrxn1α Deficient Juveniles Have Normal Olfaction.
Given the deficits we observed in the door-opening task when rats were tasked with removing a food treat, we assessed their olfactory function. Rats were placed in a novel cage with corn cob bedding and a cereal treat shallowly buried under the bedding in the center of the cage as a training trial, and a subsequent test in which the cereal treat was buried under a large pile of TekFresh bedding. During the training trial all rats found the cereal treat and began eating it within 10min. With the subsequent test trial, all rats found the food treat and began eating it in less than 1min, confirming normal olfaction (Figure 7).
Figure 7: Nrxn1α deficiency does not impact olfaction in juvenile rats.
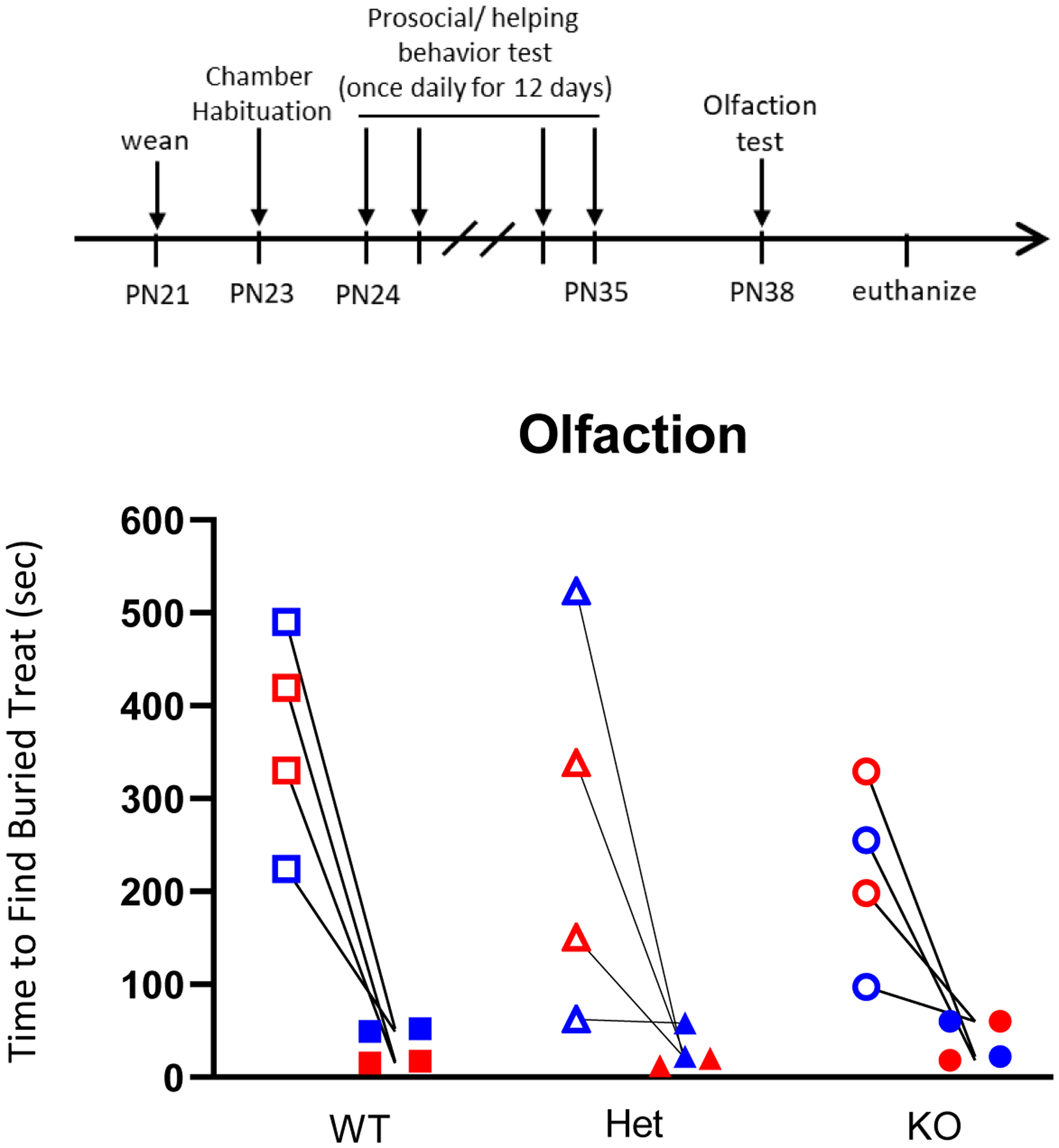
Olfaction of SD-Nrxn1tm1Sage juveniles was determined by the ability to uncover a buried food treat. Open symbols indicate the amount of time, in seconds, required for each juvenile to find and begin eating a shallowly-buried treat during the training phase, and filled symbols indicate the amount of time required for each juvenile to find and begin eating a deeply-buried treat in a subsequent test phase. Red = females, blue = males (N= 2 rats each genotype/sex).
Onset of Nurturing Behavior Towards Unfamiliar Pups is Altered in SD-Nrxn1tm1sage KO Juveniles.
Because Nrxn1α deficiency was associated with impaired performance in the door-opening task to retrieve a food treat, the effects of Nrxn1α deficiency on the prosocial task to free a trapped social partner from the same apparatus may be confounded by learning or cognitive deficits. We therefore sought to further probe prosocial behavior using a test that does not require learning a complex task. When exposed to neonatal pups isolated from the dam, prepubertal rats will eventually overcome an innate neophobic response to the pups and engage in nurturing behavior by nesting and huddling with the pups. We examined the onset of nurturing behavior in SD-Nrxn1tm1sage in a juvenile sensitization paradigm, and predicted that nurturing behavior would be reduced in Nrxn1α knockout rats. Juveniles were exposed to pups daily for 14 days and latency to the first display of nurturing behavior and the total number of days spent nurturing were recorded. Nurturing behavior, either evidence of nesting of the pups or observation of juveniles huddling with the pups, was often not observed on every subsequent day after the first observation of nurturing behavior, creating a fragmented picture of this behavior (Figure 8A). There was no effect of sex on number of days to first nurturing behavior or total days displaying nurturing behavior, therefore data for males and females were combined. Contrary to expectation, juveniles with a biallelic mutation in Nrxn1α exhibited a shorter latency to the onset of nurturing behavior, compared to WT rats, showing nurturing behavior toward the pups an average of 4 days earlier, compared to WTs (Figure 8B; Kruskal Wallis: H(3) = 6.8, P = .0328, η2(H) = 0.155). There was no difference between KO and Het or Het and WT rats in the onset of nurturing behavior. When the total number of days each juvenile spent nurturing the pups was tabulated, there was no difference among genotypes (Figure 8C; Kruskal-Wallis: H(3) = 1.2, P = .5502).
Figure 8: SD-Nrxn1tm1Sage KO juveniles have a decreased latency to display nurturing behavior in a pup sensitization paradigm.
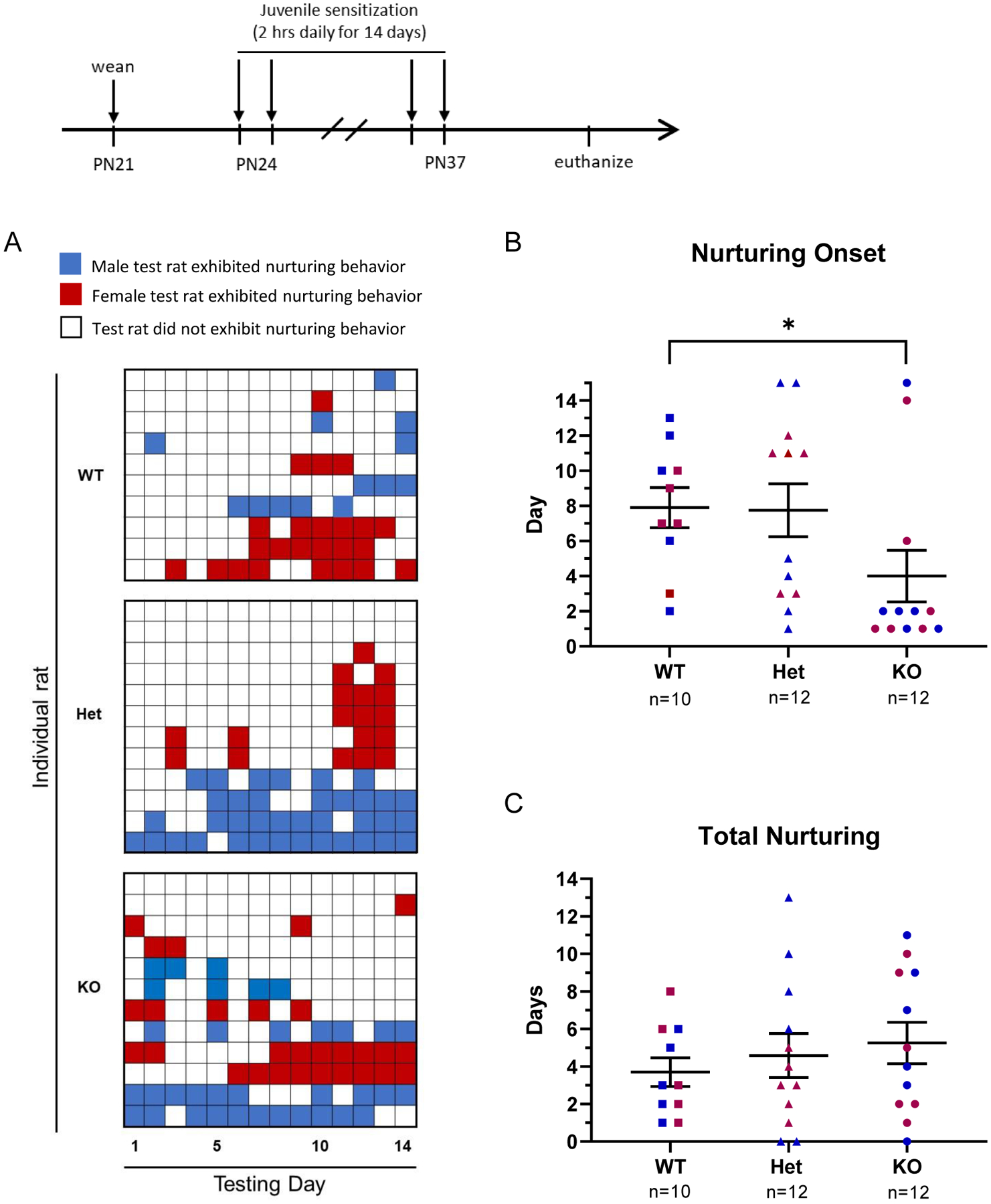
There was no effect of sex on the nurturing behavior of SD-Nrxn1tm1Sage WT, Het or KO juveniles toward neonatal pups, therefore data for males and females were combined. (A) Raster plot of daily occurrence of nurturing behavior toward unfamiliar pups over the 14 day test period. The presence of nurturing behavior, scored at the end of a daily 2 hr exposure to pups, is indicated with filled squares in red (for female test rats) or blue (for male test rats). White squares indicate no nurturing behavior was observed at the end of the 2 hr pup exposure period on that day. Each row tabulates daily observations for an individual test rat over the 14 day test period. The top block tabulates WT rats (5 males/5 females), the middle block tabulates Het rats (6 males/6 females) and the bottom block tabulates KO rats (6 males/6 females). Note that 1 KO and 2 Het males did not demonstrate pup-directed nurturing behavior on any of the 14 test days. (B) Onset of nurturing behavior, scored as the day on which juveniles first displayed nurturing behavior toward unfamiliar pups. Juvenile KO males and females nested pups earlier than WT or Het juveniles. 3 male rats that did not exhibit nurturing behavior on any of the 14 test days were assigned a score of 15 for nurturing onset. ANOVA with Tukey’s posthoc, * = p < 0.05. (C) The total number of days SD-Nrxn1tm1Sage juvenile rats exhibited nurturing behavior throughout the 15 days of exposure to pups. There was no difference in total days nurturing among genotypes. Red = females, blue = males (N = 5 WT Males, 6 Het males, 6 KO males, 5 WT females, 6 Het females, 6 KO females).
Acquisition of the Conditioned Eyeblink Response is Impaired in Male SD-Nrxn1tm1Sage Het Rats.
It has become increasingly clear that the cerebellum has an important role in the development of social behavior (Sathyanesan et al., 2019). Injury to the cerebellum during early childhood has long-lasting consequences for social cognition and is strongly associated with neurodevelopmental disorders such as ASD, ADHD and schizophrenia (Stoodley, 2016; Stoodley and Limperopoulos, 2016; Wang et al., 2014), and animal studies demonstrate direct influence of cerebellar output on reward and social circuitry (Badura et al., 2018; Carta et al., 2019). Given the importance of Nrxn1α in the formation of intracerebellar circuits (Uemura et al., 2010; Zhang et al., 2015), we sought to assess the effect of Nrxn1α deficiency on cerebellar function using a simple Pavlovian learning paradigm, the conditioned eyeblink response. The number of well-timed blinks in response to a conditioned stimulus was tabulated for a 100-trial session each day for 4 consecutive days in WT, Het and KO SD-Nrxn1tm1Sage juvenile rats (Figure 9). Repeated measures ANOVA with genotype and sex as between subjects variables and session as the within subjects variable found a significant effect of session, as rats increased the number of conditioned responses as training progressed (2Way ANOVA with Repeated Measures, F(3,72) = 38.4, p = .0001). There was no interaction between sex and session. There was a significant main effect of genotype (2Way ANOVA with Repeated Measures, F(2,24) = 6.44, p = .006, η2(P) = .349) and also a main effect of sex (2Way ANOVA with Repeated Measures, F(1,24) = 5.81, p = .02, η2(P) = .195) on acquisition of the conditioned learning response, but no interaction between genotype and sex. When males and females were analyzed separately, the effect of genotype was present for males (Figure 9A, within-sex ANOVA with Repeated Measures, F(2,13) = 8.9, p = .0210, η2(P) = .578), but not females (Figure 9B; within-sex ANOVA with Repeated Measures, F(2,12) = 1.2, p = .4651, η2p = .167). Contrary to expectation, Het SD-Nrxn1tm1Sage males exhibited a deficit in the rate of acquisition of the conditioned learning response, but KO males were not different from WT.
Figure 9: Nrxn1α deficiency alters Pavlovian learning in the conditioned eyeblink response paradigm.
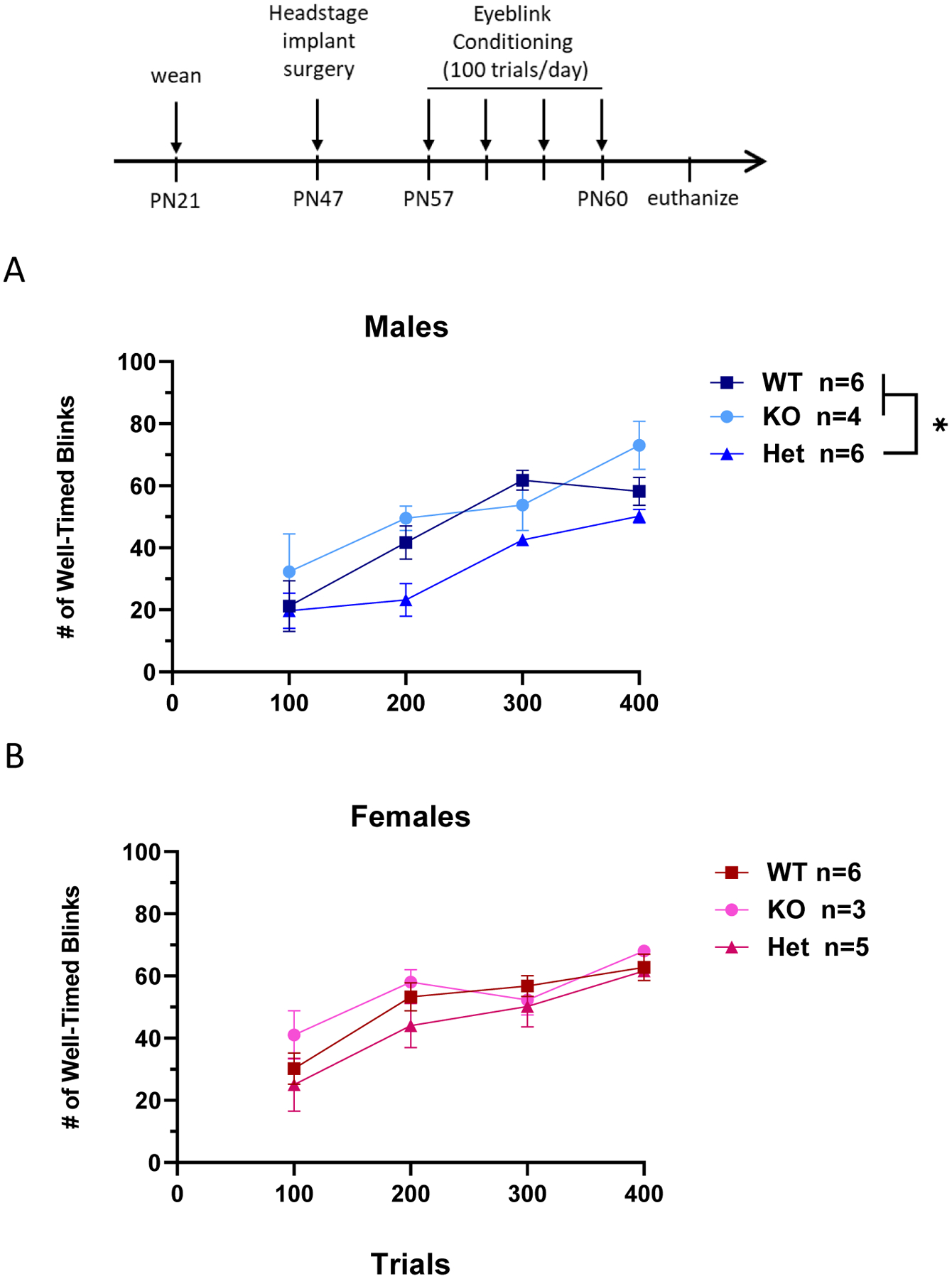
The percentage of successful conditioned responses in the delay eyeblink paradigm over 4 days of trials. SD-Nrxn1tm1Sage rats underwent 100 trials of conditioned eyeblink learning each day. (A) Acquisition of conditioned response over 4 days in males. There was no difference between WT and KO males in the acquisition of well-timed blinks over 4 days of conditioned learning trials. Both WT and KO males exhibited greater acquisition of the conditioned response over 4 days than Het males. (B) Acquisition of conditioned response over 4 days in females. There was no difference among genotype in females (ANOVA with Repeated Measures and Tukey’s posthoc, * = p < 0.05) (N = 6 WT males, 6 Het males, 4 KO males, 6 WT females, 5 Het females, 3 KO females).
Nrxn1α Deficiency Does Not Alter Baseline Sociability or Preference for Social Novelty.
When SD-Nrxn1tm1sage juveniles were presented with a choice between an unfamiliar conspecific and a novel object in the 3-chamber social interaction test, males and females of each genotype preferred exploring the social versus the non-social chamber (Figure 10A; (2Way ANOVA across all groups; chamber: F(1,64) = 272.9, p < .0001, η2p = .810; sex/genotype: F(5,64) = .02, p = .9998). There was no effect of sex or genotype on the amount of time juvenile rats spent in the social chamber (2Way ANOVA, sex: F(1,32) = .46, p = .5006; η2p = .009; genotype: F(2,32) = .21, p = .8126, η2p = .013), nor was there an effect of sex or genotype on the amount of time spent in the non-social chamber (sex: F(1,32) = .30, p = .585, η2p = .009; genotype: F(2,32) = .14, p = .8702; η2p = .009).
Figure 10: Social preference and social discrimination are not different among genotypes of SD-Nrxn1tm1Sage juveniles; object investigation is altered in KO males.
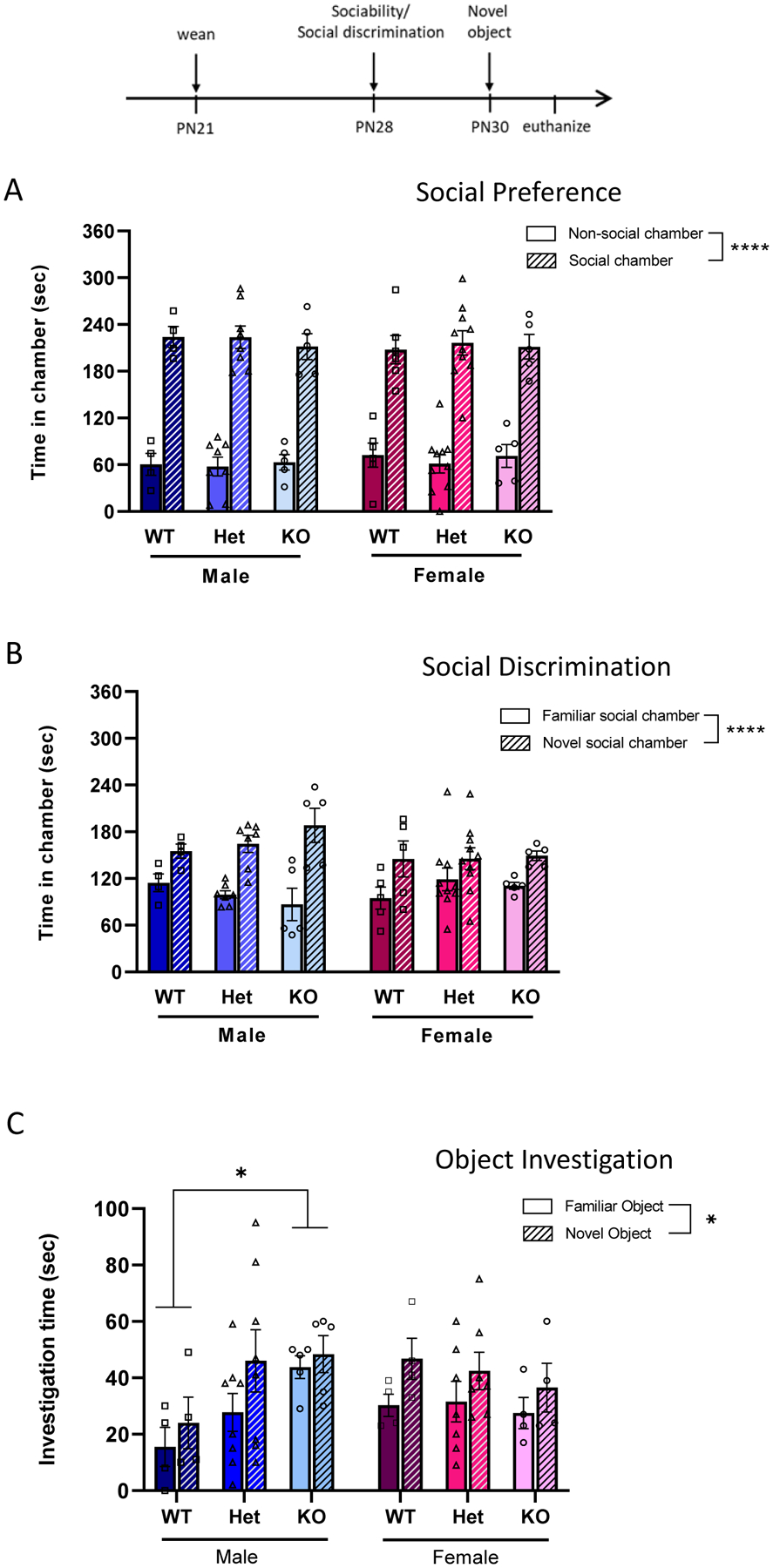
Juvenile rats were tested in the 3-chamber test of sociability/social discrimination (A,B) and novel object recognition (C, D). (A) Social preference. Time spent in each chamber containing either a novel object or an unfamiliar juvenile rat. No effect of sex or genotype was seen in the amount of time spent with a conspecific versus a novel object. (B) Social discrimination. Time spent in each chamber containing either a novel or a familiar juvenile rat. There was no effect of sex or genotype on the preference for juveniles to investigate a novel versus familiar conspecific. (C) Object investigation, as shown by the amount of time spent investigating a familiar versus a novel object. KO males spent more time investigating the familiar object than WTs, contributing to an overall increase in object investigation. Within-sex ANOVA and Tukey’s posthoc, * = p < 0.05 (N = 4 WT males, 8 Het males, 5 KO males, 6 WT females, 10 Het females, 5 KO females. 2 WT females, 3 Het females and 1 KO female were not tested in Object Investigation due to equipment failure).
When presented with a familiar conspecific in one chamber and a novel conspecific in the other chamber, juvenile rats spent more time in the chamber containing the novel conspecific, regardless of sex or genotype (Figure 10B; 2Way ANOVA across all groups; chamber: F(1,64) = 36.14, p < .0001, η2p = .361; sex/genotype: F(5,64) = .28, p = .9241). There was no effect of genotype on the amount of time spent in either the social or non-social chambers for either males or females (Within-sex ANOVA: males, familiar social chamber: F(2,13) = .97, p = .4031, η2p = .129; males, novel social chamber: F(2,13) = 1.16, p = .3452, η2p = .151; females, familiar social chamber: F(2,17) = .70, p = .5097, η2p = .076; females, novel social chamber: F(2,17) = .07, p = .9835, η2p = .008).
Complete Nrxn1α Deficiency Alters Object Investigation in Juvenile Rats.
When presented with a novel and a familiar object in an object investigation test, there was a sex-specific effect of genotype on the total time spent in object investigation (Figure 10C). KO males, but not females, spent more time in total object investigation, compared to WT rats (Within-sex ANOVA: males, combined object exploration: F(2,28) = 3.63, p = .0397, η2(P) = .206; females, combined object exploration: F(2,24) = .38, p = .6887, η2p = .031). This was driven by significantly more investigation of the familiar object by KO males, compared to WT males, when presented with both the familiar and novel objects (Within-sex ANOVA: males, familiar object investigation: F(2,14) = 3.73, p = .0434, η2(P) = .347; males, novel object investigation: F(2,14) = 1.31, p = .3008, η2p = .157). Females spent more time investigating the novel object, regardless of genotype (Females, 2Way ANOVA, object: F(1,24) = 4.06, p = .0453, η2p = .146; genotype: F(2,24) = .38, p = .6887, η2p = .031), and there was no difference among genotypes in the amount of time they spent investigating the familiar object (Within-sex ANOVA: familiar object investigation: F(2,12) = .09, p = .9116, η2p = .015), or the amount of time they spent investigating the novel object (Within-sex ANOVA: familiar object investigation: F(2,12) = .34, p = .6921, η2p = .054).
Discussion
The data presented here characterize a range of social and cognitive behaviors in rats harboring a loss-of-function mutation in Nrxn1α, a gene highly associated with ASD, schizophrenia, and other neurodevelopmental disorders. With the exception of neonatal USVs, an important aspect of this study is the focus on the juvenile lifestage. This enabled us to assess the effects of Nrxn1α deficiency after developmental sexual differentiation of the brain and prior to the rise in gonadal hormones at puberty, thereby more closely matching the developmental onset of symptoms associated with mutations in this gene. Performing these studies in a rat model of Nrxn1α knockout enabled us to examine complex social behaviors that are relevant to core deficits in neurodevelopmental disorders associated with Nrxn1 deficiency but are not readily modeled in mice.
Social Play and Ultrasonic Vocalizations
We observed a sex-specific deficit in juvenile social play behavior associated with Nrxn1α deficiency, in which fewer play events were seen in KO males. Interestingly, we did not observe higher rates of rough-and-tumble play by WT males compared to WT females, which is a conserved feature of play observed by our lab and others in the Sprague Dawley strain of rat (Argue et al., 2017; Argue and McCarthy, 2015; Hoffman et al., 2016; Pellis and Pellis, 1990; Stockman and McCarthy, 2017; VanRyzin et al., 2019; and see Northcutt and Nwankwo, 2018 and references therein). Because we were testing the WT littermates resulting from heterozygous crosses, we speculate there could be genetic drift between our colony of SD rats and those maintained by Sage Pharmaceuticals, or there could be an impact of being raised by a dam that is haplodeficient in Nrxn1α. Deciphering between these possibilities is beyond the scope of the current study. Nevertheless, the reduction in the number of play events in KO males, due to reduced instances of pouncing, pinning and boxing suggests that both the initiation and response to playful social interaction is affected with Nrxn1α deficiency. Sex-specific alterations in social play behavior is observed in rats harboring other mutations associated with ASD and schizophrenia. In juvenile rats completely deficient in the postsynaptic scaffolding protein Shank3, males, but not females exhibit decreased social interaction, although this was quantified as instances of allogrooming and sniffing between two unfamiliar conspecifics, rather than specific components of play behavior (Berg et al., 2018). A recent study examined juvenile social play behavior in rats harboring a null mutation in in the calcium channel protein CACNA1C, which is associated with ASD, schizophrenia and bipolar disorder (Kisko et al., 2020). In these animals, haploinsufficiency of CACNA1C results in no changes in male play behavior, compared to wild-type males, but female play behavior is hypermasculinized, driven mainly by an increase in pinning. Kisko and colleagues (2020) also found that CACNA1C haploinsufficiency results in fewer prosocial 50 kHz ultrasonic vocalizations during play and decreased social approach behavior toward recorded 50 kHz USVs in juvenile males, but not females. We did not measure prosocial USVs emitted during play or the affiliative response to prosocial USVs in juvenile Nrxn1α rats, although this may be an avenue worth exploring in future studies, as the production and perception of 50 kHz calls between play partners regulates play behavior (Webber et al., 2012).
In addition to the 50 kHz prosocial calls which are associated with social interactions and positive affect, juvenile and adult rats emit 22 kHz calls to signal distress or negative affect, and during early postnatal life rats will emit 40 kHz distress calls when separated from the dam and other littermates. These isolation-induced USVs have a function in thermogenic regulation, but there is also a social and affective component to this behavior (Hofer et al, 1993; Knutson et al., 2002). Deficits in social interaction seen in rodent models of ASD-associated gene mutations are often preceded by reduced ultrasonic vocalizations during early postnatal development (Wohr et al., 2013; Sungur et al., 2016; Berg et al. 2018). In a Nrxn1α knockout mouse model, both male and female pups emit fewer and less complex isolation-induced USVs during the first two weeks of life, yet only males exhibit decreased social investigation as juveniles and adults (Armstrong et al., 2020). We also observed a severe deficit in isolation-induced USVs during the first two postnatal weeks in both male and female Nrxn1α KO rats, with a less severe reduction in haploinsufficient pups, while only male KO rats are significantly affected in juvenile play behavior. Whether the deficit in isolation-induced USVs in neonatal Nrxn1α deficient rodents seen in this study reflects altered USV production during social interactions in juvenile rats is not known. Of particular relevance to this question is a study by Brunelli et al., (2006) which selectively bred lines of rats that emitted high and low rates of USVs at postnatal day 10, and observed that the ‘low emitter’ rats engaged in fewer instances of pouncing and pinning and emitted fewer USVs during juvenile social play. On the other hand, although USVs are associated with the anticipation of play behavior (Knutson et al., 1998), they are also dissociable, as changes in USV production are not always associated with altered play behavior (Kisko et al, 2020; Manduca et al, 2014). Further studies might indicate whether the male-biased deficit in social play is due to a dysfunction in social communication through USVs, or if there are other sex-specific factors involved.
Nurturing Behavior
In spite of potential communication deficits in Nrxn1α KO rats, and contrary to what we predicted, we observed a striking alteration in nurturing behavior toward unfamiliar pups in juvenile rats with biallelic Nrxn1α mutation. In most mammals, males exhibit little to no spontaneous care of infants and females do so only after giving birth or when continuously exposed to young animals. The induction of maternal behavior in juvenile and adult rats outside the context of pregnancy hormones after exposure to pups is well characterized (Numan, 1994), and the process of sensitization and display of nurturing behavior toward unfamiliar pups involves the suppression of an innate aversion to pup odor (Mayer et al., 1979). Our observation of a decreased latency for juvenile Nrxn1α deficient rats to acquire nurturing behavior toward unfamiliar pups in the juvenile sensitization paradigm, in the face of normal olfaction for these animals, suggests a disinhibition of the innate neophobic response to unfamiliar pups (Fleming and Rosenblatt, 1974a,b). The primary site of inhibitory control of maternal behavior in nulliparous and juvenile females is the amygdala via its efferent projections to the BNST (Fleming et al., 1980; Oxley and Fleming, 2000), and the valuation of social threat is dependent upon an intra-amygdala circuit that is impaired in Nrxn1α KO rats (Twining et al., 2017). The neurophysiological consequences of Nrxn1α deficiency in these areas of the brain in juveniles will be of interest for future studies.
Prosocial Helping Behavior
We tested a more complex social interaction meant to probe prosocial helping behavior, a trait which is well modeled in rats (Mason, 2021). Rats will readily learn to free a distressed conspecific, but generally will not open a chamber for a non-distressed conspecific or inanimate object (Ben-Ami Bartal et al., 2011, 2014; Sato et al., 2015; Cox and Reichel, 2020). This helping behavior is not motivated by subsequent social interaction, and when given a choice rats will free a conspecific before accessing food, and will learn a helping behavior more readily when they have prior experience of the same stressful situation as their social partner, further indicating an empathy-like rather than social motivation. Using the prosocial helping test of Ben-Ami Bartal et al (2011) we found a severe deficit in performance when tasked with freeing a confined social partner, in both male and female juvenile rats with biallelic Nrxn1α mutations and a less severe impairment in haploinsufficient rats. However, interpretation of this is confounded by the significant percentage of Nrxn1α deficient juveniles that did not learn the task for a food treat in the absence of a social partner. Opening of the confinement box door in the prosocial version of the test is aided by cooperation with the trapped social partner, pushing the door open after it is unlatched, and this cooperative assistance is lacking in the treat-retrieval version of the test. An accommodation is made for this in the experimental design, wherein the latch and door are opened by the experimenter if the test rat is not successful within several minutes of testing time. Thus, what is really being scored is the success in opening the door latch, a task that is not aided by the prosocial partner, and completion of the task is motivated differently in the prosocial and treat-retrieval tests. Notably, KO rats failed to open the door latch without experimenter assistance in either versions of the test. Because olfaction is normal in Nrxn1α deficient rats, this points to at least a partial cognitive impairment affecting the ability of these rats to learn the complex door-opening task. Nonetheless, to our knowledge, this is the first description of prosocial helping behavior in the juvenile lifestage of rats, and the first characterization of this behavior in a genetic model of neurodevelopmental disorders. A recent study assessed helping behavior using the same test in an environmental model of ASD. Treating pregnant rodents with the anti-seizure drug valproic acid results in behavioral phenotypes in the offspring having face and construct validity with the core deficits of ASD (Nicolini and Fahnestock, 2018). Male Wistar rats exposed to valproic acid in utero have a longer latency to release a trapped conspecific (Fontes-Dutra et al., 2019), similar to what we found in juvenile rats with Nrxn1α haploinsufficiency.
Sociability
In contrast to the deficits in social play and cognition in the prosocial helping task, we found no alterations in social preference or in the preference for social novelty associated with Nrxn1α deficiency in either males or females, using a 3-chamber social interaction test. This is consistent with other studies which assessed social behavior in Nrxn1 deficient adult mice using this paradigm and also found no effect of genotype or sex (Armstrong et al., 2020; Etherton et al., 2009), yet differs from Grayton et al (2013), which found a small increase in social preference in male and female Nrxn1 KO mice. The main finding consistent among all studies is that Nrxn1α deficiency does not impair the normally robust preference for social interaction found in rodents, nor does it decrease the preference for a novel social stimulus. A detailed study examining the effects of sensory cues in the 3-chamber social interaction test reveals that social olfactory cues are sufficient to elicit normal social investigation behavior in this test, while visual, tactile, auditory and non-social scents have little relevance (Ryan et al., 2008). Given that olfaction is normal in Nrxn1 KO mice (Armstrong et al., 2020; Etherton et al., 2009; Grayton et al., 2013) and rats (Twining et al., 2017; this study), it is perhaps not surprising that normal sociability and social discrimination are seen with Nrxn1 KO rats in the 3-chamber test, while deficits are found in more complex social interactions that involve vocal communication, auditory and tactile sensory input, and social cognition.
Object Investigation
In contrast to the normal sociability and social discrimination exhibited by Nrxn1α deficient rats, we observed an apparent alteration in object investigation in KO males. This corresponds to a male-specific increase in mobility in the open field in KO rats, and this hyperactive phenotype may be driving the increased object investigation in these rats, rather than a deficit in discrimination. In adult Nrxn1α mice, a test of object discrimination by Dachtler et al (2015) report no effect on long-term (24 hrs) object discrimination with Nrxn1α knockout, while Laarakker and colleagues (2012) find that the length of the discrimination trial has an effect on the results, as Nrxn1α+/− males actually discriminate better than WT males during the first 5 minutes of the trial, but during the last 5 minutes of the trial they explore both objects equally. In general, cognitive tests in rodents harboring Nrxn1α mutation indicate only mild impairments (Etherton, 2009; Dachtler et al., 2015; Esclassan et al., 2015).
Pavlovian Learning
We found a sex-specific deficit in a test of associative motor learning, the delay eyeblink conditioning paradigm. This cerebellum-dependent conditioned response is impaired in children with ASD (Sears et al., 1994; Oristaglio et al., 2013) and schizophrenia (Brown et al., 2005; Sears et al., 2000). Pavlovian eyeblink conditioning using a delay stimulus arrangement with the temporal parameters used here requires the cerebellum and its connections with motor nuclei in the brainstem (Lavond et al., 1985; Lavond et al., 1993). The deep interpositus nucleus of the cerebellum is the site of convergence of stimuli necessary for successful eyeblink conditioning (Freeman & Steinmetz, 2011; McCormick & Thompson, 1984; Lavond et al., 1985). The cerebellar cortex also receives inputs from CS and US processing pathways, where granule cells integrate mossy fiber inputs (D’Angelo & De Zeeuw, 2009; Freeman & Steinmetz, 2011). Granule cells give rise to parallel fibers, which contact many Purkinje cell dendrites (Apps & Hawkes, 2009), and this is a site of critical plasticity in acquisition of the learned eyeblink response (Garcia & Mauk, 1998; Gilbert & Thach, 1977; Green & Steinmetz, 2005; Ito & Kano, 1982). Nrxn1α is an integral presynaptic component of the parallel fiber-Purkinje cell synapse and is required for synapse maintenance in this pathway through interaction with the δ2 subunit of glutamate receptors (Uemura et al., 2010; Mishina et al., 2012; Pregno et al., 2013). Given this, one would expect altered learning in the conditioned eyeblink response with Nrxn1α deficiency, however to our surprise this was observed in Het but not KO males. Whether this reflects a compensatory mechanism of other Neurexin isoforms during development of these synapses is not known.
Sex Differences in the Effects of Nrxn1α Deficiency
In spite of the lack of studies addressing the effects of Nrxn1 deficiency on prosocial or juvenile play behavior, multiple groups identify sex differences in other areas of behavioral performance in Nrxn1α Het or KO mice. Laarakker et al. (2012) described a sex difference in Nrxn1α mice where only heterozygous males exhibited enhanced exploratory behavior and faster familiarization to a new environment. Grayton et al. (2013) studied knock-out mice from the same line and found increased aggression towards intruders in males and reduced locomotion, a deficit in spatial memory acquisition in the late hidden platform trials of the Morris Water Maze test, and increased social preference for a stranger mouse vs. a familiar mouse in the 3-chambered test in females. Yet, studies in other laboratories testing the same mouse line in either knock-out (Etherton et al., 2009) or heterozygous genotypes (Dachtler et al., 2015) found no sex differences, no changes in locomotion (Dachtler et al., 2015), no alterations in social behavior, spatial learning or anxiety (Etherton et al., 2009; Dachtler et al., 2015). This can be a common occurrence when mice are investigated behaviorally and could be due to differences in the timing of testing or handling of the animals, but also to the genetic background of the mice, a mixed C57BL/6-Sv129 background for Etherton et al. (2009), a pure C57BL/6J for Grayton et al. (2013) and Laarakker et al. (2012), and a pure C57BL/6N for Dachtler et al. (2015). Genetic modifiers in the background strain could contribute to changes in behavioral performance leading to conflicting results as shown by multiple studies comparing behavior in different mouse strains (Cook et al., 2002; Bothe et al., 2004; Sittig et al., 2016) and even among C57BL/6N substrains maintained by different vendors (Bryant et al., 2008).
The fact that loss of Nrxn1α may lead to sex-specific effects is also supported by the studies using knock-out Nrxn1α−/− Sprague Dawley rats reported by Esclassen et al (2015), where mutant male rats were impaired in spatial acquisition and discrimination and in the spatial reversal test in a novel object recognition task, while mutant female rats only showed acquisition deficits on Day 1 and performed as well as wild-type littermates in all successive trials. Interestingly, while Esclassen and colleagues found increased locomotive behavior in Nrxn1α knockout rats of both sexes, we observed a hyperactive phenotype only in males. This difference may be due to rearing or environmental conditions but may also be related to developmental stage. Esclassen et al (2015) tested adult rats, while our experiments used prepubertal animals. The mechanisms underlying sex-specific differences in cognitive and social behaviors following Nrxn1α loss of function or haploinsufficiency remain unclear and additional studies will be necessary to address whether sex-specific circuit alterations are present and if they depend on the genetic background or hormonal status of the animal. The use of juvenile animals, as we have done here, removes the potentially confounding effects of adult steroid hormones on social behaviors so that the developmental underpinnings of Nrxn1α deficiency in sex-biased disorders can be more easily discerned.
In conclusion, we have characterized here the performance of Nrxn1α deficient rats in a range of cognitive and social tasks, representing both simple and complex behaviors. Driven by the importance of Nrxn1α mutation in ASD, schizophrenia, and other neurodevelopmental disorders, previous studies have reported on behavioral deficits in rodent models of Nrxn1α deficiency. Because we have used a rat model we were able to examine complex social behaviors that are not readily studied in mice, including social play, experience-induced nurturing, and prosocial helping behavior. Each of these complex behaviors was impacted by Nrxn1α deficiency, although measures of baseline sociability and social discrimination were not. As reported in other studies, we also found a deficit in USVs in neonatal rats that suggests deficits in complex social interactions may be due in part to communication deficits. In most of our data Nrxn1α deletion impacted both males and females, although a male-specific effect on the impact of social play was detected, and male-specific effects on object discrimination, likely driven by a hyperactive phenotype, and Pavlovian learning were indicated. This study offers a unique perspective on the impact of Nrxn1α deficiency on complex social behaviors during the juvenile lifestage, and suggests the rat may offer a more relevant behavioral model of Nrxn1α deficiency in neurodevelopmental disorders. The well-characterized social play, nurturing and pro-social behaviors seen in rats make them an extremely attractive model for the social deficits seen in neurodevelopmental disorders associated with Nrxn1 mutations, especially since these complex social behaviors are not observed in laboratory mice.
Funding:
Simons Foundation Pilot Award #402378 (MMM)
NIMH Award R01MH091424 (MMM)
NICHD Award P01HD085928 (MMM, JW)
Footnotes
The authors declare no conflicts of interest.
References
- Abel KM, Drake R, & Goldstein JM (2010). Sex differences in schizophrenia. Int Rev Psychiatry, 22(5), 417–428. 10.3109/09540261.2010.515205 [DOI] [PubMed] [Google Scholar]
- Al Shehhi M, Forman EB, Fitzgerald JE, McInerney V, Krawczyk J, Shen S, Betts DR, Ardle LM, Gorman KM, King MD, Green A, Gallagher L, & Lynch SA (2019). NRXN1 deletion syndrome; phenotypic and penetrance data from 34 families. Eur J Med Genet, 62(3), 204–209. 10.1016/j.ejmg.2018.07.015 [DOI] [PubMed] [Google Scholar]
- Apps R, & Hawkes R (2009). Cerebellar cortical organization: a one-map hypothesis. Nat Rev Neurosci, 10(9), 670–681. 10.1038/nrn2698 [DOI] [PubMed] [Google Scholar]
- Argue KJ, & McCarthy MM (2015a). Characterization of juvenile play in rats: importance of sex of self and sex of partner. Biol Sex Differ, 6, 16. 10.1186/s13293-015-0034-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argue KJ, & McCarthy MM (2015b). Utilization of same- vs. mixed-sex dyads impacts the observation of sex differences in juvenile social play behavior. Curr Neurobiol, 6(1), 17–23. 10.4172/0975-9042.000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argue KJ, VanRyzin JW, Falvo DJ, Whitaker AR, Yu SJ, & McCarthy MM (2017). Activation of both cb1 and cb2 endocannabinoid receptors is critical for masculinization of the developing medial amygdala and juvenile social play behavior. eNeuro, 4(1). 10.1523/ENEURO.0344-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong EC, Caruso A, Servadio M, Andreae LC, Trezza V, Scattoni ML, & Fernandes C (2020). Assessing the developmental trajectory of mouse models of neurodevelopmental disorders: Social and communication deficits in mice with Neurexin 1α deletion. Genes Brain Behav, 19(4), e12630. 10.1111/gbb.12630 [DOI] [PubMed] [Google Scholar]
- Auger AP, & Olesen KM (2009). Brain sex differences and the organisation of juvenile social play behaviour. J Neuroendocrinol, 21(6), 519–525. 10.1111/j.1365-2826.2009.01871.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auyeung B, Baron-Cohen S, Ashwin E, Knickmeyer R, Taylor K, Hackett G, & Hines M (2009). Fetal testosterone predicts sexually differentiated childhood behavior in girls and in boys. Psychol Sci, 20(2), 144–148. 10.1111/j.1467-9280.2009.02279.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badura A, Verpeut JL, Metzger JW, Pereira TD, Pisano TJ, Deverett B, Bakshinskaya DE, Wang SS (2018). Normal cognitive and social development require posterior cerebellar activity. Elife, 7(e36401). 10.7554/eLife.36401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami Bartal I, Decety J, & Mason P (2011). Empathy and pro-social behavior in rats. Science, 334(6061), 1427–1430. 10.1126/science.1210789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami Bartal I, Rodgers DA, Bernardez Sarria MS, Decety J, & Mason P (2014). Pro-social behavior in rats is modulated by social experience. Elife, 3, e01385. 10.7554/eLife.01385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami Bartal I, Shan H, Molasky NM, Murray TM, Williams JZ, Decety J, & Mason P (2016). Anxiolytic treatment impairs helping behavior in rats. Front Psychol, 7, 850. 10.3389/fpsyg.2016.00850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg EL, Copping NA, Rivera JK, Pride MC, Careaga M, Bauman MD, Berman RF, Lein PJ, Harony-Nicolas H, Buxbaum JD, Ellegood J, Lerch JP, Wöhr M, & Silverman JL (2018). Developmental social communication deficits in the Shank3 rat model of phelan-mcdermid syndrome and autism spectrum disorder. Autism Res, 11(4), 587–601. 10.1002/aur.1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothe GW, Bolivar VJ, Vedder MJ, & Geistfeld JG (2004). Genetic and behavioral differences among five inbred mouse strains commonly used in the production of transgenic and knockout mice. Genes Brain Behav, 3(3), 149–157. 10.1111/j.1601-183x.2004.00064.x [DOI] [PubMed] [Google Scholar]
- Bradley WE, Raelson JV, Dubois DY, Godin E, Fournier H, Privé C, Allard R, Pinchuk V, Lapalme M, Paulussen RJ, & Belouchi A (2010). Hotspots of large rare deletions in the human genome. PLoS One, 5(2), e9401. 10.1371/journal.pone.0009401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RS, Zarrow MX, Goldman BD, & Denenberg VH (1974). A developmental study of maternal responsiveness in the rat. Physiol Behav, 12(1), 149–151. 10.1016/0031-9384(74)90082-1 [DOI] [PubMed] [Google Scholar]
- Brignell A, St John M, Boys A, Bruce A, Dinale C, Pigdon L, Hildebrand MS, Amor DJ, & Morgan AT (2018). Characterization of speech and language phenotype in children with NRXN1 deletions. Am J Med Genet B Neuropsychiatr Genet, 177(8), 700–708. 10.1002/ajmg.b.32664 [DOI] [PubMed] [Google Scholar]
- Brown SM, Kieffaber PD, Carroll CA, Vohs JL, Tracy JA, Shekhar A, O’Donnell BF, Steinmetz JE, & Hetrick WP (2005). Eyeblink conditioning deficits indicate timing and cerebellar abnormalities in schizophrenia. Brain Cogn, 58(1), 94–108. 10.1016/j.bandc.2004.09.011 [DOI] [PubMed] [Google Scholar]
- Brüne M (2005). Emotion recognition, ‘theory of mind,’ and social behavior in schizophrenia. Psychiatry Res, 133(2–3), 135–147. 10.1016/j.psychres.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Brunelli SA, Nie R, Whipple C, Winiger V, Hofer MA, & Zimmerberg B (2006). The effects of selective breeding for infant ultrasonic vocalizations on play behavior in juvenile rats. Physiol Behav, 87(3), 527–536. 10.1016/j.physbeh.2005.11.020 [DOI] [PubMed] [Google Scholar]
- Béna F, Bruno DL, Eriksson M, van Ravenswaaij-Arts C, Stark Z, Dijkhuizen T, Gerkes E, Gimelli S, Ganesamoorthy D, Thuresson AC, Labalme A, Till M, Bilan F, Pasquier L, Kitzis A, Dubourgm C, Rossi M, Bottani A, Gagnebin M, Sanlaville D, Gilbert-Dussardier B, Guipponi M, van Haeringen A, Kriek M, Ruivenkamp C, Antonarakis SE, Anderlid BM, Slater HR, & Schoumans J (2013). Molecular and clinical characterization of 25 individuals with exonic deletions of NRXN1 and comprehensive review of the literature. Am J Med Genet B Neuropsychiatr Genet, 162B(4), 388–403. 10.1002/ajmg.b.32148 [DOI] [PubMed] [Google Scholar]
- Bryant CD, Zhang NN, Sokoloff G, Fanselow MS, Ennes HS, Palmer AA, & McRoberts JA (2008). Behavioral differences among C57BL/6 substrains: implications for transgenic and knockout studies. J Neurogenet, 22(4), 315–331. 10.1080/01677060802357388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta I, Chen CH, Schott AL, Dorizan S, & Khodakhah K (2019). Cerebellar modulation of the reward circuitry and social behavior. Science, 363(6424). 10.1126/science.aav0581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castronovo P, Baccarin M, Ricciardello A, Picinelli C, Tomaiuolo P, Cucinotta F, Frittoli M, Lintas C, Sacco R, & Persico AM (2020). Phenotypic spectrum of NRXN1 mono- and bi-allelic deficiency: A systematic review. Clin Genet, 97(1), 125–137. 10.1111/cge.13537 [DOI] [PubMed] [Google Scholar]
- Ching MS, Shen Y, Tan WH, Jeste SS, Morrow EM, Chen X, Mukaddes NM, Yoo SY, Hanson E, Hundley R, Austin C, Becker RE, Berry GT, Driscoll K, Engle EC, Friedman S, Gusella JF, Hisama FM, Irons MB, Lafiosca T, LeClair E, Miller DT, Neessen M, Picker JD, Rappaport L, Rooney CM, Sarco DP, Stoler JM, Walsh CA, Wolff RR, Zhang T, Nasir RH, Wu BL, & Group, C. s. H. B. G. P. S. (2010). Deletions of NRXN1 (neurexin-1) predispose to a wide spectrum of developmental disorders. 153B(4), 937–947. 10.1002/ajmg.b.31063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Bendahan CC, Buitelaar JK, van Goozen SH, Orlebeke JF, & Cohen-Kettenis PT (2005). Is there an effect of prenatal testosterone on aggression and other behavioral traits? A study comparing same-sex and opposite-sex twin girls. Horm Behav, 47(2), 230–237. 10.1016/j.yhbeh.2004.10.006 [DOI] [PubMed] [Google Scholar]
- Cook MN, Bolivar VJ, McFadyen MP, & Flaherty L (2002). Behavioral differences among 129 substrains: implications for knockout and transgenic mice. Behav Neurosci, 116(4), 600–611. [PubMed] [Google Scholar]
- Cox SS, & Reichel CM (2020). Rats display empathic behavior independent of the opportunity for social interaction. Neuropsychopharmacology, 45(7), 1097–1104. 10.1038/s41386-019-0572-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristino AS, Williams SM, Hawi Z, An JY, Bellgrove MA, Schwartz CE, Costa L. a. F., & Claudianos C (2014). Neurodevelopmental and neuropsychiatric disorders represent an interconnected molecular system. Mol Psychiatry, 19(3), 294–301. 10.1038/mp.2013.16 [DOI] [PubMed] [Google Scholar]
- D’Angelo E, & De Zeeuw CI (2009). Timing and plasticity in the cerebellum: focus on the granular layer. Trends Neurosci, 32(1), 30–40. 10.1016/j.tins.2008.09.007 [DOI] [PubMed] [Google Scholar]
- Dabell MP, Rosenfeld JA, Bader P, Escobar LF, El-Khechen D, Vallee SE, Dinulos MB, Curry C, Fisher J, Tervo R, Hannibal MC, Siefkas K, Wyatt PR, Hughes L, Smith R, Ellingwood S, Lacassie Y, Stroud T, Farrell SA, Sanchez-Lara PA, Randolph LM, Niyazov D, Stevens CA, Schoonveld C, Skidmore D, MacKay S, Miles JH, Moodley M, Huillet A, Neill NJ, Ellison JW, Ballif BC, & Shaffer LG (2013). Investigation of NRXN1 deletions: clinical and molecular characterization. Am J Med Genet A, 161A(4), 717–731. 10.1002/ajmg.a.35780 [DOI] [PubMed] [Google Scholar]
- Dachtler J, Ivorra JL, Rowland TE, Lever C, Rodgers RJ, & Clapcote SJ (2015). Heterozygous deletion of α-neurexin I or α-neurexin II results in behaviors relevant to autism and schizophrenia. Behav Neurosci, 129(6), 765–776. 10.1037/bne0000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudanova I, Sedej S, Ahmad M, Masius H, Sargsyan V, Zhang W, Riedel D, Angenstein F, Schild D, Rupnik M, & Missler M (2006). Important contribution of alpha-neurexins to Ca2+-triggered exocytosis of secretory granules. J Neurosci, 26(41), 10599–10613. 10.1523/JNEUROSCI.1913-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac C, O’Connell LA, & Wu Z (2014). Neural control of maternal and paternal behaviors. Science, 345(6198), 765–770. 10.1126/science.1253291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge R, Sweet R, Lake R, Ziegler M, & Shapiro AK (1977). Gilles de la Tourette’s syndrome: clinical, genetic, psychologic, and biochemical aspects in 21 selected families. Neurology, 27(2), 115–124. 10.1212/wnl.27.2.115 [DOI] [PubMed] [Google Scholar]
- Ennaceur A, & Delacour J (1988). A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res, 31(1), 47–59. 10.1016/0166-4328(88)90157-x [DOI] [PubMed] [Google Scholar]
- Esclassan F, Francois J, Phillips KG, Loomis S, & Gilmour G (2015). Phenotypic characterization of nonsocial behavioral impairment in neurexin 1α knockout rats. Behav Neurosci, 129(1), 74–85. 10.1037/bne0000024 [DOI] [PubMed] [Google Scholar]
- Etherton MR, Blaiss CA, Powell CM, & Südhof TC (2009). Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc Natl Acad Sci U S A, 106(42), 17998–18003. 10.1073/pnas.0910297106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AS, & Rosenblatt JS (1974a). Maternal behavior in the virgin and lactating rat. J Comp Physiol Psychol, 86(5), 957–972. 10.1037/h0036414 [DOI] [PubMed] [Google Scholar]
- Fleming AS, & Rosenblatt JS (1974b). Olfactory regulation of maternal behavior in rats. I. Effects of olfactory bulb removal in experienced and inexperienced lactating and cycling females. J Comp Physiol Psychol, 86(2), 221–232. 10.1037/h0035937 [DOI] [PubMed] [Google Scholar]
- Fleming AS, Vaccarino F, & Luebke C (1980). Amygdaloid inhibition of maternal behavior in the nulliparous female rat. Physiol Behav, 25(5), 731–743. 10.1016/0031-9384(80)90377-7 [DOI] [PubMed] [Google Scholar]
- Fontes-Dutra M, Della-Flora Nunes G, Santos-Terra J, Souza-Nunes W, Bauer-Negrini G, Hirsch MM, Green L, Riesgo R, Gottfried C, & Bambini-Junior V (2019). Abnormal empathy-like pro-social behaviour in the valproic acid model of autism spectrum disorder. Behav Brain Res, 364, 11–18. 10.1016/j.bbr.2019.01.034 [DOI] [PubMed] [Google Scholar]
- Freeman JH, & Steinmetz AB (2011). Neural circuitry and plasticity mechanisms underlying delay eyeblink conditioning. Learn Mem, 18(10), 666–677. 10.1101/lm.2023011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia KS, & Mauk MD (1998). Pharmacological analysis of cerebellar contributions to the timing and expression of conditioned eyelid responses. Neuropharmacology, 37(4–5), 471–480. 10.1016/s0028-3908(98)00055-0 [DOI] [PubMed] [Google Scholar]
- Gaub M, & Carlson CL (1997). Gender differences in ADHD: a meta-analysis and critical review. J Am Acad Child Adolesc Psychiatry, 36(8), 1036–1045. 10.1097/00004583-199708000-00011 [DOI] [PubMed] [Google Scholar]
- Gilbert PF, & Thach WT (1977). Purkinje cell activity during motor learning. Brain Res, 128(2), 309–328. 10.1016/0006-8993(77)90997-0 [DOI] [PubMed] [Google Scholar]
- Grayton HM, Missler M, Collier DA, & Fernandes C (2013). Altered social behaviours in neurexin 1α knockout mice resemble core symptoms in neurodevelopmental disorders. PLoS One, 8(6), e67114. 10.1371/journal.pone.0067114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JT, & Steinmetz JE (2005). Purkinje cell activity in the cerebellar anterior lobe after rabbit eyeblink conditioning. Learn Mem, 12(3), 260–269. 10.1101/lm.89505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsson OO, Walters GB, Ingason A, Johansson S, Zayats T, Athanasiu L, Sonderby IE, Gustafsson O, Nawaz MS, Jonsson GF, Jonsson L, Knappskog PM, Ingvarsdottir E, Davidsdottir K, Djurovic S, Knudsen GPS, Askeland RB, Haraldsdottir GS, Baldursson G, Magnusson P, Sigurdsson E, Gudbjartsson DF, Stefansson H, Andreassen OA, Haavik J, Reichborn-Kjennerud T, & Stefansson K (2019). Attention-deficit hyperactivity disorder shares copy number variant risk with schizophrenia and autism spectrum disorder. Transl Psychiatry, 9(1), 258. 10.1038/s41398-019-0599-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill EL, & Frith U (2003). Understanding autism: insights from mind and brain. Philos Trans R Soc Lond B Biol Sci, 358(1430), 281–289. 10.1098/rstb.2002.1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M, & Kaufman FR (1994). Androgen and the development of human sex-typical behavior: rough-and-tumble play and sex of preferred playmates in children with congenital adrenal hyperplasia (CAH). Child Dev, 65(4), 1042–1053. [PubMed] [Google Scholar]
- Hofer MA, Brunelli SA, & Shair HN (1993). The effects of 24-hr maternal separation and of litter-size reduction on the isolation-distress response of 12-day-old rat pups. Dev Psychobiol, 26(8), 483–497. 10.1002/dev.420260806 [DOI] [PubMed] [Google Scholar]
- Hoffman JF, Wright CL, & McCarthy MM (2016). A Critical Period in Purkinje Cell Development Is Mediated by Local Estradiol Synthesis, Disrupted by Inflammation, and Has Enduring Consequences Only for Males. J Neurosci, 36(39), 10039–10049. 10.1523/JNEUROSCI.1262-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Xiao X, Zhang Z, & Li M (2019). Genetic insights and neurobiological implications from NRXN1 in neuropsychiatric disorders. Mol Psychiatry, 24(10), 1400–1414. 10.1038/s41380-019-0438-9 [DOI] [PubMed] [Google Scholar]
- Huang AY, Yu D, Davis LK, Sul JH, Tsetsos F, Ramensky V, Zelaya I, Ramos EM, Osiecki L, Chen JA, McGrath LM, Illmann C, Sandor P, Barr CL, Grados M, Singer HS, Nöthen MM, Hebebrand J, King RA, Dion Y, Rouleau G, Budman CL, Depienne C, Worbe Y, Hartmann A, Müller-Vahl KR, Stuhrmann M, Aschauer H, Stamenkovic M, Schloegelhofer M, Konstantinidis A, Lyon GJ, McMahon WM, Barta C, Tarnok Z, Nagy P, Batterson JR, Rizzo R, Cath DC, Wolanczyk T, Berlin C, Malaty IA, Okun MS, Woods DW, Rees E, Pato CN, Pato MT, Knowles JA, Posthuma D, Pauls DL, Cox NJ, Neale BM, Freimer NB, Paschou P, Mathews CA, Scharf JM, Coppola G, (TSAICG), T. S. A. I. C. f. G., & (GGRI), G. d. l. T. S. G. R. I. (2017). Rare Copy Number Variants in NRXN1 and CNTN6 Increase Risk for Tourette Syndrome. Neuron, 94(6), 1101–1111.e1107. 10.1016/j.neuron.2017.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, & Kano M (1982). Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci Lett, 33(3), 253–258. 10.1016/0304-3940(82)90380-9 [DOI] [PubMed] [Google Scholar]
- Jalbrzikowski M, Krasileva KE, Marvin S, Zinberg J, Andaya A, Bachman P, Cannon TD, & Bearden CE (2013). Reciprocal social behavior in youths with psychotic illness and those at clinical high risk. Dev Psychopathol, 25(4 Pt 1), 1187–1197. 10.1017/S095457941300045X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AP, Happé FG, Gilbert F, Burnett S, & Viding E (2010). Feeling, caring, knowing: different types of empathy deficit in boys with psychopathic tendencies and autism spectrum disorder. J Child Psychol Psychiatry, 51(11), 1188–1197. 10.1111/j.1469-7610.2010.02280.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan R (2003). Social play and autistic spectrum disorders: a perspective on theory, implications and educational approaches. Autism, 7(4), 347–360. 10.1177/1362361303007004002 [DOI] [PubMed] [Google Scholar]
- Kattenstroth G, Tantalaki E, Südhof TC, Gottmann K, & Missler M (2004). Postsynaptic N-methyl-D-aspartate receptor function requires alpha-neurexins. Proc Natl Acad Sci U S A, 101(8), 2607–2612. 10.1073/pnas.0308626100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keum S, & Shin HS (2016). Rodent models for studying empathy. Neurobiol Learn Mem, 135, 22–26. 10.1016/j.nlm.2016.07.022 [DOI] [PubMed] [Google Scholar]
- Kirov G, Rujescu D, Ingason A, Collier DA, O’Donovan MC, & Owen MJ (2009). Neurexin 1 (NRXN1) deletions in schizophrenia. Schizophr Bull, 35(5), 851–854. 10.1093/schbul/sbp079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisko TM, Braun MD, Michels S, Witt SH, Rietschel M, Culmsee C, Schwarting RKW, & Wöhr M (2020). Sex-dependent effects of Cacna1c haploinsufficiency on juvenile social play behavior and pro-social 50-kHz ultrasonic communication in rats. Genes Brain Behav, 19(2), e12552. 10.1111/gbb.12552 [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, & Panksepp J (1998). Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. J Comp Psychol, 112(1), 65–73. 10.1037/0735-7036.112.1.65 [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, & Panksepp J (2002). Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull, 128(6), 961–977. 10.1037/0033-2909.128.6.961 [DOI] [PubMed] [Google Scholar]
- Laarakker MC, Reinders NR, Bruining H, Ophoff RA, & Kas MJ (2012). Sex-dependent novelty response in neurexin-1α mutant mice. PLoS One, 7(2), e31503. 10.1371/journal.pone.0031503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavond DG, Hembree TL, & Thompson RF (1985). Effect of kainic acid lesions of the cerebellar interpositus nucleus on eyelid conditioning in the rabbit. Brain Res, 326(1), 179–182. 10.1016/0006-8993(85)91400-3 [DOI] [PubMed] [Google Scholar]
- Lavond DG, Kim JJ, & Thompson RF (1993). Mammalian brain substrates of aversive classical conditioning. Annu Rev Psychol, 44, 317–342. 10.1146/annurev.ps.44.020193.001533 [DOI] [PubMed] [Google Scholar]
- Li J, Zhao L, You Y, Lu T, Jia M, Yu H, Ruan Y, Yue W, Liu J, Lu L, Zhang D, & Wang L (2015). Schizophrenia Related Variants in CACNA1C also Confer Risk of Autism. PLoS One, 10(7), e0133247. 10.1371/journal.pone.0133247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Tian Y, Li Q, Chen H, Lv H, Xie W, & Han J (2015). The Neurexin/N-Ethylmaleimide-sensitive Factor (NSF) Interaction Regulates Short Term Synaptic Depression. J Biol Chem, 290(29), 17656–17667. 10.1074/jbc.M115.644583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Barnes JL, Wheelwright SJ, & Baron-Cohen S (2007). Self-referential cognition and empathy in autism. PLoS One, 2(9), e883. 10.1371/journal.pone.0000883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manduca A, Campolongo P, Palmery M, Vanderschuren LJ, Cuomo V, & Trezza V (2014). Social play behavior, ultrasonic vocalizations and their modulation by morphine and amphetamine in Wistar and Sprague-Dawley rats. Psychopharmacology (Berl), 231(8), 1661–1673. 10.1007/s00213-013-3337-9 [DOI] [PubMed] [Google Scholar]
- Mason P (2021). Lessons from helping behavior in rats. Curr Opin Neurobiol, 68, 52–56. 10.1016/j.conb.2021.01.001 [DOI] [PubMed] [Google Scholar]
- Matsuda K, & Yuzaki M (2011). Cbln family proteins promote synapse formation by regulating distinct neurexin signaling pathways in various brain regions. Eur J Neurosci, 33(8), 1447–1461. 10.1111/j.1460-9568.2011.07638.x [DOI] [PubMed] [Google Scholar]
- Mayer AD, Freeman NC, & Rosenblatt JS (1979). Ontogeny of maternal behavior in the laboratory rat: factors underlying changes in responsiveness from 30 to 90 days. Dev Psychobiol, 12(5), 425–439. 10.1002/dev.420120503 [DOI] [PubMed] [Google Scholar]
- Mayer AD, & Rosenblatt JS (1979). Ontogeny of maternal behavior in the laboratory rat: early origins in 18- to 27-day-old young. Dev Psychobiol, 12(5), 407–424. 10.1002/dev.420120502 [DOI] [PubMed] [Google Scholar]
- McCormick DA, & Thompson RF (1984). Neuronal responses of the rabbit cerebellum during acquisition and performance of a classically conditioned nictitating membrane-eyelid response. J Neurosci, 4(11), 2811–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina M, Uemura T, Yasumura M, & Yoshida T (2012). Molecular mechanism of parallel fiber-Purkinje cell synapse formation. Front Neural Circuits, 6, 90. 10.3389/fncir.2012.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, & Südhof TC (2003). Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature, 423(6943), 939–948. 10.1038/nature01755 [DOI] [PubMed] [Google Scholar]
- Nicolini C, & Fahnestock M (2018). The valproic acid-induced rodent model of autism. Exp Neurol, 299(Pt A), 217–227. 10.1016/j.expneurol.2017.04.017 [DOI] [PubMed] [Google Scholar]
- Numan M, & Numan MJ (1997). Projection sites of medial preoptic area and ventral bed nucleus of the stria terminalis neurons that express Fos during maternal behavior in female rats. J Neuroendocrinol, 9(5), 369–384. 10.1046/j.1365-2826.1997.t01-1-00597.x [DOI] [PubMed] [Google Scholar]
- Oristaglio J, Hyman West S, Ghaffari M, Lech MS, Verma BR, Harvey JA, Welsh JP, & Malone RP (2013). Children with autism spectrum disorders show abnormal conditioned response timing on delay, but not trace, eyeblink conditioning. Neuroscience, 248, 708–718. 10.1016/j.neuroscience.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxley G, & Fleming AS (2000). The effects of medial preoptic area and amygdala lesions on maternal behavior in the juvenile rat. Dev Psychobiol, 37(4), 253–265. [PubMed] [Google Scholar]
- Pak C, Danko T, Zhang Y, Aoto J, Anderson G, Maxeiner S, Yi F, Wernig M, & Südhof TC (2015). Human Neuropsychiatric Disease Modeling using Conditional Deletion Reveals Synaptic Transmission Defects Caused by Heterozygous Mutations in NRXN1. Cell Stem Cell, 17(3), 316–328. 10.1016/j.stem.2015.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, & Penn DL (2008). Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophr Res, 99(1–3), 164–175. 10.1016/j.schres.2007.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portfors CV (2007). Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci, 46(1), 28–34. [PubMed] [Google Scholar]
- Pregno G, Frola E, Graziano S, Patrizi A, Bussolino F, Arese M, & Sassoè-Pognetto M (2013). Differential regulation of neurexin at glutamatergic and GABAergic synapses. Front Cell Neurosci, 7, 35. 10.3389/fncel.2013.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner C, Runkel F, & Missler M (2013). Neurexins. Genome Biol, 14(9), 213. 10.1186/gb-2013-14-9-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt JS (1967). Nonhormonal basis of maternal behavior in the rat. Science, 156(3781), 1512–1514. 10.1126/science.156.3781.1512 [DOI] [PubMed] [Google Scholar]
- Rujescu D, Ingason A, Cichon S, Pietiläinen OP, Barnes MR, Toulopoulou T, Picchioni M, Vassos E, Ettinger U, Bramon E, Murray R, Ruggeri M, Tosato S, Bonetto C, Steinberg S, Sigurdsson E, Sigmundsson T, Petursson H, Gylfason A, Olason PI, Hardarsson G, Jonsdottir GA, Gustafsson O, Fossdal R, Giegling I, Möller HJ, Hartmann AM, Hoffmann P, Crombie C, Fraser G, Walker N, Lonnqvist J, Suvisaari J, Tuulio-Henriksson A, Djurovic S, Melle I, Andreassen OA, Hansen T, Werge T, Kiemeney LA, Franke B, Veltman J, Buizer-Voskamp JE, Sabatti C, Ophoff RA, Rietschel M, Nöthen MM, Stefansson K, Peltonen L, St Clair D, Stefansson H, Collier DA, & Investigators G (2009). Disruption of the neurexin 1 gene is associated with schizophrenia. Hum Mol Genet, 18(5), 988–996. 10.1093/hmg/ddn351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan BC, Young NB, Moy SS, & Crawley JN (2008). Olfactory cues are sufficient to elicit social approach behaviors but not social transmission of food preference in C57BL/6J mice. Behav Brain Res, 193(2), 235–242. 10.1016/j.bbr.2008.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanesan A, Zhou J, Scafidi J, Heck DH, Sillitoe RV, & Gallo V (2019). Emerging connections between cerebellar development, behaviour and complex brain disorders. Nat Rev Neurosci, 20(5), 298–313. 10.1038/s41583-019-0152-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Tan L, Tate K, & Okada M (2015). Rats demonstrate helping behavior toward a soaked conspecific. Anim Cogn, 18(5), 1039–1047. 10.1007/s10071-015-0872-2 [DOI] [PubMed] [Google Scholar]
- Schaaf CP, Boone PM, Sampath S, Williams C, Bader PI, Mueller JM, Shchelochkov OA, Brown CW, Crawford HP, Phalen JA, Tartaglia NR, Evans P, Campbell WM, Tsai AC, Parsley L, Grayson SW, Scheuerle A, Luzzi CD, Thomas SK, Eng PA, Kang SH, Patel A, Stankiewicz P, & Cheung SW (2012). Phenotypic spectrum and genotype-phenotype correlations of NRXN1 exon deletions. Eur J Hum Genet, 20(12), 1240–1247. 10.1038/ejhg.2012.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears LL, Andreasen NC, & O’Leary DS (2000). Cerebellar functional abnormalities in schizophrenia are suggested by classical eyeblink conditioning. Biol Psychiatry, 48(3), 204–209. 10.1016/s0006-3223(00)00247-x [DOI] [PubMed] [Google Scholar]
- Sears LL, Finn PR, & Steinmetz JE (1994). Abnormal classical eye-blink conditioning in autism. Journal of Autism and Developmental Disorders, 24(6), 737–751. 10.1007/BF02172283 [DOI] [PubMed] [Google Scholar]
- Sittig LJ, Carbonetto P, Engel KA, Krauss KS, Barrios-Camacho CM, & Palmer AA (2016). Genetic Background Limits Generalizability of Genotype-Phenotype Relationships. Neuron, 91(6), 1253–1259. 10.1016/j.neuron.2016.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siviy SM (2016). A Brain Motivated to Play: Insights into the Neurobiology of Playfulness. Behaviour, 153(6–7), 819–844. 10.1163/1568539X-00003349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Wilkins KB, Mogavero JN, & Veenema AH (2015). Social Novelty Investigation in the Juvenile Rat: Modulation by the μ-Opioid System. J Neuroendocrinol, 27(10), 752–764. 10.1111/jne.12301 [DOI] [PubMed] [Google Scholar]
- Stockman SL, & McCarthy MM (2017). Predator odor exposure of rat pups has opposite effects on play by juvenile males and females. Pharmacol Biochem Behav, 152, 20–29. 10.1016/j.pbb.2016.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzenberg DS, & Champagne FA (2016). Hormonal and non-hormonal bases of maternal behavior: The role of experience and epigenetic mechanisms. Horm Behav, 77, 204–210. 10.1016/j.yhbeh.2015.07.005 [DOI] [PubMed] [Google Scholar]
- Stoodley CJ (2016). The Cerebellum and Neurodevelopmental Disorders. Cerebellum, 15(1), 34–37. 10.1007/s12311-015-0715-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ & Limperopoulos C (2016). Structure-function relationships in the developing cerebellum: Evidence from early-life cerebellar injury and neurodevelopmental disorders. Semin Fetal Neonatal Med, 21(5), 356–364. 10.1016/j.siny.2016.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungur A, Schwarting RK, & Wöhr M (2016). Early communication deficits in the Shank1 knockout mouse model for autism spectrum disorder: Developmental aspects and effects of social context. Autism Res, 9(6), 696–709. 10.1002/aur.1564 [DOI] [PubMed] [Google Scholar]
- Südhof TC (2008). Neuroligins and neurexins link synaptic function to cognitive disease. Nature, 455(7215), 903–911. 10.1038/nature07456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H, Li Q, Zhang ZC, Li Y, & Han J (2016). Neurexin regulates nighttime sleep by modulating synaptic transmission. Sci Rep, 6, 38246. 10.1038/srep38246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T, Lee SJ, Yasumura M, Takeuchi T, Yoshida T, Ra M, Taguchi R, Sakimura K, & Mishina M (2010). Trans-synaptic interaction of GluRdelta2 and Neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell, 141(6), 1068–1079. 10.1016/j.cell.2010.04.035 [DOI] [PubMed] [Google Scholar]
- VanRyzin JW, Marquardt AE, Argue KJ, Vecchiarelli HA, Ashton SE, Arambula SE, Hill MN, & McCarthy MM (2019). Microglial Phagocytosis of Newborn Cells Is Induced by Endocannabinoids and Sculpts Sex Differences in Juvenile Rat Social Play. Neuron, 102(2), 435–449.e436. 10.1016/j.neuron.2019.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRyzin JW, Marquardt AE, & McCarthy MM (2020). Developmental origins of sex differences in the neural circuitry of play. International Journal of Play, 9(1), 58–75. 10.1080/21594937.2020.1723370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner RE, Zhang Y, Gray T, Abbacchi A, Cormier D, Todorov A, & Constantino JN (2019). Autism-Related Variation in Reciprocal Social Behavior: A Longitudinal Study. Child Dev, 90(2), 441–451. 10.1111/cdev.13170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SS, Kloth AD & Badura A (2014). The cerebellum, sensitive periods, and autism. Neuron, 83(3), 518–532. 10.1016/j.neuron.2014.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber ES, Harmon KM, Beckwith TJ, Peña S, Burgdorf J, Panksepp J, & Cromwell HC (2012). Selective breeding for 50 kHz ultrasonic vocalization emission produces alterations in the ontogeny and regulation of rough-and-tumble play. Behav Brain Res, 229(1), 138–144. 10.1016/j.bbr.2012.01.012 [DOI] [PubMed] [Google Scholar]
- Werling DM, & Geschwind DH (2013). Sex differences in autism spectrum disorders. Curr Opin Neurol, 26(2), 146–153. 10.1097/WCO.0b013e32835ee548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiśniowiecka-Kowalnik B, Nesteruk M, Peters SU, Xia Z, Cooper ML, Savage S, Amato RS, Bader P, Browning MF, Haun CL, Duda AW, Cheung SW, & Stankiewicz P (2010). Intragenic rearrangements in NRXN1 in three families with autism spectrum disorder, developmental delay, and speech delay. Am J Med Genet B Neuropsychiatr Genet, 153B(5), 983–993. 10.1002/ajmg.b.31064 [DOI] [PubMed] [Google Scholar]
- Wood GE, & Shors TJ (1998). Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci U S A, 95(7), 4066–4071. 10.1073/pnas.95.7.4066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhr M, Engelhardt KA, Seffer D, Sungur A, & Schwarting RK (2017). Acoustic Communication in Rats: Effects of Social Experiences on Ultrasonic Vocalizations as Socio-affective Signals. Curr Top Behav Neurosci, 30, 67–89. 10.1007/7854_2015_410 [DOI] [PubMed] [Google Scholar]
- Wöhr M, Silverman JL, Scattoni ML, Turner SM, Harris MJ, Saxena R, & Crawley JN (2013). Developmental delays and reduced pup ultrasonic vocalizations but normal sociability in mice lacking the postsynaptic cell adhesion protein neuroligin2. Behav Brain Res, 251, 50–64. 10.1016/j.bbr.2012.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Cheng L, Qian Y, Alliey-Rodriguez N, Kelsoe JR, Greenwood T, Nievergelt C, Barrett TB, McKinney R, Schork N, Smith EN, Bloss C, Nurnberger J, Edenberg HJ, Foroud T, Sheftner W, Lawson WB, Nwulia EA, Hipolito M, Coryell W, Rice J, Byerley W, McMahon F, Schulze TG, Berrettini W, Potash JB, Belmonte PL, Zandi PP, McInnis MG, Zöllner S, Craig D, Szelinger S, Koller D, Christian SL, Liu C, & Gershon ES (2009). Singleton deletions throughout the genome increase risk of bipolar disorder. Mol Psychiatry, 14(4), 376–380. 10.1038/mp.2008.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Rohlmann A, Sargsyan V, Aramuni G, Hammer RE, Südhof TC, & Missler M (2005). Extracellular domains of alpha-neurexins participate in regulating synaptic transmission by selectively affecting N- and P/Q-type Ca2+ channels. J Neurosci, 25(17), 4330–4342. 10.1523/JNEUROSCI.0497-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Chen LY, Liu X, Maxeiner S, Lee SJ, Gokce O & Südhof TC (2015). Neuroligins Sculpt Cerebellar Purkinje-Cell Circuits by Differential Control of Distinct Classes of Synapses. Neuron, 87(4), 781–796. 10.1016/j.neuron.2015.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]


