Abstract
Objective
A close relationship of microRNAs (miRNAs) with various human diseases has been widely reported, including cardiovascular disease. The current study attempted to examine the abnormal expression of miR-27b in asymptomatic carotid artery stenosis (ACAS), its diagnostic value and predictive value for the development of ACAS were also assessed.
Methods
Clinical serum samples were collected from both ACAS patients and healthy individuals, and levels of miR-27b in the clinical samples were detected using Real-time quantitative PCR. Cerebral ischemia events (CIEs) of patients during the 5-year follow-up were collected. The diagnostic and predictive values of serum miR-27b was assessed via plotting Receiver operating characteristic (ROC) and Kaplan-Meier curves. Multivariate cox regression analysis was performed for clinical independent index analysis.
Results
ACAS patients had higher levels of miR-27b than the healthy subjects. There were close association of serum miR-27b levels with total cholesterol (TC) level, absence of hypertension and degree of carotid stenosis. High levels of miR-27b could differentiate ACAS cases from healthy subjects, and predicted the high incidence of CIEs. MiR-27b could be used as an independent predictor of cerebrovascular events via multiple Cox regression analysis (P = .031).
Conclusion
The high level of miR-27b can predict the occurrence of ACAS, and is closely related to the subsequent occurrence of CIEs. The present results provide evidence for circulating miR-27b as a diagnostic and prognostic marker in patients with ACAS.
Keywords: miR-27b, carotid artery stenosis, diagnosis, cerebral ischemic events, prognosis
Introduction
Carotid artery stenosis (CAS) is a common disease, mainly due to arteriosclerotic stenosis.1,2 CAS is one of the major causes of cerebral ischemic events (CIE), contributing to the mortality associated with cerebrovascular diseases. 3 CAS has become one of the “top killers” endangering people's health in today's society. Asymptomatic ACAS is termed asymptomatic carotid stenosis when there are pathological changes in carotid artery extract but no clinical symptoms arise. Patients with ACAS have a higher risk of ischemic stroke with higher mortality.4,5 However, delayed treatment due to lack of attention in the early stages can be fatal once it strikes. Therefore, early diagnosis of ACAS is very important.
In recent years, with the in-depth study of microRNA, its clinical diagnostic and prognostic value in diseases has been reported radially. 6 Because of its non-invasive and convenient nature, it is expected to bring new and effective strategies for the diagnosis of diseases. 7 MicroRNA (miRNA) is a class of highly conserved small non-coding RNAs about 18∼24 nucleotides long, which regulate gene expression mainly by binding to the specific target gene mRNA 3’-UTR region.8,9 miRNAs are closely related to cell proliferation, differentiation, apoptosis, embryonic development, tissue and organ formation, and the occurrence and development of various diseases. 10 Studies have shown that there are significant differences in miRNA levels in the blood of patients with CAS compared with healthy people. Such as miR-145, miR-214.11,12 Recently, the role of miR-27b in atherosclerosis is put forward, its diagnostic and predictive potential in atherosclerosis are proved. 13 Serum miR-27b levels are significantly higher in the hypertensive patients with left ventricular hypertrophy (LVH) than in the hypertensive patients without LVH. 14 In vivo, miR-27b inhibition can improve the cardiac function of the mice with cardiac hypertrophy. 13 In addition, inhibition of miR-27b can abrogate the coronary artery ligation (CAL) induced cardiac fibrosis in rats. Considering important role of miR-27b in heart diseases, its potential role in ACAS attracts our concern. In addition, a high level of miR-27b in circulating exosomes of ACAS patients is detected by Affymetrix microarrays, which supported our hypothesis. 15 But the regulatory effect of miR-27b in ACAS has not been elucidated.
To test our hypothesis, ACAS patients were enrolled in the study, and the serum levels of miR-27b in patients were measured and compared. The Receiver operating characteristic (ROC) curve was plotted according to the level of serum miR-27b to evaluate the diagnostic value. In addition, the prognostic value of miR-27b in ACAS was explained according to the CIES during the follow-up period.
Materials and Methods
Collection of Clinical Samples
71 patients with ACAS who were admitted to Children's Hospital of Chongqing Medical University were prospectively collected. All subjects underwent a physical examination and their medical histories were reviewed. Inclusion criteria are as follows: (1) >18 years old; (2) more than 50% stenosis; (3) No history of stroke, transient ischemic attack, or other cardiovascular and cerebrovascular diseases; (4) No tumors; (5) No serious liver and kidney dysfunction and other systemic diseases; (6) understand and signed informed consent. In addition, 58 healthy subjects were raised as a control group. Basic clinical information was collected, including age, sex, smoking status, blood lipid, blood glucose and blood pressure index. Hypertension refers to systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm. This study was approved by the Ethics Committee of Children's Hospital of Chongqing Medical University. Fasting blood samples were taken from each individual, then the serum was collected after centrifugation, and all serum samples were stored at − 80°C for further experiments.
Follow-up Survey
All ACAS patients were followed for 5 years to record the onset of ipsilateral cerebral ischemic events, including strokes, transient ischemic attack (TIA) or sudden death.
Quantitative Real Time-Polymerase Chain Reaction (qRT-PCR) Assay
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was added into the serum samples to extract total RNA following the protocol description. Reverse-transcribed to cDNA was prepared with a TaqMan miRNA reverse transcription kit (Thermo Fisher Scientific). Real-time quantitative PCR (qRT-PCR) was performed for the quantification of miR-27b expression with SYBR-Green I Master mix kit (Invitrogen; Thermo Fisher Scientific, Waltham, USA), and the miRNA relative expression level was normalized by U6 and the calculation of miR-27b expressing changes was performed using the 2−ΔΔCT method.
Statistical Analysis
SPSS 20.0 software (IBM, Armonk, NY, USA) and GraphPad 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) were used to process clinical data. The clinical sample size was based on preliminary data, and calculated by using a two independent proportions power analysis with alpha = .05 and a power of 90% (beta = .1). And results indicated 52 individuals are needed for each group. Measurement data were expressed as mean value and standard deviation, and student's t-test was used for comparison between groups. The measurement data were compared by chi-square test. The diagnostic and predictive values of serum miR-27b were assessed via plotting Receiver operating characteristic (ROC) and Kaplan-Meier curves. Multivariate cox regression analysis was performed on the clinical indicators. The P < .05 was considered statistically significant.
Results
Comparison of Baseline Data Between Healthy Control and ACAS Patients’ Groups
The clinical characteristics of the study subjects were shown in Table 1, the results displayed that more hypertensive cases were observed in ACAS group compared with the control group (P < .05). There were no significant differences among other indicators, including age, sex, smoking, BMI and other blood lipid and glucose index (P > .05).
Table 1.
Comparison of clinical data between healthy control and ACAS patients
| Parameters | Healthy control | ACAS | P values |
|---|---|---|---|
| Age (years) | 63.05 ± 7.85 | 65.10 ± 6.62 | .111 |
| Sex (female/male) | 28/30 | 33/38 | .993 |
| Smoking (n, %) | 22 (37.93) | 34(47.89) | .353 |
| BMI (kg/m2) | 24.89 ± 1.61 | 25.21 ± 1.73 | .282 |
| TC (mmol/L) | 4.62 ± 0.73 | 4.83 ± 0.59 | .079 |
| TG (mmol/L) | 1.56 ± 0.56 | 1.74 ± 0.76 | .114 |
| HDL (mmol/L) | 1.34 ± 0.18 | 1.26 ± 0.35 | .090 |
| LDL (mmol/L) | 2.77 ± 0.97 | 3.04 ± 0.93 | .115 |
| FBG (mmol/L) | 5.64 ± 1.09 | 5.84 ± 1.20 | .330 |
| Hypertension (n, %) | 9 (15.52) | 30 (42.25) | s.001 |
Abbreviations: BMI, body mass index; TC: total cholesterol; TG: Triglycerides; HDL, high density lipoprotein; LDL, low density lipoprotein; FBG: fasting blood glucose.
Serum Level of miR-27b in ACAS Patients
A histogram was drawn based on qRT-PCR data to analyze the significance of the differences. As shown in Figure 1, the relative expression of miR-27b in ACAS patients was higher than that in healthy individuals (P < .001).
Figure 1.
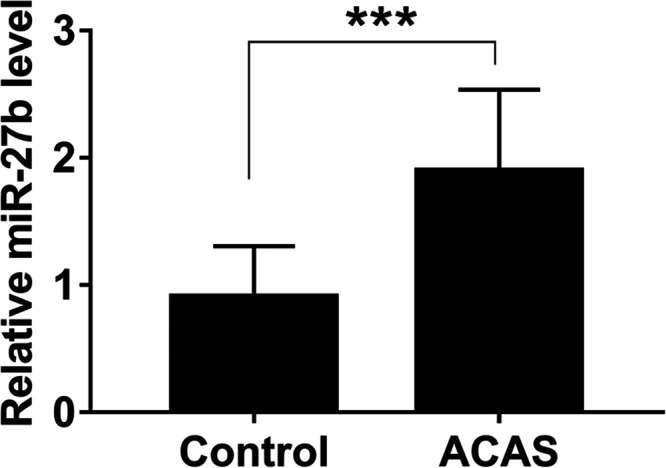
Through qRT-PCR, elevated miR-27b was detected in the serum of ACAS cases. *** P < 0.001.
Association of miR-27b with the Clinicopathological Features of ACAS Patients
To better understand the potential clinical utility of high miR-27b expression, all ACAS patients were divided into low expression group (n = 35) and high expression group (n = 36) based on the median value expression level of miR-27b. The results showed that the expression levels of miR-27b were significantly correlated with total cholesterol (P = .024), hypertension (P = .021) and degree of carotid stenosis (P = .006), and there was no obvious relationship of serum miR-27b level with age, sex, smoking history, body mass index thickness, triglycerides, high-density lipoprotein, low-density lipoprotein, and fasting blood glucose (Table 2).
Table 2.
Relationship between miR-27b and clinical parameters in ACAS patients
| Parameters | Total No. n = 71 | miR-27b expression | P values | |
|---|---|---|---|---|
| Low (n = 35) | High (n = 36) | |||
| Age (Years) | ||||
| <65 | 35 | 18 | 17 | .723 |
| ≥65 | 36 | 17 | 19 | |
| Sex | ||||
| Female | 33 | 16 | 17 | .899 |
| Male | 38 | 19 | 19 | |
| Smoking history | ||||
| No | 37 | 21 | 16 | .190 |
| Yes | 34 | 14 | 20 | |
| BMI | ||||
| <25.13 | 35 | 16 | 19 | .552 |
| ≥25.13 | 36 | 19 | 17 | |
| TC (mmol/L) | ||||
| <4.8 | 35 | 22 | 13 | .024 |
| ≥4.8 | 36 | 13 | 23 | |
| TG (mmol/L) | ||||
| <1.75 | 36 | 20 | 16 | .285 |
| ≥1.75 | 35 | 15 | 20 | |
| HDL (mmol/L) | ||||
| <1.30 | 35 | 20 | 15 | .192 |
| ≥1.30 | 36 | 15 | 21 | |
| LDL (mmol/L) | ||||
| <3.08 | 35 | 15 | 20 | .285 |
| ≥3.08 | 36 | 20 | 16 | |
| FBG (mmol/L) | ||||
| <5.70 | 33 | 20 | 13 | .076 |
| ≥5.70 | 38 | 15 | 23 | |
| Hypertension | ||||
| No | 41 | 25 | 16 | .021 |
| Yes | 30 | 10 | 20 | |
| Degree of carotid stenosis | ||||
| 50%-69% | 39 | 25 | 14 | .006 |
| 70%-99% | 32 | 10 | 22 | |
Abbreviations: BMI, body mass index; TC: total cholesterol; TG: Triglycerides; HDL, high density lipoprotein; LDL, low density lipoprotein; FBG: fasting blood glucose.
Diagnostic Value of miR-27b Expression for ACAS Patients
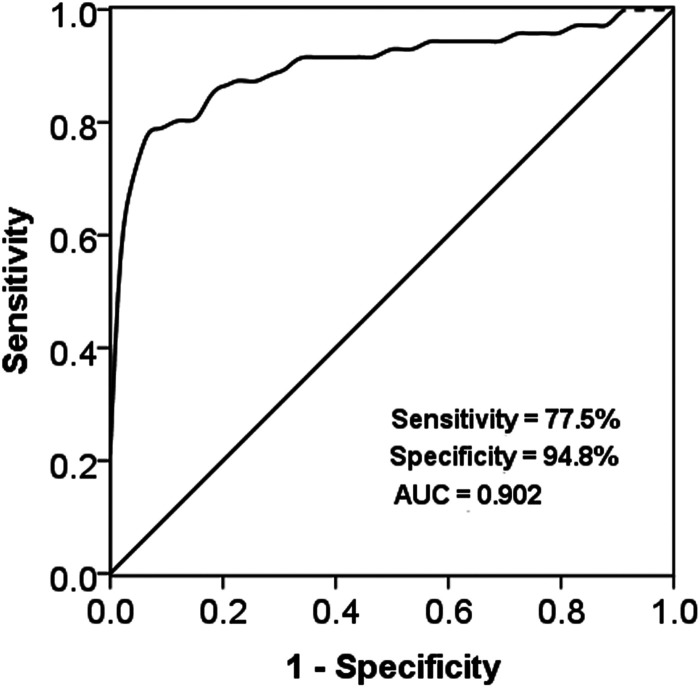
Based on the serum miR-27b levels in case and control groups, a ROC curve was plotted (Figure 2). The results showed that the AUC curve was 0.902, with a sensitivity of 77.5% and specificity of 94.8% at the cutoff value of 1.491. The data indicated that miR-27b had high diagnostic accuracy in differentiation between ACAS patients and healthy individuals.
Figure 2.
Receiver operating characteristic (ROC) curve constructed based on miR-27b in ACAS patients and healthy controls. The AUC curve was 0.902, with a sensitivity of 77.5% and specificity of 94.8% at the cutoff value of 1.491.
Predictive Value Analysis of miR-27b Expression for CIEs
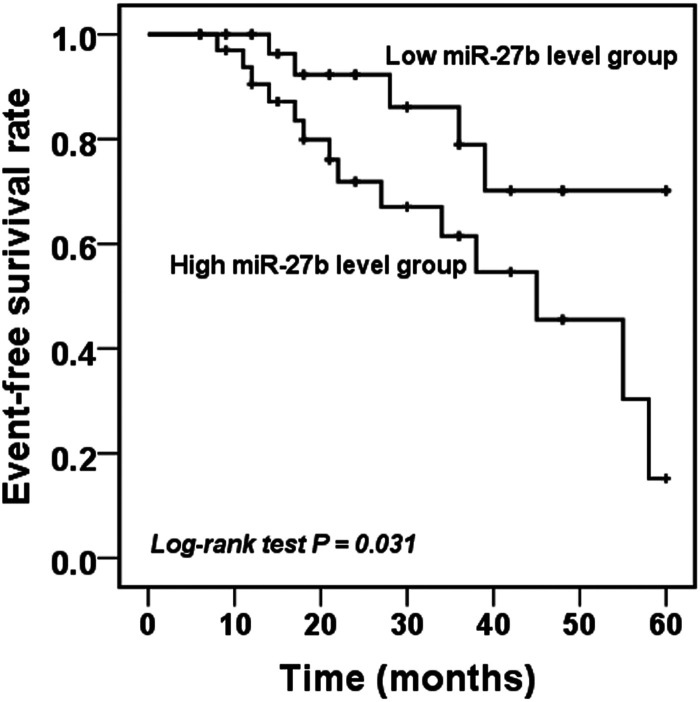
To evaluate the predictive value of miR-27b for the occurrence of CIEs in ACAS patients, the Kaplan-Meier method was applied. There were 19 patients underwent CIEs, including 12 TIAs and 7 strokes. Among 19 CIE patients, 14 cases were at high miR-27b expression and 5 were at low miR-27b expression. As shown in Kaplan-Meier curve, the patients with high miR-27b expression had a greater risk to suffer from CIEs (log-rank P = .031, Figure 3). Multivariate Cox regression analysis was performed to further investigate all risk factors related to the occurrence of CIEs. The analysis revealed that high levels of miR-27b (HR = 5.067, 95% CI = 1.170-21.943, P = .030) and high degree of carotid stenosis (HR = 3.366, 95% CI = 1.063-10.656, P =.039) were independent factors for the onset of CIEs in ACAS patients (Table 3).
Figure 3.
As shown in Kaplan-Meier curve, patients in the group with high miR-27b expression had a higher probability to suffer from CIES during the follow-up period (log-rank P = 0.031).
Table 3.
Multivariate Cox regression analysis for risk factors in ACAS patients
| Variables | Multivariate analysis | P value | |
|---|---|---|---|
| HR | 95% CI | ||
| miR-27b | 5.067 | 1.170 to 21.943 | .030 |
| Age | 1.532 | 0.347 to 6.763 | .573 |
| Sex | 2.188 | 0.460 to 10.404 | .325 |
| Smoking | 2.232 | 0.593 to 8.394 | .235 |
| BMI | 2.992 | 0.660 to 13.559 | .155 |
| TC (mmol/L) | 3.024 | 0.645 to 14.181 | .160 |
| TG (mmol/L) | 3.630 | 0.921 to 14.307 | .065 |
| LDL (mmol/L) | 4.259 | 0.957 to 18.948 | .057 |
| FBG (mmol/L) | 2.487 | 0.419 to 14.761 | .316 |
| Hypertension | 1.798 | 0.417 to 7.752 | .431 |
| Degree of carotid stenosis | 3.366 | 1.063 to 10.656 | .039 |
Abbreviations: BMI, body mass index; TC: total cholesterol; TG: Triglycerides; HDL, high density lipoprotein; LDL, low density lipoprotein; FBG: fasting blood glucose.
Discussion
CAS is an important independent risk factor for ischemic stroke, which is characterized by a high disability rate, high recurrence rate and high mortality rate. 16 Due to lack of obvious clinical symptoms at the early stage, patients with ACAS are likely to develop ischemic stroke, exacerbating cardiovascular and cerebrovascular disease related deaths. The current methods used to diagnose ACAS include carotid vascular ultrasound, transcranial Doppler ultrasound, CT angiography (CTA), MRI angiography (MRA), or whole brain digital angiography (DSA).17,18 However the development of ACAS is a slower and less obvious process, which may affect the early diagnosis, and the optimal treatment time would be missed, thus affecting the prognosis of patients. 19 In addition, traditional diagnosis and treatment methods also have disadvantages such as high cost, high technical requirements for operating equipment and personnel, and invasive operations, which will bring huge pain and heavy economic burden to patients and patients’ families.15,20 Therefore, the discovery of new and easier diagnostic markers is crucial.
MiRNA is a component of the epigenetic regulation of genes and plays an important role in the occurrence and development of cardiovascular and cerebrovascular diseases.21,22 Previous studies have shown that miRNA participates in the regulation of atherosclerosis through the regulation of lipid metabolism, and plays a very important role in the migration and proliferation of vascular smooth muscle cells, 23 the synthesis and degradation of the extracellular matrix, and the pathophysiological process of carotid artery stenosis. 24 For example, miRNA-181a, 25 miRNA-126, 26 and miR-330 27 have been shown to be closely related to atherosclerosis. In the present study, we displayed the important role of miR-27b in ACAS.
In the present study, high levels of miR-27b in the serum samples of ACAS were detected, indicating its potential role in the disease progression. Besides, there were close relationships for serum miR-27b with total cholesterol and hypertension. Supportably, compared with the healthy individuals, miR-27b is identified to be highly expressed in the serum of hypertension patients. 28 In addition, miR-27b has been reported to be an important regulator of cholesterol and lipid metabolism. 29 In obese people, there is close relationship for blood miR-27b level with higher levels of total cholesterol. 30 Hyperlipidemia and hypertension are important contributors to cerebrovascular diseases. The above results suggest that the up-regulation of miR-27b is involved in ACAS progression, its regulatory role in lipid metabolism and blood pressure may be the potential underlying mechanism.
Similar results have also been found in previous studies. A study by Li T and co-workers proposed that the expression of miR-27b was significantly increased in serum samples from patients with atherosclerosis obliterans, and miR-27b could be used as a serum biomarker for atherosclerosis obliterans. 31 In congenital heart disease associated pulmonary artery hypertension, elevated serum miR-27b is considered to be of great significance in the diagnosis and evaluation of congenital heart disease associated pulmonary artery hypertension. 32 Due to the significant difference in expression of miR-27b between normal individuals and ACAS patients, its diagnostic value for ACAS was evaluated. A ROC curve based on serum miR-27b was constructed. The results showed that miR-27b can differentiate ACAS patients from healthy individuals with relatively high sensitivity and specificity, indicating that serum miR-27b could serve as a promising diagnostic biomarker to screen ACAS patients.
Considering the abnormal expression and diagnostic value of miR-27b in ACAS, the clinical predictive value of miR-27b was further evaluated. All ACAS cases were followed up for five years, and during the follow-up time, 19 cases were recorded to suffer from CIEs. Among them, more cases showed high miR-27b levels, demonstrating that elevated miR-27b might be associated with the clinical outcome. Based on the follow-up results, KM curves were drawn, and the results indicated that patients with high miR-27b expression had a greater risk to suffer from CIEs. In addition, miR-27b was proved to be an independent factor for the onset of CIEs in ACAS patients via multivariate Cox regression analysis. Supportably, overexpression of miR-27b has been detected in ischemic stroke patients, and its clinical diagnostic value for the disease has also been proposed. 33 These results support our clinical findings.
In conclusion, elevated miR-27b level might be a promising biomarker for the early diagnosis of CAS. ACAS patients with elevated miR-27b levels have greater risk to suffer from CIEs, special attention together with early prevention should be considered for those at high risk. The present findings attempt to provide new potential markers for early diagnosis and prevention of ACAS. However, despite an initial enthusiasm in the field, miRNA-based biomarkers have not yet entered clinical routine due to incoherent normalization and non-standardized sample handling protocols. There is a long way to go before these results can be applied to routine clinical practice.
Acknowledgements
We deeply thank the help from Jianjun Zhou, Xuanxuan Wu, Junjun Guo, and Xia Qing for the qRT-PCR experiments and statistical analysis, and thanks to Zhang Ping and Liangying Yu for their contributions to data collation, analysis and revised the manuscript.
Footnotes
Research Ethics and Patient Consent: This study was approved by the Ethics Committee of Children's Hospital of Chongqing Medical University, and all participants signed informed consent before blood sampling.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Linjun Jiang https://orcid.org/0000-0002-2216-9953
References
- 1.Aday AW, Beckman JA. Medical management of asymptomatic carotid artery stenosis. Prog Cardiovasc Dis. 2017;59(6):585-590. [DOI] [PubMed] [Google Scholar]
- 2.Dharmakidari S, Bhattacharya P, Chaturvedi S. Carotid artery stenosis: medical therapy, surgery, and stenting. Curr Neurol Neurosci Rep. 2017(10);17:77. [DOI] [PubMed] [Google Scholar]
- 3.Park YJ, Kim DI, Kim GM, Kim DK, Kim YW. Natural history of asymptomatic moderate carotid artery stenosis in the Era of medical therapy. World Neurosurg. 2016;91:247-253. [DOI] [PubMed] [Google Scholar]
- 4.Lanzino G, Tallarita T, Rabinstein AA. Internal carotid artery stenosis: natural history and management. Semin Neurol. 2010;30(5):518-527. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Ma X, Lin J, He X, Tian F, Kong D. Severe carotid artery stenosis evaluated by ultrasound is associated with post stroke vascular cognitive impairment. Brain Behav. 2017;7(1):e00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye Y, Peng Y, He P, Zhang Q, Xu D. Urinary miRNAs as biomarkers for idiopathic osteonecrosis of femoral head: a multicentre study. J Orthop Translat. 2021;26:54-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosar E, Mamillapalli R, Moridi I, Duleba A, Taylor HS. Serum MicroRNA biomarkers regulated by simvastatin in a primate model of endometriosis. Reprod Sci. 2019;26(10):1343-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohr AM, Mott JL. Overview of microRNA biology. Semin Liver Dis. 2015;35(1):3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simonson B, Das S. MicroRNA therapeutics: the next magic bullet? Mini Rev Med Chem. 2015;15(6):467-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11(9):597-610. [DOI] [PubMed] [Google Scholar]
- 11.Zeng L, Liu J, Wang Y, et al. MicroRNA-210 as a novel blood biomarker in acute cerebral ischemia. Front Biosci(Elite edition). 2011;3:1265-1272. [DOI] [PubMed] [Google Scholar]
- 12.Chen YL, Sheu JJ, Sun CK, Huang TH, Lin YP, Yip HK. MicroRNA-214 modulates the senescence of vascular smooth muscle cells in carotid artery stenosis. Mol Med. 2020;26(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Song Y, Zhang Y, et al. Cardiomyocyte overexpression of miR-27b induces cardiac hypertrophy and dysfunction in mice. Cell Res. 2012;22(3):516-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Chen S, Gao Y, Zhang S. Serum MicroRNA-27b as a screening biomarker for left ventricular hypertrophy. Tex Heart Inst J. 2017;44(6):385-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolz S, Górriz D, Tembl JI, et al. Circulating MicroRNAs as novel biomarkers of stenosis progression in asymptomatic carotid stenosis. Stroke. 2017;48(1):10-16. [DOI] [PubMed] [Google Scholar]
- 16.Naylor AR. Asymptomatic carotid artery stenosis: state of the art management. J Cardiovasc Surg (Torino). 2013;54(1 supp 1):1-7. [PubMed] [Google Scholar]
- 17.Mannheim D, Falah B, Karmeli R. Endarterectomy or stenting in severe asymptomatic carotid stenosis. Isr Med Assoc J: IMAJ. 2017;19(5):289-292. [PubMed] [Google Scholar]
- 18.Paraskevas KI, Veith FJ, Ricco JB. Best medical treatment alone may not be adequate for all patients with asymptomatic carotid artery stenosis. J Vasc Surg. 2018;68(2):572-575. [DOI] [PubMed] [Google Scholar]
- 19.Gaba K, Ringleb PA, Halliday A. Asymptomatic carotid stenosis: intervention or best medical therapy? Curr Neurol Neurosci Rep. 2018;18(11):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badacz R, Przewłocki T, Gacoń J, et al. Circulating miRNA levels differ with respect to carotid plaque characteristics and symptom occurrence in patients with carotid artery stenosis and provide information on future cardiovascular events. Adv Interven Cardiol. 2018;14(1):75-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding S, Huang H, Xu Y, Zhu H, Zhong C. MiR-222 in cardiovascular diseases: physiology and pathology. BioMed Res Int. 2017;2017:4962426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vacante F, Denby L, Sluimer JC, Baker AH. The function of miR-143, miR-145 and the MiR-143 host gene in cardiovascular development and disease. Vasc Pharmacol. 2019;112:24-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horie T, Baba O, Kuwabara Y, et al. MicroRNAs and lipoprotein metabolism. J Atheroscler Thromb. 2014;21(1):17-22. [DOI] [PubMed] [Google Scholar]
- 24.Tan JR, Koo YX, Kaur P, et al. microRNAs in stroke pathogenesis. Curr Mol Med. 2011;11(2):76-92. [DOI] [PubMed] [Google Scholar]
- 25.Su Y, Yuan J, Zhang F, et al. MicroRNA-181a-5p and microRNA-181a-3p cooperatively restrict vascular inflammation and atherosclerosis. Cell Death Dis. 2019;10(5):365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schober A, Nazari-Jahantigh M, Wei Y, et al. MicroRNA-126–5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med. 2014;20(4):368-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei X, Sun Y, Han T, et al. Upregulation of miR-330–5p is associated with carotid plaque’s stability by targeting talin-1 in symptomatic carotid stenosis patients. BMC Cardiovasc Disord. 2019;19(1):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu L, Liu Z. Serum from patients with hypertension promotes endothelial dysfunction to induce trophoblast invasion through the miR27b3p/ATPase plasma membrane Ca(2 + ) transporting 1 axis. Mol Med Rep. 2021;23(5):319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Lu Y, Zhu L, Zhang H, Feng L. Inhibition of miR-27b regulates lipid metabolism in skeletal muscle of obese rats during hypoxic exercise by increasing PPARgamma expression. Front Physiol. 2020;11:1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Can U, Buyukinan M, Yerlikaya FH. The investigation of circulating microRNAs associated with lipid metabolism in childhood obesity. Pediatr Obes. 2016;11(3):228-234. [DOI] [PubMed] [Google Scholar]
- 31.Li T, Cao H, Zhuang J, et al. Identification of miR-130a, miR-27b and miR-210 as serum biomarkers for atherosclerosis obliterans. Clin Chim Acta. 2011;412(1-2):66-70. [DOI] [PubMed] [Google Scholar]
- 32.Long L, Xiao Y, Yin X, Gao S, Zhou L, Liu H. Expression of serum miR-27b and miR-451 in patients with congenital heart disease associated pulmonary artery hypertension and risk factor analysis. Exp Ther Med. 2020;20(4):3196-3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng X, Kan P, Ma Z, et al. Exploring the potential value of miR-148b-3p, miR-151b and miR-27b-3p as biomarkers in acute ischemic stroke. Biosci Rep. 2018:38(6):BSR20181033. [DOI] [PMC free article] [PubMed] [Google Scholar]