Abstract
Atherosclerosis (AS) is a common vascular disease with great harm. The current study examined the expression pattern of miR-18a-5p in AS patients, and explored its clinical values. 110 AS patients and 68 healthy controls were collected clinically, and the expression pattern of miR-18a-5p in the serum of AS patients was detected using qRT-PCR. All AS patients were followed up for five years to record the adverse cardiovascular events. ROC and Kaplan-Meier (K-M) curve were plotted to assess the diagnostic ability. The multiple Cox regression analysis was performed for independent influencing factors analysis. MiR-18a-5p was at high expression in AS patients, and showed positive correlation with the CIMT value (r = 0.789, P < .001). ROC curve suggested the high diagnostic value of serum miR-18a-5p for AS, with the AUC of 0.894. The diagnostic specificity and sensitivity were 86.8% and 79.1%, respectively. K-M plot demonstrated that cases with high miR-18a-5p levels were more likely to suffer from cardiovascular events, and it is an independent influence factor for the poor clinical outcome. Serum miR-18a-5p serves as a promising biomarker for AS diagnosis, and is related to the occurrence of adverse cardiovascular events.
Keywords: atherosclerosis, miR-18a-5p, diagnosis, prognosis
Introduction
Atherosclerosis (AS) is a common disease that seriously harms human health, it is the main pathological basis of heart ischemic and cerebrovascular diseases.1,2 Up to now, the pathogenesis of AS has not been fully understood. Many risk factors have been identified to be associated with the occurrence of AS, leading to the lack of effective prevention and treatment drugs in clinical practice.3,4 The progression of AS mainly includes four stages, the initial stage is vascular endothelial cell (VEC) injury. The progression stage was continuous proliferation and migration of vascular smooth muscle cells (VSMC), the plaque area increased continuously. Acute coronary events occurred during the rupture of unstable plaques.5–7 AS has no clinical manifestations in the early stage and is relatively insidious. Without effective intervention, AS consequently causes coronary heart disease (CHD), which leads to a great risk of sudden death. Therefore, early diagnosis and intervention of patients with AS are particularly important.
MicroRNA (miRNA) is a type of small non-coding RNA with a length of about 18 to 25 nucleotides. The main function of miRNAs is to inhibit the translation and transcription of the target genes via binding to the 3′ untranslated regions (UTR) of the target gene mRNA, thus changing the gene expression at the post-transcriptional level.8,9 MiRNA plays an important role in various biological processes, such as cell apoptosis, autophagy, proliferation and differentiation.10,11 It also has been reported that miRNAs participate in the regulation of pathogenesis of cardiovascular disease.12–14 MiR-18a-5p is located at chromosome 13q31.3 and belongs to the miRNA 17 to 92 cluster. 15 It has been reported to promote the VSMCs proliferation, migration and differentiation in several studies.16,17 It is known that the abnormal behavior of VSMCs contributes to the occurrence and development of AS. However, the clinical value of miR-18a-5p in AS has not been examined.
In the study, 110 asymptomatic AS patients were recruited, and the levels of miR-18a-5p in the serum samples were detected. Besides, the adverse cardiovascular events were recorded during the 5-year follow-up, and the clinical diagnostic and predictive values of serum miR-18a-5p were also examined.
Materials and Methods
Study Population and Sample Collection
110 asymptomatic AS patients and 68 healthy controls were recruited in Yidu Central Hospital of Weifang from January 2014 to January 2016. People with serious cardiovascular diseases, such as stroke, acute myocardial infarction, and heart failure were not included in this study. Carotid intima-media thickness (CIMT) was measured by ultrasonography, individuals with CIMT thickness ranging from 0.9 mm to 1.2 mm were included in the study as the patient's group. Physical examination and record-keeping were performed on all subjects. Besides, serum samples from all subjects were collected for subsequent testing. This study was approved by the Ethics Committee of Yidu Central Hospital of Weifang. A 5-year follow-up was conducted to obtain the prognosis of the patients.
RNA Extraction and Quantitative Real-Time PCR
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was added to extract total RNA from the serum samples, and the RNA was reversed into cDNA based on the instructions. The expression levels of miR-18a-5p were determined by quantitative real-time PCR (qRT-PCR). qRT-PCR was performed with the SYBR Green I Master Mix reagent kit (Invitrogen, USA) according to the kit instructions on an ABI 7300 PCR System. The expression levels were calculated by the 2−ΔΔCT method. U6 was used as internal control for miRNA quantification. Three repeat holes were set for each sample.
Follow-up Plan and Contents
According to the newly released definition of cardiovascular key events, hospitalization and death caused by myocardial infarction, stroke, acute heart failure, unstable angina pectoris, transient ischemic attack, coronary intervention and other cardiovascular events are defined as cardiovascular end events. All cases with AS were followed up for five years, the time of subjects’ cardiovascular endpoints was recorded on time.
Statistical Analysis
All statistical analysis of clinical data was done using SPSS 21.0 software and GraphPad 5.0. The comparison between groups was performed using Student's t-test and one-way analysis of variance (ANOVA). The receiver-operating-characteristic (ROC) curve was used to assess the diagnostic value of miR-18a-5p in AS patients. The link between two clinical variables was determined by Pearman correlation coefficient analysis. Kaplan-Meier method and the log-rank method were used to detect the predictability of miR-18a-5p for adverse cardiovascular events in patients. Prognostic value was assessed by Cox regression model. P < .05 was considered statistically significant.
Results
Clinicopathological Data in AS Patients and Healthy Controls
The demographic characteristics and clinical data of AS patients and healthy controls were shown in Table 1, the results indicated that the C-reactive protein (CRP) value in the AS group was significantly higher than that in the healthy control group (P < .05), while there was no significant difference in body mass index (BMI), age, gender, total cholesterol, high-density lipoprotein (HDL-C), low-density lipoprotein (LDL-C), systolic blood pressure (SBP), diastolic blood pressure (DBP), triglyceride (P > .05). There were individuals on medication in both groups, including ACE-I/ARB, β-Blockers and Statins. It was found that more patients in the AS groups used β -blockers compared with the control group, and the difference was significant (P < .05). There was no significant difference between the two groups in the usage of ACE-I/ARB and Statins (P > .05).
Table 1.
Clinical Data of the Study Population.
| Features | Healthy controls (n = 68) | AS patients (n = 110) | P value |
|---|---|---|---|
| Age (years) | 52.69 ± 3.74 | 53.43 ± 4.59 | .239 |
| Gender (male/female) | 35/33 | 59/51 | .780 |
| BMI (kg/m2) | 24.41 ± 3.06 | 25.09 ± 2.51 | .128 |
| Total cholesterol (mg/dl) | 199.25 ± 9.76 | 201.39 ± 12.21 | .221 |
| HDL-C (mg/dl) | 46.69 ± 7.36 | 48.50 ± 10.87 | .187 |
| LDL-C (mg/dl) | 115.97 ± 14.29 | 117.44 ± 12.33 | .469 |
| Triglyceride (mg/dl) | 168.56 ± 22.53 | 173.58 ± 20.68 | .130 |
| Heart rate (beats/min) | 74.05 ± 16.67 | 76.60 ± 15.38 | .299 |
| SBP (mm Hg) | 131.43 ± 9.91 | 134.47 ± 16.42 | .125 |
| DBP (mm Hg) | 88.75 ± 6.50 | 88.27 ± 5.27 | .593 |
| CRP (mg/l) | 6.24 ± 1.53 | 12.19 ± 2.43 | .000 |
| Medication | |||
| ACE-I/ARB | 15 | 38 | .077 |
| β-Blockers | 3 | 21 | .005 |
| Statins | 10 | 18 | .768 |
Abbreviations: AS, atherosclerosis; BMI, Body Mass Index; CIMT, Carotid Intima Media Thickness; CRP, C-reactive protein; DBP, Diastolic Blood Pressure; HDL-C, high-density lipoproteincholesterol; LDL-C, low-density lipoproteincholesterol; SBP, Systolic Blood Pressure.
Serum miR-18a-5p Level in AS Patients and the Correlation Analysis with CIMT
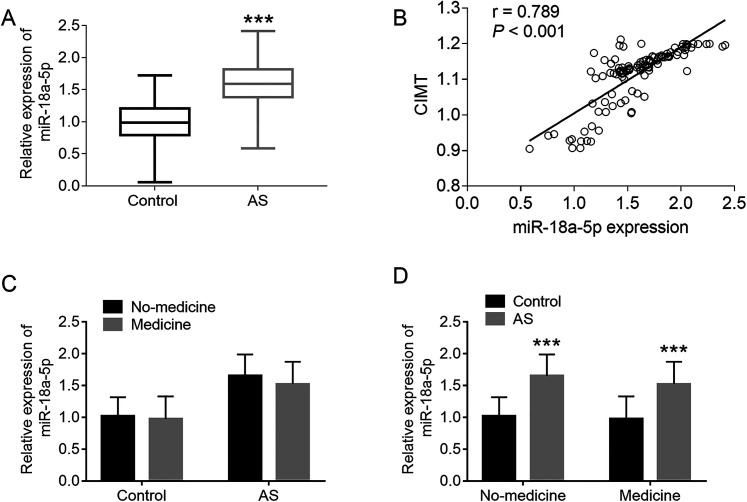
MiR-18a-5p levels in all clinical serum samples were investigated by qRT-PCR. It was observed that miR-18a-5p was at the high expression in the serum of AS patients compared with the control individuals, and the difference was statistically significant (P < .001, Figure 1A).
Figure 1.
The expression level of Serum miR-18a-5p in AS patients and the correlation study between miR-18a-5p expression and CIMT. (A) Relative expression levels of serum miR-18a-5p in healthy controls and AS patients, ***P < .001. (B) Correlation between miR-18a-5p expression level and CIMT in AS patients, r = 0.789 ***P < .001. (C) Comparison of miR-18a-5p levels between medicine and no-medicine groups for both control and AS groups. (D) In medicine or no-medicine group, comparison of miR-18a-5p levels between control group and AS group. ***P < .001, compared with control group.
CIMT is recommended by the American heart association and the American college of cardiology as the most scholastic and useful method to identify AS. Based on the Spearman correlation analysis results, a remarkable positive correlation was detected for serum miR-18a-5p with CIMT value in the patients’ group (r = 0.789, P < .001, Figure 1B).
Given the medication in both groups, all cases were divided into medication group and non-medication group according to medication status. As shown in Figure 1C, miR-18a-5p levels were indeed slightly lower in the medicine group than in the no-medicine group, but the difference was not significant (P > .05, Figure 1C). It was found that no matter in medicine group and no-medicine group, miR-18a-5p levels increased significantly in AS group compared with the control group (P < .001, Figure 1D).
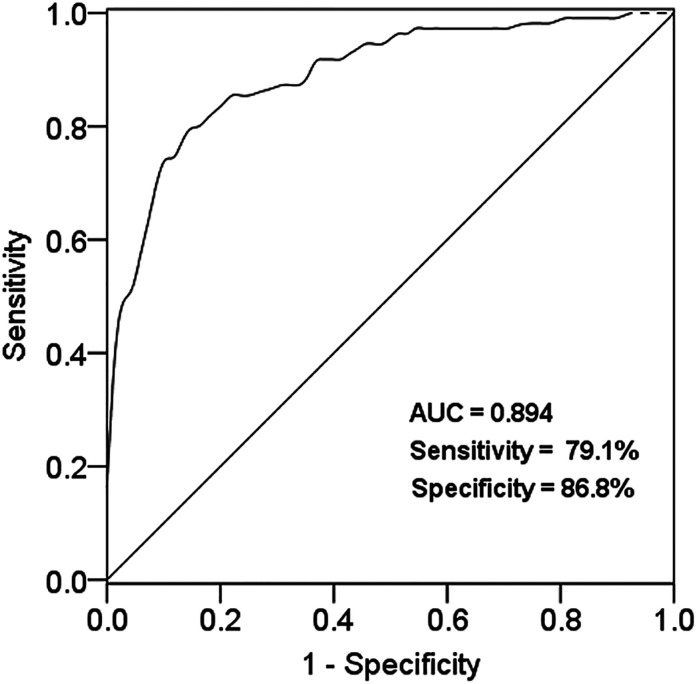
Diagnostic Value of miR-18a-5p in serum of AS Patients
As shown in Figure 2, a ROC curve was plotted based on the serum miR-18a-5p level in both case and control groups. The AUC was 0.892, the sensitivity was 79.1% and the specificity was 86.8% at the cut-off value of 1.34. The results indicated that miR-18a-5p had a good diagnostic value for AS.
Figure 2.
ROC curve was established according to the expression level of miR-18a-5p in AS patients, with the AUC score of 0.894.
miR-18a-5p Expression is Correlated with Clinical Outcomes of AS Patients
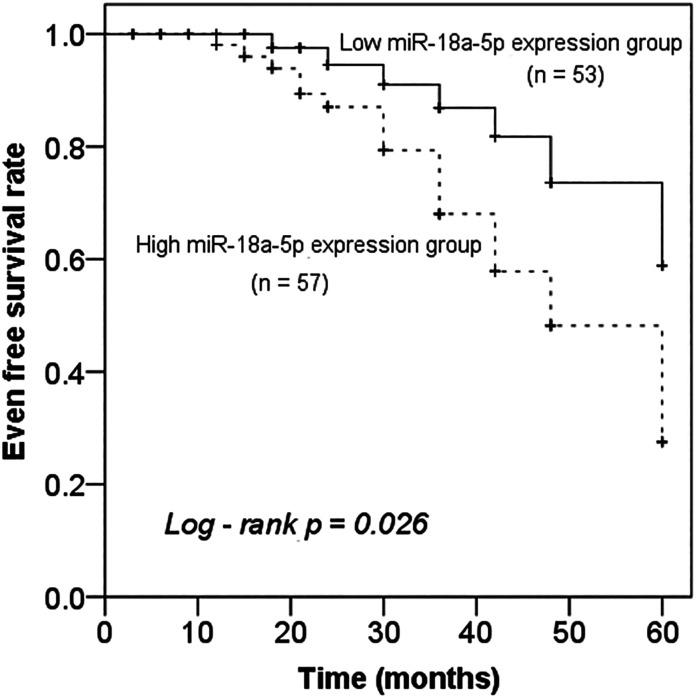
According to the mean value of serum miR-18a-5p in AS patients’ group, 110 AS patients were divided into the high expression group (n = 57) and low expression group (n = 53). The Kaplan-Meier curve was drawn based on the follow-up results, it was found that patients with elevated miR-18a-5p exhibited a significantly shorter 5-year event-free survival rate (Figure 3, P = .026). In addition, multivariate Cox regression analysis was performed to assess the predictive value for future poor clinical outcome, miR-18a-5p (P = .026, HR = 2.844) and CIMT (P = .043, HR = 3.763) were determined to be independent prognostic factors for the occurrence of poor cardiovascular events in AS patients (Table 2).
Figure 3.
Kaplan-Meier survival curves of AS patients with different miR-18a-5p expression levels, Log-rank P = .026.
Table 2.
Multivariate Cox Regression Analysis of Factors for Survival of AS Patients.
| Variables | Multivariate Cox regression analysis | ||
|---|---|---|---|
| HR | 95%CI | P value | |
| miR-18a-5p | 2.844 | 1.135 to 7.123 | .026 |
| Age (years) | 1.460 | 0.544 to 3.916 | .452 |
| Gender (male/female) | 0.744 | 0.291 to 1.905 | .537 |
| BMI (kg/m2) | 1.487 | 0.583 to 3.797 | .406 |
| Total cholesterol (mg/dl) | 1.422 | 0.590 to 3.429 | .433 |
| HDL-C (mg/dl) | 1.350 | 0.326 to 5.591 | .679 |
| LDL-C (mg/dl) | 1.175 | 0.486 to 2.840 | .720 |
| Triglyceride (mg/dl) | 1.565 | 0.4665.253 | .469 |
| Heart rate (beats/min) | 1.916 | 0.692 to 5.299 | .211 |
| SBP (mm Hg) | 1.471 | 0.562 to 3.849 | .431 |
| DBP (mm Hg) | 1.685 | 0.671 to 4.228 | .267 |
| CRP (mg/l) | 1.368 | 0.154 to 12.148 | .778 |
| CIMT (mm) | 3.763 | 1.041 to 13.605 | .043 |
| Medicine | 0.812 | 0.354 to 1.861 | .622 |
Abbreviations: AS, atherosclerosis; BMI, Body Mass Index; 95% CI: 95% confidence intervals; CIMT, Carotid Intima Media Thickness; CRP, C-reactive protein; DBP, Diastolic Blood Pressure; HDL-C, high-density lipoproteincholesterol; LDL-C, low-density lipoproteincholesterol; SBP, Systolic Blood Pressure.
Discussion
AS is the pathological basis of many cardiovascular and cerebrovascular diseases.18,19 As a result of the changes in diet structure and lifestyle, AS tends to be more frequent and younger in China. 7 In recent years, correlation of AS with the occurrence of cardiovascular events has attracted wide attention.20–22 Therefore, early diagnosis and intervention of AS are crucial to the prevention of cardiovascular and cerebrovascular diseases. Recent studies have provided new insights about the diagnostic value of expression patterns of miRNAs in serum in various diseases.23–25 In cardiovascular diseases, many miRNAs have been identified to be abnormally expressed, and their value in clinical diagnosis and prognosis has also attracted attention.26,27
MiR-18a-5p belongs to the miR-17 to 92 family, 28 which has been suggested to serve as a regulator of angiogenesis. 29 The research of Hae Jin Kee reported the crucial role of miR-18a-5p in VSMC differentiation. 16 It is well known that abnormal behaviors of VSMCs are important events in the occurrence and development of AS.30,31 In view of the regulatory effect of miR-18a-5p in VSMCs, its clinical value in AS patients attracts our interest. In the present study, clinical serum samples were collected from 110 AS patients and 68 healthy individuals, and the expression of miR-18a-5p was demonstrated to be enhanced in AS patients. Consistently, a high level of miR-18a-5p is identified in the peripheral blood of women with coronary heart disease, and it could regulate coronary heart disease development through targeting estrogen receptor (ER). 32 Combined with our clinical findings, we concluded that miR-18a-5p may play an important role in the development of AS. Given the medication in both groups, all cases were divided into medication group and non-medication group according to medication status. It was found that miR-18a-5p levels were indeed slightly lower in the medicine group than in the no-medicine group, but the difference was not significant. As for the effect of drugs on miR-18a-5p levels, further studies are needed to expand the sample. We described this prospect in the discussion section. In addition, it was also found that no matter in medicine group and no-medicine group, miR-18a-5p levels increased significantly in AS group compared with the control group. These data demonstrated the changes of miR-18a-5p in AS cases, and supported our hypothesis about its crucial role in the development of AS.
AS is chronic systemic inflammation, and the inflammatory process contributes to the occurrence of acute AS thrombotic events.18,33 CRP is an acute protein, and it is also an important clinical marker for inflammation and AS.34,35 By analyzing the clinicopathological characteristics in AS patients and healthy controls, we concluded that the concentration of CRP is usually higher in AS patients. In addition, CIMT can be reliably measured in vivo by carotid ultrasound, which is a simple and reliable method for subclinical AS evaluation.36,37 This study showed that miR-18a-5p is highly expressed in AS patients with high CIMT, and is positively correlated with CIMT value. ROC curve was used to detect the diagnostic sensitivity and specificity of miR-18a-5p. It was observed that miR-18a-5p can distinguish AS cases from healthy individuals. Therefore, the up-regulation expression of miR-18a-5p can be used as a promising marker for the diagnosis of AS. Similar results were found in acute myocardial infarction. Plasma miR-18a-5p levels were significantly higher in patients with acute myocardial infarction, and also can be used to screen patients with acute myocardial infarction from healthy controls with high sensitivity and specificity by ROC analysis. 38 Although the present study determined the aberrant expression of miR-18a-5p in the serum of AS cases, its original from AS was not explored. Furthermore, the association of miR-18a-5p with the vulnerable plaque is also needed for further exploration.
To further investigate the prognostic significance of miR-18a-5p in AS, all AS patients were followed up for five years and the clinical outcomes of each patient were recorded during the follow-up time. According to the clinical data of follow-up, the Kaplan-Meier curve was plotted for the prognostic value evaluation. It demonstrated that patients with elevated miR-18a-5p exhibited a significantly shorter 5-year event-free survival rate. In addition, multivariate Cox analysis also proved that miR-18a-5p was an independent prognostic factor for the occurrence of poor cardiovascular events in AS. This suggests that miR-18a-5p is a good prognostic marker for AS prognosis and is closely related to the occurrence of cardiovascular and cerebrovascular events in patients with 5-year follow-up. The adverse effects of miR-18a-5p on acute myocardial infarction are also presented by Bin Lin and co-workers, which may be completed by promoting cardiomyocyte autophagy and suppressing cellular senescence via brain-derived neurotrophic factor. Besides, an elevated level of miR-18a was also identified in rats models with acute myocardial infarction. 29 This also verified our results that miR-18a-5p plays an important role in the occurrence and development of cardiovascular and cerebrovascular events. Although the clinical data analysis results proved that miR-18a-5p may be a potential diagnostic and prognostic marker of AS, there are still some limitations in this study. Firstly, the sample size is relatively small, which may reduce the statistical value of our study; secondly, the mechanism of miR-18a-5p in the occurrence and development of AS is still unclear, and we will carry out a further study in this aspect. In addition, it has been previously reported that hypertension, hyperlipidemia and overweight were risk factors for AS and long-term adverse cardiovascular events. 39 But in our study, hypertension, hyperlipidemia and BMI showed no independent influence for the occurrence of cardiovascular end events, it might be because of the small sample size. Studies with larger sample sizes are needed to validate the results.
Taken together, miR-18a-5p was at high expression in the serum of AS patients, and serves as a promising biomarker for the early diagnosis of AS. The elevated level of serum miR-18a-5p is related to the poor prognosis of AS patients. MiR-18a-5p inhibition can provide a new idea for a more effective treatment of AS. The present findings provide an improvement of our knowledge regarding the mechanism and treatment of AS.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Ethical Approval: This study was approved by the Ethics Committee of Yidu Central Hospital of Weifang.
Informed Consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iDs: Yonglei Liu https://orcid.org/0000-0003-0976-3859
References
- 1.Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006;47(8):C7-C12. [DOI] [PubMed] [Google Scholar]
- 2.Zhu Y, Xian X, Wang Z, et al. Research progress on the relationship between atherosclerosis and inflammation. Biomolecules. 2018;8(3):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li B, Li W, Li X, Zhou H. Inflammation: a novel therapeutic target/direction in atherosclerosis. Curr Pharm Des. 2017;23(8):1216-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libby P, Bornfeldt KE, Tall AR. Atherosclerosis: successes, surprises, and future challenges. Circ Res. 2016;118(4):531-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Vietinghoff S, Koltsova EK. Inflammation in atherosclerosis: a key role for cytokines. Cytokine. 2019;122:154819. [DOI] [PubMed] [Google Scholar]
- 6.Wu C, Daugherty A, Lu HS. Updates on approaches for studying atherosclerosis. Arterioscler, Thromb, Vasc Biol. 2019;39(4):e108-e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimmer S, Grebe A, Latz E. Danger signaling in atherosclerosis. Circ Res. 2015;116(2):323-340. [DOI] [PubMed] [Google Scholar]
- 8.Feinberg MW, Moore KJ. MicroRNA regulation of atherosclerosis. Circ Res. 2016;118(4):703-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu Y, Thavarajah T, Gu W, Cai J, Xu Q. Impact of miRNA in atherosclerosis. Arterioscler, Thromb, Vasc Biol. 2018;38(9):e159-e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song H, Li D, Wu T, et al. MicroRNA-301b promotes cell proliferation and apoptosis resistance in triple-negative breast cancer by targeting CYLD. BMB Rep. 2018;51(11):602-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdelhalim DA, Elgamal BM, ElKafoury MR, et al. MicroRNA-150 down regulation in acute myeloid leukaemia patients and Its prognostic implication. Open Access Maced JMed Sci. 2018;6(11):1993-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Churov A, Summerhill V, Grechko A, Orekhova V, Orekhov A. MicroRNAs as potential biomarkers in atherosclerosis. Int J Mol Sci. 2019;20(22):5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laffont B, Rayner KJ. MicroRNAs in the pathobiology and therapy of atherosclerosis. Can J Cardiol. 2017;33(3):313-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu X. The role of exosomes and exosome-derived microRNAs in atherosclerosis. Curr Pharm Des. 2017;23(40):6182-6193. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Wei X, Wu B, Su J, Tan W, Yang K. Tumor-educated platelet miR-34c-3p and miR-18a-5p as potential liquid biopsy biomarkers for nasopharyngeal carcinoma diagnosis. Cancer Manag Res. 2019;11:3351-3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kee HJ, Kim GR, Cho SN, et al. miR-18a-5p MicroRNA increases vascular smooth muscle cell differentiation by downregulating Syndecan4. Korean Circ J. 2014;44(4):255-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Chen X. miR-18a-5p promotes proliferation and migration of vascular smooth muscle cells by activating the AKT/extracellular regulated protein kinases (ERK) signaling pathway. Med Sci Monit. 2020;26:e924625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. 2016;118(4):535-546. [DOI] [PubMed] [Google Scholar]
- 19.Xu S, Kamato D, Little PJ, Nakagawa S, Pelisek J, Jin ZG. Targeting epigenetics and non-coding RNAs in atherosclerosis: from mechanisms to therapeutics. Pharmacol Ther. 2019;196:15-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canfrán-Duque A, Rotllan N, Zhang X, et al. Macrophage deficiency of miR-21 promotes apoptosis, plaque necrosis, and vascular inflammation during atherogenesis. EMBO Mol Med. 2017;9(9):1244-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Gregoli K, Mohamad Anuar NN, Bianco R, et al. MicroRNA-181b controls atherosclerosis and aneurysms through regulation of TIMP-3 and elastin. Circ Res. 2017;120(1):49-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hajibabaie F, Kouhpayeh S, Mirian M, et al. MicroRNAs as the actors in the atherosclerosis scenario. J Physiol Biochem. 2020;76(1):1-12. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Teng C, Ma J, et al. miR-19 family: a promising biomarker and therapeutic target in heart, vessels and neurons. Life Sci. 2019;232:116651. [DOI] [PubMed] [Google Scholar]
- 24.Gryshkova V, Fleming A, McGhan P, et al. miR-21-5p as a potential biomarker of inflammatory infiltration in the heart upon acute drug-induced cardiac injury in rats. Toxicol Lett. 2018;286:31-38. [DOI] [PubMed] [Google Scholar]
- 25.Quan X, Ji Y, Zhang C, et al. Circulating MiR-146a may be a potential biomarker of coronary heart disease in patients with subclinical hypothyroidism. Cell Physiol Biochem. 2018;45(1):226-236. [DOI] [PubMed] [Google Scholar]
- 26.Hulsmans M, Holvoet P. MicroRNA-containing microvesicles regulating inflammation in association with atherosclerotic disease. Cardiovasc Res. 2013;100(1):7-18. [DOI] [PubMed] [Google Scholar]
- 27.Kucher AN, Nazarenko MS. The role of MicroRNA in atherogenesis. Kardiologiia. 2018;57(9):65-76. [DOI] [PubMed] [Google Scholar]
- 28.Kolenda T, Guglas K, Kopczyńska M, et al. Good or not good: role of miR-18a in cancer biology. Rep Pract Oncol Radiother: J Greatpoland Cancer Center Poznan Polish Soc Radiat Oncol. 2020;25(5):808-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin B, Feng D, Xu J. Cardioprotective effects of microRNA-18a on acute myocardial infarction by promoting cardiomyocyte autophagy and suppressing cellular senescence via brain derived neurotrophic factor. Cell Biosci. 2019;9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen Tervaert JW. Cardiovascular disease due to accelerated atherosclerosis in systemic vasculitides. Best Pract Res Clin Rheumatol. 2013;27(1):33-44. [DOI] [PubMed] [Google Scholar]
- 31.Nelson AJ, Peterson ED, Pagidipati NJ. Atherosclerotic cardiovascular disease and heart failure: determinants of risk and outcomes in patients with diabetes. Prog Cardiovasc Dis. 2019;62(4):306-314. [DOI] [PubMed] [Google Scholar]
- 32.Yuan L, Tang C, Li D, Yang Z. MicroRNA-18a expression in female coronary heart disease and regulatory mechanism on endothelial cell by targeting estrogen receptor. J Cardiovasc Pharmacol. 2018;72(6):277-284. [DOI] [PubMed] [Google Scholar]
- 33.Kulbertus H, Lancellotti P. Atherosclerosis: a complex disease. Rev Med Liege. 2012;67(5-6):273-278. [PubMed] [Google Scholar]
- 34.Ballantyne CM, Nambi V. Markers of inflammation and their clinical significance. Atheroscler Suppl. 2005;6(2):21-29. [DOI] [PubMed] [Google Scholar]
- 35.Soeki T, Sata M. Inflammatory biomarkers and atherosclerosis. Int Heart J. 2016;57(2):134-139. [DOI] [PubMed] [Google Scholar]
- 36.Nezu T, Hosomi N, Aoki S, Matsumoto M. Carotid intima-media thickness for atherosclerosis. J Atheroscler Thromb. 2016;23(1):18-31. [DOI] [PubMed] [Google Scholar]
- 37.Polak JF, O’Leary DH. Carotid intima-media thickness as surrogate for and predictor of CVD. Glob Heart. 2016;11(3):295-312. e3. [DOI] [PubMed] [Google Scholar]
- 38.Jiang M, Yin Y, Xie L, He H. Plasma miR-18 screens acute myocardial infarction from healthy controls by targeting hypoxia inducible factor 1α. Clin Lab. 2018;64(7):1207-1212. [DOI] [PubMed] [Google Scholar]
- 39.Rustempasic N, Gengo M. Assesment of carotid stenosis with CT angiography and color Doppler ultrasonography. Med Arch. 2019;73(5):321-325. [DOI] [PMC free article] [PubMed] [Google Scholar]