Abstract
Oral booster-single strain probiotic bifidobacteria could be a potential strategy for SARS-CoV-2. This study aims to evaluate the role of oral probiotic Bifidobacterium on moderate/severe SARS-CoV-2 inpatients. In this single-center study, we analyzed data of 44 moderate/severe inpatients with diagnosed COVID-19 in Istanbul Maltepe University Medical Faculty Hospital, 2020 from 1 November 2020 to 15 December 2020. Clinical and medication features were compared and analyzed between patients with or without probiotic. In result, 19 of the 44 patients (43.18%) who were administrated with oral booster-single strain probiotic were discharged with the median inpatient day of 7.6 days which were significantly shorter than those of patients without probiotic. There were significant differences in inpatient days, radiological improvement at day 6 and week 3, and reduction in interleukin-6 levels in those receiving oral probiotic therapy. Although the mortality rate was 5% in the probiotic group, it was 25% in the non-probiotic group. Booster-single strain probiotic bifidobacteria could be an effective treatment strategy for moderate/severe SARS-CoV-2 inpatients to reduce the mortality and length of stay in hospital.
Keywords: SARS-CoV-2, probiotic, bifidobacteria
Introduction
SARS-CoV-2 is a pandemic virus that exists itself with lung disease as well as leading to symptoms and signs associated with the gastrointestinal tract. It is especially manifested by the disturbed immune status in lung and intestinal tissues. Although the pathophysiology of the SARS-CoV-2 in lung and gut remains to be defined, it is evident that its pathological impacts include both direct effects of viral invasion and a complex immunological response. 1 Increased endoplasmic reticulum stress and cellular autophagy, which play key roles for the cellular replication of the virus, are mediated by the enhanced adaptive Th17/IL-6 immune system and this leads to an uncontrolled immune response. 2 Currently, there are no proven medical treatment developed for this uncontrolled immune response. Anti-inflammatory agents (corticosteroids), anticoagulation agents (enoxaparin), Favipiravir (anti-viral treatment), and specific immune treatment (Anti-Il-6, anti-Il-1, and Immune plasma) are considered for the medical therapy of moderate/severe SARS-CoV-2 inpatients in the Turkish Health of Ministry guidelines for COVID-19. 3
Probiotics are living microorganisms that have been shown to have modulatory effects on adaptive immunity. The beneficial immune modulation effects of some single strain probiotic bacteria have been shown. 4 The pathogenesis of the immune response to coronavirus bears a strong resemblance with TH17-Th1driven autoimmune diseases and these TH17-Th1 immune interactions appear to play an important role in virus replication. The immune beneficial effects of bifidobacteria strains include an anti-IL-17 effect which could play important role in SARS-CoV-2 treatment. 2 Bifidobacterium animalis sp. Lactis provides to rapid mucosal healing in inflammatory bowel disease (IBD) and this effect was related to the IL-17 inhibitory effect of the Bifidobacterium animalis sp. Lactis strain.2,4 Cytokine storm can be controlled through these immune modulating effects of probiotic bifidobacteria. In a current analysis, it has been shown that reduced Bifidobacterium abundance is likely associated with increased SARS-CoV-2 infection severity, 5 so Bifidobacterium and other probiotic supplementation or refloralization via booster Bifidobacterium administration might provide a therapeutic benefit, particularly for patients hospitalized with severe disease. Here, we offer a new approach with booster dose oral single strain probiotic Bifidobacterium administration in moderate/severe SARS-CoV-2 patients.
Method
Patients
In this retrospective study, 44 moderate/severely ill adult inpatients (≥18 years old), who were admitted to Istanbul Maltepe University Medical Faculty Hospital, the designated hospital for Covid-19 patients, 2020 from 1 November 2020 to 15 December 2020 have been included. The patients were confirmed as COVID-19 positive according to World Health Organisation guidance, and for non-probiotic group, the treatment was given according to Turkish Health Ministry guidelines for COVID-19. It has been scanned retrospectively from the hospital registry system in accordance with the Helsinki Declaration Principles. The patients were followed up to 15 January 2021.
Medication
In this retrospective study, 44 moderate/severely ill adult inpatients (≥18 years old), who were admitted to Istanbul Maltepe University Medical Faculty Hospital, the designated hospital for Covid-19 patients, 2020 from 1 November 2020 to 15 December 2020 have been included. The inpatients were divided into probiotic group (n:20) and nonprobiotic (n:24) group. The probiotic group consisted of those who deny and do not tolerate the (Tables 1 and 2) Ministry of Health guide treatment for SARS-CoV-2. Oral administration of probiotic treatment included one trillion CFUs Bifidobacterium BB-12 strain which were dissolved in 250 mL water. Total dose were divided into three parts and administrated to patients for 3 days. Inpatient groups were not consumed probiotic-containing foods or any other probiotic supplements during hospitalization.
Table 1.
Clinical features of non-probiotic group.
| Moderate (M) and severe(S) pneumonia | Steroid and LWMAH | Hospitalization period (Day) | Specific treatment (Sp) (anti-IL-1,Anti-IL-6,Plazma) | Sp treatment type | Sp. Treatment type | Sp.treatment type | Antibiotic | Antibiotic type | Antibiotic type | Dead | Thorax CT resolution 6 day of hospitalization | Thorax CT resolution on 3 weeks | Hospitalization IL-6 levels (pg/ml) | 3 weeks ıl-6 level |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | Yes | 14 | No | No | No | No | No | 80 | 50 | |||||
| M | Yes | 13 | No | No | No | No | No | 58 | N/A | |||||
| M | Yes | 11 | No | No | No | Yes | Yes | 36 | 15 | |||||
| S | Yes | 12 | Yes | Tocilizumab | Yes | Ceftriaxone | No | No | N/A | 77 | 36 | |||
| M | Yes | 14 | No | No | No | No | Yes | 48 | 10 | |||||
| S | Yes | 10 | Yes | TCZ | Yes | Meropenem | No | No | N/A | 35 | N/A | |||
| S | Yes | 10 | No | Yes | Gentamycine | No | No | N/A | 64 | N/A | ||||
| S | Yes | 10 | No | Yes | Ceftriaxone | No | No | No | 44 | 24 | ||||
| S | Yes | 14 | Yes | TCZ | Anti-IL-1 | Plasma | Yes | Ceftriaxone | Meropenem | No | No | No | 96 | 46 |
| M | Yes | 13 | No | Yes | Ceftriaxone | No | Yes | No | 84 | 28 | ||||
| S | Yes | 12 | Yes | TCZ | Yes | Ceftriaxone | No | No | No | 45 | 36 | |||
| S | Yes | 11 | Yes | TCZ | Anti-IL-1 | Yes | Ceftriaxone | No | No | N/A | 63 | N/A | ||
| S | Yes | 22 | Yes | TCZ | No | No | No | No | 180 | N/A | ||||
| S | Yes | 10 | Yes | No | No | Yes | Yes | 106 | 16 | |||||
| S | Yes | 13 | Yes | İmmun plazma | Yes | Ceftriaxone | Meropenem | Yes | No | N/A | 74 | N/A | ||
| S | Yes | 23 | Yes | TCZ | Yes | Meronem | Yes | No | N/A | 256 | N/A | |||
| M | Yes | 11 | Yes | Anti-IL-1 | Yes | Ceftriaxone | Yes | No | N/A | 127 | N/A | |||
| S | Yes | 17 | Yes | TCZ | ACT | Plasma | Yes | Ceftriaxone | Meropenem | Yes | No | N/A | 345 | N/A |
| S | Yes | 14 | Yes | TCZ | ACT | Yes | Ceftriaxone | Yes | No | N/A | 156 | N/A | ||
| S | Yes | 17 | No | Yes | Meronem | No | No | No | 106 | 60 | ||||
| S | Yes | 10 | Yes | Anti-IL-1 | Yes | Ceftriaxone | No | No | 62 | 36 | ||||
| S | Yes | 8 | Yes | ACT | Yes | Ceftriaxone | No | No | Yes | 24 | 4 | |||
| S | Yes | 24 | Yes | Anti-IL-1 | Yes | Ceftriaxone | Meropenem | No | No | No | 124 | 76 |
Table 2.
Clinical features of probiotic group (N/A: Not applicable).
| Treatment case group | Gender | Age | Moderate and severe pneumonia | Steroid and LWMAH | Hospitalization period (Day) | Specific treatment (Sp) (anti-IL-1,Anti-IL-6,Plazma) | Antibiotic | Antibiotic type | Dead | Thorax CT resolution 6 days of hospitalization (posttreatment 3 day) | Thorax CT resolution on 3 weeks | Hospitalization IL-6 levels (pg/ml) | 3 weeks ıl-6 level |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 45 | Moderate | Yes | 7 | No | No | No | Yes | Yes | 62 | 5 | |
| 2 | F | 47 | Moderate | Yes | 6 | No | No | No | Yes | Yes | 79 | 7 | |
| 3 | M | 51 | Moderate | Yes | 7 | No | No | No | Yes | Yes | 27 | 3 | |
| 4 | F | 36 | Severe | Yes | 8 | No | Yes | Levofloksasin | No | Yes | Yes | 156 | 4 |
| 5 | M | 41 | Moderate | Yes | 6 | No | No | No | Yes | Yes | 124 | 7 | |
| 6 | M | 71 | Severe | Yes | 9 | No | Yes | Levofloksasin | No | Yes | N/A | 78 | N/A |
| 7 | F | 60 | Moderate | Yes | 7 | No | No | No | No | N/A | 56 | N/A | |
| 8 | F | 61 | Severe | Yes | 8 | No | Yes | Levofloksasin | No | No | Yes | 188 | 8 |
| 9 | F | 45 | Moderate | Yes | 9 | No | Yes | Levofloksasin | No | Yes | Yes | 94 | 4 |
| 10 | M | 66 | Moderate | Yes | 7 | No | Yes | Levofloksasin | No | Yes | Yes | 116 | 6 |
| 11 | M | 44 | Moderate | Yes | 6 | No | No | No | Yes | Yes | 246 | 6 | |
| 12 | F | 50 | Moderate | Yes | 6 | No | No | No | Yes | N/A | 135 | N/A | |
| 13 | F | 50 | Severe | Yes | 9 | No | Yes | Levofloksasin | Yes | No | N/A | 486 | N/A |
| 14 | M | 65 | Severe | Yes | 8 | No | No | No | No | Yes | 106 | 10 | |
| 15 | F | 45 | Moderate | Yes | 7 | No | Yes | No | Yes | N/A | 55 | N/A | |
| 16 | M | 44 | Moderate | Yes | 7 | No | Yes | No | Yes | N/A | 79 | N/A | |
| 17 | M | 73 | Moderate | Yes | 8 | No | Yes | Levofloksasin | No | Yes | N/A | 24 | N/A |
| 18 | M | 55 | Severe | Yes | 9 | No | Yes | Levofloksasin | No | No | Yes | 110 | 9 |
| 19 | M | 49 | Moderate | Yes | 8 | No | Yes | No | Yes | Yes | 56 | 6 | |
| 20 | M | 73 | Severe | Yes | 9 | No | Yes | Levofloksasin | No | No | Yes | 88 | 5 |
Additional treatment included the following levofloksasin in probiotic group, ceftriaxone-meropenem in non-probiotic group and specific treatment (Anti-Interleukin-6, Anti-Interleukin-1, and Immune plasma) in non-probiotic group.
Procedures
The clinical features, medical therapy, and laboratory findings of diagnosed moderate/severe cases of SARS-CoV-2 were collected from hospital electronic medical records. The diagnosis of SARS-CoV-2 is to confirm real-time reverse-transcriptase polymerase-chain-reaction (RTPCR) assay tests using throat-swab specimens that were obtained from upper respiratory tracts after clinical symptoms of fever, cough, and dyspnea.
The lung disease severity of SARS-CoV-2 was defined according to the clinical and thorax CT radiological criteria:
Moderate Disease/Pneumonia: Patients who describe shortness of breath in addition to clinical findings, SpO> 90 and above, need intermittent nasal oxygen and with ground-glass, uncommon local, or multi-focal infiltration findings on tomography.
Severe Disease/Pneumonia: In addition to clinical findings, patients with significant shortness of breath, SpO: <90 and in need of oxygen with a continuous nasal and/or mask, and diffuse ground-glass in tomography, diffuse multi-focal infiltrations and consolidated areas.
The specific treatment consists of monoclonal antibodies [anti-Il-6 (TCZ), anti-Il-1] and immune plasma therapies. According to Turkish management guidelines for COVID-19, Oral Favipiravir treatment 2000 mg/total dose in Mild/Moderate inpatients and specific treatment who does not respond the Favipiravir treatment in Moderate/Severe inpatients was given non-probiotic group.
Thoracic tomography was taken on hospital admission, on the sixth day and 3 weeks after hospitalization. Radiological improvement was considered as significant improvement in lung condition with regression of diffuse airspace consolidations, ground-glass opacities and septal thickening.
Plasma levels of interleukin-6 were collected after hospitalization and at 3 weeks.
The criteria for discharge were considered; absence of fever for at least 3 days, substantial improvement in both lungs on chest CT, clinical remission of respiratory symptoms, and two throat-swab samples negative for the SARS-CoV-2 test obtained at least 24 h.
Statistical analysis
Chi-square independence test was used to get the dependency relation between variables which are at nominal and/or ordinal scale. If the prerequisite of chi-square independence test was not provided, fisher exact test used for 2*2 contingency tables. To test the mean equality for two groups, Man–Whitney U test was used because of normality condition. Frequency distribution, Percentage was used as descriptive statistics for nominal and ordinal scale variables. Mean, standard deviation, and frequency were used as descriptive statistics for interval/ratio scale variables. SPSS Version 25 were used for analysis. Power of test was calculated as post-hoc.
Results
Outcome
This retrospective single-center study analyzed 44 moderate/severe inpatients with SARS-CoV-2 who were hospitalized in Istanbul Maltepe University Medical Faculty Hospital 2020 from 1 November 2020 to 15 December 2020. The clinical features were followed up to 15 January 2021. In result, 19 of the 20 patients (95%) who were treated with probiotic discharged with the median inpatient day of 7.6 days and lower mortality rate 5%. 19 of the 24 (79%) patients without the treatment of probiotic discharged with the median inpatient day of 13.6 days and higher mortality rate 20.83%. Significant differences existed across the treatment of probiotic with regards to inpatient days (p < 0.001), sixth day and at 3 weeks radiological improvement (p < 0.001) and decrease in interleukin-6 levels (p < 0.001). No adverse event was observed in probiotic group.
Clinical features
Probiotic and non-probiotic groups
Since the number of successful treatment for death rates is below 5, independent samples t test could not be performed for rates. The chi-square test could not be performed because the ratio of cells with an expected value less than five is more than 50%. According to Fisher Exact test results in 2 * 2 tables, the death status was not dependent on the group (Chi-square = 2.497, df = 1, p = 0.192). Although the tests used for group comparisons cannot be used due to sample size and Fisher’s exact test results do not show dependence, the mortality rate in the treatment group is one fourth of the control group (Table 3).
Table 3.
Mortality rate in non-probiotic and probiotic group (p = 0.192) power 0.185.
| Dead * group Crosstabulation | |||||
|---|---|---|---|---|---|
| Group | Total | ||||
| Non-probiotic | Probiotic | ||||
| Dead | Yes | Count | 5 | 1 | 6 |
| % Within group | 21.7% | 5.0% | 14.0% | ||
| No | Count | 18 | 19 | 37 | |
| % Within group | 78.3% | 95.0% | 86.0% | ||
| Total | Count | 23 | 20 | 43 | |
| % Within group | 100% | 100% | 100% | ||
Inpatient day
Hospitalization period (Day) values differ at 99% confidence level between control and treatment groups (μNon-probiotic=13.6, μProbiotic=7.6, p = 0.000) (Table 4).
Table 4.
Inpatient days in probiotic and non-probiotic group (p < 0.001) power 0.999.
| Group | N | Mean | Std. Deviation | Std. Error mean | Man UZ | p | |
|---|---|---|---|---|---|---|---|
| Hospitalization period (Day) | Non-probiotic | 23 | 13.6 | 4.34 | 0.90 | 7500 | 0.000 |
| Probiotic | 20 | 7.6 | 1.10 | 0.25 | −5447 | ||
Thoracic findings
Thorax CT resolution on 6 days of hospitalization (Posttreatment 3 day for probiotic group) results are dependent on treatment groups at 99% confidence level (Table 5). Although the early response rate was 13.6% in the nonprobiotic group, this rate increased to 70.0% in the probiotic group.
Table 5.
Early thoracic findings in probiotic and non-probiotic group (p < 0.001) power 0.964.
| Crosstab | |||||
|---|---|---|---|---|---|
| Group | Total | ||||
| Non-probiotic | Probiotic | ||||
| Thorax CT resolution 6 days of hospitalization (Posttreatment 3 day) | Yes | Count | 3 | 14 | 17 |
| % Within group | 13.6% | 70.0% | 40.5% | ||
| No | Count | 19 | 6 | 25 | |
| % Within group | 86.4% | 30.0% | 59.5% | ||
| Total | Count | 22 | 20 | 42 | |
| % Within group | 100% | 100% | 100% | ||
Thorax CT resolution on 3 weeks results are dependent on groups at 99% confidence level. Although the response rate was 28.6% in the nonprobiotic group, this rate increased to 100.0% in the probiotic group (Table 6) (Figures 1 and 2).
Table 6.
Thorax CT resolution results at week three in probiotic and non-probiotic group (p<0.01) power 0.998.
| Crosstab | |||||
|---|---|---|---|---|---|
| Group | Total | ||||
| Non-probiotic | Probiotic | ||||
| Thorax CT resolution on 3 weeks | Yes | Count | 4 | 13 | 17 |
| % Within group | 28.6% | 100.0% | 63.0% | ||
| No | Count | 10 | 0 | 10 | |
| % Within group | 71.4% | 0.0% | 37.0% | ||
| Total | Count | 14 | 13 | 27 | |
| % Within group | 100% | 100% | 100% | ||
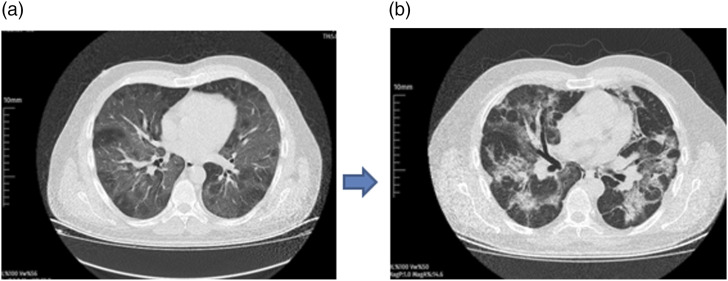
Figure 1.
Non-probiotic case with severe disease thorax CT at hospital admission (a) and at 3 weeks (b).
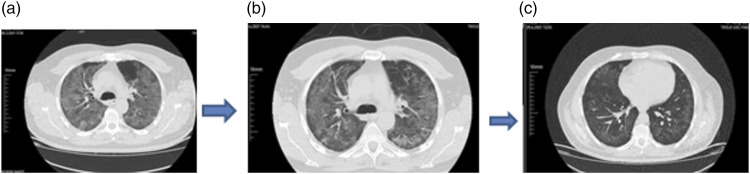
Figure 2.
Probiotic case with severe disease thorax CT at hospital admission (a), on sixth day of hospitalization (posttreatment 3th day) (b) and at 3 weeks (b).
Plasma levels of interleukin-6
Third week’s plasma Il-6 level values differ at 99% confidence level between control and treatment groups (μNon-probiotic = 33.6, μProbiotic = 6.2, p = 0.000) (Table 7).
Table 7.
Plasma Il-6 levels in probiotic and non-probiotic group (p=0.000) power 0.993.
| Group | N | Mean | Std. Deviation | Std. Error mean | Man U Z | p | |
|---|---|---|---|---|---|---|---|
| 3 weeks ıl-6 level | Non-probiotic | 13 | 33.6 | 20.71 | 5.74 | 11,50000 | 0.000 |
Discussion
In current, there is no specific anti-viral medical therapy for SARS-CoV-2, 6 and often supportive treatments are considered. Probiotics could be considered as a therapy for SARS-CoV-2.2,7–9 However, there is a lack of scientific report about the effect of single strain probiotic bacteria on SARS-CoV-2. In our study, we evaluated 44 moderate/severe SARS-CoV-2 inpatients, providing clinical evidence that single strain probiotic bifidobacteria can reduce mortality and hospital stay in moderate/severe SARS-CoV-2 patients. Early resolution in thorax CT and decrease in interleukin six level can be predictors of healing.
SARS-CoV-2 causes Endoplasmic Reticulum (ER) stress condition leads to the inositol-requiring enzyme 1(IRE1) pathway 2 mediated autophagy. In a report, viral replication could be decreased by blocking the inositol-requiring enzyme one in SARS-CoV patients with bronchitis. 10 Also, Kong et al. 11 showed that IL-6 induces autophagy via the IRE1. Interleukin-17 is a proinflammatory cytokine that reveals an important role in the adaptive immune system. IL-17 (originating mainly from Th17) is also a strong cytokine by increasing of ER stress and autophagy via IRE1. 4 Besides these, IL-17 plays a potent inducer of chronic inflammation in IBD. 4 Hou et al. 12 reported that the increasing of IL-6 stimulates the production of Th17 cells and that IL-6 and IL-17 association promotes the viral replication and this synergistically communication could be important therapeutic strategy for SARS-CoV therapies. Increased IL-6 levels were reported in patients with SARS and were correlated with disease severity. 13 Zhou et al. 14 showed that IL-6 levels were correlated with mortality in patients with SARS-CoV-2. The over expression of IL-6 might explain the excessive activated Th17/Il-17 cells observed in SARS-CoV-2 patients. 15 Grifoni et al. 16 cited that blood IL-6 level at hospital admission might be considered as a predictive factor for the combined endpoint progression to moderate/severe disease and/or in-hospital mortality, and it seems to be the best prognostic factor for negative outcome. Although cytokine storm occurred due to the uncontrolled adaptive immune response that occurs during the virus cell invasion and cellular replication, treatment responses could not be shown with specific anti-interleukin treatments. Rosas et al. showed that there is no effect of anti-interleukin six treatment on mortality and morbidity in SARS-CoV-2 patients. 17 Also, Cavalli et al. presented that interleukin-1 inhibition has no effect on disease mortality and morbidity. 18
There are currently limited scientific studies with probiotics in SARS-CoV-2. Li et al. reported that oral probiotics might be an effective therapeutic approach for the treatment of SARS-CoV-2 patients to reduce the secondary infection and modulated the adaptive immunity. 19 In accordance to Santacroce et al. 20 probiotics might have an important role against the SARS-CoV-2 infection. Some strains of Bifidobacterium such as BB-12, Infantis show anti- IL-17 effect. A booster-single dose administration of Bifidobacterium strain such as BB-12, Infantis might be considered as a therapeutic method in SARS-CoV-2 patients. 2 Bifidobacterium animalis might provide blocking the replication of SARS-CoV by reducing ER stress-related autophagy over the anti-interleukin-17 effect. Oral booster dose administration of Bifidobacterium animalis subsp. Lactis in SARS-CoV-2 patients’ effectiveness on intestinal and lung recovery could be attributed to the enhancement/control of immune modulatory status and possible anti-IL-17/IL six effect in intestinal mucosa. B. animalis subsp. Lactis BB-12 does not belong to the human gut microbiota system. However, BB-12 strain has been reported to be effective and safe for human intestinal microbiota. 21 Also, BB-12 strain shows strong growth ability under gastric pH and bile acid conditions. 22 Larsen et al showed that the dysbiosis recovery of BB-12 increased significantly with increasing dose. 23 According to them, up to 1012 CFU of Bifidobacterium BB-12 can be considered as a booster dose. Some of single strain probiotic bifidobacteria such as BB-12, Infantis have an anti-viral exopolysaccharide cell feature that can block SARS-CoV-2 adhesion on tissue. Baindara et al. 24 cited that Lactobacillus casei and Bifidobacterium lactis probiotics have anti-viral activity against the respiratory viral diseases. Although it has been shown that probiotics containing multiple species taken from the mouth reduce the severity of the infection, 20 the cause and effect relationship cannot be clearly revealed due to the various of probiotic bacterial species, at this point, the high dose single strain of probiotic bifidobacteria approach maybe more suitable method. However, our study group was small and our initial results must further be supported by large randomized studies. There is also a need for more comprehensive analytic calculation studies by confirming gut microbial analysis methods in those with dysbiosis in large t randomized studies
Conclusion
Oral booster dose Bifidobacterium animalis subsp. Lactis administration could provide lower mortality, shortening the length of stay in hospital, early radiologic improvement and decrease plasma IL-6 level in moderate/severe SARS-CoV-2 patients. It is still necessary to develop diagnostic strategies to determine the patients to whom this method would be the most applicable.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Hüseyin S Bozkurt https://orcid.org/0000-0003-2097-2950
Ömer Bilen https://orcid.org/0000-0001-7198-8421
References
- 1.Wong SH, Lui RN, Sung JJ. (2020) Covid-19 and the digestive system. J ournal of Gastroenterology and Hepatology 35(5): 744–748. [DOI] [PubMed] [Google Scholar]
- 2.Bozkurt HS, Quigley EM. (2020) The probiotic Bifidobacterium in the management of Coronavirus: A theoretical basis. International Journal of Immunopathology and Pharmacology 34: 2058738420961304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Republic Of Turkey Ministry Of Health (2020) COVID-19 (SARS-CoV-2 infection) guide. Turkey: General Directorate of Public Health, Turkish Ministry of Health. Available at: https://hsgm.saglik.gov.tr/depo/birimler/goc_sagligi/covid19/rehber/COVID-19_Rehberi20200414_eng_v4_002_14.05.2020.pdf. [Google Scholar]
- 4.Bozkurt HS, Kara B. (2020) A new treatment for ulcerative colitis: intracolonic Bifidobacterium and xyloglucan application. European Journal of Inflammation 158(3): S57. [Google Scholar]
- 5.Hazan S, Stollman N, Bozkurt H. (2021) The missing microbes: bifidobacterium and faecalibacterium depletion and loss of microbiome diversity as potential susceptibility markers for SARS-CoV-2 infection and severity. British Medical Journal 21262832. [Google Scholar]
- 6.Lu HZ. (2020) Drug treatment options for the 2019-new coronavirus (2019-nCoV). BioScience Trends 14(1): 69–71. [DOI] [PubMed] [Google Scholar]
- 7.Rozga M, Cheng FW, Handu D. (2020) Effects of probiotics in conditions or infections similar to COVID-19 on health outcomes: an evidence analysis center scoping review. Journal of the Academy Nutrition and Dietetics 121(9): 1841–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahooti M, Miri SM, Abdolalipour E, et al. (2020) The immunomodulatory effects of probiotics on respiratory viral infections: a hint for COVID-19 treatment? Microbial Pathogenesis 148: 104452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sundararaman A, Ray M, Ravindra PV, et al. (2020) Role of probiotics to combat viral infections with emphasis on COVID-19. Applied Microbiology and Biotechnology 104: 8089–8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fung S, Liu DX. (2019) The ER stress sensor IRE1 and MAP kinase ERK modulate autophagy induction in cells infected with coronavirus infectious bronchitis virus. Virology 533: 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong EY, Cheng SH, Yu KN. (2018) Induction of autophagy and interleukin 6 secretion in bystander cells: metabolic cooperation for radiation-induced rescue effect? Journal of Radiation Research 59(2): 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou W, Jin YH, Kang HS, Kim BS. (2014) Interleukin-6 (IL-6) and IL-17 synergistically promote viral persistence by inhibiting cellular apoptosis and cytotoxic T cell function. Journal of Virology 88(15): 8479–8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Li J, Zhan Y, et al. (2004) Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infection and Immunity 72: 4410–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou F, Yu T, Du R, et al. (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan. Lancet 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Z, Shi L, Wang Y, et al. (2020) Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory Medicine 8: 420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grifoni E, Valoriani A, Cei F, et al. (2020) Interleukin-6 as prognosticator in patients with COVID-19. The Journal of Infection 81(3): 452–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosas IO, Bräu N, Waters M, et al. (2021) Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. The New England Journal of Medicine 384: 1503–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavalli G, Dagna L. (2021). The right place for IL-1 inhibition in COVID-19. The Lancet Respiratory Medicine 9: 223–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, Cheng F, Xu Q, et al. (2021) The role of probiotics in coronavirus disease-19 infection in Wuhan: A retrospective study of 311 severe patients. International Immunopharmacology 95: 107531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santacroce L, Inchingolo F, Topi S, et al. (2021) Potential beneficial role of probiotics on the outcome of COVID-19 patients: an evolving perspective. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 15(1): 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jungersen M, Wind A, Johansen E, et al. (2014). The science behind the probiotic strain Bifidobacterium animalis subsp. lactis BB-12®. Microorganisms; 2(2): 92–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bozkurt K, Denktas C, Ozdemir O, et al. (2019) Charge transport in bifidobacterium animalis subsp.lactis BB-12 under various atmospheres. Open Journal of Applied Sciences 9: 506–514. [Google Scholar]
- 23.Larsen C, Nielsen S, Kæstel P, et al. (2006) Dose–response study of probiotic bacteria Bifidobacterium animalis subsp lactis BB-12 and Lactobacillus paracasei subsp paracasei CRL-341 in healthy young adults. European Journal of Clinical Nutrition 60: 1284–1293. [DOI] [PubMed] [Google Scholar]
- 24.Baindara P, Chakraborty R, Holliday ZM, et al. (2021) Oral probiotics in coronavirus disease 2019: connecting the gut–lung axis to viral pathogenesis, inflammation, secondary infection and clinical trials. New Microbes and New Infections 40: 100837. [DOI] [PMC free article] [PubMed] [Google Scholar]