Abstract
Elevation of intracellular 8-bromo-cyclic AMP (cAMP) can activate certain steroid receptors and enhance the ligand-dependent activation of most receptors. During ligand-independent activation of the chicken progesterone receptor (cPRA) with the protein kinase A (PKA) activator, 8-bromo-cAMP, we found no alteration in cPRA phosphorylation (W. Bai, B. G. Rowan, V. E. Allgood, B. W. O'Malley, and N. L. Weigel, J. Biol. Chem. 272:10457–10463, 1997). To determine if other receptor-associated cofactors were targets of cAMP-dependent signaling pathways, we examined the phosphorylation of steroid receptor coactivator 1 (SRC-1). We detected a 1.8-fold increase in SRC-1 phosphorylation in transfected COS-1 cells incubated with 8-bromo-cAMP. Phosphorylation was increased on two mitogen-activated protein kinase (MAPK) sites, threonine 1179 and serine 1185. PKA did not phosphorylate these sites in vitro. However, blockage of PKA activity in COS-1 cells with the PKA inhibitor (PKI) prevented the 8-bromo-cAMP-mediated phosphorylation of these sites. Incubation of COS-1 cells with 8-bromo-cAMP resulted in activation of the MAPK pathway, as determined by Western blotting with antibodies to the phosphorylated (active) form of Erk-1/2, suggesting an indirect pathway to SRC-1 phosphorylation. Mutation of threonine 1179 and serine 1185 to alanine in COS-1 cells coexpressing cPRA and the GRE2E1bCAT reporter resulted in up to a 50% decrease in coactivation during both ligand-independent activation and ligand-dependent activation. This was due, in part, to loss of functional cooperation between SRC-1 and CREB binding protein for coactivation of cPRA. This is the first demonstration of cross talk between a signaling pathway and specific phosphorylation sites in a nuclear receptor coactivator that can regulate steroid receptor activation.
Steroid receptors are members of a superfamily of ligand-activated transcription factors that bind to sequence-specific DNA binding sites in the promoter of target genes to regulate transcription. In addition to the regulation by ligand, steroid receptor action is modulated by cellular signaling pathways that activate intracellular kinases that, in turn, target receptors or other proteins relevant to the receptor activation process. Steroid receptors are phosphoproteins, and receptor phosphorylation has been shown to regulate the activity of the progesterone receptor (PR) (9–11, 13, 21), estrogen receptor (ER) (7, 15, 16, 20, 23, 34), the glucocorticoid receptor (GR) (5, 29, 36, 56), and the androgen receptor (AR) (62).
In some cases, activation of a cellular signaling pathway is sufficient to activate a receptor in the absence of hormone. Elevation of intracellular cyclic AMP (cAMP), a common second messenger for a number of hormones and a direct activator of protein kinase A (PKA), can induce a ligand-independent activation of chicken PR (cPR) (22), AR (44), and the ER (8), as well as enhance steroid-dependent activation of a broader range of receptors, including PR (13, 35), ER (8), GR (45), AR (19, 30), and the mineralocorticoid receptor (41). Previously, we found that activation of cAMP-dependent signaling pathways with the agent 8-bromo-cAMP caused no change in receptor phosphorylation during ligand-independent activation of cPR (9) or cAMP enhancement of steroid-dependent activation of human PR (hPR) (13). This suggested that other receptor-associated proteins, such as the recently discovered coactivators for the steroid receptor superfamily, might be targets of kinase pathways that activate the PR, but do not alter receptor phosphorylation.
Steroid receptor coactivator 1 (SRC-1) (47) was the first-identified member of a family of coactivators that regulate steroid and nuclear receptor action. Coregulators, both coactivators and corepressors, interact with steroid and nuclear receptors and, respectively, enhance or block receptor-dependent transcription. This has provided an additional regulatory mechanism for steroid receptor action (for a review, see reference 42). Recent evidence has suggested that the steroid-nuclear receptor coregulator proteins may be targets of cellular signaling pathways. The activities of the corepressors N-CoR and SMRT (28, 38, 54) and the coactivator CREB binding protein (CBP) (2, 3, 17, 31, 32, 39, 40, 59, 61) are regulated by cell signaling pathways. The potential for regulating coactivator function through signal pathway-mediated phosphorylation was realized by our laboratory with the first identification of the major phosphorylation sites in a nuclear receptor coactivator, SRC-1 (50) (Fig. 1). Furthermore, we showed that SRC-1 is a target of the mitogen-activated protein kinase (MAPK) pathway (50), providing a direct link to a pathway known to regulate steroid receptor-dependent gene transcription (1, 15, 34, 36, 60). The SRC-1 phosphorylation sites, all of which contain consensus sequences for the serine/threonine-proline-directed protein kinases, suggested a means by which signaling pathways that target specific coactivator phosphorylation sites could regulate nuclear receptor action. However, there have been no reports of specific phosphorylation sites in nuclear receptor coregulators that modulate steroid receptor action. In this report, experiments were undertaken to determine if SRC-1 phosphorylation was regulated during 8-bromo-cAMP-induced ligand-independent activation of cPR A form (cPRA), to determine if SRC-1 phosphorylation modulates PR activation, and to identify the mechanisms by which altered phosphorylation modulates receptor activation.
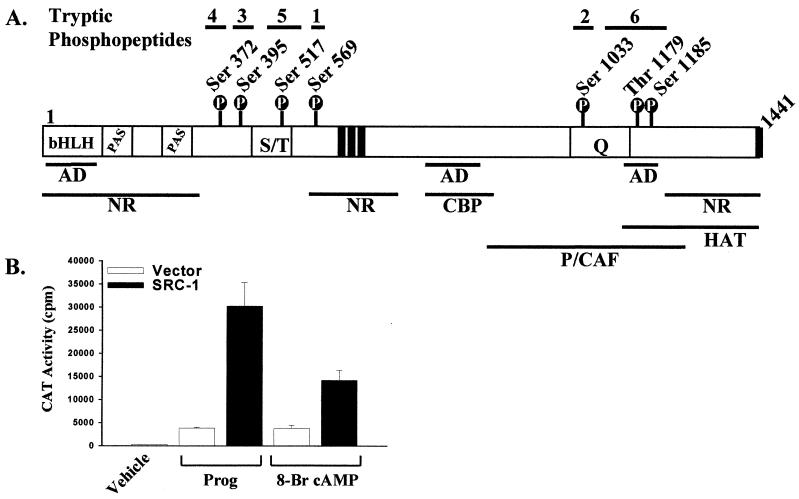
FIG. 1.
SRC-1 phosphorylation sites and tryptic phosphopeptides. (A) Locations of seven phosphorylation sites in SRC-1 and locations of tryptic phosphopeptides 1 to 6 corresponding to phosphorylation sites Ser-569, Ser-395, Ser-1033, Ser-372, Ser-517, and Thr-1179 and Ser-1185, respectively. Functional domains of SRC-1 are also indicated. P, phosphorylation site; bHLH, basic helix-loop-helix motif; PAS, Per-Arnt-Sim domain; S/T, serine/threonine-rich region; AD, putative activation domain; Q, glutamine-rich region; NR, nuclear receptor interaction domains; CBP, CBP interaction domain; P/CAF, P300/CBP-associated factor interaction domain; HAT, HAT domain. Black vertical bars indicate LXXLL receptor interaction motifs. (B) SRC-1 coactivates the ligand-dependent and ligand-independent activation of cPRA. COS-1 cells plated in six-well plates (2 × 105 cells/well) were transfected with cPRA (0.005 μg/well), GRE2E1bCAT reporter (0.4 μg/well), and either pCR3.1 vector or wild-type SRC-1 (0.4 μg/well) by using Lipofectamine as described in Materials and Methods. Twenty-four hours posttransfection, cells were incubated with either vehicle, 8-bromo-cAMP (1 mM), or progesterone (10−8 M) for 24 h prior to preparation of cell extracts for CAT assays of triplicate samples.
MATERIALS AND METHODS
Materials.
Cell culture reagents and Lipofectamine were purchased from GIBCO/BRL Life Technologies (Grand Island, N.Y.). Carrier-free [32P]H3PO4 and [γ-32P]ATP were purchased from DuPont-NEN (Boston, Mass.). High-performance liquid chromatography (HPLC) reagents were purchased from J. T. Baker Chemical Corp. (Phillipsburg, N.J.). 8-Methoxy-psoralen, poly-l-lysine, protease inhibitors, PKA inhibitor (PKI), and monothioglycerol were purchased from Sigma (St. Louis, Mo.). Xylene was purchased from Fisher Scientific (Pittsburgh, Pa.). Tosylphenylalanyl chloromethyl ketone-treated trypsin was obtained from Worthington Biochemical Corp. (Freehold, N.J.). Anti SRC-1 immunoglobulin G (IgG) (clone 1135) was prepared as previously described (52). Rabbit anti-mouse IgG (H+L) antibody was obtained from Zymed (San Francisco, Calif.). Epidermal growth factor (EGF) was purchased from Collaborative Biomedical Products, Bedford, Mass.). The oligonucleotides used in the plasmid construction and sequencing were purchased from GenoSys (The Woodlands, Tex.) and from GIBCO/BRL Life Technologies. The CheckMate mammalian two-hybrid system and the MEK inhibitor U0126 were purchased from Promega (Madison, Wis.). Erk-1/2 and phospho-Erk-1/2 antibodies were purchased from New England Biolabs (Beverly, Mass.).
Plasmid construction.
Plasmid expression vectors for SRC-1, P/CAF (P300/CBP-associated factor), and CBP were prepared by subcloning into the pCR3.1 vector as described previously (33, 50). Expression vectors for the mammalian two-hybrid studies were prepared by subcloning SRC-1, P/CAF, and CBP in frame with Gal DNA binding domain and VP16 activation domain vectors from the CheckMate mammalian Two-hybrid system (Promega). Mutagenesis was performed with the Stratagene Quick Change kit. The SRC-1 sequence and mutations were confirmed by DNA sequencing.
Transfection of SRC-1 in COS-1 cells and metabolic labeling.
Expression of SRC-1 in COS-1 cells and metabolic labeling with [32P]H3PO4 were performed as described previously (50). Briefly, 2 × 107 COS-1 cells were plated on 150-mm-diameter dishes (2 × 106 cells/dish) in Dulbecco's modified Eagle's medium (DMEM) with 5% fetal bovine serum (FBS) that had been treated with dextran-coated charcoal. Twenty-four hours after plating, the cells were infected with expression vectors for SRC-1 (0.1 μg/dish), cPRA (0.01 μg/dish), and GRE2E1bCAT reporter (4 μg/dish) by a nonrecombinant adenovirus DNA transfer technique (4) at an adenovirus/cell ratio of 400 to 1. Thirty-six hours postinfection, DMEM was removed and replaced with phosphate-free DMEM containing 1% dialyzed, stripped FBS. After incubation of cells for 1 h at 37°C, the medium was removed and replaced with phosphate-free DMEM containing [32P]H3PO4 (0.13 to 0.26 mCi/ml). After 1 h of incubation at 37°C, cells were incubated with 8-bromo-cAMP (1 mM), PKI (10−6 M), or vehicle. In other experiments, cells were treated with U0126 (25 μM) or vehicle 30 min prior to incubation with 8-bromo-cAMP. Following these treatments, cells were cultured for 12 to 14 h (overnight incubations) at 37°C prior to harvest. Immunoblotting analysis for SRC-1 in these experiments was performed as described below with an anti-SRC-1 monoclonal antibody.
Purification of SRC-1 and preparation of tryptic phosphopeptides.
Immunopurification of SRC-1 and digestion with trypsin were performed as described previously (50). Briefly, immunopurified SRC-1 was electrophoresed on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE [6.5% polyacrylamide]) gels, and the 32P-phosphorylated SRC-1 was visualized by autoradiography. The phosphorylated SRC-1 was excised from the gel and incubated with 10 to 40 μg of trypsin for 24 h to extensively digest SRC-1, and the supernatant containing the SRC-1 phosphopeptides was evaporated with a SpeedVac (Savant). The dried phosphopeptides were resuspended in 150 μl of 50% acetonitrile in HPLC-grade water containing 0.1% trifluoroacetic acid for injection into the HPLC.
Separation of SRC-1 tryptic phosphopeptides by HPLC and alkaline polyacrylamide gels.
Tryptic phosphopeptides of SRC-1 were separated by HPLC and alkaline PAGE as previously described (50). Briefly, [32P]phosphopeptides were separated on a C18 reversed-phase HPLC column using a 0 to 45% gradient of acetonitrile containing 0.1% trifluoroacetic acid. Radioactive peaks were detected with a Packard model IC Flo-One radioactive flow detector. Fractions from the HPLC were electrophoresed on 25% alkaline polyacrylamide gels (58) and visualized by autoradiography.
Immunoblotting analysis with Erk-1/2 and phospho-Erk-1/2 antibodies.
To mimic the conditions used for SRC-1 analysis, COS-1 cells plated on six-well plates (2 × 105 cells/well) in DMEM containing 5% stripped fetal calf serum (FCS) were transfected with expression vectors for SRC-1 (0.01 μg/well), cPRA (0.005 μg/well), and GRE2E1bCAT receptor (0.4 μg/well) by a nonrecombinant adenovirus DNA transfer technique as described above. The DNA levels were equivalent, on a per cell basis, to the DNA levels used in the [32P]H3PO4 labeling experiments described above. Thirty-six hours later, the medium was removed and replaced with phosphate-free DMEM containing nonradioactive H3PO4 and incubated for 1 h at 37°C. Subsequently, cells were incubated with either 1 mM 8-bromo-cAMP, 10 ng of EGF per ml, or vehicle for 0 to 30 min. Cells were harvested at 0, 5, 15, and 30 min as follows. The medium was removed, and the cells were scraped from the plates and collected by centrifugation. Proteins were extracted from the cells by adding lysis buffer (10 mM Tris [pH 8], 50 mM potassium phosphate, 50 mM sodium fluoride, 1 mM sodium vanadate, 2 mM EDTA, 2 mM EGTA, 0.4 M sodium chloride, 5 mM α-monothioglycerol, protease inhibitor mix) to the cell pellets and vortexing for 10 to 15 s followed by centrifugation at 15,000 × g for 5 min. Protein content was measured, and equal amounts of protein from each sample were electrophoresed on SDS-PAGE (6.5 or 10% polyacrylamide) gels followed by transfer to a nitrocellulose membrane for Western blotting. SRC-1, Erk-1/2, and phospho-Erk-1/2 were detected with anti-SRC-1, anti-Erk-1/2 or anti-phospho-Erk-1/2 antibodies, respectively, followed by chemiluminescent detection with the ECL enhanced chemiluminescence reagent (Amersham).
In vitro phosphorylation of SRC-1.
In vitro phosphorylation of SRC-1 was performed as described previously (50). Briefly, immunopurified baculovirus-expressed SRC-1 (14) was incubated with 0.5 ng of PKA per μl (Upstate Biotechnology, Lake Placid, N.Y.) in kinase reaction buffer (20 mM Tris [pH 7.5], 1 mM EGTA, 5 mM MgCl2) with a final specific activity of [γ-32P]ATP of 33,000 dpm/pmol of ATP, in a final reaction volume of 40 μl. The reaction mixture was incubated for 30 min at 37°C, and the reaction was terminated by addition of 4× Laemmli sample buffer. The samples were electrophoresed on an SDS-PAGE (10% polyacrylamide) gel, and phosphorylated SRC-1 was visualized by autoradiography. Preparation, separation, and visualization of tryptic phosphopeptides were performed as described above.
Transfection of cPRA, SRC-1, SRC-1-VP16, and CBP for CAT assays.
COS-1 or HeLa cells plated on six-well plates (2 × 105 cells/well) in DMEM containing 5% stripped FCS were transfected with expression vectors for cPRA (0.005 μg/well) and GRE2E1bCAT reporter (0.4 μg/well), along with combinations of expression vectors for SRC-1, SRC-VP16, SRC-1 phosphorylation mutants, and CBP with 2 μl of Lipofectamine (GIBCO/BRL) per well. pCR3.1 vector was transfected into cells to normalize the total level of DNA between samples. Cells were cultured at 37°C with 5% CO2 for 24 h. Following this incubation, cells were incubated with either 8-bromo-cAMP (1 mM), progesterone (10−8 M), or vehicle for 24 h. Subsequent to this, cells were harvested, protein was extracted from the cells, and chloramphenicol acetyltransferase (CAT) assays normalized to protein content were performed as described previously (50).
Mammalian two-hybrid interaction assays and luciferase assays.
HeLa cells plated on six-well plates (2 × 105 cells/well) were transfected with the 17-mer TATA-luciferase reporter (0.4 μg/well; Promega) along with combinations of expression vectors for Gal, Gal-SRC-1, and Gal-SRC-1 phosphorylation mutants, VP16, P/CAF-VP16, or CBP-VP16 with 2 μl of Lipofectamine per well. pCR3.1 vector was used to equalize the total level of DNA among samples. Cells were cultured at 37°C with 5% CO2 in DMEM with 5% FBS that had been treated with dextran-coated charcoal. Twenty-four hours following transfection, cells were harvested, and proteins were extracted from cell pellets by using 1× lysis buffer provided in the Promega luciferase assay kit. Standard luciferase assays were performed normalized to protein.
RESULTS
SRC-1 stimulates ligand-independent activation of the PR.
To examine whether SRC-1 phosphorylation is altered during ligand-independent activation of steroid receptors, and to determine whether SRC-1 phosphorylation contributes to ligand-independent activation, we took advantage of a model system in which 8-bromo-cAMP induces ligand-independent activation of cPRA. In this model, receptor phosphorylation is unaltered (50). This allowed us to exclude alterations in receptor phosphorylation as contributing to the ligand-independent activation and to examine the role of 8-bromo-cAMP-regulated phosphorylation of SRC-1 in mediating ligand-independent activation. SRC-1 was identified in a yeast two-hybrid screen based on its hormone-dependent interaction with hPR, and initial studies showed that it stimulates hormone-dependent activity of PR (47). To determine whether SRC-1 also serves as a coactivator for 8-bromo-cAMP-induced PR activation, cells were transfected with cPRA and reporter and treated or not with progesterone or 8-bromo-cAMP. As shown in Fig. 1B, SRC-1 serves as a coactivator for both hormone-dependent and ligand-independent activation of cPR. These data support the hypothesis that regulated phosphorylation of SRC-1 could be a candidate target for the effects of cell signaling pathways on steroid receptor activity.
8-Bromo-cAMP increases the phosphorylation of specific SRC-1 phosphopeptides in COS-1 cells.
Plasmids for SRC-1, cPRA, and the GRE2E1bCAT reporter were transfected into COS-1 cells; the cells were labeled in vivo with [32P]H3PO4 and subsequently incubated with 8-bromo-cAMP (1 mM) or vehicle. Phosphorimage analysis of immunopurified phosphorylated SRC-1 run on SDS-PAGE gels revealed that 8-bromo-cAMP treatment resulted in an increase (1.8-fold ± 0.1 [mean ± standard error for three experiments]) in SRC-1 phosphorylation when normalized to protein content by densitometric scans of the Western blot (Fig. 2A). This is in contrast to progesterone treatment, which does not alter SRC-1 phosphorylation (50).
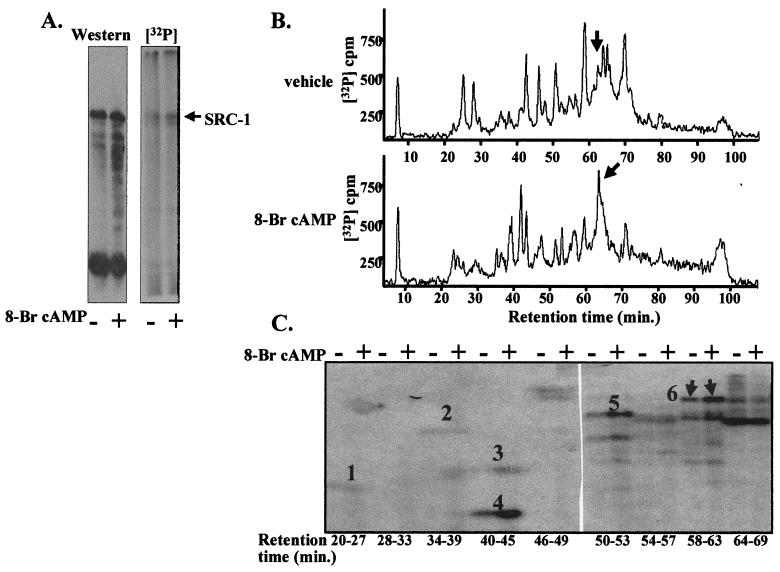
FIG. 2.
Treatment of COS-1 cells with 8-bromo-cAMP increases the phosphorylation of SRC-1. (A) 8-bromo-cAMP increases SRC-1 phosphorylation. COS-1 cells were transfected with expression vectors for SRC-1 (0.1 μg/dish), cPRA (0.01 μg/dish), and GRE2E1bCAT (4 μg/dish) and metabolically labeled with [32P]H3PO4 as described in Materials and Methods. SRC-1 was immunopurified and then electrophoresed on an SDS-PAGE (6.5% polyacrylamide) gel. The protein was transferred to a nitrocellulose membrane, and the membrane was exposed to X-AR film for autoradiography (2 h). Following autoradiography, the membrane was subjected to Western blotting with anti-SRC-1 antibody. (B) 8-bromo-cAMP treatment increases phosphorylation on phosphopeptide 6. Following electrophoresis on the SDS-PAGE (6.5% polyacrylamide) gel, the SRC-1 band from a separate gel was cut out, and the gel slice was subjected to trypsin digestion. The released phosphotryptic peptides of SRC-1 were separated by C18 reversed-phase HPLC as described in Materials and Methods. Elution of the 8-bromo-cAMP-regulated phosphopeptide is indicated by the arrows. A representative profile of more than three experiments is shown. (C) SRC-1 phosphopeptides purified from cells treated with either 1 mM 8-bromo-cAMP or vehicle were separated by C18 reversed-phase HPLC, and identical HPLC fractions from the two samples were electrophoresed side by side on a 25% alkaline polyacrylamide gel to compare the intensity of the [32P]phosphopeptide signal. The gel was dried and then exposed to X-AR film for autoradiography (72 h) as described in Materials and Methods. The arrows indicate migration of the 8-bromo-cAMP-regulated phosphopeptide (6). Migrations of phosphopeptides 1 to 5 (see Fig. 1 for locations of peptides in SRC-1) are also indicated. A representative profile of more than three experiments is shown.
The 8-bromo-cAMP-enhanced phosphorylation of SRC-1 was not dependent on whether PR was coexpressed with SRC-1 in COS-1 cells (data not shown), indicating that enhanced phosphorylation was not dependent on the recruitment of SRC-1 by receptor. Moreover, the enhanced phosphorylation of SRC-1 was not simply an increase in net phosphorylation, but represents alterations at specific sites. Examination of the tryptic phosphopeptide maps of SRC-1 by C18 reversed-phase HPLC (Fig. 2B) showed a complex and somewhat variable alteration in the pattern of the phosphopeptides following incubation of cells with 8-bromo-cAMP. However, there was one phosphopeptide peak that showed a reproducible increase in phosphorylation that was identified in three experiments (Fig. 2B, arrow). In an identical experiment, phosphopeptides were visualized by electrophoresis of HPLC fractions on alkaline polyacrylamide gels. Autoradiography of the gels revealed an 8-bromo-cAMP-enhanced phosphopeptide (Fig. 2C, numbered 6). Other phosphopeptides (Fig. 2C, numbered 1 to 5) corresponded to the major SRC-1 phosphopeptides identified in COS-1 cells (50). Although the phosphorylation of phosphopeptides 4 and 5 also appeared to be increased following treatment with 8-bromo-cAMP (Fig. 2C), this increase was not reproducible over three experiments.
PKA does not directly phosphorylate the 8-bromo-cAMP enhanced SRC-1 phosphopeptide in vitro.
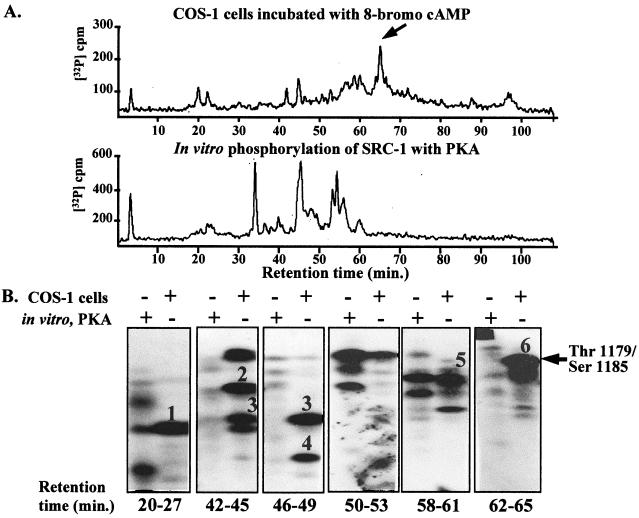
Because 8-bromo-cAMP is a direct activator of PKA, it was likely that 8-bromo-cAMP-enhanced phosphorylation of SRC-1 occurred via a direct phosphorylation by PKA. To determine if PKA could directly phosphorylate SRC-1, in vitro phosphorylation of immunopurified SRC-1 with purified PKA was performed. PKA phosphorylated SRC-1, as indicated by several phosphopeptide peaks detected on the HPLC profile (Fig. 3A, bottom). However, the 8-bromo-cAMP-enhanced phosphopeptide in COS-1 cells (Fig. 3A, top, arrow) was not phosphorylated by PKA in vitro, as evidenced by the lack of a phosphopeptide peak in this region. This was further confirmed by alkaline PAGE of SRC-1 phosphopeptides in an identical experiment. The migration of the 8-bromo-cAMP-enhanced phosphopeptide from COS-1 cells in HPLC fractions 58 to 63 (Fig. 3B, arrow) was absent in identical fractions from SRC-1 phosphorylated in vitro with PKA (Fig. 3B, 58 to 63 min). Although PKA did phosphorylate tryptic peptides comigrating with phosphopeptides 1 and 5 (Fig. 3B, 20 to 27 and 54 to 57 min), further analysis by modified manual Edman degradation revealed that these PKA phosphopeptides did not correspond to the authentic phosphorylation sites identified in COS-1 cells (50; data not shown).
FIG. 3.
PKA does not directly phosphorylate the 8-bromo-cAMP-regulated phosphopeptide in vitro. (A) Baculovirus-expressed SRC-1 was immunoprecipitated under native conditions, and SRC-1 bound to the protein A beads was incubated with purified PKA and [γ-32P]ATP as described in Materials and Methods. SDS-PAGE sample buffer was added, and the reaction mixture was heated to 100°C for 5 min to stop the reaction. Following electrophoresis on an SDS-PAGE (6.5% polyacrylamide) gel, the SRC-1 band was cut out, and the gel slice was subjected to trypsin digestion. The released phosphotryptic peptides of SRC-1 were separated by C18 reversed-phase HPLC, as described in Materials and Methods, and compared to the phosphopeptide map of SRC-1 phosphorylated in COS-1 cells by the procedure described in the legend to Fig. 2A. The arrow indicates the 8-bromo-cAMP-regulated phosphopeptide. (B) SRC-1 phosphopeptides purified from cells treated with 1 mM 8-bromo-cAMP or purified following in vitro phosphorylation with PKA were separated by C18 reversed-phase HPLC, and identical HPLC fractions from both samples were electrophoresed side by side on 25% alkaline polyacrylamide gels as described in Materials and Methods. Polyacrylamide gels were exposed to X-AR film for autoradiography.
We previously identified two phosphorylation sites in phosphopeptide 6, threonine 1179 and serine 1185 (boldface letters below) which are located within perfect and imperfect consensus phosphorylation sequences, respectively, for the MAPK family members Erk-1/2 (LPQGAPQQFPYPPNYGTNPGTPPASTSPFSQLAANPEASLANR). Both sites were phosphorylated in vitro by Erk-2; however, neither phosphorylation site contains a consensus sequence for PKA (50). This is consistent with the data shown in Fig. 3, in which PKA did not phosphorylate threonine 1179 and serine 1185 in vitro, although 8-bromo-cAMP treatment enhanced phosphorylation of these sites in vivo.
Stimulation of threonine 1179 and serine 1185 phosphorylation requires both the PKA and MAPK pathways.
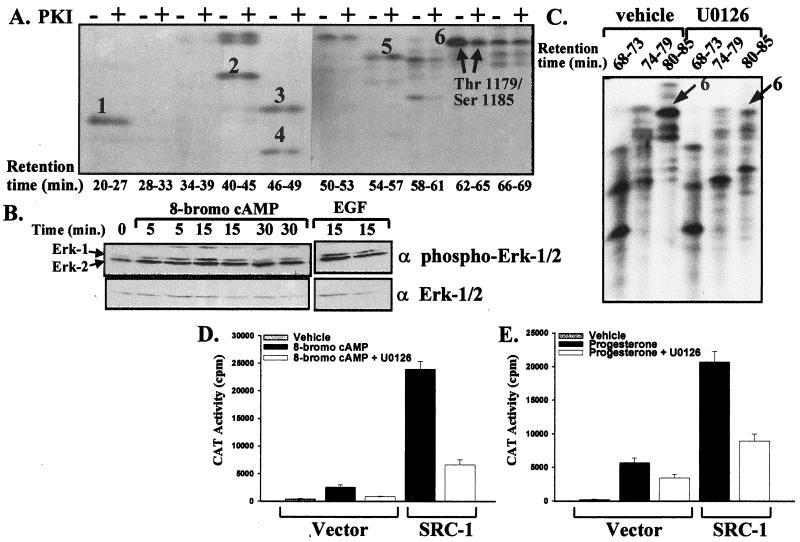
Although PKA did not directly phosphorylate Thr-1179 and Ser-1185 of SRC-1 in vitro, PKA activity was required for the 8-bromo-cAMP-mediated phosphorylation of these sites. In COS-1 cells cotreated with 8-bromo-cAMP and PKI, the most dramatic effect was the loss of the 8-bromo-cAMP-mediated increase in SRC-1 phosphorylation at Thr-1179 and Ser-1185 (Fig. 4A, arrows). (Note that phosphopeptide 6 eluted at a slightly later retention time in this experiment than in Fig. 2 and 3, due to slight variations between different HPLC runs.)
FIG. 4.
Activation and inhibition of the PKA and MAPK pathways regulate SRC-1 phosphorylation and activity. (A) PKI treatment reduces phosphorylation at the Thr-1179–Ser-1185 phosphopeptide. The method was identical to that described in the legend to Fig. 2C, except treatment was with 1 mM 8-bromo-cAMP with or without 10−6 M PKI for 12 to 14 h. The migration of phosphopeptides 1 to 6 is shown. Arrows indicate that SRC-1 phosphopeptide 6 shows reduced phosphorylation following treatment with PKI. (B) Activation of Erk-1/2 by 8-bromo-cAMP. COS-1 cells plated in six-well plates (2 × 105 cells/well) were transfected with DNA levels that were equivalent to the levels used per cell described in the legend to Fig. 2. Thirty-six hours later, the medium was removed and replaced with phosphate-free DMEM as described in Materials and Methods and subsequently incubated with either 1 mM 8-bromo-cAMP, 10 ng of EGF per ml as a positive control, or vehicle for 0 to 30 min. Cells were harvested at 0, 5, 15, and 30 min following treatment, and cell extracts were prepared for Western blotting with anti-Erk-1/2 and anti-phospho-Erk-1/2 antibodies. (C) Treatment with U0126 reduces the 8-bromo-cAMP-enhanced phosphorylation at Thr-1179 and Ser-1185. The method for transfection and labeling cells with [32P]H3PO4 was identical to that described in the legend to Fig. 2. Cells were treated with 1 mM 8-bromo-cAMP with or without 30 μM U0126 for 12 to 14 h prior to cell harvest. SRC-1 was purified and subjected to trypsin digestion, and phosphopeptides were separated by reversed-phase HPLC as described in Fig. 2. Identical HPLC fractions from both samples (U0126 or vehicle treatment) were electrophoresed side by side on 25% alkaline polyacrylamide gels as described in Materials and Methods. Polyacrylamide gels were exposed to X-AR film for autoradiography. Arrows indicate phosphopeptide 6 containing Thr-1179 and Ser-1185. (D and E) U0126 inhibits 8-bromo-cAMP-dependent and progesterone-dependent activation of cPRA. COS-1 cells were transfected as described in the legend to Fig. 1. Twenty-four hours posttransfection, cells were pretreated with or without 30 μM U0126 for 30 min, followed by incubation with 8-bromo-cAMP (1 mM) (D) or progesterone (10−8 M) (E) for 24 h prior to preparation of cell extracts for CAT assays of triplicate samples.
The effect of PKI suggested that 8-bromo-cAMP-regulated phosphorylation of SRC-1 occurred through an indirect pathway in which PKA induced the activity of another kinase, such as MAPK, which, in turn, directly phosphorylates SRC-1. Elevation of intracellular cAMP and concomitant activation of PKA can result in either an activation (6, 12, 25, 26, 48) or an inhibition (18, 37, 55) of the MAPK pathway, depending on the cell or tissue origin. To assess whether treatment of transfected COS-1 cells with 8-bromo-cAMP would activate the MAPK pathway, an antibody to the activated, phosphorylated form of Erk-1/2 was used in Western blotting experiments. Within 5 min of incubation with 8-bromo-cAMP (1 mM), an increase in the activated, phosphorylated form of Erk-1/2 was detected (Fig. 4B) with no change in total Erk, showing that 8-bromo-cAMP treatment of COS-1 cells activates the MAPK pathway.
To determine whether activation of the MAPK pathway is required for the 8-bromo-cAMP-regulated phosphorylation at Thr-1179 and Ser-1185, U0126, a specific inhibitor of the MAPK pathway, was used. U0126 blocks the action of MEK1/2 upstream of Erk-1/2. When metabolically labeled COS-1 cells expressing SRC-1 were preincubated with U0126 (30 μM) for 30 min prior to addition of 8-bromo-cAMP, the 8-bromo-cAMP-enhanced phosphorylation of Thr-1179 and Ser-1185 was markedly reduced (Fig. 4C, arrow). (Note that the Thr-1179–Ser-1185 phosphopeptide eluted at a later retention time [80 to 85 min] because a different HPLC system was used for these experiments.) This reduction was at least comparable to the inhibition by PKI (Fig. 4A). Moreover, this treatment caused a significant reduction in both the ligand-independent and ligand-dependent activation of cPRA in both the presence and absence of cotransfected SRC-1 (Fig. 4D and E).
Phosphorylation at threonine 1179 and serine 1185 of SRC-1 is required for optimal stimulation of ligand-independent activation and ligand-dependent activation of the PR.
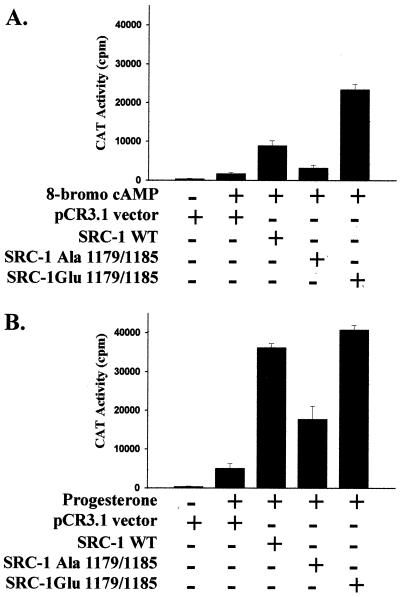
To determine if the 8-bromo-cAMP-regulated phosphorylation at Thr-1179 and Ser-1185 is important for ligand-independent activation of cPRA, Thr-1179 and Ser-1185 were mutated to either alanine or glutamic acid, and the activity of the mutated SRC-1 was compared to that of wild-type SRC-1 in COS-1 cells. Mutation of Thr-1179 and Ser-1185 to alanine markedly reduced the 8-bromo-cAMP-mediated activation of cPRA (Fig. 5A), whereas mutation of Thr-1179 and Ser-1185 to glutamic acid restored SRC-1 activity to levels comparable to or higher than those of wild-type SRC-1. The nonphosphorylatable alanine residue mimics loss of phosphorylation, whereas a glutamic acid residue maintains a negative charge that mimics the negative charge of a phosphorylation site. These data demonstrate that phosphorylation at Thr-1179 and Ser-1185 is important for 8-bromo-cAMP-induced activation. Mutation of Thr-1179 and Ser-1185 to alanine also reduced the progesterone-mediated activation of cPRA (Fig. 5B). Note that Thr-1179 and Ser-1185 are partially phosphorylated in the absence of 8-bromo-cAMP treatment (Fig. 2C), consistent with their mutation modulating progesterone-dependent transcription.
FIG. 5.
Phosphorylation at threonine 1179 and serine 1185 regulates ligand-dependent and ligand-independent activation of cPRA. Mutation of Thr-1179 and Ser-1185 to alanine reduces SRC-1 coactivation of cPRA. Threonine 1179 and serine 1185 of SRC-1 were mutated to either alanine or glutamic acid by oligonucleotide-directed mutagenesis as described in Materials and Methods. COS-1 cells plated in six-well plates (2 × 105 cells/well) were transfected with cPRA (0.005 μg/well), GRE2E1bCAT reporter (0.4 μg/well), and either pCR3.1 vector, wild-type (WT) SRC-1, SRC-1-Ala-1179/1185, or SRC-1-Glu-1179/1185 (all at 0.4 μg/well) by using Lipofectamine as described in Materials and Methods. Twenty-four hours posttransfection, cells were incubated with either 1 mM 8-bromo-cAMP or vehicle (A) or with 10−8 M progesterone or vehicle (B) for 24 h prior to preparation of cell extracts for CAT assays or triplicate samples.
Phosphorylation at threonine 1179 and serine 1185 of SRC-1 does not affect binding of SRC-1 to cPRA or CBP or affect the intrinsic activation domains of SRC-1.
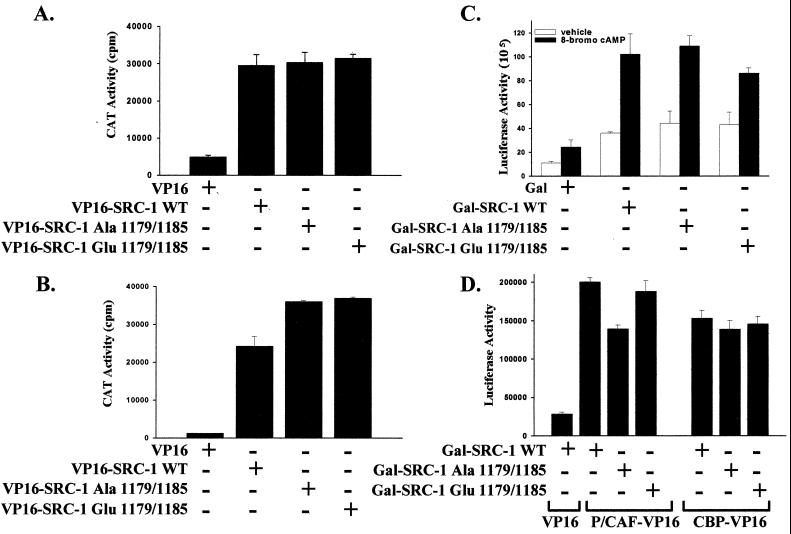
Threonine 1179 and serine 1185 reside in the carboxy-terminal region of SRC-1 overlapping or adjacent to interaction domains for steroid receptors, P/CAF and CBP, as well as residing within an SRC-1 activation domain, a region defined by its ability to activate transcription (46) (Fig. 1). To determine what effect phosphorylation of SRC-1 would have on binding to cPRA, a modified mammalian two-hybrid assay was performed in which the VP16 activation domain was fused to SRC-1 to overcome its intrinsic activation domains. We found that phosphorylation at Thr-1179 and Ser-1185 did not alter the interaction of SRC-1 with cPRA. Mutation of these sites to alanine did not reduce the interaction of SRC-1-VP16 with cPRA in response to progesterone (Fig. 6A) or 8-bromo-cAMP (Fig. 6B). Mutation of Thr-1179 and Ser-1185 to glutamic acid showed similar activity to the alanine mutation, indicating that these sites do not regulate the interaction of SRC-1 with receptor. Note that these sites do not overlap any of the LXXLL motifs in SRC-1 that are the major interaction regions for nuclear receptors (27, 53). Using coimmunoprecipitation assays, we also found that the alanine and glutamic acid mutations did not alter the interaction of SRC-1 with the receptor (data not shown). The transcriptional activity of SRC-1 was unaltered by phosphorylation at these sites. Using Gal–SRC-1 fusion proteins coexpressed with a Gal reporter, we found that mutation of Thr-1179 and Ser-1185 to alanine or glutamic acid did not alter the intrinsic activation domains of SRC-1 in the presence or absence of 8-bromo-cAMP (Fig. 6C). However, 8-bromo-cAMP did increase the intrinsic transactivation function of SRC-1.
FIG. 6.
Effect of mutation at threonine 1179 and serine 1185 on SRC-1 activation domains and interaction of SRC-1 with cPRA, P/CAF, and CBP. (A and B) Modified mammalian two-hybrid interaction shows that mutation at Thr-1179 and Ser-1185 has no effect on interaction of SRC-1 with PR. HeLa cells plated in six-well plates (2 × 105 cells/well) were transfected with cPRA (0.005 μg/well), GRE2E1bCAT (0.4 μg/well), and either VP16, wild-type (WT) SRC-1-VP16, SRC-1-VP16-Ala-1179/1185, or SRC-1-VP16-Glu-1179/1185 (0.3 μg/well). Twenty-four hours posttransfection, cells were treated with progesterone (10−8 M) (A) or 8-bromo-cAMP (1 mM) (B). Twenty-four hours after treatment, cells were harvested and prepared for standard CAT assays, in triplicate, normalized to protein content. (C) Mutation at Thr-1179 and Ser-1185 has no effect on the intrinsic activation of Gal-SRC-1. HeLa cells plated in six-well plates (2 × 105 cells/well) were transfected with a 17-mer TATA-luciferase reporter (0.4 μg/well; Promega) and expression vectors for either Gal, wild-type Gal-SRC-1, Gal-SRC-1-Ala-1179/1185, or Gal-SRC-1-Glu-1179/1185 (0.2 μg/well). Twenty-four hours posttransfection, cells were incubated with 1 mM 8-bromo-cAMP or vehicle for 24 h prior to preparation of cell extracts for standard luciferase assays, in triplicate, normalized to protein levels as described in Materials and Methods. (D) Mammalian two-hybrid assay shows that mutation of Thr-1179 and Ser-1185 to alanine reduces the interaction of Gal-SRC-1 with P/CAF-VP16, but not with CBP-VP16. HeLa cells plated in six-well plates (2 × 105 cells/well) were transfected with 17-mer TATA-luciferase (0.4 μg/well), and either wild-type Gal-SRC-1, Gal-SRC-1-Ala-1179/1185, or Gal-SRC-1-Glu-1179/1185 (0.3 μg/well) in combination with either VP16, P/CAF-VP16, or CBP-VP16 (0.3 μg/well). Cells were harvested 48 h after transfection, and cell extracts were prepared for luciferase assays, in triplicate, normalized to protein content.
To look at the interaction of SRC-1 with other associated proteins, a mammalian two-hybrid assay was performed in which Gal–SRC-1 was coexpressed with either P/CAF-VP16 or CBP-VP16 and a Gal reporter. There was a reproducible modest reduction in the interaction of Gal–SRC-1 with P/CAF-VP16 when Thr-1179 and Ser-1185 were mutated to alanine, and this interaction was restored with the glutamic acid mutation (Fig. 6D). As shown in Fig. 1, these sites overlap with the region that interacts with P/CAF (52). In contrast, there was no effect of Thr-1179–Ser-1185 phosphorylation on the interaction of SRC-1 with CBP (Fig. 6D). Consistent with this finding, these sites do not overlap the region of interaction of SRC with CBP (Fig. 1).
Phosphorylation at threonine 1179 and serine 1185 of SRC-1 regulates the functional interaction between SRC-1 and CBP for cPRA activation.
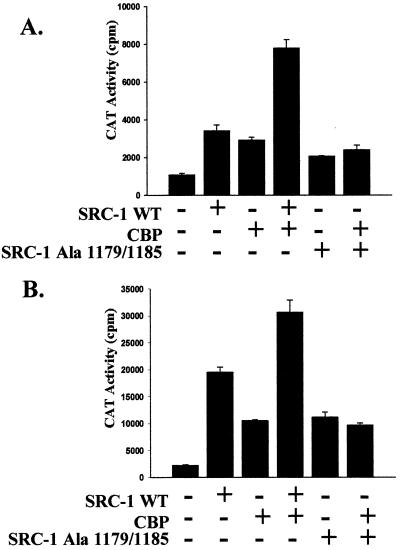
SRC-1 and CBP can functionally synergize to enhance steroid receptor target gene transcription (51). To determine whether phosphorylation at Thr-1179 and Ser-1185 regulates this functional synergy, activation of cPRA in the presence of either wild-type SRC-1 or Ala-1179/1185-SRC-1 and CBP was compared in COS-1 cells. SRC-1 and CBP expressed separately enhance both the ligand-dependent and ligand-independent activation of cPRA (Fig. 7A and B, 2nd and 3rd bars). When wild-type SRC-1 and CBP are coexpressed, the coactivators functionally cooperate to induce at least an additive enhancement of receptor activation when compared to either coactivator expressed singly (Fig. 7A and B, compare 4th bar to 2nd and 3rd bars). However, mutation of Thr-1179 and Ser-1185 to alanine markedly inhibits the functional cooperation between SRC-1 and CBP, with activation being similar to that of either singly expressed coactivator (Fig. 7A and B, compare 6th bar to 5th bar). Mutation of Thr-1179 and Ser-1185 to glutamic acid showed functional cooperation with CBP that was comparable to that of wild-type SRC-1 (data not shown).
FIG. 7.
Mutation of threonine 1179 and serine 1185 to alanine reduces the cooperative interaction of SRC-1 and CBP for cPRA activation. Threonine 1179 and serine 1185 of SRC-1 were mutated to alanine by oligonucleotide-directed mutagenesis as described in Materials and Methods. COS-1 cells plated in six-well plates (2 × 105 cells/well) were transfected with cPRA (0.005 μg/well), GRE2E1bCAT (0.4 μg/well), and combinations of wild-type (WT) SRC-1, SRC-1-Ala-1179/1185, or CBP (each at 0.3 μg/well) with Lipofectamine as described in Materials and Methods. pCR3.1 vector was used to equalize the total level of DNA in the samples. Twenty-four hours posttransfection, cells were incubated with either 1 mM 8-bromo-cAMP (A) or 10−8 M progesterone (B) for 24 h prior to preparation of cell extracts for CAT assays of triplicate samples. Comparable results were obtained in HeLa cells (data not shown).
DISCUSSION
cPR is a phosphoprotein, and phosphorylation is enhanced following treatment with progesterone (21, 49). We had previously described transcriptional activation of cPR as a result of treatment of cells with 8-bromo-cAMP and inhibition of this response as well as hormone-dependent transcriptional activity by PKI (22). Notably, during the ligand-independent activation of cPRA with 8-bromo-cAMP, a direct activator of PKA, no alteration in receptor phosphorylation was detected (9). This led us to speculate that other proteins involved in receptor activation by 8-bromo-cAMP could be targets of elevated kinase activity, including the recently discovered steroid receptor coactivator family. Here we show that SRC-1 phosphorylation and function are regulated by 8-bromo-cAMP through phosphorylation of specific sites in the C-terminal region. Furthermore, this phosphorylation did not require cPRA expression, indicating that association of SRC-1 with the receptor is not a prerequisite. Unexpectedly, the 8-bromo-cAMP-induced phosphorylation of SRC-1 occurred through an indirect pathway that resulted in activation of MAPK and phosphorylation of Thr-1179 and Ser-1185. This is consistent with our finding that EGF, an upstream activator of MAPK, enhances the effect of SRC-1 on PR-dependent gene transcription (50). Phosphorylation at these sites was required for optimal PR-dependent transcription and for functional cooperation with CBP to elicit maximal target gene transcription. These findings are a direct demonstration of cross talk between cellular signaling pathways and steroid receptor signaling that occurs, not at the level of receptor phosphorylation, but through phosphorylation of specific sites in a coactivator protein. However, we did not find a direct role for PKA in phosphorylating SRC-1, because PKA did not phosphorylate any of the identified sites in vitro, and none of these sites contains a consensus sequence for PKA.
Because cPRA phosphorylation in unchanged with 8-bromo-cAMP treatment (9), we originally speculated that the 8-bromo-cAMP-regulated phosphorylation of SRC-1 might trigger ligand-independent activation of the receptor. Although loss of phosphorylation at Thr-1179 and Ser-1185 markedly diminishes PR-dependent gene transcription, these sites are not molecular switches that trigger the ligand-independent activation pathway. Rather, phosphorylation at Thr-1179 and Ser-1185 is an important modulatory signal, not just for ligand-independent activation, but also for ligand-dependent activation of the PR. Changes in phosphorylation at these sites serve as a sensor for nonsteroidal signals. The reduction in activity observed as a result of mutation of these two sites is likely to be an underestimate of the total contribution of SRC-1 phosphorylation to receptor activation for two reasons. First, the cells contain endogenous SRC-1 as well as other coactivators that can substitute for SRC-1. Second, although Thr-1179 and Ser-1185 are the sites that are most dramatically regulated by elevation of intracellular cAMP and are required for functional cooperation with CBP, other sites may be regulated to a smaller degree (or their regulation may be more difficult to detect due to difficulties in obtaining complete tryptic digests of such large peptides) and may also contribute to ligand-independent activation. We speculate that this is possible because all seven SRC-1 phosphorylation sites identified in COS-1 cells contain consensus sequences for the serine/threonine-proline-directed protein kinases, and six of these were phosphorylated by Erk-2 in vitro (50). Thus, steroid receptor-dependent gene transcription can be regulated by cross talk mechanisms that target the coregulator proteins as well as steroid receptors.
It is likely that 8-bromo-cAMP-dependent signaling pathways target other proteins in the multicomponent steroid receptor complex at the promoter. It is well established that steroid receptors themselves are targets of signaling pathways (for review, see reference 57). Furthermore, other coregulator proteins are targets for phosphorylation and dephosphorylation mechanisms that may regulate their activity. Phosphorylation of CBP by MAPK was shown to enhance CBP histone acetyltransferase (HAT) activity (2) and transcriptional activity (31). CBP is also potentially phosphorylated by PKA (32), and its activity is regulated by the PKA pathway (17, 39, 59, 61). Recently AIB-1, another member of the steroid receptor coactivator family, was shown to be a phosphoprotein in MCF-7 cells; in vitro it is a substrate for phosphorylation by Erk-2. The transcriptional activity of Gal4–AIB-1 fusion proteins expressed in COS-1 cells was enhanced by coexpression of constitutively active MEK1. This suggested a role for the MAPK pathway in regulating AIB-1 transcriptional activity (24).
Given that cAMP-dependent signaling pathways target several proteins important in steroid receptor activation, the mechanisms regulating target gene transcription are likely complex and may involve altered phosphorylation of several proteins and regulation of multiple interactions. Phosphorylation at Thr-1179 and Ser-1185 did not alter the binding of SRC-1 to cPRA, even though these phosphorylation sites are adjacent to an interaction domain for the PR (46, 47). However, these sites are not in the vicinity of the major interaction domain for steroid receptors, the NR box, toward the central region of the protein (27, 53) (Fig. 1). Mutation of these sites reduced the interaction of SRC-1 with P/CAF. P/CAF is a HAT, and the absence of this protein at the promoter would result in a less “open” chromatin conformation reducing the degree of gene transcription. Although phosphorylation at Thr-1179 and Ser-1185 did not directly regulate physical interaction between SRC-1 and CBP, it was required for functional cooperation. In a recently published study (24), coexpression of truncated forms of Gal4–AIB-1 with constitutively active MEK1 resulted in enhanced interaction of the CBP paralog, P300, and its associated HAT activity with AIB-1. Whether this altered interaction is a result of altered phosphorylation of AIB-1 or P300 remains to be determined. Although the phosphorylation sites in AIB-1 have not been identified, a sequence comparison between AIB-1 and SRC-1 revealed that Thr-1179 and Ser-1185 are not conserved in AIB-1 (24). This suggests that activation of the MAPK pathway may target unrelated phosphorylation sites in AIB-1, which, in turn, may explain the different mechanisms by which these related coactivators regulate gene transcription. With regard to SRC-1, more detailed analysis of the other phosphorylation sites and phosphorylation sites in CBP and P300 will be necessary to determine whether phosphorylation regulates a direct interaction between the two proteins.
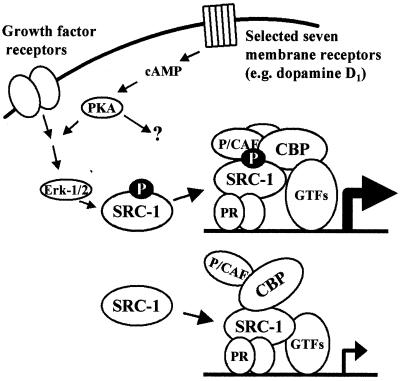
The major effect of phosphorylation at Thr-1179 and Ser-1185 was in facilitating the functional cooperation between SRC-1 and CBP. We favor a model in which elevation of intracellular cAMP, and consequent phosphorylation of SRC-1 at Thr-1179 and Ser-1185, alters the dynamic complexes at the promoter (43) in such a way that CBP and SRC-1 can functionally cooperate to elicit maximal target gene transcription (Fig. 8). In the absence of this phosphorylation, SRC-1 is still recruited to the promoter through its interaction with the PR and may still interact with CBP and other cofactors, but at the functional level, SRC-1 has lost a significant degree of its ability to enhance PR-dependent gene transcription.
FIG. 8.
Model for 8-bromo-cAMP-mediated phosphorylation of SRC-1 and progesterone receptor activation in COS-1 cells. Elevation of intracellular cAMP results in rapid PKA-dependent activation of Erk-1/2 that, in turn, phosphorylates SRC at Thr-1179 and Ser-1185. When these sites are fully phosphorylated, SRC-1 is able to form a functional complex at the promoter that results in maximal gene transcription (top). In the absence or reduction in phosphorylation at Thr-1179 and Ser-1185, SRC-1 can no longer form a fully functional complex with CBP, and transcriptional synergism is lost (bottom). Activation of PKA and MAPK will also target other cofactors important for PR activation. P/CAF, P300/CBP-associated factor; GTFs, general transcription factors; P, phosphorylation site.
We have uncovered a novel, indirect pathway for cross talk between kinase signaling pathways and PR activation that results in phosphorylation of specific sites in a nuclear receptor coactivator. Our study necessitates a broader view of nuclear receptor action to incorporate not just the temporal and tissue-specific expression patterns of coactivators and corepressors, but also a detailed examination of specific phosphorylation sites in coregulator proteins and the role this plays in regulating steroid receptor action.
ACKNOWLEDGMENTS
This work was supported by Postdoctoral Fellowship PF-4273 from the American Cancer Society (to B.G.R.) and by a grant from NIH (NICHD) (to B.W.O.).
REFERENCES
- 1.Abreu-Martin M T, Chari A, Palladino A A, Craft N A, Sawyers C L. Mitogen-activated protein kinase kinase kinase 1 activates androgen receptor-dependent transcription and apoptosis in prostate cancer. Mol Cell Biol. 1999;19:5143–5154. doi: 10.1128/mcb.19.7.5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ait S A, Carlisi D, Ramirez S, Upegui-Gonzalez L C, Duquet A, Robin P, Rudkin B, Harel-Bellan A, Trouche D. Phosphorylation by p44 MAP kinase/ERK1 stimulates CBP histone acetyl transferase activity in vitro. Biochem Biophys Res Commun. 1999;262:157–162. doi: 10.1006/bbrc.1999.1132. [DOI] [PubMed] [Google Scholar]
- 3.Ait S A, Ramirez S, Barre F X, Dkhissi F, Magnaghi-Jaulin L, Girault J A, Robin P, Knibiehler M, Pritchard L L, Ducommun B, Trouche D, Harel-Bellan A. Histone acetyltransferase activity of CBP is controlled by cycle-dependent kinases and oncoprotein E1A. Nature. 1998;396:184–186. doi: 10.1038/24190. [DOI] [PubMed] [Google Scholar]
- 4.Allgood V E, Zhang Y, O'Malley B W, Weigel N L. Analysis of chicken progesterone receptor function and phosphorylation using an adenovirus-mediated procedure for high-efficiency DNA transfer. Biochemistry. 1997;36:224–232. doi: 10.1021/bi961125c. [DOI] [PubMed] [Google Scholar]
- 5.Alnemri E S, Maksymowych A B, Robertson N M, Litwack G. Characterization and purification of a functional rat glucocorticoid receptor overexpressed in a baculovirus system. J Biol Chem. 1991;266:3925–3936. [PubMed] [Google Scholar]
- 6.Ambrosini A, Tininini S, Barassi A, Racagni G, Sturani E, Zippel R. cAMP cascade leads to Ras activation in cortical neurons. Brain Res Mol Brain Res. 2000;75:54–60. doi: 10.1016/s0169-328x(99)00294-6. [DOI] [PubMed] [Google Scholar]
- 7.Arnold S F, Obourn J D, Jaffe H, Notides A C. Phosphorylation of the human estrogen receptor on tyrosine 537 in vivo and by src family tyrosine kinases in vitro. Mol Endocrinol. 1995;9:24–33. doi: 10.1210/mend.9.1.7539106. [DOI] [PubMed] [Google Scholar]
- 8.Aronica S M, Katzenellenbogen B S. Stimulation of estrogen receptor-mediated transcription and alteration in the phosphorylation state of the rat uterine estrogen receptor by estrogen, cyclic adenosine monophosphate, and insulin-like growth factor-I. Mol Endocrinol. 1993;7:743–752. doi: 10.1210/mend.7.6.7689695. [DOI] [PubMed] [Google Scholar]
- 9.Bai W, Rowan B G, Allgood V E, O'Malley B W, Weigel N L. Differential phosphorylation of chicken progesterone receptor in hormone-dependent and ligand-independent activation. J Biol Chem. 1997;272:10457–10463. doi: 10.1074/jbc.272.16.10457. [DOI] [PubMed] [Google Scholar]
- 10.Bai W, Tullos S, Weigel N L. Phosphorylation of Ser530 facilitates hormone-dependent transcriptional activation of the chicken progesterone receptor. Mol Endocrinol. 1994;8:1465–1473. doi: 10.1210/mend.8.11.7877616. [DOI] [PubMed] [Google Scholar]
- 11.Bai W, Weigel N L. Phosphorylation of Ser211 in the chicken progesterone receptor modulates its transcriptional activity. J Biol Chem. 1996;271:12801–12806. doi: 10.1074/jbc.271.22.12801. [DOI] [PubMed] [Google Scholar]
- 12.Barge R M, Falkenburg J H, Willemze R, Maassen J A. 8-Bromo-cAMP induces a proliferative response in an IL-3 dependent leukemic cell line and activates Erk 1,2 via a Shc-independent pathway. Biochim Biophys Acta. 1997;1355:141–146. doi: 10.1016/s0167-4889(96)00130-9. [DOI] [PubMed] [Google Scholar]
- 13.Beck C A, Weigel N L, Edwards D P. Effects of hormone and cellular modulators of protein phosphorylation on transcriptional activity, DNA binding, and phosphorylation of human progesterone receptors. Mol Endocrinol. 1992;6:607–620. doi: 10.1210/mend.6.4.1316549. [DOI] [PubMed] [Google Scholar]
- 14.Boonyaratanakornkit V, Melvin V, Prendergast P, Altmann M, Ronfani L, Bianchi M E, Taraseviciene L, Nordeen S K, Allegretto E A, Edwards D P. High-mobility group chromatin proteins 1 and 2 functionally interact with steroid hormone receptors to enhance their DNA binding in vitro and transcriptional activity in mammalian cells. Mol Cell Biol. 1998;18:4471–4487. doi: 10.1128/mcb.18.8.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bunone G, Briand P A, Miksicek R J, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J. 1996;15:2174–2183. [PMC free article] [PubMed] [Google Scholar]
- 16.Castoria G, Migliaccio A, Green S, Di Domenico M, Chambon P, Auricchio F. Properties of a purified estradiol-dependent calf uterus tyrosine kinase. Biochemistry. 1993;32:1740–1750. doi: 10.1021/bi00058a007. [DOI] [PubMed] [Google Scholar]
- 17.Chawla S, Hardingham G E, Quinn D R, Bading H. CBP: a signal-regulated transcriptional coactivator controlled by nuclear calcium and CaM kinase IV. Science. 1998;281:1505–1509. doi: 10.1126/science.281.5382.1505. [DOI] [PubMed] [Google Scholar]
- 18.Cook S J, McCormick F. Inhibition by cAMP of Ras-dependent activation of Raf. Science. 1993;262:1069–1072. doi: 10.1126/science.7694367. [DOI] [PubMed] [Google Scholar]
- 19.Culig Z, Hobisch A, Hittmair A, Cronauer M V, Radmayr C, Zhang J, Bartsch G, Klocker H. Synergistic activation of androgen receptor by androgen and luteinizing hormone-releasing hormone in prostatic carcinoma cells. Prostate. 1997;32:106–114. doi: 10.1002/(sici)1097-0045(19970701)32:2<106::aid-pros5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 20.Dayani N, McNaught R W, Shenolikar S, Smith R G. Receptor interconversion model of hormone action. 2. Requirement of both kinase and phosphatase activities for conferring estrogen binding activity to the estrogen receptor. Biochemistry. 1990;29:2691–2698. doi: 10.1021/bi00463a011. [DOI] [PubMed] [Google Scholar]
- 21.Denner L A, Schrader W T, O'Malley B W, Weigel N L. Hormonal regulation and identification of chicken progesterone receptor phosphorylation sites. J Biol Chem. 1990;265:16548–16555. [PubMed] [Google Scholar]
- 22.Denner L A, Weigel N L, Maxwell B L, Schrader W T, O'Malley B W. Regulation of progesterone receptor-mediated transcription by phosphorylation. Science. 1990;250:1740–1743. doi: 10.1126/science.2176746. [DOI] [PubMed] [Google Scholar]
- 23.Denton R R, Koszewski N J, Notides A C. Estrogen receptor phosphorylation. Hormonal dependence and consequence on specific DNA binding. J Biol Chem. 1992;267:7263–7268. [PubMed] [Google Scholar]
- 24.Font de Mora J, Brown M. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol Cell Biol. 2000;20:5041–5047. doi: 10.1128/mcb.20.14.5041-5047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Z, Chen T, Weber M J, Linden J. A2B adenosine and P2Y2 receptors stimulate mitogen-activated protein kinase in human embryonic kidney-293 cells. Cross-talk between cyclic AMP and protein kinase C pathways. J Biol Chem. 1999;274:5972–5980. doi: 10.1074/jbc.274.9.5972. [DOI] [PubMed] [Google Scholar]
- 26.Han X B, Conn P M. The role of protein kinases A and C pathways in the regulation of mitogen-activated protein kinase activation in response to gonadotropin-releasing hormone receptor activation. Endocrinology. 1999;140:2241–2251. doi: 10.1210/endo.140.5.6707. [DOI] [PubMed] [Google Scholar]
- 27.Heery D M, Kalkhoven E, Hoare S, Parker M G. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387:733–736. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- 28.Hong S H, Wong C W, Privalsky M L. Signaling by tyrosine kinases negatively regulates the interaction between transcription factors and SMRT (Silencing Mediator of Retinoic acid and Thyroid hormone receptor) corepressor. Mol Endocrinol. 1998;12:1161–1171. doi: 10.1210/mend.12.8.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu J M, Bodwell J E, Munck A. Control by basal phosphorylation of cell cycle-dependent, hormone-induced glucocorticoid receptor hyperphosphorylation. Mol Endocrinol. 1997;11:305–311. doi: 10.1210/mend.11.3.9896. [DOI] [PubMed] [Google Scholar]
- 30.Ikonen T, Palvimo J J, Kallio P J, Reinikainen P, Janne O A. Stimulation of androgen-regulated transactivation by modulators of protein phosphorylation. Endocrinology. 1994;135:1359–1366. doi: 10.1210/endo.135.4.7925097. [DOI] [PubMed] [Google Scholar]
- 31.Janknecht R, Nordheim A. MAP kinase-dependent transcriptional coactivation by Elk-1 and its cofactor CBP. Biochem Biophys Res Commun. 1996;228:831–837. doi: 10.1006/bbrc.1996.1740. [DOI] [PubMed] [Google Scholar]
- 32.Janknecht R, Nordheim A. Regulation of the c-fos promoter by the ternary complex factor Sap-1a and its coactivator CBP. Oncogene. 1996;12:1961–1969. [PubMed] [Google Scholar]
- 33.Jenster G, Spencer T E, Burcin M M, Tsai S Y, Tsai M J, O'Malley B W. Steroid receptor induction of gene transcription—a two step model. Proc Natl Acad Sci USA. 1997;94:7879–7884. doi: 10.1073/pnas.94.15.7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 35.Kazmi S M, Visconti V, Plante R K, Ishaque A, Lau C. Differential regulation of human progesterone receptor A and B form-mediated trans-activation by phosphorylation. Endocrinology. 1993;133:1230–1238. doi: 10.1210/endo.133.3.8365365. [DOI] [PubMed] [Google Scholar]
- 36.Krstic M D, Rogatsky I, Yamamoto K R, Garabedian M J. Mitogen-activated and cyclin-dependent protein kinases selectively and differentially modulate transcriptional enhancement by the glucocorticoid receptor. Mol Cell Biol. 1997;17:3947–3954. doi: 10.1128/mcb.17.7.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lange-Carter C A, Johnson G L. Ras-dependent growth factor regulation of MEK kinase in PC12 cells. Science. 1994;265:1458–1461. doi: 10.1126/science.8073291. [DOI] [PubMed] [Google Scholar]
- 38.Lavinsky R M, Jepsen K, Heinzel T, Torchia J, Mullen T M, Schiff R, Delrio A L, Ricote M, Ngo S, Gemsch J, Hilsenbeck S G, Osborne C K, Glass C K, Rosenfeld M G, Rose D W. Diverse signaling pathways modulate nuclear receptor recruitment of N-CoR and SMRT complexes. Proc Natl Acad Sci USA. 1998;95:2920–2925. doi: 10.1073/pnas.95.6.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y Z, Chrivia J C, Latchman D S. Nerve growth factor up-regulates the transcriptional activity of CBP through activation of the p42/p44(MAPK) cascade. J Biol Chem. 1998;273:32400–32407. doi: 10.1074/jbc.273.49.32400. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y Z, Thomas N S, Latchman D S. CBP associates with the p42/p44 MAPK enzymes and is phosphorylated following NGF treatment. Neuroreport. 1999;10:1239–1243. doi: 10.1097/00001756-199904260-00016. [DOI] [PubMed] [Google Scholar]
- 41.Massaad C, Houard N, Lombes M, Barouki R. Modulation of human mineralocorticoid receptor function by protein kinase A. Mol Endocrinol. 1999;13:57–65. doi: 10.1210/mend.13.1.0226. [DOI] [PubMed] [Google Scholar]
- 42.McKenna N J, Lanz R B, O'Malley B W. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 43.McKenna N J, Nawaz Z, Tsai S Y, Tsai M J, O'Malley B W. Distinct steady-state nuclear receptor coregulator complexes exist in vivo. Proc Natl Acad Sci USA. 1998;95:11697–11702. doi: 10.1073/pnas.95.20.11697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nazareth L V, Weigel N L. Activation of the human androgen receptor through a protein kinase A signaling pathway. J Biol Chem. 1996;271:19900–19907. doi: 10.1074/jbc.271.33.19900. [DOI] [PubMed] [Google Scholar]
- 45.Nordeen S K, Moyer M L, Bona B J. The coupling of multiple signal transduction pathways with steroid response mechanisms. Endocrinology. 1994;134:1723–1732. doi: 10.1210/endo.134.4.8137736. [DOI] [PubMed] [Google Scholar]
- 46.Onate S A, Boonyaratanakornkit V, Spencer T E, Tsai S Y, Tsai M J, Edwards D P, O'Malley B W. The steroid receptor coactivator-1 contains multiple receptor interacting and activation domains that cooperatively enhance the activation function 1 (AF1) and AF2 domains of steroid receptors. J Biol Chem. 1998;273:12101–12108. doi: 10.1074/jbc.273.20.12101. [DOI] [PubMed] [Google Scholar]
- 47.Onate S A, Tsai S Y, Tsai M J, O'Malley B W. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 48.Parameswaran N, Disa J, Spielman W S, Brooks D P, Nambi P, Aiyar N. Activation of multiple mitogen-activated protein kinases by recombinant calcitonin gene-related peptide receptor. Eur J Pharmacol. 2000;389:125–130. doi: 10.1016/s0014-2999(99)00874-2. [DOI] [PubMed] [Google Scholar]
- 49.Poletti A, Weigel N L. Identification of a hormone-dependent phosphorylation site adjacent to the DNA-binding domain of the chicken progesterone receptor. Mol Endocrinol. 1993;7:241–246. doi: 10.1210/mend.7.2.8469237. [DOI] [PubMed] [Google Scholar]
- 50.Rowan B G, Weigel N L, O'Malley B W. Phosphorylation of steroid receptor coactivator-1. Identification of the phosphorylation sites and phosphorylation through the mitogen-activated protein kinase pathway. J Biol Chem. 2000;275:4475–4483. doi: 10.1074/jbc.275.6.4475. [DOI] [PubMed] [Google Scholar]
- 51.Smith C L, Onate S A, Tsai M J, O'Malley B W. CREB binding protein acts synergistically with steroid receptor coactivator-1 to enhance steroid receptor-dependent transcription. Proc Natl Acad Sci USA. 1996;93:8884–8888. doi: 10.1073/pnas.93.17.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spencer T E, Jenster G, Burcin M M, Allis C D, Zhou J X, Mizzen C A, McKenna N J, Onate S A, Tsai S Y, Tsai M J, O'Malley B W. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389:194–198. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- 53.Torchia J, Rose D W, Inostroza J, Kamei Y, Westin S, Glass C K, Rosenfeld M G. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature. 1997;387:677–684. doi: 10.1038/42652. [DOI] [PubMed] [Google Scholar]
- 54.Wagner B L, Norris J D, Knotts T A, Weigel N L, McDonnell D P. The nuclear corepressors NCoR and SMRT are key regulators of both ligand- and 8-bromo-cyclic AMP-dependent transcriptional activity of the human progesterone receptor. Mol Cell Biol. 1998;18:1369–1378. doi: 10.1128/mcb.18.3.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Webster J, Prager D, Melmed S. Insulin-like growth factor-1 activation of extracellular signal-related kinase-1 and -2 in growth hormone-secreting cells. Mol Endocrinol. 1994;8:539–544. doi: 10.1210/mend.8.5.8058064. [DOI] [PubMed] [Google Scholar]
- 56.Webster J C, Jewell C M, Bodwell J E, Munck A, Sar M, Cidlowski J A. Mouse glucocorticoid receptor phosphorylation status influences multiple functions of the receptor protein. J Biol Chem. 1997;272:9287–9293. doi: 10.1074/jbc.272.14.9287. [DOI] [PubMed] [Google Scholar]
- 57.Weigel N L. Steroid hormone receptors and their regulation by phosphorylation. Biochem J. 1996;319:657–667. doi: 10.1042/bj3190657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.West M H P, Wu R S, Bonner W M. Polyacrylamide gel electrophoresis of small peptides. Electrophoresis. 1984;5:133–138. [Google Scholar]
- 59.Xu L, Lavinsky R M, Dasen J S, Flynn S E, McInerney E M, Mullen T M, Heinzel T, Szeto D, Korzus E, Kurokawa R, Aggarwal A K, Rose D W, Glass C K, Rosenfeld M G. Signal-specific co-activator domain requirements for Pit-1 activation. Nature. 1998;395:301–306. doi: 10.1038/26270. [DOI] [PubMed] [Google Scholar]
- 60.Yeh S, Lin H K, Kang H Y, Thin T H, Lin M F, Chang C. From HER2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proc Natl Acad Sci USA. 1999;96:5458–5463. doi: 10.1073/pnas.96.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zanger K, Cohen L E, Hashimoto K, Radovick S, Wondisford F E. A novel mechanism for cyclic adenosine 3′,5′-monophosphate regulation of gene expression by CREB-binding protein. Mol Endocrinol. 1999;13:268–275. doi: 10.1210/mend.13.2.0245. [DOI] [PubMed] [Google Scholar]
- 62.Zhou Z X, Kemppainen J A, Wilson E M. Identification of three proline-directed phosphorylation sites in the human androgen receptor. Mol Endocrinol. 1995;9:605–615. doi: 10.1210/mend.9.5.7565807. [DOI] [PubMed] [Google Scholar]